Abstract
Background
Patients with pulmonary atresia with intact ventricular septum and critical pulmonary stenosis (PAIVS/CPS) have wide variation in right ventricle (RV) size, systolic function, and diastolic function at birth. Establishment of antegrade pulmonary blood flow creates the potential for RV dilation from chronic pulmonary insufficiency. Future surgical decisions are based on RV size and function, largely supported by longitudinal studies of patients with Tetralogy of Fallot (TOF). Given potential differences in RV physiology and lack of similar data in PAIVS/CPS, the objective of this study was to determine differences in RV size, systolic function, and diastolic function between patients with PAIVS/CPS versus TOF.
Methods
We retrospectively collected cardiovascular magnetic resonance (CMR) data in 27 patients with PAIVS/CPS (ages 13.3 ± 8.8 years) and 78 with TOF (11.4 ± 5.4 years). RV volumes, ejection fraction (EF), regurgitant fraction, end-diastolic forward flow across the pulmonary valve, and right atrial cross-sectional area were calculated.
Results
There was no difference between the groups in RV end-diastolic volume (RVEDVi), RVEF, or pulmonary regurgitation. RVEF tended to decrease in TOF when RVEDVi exceeded 164 ml/m2. In PAIVS/CPS, RVEDVi less frequently reached 164 ml/m2 and was not associated with RVEF. There was worse RV diastolic dysfunction in PAIVS/CPS, with 1.5 times larger right atrial area and two times higher pulmonary end-diastolic forward flow (p < 0.0001).
Conclusions
Patients with PAIVS/CPS have similar RV size, systolic function, and pulmonary regurgitation as TOF. However, impaired RV diastolic function may limit extremes of RV dilatation and impact long-term management of PAIVS/CPS.
Keywords: Pulmonary atresia, Pulmonary stenosis, Tetralogy of Fallot, Cardiovascular magnetic resonance, Echocardiography
Introduction
Pulmonary atresia with intact ventricular septum (PAIVS) and critical pulmonary stenosis (CPS) are two forms of congenital heart disease with severe right ventricular (RV) outflow tract obstruction. In utero, limited egress of blood from the RV leads to RV hypertension, RV hypertrophy, tricuspid valve dysplasia or hypoplasia, and often RV hypoplasia [1]. Postnatally, surgical and/or transcatheter interventions including modified Blalock–Taussig shunt (mBTS), right ventricular outflow tract (RVOT) patch, balloon pulmonary valvuloplasty, or combinations of these procedures are required to replace the ductus arteriosus as a source of stable pulmonary blood flow [2–6]. The goals of RVOT reconstruction are to re-establish antegrade pulmonary blood flow and to encourage RV dilation. This dilation is thought to result from both antegrade flow and residual volume-loading lesions, namely tricuspid regurgitation (TR) and pulmonary insufficiency (PI) [7–9]. The ideal outcome is for the RV is to be able to maintain normal cardiac output at rest and increase pulmonary blood flow during periods of high demand, such as during exercise [5, 10].
In contrast to patients with PAIVS/CPS, those with Tetralogy of Fallot (TOF) have a large ventricular septal defect that provides an alternate route for blood to exit the RV and prevents the same degree of RV hypoplasia and hypertrophy despite potentially as severe RVOT obstruction. After surgical repair of TOF, patients commonly develop progressive RV dilation (i.e., RV dilation out of proportion to somatic growth) and eventually both diastolic and systolic dysfunction due to chronic PI [11]. The adverse long-term effects of RV volume overload have been well studied in TOF, and cardiovascular magnetic resonance (CMR) imaging has been an essential tool for guiding the timing of pulmonary valve replacement in this population [11, 12]. Despite significant fundamental differences in RV morphology between PAIVS/CPS and TOF, to our knowledge a similar systematic evaluation of RV dilation has not been performed in patients with repaired PAIVS/CPS. In particular, the effect of chronic volume-loading on RV size and function over time is not well understood. Since PAIVS/CPS patients often have much more severe RV hypoplasia and hypertrophy than those with TOF, chronic PI could theoretically lead to less pathologic dilation but more significant diastolic dysfunction in the former. Knowledge of how the RV adapts differently in PAIVS/CPS in turn may better inform clinicians of risk stratification and guide timing of pulmonary valve replacement.
To study how chronic volume-loading affects RV size and function, we compared CMR data in a cohort of PAIVS/CPS patients with a similarly aged cohort of TOF patients. Our primary aim was to evaluate factors associated with serial dilation of the RV and their associations with functional changes. We hypothesized that the hypoplastic and hypertrophic RV generally observed in PAIVS/CPS newborns would have less potential to pathologically dilate compared to that of TOF patients.
Materials and Methods
Study Design and Patient Population
We performed a retrospective cross-sectional analysis of patients with PAIVS/CPS or TOF who underwent CMR for assessment of RV volumes and function. Due to the rare nature of PAIVS/CPS, the study utilized data from two centers, Children’s Hospital Los Angeles and C.S. Mott Children’s Hospital at the University of Michigan. CMR databases were reviewed for studies performed between January 1999 and January 2014. All patients were referred for evaluation of RV size, RV function, and PI after initial TOF repair, 1.5 ventricle palliation of PAIVS/CPS, or 2 ventricle repair of PAIVS/CPS. In addition to the cross-sectional analysis of the entire cohort, a longitudinal analysis of RV dilation was performed for the subset of patients who had multiple CMR studies.
We defined CPS as severe pulmonary stenosis requiring a patent ductus arteriosus to maintain adequate pulmonary blood flow. Among PAIVS patients, we only included those who had 2 ventricle repair or 1.5 ventricle palliation (i.e., we excluded patients who had Fontan palliation), as only these patients had the potential for RV enlargement from chronic PI. We also excluded individual studies if CMR was performed after pulmonary valve replacement, since this procedure eliminated volume-loading from PI.
We excluded TOF patients or studies if (1) CMR occurred prior to complete repair of TOF; (2) CMR was performed after pulmonary valve replacement; (3) CMR data sets were incomplete; or (4) other significant congenital heart defects (e.g., atrioventricular canal, Ebstein’s anomaly, total anomalous venous drainage, residual atrial septal defect, multiple aorticopulmonary collaterals, and absent pulmonary valve) were present. Since the initial TOF cohort had a slightly older median age due to four patients in their fifth and sixth decade, we also excluded these patients to better match age ranges between the two cohorts.
While surgical approaches for PAIVS/CPS vary by center and surgeon, general approaches in our institutions are as follows. For CPS patients, either transcatheter balloon pulmonary valvuloplasty or surgical RVOT patch is performed. For PAIVS patients, those with adequate tricuspid valve size (Z-score > − 2.5) and no concern for RV dependent coronary circulation may get either a transcatheter pulmonary valve perforation and balloon valvuloplasty or surgical RVOT patch with or without mBTS. After initial palliation, PAIVS patients may undergo single ventricle palliation with bidirectional Glenn and Fontan procedures, 1.5 ventricle palliation with bidirectional Glenn shunt and atrial septal defect closure, or full 2 ventricle repair.
All PAIVS/CPS and TOF patients had PI after their initial repair or palliative surgeries. None of the patients had a significant left to right shunt at the atrial level that could affect right atrial (RA) or RV volumes. None of the patients had significant mitral or aortic regurgitation that could affect LV stroke volume.
CMR Measurements
We performed all CMR examinations on either a 1.5 T General Electric scanner (General Electric Medical Systems, Milwaukee, WI) or a 1.5 T Philips scanner (Achieva, Philips Healthcare, The Netherlands). Our standard imaging protocol included axial and short-axis cine steady-state free precession (SSFP) sequences for chamber size and ventricular function quantification, and phase contrast velocity sequences in the main and branch pulmonary arteries for flow quantification. We utilized vectorcardiographic gating on all scans to control for cardiac motion. Patients who were able to adequately hold their breath were asked to do so for short-axis SSFP sequences to minimize artifacts from respiratory motion. Otherwise we used three signal averages to compensate for respiratory motion. We performed all flow sequences free-breathing with multiple signal averages.
We performed volume and flow quantification with the GE or Philips cardiac analysis packages at Children’s Hospital Los Angeles, and with the commercial software, Qmass (Medis, Leiden, The Netherlands), at the University of Michigan. We calculated RV and LV end-diastolic volume (EDV) and end-systolic volume (ESV) using Simpson’s method of stacking discs and used body surface area (BSA) to index the volumes (EDVi and ESVi). Height and/or weight were not available for eight CMR studies. Those with both height and weight missing had a BSA documented on the report (two studies), while those missing only height had BSA calculated from weight only (seven studies). Of the seven studies with weight only, six were TOF and one was PAIVS; three of the TOF patients had weight < 5% and one had weight > 95%, while the PAIVS patient had a normal weight. We calculated RV and LV ejection fraction (EF) using the formula: stroke volume (SV)/EDV.
In the subset of patients that had serial CMR studies, we longitudinally assessed RV dilation. We defined accelerated dilation as an average of > 30 ml/m2/year or > 40% change per year. This threshold was defined based on visual inspection of the longitudinal data, but the same threshold of 30 ml/m2 has been used in prior longitudinal studies of TOF as an indicator of “disease progression” [13].
We measured PI using phase contrast, velocity-encoded imaging in the main pulmonary artery just above the RVOT. We calculated PI regurgitant fraction using the formula: main pulmonary artery regurgitant flow/main pulmonary artery forward flow. Using RV and LV ventricular SV measurements, we also calculated regurgitant fraction using the formula: (RVSV – LVSV)/RVSV. This latter formula is a measurement of total RV regurgitant volume, accounting for both TR and PI. Four patients (15%) with PAIVS/CPS had negative total RV regurgitant fractions due to a hypoplastic right ventricle (RV) and right to left shunting across an atrial septal defect.
As a surrogate for RV diastolic dysfunction, we measured RA cross-sectional area just superior to the entrance of the coronary sinus on an axial cine sequence at end-systole. We indexed this measurement to BSA as we did with RV volumes to account for differences in patient sizes. We used this in lieu of RA volume due to inadequate coverage to perform accurate RA volume measurement on all studies. A similar estimate of RA volume, using Simpson’s method on a single RA slice from an apical four-chamber view on echocardiogram, has been demonstrated to correlate with both RV systolic and diastolic dysfunction [14]. Additionally, RA volume has been shown to be larger in patients with repaired TOF compared to healthy controls even in the absence of significant TR, suggesting that RA enlargement is instead due to decreased RV compliance [15]. Again, none of the patients had a significant left to right shunt at the atrial level that could affect RA size.
For a second marker of RV diastolic dysfunction, we also measured end-diastolic forward flow (EDFF) across the pulmonary valve. The presence of this abnormal antegrade pulmonary blood flow during RA contraction is thought to be due to “restrictive RV physiology” [16]. EDFF was assessed in two ways. The first measure was a binary variable given as the presence or absence of EDFF on phase contrast images. The second measure, EDFF peak flux, was a continuous variable quantifying the maximum EDFF flow rate. In conjunction with RA cross-sectional area/BSA, the latter measurement was used to better assess the severity of diastolic dysfunction rather just the presence or absence of diastolic dysfunction.
All ventricular volume, ventricular function, and PI measurements were extracted from the clinical CMR report associated with each study. RA cross-sectional area and EDFF were retrospectively measured on all studies by one investigator (JAD).
Echocardiographic Measurements
Given the wide range of RV sizes in PAIVS/CPS infants, we evaluated initial tricuspid valve size on newborn echocardiograms in this cohort. One investigator confirmed the tricuspid valve size in the apical four-chamber view on all studies (JAD). We determined the tricuspid valve Z-score using an online calculator (http://zscore.chboston.org) that accounts for child’s BSA and the valve annulus diameter as measured using the American Society of Echocardiography measurement standards [17]. This does not account for an effective orifice size.
A single investigator (JAD) graded TR severity qualitatively by echocardiography on the study performed closest to the time of the CMR (within 12 months).
Statistical Analysis
Variables are expressed as mean and standard deviation for normally distributed data or median and interquartile range for non-parametric data. Since a subset of patients had multiple CMR studies, we used statistical weighting to account for multiple data points. For patients with multiple studies, we weighted measurements by the reciprocal of the number of studies (i.e., measurements for a patient with three studies are weighted by a factor of 1/3 when calculating summary statistics). We performed comparisons between groups with Student’s t test for normally distributed data, Wilcoxon rank sum test for non-normally distributed data, or Z-statistic for comparison of Z-scores to the general population. We used linear regression to explore the associations between RVEDVi and age, RVEDVi and EF, as well as RVEDVi and regurgitant fraction. For these analyses, we used ANCOVA to compare linear regression slopes between groups. To determine the RVEDVi threshold past which EF decreases in the TOF group, we used receiver operating characteristic (ROC) curve analysis. We performed all statistics using JMP Pro version 12.1 (SAS Institute Inc. Cary, NC, USA).
Results
Patient Demographics and Initial Tricuspid Valve Size
We identified a total of 193 CMR studies (44 PAIVS/CPS, 149 TOF) in our database. Of these, we excluded 38 due to other significant congenital heart defects, 8 for prior pulmonary valve replacement, 4 for incomplete CMR data, and 1 for timing prior to TOF repair. This yielded 27 PAIVS/CPS patients with 42 CMR studies, and 78 TOF patients with 100 CMR studies that met both inclusion and exclusion criteria. As shown in Table 1, the two groups had a similar distribution of age and sex. PAIVS/CPS patients had earlier initial interventions, which are detailed in Table 2. Initial tricuspid valve Z-scores for PAIVS/CPS patients were generally within normal limits with only 7% having a tricuspid valve Z-score < − 2. Among the PAIVS/CPS cohort, there was no difference in tricuspid valve Z-score nor age at CMR between those who underwent 1.5 ventricle palliation versus 2 ventricle repair.
Table 1.
Patient demographics
| PAIVS/CPS (n = 27) | TOF (n = 78) | p value | |
|---|---|---|---|
| Diagnosis (n) | N/A | ||
| PAIVS | 21 (78%) | N/A | |
| CPS | 6 (22%) | ||
| Age at RVOT intervention (months) | 0.1 (0.1–1) | 7 (1–74) | 0.0001 |
| Type of RVOT intervention (n) | |||
| Surgical | 20 (74%) | 78 (100%) | |
| Catheter | 7 (26%) | ||
| 1.5 ventricle palliation (n) | 6 (22%) | N/A | N/A |
| Newborn tricuspid valve annulus diameter (mm) | 11.4 ± 2.5 | N/A | N/A |
| Newborn tricuspid valve annulus Z-score | 0.05 ± 1.3 | N/A | N/A |
| Male (%) | 48% | 55% | 0.53 |
| Age at CMR (years) | 13.3 (0.04–34) | 11.4 (0.6–29.2) | 0.21 |
| Weight at CMR (kg) | 53.8 (19.6–64.6) | 35.8 (22–54.5) | 0.18 |
| Height at CMR (cm)a | 157 (108.5–170) | 136.5 (117.1–159) | 0.1 |
| Body surface area at CMR (m2) | 1.5 (0.76–1.75) | 1.2 (0.86–1.56) | 0.14 |
Data are presented as either percent of total, median (interquartile range), or mean ± standard deviation PAIVS/CPS pulmonary atresia with intact ventricular septum/critical pulmonary stenosis, TOF Tetralogy of Fallot, RVOT right ventricular outflow tract
Height data exclude seven patients who did not have height recorded
Table 2.
Surgical and catheter interventions in PAIVS/CPS cohort
| Patient ID | Ventricles | Diagnosis | Initial newborn intervention | Second intervention | Third intervention |
|---|---|---|---|---|---|
| CHLA 1 | 2 | CPS | Balloon pulmonary valvotomy | ||
| CHLA 2 | 2 | CPS | BTS + RVOT reconstruction | ||
| CHLA 3 | 2 | CPS | BTS + RVOT reconstruction | ||
| CHLA 4 | 2 | CPS | BTS + RVOT reconstruction | ||
| CHLA 5 | 2 | CPS | Balloon pulmonary valvotomy then BTS + pulmonary valvectomy | TV repair + pulmonary homograft replacement + partial ASD closure | |
| CHLA 6 | 1.5 | CPS | BTS + RVOT reconstruction | Glenn + RVOT reconstruction | |
| CHLA 7 | 2 | PAIVS | BTS + RVOT reconstruction | BTS device occlusion | |
| CHLA 8 | 2 | PAIVS | BTS + RVOT reconstruction | Pulmonary homograft replacement | |
| CHLA 9 | 2 | PAIVS | BTS + RVOT reconstruction | BTS device occlusion + ASD closure | |
| CHLA 10 | 1.5 | PAIVS | BTS + RVOT reconstruction | Glenn + partial ASD closure | |
| CHLA 11 | 2 | PAIVS | BTS + RVOT reconstruction | Right pulmonary arterioplasty + BTS takedown | |
| CHLA 12 | 2 | PAIVS | BTS + RVOT reconstruction | ||
| CHLA 13 | 2 | CPS | Balloon pulmonary valvotomy then BTS + pulmonary valvectomy | ||
| CHLA 14 | 1.5 | PAIVS | BTS + RVOT reconstruction | Glenn + partial ASD closure | |
| CHLA 15 | 2 | CPS | BTS + RVOT reconstruction | BTS device occlusion | |
| CHLA 16 | 2 | PAIVS | Balloon pulmonary valvotomy | ||
| CHLA 17 | 2 | PAIVS | BTS + RVOT reconstruction | ||
| CHLA 18 | 1.5 | PAIVS | BTS + RVOT reconstruction | Glenn | |
| CHLA 19 | 1.5 | PAIVS | BTS | Glenn + RVOT reconstruction + partial ASD closure | |
| UM 1 | 2 | PAIVS | Balloon pulmonary valvotomy | Open pulmonary valvotomy + PFO closure | RVOT patch |
| UM 2 | 2 | PAIVS | BTS + RVOT reconstruction | Transannular patch + BTS takedown | Surgical ASD closure |
| UM 3 | 2 | PAIVS | Balloon pulmonary valvotomy then BTS + pulmonary valvectomy | Transannular patch + BTS takedown | |
| UM 4 | 2 | PAIVS | Central shunt + RVOT reconstruction | ASD closure + BTS takedown | |
| UM 5 | 2 | PAIVS | Central shunt + RVOT reconstruction | Transannular patch + BTS takedown | Device ASD closure |
| UM 6 | 2 | PAIVS | Balloon pulmonary valvotomy (periventricular) | Balloon pulmonary valvotomy | |
| UM 7 | 2 | PAIVS | Balloon pulmonary valvotomy | ||
| UM 8 | 1.5 | PAIVS | BTS + periventricular pulmonary valvotomy | Glenn + TV annuloplasty + RVOT reconstruction + partial ASD closure |
The variability in interventional strategies highlights the heterogeneity in PAIVS/CPS pathophysiology
CHLA Children’s Hospital Los Angeles, UM University of Michigan, PAIVS pulmonary atresia with intact ventricular septum, CPS critical pulmonary stenosis, BTS Blalock–Taussig shunt, RVOT right ventricular outflow tract, ASD atrial septal defect, PFO patent foramen ovale
RV Size
Comparisons of cardiac CMR and echocardiography data for both groups are shown in Table 3. Pulmonary regurgitant fraction by CMR was similar between the two groups, but TR grade by echocardiography was significantly higher in PAIVS/CPS patients. Neither TR nor PI severity was associated with age. PAIVS/CPS patients with 2 ventricle repair had similar RVEDVi compared to TOF patients, while PAIVS/CPS patients with 1.5 ventricle palliation had a significantly lower RVEDVi (Fig. 1). Although the median RVEDVi was similar between PAIVS/CPS and TOF, the TOF distribution was more skewed, with 10% exceeding 200 ml/m2. However, when we examined the association between RVEDVi versus age (Fig. 2a) and RVEDVi versus pulmonary regurgitant fraction (Fig. 2b), we did not observe a difference between PAIVS/CPS and TOF patients.
Table 3.
CMR and echocardiographic data
| Parameter | PAIVS/CPS (n = 42) | TOF (n = 100) | p value |
|---|---|---|---|
| RVEDVi (ml/m2) | 108.5 (87.4–145.9) | 120.7 (96.0–153.2) | 0.12 |
| RVESVi (ml/m2) | 50.4 (38.8–66.5) | 56.0 (38.7–66.5) | 0.48 |
| RVEF (%) | 52.5 (48.6–57.0) | 54.8 (50.0–61.1) | 0.18 |
| RV total regurgitant fraction [TR + PI] (%) | 33.3 (18.9–43.3) | 36.2 (26.8–44.6) | 0.42 |
| RA cross-sectional area/body surface area (cm2/m2) | 19.2 (16.1–23.8) | 12.6 (10.9–14.6) | < 0.0001 |
| LVEDVi (ml/m2) | 69.3 (63.2–76.0) | 69.2 (59.0–84.9) | 0.91 |
| LVESVi (ml/m2) | 29.8 (24.0–35.2) | 27.9 (22.6–33.9) | 0.22 |
| LVEF (%) | 55.5 (53.4–61.0) | 60.7 (57.1–64.1) | 0.002 |
| Main pulmonary artery regurgitant fraction (%) | 37.3 ± 15.4 | 37.4 ± 14.6 | 0.99 |
| Presence of EDFF (n) | 33 (78%) | 15 (39%) | 0.0006 |
| EDFF peak flux (ml/s) | 60 (41–96) | 29 (16–48) | 0.002 |
| Echocardiogram tricuspid regurgitation severity (n) | 0.03 | ||
| None to trivial | 8 (28%) | 24 (59%) | |
| Mild to moderate | 18 (62%) | 16 (39%) | |
| Severe | 3 (10%) | 1 (2%) |
Main pulmonary artery regurgitant fraction was only available for 38 PAIVS/CPS and 90 TOF studies, EDFF for 41 PAIVS/CPS and 38 TOF studies, and echocardiogram data for 29 PAIVS/CPS and 59 TOF studies
Data are presented as either percent of total, median (interquartile range), or mean ± standard deviation. PAIVS/CPS pulmonary atresia with intact ventricular septum/critical pulmonary stenosis, TOF Tetralogy of Fallot, RV right ventricle, LV left ventricle, EDVi end-diastolic volume indexed to body surface area, ESVi end-systolic area indexed to body surface area, EF ejection fraction, RA right atrium, TR tricuspid regurgitation, PI pulmonary insufficiency, EDFF end-diastolic forward flow in main pulmonary artery
Fig. 1.
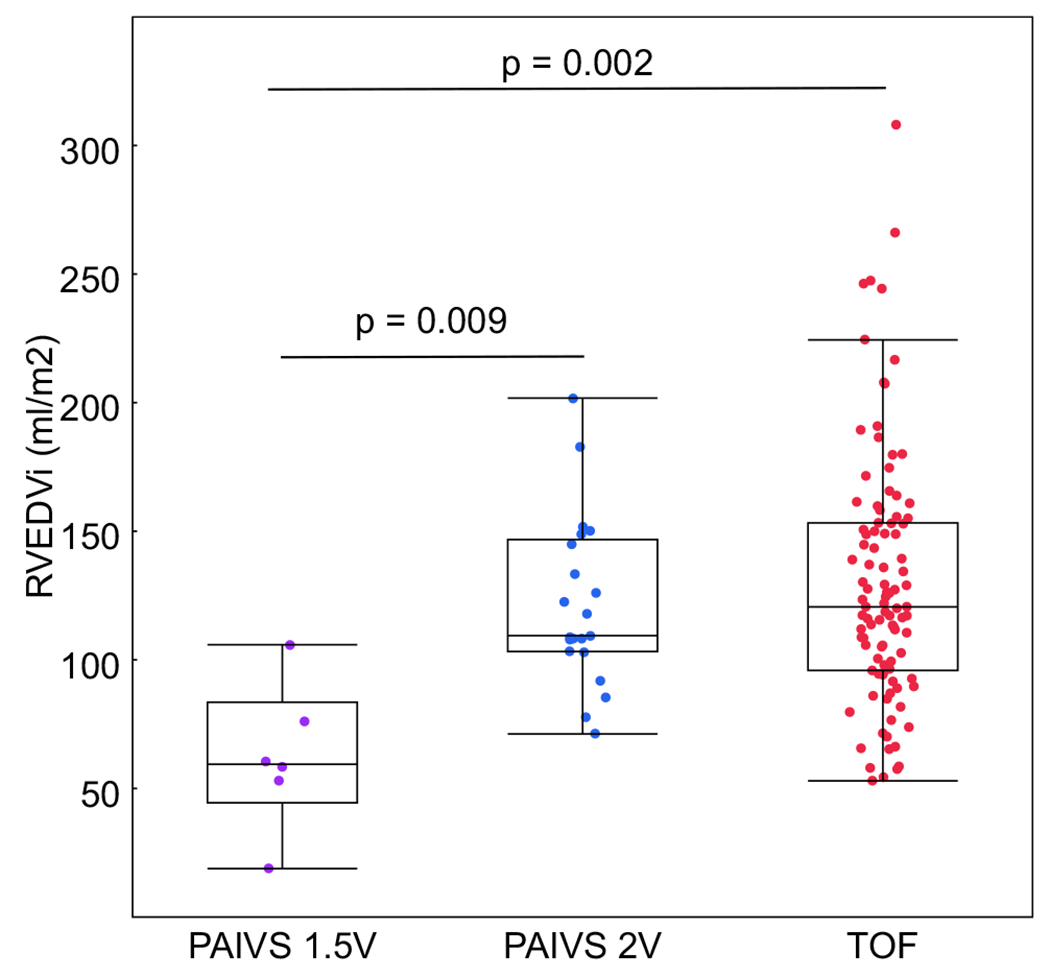
RVEDVi is similar between patients with 2-ventricle PAIVS repair (PAIVS 2 V) and TOF; however, patients with 1.5 ventricle palliation (PAIVS 1.5 V) have significantly smaller RVEDVi compared to both 2-ventricle groups
Fig. 2.
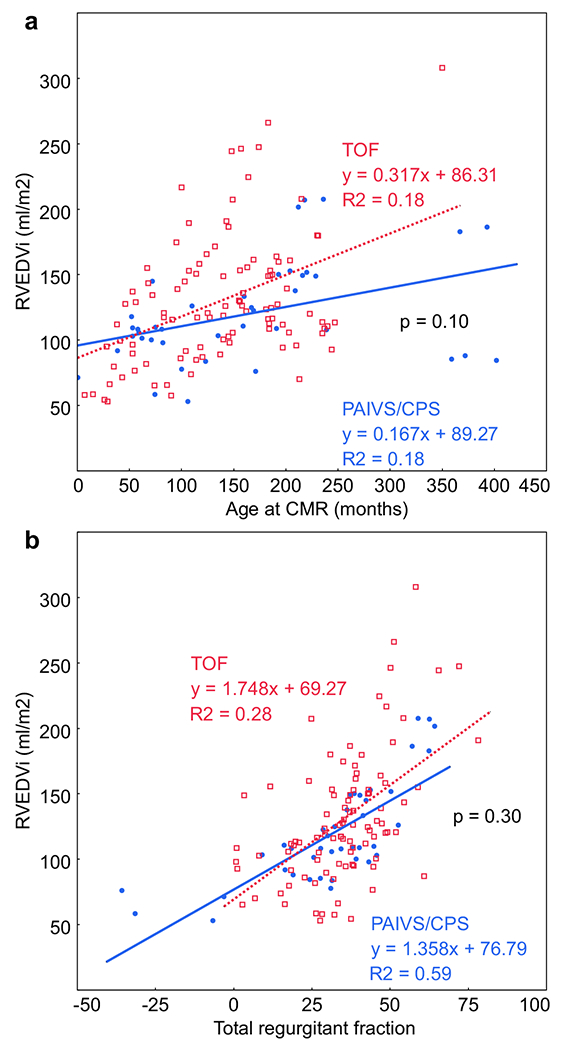
a The association between RVEDVi and age is similar between PAIVS/CPS (blue circles) and TOF (red squares). b RVEDVi increases with regurgitant fraction similarly between PAIVS/CPS and TOF. Negative regurgitant fraction in some PAIVS/CPS patients is due to right to left shunting across an atrial septal defect
Longitudinal RV Dilation
Longitudinal RV dilation data were available in a subset of patients (10 PAIVS/CPS and 15 TOF), as shown in Fig. 3a. While the association between RV size and age and the average longitudinal dilation over time were similar between the two cohorts (Figs. 2a, 3b, respectively), a subset (27%) of TOF patients had accelerated RV dilation. This subgroup had an average dilation rate of 45.8 ml/m2/year (48.5% change per year), while those without accelerated dilation had an average dilation rate of 5.6 ml/m2/year (5% change per year). In contrast, the average dilation rate in the PAIVS/CPS group was only 1.8 ml/m2/year (1.6% change per year). Accelerated dilation was not seen in any of the PAIVS/CPS patients (maximum dilation rate 16.2 ml/m2/year or 15.1% change per year). In both cohorts, a few patients had a decrease in RVEDVi over time, suggesting that somatic growth outpaced RV dilation.
Fig. 3.
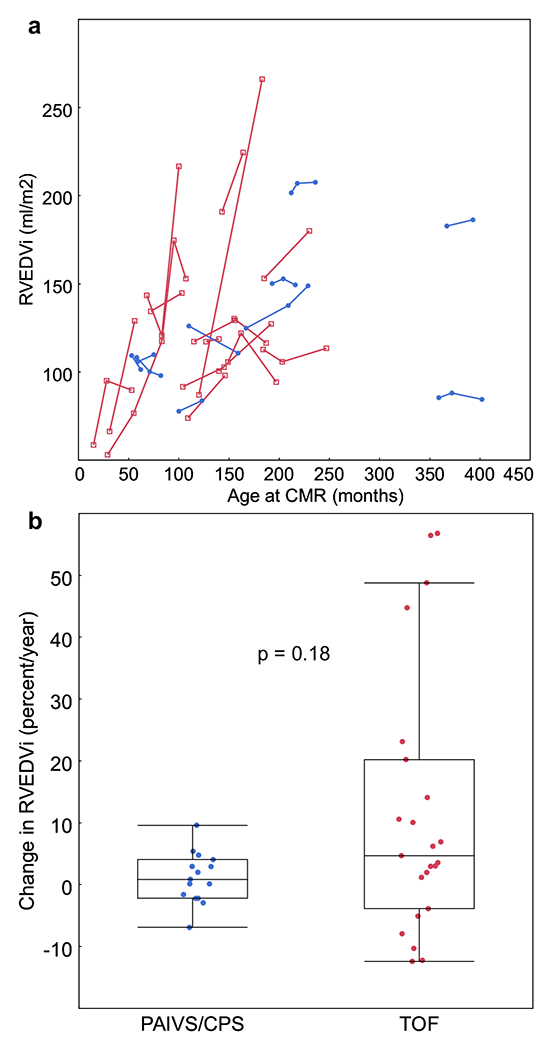
a RVEDVi increases over time in a subset of PAIVS/CPS (blue circles) and TOF patients (red squares). b There are two distinct subgroups within the TOF cohort, one with dilation of > 40% per year and one with dilation < 20% per year, suggesting acceleration of RV dilation above somatic growth in the former
RV Function
RV systolic function was comparable between the PAIVS/CPS and TOF cohorts (Fig. 4a). PAIVS/CPS patients also had similar RV systolic function regardless of 1.5 ventricle palliation versus 2 ventricle repair. However, despite similar RV volume and function measurements, the relationship between RVEDVi and RVEF was quite different between the PAIVS/CPS and TOF cohorts. While RVEF inversely correlated with RVEDVi in TOF patients, RVEF was not associated with RVEDVi in PAIVS/CPS patients (Fig. 4b). By ROC analysis, we determined that the optimum RVEDVi to predict an RVEF < 50% in the TOF group was 164 ml/m2 (p = 0.03, AUC 0.62, specificity 0.9, sensitivity 0.44). There were 5 PAIVS/CPS studies (12%) versus 18 TOF studies (18%) with an RVEDVi > 164 ml/m2 (p = 0.36), and 11 PAIVS/CPS studies (26%) versus 25 TOF studies (25%) with an RVEF < 50% (p = 0.93).
Fig. 4.
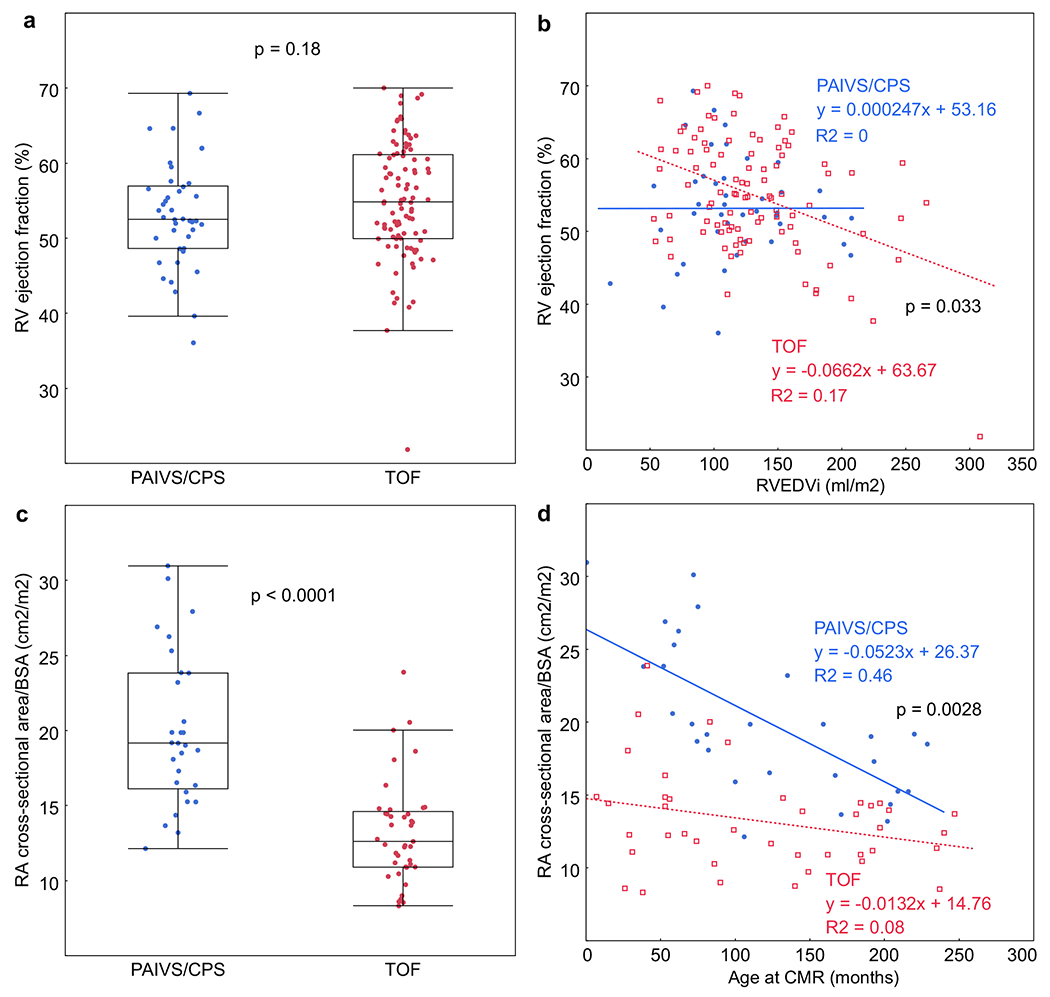
a RV systolic function in PAIVS/CPS patients is not different from TOF patients. b RVEF is not associated with RVEDVi in PAIVS/CPS patients (blue circles); however, RVEF is significantly, albeit weakly associated with RVEDVi in TOF patients (red squares), particularly with RVEDVi > 164 ml/m2. c RA cross-sectional area/BSA, a marker of chronic diastolic dysfunction, is larger in PAIVS/CPS patients. d RA cross-sectional area/BSA is larger in PAIVS/CPS patients across all ages. However, the difference decreases with increasing age, suggesting improvement in diastolic function over time
Chronic diastolic dysfunction and restrictive RV physiology, as assessed by RA cross-sectional area/BSA and EDFF, were worse in the PAIVS/CPS cohort. These were not significantly different between the 1.5 ventricle and 2 ventricle subgroups. The presence of EDFF did not correlate with LV function or size, nor did it correlate with RV size. RA cross-sectional area/BSA was 1.5 times larger (Fig. 4c, p < 0.0001) and EDFF was 2 times higher in the PAIVS/CPS group (p = 0.002). Since RA cross-sectional area/BSA could have been affected by TR severity, we evaluated the relationship between these two variables in both cohorts. While there were more patients with mild to moderate TR in the PAIVS/CPS cohort (62% vs. 39%), RA cross-sectional area/BSA was not significantly different between those with none to trivial TR versus those with mild to moderate TR in either the PAIVS/CPS cohort (19.6 ± 5.1 versus 19 ± 4.4 cm2/m2, p = 0.81) or TOF cohort (13.4 ± 3.7 versus 13.1 ± 3 cm2/m2, p = 0.83). In contrast, RA cross-sectional area/BSA was larger in those with severe TR in the PAIVS/CPS cohort (27.4 ± 2.6 cm2/m2, p = 0.024) but not in the TOF cohort (10.3 ± 3.5 cm2/m2, p = 0.68). Although this biased the PAIVS/CPS cohort toward a larger average RA cross-sectional area/BSA, since only a minority of patients in either group had severe TR (10% PAIVS/CPS, 2% TOF), the magnitude of this effect was minimal. As illustrated in Fig. 4d, RA cross-sectional area/BSA was larger in the PAIVS/CPS cohort than in the TOF cohort across all ages, although the difference between the two groups decreased with age. Neither RA cross-sectional area/BSA nor EDFF correlated with systolic function in patients with PAIVS/CPS. Furthermore, the linear association between RA cross-sectional area and RVEDVi was not different for PAIVS/CPS versus TOF (data not shown).
Discussion
Patients with PAIVS/CPS are born with varying degrees of tricuspid valve and RV hypoplasia. The RV can also be significantly hypertrophied with myocardial disarray and fibrosis, which may lead to differences in response to chronic volume load [18, 19]. Our study demonstrates that despite this variability, RVEDVi and RVEF are similar in patients with repaired or palliated PAIVS/CPS versus repaired TOF. However, while RV volume and systolic function are similar, RV diastolic function is significantly worse in PAIVS/CPS patients. Although both groups have features of restrictive RV physiology, it is more prominent in PAIVS/CPS and not associated with systolic dysfunction. This is an important distinction for patients with PAIVS/CPS, in whom the initial surgical approach is based on speculated RV dilation potential. Furthermore, since the timing of pulmonary valve replacement is typically based on data derived from studies of patients with TOF, this difference in physiology suggests that application of the TOF guidelines to PAIVS/CPS may not be ideal.
Unlike with TOF, adaptive versus pathologic changes in RV size and function after RVOT reconstruction have not been well described in PAIVS/CPS during the modern era using CMR [7–9, 20]. This presents a clinical challenge for determining timing of pulmonary or tricuspid valve replacement for patients with PAIVS/CPS. In patients with TOF, RVEDVi, RVESVi, and RVEF have been shown to predict adverse outcomes, including decreased exercise capacity, ventricular arrhythmias, and mortality [21]. These measurements have also been used to establish guidelines for timing of pulmonary valve replacement [22, 23]. Unfortunately, similar outcomes data are not available for patients with PAIVS/CPS.
Based on the initial tricuspid valve Z-scores, our PAIVS/CPS cohort had relatively mild RV hypoplasia, which is a reflection of how the sample was obtained (i.e., those with severe RV hypoplasia would have undergone single ventricle palliation and thus would not have been included in this study). While RV dilation and its relationship with age were similar between our two cohorts, markedly accelerated dilation was seen in a subset of TOF patients but not in any of the PAIVS/CPS patients. Despite the limitations of a small sample size in the subgroup analysis, the acceleration in dilation was striking and appeared to occur over a broad but young age range.
Concordant with prior echocardiographic studies, RV systolic function was maintained in the PAIVS/CPS cohort [24]. In contrast, TOF patients with RVEDVi greater than 164 ml/m2 exhibited declining RV systolic function. Though not statistically different, there was a trend toward fewer PAIVS/CPS patients reaching this volume threshold; whether this is due to an intrinsic limit to RV dilation or a different response to chronic volume load cannot be determined with our study design. Similarly, several studies comparing patients with PS treated with surgical valvotomy or balloon valvuloplasty to age-and health-matched patients with surgically corrected TOF demonstrated higher RVEF in the former, although these patients were generally older and had a larger average RVEDVi than our cohort [25–27].
While systolic function is preserved in PAIVS/CPS, diastolic function is significantly impaired. Recent reports from Lam and colleagues demonstrated restrictive RV physiology was not only common in adult patients with PS, but that it also correlated with worse functional status, despite preserved EF [28]. Furthermore, increased RA size has been associated with chronic RV diastolic dysfunction, particularly in cases of long-standing restrictive RV physiology [15, 29]. When we evaluated predictors of RA cross-sectional area/BSA, the diagnosis of PAIVS/CPS was the best predictor, with additional influence by EDFF and TR grade. This, in conjunction with our finding that diastolic dysfunction exists independent of systolic dysfunction, suggests that PAIVS/CPS is primarily a diastolic disease process. Therefore, RV diastolic dysfunction may mediate clinical outcomes in patients with PAIVS/CPS. Interestingly, while RA cross-sectional area/BSA decreased with age in both cohorts, it decreased more rapidly in the PAIVS/CPS cohort, suggesting some degree of improvement in diastolic function over time.
Our study had several important limitations including its retrospective nature and the limited number of cases with longitudinal data available. Since ventricular volume and function measurements were extracted from clinical reports, we did not account for interobserver and interinstitutional variability. However, based on prior studies of CMR reproducibility, we expect this variability to be low (5–10%) [30]. There was variation in surgical management for initial repair, palliation, and pulmonary valve replacement for both cohorts. There may also be a selection/institutional bias in the PAIVS/CPS group regarding the decision to pursue a single ventricle, 1.5 ventricle, or 2 ventricle pathway. However, the aim of this study was not to determine which pathway is optimal and the similarity in data collected from the two institutions partially compensated for this bias. Many TOF patients in the early part of the study period never had a CMR prior to pulmonary valve replacement, which may have also lead to a selection bias in our sample. An important limitation was the potential for a cohort effect based on age and surgical strategies at the time of initial TOF repair. Much of the RV volume and function data in the literature included patients who were repaired “late,” after 2–3 years of age, whereas our data included very few of those patients. Most of our patients were repaired before 1 year of age, resulting in less RV hypertrophy and longer standing PI. Also, many of our patients had a transannular patch with extensive RVOT reconstruction, a strategy employed to prevent outflow tract stenosis. Finally, since four (15%) of the PAIVS/CPS patients had right to left shunting across a residual ASD, RA cross-sectional area may not have been an accurate measure of RV compliance in this subgroup.
Conclusions
RV size and systolic function are similar in patients with repaired/palliated PAIVS/CPS compared to those with repaired TOF. However, restrictive right ventricular physiology and chronic diastolic dysfunction are much more common in patients with pulmonary atresia intact ventricular septum/critical pulmonary stenosis. Future prospective studies aimed at determining optimal timing of pulmonary valve replacement in this population should include measures of diastolic function in addition to longitudinal measures of RV size and systolic function. These data will provide a better understanding of what defines normal growth versus pathologic RV dilation and how it affects long-term functional outcomes.
Acknowledgements
Jon Detterich was supported by a grant from the National Institutes of Health (5 K23 HL119627-03) during the conduct of this study.
Footnotes
Conflict of interest All authors declare no conflicts of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent For this type of study formal consent is not required.
References
- 1.Gersony WM, Bernhard WF, Nadas AS, Gross RE (1967) Diagnosis and surgical treatment of infants with critical pulmonary outflow obstruction. Study of thirty-four infants with pulmonary stenosis or atresia, and intact ventricular septum. Circulation 35:765–776. 10.1161/01.CIR.35.4.765 [DOI] [PubMed] [Google Scholar]
- 2.Ashburn DA, Blackstone EH, Wells WJ et al. (2004) Determinants of mortality and type of repair in neonates with pulmonary atresia and intact ventricular septum. J Thorac Cardiovasc Surg 127:1000–1008. 10.1016/jjjtcvs.2003.11.057 [DOI] [PubMed] [Google Scholar]
- 3.Jahangiri M, Zurakowski D, Bichell D et al. (1999) Improved results with selective management in pulmonary atresia with intact ventricular septum. J Thorac Cardiovasc Surg 118:1046–1055 [DOI] [PubMed] [Google Scholar]
- 4.Sano S, Ishino K, Kawada M et al. (2000) Staged biventricular repair of pulmonary atresia or stenosis with intact ventricular septum. Ann Thorac Surg 70:1501–1506 [DOI] [PubMed] [Google Scholar]
- 5.Yoshimura N, Yamaguchi M (2009) Surgical strategy for pulmonary atresia with intact ventricular septum: initial management and definitive surgery. Gen Thorac Cardiovasc Surg 57:338–346 [DOI] [PubMed] [Google Scholar]
- 6.Alwi M, Geetha K, Bilkis AA et al. (2000) Pulmonary atresia with intact ventricular septum percutaneous radiofrequency-assisted valvotomy and balloon dilation versus surgical valvotomy and Blalock Taussig shunt. J Am Coll Cardiol 35:468–476. 10.1016/S0735-1097(99)00549-5 [DOI] [PubMed] [Google Scholar]
- 7.Lewis AB, Wells W, Lindesmith GG (1986) Right ventricular growth potential in neonates with pulmonary atresia and intact ventricular septum. J Thorac Cardiovasc Surg 91:835–840 [PubMed] [Google Scholar]
- 8.Ovaert C, Qureshi SA, Rosenthal E et al. (1998) Growth of the right ventricle after successful transcatheter pulmonary valvotomy in neonates and infants with pulmonary atresia and intact ventricular septum. J Thorac Cardiovasc Surg 115:1055–1062. 10.1016/S0022-5223(98)70405-2 [DOI] [PubMed] [Google Scholar]
- 9.Rao PS, Liebman J, Borkat G (1976) Right ventricular growth in a case of pulmonic stenosis with intact ventricular septum and hypoplastic right ventricle. Circulation 53:389–394. 10.1161/01.CIR.53.2.389 [DOI] [PubMed] [Google Scholar]
- 10.Karamlou T, Poynter JA, Walters HL et al. (2013) Long-term functional health status and exercise test variables for patients with pulmonary atresia with intact ventricular septum: a Congenital Heart Surgeons Society study. J Thorac Cardiovasc Surg 145:1018–1027.e3. 10.1016/jotcvs.2012.11.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geva T (2013) Indications for pulmonary valve replacement in repaired tetralogy of Fallot: the quest continues. Circulation 128:1855–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geva T (2011) Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Magn Reson 13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wald RM, Valente AM, Gauvreau K et al. (2015) Cardiac magnetic resonance markers of progressive RV dilation and dysfunction after tetralogy of Fallot repair. Heart 101:1724–1730. 10.1136/heartjnl-2015-308014 [DOI] [PubMed] [Google Scholar]
- 14.Sallach JA, Tang WHW, Borowski AG et al. (2009) Right atrial volume index in chronic systolic heart failure and prognosis. JACC Cardiovasc Imaging 2:527–534. 10.1016/j.jcmg.2009.01.012 [DOI] [PubMed] [Google Scholar]
- 15.Friedberg MK, Fernandes FP, Roche SL et al. (2012) Impaired right and left ventricular diastolic myocardial mechanics and filling in asymptomatic children and adolescents after repair of tetralogy of Fallot. Eur Heart J Cardiovasc Imaging 13:905–913. 10.1093/ehjci/jes067 [DOI] [PubMed] [Google Scholar]
- 16.Helbing WA, Niezen RA, Cessie SLE et al. (1997) Right ventricular diastolic function in children with pulmonary regurgitation after repair of tetralogy of Fallot: volumetric evaluation by magnetic resonance velocity mapping. J Am Coll Cardiol 28:1827–1835. 10.1016/S0735-1097(96)00387-7 [DOI] [PubMed] [Google Scholar]
- 17.Lai WW, Geva T, Shirali GS et al. (2006) Guidelines and standards for performance of a pediatric echocardiogram: a report from the task force of the pediatric council of the American Society of Echocardiography. J Am Soc Echocardiogr 19:1413–1430. 10.1016/j.echo.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 18.Oosthoek PW, Moorman AFM, Sauer U, Gittenberger-de Groot AC (1995) Capillary distribution in the ventricles of hearts with pulmonary atresia and intact ventricular septum. Circulation 91:1790–1798. 10.1161/01.CIR.91.6.1790 [DOI] [PubMed] [Google Scholar]
- 19.Bulkley BH, D’Amico B, Taylor AL (1983) Extensive myocardial fiber disarray in aortic and pulmonary atresia. Relevance to hypertrophic cardiomyopathy. Circulation 67:191–198. 10.1161/01.CIR.67.1.191 [DOI] [PubMed] [Google Scholar]
- 20.Patel RG, Freedom RM, Moes CA et al. (1980) Right ventricular volume determinations in 18 patients with pulmonary atresia and intact ventricular septum. Analysis of factors influencing right ventricular growth. Circulation 61:428–440 [DOI] [PubMed] [Google Scholar]
- 21.Knauth AL, Gauvreau K, Powell AJ et al. (2008) Ventricular size and function assessed by cardiac MRI predict major adverse clinical outcomes late after tetralogy of Fallot repair. Heart 94:211–216. 10.1136/hrt.2006.104745 [DOI] [PubMed] [Google Scholar]
- 22.Geva T, Sandweiss BM, Gauvreau K et al. (2004) Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol 43:1068–1074. 10.1016/j.jacc.2003.10.045 [DOI] [PubMed] [Google Scholar]
- 23.Valente AM, Cook S, Festa P et al. (2014) Multimodality imaging guidelines for patients with repaired tetralogy of Fallot: a report from the American society of echocardiography: developed in collaboration with the society for cardiovascular magnetic resonance and the society for pediatric radiol. J Am Soc Echocardiogr 27:111–141. 10.1016/jjecho.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 24.Hoashi T, Kagisaki K, Kitano M et al. (2012) Late clinical features of patients with pulmonary atresia or critical pulmonary stenosis with intact ventricular septum after biventricular repair. Ann Thorac Surg 94:833–841. 10.1016/j.athoracsur.2012.04.071 [DOI] [PubMed] [Google Scholar]
- 25.Puranik R, Tsang V, Lurz P et al. (2012) Long-term importance of right ventricular outflow tract patch function in patients with pulmonary regurgitation. J Thorac Cardiovasc Surg 143:1103–1107. 10.1016/j.jtcvs.2011.09.039 [DOI] [PubMed] [Google Scholar]
- 26.Zdradzinski MJ, Qureshi AM, Stewart R et al. (2014) Comparison of long-term postoperative sequelae in patients with tetralogy of Fallot versus isolated pulmonic stenosis. Am J Cardiol 114:300–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joynt MR, Yu S, Dorfman AL et al. (2016) Differential impact of pulmonary regurgitation on patients with surgically repaired pulmonary stenosis versus tetralogy of Fallot. Am J Cardiol 117:289–294. 10.1016/j.amjcard.2015.10.025 [DOI] [PubMed] [Google Scholar]
- 28.Lam YY, Kaya MG, Goktekin O et al. (2007) Restrictive right ventricular physiology. Its presence and symptomatic contribution in patients with pulmonary valvular stenosis. J Am Coll Cardiol 50:1491–1497. 10.1016/j.jacc.2007.06.042 [DOI] [PubMed] [Google Scholar]
- 29.Luijnenburg SE, Peters RE, Van Der Geest RJ et al. (2013) Abnormal right atrial and right ventricular diastolic function relate to impaired clinical condition in patients operated for tetralogy of Fallot. Int J Cardiol 167:833–839. 10.1016/j.ijcard.2012.02.011 [DOI] [PubMed] [Google Scholar]
- 30.Geva T (2014) MRI is the preferred method for evaluating right ventricular size and function in patients with congenital heart disease. Circ Cardiovasc Imaging 7:190–197. 10.1161/CIRCIMAGING.113.000553 [DOI] [PMC free article] [PubMed] [Google Scholar]


