Abstract
Introduction:
The prognostic impact of tumor infiltrating lymphocytes (TILs) on outcomes and treatment efficacy for patients with melanoma in the contemporary era remains poorly characterized.
Methods:
Consecutive patients who underwent wide excision and sentinel lymph node (SLN) biopsy for cutaneous melanoma ≥1.0mm thick at a single institution were identified (2006–2019). Patients were stratified based on primary tumor TIL status as brisk (bTILs), non-brisk (nbTILs) or absent (aTILs). Associations between patient factors and outcomes were analyzed using multivariable analysis.
Results:
Of the 1,017 patients evaluated, 846 (83.2%) had primary TILs: nbTILs (n=759 [89.7%]), bTILs (n=87 [10.3%]). On multivariable analysis, compared to those without TILs, patients with any-type TILs had higher rates of regression (OR 1.86, p=0.016), lower rates of acral lentiginous histology (OR 0.22, p<0.001), and lower rates of SLN positivity (OR 0.64, p=0.042). On multivariable analysis, no association was found between disease-specific survival and bTILs (HR 1.04, p=0.927) or nbTILs (HR 0.89, p=0.683). bTILs were associated with a recurrence-free survival (RFS) advantage (bTILs [HR 0.46, p=0.047]; nbTILs [HR 0.71, p=0.088]), with 5-year RFS being 84.0%, 71.8%, and 68.4% for bTILs, nbTILs, and aTILs, respectively (p=0.044). For the 114 immune checkpoint blockade (ICB) naïve patients who developed a recurrence treated with ICB therapy, no association was seen between progression-free survival and bTILs (HR 0.64, p=0.482) or nbTILs (0.58, p=0.176).
Conclusions:
The prognostic significance of primary TILs in the contemporary melanoma era appears complex. Further studies characterizing TIL phenotype and their association with regional metastasis and responsiveness to ICB therapy are warranted.
INTRODUCTION
Although not a component of the current 8th edition of the American Joint Committee on Cancer (AJCC) staging system,1 tumor infiltrating lymphocytes (TILs) were included within the original Clark model predicting survival for patients with early-stage melanoma,2 and are commonly classified as absent, non-brisk, or brisk based on their density and distribution within a primary melanoma lesion. Since that time, their prognostic significance has been variably reported in the literature. Many studies have evaluated the association between TIL status and other primary melanoma features finding that denser TIL infiltrates are commonly associated with younger patients and thinner melanomas without ulceration and with low mitotic rates.3–6 While TIL status has been shown in many studies to be independently associated with sentinel lymph node (SLN) positivity, the association between TILs and survival (when SLN status is accounted for) remains controversial and less well defined.2,5,7–11 Furthermore, data suggest that certain TIL cell populations may be associated with the effectiveness of modern systemic melanoma therapies for patients who develop metastatic disease, specifically immune checkpoint blockade (ICB) agents such as ipilimumab, nivolumab or pembrolizumab, although evidence regarding whether a correlation exists between TIL distribution – particularly in the primary lesion – and responsiveness to these therapies is not well studied.12–15
Utilizing data from a high-volume cutaneous malignancy referral center, the current retrospective observational study sought to determine the prognostic significance of TILs on melanoma recurrence, survival, and treatment response to ICB therapy among a contemporary cohort of patients with clinically localized primary cutaneous melanoma ≥1.0mm thick who underwent SLN staging.
METHODS
Data source and patient selection
Between January 1, 2006, and December 31, 2019, patients with clinically localized (AJCC 8th edition clinical stage I and II)1 primary cutaneous melanoma ≥1.0mm thick who underwent wide excision and SLN biopsy at a single institution were identified. A comprehensive retrospective review of medical records was conducted, and patients with the presence of any-type TILs within their primary tumor were compared to those with absent TILs (aTILs) within their primary tumor. Patients were further stratified based on primary tumor TIL status as either brisk (bTILs), non-brisk (nbTILs) or aTILs. bTILs were defined as TILs that were present along the entire base of the tumor or throughout it, and nbTILs were defined as TILs that did not meet these criteria. aTILs were defined as tumors without any TILs present. Focally brisk TILs were defined as numerous TILs not meeting criteria for the brisk category, and patients with focally brisk TILs were included within the nbTILs group.2,16 All patients’ primary tumor specimens were reviewed at the time of diagnosis by specialized dermatopathologists at the participating institution. Patients with unknown TIL status, who did not undergo wide excision and SLN staging, or with tumors <1.0mm were excluded. The final cohort consisted of 1,017 patients. This study was reviewed and approved by the University of Pennsylvania Institutional Review Board.
Variables and outcomes
Patient variables evaluated included age, sex, race (White, Black, or Asian American, Pacific Islander [AAPI]), performance of completion lymph node dissection (CLND), and treatment with adjuvant systemic therapy. Tumor variables evaluated included location (trunk, extremity, or head/neck), histologic subtype (superficial spreading, nodular, lentigo maligna, acral lentiginous, or not otherwise specified [NOS]), Breslow depth, ulceration, mitotic count per mm2, regression, vertical growth phase, lymphovascular invasion (LVI), neurotropism, microsatellitosis, and SLN status. Sites of disease recurrence included local, in-transit, regional lymph node basin, and distant sites. Variables with unknown results were recorded as unknown. The primary study outcome was 5-year disease-specific survival (DSS). Secondary study outcomes included identification of factors associated with the presence of TILs, 5-year recurrence-free survival (RFS), and 3-year progression-free survival (PFS) among the subgroup of patients who were immune checkpoint blockade (ICB) naïve that developed a melanoma recurrence which was treated with ICB therapy. ICB therapy included the agents ipilimumab, nivolumab or pembrolizumab.
Statistical methods
Univariable analyses were performed using the Pearson’s chi-squared or Fisher’s exact test, as appropriate, for categorical variables, and using the Wilcoxon rank-sum test for continuous variables. Multivariable logistic regression analysis was performed incorporating the factors with a p-value of ≤0.10 univariable analysis to identify clinicopathologic factors associated with the presence of TILs, and the goodness of fit of the model was assessed using the Hosmer-Lemeshow test.17 Cox proportional hazards regression analysis was performed to identify associations between patients and outcomes, and the proportionality of each model was confirmed using the Schoenfeld residuals test.18 Five-year recurrence and survival outcomes were evaluated using the Kaplan-Meier method and compared using the log-rank test. Recurrence and survival analyses were calculated from the date of definitive treatment of the melanoma. RFS was defined as the time-period between the date of melanoma excision and the date of diagnosis of first recurrence at any site. PFS was defined as the time-period between the date of initiation of ICB therapy for treatment of a melanoma recurrence and the date of diagnosis of a subsequent recurrence or disease progression at any site. Follow-up time was calculated as the time-period between the date of melanoma excision and the date of last follow-up or death using the reverse Kaplan-Meier method.19 All tests were two sided. P-values <0.05 were considered statistically significant. All statistical analyses were performed using Stata Version 17.20
RESULTS
Patient demographics and factors associated with TILs
Of the 1,017 patients evaluated, 846 (83.2%) had TILs within their primary melanoma; of these, the majority (n=759 [89.7%]) were nbTILs, and 87 (10.3%) were bTILs. The median age of the cohort was 61 (interquartile range [IQR] 52–71) years, 627 patients (61.7%) were male, and 900 patients (88.5%) were white. Twenty-five patients (2.5%) received adjuvant treatment with ICB therapy. Baseline patient characteristics and univariable analysis comparing patients with and without primary tumor TILs are shown in Table 1.
Table 1.
Patient cohort and univariable analysis.
| No TILs N=171 (16.8%) | TILs N=846 (83.2%) | nbTILs N=759 (74.6%) | bTILs N=87 (8.6%) | ||
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | p-value* | |
| Age, years, median (IQR) | 60 (49–68) | 62 (53–71) | 62 (53–71) | 60 (49–72) | 0.04 |
| Sex | |||||
| Male | 106 (62.0) | 521 (61.6) | 462 (60.9) | 59 (67.8) | 0.92 |
| Female | 65 (38.0) | 325 (38.4) | 297 (39.1) | 28 (32.2) | |
| Race | |||||
| White | 149 (87.1) | 751 (88.8) | 674 (88.8) | 77 (88.5) | 0.15 |
| Black | 6 (3.5) | 12 (1.4) | 11 (1.5) | 1 (1.2) | |
| AAPI | 9 (5.3) | 32 (3.8) | 28 (3.7) | 4 (4.6) | |
| Not reported | 7 (4.1) | 51 (6.0) | 46 (6.1) | 5 (5.8) | |
| Location | |||||
| Head/neck | 24 (14.0) | 103 (12.2) | 95 (12.5) | 8 (9.2) | 0.01 |
| Extremity | 103 (60.2) | 419 (49.5) | 385 (50.7) | 34 (39.1) | |
| Trunk | 44 (25.7) | 324 (38.3) | 279 (36.8) | 45 (51.7) | |
| Histology | |||||
| Superficial spreading | 66 (38.6) | 418 (49.4) | 370 (48.8) | 48 (55.2) | <0.01 |
| Nodular | 46 (26.9) | 267 (31.6) | 246 (32.4) | 21 (24.1) | |
| Lentigo maligna | 8 (4.7) | 43 (5.1) | 40 (5.3) | 3 (3.5) | |
| Acral lentiginous | 19 (11.1) | 23 (2.7) | 19 (2.5) | 4 (4.6) | |
| NOS | 32 (18.7) | 95 (11.2) | 84 (11.1) | 11 (12.6) | |
| Thickness, mm, median (IQR) | 2.0 (1.2–3.4) | 1.9 (1.3–3.0) | 1.9 (1.3–3.2) | 1.5 (1.1–2.2) | 0.32 |
| Ulceration | |||||
| Absent | 126 (73.7) | 576 (68.1) | 514 (67.7) | 62 (71.3) | 0.34 |
| Present | 43 (25.2) | 255 (30.1) | 232 (30.6) | 23 (26.4) | |
| Unknown | 2 (1.2) | 15 (1.8) | 13 (1.7) | 2 (2.3) | |
| Mitotic count/mm2, median (IQR) | 3.4 (1.3–7.7) | 4.0 (1.2–8.0) | 4.0 (1.5–8.0) | 3.3 (1.0–6.0) | 0.74 |
| Regression | |||||
| Absent | 136 (79.5) | 613 (72.5) | 564 (74.3) | 49 (56.3) | <0.01 |
| Present | 21 (12.3) | 200 (23.6) | 164 (21.6) | 36 (41.4) | |
| Unknown | 14 (8.2) | 33 (3.9) | 31 (4.1) | 2 (2.3) | |
| Vertical growth phase | |||||
| Absent | 40 (23.4) | 213 (25.2) | 190 (25.0) | 23 (26.4) | 0.62 |
| Present | 127 (74.3) | 621 (73.4) | 560 (73.8) | 61 (70.1) | |
| Unknown | 4 (2.3) | 12 (1.4) | 9 (1.2) | 3 (3.5) | |
| Lymphovascular invasion | |||||
| Absent | 132 (77.2) | 739 (87.4) | 656 (86.4) | 83 (95.4) | <0.01 |
| Present | 26 (15.2) | 80 (9.5) | 76 (10.0) | 4 (4.6) | |
| Unknown | 13 (7.6) | 27 (3.2) | 27 (3.6) | 0 (0.0) | |
| Neurotropism | |||||
| Absent | 99 (57.9) | 552 (65.3) | 493 (65.0) | 59 (67.8) | 0.19 |
| Present | 10 (5.9) | 39 (4.6) | 37 (4.9) | 2 (2.3) | |
| Unknown | 62 (36.3) | 255 (30.1) | 229 (30.2) | 26 (29.9) | |
| Microsatellitosis | |||||
| Absent | 145 (84.8) | 746 (88.2) | 664 (87.5) | 82 (94.3) | 0.07 |
| Present | 11 (6.4) | 62 (7.3) | 60 (7.9) | 2 (2.3) | |
| Unknown | 15 (8.8) | 38 (4.5) | 35 (4.6) | 3 (3.5) | |
| Sentinel lymph node status | |||||
| Negative | 129 (75.4) | 710 (83.9) | 630 (83.0) | 80 (92.0) | <0.01 |
| Positive | 42 (24.6) | 136 (16.1) | 129 (17.0) | 7 (8.1) | |
| Completion lymph node dissection | |||||
| No | 160 (93.6) | 789 (93.3) | 704 (92.8) | 85 (97.7) | 0.88 |
| Yes | 11 (6.4) | 57 (6.7) | 55 (7.3) | 2 (2.3) | |
| Adjuvant therapy | |||||
| No | 160 (93.6) | 810 (95.7) | 725 (95.5) | 85 (97.7) | 0.22 |
| Yes | 11 (6.4) | 36 (4.3) | 34 (4.5) | 2 (2.3) | |
| Interferon | 7 (63.6) | 11 (30.6) | 10 (29.4) | 1 (50.0) | |
| Temozolomide | 2 (18.2) | 2 (5.6) | 1 (2.9) | 1 (50.0) | |
| Ipilimumab | 0 (0.0) | 2 (5.6) | 2 (5.9) | 0 (0) | |
| Nivolumab | 1 (9.1) | 19 (52.8) | 19 (55.9) | 0 (0) | |
| Pembrolizumab | 1 (9.1) | 2 (5.6) | 2 (5.9) | 0 (0) | |
P-values corresponding to univariable analysis performed between absent and present TILs.
TILs: tumor infiltrating lymphocytes; nbTILs: non-brisk TILs; bTILs; brisk TILs; IQR: interquartile range; AAPI: Asian American, Pacific Islander; NOS: not otherwise specified; mm: millimeter
On multivariable logistic regression analysis, compared to those with aTILs, patients with any-type TILs had significantly higher rates of regression of their primary tumor (odds ratio [OR] 1.86, 95% confidence interval [CI] 1.12–3.09, p=0.016), significantly lower rates of acral lentiginous histology (OR 0.22, 95% CI 0.11–0.45, p<0.001), and significantly lower rates of SLN positivity (OR 0.64, 95% CI 0.42–0.98, p=0.042) (Table 2). Specifically, the rate of regression for patients with bTILs, nbTILs and aTILs was 41.4% (n=36 of 87), 21.6% (n=164 of 759), and 12.3% (n=21 of 171), respectively (p<0.001); the rate of acral lentiginous histology for patients with bTILs, nbTILs and aTILs was 4.6% (n=4 of 87), 2.5% (n=19 of 759), and 11.1% (n=19 of 171), respectively (p<0.001); and the SLN positivity rate for patients with bTILs, nbTILs and aTILs was 8.1% (n=7 of 87), 17.0% (129 of 759), and 24.6% (42 of 171), respectively (p=0.003). Increasing age correlated with a higher presence of TILs (OR 1.01, 95% CI 0.99–1.02), but this association did not reach statistical significance (p=0.066). Although LVI status was not significantly associated with the presence or absence of any-type TILs on multivariable analysis, the proportion of patients with LVI was highest among those with aTILs (4.6% [n=4 of 87] bTILs vs. 10.0% [n=76 of 759] nbTILs vs. 15.2% [n=26 of 171] aTILs, p=0.001).
Table 2.
Logistic regression analysis of factors associated with presence of any-type TILs.
| Odds Ratio (95% Confidence Interval) | p-value | |
|---|---|---|
| Age | 1.01 (0.99–1.02) | 0.066 |
| Location | ||
| Head/neck | 1.00 (Reference) | |
| Extremity | 1.00 (0.58–1.70) | 0.988 |
| Trunk | 1.60 (0.89–2.86) | 0.144 |
| Histology | ||
| Superficial spreading | 1.00 (Reference) | |
| Nodular | 1.02 (0.70–1.55) | 0.926 |
| Lentigo maligna | 0.72 (0.31–1.67) | 0.441 |
| Acral lentiginous | 0.22 (0.11–0.45) | <0.001 |
| NOS | 0.52 (0.31–0.86) | 0.010 |
| Regression | ||
| Absent | 1.00 (Reference) | |
| Present | 1.86 (1.12–3.09) | 0.016 |
| Unknown | 0.78 (0.35–1.74) | 0.548 |
| Lymphovascular invasion | ||
| Absent | 1.50 (0.89–2.54) | 0.130 |
| Present | 1.00 (Reference) | |
| Unknown | 0.83 (0.32–2.16) | 0.703 |
| Microsatellitosis | ||
| Absent | 1.00 (Reference) | |
| Present | 1.43 (0.71–2.90) | 0.320 |
| Unknown | 0.75 (0.35–1.61) | 0.465 |
| Sentinel lymph node status | ||
| Negative | 1.00 (Reference) | |
| Positive | 0.64 (0.42–0.98) | 0.042 |
NOS: not otherwise specified
Recurrence and survival outcomes
Median follow-up time for patients alive at last follow-up was 63 (IQR 28–105) months and did not significantly differ based on TIL status: 61 (IQR 26–115) months for those with aTILs, 62 (IQR 29–105) months for those with nbTILs, and 68 (IQR 26–104) months for those with bTILs (log-rank p=0.804).
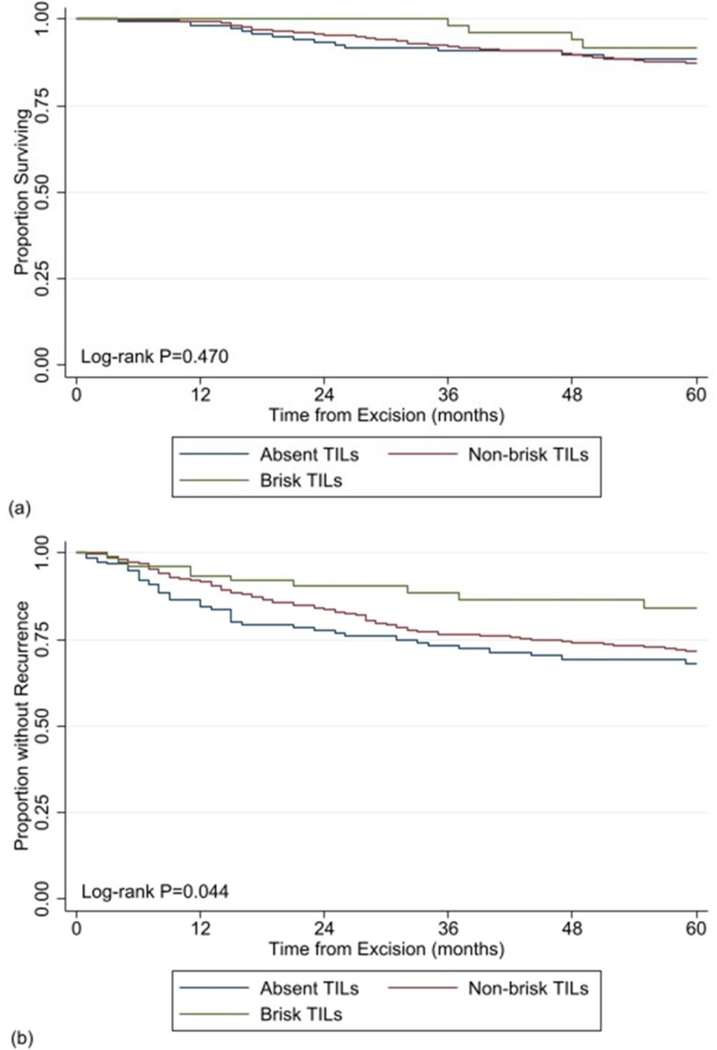
On multivariable survival analysis, as compared to those with aTILs, there was no association between the presence of any-type TILs and DSS (hazards ratio [HR] 0.90, 95% CI 0.51–1.58, p=0.710), and 5-year DSS did not differ based on the presence or absence of any-type TILs (87.7% TILs vs. 88.6% aTILs, log-rank p=0.560). Likewise, as compared to patients with aTILs, no association with DSS was observed upon stratification by TILs type (bTILs [HR 1.04, 95% CI 0.44–2.49, p=0.927)]; nbTILs [HR 0.89, 95% CI 0.50–1.57, p=0.683]), and 5-year DSS rates were similar between these groups (91.8% bTILs vs. 87.3% nbTILs vs. 88.6% aTILs, log-rank p=0.470) (Table 3, Figure 1a).
Table 3.
Cox proportional hazards regression analysis of factors associated with disease-specific survival.
| Hazards Ratio (95% Confidence Interval) | p-value | |
|---|---|---|
| Tumor infiltrating lymphocytes | ||
| Absent | 1.00 (Reference) | |
| Non-brisk | 0.89 (0.50–1.60) | 0.683 |
| Brisk | 1.04 (0.44–2.49) | 0.927 |
| Age | 1.01 (0.99–1.03) | 0.131 |
| Sex | ||
| Male | 1.46 (0.92–2.31) | 0.108 |
| Female | 1.00 (Reference) | |
| Race | ||
| White | 1.00 (Reference) | |
| Black | 1.00 (0.28–3.55) | 0.999 |
| AAPI | 1.06 (0.40–2.79) | 0.901 |
| Unknown | 1.64 (0.71–3.79) | 0.250 |
| Location | ||
| Head/neck | 1.87 (1.03–3.40) | 0.039 |
| Extremity | 1.00 (Reference) | |
| Trunk | 1.44 (0.89–2.33) | 0.133 |
| Histology | ||
| Superficial spreading | 1.00 (Reference) | |
| Nodular | 1.41 (0.87–2.30) | 0.167 |
| Lentigo maligna | 1.12 (0.37–3.37) | 0.835 |
| Acral lentiginous | 2.19 (0.87–5.51) | 0.095 |
| NOS | 1.09 (0.53–2.23) | 0.813 |
| Thickness, mm | 1.07 (0.99–1.15) | 0.072 |
| Vertical growth phase | ||
| Absent | 1.00 (Reference) | |
| Present | 0.97 (0.41–2.28) | 0.945 |
| Unknown | Colinear | |
| Regression | ||
| Absent | 1.00 (Reference) | |
| Present | 1.39 (0.86–2.25) | 0.176 |
| Unknown | 1.19 (0.36–3.98) | 0.776 |
| Ulceration | ||
| Absent | 1.00 (Reference) | |
| Present | 1.46 (0.93–2.29) | 0.097 |
| Unknown | Colinear | |
| Lymphovascular invasion | ||
| Absent | 1.00 (Reference) | |
| Present | 2.14 (1.29–3.56) | 0.003 |
| Unknown | 0.37 (0.04–3.06) | 0.354 |
| Neurotropism | ||
| Absent | 1.00 (Reference) | |
| Present | 1.30 (0.59–2.89) | 0.515 |
| Unknown | 0.78 (0.34–1.80) | 0.560 |
| Microsatellitosis | ||
| Absent | 1.00 (Reference) | |
| Present | 2.11 (1.20–3.71) | 0.010 |
| Unknown | 0.91 (0.30–2.73) | 0.871 |
| Mitotic count/mm2 | 1.00 (0.97–1.02) | 0.794 |
| Sentinel lymph node status | ||
| Negative | 1.00 (Reference) | |
| Positive | 2.93 (1.60–5.36) | <0.001 |
| Completion lymph node dissection | ||
| No | 1.00 (Reference) | |
| Yes | 1.18 (0.60–2.33) | 0.628 |
| Adjuvant therapy | ||
| No | 1.00 (Reference) | |
| Yes | 0.51 (0.23–1.17) | 0.112 |
AAPI: Asian American, Pacific Islander; NOS: not otherwise specified; mm: millimeter
Figure 1:
Comparison of Kaplan-Meier estimates of a) 5-year disease-specific survival curves and b) 5-year recurrence free survival curves between patients with brisk versus non-brisk versus absent tumor infiltration lymphocytes within their primary melanoma specimen.
Two hundred and thirty-five patients (23.1%) developed a melanoma recurrence. On multivariable analysis, as compared to those with aTILs, the presence of any-type TILs correlated, but was not significantly associated with, improved RFS (HR 0.69, 95% CI 0.46–1.03, p=0.067), and 5-year RFS was not significantly different based on the presence or absence of any-type TILs (72.9% TILs vs. 68.2% aTILs, log-rank p=0.103). However, upon stratification by TILs type, as compared to aTILs, bTILs specifically were associated with improved RFS (bTILs [HR 0.46, 95% CI 0.21–0.98, p=0.047]; nbTILs [HR 0.71, 95% CI 0.48–1.05, p=0.088]), and 5-year RFS was significantly greater for patients with bTILs (84.0% bTILs vs. 71.8% nbTILs vs. 68.4% aTILs, log-rank p=0.044) (Table 4, Figure 1b). Median time to recurrence for patients with bTILs, nbTILs and aTILs was 21 (IQR 11–70) months, 18 (IQR 9–31) months, and 12 (IQR 6–26) months, respectively. The majority of recurrences, regardless of TIL type, occurred within the first 3 years following melanoma excision (n=8 [53.3%] bTILs vs. n=140 [80.4%] nbTILs vs. n=37 [80.4%] aTILs). The majority of recurrences overall were at distant sites (n=122 [51.9%]), and distant recurrences remained the most common site of recurrence when stratified by TIL status (n=10 of 15 [66.7%] bTILs vs. n=94 of 174 [54.0%] vs. n=18 of 46 [39.1%]).
Table 4.
Cox proportional hazards regression analysis of factors associated with recurrence-free survival.
| Hazards Ratio (95% Confidence Interval) | p-value | |
|---|---|---|
| Tumor infiltrating lymphocytes | ||
| Absent | 1.00 (Reference) | |
| Non-brisk | 0.71 (0.48–1.05) | 0.088 |
| Brisk | 0.46 (0.21–0.98) | 0.047 |
| Age | 1.01 (0.99–1.02) | 0.272 |
| Sex | ||
| Male | 1.12 (0.81–1.54) | 0.504 |
| Female | 1.00 (Reference) | |
| Race | ||
| White | 1.00 (Reference) | |
| Black | 1.02 (0.43–2.42) | 0.965 |
| AAPI | 0.55 (0.25–1.23) | 0.145 |
| Unknown | 1.22 (0.66–2.28) | 0.529 |
| Location | ||
| Head/neck | 2.10 (1.37–3.22) | 0.001 |
| Extremity | 1.00 (Reference) | |
| Trunk | 1.30 (0.93–1.84) | 0.130 |
| Histology | ||
| Superficial spreading | 1.00 (Reference) | |
| Nodular | 1.08 (0.77–1.52) | 0.654 |
| Lentigo maligna | 2.26 (1.22–4.18) | 0.010 |
| Acral lentiginous | 2.23 (1.19–4.19) | 0.013 |
| NOS | 0.92 (0.54–1.58) | 0.766 |
| Thickness, mm | 1.04 (0.98–1.10) | 0.207 |
| Vertical growth phase | ||
| Absent | 1.00 (Reference) | |
| Present | 1.40 (0.67–2.92) | 0.364 |
| Unknown | 5.01 (1.33–18.92) | 0.017 |
| Regression | ||
| Absent | 1.00 (Reference) | |
| Present | 1.02 (0.71–1.46) | 0.909 |
| Unknown | 0.59 (0.23–1.51) | 0.273 |
| Ulceration | ||
| Absent | 1.00 (Reference) | |
| Present | 1.76 (0.26–2.46) | 0.001 |
| Unknown | 2.24 (0.66–7.55) | 0.194 |
| Lymphovascular invasion | ||
| Absent | 1.00 (Reference) | |
| Present | 1.64 (1.12–2.40) | 0.010 |
| Unknown | 1.24 (0.42–3.64) | 0.691 |
| Neurotropism | ||
| Absent | 1.00 (Reference) | |
| Present | 0.88 (0.47–1.66) | 0.695 |
| Unknown | 0.58 (0.29–1.15) | 0.121 |
| Microsatellitosis | ||
| Absent | 1.00 (Reference) | |
| Present | 1.76 (1.11–2.78) | 0.016 |
| Unknown | 0.41 (0.16–1.06) | 0.065 |
| Mitotic count/mm2 | 1.02 (0.99–1.03) | 0.116 |
| Sentinel lymph node status | ||
| Negative | 1.00 (Reference) | |
| Positive | 4.11 (2.74–6.18) | <0.001 |
| Completion lymph node dissection | ||
| No | 1.00 (Reference) | |
| Yes | 0.78 (0.48–1.27) | 0.322 |
| Adjuvant therapy | ||
| No | 1.00 (Reference) | |
| Yes | 0.56 (0.32–0.98) | 0.042 |
AAPI: Asian American, Pacific Islander; NOS: not otherwise specified; mm: millimeter
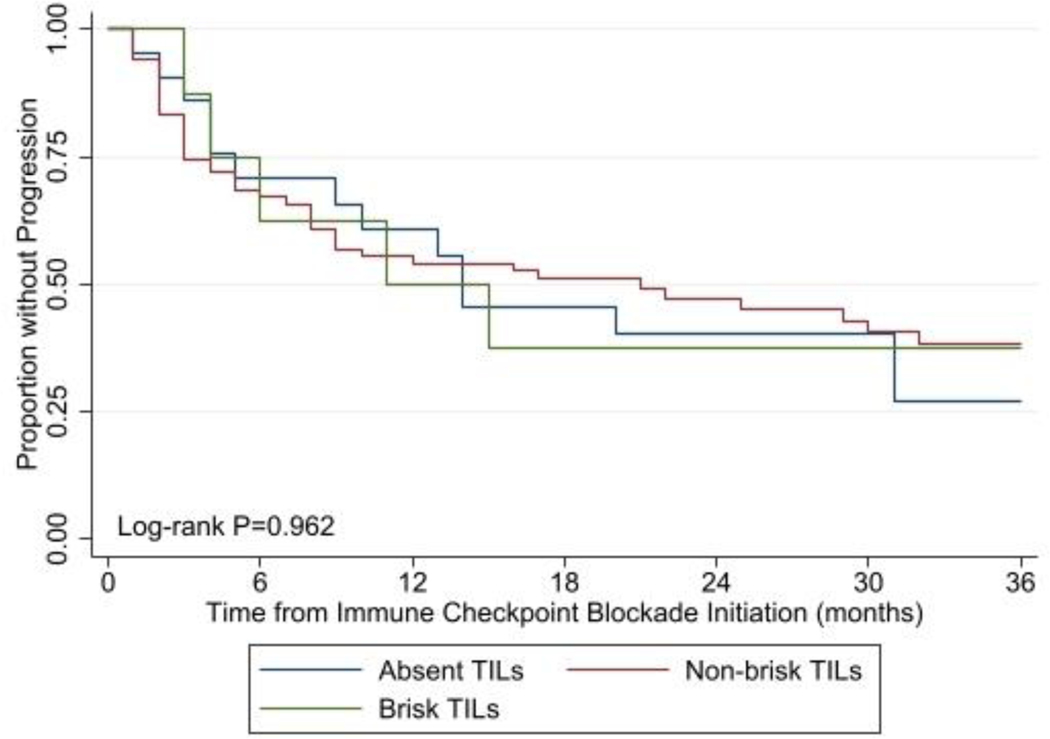
One-hundred and fourteen patients who did not receive adjuvant ICB treatment developed a melanoma recurrence and were subsequently treated with ICB agents. Of these patients, 67 patients (58.8%) developed disease progression following ICB therapy initiation, and the median time to progression was 11 months, 21 months, and 14 months for patients with bTILs, nbTILs, and aTILs, respectively. Among this cohort, as compared to those with aTILs, there was no association between the presence of any-type TILs and PFS (HR 0.59, 95% CI 0.27–1.27, p=0.178), and 3-year PFS was not significantly different based on the presence or absence of any-type TILs (38.6% TILs vs. 27.0% aTILs, log-rank p=0.782). Likewise, as compared to patients with aTILs, no association with PFS was observed upon stratification by TILs type (bTILs [HR 0.64, 95% CI 0.18–2.23, p=0.482]; nbTILs [0.58, 95% CI 0.27–1.27, p=0.176), and 3-year PFS was similar between groups (bTILs 37.5% vs. nbTILs 38.3% vs. aTILs 27.0%, log-rank p=0.962) (Figure 2).
Figure 2:
Kaplan-Meier estimate of 3-year progression free survival curves among patients who developed a melanoma recurrence and were treated with immune checkpoint blockade therapy comparing patients with brisk versus non-brisk versus absent tumor infiltration lymphocytes within their primary melanoma specimen.
DISCUSSION
While studies have reliably demonstrated an association between TILs and SLN status, their association with survival outcomes has been inconsistent. Moreover, limited data exist describing the association between TILs and progression for patients treated with ICB therapy for melanoma recurrence.7–9,11 Among this large contemporary cohort of patients with primary cutaneous melanoma ≥1.0mm thick who underwent wide excision and SLN biopsy, patients presenting with TILs were significantly more likely to have regression of their primary tumor, and significantly less likely to have a positive SLN biopsy and acral lentiginous histology. Furthermore, an inverse relationship was seen between the briskness of TIL populations and SLN status, with patients with bTILs being significantly less likely to have a positive sentinel node than those with nbTILs. Our results are consistent with those of Azimi et al. who also demonstrated that increasing TIL grade (based on the density and distribution of TILs within the primary tumor) was inversely associated with SLN positivity.5 Additionally, patients with bTILs in the present study were also found to have the lowest rates of LVI, which may be a contributing factor to the association seen between TILs presence and SLN status.21 TILs are an integral component of the immune tumor microenvironment and a representation of the host immune response against the tumor.22 Although the types of lymphocytes making up TIL populations within a melanoma vary and the interplay between the tumor and these TILs is complex, the general principle remains that denser and/or brisker TIL infiltrates represent a more robust host immune response against the tumor.23 Melanoma is known to be a highly immunogenic tumor, and a stronger anti-tumor reaction in patients with bTILs is likely one of the main mechanisms responsible for the finding that these patients were most likely to have pathologically localized disease at diagnosis.24 The lower incidence of TILs observed for patients with acral lentiginous melanoma in the present study may be explained by the lower tumor mutational burden seen with this specific histologic subtype as compared to other cutaneous melanomas.25 Finally, regression is thought to occur secondary to host immune-mediated responses, as well, and has been shown to be associated with TIL status in prior studies, with some data suggesting that the combination of regression and TIL presence may have particularly favorable prognostic implications, although the prognostic significance of regression in general remains controversial, with some studies demonstrating a positive influence between regression and outcomes while others show a negative influence.26–29
In the present study, patients with bTIL infiltrates were found to have significantly improved RFS, but not DSS, compared to those without bTILs. The association between TILs and melanoma survival has been variably reported. Some early studies demonstrated that bTILs were associated with improved survival compared to nbTILs or aTILs, while others found no correlation between TIL status and survival, or an association only under certain clinical contexts.2,4,10,30,31 In contrast, more recent studies since the advent of SLN biopsy have not consistently found TILs to be independently associated with survival when SLN status is accounted for.3,8,11,31,32 While Burton et al. and Mandalà et al. found TILs to be favorable for survival on univariable analysis, this association did not remain evident when SLN status was incorporated into a multivariable model.3,8 Similarly, in the current study, SLN status was strongly associated with DSS, which may have mitigated any direct association between TILs and DSS. However, bTILs remained associated with improved RFS even when accounting for SLN status, suggesting that TILs may be important for predicting recurrence, a finding that is in concordance with data reported from more contemporary analyses.31 Additionally, some of the contradictory findings reported by others may be the result of variability in the histopathologic characterization of TIL infiltrates, or due to inconsistent or incomplete SLN staging among all patients in the studies.31 In the current study, all included patients underwent SLN staging, and histopathologic assessment of TIL status was performed by specialized dermatopathologists.
The present study did not find an association between TIL status and PFS among ICB therapy naïve patients treated for a melanoma recurrence with ICB agents. Although limited data exist evaluating the prognostic impact of TIL density on ICB treatment response in melanoma, an association between higher TIL density and improved treatment response among patients with metastatic disease who are treated with ICB agents has been observed with other cancer types.33–36 As previously mentioned, the specific subtypes of lymphocytes comprising a TIL infiltrate, and the interaction between these cells and the overall immune tumor microenvironment, is highly complex.12 The immune infiltrate of most cancers is composed predominantly of macrophages and differing types of T cells, including CD4+ memory cells, CD4+ regulatory cells, and CD8+ effector cells. However, the proportions of each of these cell populations present within a given tumor varies between different cancer types and even between different patients with the same malignant histology.12 Melanoma is known to have a high tumor mutational burden, which is thought to be one reason why ICB therapy is so efficacious in these patients, but data have also shown that tumor mutational burden may correlate with TIL density, as well.13 Additionally, recent reports have demonstrated that higher densities of CD8+ T cells within pretreatment primary melanoma specimens is strongly associated with a positive treatment response to anti-programmed cell death-1 monotherapy.34,35 The lack of association identified between TILs and PFS in the current study could reflect heterogeneity in the specific composition of lymphocyte infiltrations in the study cohort, or potentially an evolution of tumor biology and immune response between primary tumor and subsequent recurrences. Future studies should further evaluate the interplay between specific types of TILs and their densities within primary melanoma specimens, and whether this can be utilized to detect novel prognostic biomarkers for treatment response to ICB therapy in patients who develop melanoma recurrence.
It should be noted that in the current study, tumor thickness and ulceration status correlated, but were not significantly associated, with DSS. This finding is in contrast to consistent data showing a strong association between these two factors and DSS, independent of SLN status.37,38 It is likely that these associations were not found to be statistically significant due to the restriction of the study cohort to patients with tumors ≥1.0mm thick. However, given the accepted association between TILs and thinner lesions, and the overall good prognosis for patients with early melanomas regardless of other tumor features, the inclusion of only patients with melanomas ≥1.0mm thick was employed to try to determine the prognostic significance of TILs among a group of patients with higher risk of recurrence, and moreover among a group of patients who would routinely be recommended for SLN biopsy (which is one of the most important prognostic factors in clinically localized melanoma).
In addition to those already discussed, there are several limitations which should be considered when interpreting the results of this study. No data was included on the specific immunophenotyping (i.e. molecular characterization) of the lymphocyte infiltrates, which could have important prognostic implications; nonetheless, this does not detract from the associations identified between the histopathologic assessment and clinical outcomes. Additionally, TIL response could be variably reported among histopathologists thus biasing the results; however, this would likely not have had a significant impact on the current study in which a small group of specialized dermatopathologists reviewed the specimens. As a retrospective analysis, it is possible that potential confounding variables were not captured and could have impacted the results in undefined ways. Additionally, inclusion of patients with unknown results for variables analyzed could have also confounded the findings of this study. Finally, some patients may have developed recurrent disease but did not return to the participating institution for further care, thus resulting in misclassification of these patients as having no melanoma recurrence; however, it should be noted that prolonged follow-up was observed among all patient cohorts within the study.
CONCLUSION
Among this large contemporary cohort of patients undergoing wide excision and SLN biopsy for intermediate to thick primary cutaneous melanoma, primary TILs were associated with SLN status and improved RFS, but not DSS. Additionally, in the setting of disease recurrence, primary TILs were not found to confer predictive value for ICB therapy responsiveness. The prognostic influence of primary TILs in the contemporary melanoma era appears complex, and further studies characterizing TIL phenotype and their association with regional metastasis and responsiveness to ICB therapy are warranted.
SYNOPSIS.
The prognostic role of tumor infiltrating lymphocytes in melanoma has been variably reported. We evaluated the significance of primary tumor infiltrating lymphocytes on recurrence, survival, and treatment response in a contemporary cohort of melanoma patients who underwent sentinel node biopsy.
Acknowledgments
All authors have reviewed and approved the submitted manuscript, and agree to be accountable for all aspects of the work submitted.
Footnotes
IRB approval status: Reviewed and approved by the University of Pennsylvania (protocol #: 850510).
Conflicts of interest: None declared. This study has been accepted as a Virtual Poster Grand Rounds presentation at the 2022 Society of Surgical Oncology virtual meeting week, scheduled for February 28-March 3, 2022.
REFERENCES
- 1.Amin M, Edge S, Greene F, et al. AJCC Cancer Staging Manual (8th edition). Springer International Publishing: American Joint Commission on Cancer; 2017. [Google Scholar]
- 2.Clark WH, Elder DE, Guerry D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. Dec 20 1989;81(24):1893–904. doi: 10.1093/jnci/81.24.1893 [DOI] [PubMed] [Google Scholar]
- 3.Mandalà M, Imberti GL, Piazzalunga D, et al. Clinical and histopathological risk factors to predict sentinel lymph node positivity, disease-free and overall survival in clinical stages I-II AJCC skin melanoma: outcome analysis from a single-institution prospectively collected database. Eur J Cancer. Sep 2009;45(14):2537–45. doi: 10.1016/j.ejca.2009.05.034 [DOI] [PubMed] [Google Scholar]
- 4.Clemente CG, Mihm MC, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. Apr 1996;77(7):1303–10. doi: [DOI] [PubMed] [Google Scholar]
- 5.Azimi F, Scolyer RA, Rumcheva P, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. Jul 20 2012;30(21):2678–83. doi: 10.1200/JCO.2011.37.8539 [DOI] [PubMed] [Google Scholar]
- 6.Thomas NE, Busam KJ, From L, et al. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. J Clin Oncol. Nov 20 2013;31(33):4252–9. doi: 10.1200/JCO.2013.51.3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kruper LL, Spitz FR, Czerniecki BJ, et al. Predicting sentinel node status in AJCC stage I/II primary cutaneous melanoma. Cancer. Nov 15 2006;107(10):2436–45. doi: 10.1002/cncr.22295 [DOI] [PubMed] [Google Scholar]
- 8.Burton AL, Roach BA, Mays MP, et al. Prognostic significance of tumor infiltrating lymphocytes in melanoma. Am Surg. Feb 2011;77(2):188–92. [PubMed] [Google Scholar]
- 9.Taylor RC, Patel A, Panageas KS, Busam KJ, Brady MS. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol. Mar 01 2007;25(7):869–75. doi: 10.1200/JCO.2006.08.9755 [DOI] [PubMed] [Google Scholar]
- 10.Barnhill RL, Fine JA, Roush GC, Berwick M. Predicting five-year outcome for patients with cutaneous melanoma in a population-based study. Cancer. Aug 01 1996;78(3):427–32. doi: [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Lian JW, Chin YH, et al. Assessing the Prognostic Significance of Tumor-Infiltrating Lymphocytes in Patients With Melanoma Using Pathologic Features Identified by Natural Language Processing. JAMA Netw Open. Sep 01 2021;4(9):e2126337. doi: 10.1001/jamanetworkopen.2021.26337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linette GP, Carreno BM. Tumor-Infiltrating Lymphocytes in the Checkpoint Inhibitor Era. Curr Hematol Malig Rep. 08 2019;14(4):286–291. doi: 10.1007/s11899-019-00523-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. Mar 02 2016;8(328):328rv4. doi: 10.1126/scitranslmed.aad7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li F, Li C, Cai X, et al. The association between CD8+ tumor-infiltrating lymphocytes and the clinical outcome of cancer immunotherapy: A systematic review and meta-analysis. EClinicalMedicine. Nov 2021;41:101134. doi: 10.1016/j.eclinm.2021.101134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein S, Mauch C, Brinker K, et al. Tumor infiltrating lymphocyte clusters are associated with response to immune checkpoint inhibition in BRAF V600. Sci Rep. 01 19 2021;11(1):1834. doi: 10.1038/s41598-021-81330-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mihm MC, Mulé JJ. Reflections on the Histopathology of Tumor-Infiltrating Lymphocytes in Melanoma and the Host Immune Response. Cancer Immunol Res. Aug 2015;3(8):827–35. doi: 10.1158/2326-6066.CIR-15-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemeshow S, Hosmer DW. A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. Jan 1982;115(1):92–106. doi: 10.1093/oxfordjournals.aje.a113284 [DOI] [PubMed] [Google Scholar]
- 18.Lee M, Han J. Statistical methods and models in the analysis of time to event data. Ann Transl Med. Feb 2020;8(4):73. doi: 10.21037/atm.2019.12.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. Aug 1996;17(4):343–6. doi: 10.1016/0197-2456(96)00075-x [DOI] [PubMed] [Google Scholar]
- 20.StataCorp. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC; 2021. [Google Scholar]
- 21.Egger ME, Gilbert JE, Burton AL, et al. Lymphovascular invasion as a prognostic factor in melanoma. Am Surg. Aug 2011;77(8):992–7. [PubMed] [Google Scholar]
- 22.Vose BM, Moore M. Human tumor-infiltrating lymphocytes: a marker of host response. Semin Hematol. Jan 1985;22(1):27–40. [PubMed] [Google Scholar]
- 23.Weiss SA, Han SW, Lui K, et al. Immunologic heterogeneity of tumor-infiltrating lymphocyte composition in primary melanoma. Hum Pathol. 11 2016;57:116–125. doi: 10.1016/j.humpath.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Passarelli A, Mannavola F, Stucci LS, Tucci M, Silvestris F. Immune system and melanoma biology: a balance between immunosurveillance and immune escape. Oncotarget. Dec 01 2017;8(62):106132–106142. doi: 10.18632/oncotarget.22190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tod BM, Schneider JW, Bowcock AM, Visser WI, Kotze MJ. The tumor genetics of acral melanoma: What should a dermatologist know? JAAD International. 2020/12/01/ 2020;1(2):135–147. doi: 10.1016/j.jdin.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aivazian K, Ahmed T, El Sharouni MA, et al. Histological regression in melanoma: impact on sentinel lymph node status and survival. Mod Pathol. Nov 2021;34(11):1999–2008. doi: 10.1038/s41379-021-00870-2 [DOI] [PubMed] [Google Scholar]
- 27.Aung PP, Nagarajan P, Prieto VG. Regression in primary cutaneous melanoma: etiopathogenesis and clinical significance. Lab Invest. Feb 27 2017;doi: 10.1038/labinvest.2017.8 [DOI] [PubMed] [Google Scholar]
- 28.Ribero S, Gualano MR, Osella-Abate S, et al. Association of Histologic Regression in Primary Melanoma With Sentinel Lymph Node Status: A Systematic Review and Meta-analysis. JAMA Dermatol. Dec 01 2015;151(12):1301–1307. doi: 10.1001/jamadermatol.2015.2235 [DOI] [PubMed] [Google Scholar]
- 29.Gualano MR, Osella-Abate S, Scaioli G, et al. Prognostic role of histological regression in primary cutaneous melanoma: a systematic review and meta-analysis. Br J Dermatol. 02 2018;178(2):357–362. doi: 10.1111/bjd.15552 [DOI] [PubMed] [Google Scholar]
- 30.Sinnamon AJ, Sharon CE, Song Y, et al. The prognostic significance of tumor-infiltrating lymphocytes for primary melanoma varies by sex. J Am Acad Dermatol. Aug 2018;79(2):245–251. doi: 10.1016/j.jaad.2018.02.066 [DOI] [PubMed] [Google Scholar]
- 31.Fu Q, Chen N, Ge C, et al. Prognostic value of tumor-infiltrating lymphocytes in melanoma: a systematic review and meta-analysis. Oncoimmunology. 2019 2019;8(7):1593806. doi: 10.1080/2162402X.2019.1593806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fortes C, Mastroeni S, Mannooranparampil TJ, et al. Tumor-infiltrating lymphocytes predict cutaneous melanoma survival. Melanoma Res. Aug 2015;25(4):306–11. doi: 10.1097/CMR.0000000000000164 [DOI] [PubMed] [Google Scholar]
- 33.Gataa I, Mezquita L, Rossoni C, et al. Tumour-infiltrating lymphocyte density is associated with favourable outcome in patients with advanced non-small cell lung cancer treated with immunotherapy. Eur J Cancer. 03 2021;145:221–229. doi: 10.1016/j.ejca.2020.10.017 [DOI] [PubMed] [Google Scholar]
- 34.Wong PF, Wei W, Smithy JW, et al. Multiplex Quantitative Analysis of Tumor-Infiltrating Lymphocytes and Immunotherapy Outcome in Metastatic Melanoma. Clin Cancer Res. 04 15 2019;25(8):2442–2449. doi: 10.1158/1078-0432.CCR-18-2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. Nov 27 2014;515(7528):568–71. doi: 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schalper KA, Kaftan E, Herbst RS. Predictive Biomarkers for PD-1 Axis Therapies: The Hidden Treasure or a Call for Research. Clin Cancer Res. 05 01 2016;22(9):2102–4. doi: 10.1158/1078-0432.CCR-16-0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaikh WR, Dusza SW, Weinstock MA, Oliveria SA, Geller AC, Halpern AC. Melanoma Thickness and Survival Trends in the United States, 1989 to 2009. J Natl Cancer Inst. Jan 2016;108(1)doi: 10.1093/jnci/djv294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen W, Sakamoto N, Yang L. Melanoma-specific mortality and competing mortality in patients with non-metastatic malignant melanoma: a population-based analysis. BMC Cancer. 07 07 2016;16:413. doi: 10.1186/s12885-016-2438-3 [DOI] [PMC free article] [PubMed] [Google Scholar]