Abstract
Inhalation is the most relevant route of volatile organic chemical (VOC) exposure; however, due to unique challenges posed by their chemical properties and poor solubility in aqueous solutions, in vitro chemical safety testing is predominantly performed using direct application dosing/submerged exposures. To address the difficulties in screening toxic effects of VOCs, our cell culture exposure system permits cells to be exposed to multiple concentrations at air-liquid interface (ALI) in a 24-well format. ALI exposure methods permit direct chemical-to-cell interaction with the test article at physiological conditions. In the present study, BEAS-2B and primary normal human bronchial epithelial cells (pHBEC) are used to assess gene expression, cytotoxicity, and cell viability responses to a variety of volatile chemicals including acrolein, formaldehyde, 1,3-butadiene, acetaldehyde, 1-bromopropane, carbon tetrachloride, dichloromethane, and trichloroethylene. BEAS-2B cells were exposed to all the test agents, while pHBECs were only exposed to the latter four listed above. The VOC concentrations tested elicited only slight cell viability changes in both cell types. Gene expression changes were analyzed using benchmark dose (BMD) modeling. The BMD for the most sensitive gene set was within one order of magnitude of the threshold-limit value reported by the American Conference of Governmental Industrial Hygienists, and the most sensitive gene sets impacted by exposure correlate to known adverse health effects recorded in epidemiologic and in vivo exposure studies. Overall, our study outlines a novel in vitro approach for evaluating molecular-based points-of-departure in human airway epithelial cell exposure to volatile chemicals.
Keywords: inhalation, benchmark dose, in vitro, cell culture exposure system, VOC, transcriptomics
Introduction
Well-designed in vitro exposure models require less time and fewer resources compared to traditional in vivo toxicity testing, and in some cases better mimic human biological relevance. Still, air pollutants and inhaled toxicants remain one of the most difficult human exposures to replicate in an in vitro laboratory setting. Inhaled volatile organic chemicals (VOC) are particularly challenging to test reliably in vitro, given difficulties in generating the appropriate test agent atmospheres, relevance and transferability of the cell or tissue models used, and compatibility with high-throughput screening approaches. VOC inhalation exposure is prevalent in both occupational and environmental settings, representing a significant risk to human health (Alabdulhadi et al. 2019; Cheng et al. 2019; Chiang et al. 2010). Currently, approximately one fourth of chemicals nominated for study in the U.S. Environmental Protection Agency’s (EPA) chemical substance inventory are volatile/semi-volatile or are not soluble in DMSO or water and cannot be tested using traditional in vitro methods for evaluating chemical safety (USEPA 2020b). In vivo rodent inhalation toxicity testing for VOCs has been the gold standard for regulatory agencies but may provide limited relevance to human health effects due to differences in human physiology, such as rodents primarily being obligate nasal breathers (Bates and Irvin 2003; Harkema 1991; Harkema et al.). Additionally, biological effects of VOCs cannot be confidently evaluated in high-throughput test systems that use submerged cultures due to solubility problems and evaporation from the culture wells. Poorly soluble test agents have minimal contact with the cells in a direct-dosing application, further complicating the process of determining delivered dose to the cells. These issues have led to the exclusion of VOCs from testing within the Toxicity Forecaster (e.g. ToxCast) screening program and prevented generation of data from that collection of assays for use in risk assessment (Judson et al. 2010). Thus, new approach methods (NAMs) that include in vitro exposure technology for VOCs and associated assays for evaluating biological activity of VOCs in human cells, such as high-throughput transcriptomics, are necessary to provide comprehensive information regarding these data poor chemical species (Harrill et al. 2021).
To circumvent the difficulties in toxicity screening of volatile test agents, we developed a cell culture exposure system (CCES) that permits cells to be exposed to multiple concentrations of test agent at air-liquid interface (ALI) in a 24-well format. ALI exposure methods permit direct chemical-to-cell interaction at physiological conditions, providing a more environmentally realistic airway exposure condition, as compared to submerged cultures (Doyle et al. 2004; Dvorak et al. 2011; Ebersviller et al. 2012). Our previous studies utilizing the CCES established a consistent capacity to deliver high concentrations of volatiles to ALI cell culture inserts (Zavala et al. 2017; Zavala et al. 2018). A variety of in vitro biological effects data can be analyzed in ALI cultures exposed to VOCs using the CCES, including measures of cell viability, release of soluble factors, and gene expression. In comparative studies, ALI exposures have been shown to be a good representation of the in vivo airway epithelial transcriptome (Baldridge et al. 2015; Dvorak et al. 2011). Therefore, high-throughput transcriptome profiling using RNA-Seq or targeted RNA-Seq may provide insight into the molecular mechanisms of toxicity for VOCs. Further, the ability of the CCES to conduct concurrent exposure to six concentrations of a test agent through serial dilution allows for a concentration-response assessment to be conducted in a single experiment. By implementing a novel multiple concentration exposure system, we can apply EPA-developed NAMs for dose-response modeling of in vitro data.
Using the CCES exposure design, our data is amenable to transcriptomic benchmark dose (BMD) analysis as outlined in previously published methods for integrating BMD estimates with genomic data and the National Toxicology Program approach to genomics dose-response modeling (NTP 2018; Thomas et al. 2007; Thomas et al. 2011; Thomas et al. 2013). Research utilizing TempO-Seq high-throughput transcriptomics (HTTr) analysis has produced technically and biologically reproducible concentration-response modeling data (Gwinn et al. 2020; Harrill et al. 2019; Harrill et al. 2021). These previous studies show that transcriptome-based BMDs accurately predict points of departure (PoD) and mode of action (MoA) for both VOCs and non-VOCs (Jackson et al. 2014; Johnson et al. 2020; Thomas et al. 2007). Optimized analytical protocols utilizing BMDExpress2 consider transcriptomics data at the level of individual genes, as well as at the level of aggregated gene set collections to identify the most sensitive cellular responses to chemical exposure (Filipsson et al. 2003; Phillips et al. 2019; Thomas et al. 2007; Yang et al. 2007). For the first time, our experimental design implements HTTr BMD analysis techniques with in vitro ALI VOC exposures.
Over the course of the study, we tested eight widespread VOCs in BEAS-2B cells with four of those test agents (1-bromopropane, carbon tetrachloride, dichloromethane, and trichloroethylene) repeated in primary human bronchial epithelial cells (pHBECs). To stay within relevant concentrations and below overt cytotoxicity, the highest concentration tested was targeted as 10-fold higher than the occupational threshold limit value (TLV®). TLVs are protective occupational exposure limits as determined by the American Conference of Governmental Industrial Hygienists (ACGIH) committee review of existing published and peer-reviewed literature. (ACGIH 2018). In addition to evaluating changes to cell viability, the primary endpoint focused on using TempO-Seq to identify changes in gene expression at both the gene and aggregate gene set level to determine the biological pathway altering concentration (BPAC) for each test agent. Finally, we utilized ACGIH TLVs and in vivo toxicity data from EPA Integrated Risk Information System (IRIS) assessments as benchmarks for comparison to in vitro transcriptional PoDs. The study reported in this manuscript characterizes the impact VOCs have on human airway epithelial cells and establishes HTTr and BMD analysis as a primary diagnostic tool for ALI gas and vapor exposures. The novel approach also supports a recent research focus on in vitro methodologies to support traditional in vivo toxicity testing (USEPA 2014).
Materials and Methods:
BEAS-2B Cell Culture
The BEAS-2B cells used are a non-diseased, viral transformed human bronchial epithelial cell line acquired from ATCC (Manassas, VA, CRL-9609). Prior to cell culture, T75 flasks (Corning, One Riverfront Plaza, NY, 430641) and 6.5 mm Transwell® Inserts (Costar, Corning, NY, 3470) were coated with 100 μg/mL bovine collagen (PureCol Collagen [Advanced Biomatrix, San Diego, CA, 5005] diluted in Ultrapure Molecular (nuclease free) Water [Invitrogen, ThermoFisher Scientific, Rockford, IL, 10977023]) for 2 h at room temperature, followed by a water rinse. To prepare the cells for exposure at ALI, cells were thawed from pre-passaged frozen vials, grown on a T75 flask at 37°C 5% CO2 incubator conditions. The cells proliferated while submerged in Keratinocyte Growth Medium (KGM) (Keratinocyte Basal Medium (KBM) [Lonza, Walkersville, MD] plus KGM Gold Bullet Kit [Lonza, Walkersville, MD, 00192151]) with 25 mM HEPES Buffer (Sigma-Aldrich, St. Louis, MO) or Bronchial Epithelial Growth Medium (BEGM) (Bronchial Epithelial Basal Medium (BEBM) [Lonza, Walkersville, MD, CC-3171] plus BEGM Bullet Kit [Lonza, Walkersville, MD, CC-3170]. The utilized media type was switched to the latter (BEGM) for the experiments with dichloromethane, trichloroethylene, carbon tetrachloride, and 1-bromopropane due to vendor formulation changes and availability. Cells were grown for a minimum of two subsequent passages after thawing. Cells were passaged when approximately 75% confluent and new flasks seeded at 1 × 106 cells. The 6.5 mm inserts were seeded at 2–5 × 105 cells/insert and were maintained for 48–72 h before being used for exposure as a fully confluent monolayer. All apical media was removed at least 5 days before using BEAS-2B cells for exposure to the test agent. No FBS was used in the culture of BEAS-2B cells which were observed to have normal basal respiratory cell morphology at ALI.
Primary Human Bronchial Epithelial Cell Culture
Primary human bronchial epithelial cells (pHBEC) were obtained via bronchial brushing from healthy, non-smoking donors aged 21–40 years using a previously published method (Dailey and McCullough 2021a; 2021b; McCullough et al. 2014). Donors gave their consent after being informed of procedures and associated risks. This study utilized cells from 12 healthy non-smoking donors, 10 male and 2 female donors between age 26–41. The consent and collection protocol were approved by the University of North Carolina School of Medicine Committee on the Protection of the Rights of Human Subjects and by the US Environmental Protection Agency. After collection, pHBECs were seeded at approximately 1 × 106 cells and cultured in BEGM (BEGM Bullet Kit, Lonza, Walkersville, MD CC-3170) on plastic T75 tissue culture flasks and expanded until the third passage. For all exposures, cells were lifted using trypsin-EDTA (Gibco 25300062) and plated on uncoated 6.5 mm diameter (24-well format) Transwell™ inserts (Costar, Corning, NY, 3470) at a density of 1 × 105 cells/insert depending on the donor. Each insert of cells was initially grown submerged with 1:1 DMEM-H (Gibco, 11995–065) and BEGM with complete bullet kit of growth supplements, 50 μg/mL of bovine pituitary extract (Lonza) and 1.5 μg/mL Bovine Serum Albumin (Sigma, A9418) on both the basolateral and apical compartments. The pHBECs were then expanded and grown for 2–4 days until they reached confluency as previously described (Dailey and McCullough 2021a; Ross et al. 2007). The cells were then placed at ALI by removing all apical medium and replacing the basolateral medium with the base media described above, supplemented with 100 nM retinol. The pHBECs continue to differentiate for at least 21 days before being used in an exposure. Previous research categorizing the pHBECs we use show a columnar pseudostratified cell layer with both cilia and mucin producing cells present after day 21 of ALI culture (Ross et al. 2007). All initial pHBEC tissue culture procedures took place at EPA’s Human Studies Facility. Cells were transported to the EPA Research Triangle Park campus located approximately 15 miles away and allowed to recover for at least 20 h prior to exposure.
Cell Culture Exposure System
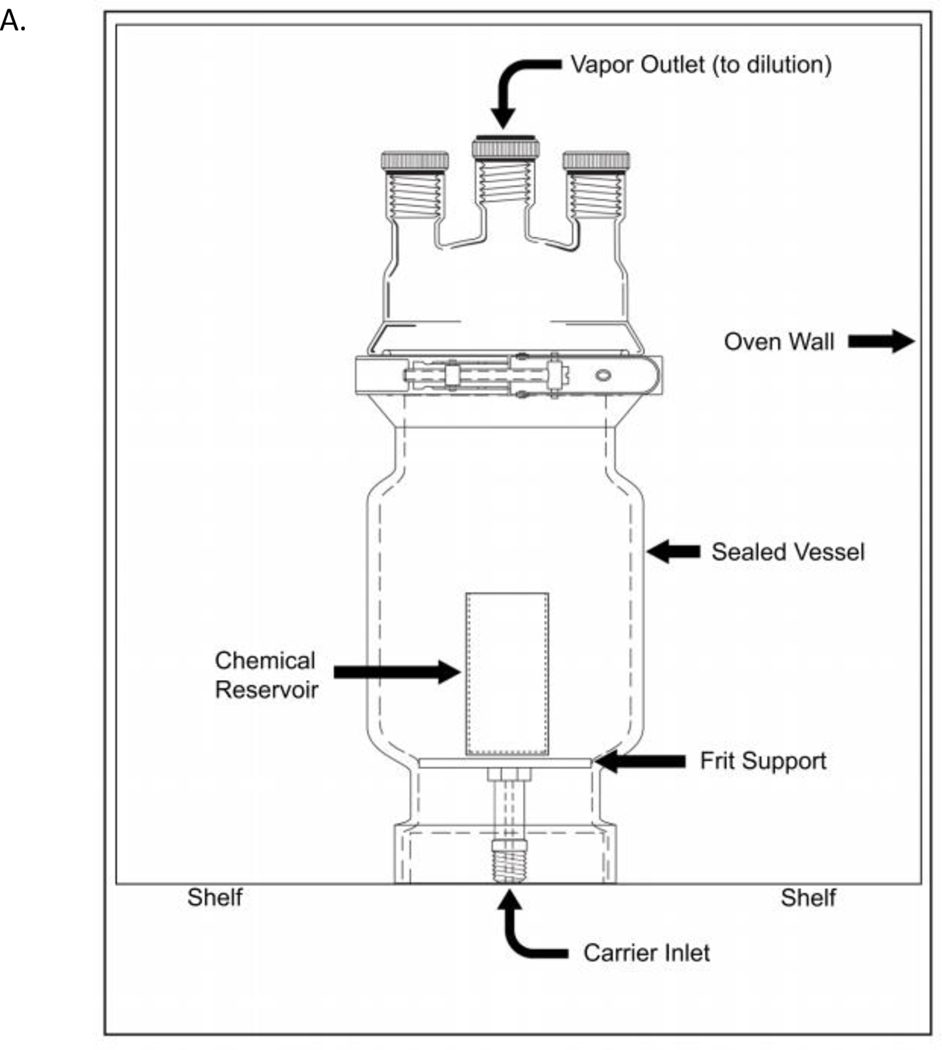
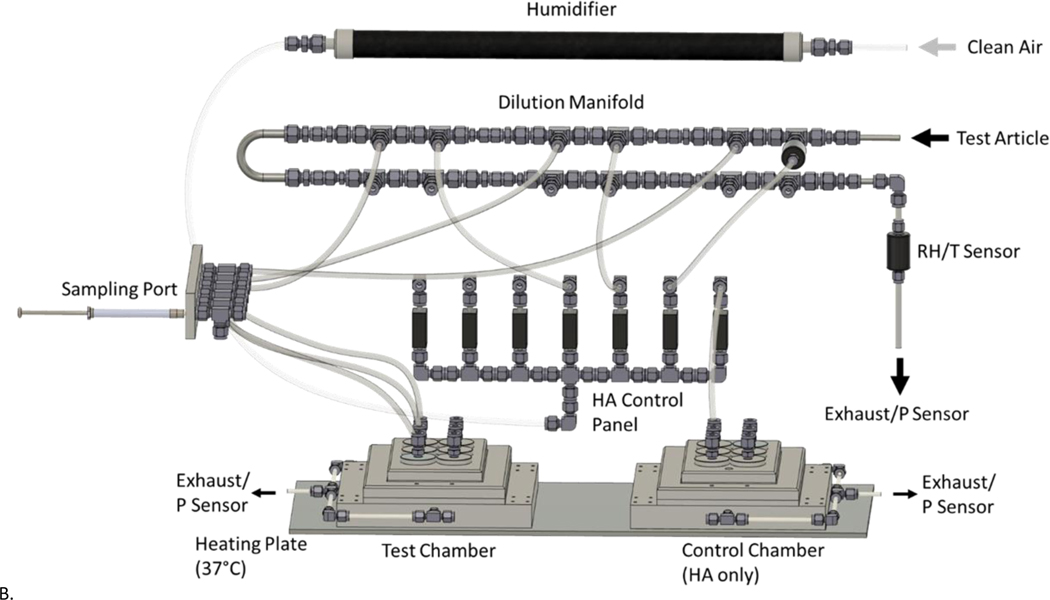
Beginning 2 h prior to test agent exposure, designated cells were prepped for exposure by performing an apical wash with Dulbecco’s Phosphate-Buffered Saline (DPBS) (Gibco, Grand Island, NY) and replacing the basolateral compartment with 0.5 mL of fresh media with 25 mM HEPES Buffer. Cells remained in the incubator (37°C, 5% CO2) to recover for 2 h before being placed in the Cell Culture Exposure System (CCES) to undergo test agent exposures. The CCES was developed at EPA’s Inhalation Toxicology Facility and is comprised of a base module that accommodates a 24-well tissue culture plate with 6.5 mm diameter Transwell® inserts (patent pending). The CCES was developed to simulate an inhalation exposure chamber design. It supplies air under positive pressure and exhausts air under negative pressure to provide a known concentration of test article over a specific time. The design allows for air flow control (slightly positive pressure) at each set of four wells on a 24-well cell culture plate while also allowing for six concentrations with replicates (four replicate wells per concentration) for each exposure regimen. The inlet nozzles are positioned 2 mm above each of the inserts. The previous study characterizing the CCES showed a uniform distribution of gasses across inserts receiving a single concentration (Zavala et al. 2018). Two CCES modules are used simultaneously. One CCES module receives the test agent at six different concentrations while the other CCES module received filtered humidified dilution air and evaluated as a sham exposure control. The CCES modules (base and top plates) were machined out of anodized aluminum, while the distribution manifolds and inlet nozzles were machined out of stainless steel and Swagelok fittings. Figure 1A shows the dynamic headspace generator, while Figure 1B provides a basic schematic of the entire CCES including the dilution manifold and exposure module chambers used for these experiments. A uniform air flow distribution was maintained through each nozzle so the cells in each Transwell® insert was exposed to either humidified air or diluted test agent at a flow rate of 12.5 mL/min/well. Optimal exposure temperature (37°C), relative humidity (85%) and air flow rates were tested and validated for the CCES in previous studies designed to optimize low variability in delivered material to the replicate wells. (Zavala et al. 2017; Zavala et al. 2018). Cells were exposed to the test agent at ALI for 2 h before being transported back to the incubator (37°C, 5% CO2) for 4 h prior to sample collection and analysis. A 2 h exposure to the test agent was optimized to avoid stressing cell cultures in initial tests using the CCES for this pilot study. All sample collection and assays were run 4 h post-exposure to complete a 6 h timepoint to reflect both standard in vivo inhalation exposure regimes of 4 to 6 h of exposure and high-throughput screening studies. A sham exposure control cell culture plate which received only humidified air and an incubator control plate were tested in parallel with the plate which received the test agent.
Figure 1.
Schematic of CCES dilution manifold and exposure plates. A) Dynamic headspace generator design used for chemical generation and transport to the exposure manifold. B) The cell culture exposure system (CCES) includes a diffusion humidifier designed to regulate relative humidity levels in the dilution air before being passed through a dilution manifold. Each diluted chemical concentration is transported to a sampling port which allows real-time concentration analysis before being delivered to four wells containing inserts (n=4 per dose). For visual clarity, only tubing for the first three doses are shown. A separate control plate receives only humidified dilution air for the duration of the exposure. Both the VOC and sham exposure plates are enclosed in a warmed, humidified enclosure, while the incubator control remains in the 37°C 5% CO2 cell culture incubator.
Test Agent Selection and Generation
As part of this pilot study, we selected eight test agents (Table 1) that are relatively common industrial chemicals and have a substantial body of research, including epidemiologic and in vivo inhalation exposure studies. Additionally, all the test agents herein have been assessed and assigned occupational exposure limits by professional associations such as the American Conference of Governmental Industrial Hygienists (ACGIH). We utilized the ACGIH TLV to determine the concentration range tested, starting at 10-fold higher than the recommended TLV. A preliminary range finding experiment was conducted in BEAS-2B cells in order to confirm the highest dose did not exceed 50% loss of cell viability. Each chemical was generated based on laboratory specifications as the state which it was procured from the manufacturer. Acrolein, acetaldehyde, and 1,3-butadiene were obtained at a certified gas concentration with the balance being air or nitrogen. 1-Bromopropane, dichloromethane, trichloroethylene and carbon tetrachloride were obtained from the vendors as liquids with certification of analysis for purity. Formaldehyde was generated from solid paraformaldehyde. All vendor information, purity, and specifics to generation methods are listed in Table 1.
Table 1.
Test agent generation parameters.
| Chemical Name | CAS # | Vendor | Catalog Number | Gas Mixture or Lot # and Purity | GC Conditions/Methodology | Generation Method | Preparation for Method |
|---|---|---|---|---|---|---|---|
| Acrolein | 107-02-8 | Airgas National Welders | Custom | Acrolein in Nitrogen at 1011 PPM | Isothermal Oven Temperature: 28 °C held for 2.8 min; Column Flow: 10 mL/min; Splitless inlet at 200 °C; FID detector at 250C | Gas Cylinder | Acrolein in Nitrogen Target = 1000 PPM, Actual = 1011 PPM, Accuracy = ±2% |
| Acetaldehyde | 75-07-0 | Airgas National Welders | Custom | Acetaldehyde in Nitrogen at 2064 PPM | Isothermal Oven Temperature: 45 °C held for 1.5 min; Column Flow: 10 mL/min; Splitless inlet at 200 °C; FID detector at 250C | Gas Cylinder | Acetaldehyde in Nitrogen, Target = 2000 PPM, Actual = 2064 PPM, Accuracy = ±2% |
| 1-Bromopropane | 106-94-5 | Sigma Aldrich | B78106 | Lot #: WXBC0691V Purity: 99.8% | Isothermal Oven Temperature: 45 °C held for 1.5 min; Column Flow: 10 mL/min; Splitless inlet at 200 °C; FID and ECD detector at 250C | Liquid in vial | Chemical placed in vial (25mL) with orifice which was placed into the dynamic headspace generator to carry VOCs in air stream into chamber |
| Formaldehyde (paraformaldhyede) | 50-00-0 (30525-89-4) | Sigma Aldrich | 158127 | Lot #: STBB9507V Purity: 94.1% | Non-dispersive infrared analyzers | Solid in vial | Chemical placed in vial (25mL) with orifice which was placed into the dynamic headspace generator to carry VOCs in air stream into chamber |
| 1,3-Butadiene | 106-99-0 | Airgas National Welders | Custom | 1,3 Butadiene in Air at 511 PPM | Isothermal Oven Temperature: 27 °C held for 1.0 min; Column Flow: 10 mL/min; Splitless inlet at 200 °C; FID detector at 250C | Gas Cylinder | 1,3 Butadiene in Air Target = 500 PPM, Actual = 511 PPM, Accuracy = ±2% |
| Dichloromethane | 75-09-2 | Burdick & Jackson | 300-4 | Lot #: C0674 Purity: 99.9% | Isothermal Oven Temperature: 45 °C held for 2.5 min; Column Flow: 10 mL/min; Splitless inlet at 200 °C; FID and ECD detector 250C | Liquid in vial | Chemical placed in vial (25mL) with orifice which was placed into the dynamic headspace generator to carry VOCs in air stream into chamber |
| Trichloroethylene | 79.01-6 | Sigma Aldrich | 251402 | Lot #: SHBJ3703 Purity: 99.98% | Isothermal Oven Temperature: 60 °C held for 4 min; Column Flow: 10 mL/min; Splitless inlet at 200 °C; FID and ECD detector at 250C | Liquid in vial | Chemical placed in vial (25mL) with orifice which was placed into the dynamic headspace generator to carry VOCs in air stream into chamber |
| Carbon Tetrachloride | 56-23-5 | Sigma Aldrich | 319961 | Lot #: SHBJ0149 Purity: 99.90% | Isothermal Oven Temperature: 60 °C held for 4 min; Column Flow: 10 mL/min; Splitless inlet at 200 °C; FID and ECD detectors at 250C | Liquid in vial | Chemical placed in vial (25mL) with orifice which was placed into the dynamic headspace generator to carry VOCs in air stream into chamber |
The gaseous test agents were used as purchased and the liquid and solid test agents were generated using our in-house dynamic headspace generator, consisting of a sealed vessel (unjacketed pressure filtering reactor, P/N-6384–235; Ace Glass, Vineland, NJ) contained in a warming oven (Heratherm general protocol oven P/N-51028873; ThermoFisher Scientific, Waltham, MA) held at a chemical specific temperature. The sealed vessel contained a reservoir of liquid or solid chemical and had both a carrier gas inlet and outlet that allowed the fixed carrier flow to transport the volatilized chemical vapors. The test agent was then diluted by our in-house compressed air system which provides breathing-quality air, according to the ASTM standards. This pressure-regulated air was delivered to three mass flow controllers, allowing the dilution of test agent (gas or vapor) to be accurately metered and delivered to the first dilution tee on the CCES dilution manifold.
The CCES dilution manifold added the desired quantity of humidified air (80–85% RH at 37°C), to the generated test agent at six points to facilitate a half-log dilution concentration curve (Fig 1b). During exposure, real-time concentrations were measured at each dilution nozzle pair prior to exposing the cells directly (Fig 1a). Concentrations were determined by injecting a 1 mL sample from each chamber inlet nozzle on a Hewlett Packard 5890II or Agilent 6890 gas chromatograph (GC) with a Supelco SBP-624 capillary GC column (30 m × 0.53 mm) coupled to flame ionization detector (FID) or electron capture detector (ECD). Both GCs used hydrogen as the carrier gas at a flowrate of 10 mL/min. The inlets, detectors and oven temperatures were test agent specific and were chosen to give a retention time (RT) of six minutes or less to allow two samples per inlet nozzle and quality control samples. A graphical representation of the actual GC-measured concentrations and the dynamic headspace generator nominal concentration based on dilution are recorded in Supplemental Figure 1.
CellTiter-Glo Luminescent Cell Viability Assay
Cell viability was measured by determining the amount of adenosine triphosphate (ATP) synthesized inside the cells using the Promega CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega, Madison, WI). Total cell material was lysed, and ATP was collected on an opaque Corning 96-well plate (Corning, NY) using the reagents from the kit (CellTiter-Glo® Substrate (lyophilized), and CellTiter-Glo® Buffer) and the cell growth media associated with the designated cell type being analyzed. Luminescence was then detected using a SpectraMax® i3 Multi-Mode Microplate Detection Platform plate reader (Molecular Devices, San Jose, CA). An ATP standard curve was used as a positive control and as a quality control parameter to quantify the amount of ATP present based on the luminescence values. This control was made by combining stock ATP standard (Sigma-Aldrich, St. Louis, MO) with the working reagent and performing a serial dilution at μM concentrations of 50, 25, 12.5, 6.25, 3.125, and 0. Data shown are mean ± SEM from 3 separate biological replicates. A one-way ANOVA and Fisher’s LSD post-hoc test was used to determine statistically meaningful differences compared to the sham control exposed cells (p≤0.05). Statistical analysis was conducted using GraphPad Prism software (San Diego, CA).
LDH Measurement of Cytotoxicity
Lactate dehydrogenase (LDH) as a measurement of cytotoxicity was determined by sampling the basolateral media from each exposed cell sample 4 h post-exposure. Fifty μL of the basolateral medium from each well were transferred in duplicate to a 96-well clear polystyrene culture plate (Corning, NY). Additionally, three sets of controls were added to the 96-well plate. First, an LDH solution provided as part of the Pierce™ LDH kit (Rockford, IL) was utilized as a positive control. Second, blanks were measured using the corresponding cell culture media. Finally, a completely lysed insert that represented a measure of total (100%) LDH was created by adding lysis buffer to a single incubator control replicate. The LDH reaction was performed using the LDH measurement kit, and the plate was read in a Spectramax i3 (San Jose, CA) with the absorbance set to read at 490 and 680 nm wavelengths. After reading the plate, the raw data was exported into an excel spreadsheet for analysis. Each sample was blank corrected and calculated as percent change from the sham control in relation to the mean of the 100% LDH lysed control inserts. Data shown are mean ± SEM from three separate biological replicates. A one-way ANOVA and Fisher’s LSD post-hoc test was used to determine statistically meaningful differences compared to the sham control exposed cells (p≤0.05). Statistical analysis was conducted using GraphPad Prism software (San Diego, CA).
Whole Cell Lysate Collection
An optimized whole cell lysis procedure was used to preserve the RNA of both BEAS-2B and pHBEC samples. After a 4 h recovery post 2 h test agent exposure, the two technical replicate inserts from each exposure condition were placed on a new 24-well plate (Corning, NY). A single replicate insert of the incubator control cells was also preserved for whole cell lysis. Prior to adding the lysis buffer, the cells were thoroughly washed twice with DPBS to remove any remaining apical mucus or surfactant which may interfere with complete lysis. When transferring the BEAS-2B cells or pHBECs, the BioSpyder lysis buffer (Carlsbad, CA) was added to each of the samples and allowed to incubate for 15 minutes at room temperature. Each sample was mixed and pipetted into an individual well in the 384-well optical imaging plate (Corning, NY). After placing each of the samples into the 384-well plate, it was sealed with ThermalSeal RT 2mil Film (Sigma-Aldrich, MO) and stored in a −80°C freezer until shipment to BioSpyder for analysis.
BioSpyder TempO-Seq Methodology
Plates were shipped to BioSpyder, Inc. frozen (on dry ice) using overnight priority shipping. BEAS-2B and pHBEC lysates were then analyzed by BioSpyder using the TempO-Seq human whole transcriptome version 2 (hWTv2) assay (Yeakley et al. 2017), which includes 22,537 probes covering 19,703 genes. Lysates were processed as described previously (House et al. 2017). In brief, 2 μL of each lysate was hybridized with 2 μL of detector oligos from the hWTv2 assay using the following thermal cycler protocol: 10 min at 70°C, followed by gradual decrease to 45°C over 49 min, terminating with 45°C incubation for 16–24 h. Excess oligos were then removed via nuclease digestion (90 min at 37°C), and hybridized detector oligos were ligated (1 h at 37°C) following respective additions of 24 μL TempO-Seq nuclease and ligation mixes. RNA/DNA duplexes were then heat-denatured, and 10 μL of each ligation product were transferred to an amplification microplate containing 10 μL of PCR master mix per well. Ligation products were then uniquely labeled during product amplification with well coordinate-specific “barcoded” primer pairs containing universal adaptors for sequencing. Samples were pooled into sequencing libraries, distributed across multiple lanes of a HiSeq dual flow cell and analyzed on a HiSeq 2500 Ultra-High-Throughput Sequencing System (Illumina, San Diego, CA). The target depth for each test sample was 6 × 106 total sequenced reads.
HTTr data processing
Raw TempO-Seq data were provided by the vendor as individual FASTQ files for each sample well and were subsequently processed through a custom bioinformatics pipeline previously described (Harrill et al. 2021). Each FASTQ file was aligned to the probe sequences in the hWTv2 assay using HISAT2 v2.1.0 (Kim et al. 2015; Kim et al. 2019) with spliced alignment disabled. Aligned reads in SAM format were processed with SAMtools v1.9 (Li et al. 2009) to compute the number of uniquely aligned reads for each probe. Probe counts and associated metadata for each well were stored for analysis using MongoDB v3.6.14. Source code can be found at https://github.com/USEPA/httrpl_pilot.
HTTr Quality Control
Sample level quality control (QC) criteria were based on the cell viability results for each concentration of the test agents, multiple well-level metrics computed from read mapping rate, and count distribution across probes, as previously described (Harrill et al. 2021). To be considered for BMD analysis, the thresholds for fraction of viable cells (FrVC) and fraction of reads uniquely mapped to probes (FMR) are simple majority cutoffs (≥ 50% of cells must be viable; ≥ 50% of reads must uniquely map to probe sequences). The threshold for number of uniquely mapped reads (NMR) was set at 10% of target per-sample read depth (≥ 600,000 genes). Additional QC metrics outlined in our previous HTTr studies, including Ncov5, Nsig80, and GiC were optimized specifically for MCF7 cells in true high-throughput screenings and could not be applied as global threshold levels for this limited pilot study design.
BMD Gene Expression Analysis
Transcriptional benchmark dose (BMD) values at the probe level were computed and aggregation of BMD values to the gene set level was performed with BMDExpress2 (Phillips et al. 2019) based on the NTP approach to genomic dose-response modeling (NTP 2018) and previously published manuscripts (Harrill et al. 2021). First, probe-level concentration response analysis was performed as follows: Probe counts for each test agent and corresponding sham exposure controls (subset to samples without QC flags, and probes with mean count > 5) were normalized to log2 counts per million (CPM) values using the sum of filtered probe counts as the sample depth and addition of a pseudo-count of 1 before converting to log scale. For each test agent, probe-level log2 CPM values were input to BMDExpress2 using the following optimized parameters: Pre-filtering was used to remove probes that cannot pass an ANOVA test (FDR p-value ≤ 0.05) with a maximal fold-change < 2x across all concentrations. Each probe passing this pre-filter was then fit to eight different dose-response models (linear, poly2, power, Hill, exp2, exp3, exp4, and exp5). The best-fit model for each probe was selected based on the lowest Akaike information criterion (AIC). Models with a goodness of fit p-value < 0.1 and Hill models with k parameter < 1/3 the lowest positive concentration were excluded from final model selection (Akaike 1974; Harrill et al. 2021). The benchmark response (BMR) was set to 1.349 × standard deviation of sham exposure control samples, corresponding to 10% tail in a normal distribution. The BMD value is the concentration at which the winning model curve crosses the benchmark response level (BMR) (Filipsson et al. 2003; Thomas et al. 2007; Yang et al. 2007).
The overall potency of a test agent was further analyzed by aggregating the probe-level BMDs from the best fit models to estimate a biological pathway altering concentration (BPAC) based on the median BMD value for the most sensitive gene set. For the purposes of this study, we used the Molecular Signatures Database v7.2 (MSigDB_C2) collection of annotated gene sets. Briefly, probe-level curve-fits were filtered to only those meeting the following criteria: best fit model produced convergent BMD, BMDL, and BMDU values; BMD < highest measured concentration; BMDU:BMDL ratio < 40; and probe annotated as measuring a single gene. If multiple probes corresponding to the same gene had valid curve fits under this criterion, the gene-level BMD/BMDL/BMDU were taken as the average of all probes with valid curve fits. BMD bounds (i.e. BMDL and BMDU) were computed in accordance with the profile likelihood method (Banga et al. 2002; NTP 2018). The BPAC was computed as the median BMD for all associated genes passing the above filters in the most sensitive gene set. Only gene sets containing at least three valid genes and 5% gene set coverage were retained in our analysis(Harrill et al. 2021; NTP 2018). Additional analysis was conducted to determine the reason probe-level responses were not always captured in gene set results (Figure 4).
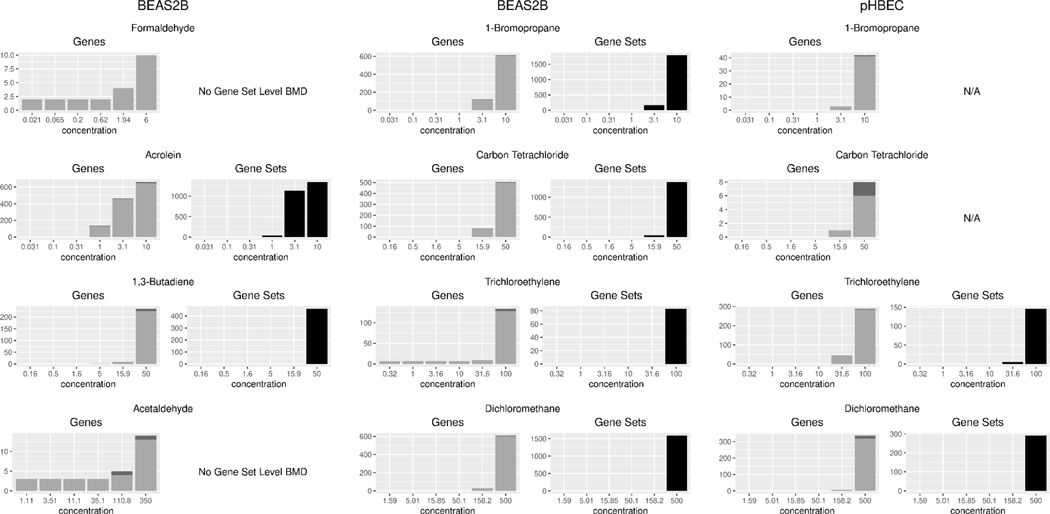
Figure 4:
Bar plot of Gene and Gene Set level accumulation as a function of VOC test concentration for each cell type and chemical combination tested. Each bar represents the number of genes or gene sets that had a BMD less than or equal to the test concentration. Light grey and black shaded bars represent genes or gene sets (respectively) which meet all criteria and were analyzed while the dark grey portion of the bar represents those gene targets which were not annotated in the MSigDB gene set collection and did not contribute to gene set level BMDs. Sample size n=3 per test condition.
Determination of Reference TLV and NOAEL/LOAEL Values
Threshold Limit Values (TLVs) were obtained from the ACGIH® 2018 Guide to Occupational Exposure Values. LOAEL and NOAEL values were obtained by examining the most recent risk assessment conducted by EPA or ATSDR. A complete list of examined studies and LOAEL/NOAEL values is captured in Table 4. When recording representative values in Table 3, acute or sub-chronic inhalation exposures performed in mice or rats with respiratory/pulmonary endpoints were prioritized, and the most sensitive finding was reported. The 6 h exposures are the most prevalent in vivo study design that can be simulated in the CCES optimized full exposure model (2 h test agent exposure, 4 h post). In the case of dichloromethane, sub-chronic 13-week respiratory LOAEL and NOAEL values were identified. Inhalation trichloroethylene studies were quite limited, but many reported changes in auditory and visual function. Immunosuppression LOAEL and NOAEL values were selected because pulmonary endpoints were assessed for immunosuppressive changes (e.g., function of alveolar macrophages). Both subchronic and chronic inhalation studies with carbon tetrachloride were identified, despite no LOAEL and NOAEL values subcategorized into organ systems and all be loosely defined as hepatic changes.
Table 4:
Representative studies obtained from the ATSDR and IRIS documentation with respective reported NOAEL and LOAEL values. Abbreviations: LOAEL = lowest-observed-adverse-effect level; NOAEL = no-observed-adverse-effect level; NR = not reported.
| 1,3-Butadiene (ASTDR 2012) | |||||
|---|---|---|---|---|---|
| System/Observations | Species | NOAEL (ppm) | LOAEL (ppm) | Exposure Conditions | References |
| Respiratory | Rat | 8000 | NR | Sub-chronic: 13 wk 5 d/wk 6 hr/d | (Crouch et al. 1979) |
| Respiratory | Mouse | 8000 | NR | Sub-chronic: 14 wk 5 d/wk 6 hr/d | (NTP 1984) |
| Respiratory: alveolar epithelial hyperplasia after 40 wks | Mouse | NR | 200 | Sub-chronic: 13-52 wk 6 hr/d 5 d/wk | (NTP 1993) |
| Respiratory: alveolar epithelial hyperplasia | Mouse | 200 | 625 | Sub-chronic: 40 wk 6 hr/d 5 d/wk | (NTP 1993) |
| Respiratory Cancer: alveolar/bronchiolar adenoma/carcinoma | Mouse | NR | 200 | Sub-chronic: 13-52 wk 6 hr/d 5 d/wk | (NTP 1993) |
| Respiratory: increased organ weight, metaplasia | Rat | 1000 | 8000 | Chronic: 105-111 wk 5 d/wk 6 hr/d | (Owen et al. 1987); (Owen and Glaister 1990) |
| Respiratory: atrophy of nasal olfactory epithelium | Mouse | 625 | 1250 | Chronic: 61 wk 5 d/wk | (NTP 1984) |
| Respiratory: alveolar epithelial hyperplasia | Mouse | 6.25 | NR | Chronic: 2 yr 6 hr/d 5 d/wk | (NTP 1993) |
| Respiratory Cancer: alveolar/bronchiolar adenoma or carcinoma | Mouse | NR | 625 | Chronic: 61 wk 5 d/wk 6 hr/d | (NTP 1984) |
| Respiratory Cancer: alveolar/bronchiolar adenoma or carcinoma | Mouse | NR | 6.25 | Chronic: 2 yr 6 hr/d 5 d/wk | (NTP 1993) |
| Dichloromethane (USEPA 2011a) | |||||
| System/Observations | Species | NOAEL (ppm) | LOAEL (ppm) | Exposure Conditions | References |
| Pulmonary: foreign body pneumonia | Rat | 4200 | 8400 | Sub-chronic: 13 wk 5 d/wk 6 hr/d | (NTP 1986a) |
| Pulmonary lesions | Mouse | 8400 | NR | Sub-chronic: 13 wk 5 d/wk 6 hr/d | (NTP 1986a) |
| Pulmonary: cara cell vacuolation | Mouse | 4000 | NR | Sub-chronic: 13 wk (5 d/wk 6 hr/d) | (Foster et al. 1992) |
| Hepatic: hepatocyte vacuolation and necrosis; hemosiderosis in liver; renal tubular degeneration | Rat | NR NR 1000 |
1000 1000 2000 |
Chronic: 2 yrs (5 d/wk, 6 hr/d) | (Mennear et al. 1988); (NTP 1986a) |
| Hepatic: hepatocyte degeneration; renal tubule casts | Mouse | NR NR |
2000 2000 |
Chronic: 2 yrs (5 d/wk, 6 hr/d) | (Mennear et al. 1988); (NTP 1986a) |
| Hepatic: hepatocyte vacuolation; hepatocyte necrosis | Rat | NR 500 |
500 1500 |
Chronic: 2 yrs (5 d/wk, 6 hr/d) | (Burek et al. 1984) |
| Hepatic: hepatocyte vacuolation | Rat | 200 | 500 | Chronic: 2 yrs (5 d/wk, 6 hr/d) | (Nitschke et al. 1988) |
| Trichloroethylene (USEPA 2011b) | |||||
| System/Observations | Species | NOAEL (ppm) | LOAEL (ppm) | Exposure Conditions | References |
| Immunosuppression: ↓ pulmonary bactericidal activity assay | Mouse | 2.6 | 5.2 | Acute and Acute Repeated: 1 or 5 d (3hr/d) | (Aranyi et al. 1986) |
| Immunosuppression: dose-dependent changes in bacterial clearance from lung and phagocytic function of alveolar macrophages | Mouse | 25 | 50 | Acute: Single 3 hr exposure | (Selgrade and Gilmour 2010) |
| Immunosuppression | Rat | 300 | 1000 | Sub-chronic: 4 wk (5 d/wk, 6 hrs/d) | (Woolhiser et al. 2006) |
| Carbon Tetrachloride: (USEPA 2010b) | |||||
| System/Observations | Species | NOAEL (ppm) | LOAEL (ppm) | Exposure Conditions | References |
| Hepatic: fatty change in liver | Rat | ND | 50 | Sub-chronic: 10.5 mos (8 hr/d, 5 d/wk) | (Smyth et al. 1936) |
| Hepatic: increased liver weight; fatty degeneration in liver | Rat | 5 | 10 | Sub-chronic: 6 mos (7 hr/d, 5 d/wk) | (Adams et al. 1952) |
| Hepatic: reduced body weight gain; enlarged liver with fatty change | Rat | 1 | 10 | Sub-chronic: 13 wks (24 hr/d, 7 d/wk) | (Prendergast et al. 1967) |
| Hepatic: increased liver weight; fatty change in liver | Rat | ND | 10 | Sub-chronic: 13 wks (6 hr/d, 5 d/wk) | (Nagano et al. 2007b) |
| Hepatic: slight cytological alterations in the liver | Mouse | ND | 10 | Sub-chronic: 13 wks (6 hr/d, 5 d/wk) | (Nagano et al. 2007b) |
| Hepatic: increased ALT, SDH; necrosis in liver | Rat | 20 | 100 | Sub-chronic: 12 wks (6 hr/d, 5 d/wk) | (Benson and Springer 1999) |
| Hepatic: increased ALT, SDH; necrosis and cell proliferation in liver | Mouse | 5 | 20 | Sub-chronic: 12 wks (6 hr/d, 5 d/wk) | (Benson and Springer 1999) |
| Hepatic: reduced body weight gain, lesions in the liver (fatty changes, fibrosis, cirrhosis) | Rat | 5 | 25 | Chronic: 2 yrs (5 d/wk, 6 hr/d) | (Nagano et al. 2007a) |
| Hepatic: reduced survival late in study (because of liver tumors); reduced body weight gain | Mouse | 5 | 25 | Chronic: 2 yrs (5 d/wk, 6 hr/d) | (Nagano et al. 2007a) |
| Acrolein: (USEPA 2003) | |||||
| System/Observations | Species | NOAEL (ppm) | LOAEL (ppm) | Exposure Conditions | References |
| Respiratory: nasal necrosis of respiratory epithelium and increased proliferation | Rat | ND | 0.25 | Acute Repeated: 3 d (6 hr/d) | (Cassee et al. 1996) |
| Respiratory: bronchial necrosis and pulmonary edema, parenchymal restriction, increase in lung collagen | Rat | ND | 0.4 | Sub-chronic: 62 d (6 hr/day 5 days/wk) | (Kutzman 1981) (Kutzman et al. 1985) (Costa et al. 1986) |
| Acetylaldehyde: (USEPA 1991) | |||||
| System/Observations | Species | NOAEL (ppm) | LOAEL (ppm) | Exposure Conditions | References |
| Respiratory: degeneration of olfactory epithelium | Rat | 150 | 400 | Acute Repeated: 5 d (6 hr/d) | (Appelman et al. 1982) (Appelman et al. 1986) |
| 1-Bromopropane: (USEPA 2020a) | |||||
| System/Observations | Species | NOAEL (ppm) | LOAEL (ppm) | Exposure Conditions | References |
| Respiratory: pulmonary edema and emphysema | Rat | 6040 | NR | Acute: 4 hr | (Elf Atochem 1997) |
| Respiratory: nasal lesions | Rat | 250 | NR | Sub-chronic: 16 d (6 hr/d, 5 d/wk) | (NTP 2011) |
| Respiratory: histopathologic changes in nasal cavities | Rat | 994 | NR | Sub-chronic: 4 wks (6 hr/d, 5 d/wk) | (ClinTrials 1997) |
| Respiratory | Rat | 800 | NR | Sub-chronic: 12 wks (8 hr/d, 7 d/wk) | (Ichihara et al. 2000) |
| Respiratory | Rat | 1800 | NR | Sub-chronic: 8 wks (6 hr/d, 5 d/wk) | (Kim et al. 1999) |
| Respiratory | Rat | 600 | NR | Sub-chronic: 13 wks (6 hr/d, 5 d/wk) | (ClinTrials 1997) |
| Respiratory | Rat | 1000 | NR | Sub-chronic: 14 wks (6.2 hr/d, 5 d/wk) | (NTP 2011) |
| Respiratory | Rat | 750 | NR | Sub-chronic: 6 hr/day during premating (≥ 70 days), mating, and until weaning of offspring | (WIL 2001) |
| Respiratory: nasal and pulmonary lesions | Mouse | 250 | NR | Sub-chronic: 17 d (6.2 hr/d, 5 d/wk) | (NTP 2011) |
| Respiratory: cytoplasmic vacuolization in the nose, larynx, trachea, and lung | Mouse | 250 | NR | Sub-chronic: 14 wks (6.2 hr/d, 5 d/wk) | (NTP 2011) |
| Respiratory: histopathological lesions in the nasal respiratory epithelium, larynx, trachea, and bronchioles | Mouse | NR | 62.5 | Chronic: 105 wks (6.2 hr/d, 5 d/wk) | (NTP 2011) |
| Respiratory: chronic nasal inflammation and squamous metaplasia in larynx | Rat | 125 | NR | Chronic: 105 wks (6.2 hr/d, 5 d/wk) | (NTP 2011) |
| Formaldehyde: (USEPA 2010a) | |||||
| System/Observations | Species | NOAEL (ppm) | LOAEL (ppm) | Exposure Conditions | References |
| Respiratory: histopathologic changes | Rat | NR | 10 | Acute: 4 hr | (Bhalla et al. 1991) |
| Respiratory: histopathologic lesions to the respiratory epithelium | Mouse | NR | 3.13 | Acute Repeated: 5d (6 hr/d, 5 d) | (Buckley et al. 1984) |
| Respiratory: histopathologic lesions to the nasal conchae, lateral wall, and ventral nasal conchae. | Rat | 2 | NR | Acute and Acute Repeated: 1, 2, or 4 d (6 hr/d) | (Monteiro-Riviere and Popp 1986) |
| Respiratory: histopathologic lesions in the nasal turbinates and trachea | Rat | NR | 15 | Acute: 6 hr | (Kamata et al. 1996b) (Kamata et al. 1997) |
| Respiratory: cellular necrosis to the nasal epithelium | Rat | NR | 6 | Acute: 6 hr | (Chang et al. 1983) |
| Respiratory: phospholipid content was reduced in lung surfactant | Rat | NR | 128.4 | Acute: 6 hr | (Kamata et al. 1996a) (Kamata et al. 1997) |
| Respiratory: biochemical changes in lung homogenates | Rat | NR | 15 | Acute: 6 hr | (Kamata et al. 1996b) (Kamata et al. 1997) |
| Respiratory: squamous metaplasia | Mouse | 4 | NR | Sub-chronic: 13 wks (6 hr/d, 5 d/wk) | (Maronpot et al. 1986) |
| Respiratory: squamous metaplasia, epithelial hyperplasia | Rat | 1 | NR | Sub-chronic: 13 wks (6 hr/d, 5 d/wk) | (Zwart et al. 1988) |
| Respiratory: rhinitis, epithelial hyperplasia, and squamous metaplasia | Rat | 1 | NR | Sub-chronic: 4, 8, or 13 wks (6 hr/d, 5 d/wk) | (Feron et al. 1988) |
| Respiratory: keratinized and nonkeratinized squamous metaplasia | Rat | 1 | NR | Sub-chronic: 13 wks (6 hr/d, 5 d/wk) | (Zwart et al. 1988) |
| Respiratory: rhinitis and nasal lesions | Rat | 1 | NR | Sub-chronic: 26 wks (22 hr/d, 7 d/wk) | (Rusch et al. 1983) |
| Respiratory: decrease in zinc content of lung, increase in iron content | Rat | NR | 5 | Sub-chronic: 4 or 13 wks (8 hr/d, 5 d/wk) | (Özen et al. 2003) |
| Respiratory: increased P450 levels | Rat | 0.5 | NR | Sub-chronic: 1-4 days, 12 wks, or 24 wks (6 hr/d, 5 d/wk) | (Dallas et al. 1989) |
| Respiratory: histological changes in tracheobronchial epithelial | Mice | NR | 41 | Chronic: up to 35 weeks (1 hr/d, 3 d/wk) | (Horton et al. 1963) |
| Respiratory: rhinitis, hyperplasia, dysplasia, and squamous metaplasia of the nasal epithelium; atrophy of the olfactory epithelium; glandular adenitis and nasolacrimal duct hyperplasia and metaplasia. | Mice | NR | 2 | Chronic: 24 mo (6 hr/d, 5 d/wk) | (Swenberg et al. 1980) (Kerns et al. 1983) (CIIT 1982) (Battelle 1981) |
| Respiratory: squamous metaplasia and dysplasia | Rat | NR | 12.4 | Chronic: 104 wks (6 hr/d, 5 d/wk) | (Holmström et al. 1989) |
| Respiratory: increased rhinitis, hyperplasia, and squamous metaplasia of the nasal respiratory epithelium | Rat | NR | 0.3 | Chronic: 28 mo (6 hr/d, 5 d/wk) | (Tobe et al. 1985) |
| Respiratory: squamous cell metaplasia and epithelial hyperplasia | Rat | NR | 0.3 | Chronic: 28 mo (6 hr/d, 5 d/wk) | (Kamata et al. 1997) |
| Respiratory: squamous metaplasia, epithelial hyperplasia, and polyps/papillomas | Rat | NR | 15 | Chronic: Lifetime (6 hr/d, 5 d/wk) | (Albert et al. 1982) (Sellakumar et al. 1985) |
| Respiratory: nasal lesions, squamous metaplasia and epithelial dysplasia, hyperkeratosis, goblet cell hyperplasia, and rhinitis | Rat | NR | 2 | Chronic: 24 mo (6 hr/d, 5 d/wk) | (Swenberg et al. 1980) (Kerns et al. 1983) (CIIT 1982) (Battelle 1981) (Morgan et al. 1986) |
| Respiratory: olfactory degeneration, squamous metaplasia, epithelial hypertrophy and hyperplasia, and mixed inflammatory cell infiltrate | Rat | NR | 2 | Chronic: 24 mo (6 hr/d, 5 d/wk) | (Monticello et al. 1996) |
| Respiratory: rhinitis, hyperplasia, and metaplasia | Rat | 1 | NR | Chronic: 13 or 52 wks (6 hr/d, 5 d/wk) | (Appelman et al. 1988) (Zwart et al. 1988) |
Table 3.
Summary of analysis showing the BPAC for each chemical tested across both cell types. Includes comparison to 2018 ACGIH TLV and reported LOAEL and NOAEL values from in vivo inhalation studies reviewed in the EPA IRIS toxicological reviews for each respective chemical (www.epa.gov/iris). Abbreviations used: N/A: no gene set met criteria to calculate a BPAC, NR: not reported in the literature reference. ‘--’ indicates that the chemical was not tested in pHPEC cells.
| Chemical Name | BEAS-2B BPAC (ppm) | pHPEC BPAC (ppm) | Representative LOAEL (ppm) | Representative NOAEL (ppm) | TLV (ppm) |
|---|---|---|---|---|---|
| Acrolein | 0.586 | -- | 0.25 | NR | 0.1 |
| 1-Bromopropane | 2.246 | N/A | NR | 6040 | 0.1 |
| Formaldehyde | N/A | -- | 6 | NR | 0.3 |
| 1,3-Butadiene | 13.979 | -- | 200 | NR | 10 |
| Carbon Tetrachloride | 9.563 | N/A | 20 | 5 | 10 |
| Acetaldehyde | N/A | -- | 400 | 150 | 25 |
| Trichloroethylene | 44.842 | 28.148 | 50 | 25 | 50 |
| Dichloromethane | 142.127 | 226.73 | 8400 | 4200 | 100 |
Results:
Evaluation of cell viability and cytotoxicity for each test agent
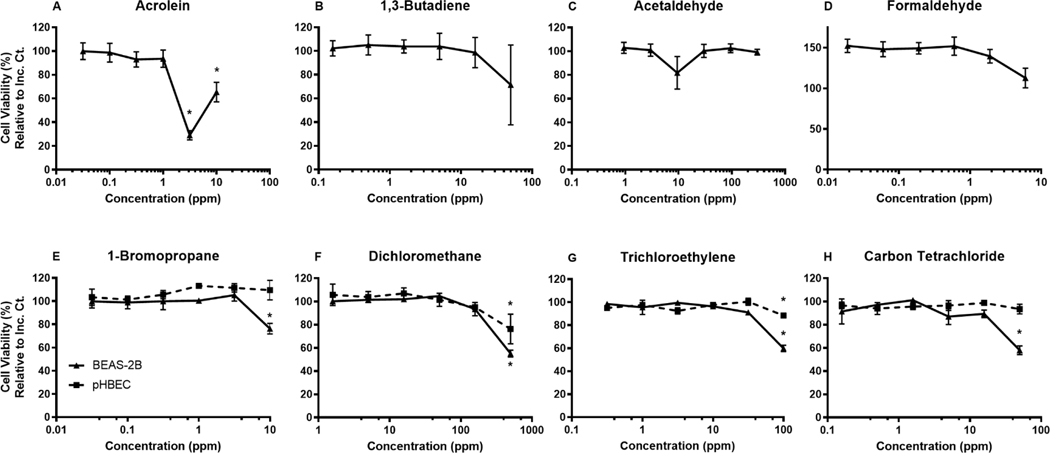
We designed our exposure conditions to elicit no greater than 50% change to cell viability when compared to the sham exposure control. As described, cell viability was measured by cellular ATP levels in the CellTiter-Glo assay and cytotoxicity was measured by LDH release. In general, the same concentrations were tested in both the BEAS-2B and the pHBECs. As a baseline, we observed no statistical difference between the sham exposed cells and the incubator control cells (Supplemental figure 2). In the four VOCs tested in both cell lines, the pHBECs exhibited less change to cytotoxicity and cell viability at the highest concentration when compared to the BEAS-2B cells exposed to the same test agent.
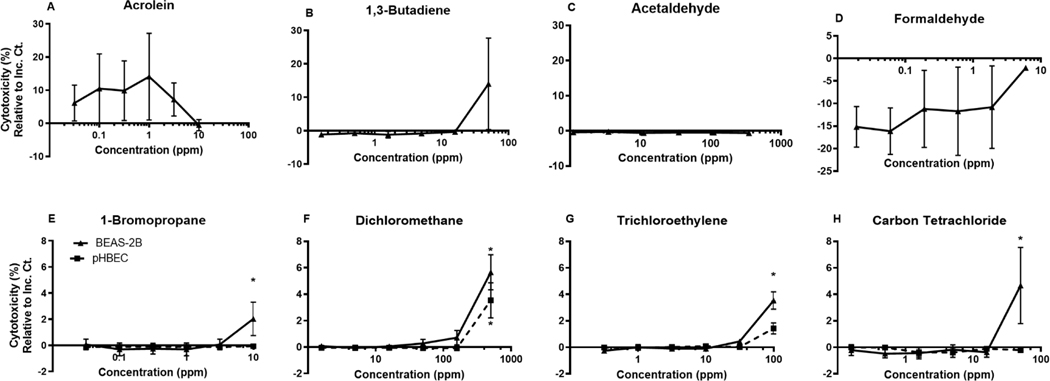
Cytotoxicity was measured by LDH present in the basolateral compartment post-exposure by percent change relative to the sham control and the 100% lysed cell insert (Figure 2). Many test agent concentrations tested yielded no significant change in cytotoxicity compared to the sham exposure control. However, 1-bromopropane exposure at 10 ppm and carbon tetrachloride at 50 ppm induced a statistically significant increase in BEAS-2B cytotoxicity. Fifty ppm dichloromethane and 100 ppm trichloroethylene caused a significant increase in cytotoxicity in both BEAS-2Bs and pHBECs. All observed significant changes to cytotoxicity fell below a 10% change compared to the sham exposure control.
Figure 2.
Dose response of each respective chemical as measured by LDH release presented as % Change in Cytotoxicity compared to the filtered air control and 100% lysed control. BEAS-2B and pHBEC cells exposed to chemicals A. Acrolein, B. 1,3-Butadiene, C. Acetaldehyde, D. Formaldehyde, E. 1-Bromopropane, F. Dichloromethane, G. Trichloroethylene, H. Carbon Tetrachloride. Mean±SEM, n=3, *p≤0.05.
In Figure 3, when measuring percent cell viability compared to the sham exposure control, BEAS-2B cells exposed to acrolein, 1-bromopropane, dichloromethane, trichloroethylene, and carbon tetrachloride yielded a significant change at the highest tested concentration. Concentrations of 10 and 3.125 ppm acrolein caused the largest observed reduction in viability for BEAS-2B cells. Acrolein at 3.125 ppm had the most impact, reducing cell viability down to below 30%. Again, pHBECs are expectedly more robust and resistant to chemical perturbations compared to the BEAS-2B cells and only displayed a significant change to cell viability when exposed to dichloromethane and trichloroethylene exposure at the highest concentration tested, 500 ppm and 100 ppm respectively.
Figure 3.
Dose response of each respective chemical as measured by CellTiter-Glo ATP availability and presented as % Cell Viability compared to the filtered air control. BEAS-2B and pHBEC cells exposed to chemicals A. Acrolein, B. 1,3-Butadiene, C. Acetaldehyde, D. Formaldehyde, E. 1-Bromopropane, F. Dichloromethane, G. Trichloroethylene, H. Carbon Tetrachloride. Mean±SEM, n=3, *p≤0.05.
BMDExpress Concentration-response Analysis
In Table 2, we report median BMD and BMDL values for the top ten most sensitive gene sets meeting our criteria for each test agent and cell type combination tested. Although several major inflammatory, stress response, and cell function gene sets were altered in all VOCs tested, few common trends and overlapping targets were observed in the topmost sensitive gene sets. Ultimately, more chemical test agents need to be tested to reveal the relevance and context activation of specific gene sets. Notably, we also did not observe any overlapping gene sets between the BEAS-2B cells and pHBECs exposed to either trichloroethylene or dichloromethane. In Figure 4, gene level BMDs were calculated for all the VOCs tested in both cell types with most probes exhibiting a concentration response expression between the highest and second highest concentrations tested. Only those gene targets meeting the criteria outlined in the above methods section were considered and analyzed at the gene and gene set level. Both 1-bromopropane and carbon tetrachloride tested in pHBECs did not pass an ANOVA p ≤ 0.05 FDR cutoff and were not analyzed further for gene expression changes or BMD. Although gene level BMDs were generated, no gene sets in the MSigDB_C2 collection met our minimum cut-offs for reporting a gene set BMD in the case of formaldehyde and acetaldehyde tested in BEAS-2B cells (Figure 4). We determined that most reported genes were annotated in MSigDB and therefore considered in gene set level analysis, calculated to show 95.13% of the unique entrez gene IDs considered, were also part of the unique entrez gene IDs included in the MSigDB collection. However, these cell type/test agent conditions had a relatively small number of probes with concentration-responsive transcription in the dose range tested, resulting in the lack of reported gene set BMDs for these cases. To be reported, a gene set must contain at least three concentration-responsive gene targets passing the prefilter criteria for inclusion and those gene targets must make up at least 5% of the gene set. In the example of BEAS-2B cells exposed to carbon tetrachloride, we obtained good annotation coverage with a BMD calculated for 584 genes, the majority of which (576) were annotated and mapped to 1429 different gene sets in MSigDB.
Table 2.
Top ten most sensitive gene set collections by median BMD for each chemical and cell type tested.
| Chemical Name | Most Sensitive Gene Sets (Pathways) | Probe IDs | Median BMD (MSigDB_C2), ppm | Median BMDL (MSigDB_C2), ppm |
|---|---|---|---|---|
| Acrolein (BEAS-2B cells) | REACTOME_REGULATION_OF_CHOLESTEROL_BIOSYNTHESIS_BY_SREBP_SREBF | SQLE_6736;INSIG2_21088;CYP51A1_92320 | 0.585727 | 0.39902 |
| NAKAMURA_ADIPOGENESIS_EARLY_DN | SERPINE2_27841,SERPINE2_27842;PDE6D_13043;LOX_21772 | 0.746618 | 0.4431965 | |
| NAKAMURA_ADIPOGENESIS_LATE_DN | SERPINE2_27841,SERPINE2_27842;PDE6D_13043;LOX_21772 | 0.746618 | 0.4431965 | |
| BIOCARTA_VDR_PATHWAY | SMARCC2_12862;BAZ1B_17476;ARID1A_26387 | 0.746681 | 0.3875 | |
| REACTOME_RUNX1_INTERACTS_WITH_CO_FACTORS_WHOSE_PRECISE_EFFECT_ON_RUNX1_TARGETS_IS_NOT_KNOWN | SMARCC2_12862;RUNX1_27815,RUNX1_6060;ARID1A_26387 | 0.746681 | 0.3875 | |
| LIU_COMMON_CANCER_GENES | NDUFS8_4528;LIMD1_17670;ATP6V0A1_11775 | 0.752497 | 0.464197 | |
| ZHAN_MULTIPLE_MYELOMA_MS_DN | OAS3_87750;MRPS31_92464;LETMD1_12057;DHPS_91625 | 0.760868 | 0.3744815 | |
| REACTOME_GAMMA_CARBOXYLATION_HYPUSINE_FORMATION_AND_ARYLSULFATASE_ACTIVATION | FN3K_92980;DPH2_91691;DHPS_91625;ARSJ_16411 | 0.7900935 | 0.343939 | |
| POMEROY_MEDULLOBLASTOMA_DESMOPLASIC_VS_CLASSIC_UP | UGCG_11621;PLEC_14082;LRP8_13959;FLT3LG_25439 | 0.828844 | 0.397706 | |
| WAKABAYASHI_ADIPOGENESIS_PPARG_BOUND_36HR | EXOC6_19851;DYNLRB1_15749;CHUK_1342 | 0.834914 | 0.360823 | |
| 1-Bromopropane (BEAS-2B cells) | GAZDA_DIAMOND_BLACKFAN_ANEMIA_PROGENITOR_UP | MAFF_27478,MAFF_28315;FTH1_92999,FTH1_93000;CD83_25945 | 2.24587 | 1.8379 |
| LEE_TARGETS_OF_PTCH1_AND_SUFU_UP | RND3_24211;MAGOHB_22392;IER5_15403 | 2.25517 | 1.88619 | |
| SCHEIDEREIT_IKK_TARGETS | NFKBIA_4568;NFKB1_4566;CYLD_17180 | 2.26819 | 1.85512 | |
| EPPERT_LSC_R | TGIF2_27952;PPP1R10_16865;NAB1_13978 | 2.40606 | 1.98632 | |
| LEE_CALORIE_RESTRICTION_NEOCORTEX_UP | RELB_28340;NFKBIA_4568;MAFF_27478,MAFF_28315;EIF2AK3_24919;CNOT2_17056 | 2.45342 | 2.0422 | |
| BIOCARTA_DEATH_PATHWAY | NFKBIA_4568;NFKB1_4566;BIRC3_11879 | 2.4806 | 2.06161 | |
| IKEDA_MIR133_TARGETS_UP | NFAT5_16375,NFAT5_27581;MAFK_26036;CYLD_17180 | 2.48805 | 2.05714 | |
| ZHAN_MULTIPLE_MYELOMA_PR_DN | JMJD1C_11504;FGF2_2405;CYLD_17180;ARID5B_88738,ARID5B_89998 | 2.50382 | 1.89154 | |
| KANG_CISPLATIN_RESISTANCE_UP | TENT5A_15343;MAFF_27478,MAFF_28315;IER5_15403;FTH1_92999,FTH1_93000 | 2.5370175 | 1.7680525 | |
| PID_HIV_NEF_PATHWAY | TRAF1_15516;NFKBIA_4568;NFKB1_4566;BIRC3_11879 | 2.579205 | 2.144825 | |
| 1,3-Butadiene (BEAS-2B cells) | TAKEDA_TARGETS_OF_NUP98_HOXA9_FUSION_6HR_DN | TM4SF1_12122;KDM6B_16198;HMOX1_3041 | 13.9794 | 7.78439 |
| ABBUD_LIF_SIGNALING_1_UP | SOCS3_15641;RGS4_20411;CEBPB_10745 | 21.3365 | 16.7624 | |
| CONRAD_STEM_CELL | KLF5_20609;KLF4_23258;CXCL5_15735 | 26.4384 | 21.1357 | |
| GROSS_HYPOXIA_VIA_ELK3_ONLY_UP | PPP1R15A_14098;CEBPB_10745;ADM_12873,ADM_140 | 28.2428 | 20.7323 | |
| LIEN_BREAST_CARCINOMA_METAPLASTIC_VS_DUCTAL_UP | HMOX1_3041;CXCL8_14324;CEBPB_10745;BMP2_22143;ADM_12873,ADM_140 | 28.2428 | 20.7323 | |
| SEMENZA_HIF1_TARGETS | HMOX1_3041;EDN1_2029;ADM_12873,ADM_140 | 28.2428 | 20.7323 | |
| ZAMORA_NOS2_TARGETS_UP | IER2_3213;HMOX1_3041;ADM_12873,ADM_140 | 28.2428 | 20.7323 | |
| SWEET_KRAS_TARGETS_DN | IRS2_21012;FOSL1_2463;DUSP4_1990;CXCL5_15735 | 28.5691 | 21.73905 | |
| HINATA_NFKB_TARGETS_KERATINOCYTE_DN | RUNX1_20078;GPRC5A_15162;DUSP4_1990 | 29.0199 | 22.4869 | |
| AMIT_SERUM_RESPONSE_240_MCF10A | TM4SF1_12122;PHLDA2_5123;ADRB2_151 | 29.6407 | 23.7457 | |
| Carbon Tetrachloride (BEAS-2B cells) | ANDERSEN_CHOLANGIOCARCINOMA_CLASS1 | TMEM156_25729;RGS4_20411;PTHLH_15144,PTHLH_5582;DCDC2_14713;CXCL6_15723 | 9.56328 | 7.98027 |
| CAFFAREL_RESPONSE_TO_THC_UP | ZFP36L1_14656;VEGFA_12596;MYC_4394;HIST1H2AC_93104;CPEB4_18785 | 11.2356 | 9.30456 | |
| MANN_RESPONSE_TO_AMIFOSTINE_UP | ZFP36L1_14656;MYC_4394;GDF15_18329 | 11.2356 | 9.30456 | |
| CHIN_BREAST_CANCER_COPY_NUMBER_UP | TGFB2_25683;PTGS2_5577;MYC_4394 | 11.6191 | 9.6667 | |
| DANG_MYC_TARGETS_DN | ZFP36L1_14656;PDGFB_5026;MYC_4394;CDKN2B_14415 | 12.3503 | 10.23368 | |
| GUENTHER_GROWTH_SPHERICAL_VS_ADHERENT_DN | TGFB2_25683;PLAUR_23941,PLAUR_5179;NR3C1_4689 | 13.0934 | 10.60316 | |
| WALLACE_PROSTATE_CANCER_UP | SOX4_6686;MYC_4394;GDF15_18329 | 13.347 | 9.58186 | |
| ZAMORA_NOS2_TARGETS_DN | ZFP36L1_14656;SOX4_6686;SOX17_89363;NFIA_18923;HSPB8_15039 | 13.347 | 11.0524 | |
| PID_LKB1_PATHWAY | TSC1_19318;SIK1_17023;MYC_4394 | 13.3507 | 9.14916 | |
| SIMBULAN_UV_RESPONSE_IMMORTALIZED_DN | MYC_4394;ID1_14943;EREG_21427 | 13.3895 | 10.833 | |
| Trichloroethylene (BEAS-2B cells) | ROVERSI_GLIOMA_LOH_REGIONS | PPFIBP1_22884;NPFFR2_90111;GPRC5A_15162;DDHD1_10888 | 44.8416 | 29.00405 |
| HEIDENBLAD_AMPLIFIED_IN_PANCREATIC_CANCER | PPFIBP1_22884;MAL2_23815;CCN3_87436 | 47.0682 | 35.7006 | |
| OLSSON_E2F3_TARGETS_DN | NFKBIA_4568;MAL2_23815;HIST1H1C_2950 | 65.4966 | 47.7346 | |
| VANASSE_BCL2_TARGETS_UP | RELN_21362;MACC1_23235;HIST1H1C_2950 | 65.4966 | 41.4074 | |
| REACTOME_HDACS_DEACETYLATE_HISTONES | HIST2H4B_16248;HIST2H4A_33956;HIST2H2BF_93215;HIST1H2BJ_92661;HIST1H2AG_93175;HIST1H2AC_93104 | 67.48565 | 54.3204 | |
| SATO_SILENCED_BY_DEACETYLATION_IN_PANCREATIC_CANCER | HIST1H1C_2950;GDF15_2621;DHRS2_1857 | 69.8154 | 60.8512 | |
| RADMACHER_AML_PROGNOSIS | MAL_21284;FHL2_2423;EZR_25288;DAPK1_1752 | 72.244 | 51.08815 | |
| SEITZ_NEOPLASTIC_TRANSFORMATION_BY_8P_DELETION_UP | TENT5A_15343;OASL_89164;GPRC5A_15162;CCN3_87436 | 72.4401 | 49.10325 | |
| BASSO_HAIRY_CELL_LEUKEMIA_UP | SUSD5_15755;MAF_13534;IGFBP3_3270;DAPK1_1752 | 72.6945 | 46.20455 | |
| AIYAR_COBRA1_TARGETS_DN | ZNF532_20212;SOX4_6686;CLDN4_89537 | 73.9443 | 51.0529 | |
| Dichloromethane (BEAS-2B cells) | REACTOME_DEPURINATION | HIST2H4A_33956;HIST1H2BJ_92661;HIST1H2AC_93104 | 142.127 | 118.71 |
| FIGUEROA_AML_METHYLATION_CLUSTER_3_DN | PPP1R15A_14098;HIST1H2BJ_92661;HIST1H2AG_93175 | 158.016 | 130.242 | |
| COLIN_PILOCYTIC_ASTROCYTOMA_VS_GLIOBLASTOMA_DN | SQSTM1_6740;CHI3L1_14316;BHLHE40_689 | 160.996 | 132.484 | |
| BOYLAN_MULTIPLE_MYELOMA_C_DN | KLF6_3626;JUN_89022;IRS2_21012 | 168.708 | 137.335 | |
| ZHAN_V2_LATE_DIFFERENTIATION_GENES | NR3C1_4689;KLF6_3626;IRF1_21247 | 168.708 | 137.335 | |
| BREUHAHN_GROWTH_FACTOR_SIGNALING_IN_LIVER_CANCER | SMAD7_12470;IRS2_21012;HBEGF_15347,HBEGF_2892 | 191.462 | 159.963 | |
| REACTOME_EGFR_DOWNREGULATION | SPRY1_18786;SH3KBP1_6296;HBEGF_15347,HBEGF_2892 | 191.462 | 159.963 | |
| REACTOME_SIGNALING_BY_EGFR | SPRY1_18786;SH3KBP1_6296;HBEGF_15347,HBEGF_2892 | 191.462 | 159.963 | |
| PID_EPO_PATHWAY | SOCS3_15641;NFKB1_4566;IRS2_21012 | 198.521 | 154.356 | |
| REACTOME_SIGNALING_BY_NON_RECEPTOR_TYROSINE_KINASES | SOCS3_15641;NR3C1_4689;HBEGF_15347,HBEGF_2892 | 198.521 | 159.963 | |
| Trichloroethylene (pHBEC cells) | KRIEG_HYPOXIA_VIA_KDM3A | NRP2_27606;KDM3A_3541;EBP_2015 | 28.1584 | 15.5176 |
| KYNG_DNA_DAMAGE_BY_GAMMA_RADIATION | PCSK5_27656;NRP2_27606;LRBA_90214;AP1G1_21853 | 30.0328 | 16.13885 | |
| PID_VEGFR1_PATHWAY | VEGFA_28053;NRP2_27606;CD2AP_13562 | 30.6881 | 16.3157 | |
| KEGG_N_GLYCAN_BIOSYNTHESIS | MAN2A1_21283;MAN1A2_89683;MAN1A1_12471;ALG14_15409 | 30.87085 | 16.32435 | |
| REACTOME_SPHINGOLIPID_DE_NOVO_BIOSYNTHESIS | SGMS2_22813;SGMS1_13935;KDSR_14120 | 31.3726 | 16.5192 | |
| LUCAS_HNF4A_TARGETS_UP | RBKS_5758;MAN1A1_12471;KIFAP3_22932;EBP_2015 | 32.1622 | 16.56735 | |
| YU_MYC_TARGETS_DN | KLF2_16192;BTG1_26906;ABCA1_22891 | 32.2274 | 16.4188 | |
| REACTOME_FOXO_MEDIATED_TRANSCRIPTION | SIRT1_21308;RBL2_5761;BTG1_26906;BCL2L11_26447 | 32.29735 | 16.61775 | |
| PID_FOXO_PATHWAY | SIRT1_21308;SGK1_22771;RBL2_5761;BCL2L11_26447 | 32.48035 | 16.893 | |
| PID_HIF2PATHWAY | VEGFA_28053;SLC2A1_14159;SIRT1_21308 | 32.5934 | 16.8167 | |
| Dichloromethane (pHBEC cells) | MATTIOLI_MULTIPLE_MYELOMA_WITH_14Q32_TRANSLOCATIONS | MAF_13534;KLF4_23258;IL6R_17769 | 266.73 | 204.336 |
| DELACROIX_RAR_TARGETS_UP | RESF1_15542;DUSP1_1978;BHLHE40_689 | 323.565 | 232.508 | |
| SWEET_KRAS_TARGETS_UP | TPM1_28000;GADD45B_2570,GADD45B_28755;CLDN4_89537;BHLHE40_689;PGLS_16643 | 325.962 | 232.508 | |
| MENSE_HYPOXIA_UP | PPP1R15A_14098;MAFF_27478,MAFF_28315;KLF4_23258;INSIG1_3385;ENO2_2153;BHLHE40_689 | 326.754 | 236.46375 | |
| BERENJENO_ROCK_SIGNALING_NOT_VIA_RHOA_DN | TPM1_28000;IGF1R_3254;IER2_3213 | 329.401 | 236.301 | |
| NAKAMURA_ADIPOGENESIS_EARLY_DN | TPM1_28000;IER2_3213;GDF15_18329 | 329.401 | 236.301 | |
| NAKAMURA_ADIPOGENESIS_LATE_DN | TPM1_28000;IER2_3213;GDF15_18329 | 329.401 | 236.301 | |
| RASHI_RESPONSE_TO_IONIZING_RADIATION_1 | IER2_3213;FOS_2461;DUSP1_1978 | 329.401 | 236.301 | |
| VANHARANTA_UTERINE_FIBROID_DN | MAFF_27478,MAFF_28315;KLF4_23258;IER2_3213;GADD45B_2570,GADD45B_28755 | 329.672 | 238.36025 | |
| AMIT_DELAYED_EARLY_GENES | MAFF_27478,MAFF_28315;FOSL1_2463;BHLHE40_689 | 329.943 | 240.4195 |
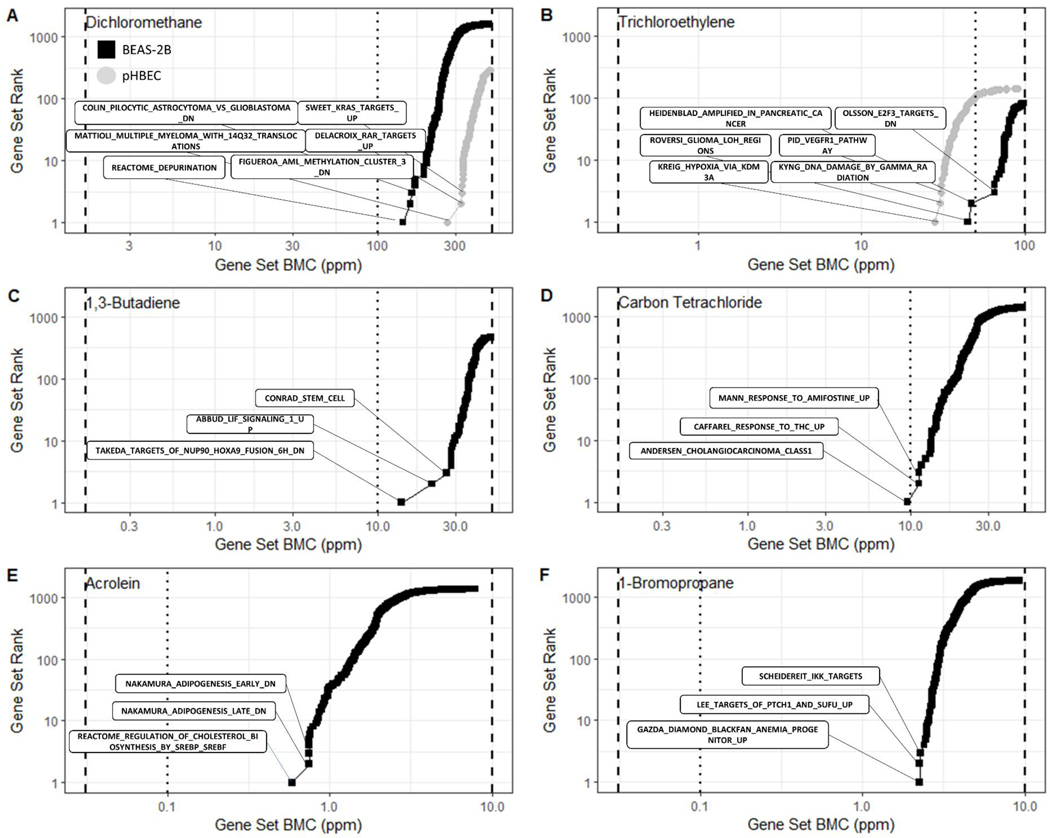
The accumulation plots shown in Figure 5 emphasize the range of response sensitivity for each test agent. Generally, the reported BPAC for each reported VOC fall between the second and third highest tested chemical concentration. Acrolein elicited the most sensitive response in a cholesterol synthesis gene set with a BPAC at 0.586 ppm. Moreover, many of the top ten sensitive gene sets recorded BMDs approaching or lower than the ACGIH TLVs, indicated by the blue dotted line in Figure 5. Trichloroethylene (Figure 5A) and carbon tetrachloride (Figure 5B) both produced a BPAC at concentrations below the TLV.
Figure 5.
Accumulation plots for the best BMD values for enriched gene sets for BEAS-2B cells (black squares) and pHBEC cells (grey circles). Each point represents the Median BMD for a gene set. Black dashed lines indicate the range of concentrations tested and the dotted line indicates the ACGIH TLV.
Discussion
Ultimately, the results presented in this manuscript provide a foundation for using in vitro ALI cultures together with human-based gene expression dose response modeling NAMs to provide quantitative PODs for VOC exposure. Evaluating in vitro transcriptomics driven gene set level BMDs for VOC ALI exposures provided a basis of comparison to both human occupational exposure guidelines, such as the ACGIH TLV and in vivo inhalation studies (Table 3). ACGIH TLVs were obtained from the 2018 TLVs and BEIs: based on the documentation of the threshold limit values for chemical substances and physical agents & biological exposure indices (ACGIH 2018). In most cases, the gene set level BMDs from VOC ALI testing were reasonable approximations of the TLVs. Except for 1-bromopropane, calculated BPACs fell within five-fold of the TLVs for each respective test agent. A five-fold difference represents a significant correlation between our exposure generated BPAC and TLV. Previous studies examining the variability of PODs for in vivo experiments show higher uncertainty levels and a larger overall POD range between studies (Paul Friedman et al. 2019; Pham et al. 2020).
In the example of dichloromethane (TLV of 100 ppm), we report a very similar BPAC of 142.1 ppm for BEAS-2B and 226.7 ppm for pHBECs. The ACGIH TLV documentation on dichloromethane includes inhalation study data associated with lung cancer progression in vivo (NTP 1986b). Interestingly, the three most sensitive gene sets for dichloromethane exposure in pHBECs (Table 2) are all associated with cell cycle dysregulation and cancer progression (Delacroix et al. 2010; Mattioli et al. 2005). The SWEET_KRAS_TARGET gene set in particular is associated with the development of lung adenocarcinoma (Sweet-Cordero et al. 2005). There is additional overlap between the most sensitive gene sets in the dichloromethane exposed BEAS-2B data and those identified in gene methylation cohort studies (Figueroa et al. 2010). We observed additional concurrence between sensitive gene sets and previously reported toxicological effects for VOCs of interest. Acrolein induced changes in cholesterol synthesis pathways, which are known targets of oxidative stress and acrolein exposure (Moghe et al. 2015). The first and second most sensitive gene set collection in 1,3-butadiene exposed BEAS-2B cells, relates to transcription modification of known markers for myeloid leukemia NUP98 and LIF (Takeda et al. 2006). The EPA hazard summary for 1,3-butadiene highlights epidemiological studies showing association between exposure and increased incidence of leukemia (Delzell et al. 1996; Macaluso et al. 1996; Struski et al.). Chronic in vivo rat carbon tetrachloride inhalation toxicity studies reveal significant fatty changes and hepatic tumor progression, mirroring our most sensitive gene set pathway reported in exposed BEAS-2B cells (Nagano et al. 2007a; Villanueva et al. 2011). Finally, prevalence of renal, bladder, and lung cancer in trichloroethylene toxicity studies is reflected in the top three most sensitive gene sets we report in BEAS-2B cells exposed to trichloroethylene and the most sensitive gene set in exposed pHBECs (Heidenblad et al. 2005; Krieg et al. 2010; Maltoni et al. 1986; Olsson et al. 2007; Roversi et al. 2006).
Broad correlations exist between TLV documentation, known chemical adverse health effects, and the determined sensitive gene sets. Taken together, these relationships provide context highlighting the practicality and promise of ALI VOC exposure and transcriptomics analysis. The representative LOAEL and NOAEL values reported in Table 3 are limited to those reported in the EPA IRIS Toxicological Review database (epa.gov/iris) reports. In general, the reported LOAEL and NOAEL value is higher than the BPAC calculated in our test agent exposures, however, they remain within an order of magnitude difference. BPACs for acrolein, carbon tetrachloride and trichloroethylene are all between the reported LOAEL and NOAEL values. The congruity of our transcriptome-based BPAC values with the TLV documentation and IRIS in vivo inhalation data provides strong evidence that our study design and analysis methods yield informative PoDs. It should be noted that, while the BPACs calculated in this study successfully approximate the respective TLVs, these findings are not intended to be predictive of any specific adverse health outcomes. The most sensitive gene sets are broad groupings of genes with similar activities that may help guide follow-up studies but should not be viewed as definitive mechanisms of action. Limitations also exist in determining dose equivalency between ALI in vitro CCES and in vivo studies. Methods to determine dosimetric uptake in vitro in comparison to in vivo is being developed and important to assess dose equivalency across studies. Despite these challenges, the preliminary evidence that BMD analysis and PoD calculation in an ALI in vitro system provides an early marker of exposure is of scientific importance.
For the purposes of this study, change to viability concentration-response was not a primary endpoint and was reserved as only a cut-off for consideration in the transcriptomics data. Despite the 50% average viability threshold to be considered for TempO-Seq, our average response at the highest concentration was below 20% change in BEAS-2B and little to no change was observed in the pHBECs. In our study, preliminary dose response testing was conducted only in the BEAS-2B cells and may have represented concentrations that did not have as strong an effect on the pHBECs. Future studies should utilize cell model specific range finding to ensure an observable dose response. Even known respiratory toxicants formaldehyde and acetaldehyde did not significantly change BEAS-2B cell viability at the concentrations they were exposed. In the case of formaldehyde, the lower tested concentrations skewed both LDH and ATP measurements above those of the sham control cells. This finding indicates the challenges behind exposing airway cells to these chemicals in a humidified system under flow at such low concentrations. Future studies should endeavor to examine the possible conversion of parent chemical species within the exposure system.
It should be noted that we did attempt to quantitate a BMD for the cell viability endpoints. Ultimately, the magnitude of response in our measurements of cytotoxicity/viability were not great enough to meet the 10% relative deviation cutoff for BMD modeling as outlined in the NTP dose response modeling approach (NTP 2018). These results support the strong ability for transcriptional BMD analysis to identify adverse effects in exposures that did not result in significant changes to cell metabolism or viability, providing a more sensitive endpoint in screening level exposure endpoints. The lack of dynamic range in our tested concentrations undoubtedly contributed to the non-viable BMD models for the viability endpoints, as well as the lack of probe and gene sets assigned BMDs. A wider range of tested concentrations and viability BMD modeling should be utilized and examined in future studies. The observed probe level assigned BMDs in Figure 4 are heavily weighted toward the highest concentration tested for most of the tested VOCs. Altering the test agent concentration range and time course in future experiments could lead to tissue remodeling, uncover different expression profiles and likely reveal gene set level changes with differential BPACs for the VOCs tested. Subsequent studies are planned to evaluate 6 and 24 h post-exposure time points and repeat exposures to simulate 14-day in vivo inhalation study designs.
In summary, the communicated study utilized a novel inhalation exposure system to investigate VOCs which were previously incapable of being tested in traditional in vitro direct-dosing methods. Second, we implemented transcriptome-based analytical techniques to describe portal of entry impacts of inhaled VOCs with many of the most sensitive gene sets correlating to known adverse effects from previous in vivo experiments. The findings outlined above serve as a primer to inform future in vitro inhalation exposure study design and risk assessment. Foremost, the BMD analysis and comparison to known LOAEL and NOAEL data, as well as ACGIH TLVs, provide a proof of concept that transcriptome-based PoD analysis can be used as a predictive tool for adverse outcomes in human exposure.
Supplementary Material
Acknowledgements
This project is supported in part by an appointment to the Research Participation Program at the Office of Research and Development, U.S. Environmental Protection Agency, administered by the Oak Ridge Institute for Science and Education and Oak Ridge Associated Universities through an interagency agreement between the U.S. Department of Energy and EPA.
Funding Information
The U.S. Environmental Protection Agency through its Office of Research and Development provided funding for this research
Footnotes
Conflict of Interest/Disclosure
The authors declare no conflict of interest. This manuscript has been reviewed by the Center for Public Health & Environmental Assessment, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Competing financial interests: The authors declare they have no actual or potential competing financial interests
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency
References
- ACGIH. 2018. 2018 tlvs® and beis® : Based on the documentation of the threshold limit values for chemical substances and physical agents & biological exposure indices. ACGIH. [Google Scholar]
- Adams EM, Spencer HC, Rowe VK, Mc CD, Irish DD. 1952. Vapor toxicity of carbon tetrachloride determined by experiments on laboratory animals. AMA Arch Ind Hyg Occup Med. 6(1):50–66. [PubMed] [Google Scholar]
- Akaike H. 1974. New look at statistical-model identification. Ieee Transactions on Automatic Control. Ac19(6):716–723. [Google Scholar]
- Alabdulhadi A, Ramadan A, Devey P, Boggess M, Guest M. 2019. Inhalation exposure to volatile organic compounds in the printing industry. J Air Waste Manag Assoc. 69(10):1142–1169. [DOI] [PubMed] [Google Scholar]
- Albert RE, Sellakumar AR, Laskin S, Kuschner M, Nelson N, Snyder CA. 1982. Gaseous formaldehyde and hydrogen chloride induction of nasal cancer in the rat. J Natl Cancer Inst. 68(4):597–603. [PubMed] [Google Scholar]
- Appelman LM, Woutersen RA, Feron VJ. 1982. Inhalation toxicity of acetaldehyde in rats. I. Acute and subacute studies. Toxicology. 23(4):293–307. [DOI] [PubMed] [Google Scholar]
- Appelman LM, Woutersen RA, Feron VJ, Hooftman RN, Notten WR. 1986. Effect of variable versus fixed exposure levels on the toxicity of acetaldehyde in rats. J Appl Toxicol. 6(5):331–336. [DOI] [PubMed] [Google Scholar]
- Appelman LM, Woutersen RA, Zwart A, Falke HE, Feron VJ. 1988. One-year inhalation toxicity study of formaldehyde in male rats with a damaged or undamaged nasal mucosa. J Appl Toxicol. 8(2):85–90. [DOI] [PubMed] [Google Scholar]
- Aranyi C, O’Shea WJ, Graham JA, Miller FJ. 1986. The effects of inhalation of organic chemical air contaminants on murine lung host defenses. Fundamental and Applied Toxicology. 6(4):713–720. [DOI] [PubMed] [Google Scholar]
- ASTDR. 2012. Toxicological profile for 1,3-butadiene. In: U.S. Department of Health and Human Services PHS, editor. Atlanta, GA: Agency for Toxic Substances and Disease Registry. [Google Scholar]
- Baldridge KC, Zavala J, Surratt J, Sexton KG, Contreras LM. 2015. Cellular rna is chemically modified by exposure to air pollution mixtures. Inhal Toxicol. 27(1):74–82. [DOI] [PubMed] [Google Scholar]
- Banga S, Patil GP, Taillie C. 2002. Direct calculation of likelihood-based benchmark dose levels for quantitative responses. Environmental and Ecological Statistics. 9(3):295–315. [Google Scholar]
- Bates JHT, Irvin CG. 2003. Measuring lung function in mice: The phenotyping uncertainty principle. Journal of Applied Physiology. 94(4):1297–1306. [DOI] [PubMed] [Google Scholar]
- Battelle. 1981. Final report on chronic inhalation toxicology study in rats and mice exposed to formaldehyde. Battelle Columbus Laboratories.
- Benson JM, Springer DL. 1999. Improved risk estimates for carbon tetrachloride. Lovelace Biomedical and Environmental Research Institute, Albuquerque, New Mexico.
- Bhalla DK, Mahavni V, Nguyen T, McClure T. 1991. Effects of acute exposure to formaldehyde on surface morphology of nasal epithelia in rats. Journal of Toxicology and Environmental Health. 33(2):171–188. [DOI] [PubMed] [Google Scholar]
- Buckley LA, Jiang XZ, James RA, Morgan KT, Barrow CS. 1984. Respiratory tract lesions induced by sensory irritants at the rd50 concentration. Toxicology and Applied Pharmacology. 74(3):417–429. [DOI] [PubMed] [Google Scholar]
- Burek JD, Nitschke KD, Bell TJ, Wackerle DL, Childs RC, Beyer JE, Dittenber DA, Rampy LW, McKenna MJ. 1984. Methylene chloride: A two-year inhalation toxicity and oncogenicity study in rats and hamsters1. Toxicological Sciences. 4(1):30–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassee FR, Groten JP, Feron VJ. 1996. Changes in the nasal epithelium of rats exposed by inhalation to mixtures of formaldehyde, acetaldehyde, and acrolein. Fundam Appl Toxicol. 29(2):208–218. [DOI] [PubMed] [Google Scholar]
- Chang JC, Gross EA, Swenberg JA, Barrow CS. 1983. Nasal cavity deposition, histopathology, and cell proliferation after single or repeated formaldehyde exposures in b6c3f1 mice and f-344 rats. Toxicol Appl Pharmacol. 68(2):161–176. [DOI] [PubMed] [Google Scholar]
- Cheng NY, Chuang HC, Shie RH, Liao WH, Hwang YH. 2019. Pilot studies of voc exposure profiles during surgical operations. Ann Work Expo Health. 63(2):173–183. [DOI] [PubMed] [Google Scholar]
- Chiang HL, Lin WH, Lai JS, Wang WC. 2010. Inhalation risk assessment of exposure to the selected volatile organic compounds (vocs) emitted from the facilities of a steel plant. J Environ Sci Health A Tox Hazard Subst Environ Eng. 45(11):1397–1405. [DOI] [PubMed] [Google Scholar]
- CIIT. 1982. Summary of final report on formaldehyde study. Chemical Industry Institute of Toxicology. [Google Scholar]
- ClinTrials. 1997. A 28-day inhalation toxicity study of a vapor formulation of albta1 in albino rat. Baton Rouge, LA: Albemarle Corporation. No. Document No. 91198. [Google Scholar]
- Costa DL, Kutzman RS, Lehmann JR, Drew RT. 1986. Altered lung function and structure in the rat after subchronic exposure to acrolein. Am Rev Respir Dis. 133(2):286–291. [DOI] [PubMed] [Google Scholar]
- Crouch CN, Pullinger DH, Gaunt IF. 1979. Inhalation toxicity studies with 1,3-butadiene – 2. 3 month toxicity study in rats. American Industrial Hygiene Association Journal. 40(9):796–802. [DOI] [PubMed] [Google Scholar]
- Dailey L, McCullough SD. 2021a. Culture of primary human tracheobronchial epithelial cells. Protocol Exchange.
- Dailey L, McCullough SD. 2021b. Establishing differentiated air-liquid interface primary human bronchial epithelial cell cultures. Protocol Exchange.
- Dallas CE, Badeaux P, Theiss JC, Fairchild EJ. 1989. The influence of inhaled formaldehyde on rat lung cytochrome p450. Environmental research. 49(1):50–59. [DOI] [PubMed] [Google Scholar]
- Delacroix L, Moutier E, Altobelli G, Legras S, Poch O, Choukrallah MA, Bertin I, Jost B, Davidson I. 2010. Cell-specific interaction of retinoic acid receptors with target genes in mouse embryonic fibroblasts and embryonic stem cells. Mol Cell Biol. 30(1):231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delzell E, Sathiakumar N, Hovinga M, Macaluso M, Julian J, Larson R, Cole P, Muir DC. 1996. A follow-up study of synthetic rubber workers. Toxicology. 113(1–3):182–189. [DOI] [PubMed] [Google Scholar]
- Doyle M, Sexton KG, Jeffries H, Bridge K, Jaspers I. 2004. Effects of 1,3-butadiene, isoprene, and their photochemical degradation products on human lung cells. Environmental health perspectives. 112(15):1488–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak A, Tilley AE, Shaykhiev R, Wang R, Crystal RG. 2011. Do airway epithelium air-liquid cultures represent the in vivo airway epithelium transcriptome? Am J Respir Cell Mol Biol. 44(4):465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersviller S, Lichtveld K, Sexton KG, Zavala J, Lin YH, Jaspers I, Jeffries HE. 2012. Gaseous vocs rapidly modify particulate matter and its biological effects - part 1: Simple vocs and model pm. Atmos Chem Phys Discuss. 12(2):5065–5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elf Atochem SA. 1997. Study of acute toxicity of n-propyl bromide administered to rats by vapour inhalation. Determination of the 50% lethal concentration (lc50/4 hours). Ineris-l.E.T.E. Study no. 95122. Study performed by laboratoire d’etudes de toxicologie experimentale.
- Feron VJ, Bruyntjes JP, Woutersen RA, Immel HR, Appelman LM. 1988. Nasal tumours in rats after short-term exposure to a cytotoxic concentration of formaldehyde. Cancer letters. 39(1):101–111. [DOI] [PubMed] [Google Scholar]
- Figueroa ME, Lugthart S, Li Y, Erpelinck-Verschueren C, Deng X, Christos PJ, Schifano E, Booth J, van Putten W, Skrabanek L et al. 2010. DNA methylation signatures identify biologically distinct subtypes in acute myeloid leukemia. Cancer Cell. 17(1):13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipsson AF, Sand S, Nilsson J, Victorin K. 2003. The benchmark dose method--review of available models, and recommendations for application in health risk assessment. Crit Rev Toxicol. 33(5):505–542. [PubMed] [Google Scholar]
- Foster JR, Green T, Smith LL, Lewis RW, Hext PM, Wyatt I. 1992. Methylene chloride—an inhalation study to investigate pathological and biochemical events occurring in the lungs of mice over an exposure period of 90 days. Toxicological Sciences. 18(3):376–388. [DOI] [PubMed] [Google Scholar]
- Gwinn WM, Auerbach SS, Parham F, Stout MD, Waidyanatha S, Mutlu E, Collins B, Paules RS, Merrick BA, Ferguson S et al. 2020. Evaluation of 5-day in vivo rat liver and kidney with high-throughput transcriptomics for estimating benchmark doses of apical outcomes. Toxicological Sciences. (1096–0929 (Electronic)). [DOI] [PMC free article] [PubMed]
- Harkema JR. 1991. Comparative aspects of nasal airway anatomy: Relevance to inhalation toxicology. Toxicol Pathol. 19(4 Pt 1):321–336. [DOI] [PubMed] [Google Scholar]
- Harkema JR, Carey Sa Fau - Wagner JG, Wagner JG. The nose revisited: A brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. (0192–6233 (Print)). [DOI] [PubMed]
- Harrill J, Shah I, Setzer RW, Haggard D, Auerbach S, Judson R, Thomas RS. 2019. Considerations for strategic use of high-throughput transcriptomics chemical screening data in regulatory decisions. Current opinion in toxicology. 15:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrill JA, Everett LJ, Haggard DE, Sheffield T, Bundy J, Willis CM, Thomas RS, Shah I, Judson RS. 2021. High-throughput transcriptomics platform for screening environmental chemicals. Toxicological Sciences. [DOI] [PMC free article] [PubMed]
- Heidenblad M, Lindgren D, Veltman JA, Jonson T, Mahlamäki EH, Gorunova L, van Kessel AG, Schoenmakers EF, Höglund M. 2005. Microarray analyses reveal strong influence of DNA copy number alterations on the transcriptional patterns in pancreatic cancer: Implications for the interpretation of genomic amplifications. Oncogene. 24(10):1794–1801. [DOI] [PubMed] [Google Scholar]
- Holmström M, Wilhelmsson B, Hellquist H. 1989. Histological changes in the nasal mucosa in rats after long-term exposure to formaldehyde and wood dust. Acta Otolaryngol. 108(3–4):274–283. [DOI] [PubMed] [Google Scholar]
- Horton AW, Tye R, Stemmer KL. 1963. Experimental carcinogenesis of the lung. Inhalation of gaseous formaldehyde or an aerosol of coal tar by c3h mice. J Natl Cancer Inst. 30:31–43. [PubMed] [Google Scholar]
- House JS, Grimm FA, Jima DD, Zhou Y-H, Rusyn I, Wright FA. 2017. A pipeline for high-throughput concentration response modeling of gene expression for toxicogenomics. Frontiers in genetics. 8:168–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara G, Kitoh J, Yu X, Asaeda N, Iwai H, Kumazawa T, Shibata E, Yamada T, Wang H, Xie Z et al. 2000. 1-bromopropane, an alternative to ozone layer depleting solvents, is dose-dependently neurotoxic to rats in long-term inhalation exposure. Toxicological Sciences. 55(1):116–123. [DOI] [PubMed] [Google Scholar]
- Jackson AF, Williams A, Recio L, Waters MD, Lambert IB, Yauk CL. 2014. Case study on the utility of hepatic global gene expression profiling in the risk assessment of the carcinogen furan. Toxicol Appl Pharmacol. 274(1):63–77. [DOI] [PubMed] [Google Scholar]
- Johnson KJ, Auerbach SS, Costa E. 2020. A rat liver transcriptomic point of departure predicts a prospective liver or non-liver apical point of departure. Toxicol Sci. 176(1):86–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson RS, Houck KA, Kavlock RJ, Knudsen TB, Martin MT, Mortensen HM, Reif DM, Rotroff DM, Shah I, Richard AM et al. 2010. In vitro screening of environmental chemicals for targeted testing prioritization: The toxcast project. Environmental health perspectives. 118(4):485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata E, Nakadate M, Ogawa Y. 1996a. Acute inhalation toxicity study of formaldehyde in rats: Effect of vapor on pulmonary surfactant. Oyo Yakuri. 51:33–37. [Google Scholar]
- Kamata E, Nakadate M, Uchida O. 1996b. Effects of formaldehyde vapor on teh nasal cavity and lungs of f-344 rats. Journal of Environmental Pathology, Toxicology and Oncology. 15:1–8. [PubMed] [Google Scholar]
- Kamata E, Nakadate M, Uchida O, Ogawa Y, Suzuki S, Kaneko T, Saito M, Kurokawa Y. 1997. Results of a 28-month chronic inhalation toxicity study of formaldehyde in male fisher-344 rats. The Journal of toxicological sciences. 22(3):239–254. [DOI] [PubMed] [Google Scholar]
- Kerns WD, Pavkov KL, Donofrio DJ, Gralla EJ, Swenberg JA. 1983. Carcinogenicity of formaldehyde in rats and mice after long-term inhalation exposure. Cancer Res. 43(9):4382–4392. [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL. 2015. Hisat: A fast spliced aligner with low memory requirements. Nature methods. 12(4):357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. 2019. Graph-based genome alignment and genotyping with hisat2 and hisat-genotype. Nature biotechnology. 37(8):907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-Y, Chung Y-H, Jeong J-H, Lee Y-M, Sur G-S, Kang J-K. 1999. Acute and repeated inhalation toxicity of 1-bromopropane in sd rats. 41(2):121–128. [Google Scholar]
- Krieg AJ, Rankin EB, Chan D, Razorenova O, Fernandez S, Giaccia AJ. 2010. Regulation of the histone demethylase jmjd1a by hypoxia-inducible factor 1 alpha enhances hypoxic gene expression and tumor growth. Mol Cell Biol. 30(1):344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzman RS. 1981. A subchronic inhalation study of fischer 344 rats exposed to 0, 0.4, 1.4 or 4.0 ppm acrolein. Brookhaven National Lab. [Google Scholar]
- Kutzman RS, Popenoe EA, Schmaeler M, Drew RT. 1985. Changes in rat lung structure and composition as a result of subchronic exposure to acrolein. Toxicology. 34(2):139–151. [DOI] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, Genome Project Data Processing S. 2009. The sequence alignment/map format and samtools. Bioinformatics (Oxford, England). 25(16):2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso M, Larson R, Delzell E, Sathiakumar N, Hovinga M, Julian J, Muir D, Cole P. 1996. Leukemia and cumulative exposure to butadiene, styrene and benzene among workers in the synthetic rubber industry. Toxicology. 113(1–3):190–202. [DOI] [PubMed] [Google Scholar]
- Maltoni C, Lefemine G, Cotti G. 1986. Experimental research on trichloroethylene carcinogenesis. Princeton, NJ: Princeton Scientific Publishing. [Google Scholar]
- Maronpot RR, Miller RA, Clarke WJ, Westerberg RB, Decker JR, Moss OR. 1986. Toxicity of formaldehyde vapor in b6c3f1 mice exposed for 13 weeks. Toxicology. 41(3):253–266. [DOI] [PubMed] [Google Scholar]
- Mattioli M, Agnelli L, Fabris S, Baldini L, Morabito F, Bicciato S, Verdelli D, Intini D, Nobili L, Cro L et al. 2005. Gene expression profiling of plasma cell dyscrasias reveals molecular patterns associated with distinct igh translocations in multiple myeloma. Oncogene. 24(15):2461–2473. [DOI] [PubMed] [Google Scholar]
- McCullough SD, Duncan KE, Swanton SM, Dailey LA, Diaz-Sanchez D, Devlin RB. 2014. Ozone induces a proinflammatory response in primary human bronchial epithelial cells through mitogen-activated protein kinase activation without nuclear factor-κb activation. Am J Respir Cell Mol Biol. 51(3):426–435. [DOI] [PubMed] [Google Scholar]
- Mennear JH, McConnell EE, Huff JE, Renne RA, Giddens E. 1988. Inhalation toxicity and carcinogenesis studies of methylene chloride (dichloromethane) in f344/n rats and b6c3f1 mice. Annals of the New York Academy of Sciences. 534:343–351. [DOI] [PubMed] [Google Scholar]
- Moghe A, Ghare S, Lamoreau B, Mohammad M, Barve S, McClain C, Joshi-Barve S. 2015. Molecular mechanisms of acrolein toxicity: Relevance to human disease. Toxicological sciences : an official journal of the Society of Toxicology. 143(2):242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro-Riviere NA, Popp JA. 1986. Ultrastructural evaluation of acute nasal toxicity in the rat respiratory epithelium in response to formaldehyde gas. Fundamental and Applied Toxicology. 6(2):251–262. [PubMed] [Google Scholar]
- Monticello TM, Swenberg JA, Gross EA, Leininger JR, Kimbell JS, Seilkop S, Starr TB, Gibson JE, Morgan KT. 1996. Correlation of regional and nonlinear formaldehyde-induced nasal cancer with proliferating populations of cells. Cancer Res. 56(5):1012–1022. [PubMed] [Google Scholar]
- Morgan KT, Jiang X-Z, Starr TB, Kerns WD. 1986. More precise localization of nasal tumors associated with chronic exposure of f-344 rats to formaldehyde gas. Toxicology and applied pharmacology. 82(2):264–271. [DOI] [PubMed] [Google Scholar]
- Nagano K, Sasaki T, Umeda Y, Nishizawa T, Ikawa N, Ohbayashi H, Arito H, Yamamoto S, Fukushima S. 2007a. Inhalation carcinogenicity and chronic toxicity of carbon tetrachloride in rats and mice. Inhalation Toxicology. 19(13):1089–1103. [DOI] [PubMed] [Google Scholar]
- Nagano K, Umeda Y, Saito M, Nishizawa T, Ikawa N, Arito H, Yamamoto S, Fukushima S. 2007b. Thirteen-week inhalation toxicity of carbon tetrachloride in rats and mice. J Occup Health. 49(4):249–259. [DOI] [PubMed] [Google Scholar]
- Nitschke KD, Burek JD, Bell TJ, Kociba RJ, Rampy LW, McKenna MJ. 1988. Methylene chloride: A 2-year inhalation toxicity and oncogenicity study in rats. Fundam Appl Toxicol. 11(1):48–59. [DOI] [PubMed] [Google Scholar]
- NTP. 1984. Ntp toxicology and carcinogenesis studies of 1,3-butadiene (cas no. 106–99-0) in b6c3f1 mice (inhalation studies). National Toxicology Program technical report series. 1984/08/01 ed. p. 1–111. [PubMed]
- NTP. 1986a. Ntp toxicology and carcinogenesis studies of dichloromethane (methylene chloride) (cas no. 75–09-2) in f344/n rats and b6c3f1 mice (inhalation studies). National Toxicology Program technical report series. 1986/01/01 ed. p. 1–208. [PubMed]
- NTP. 1986b. Toxicology and carcinogenesis studies of dichloromethane (methylene chloride) in f344/n rats and b6c3f1 mice (inhalation studies). US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institutes of Health, Research Triangle Park, NC. [Google Scholar]
- NTP. 1993. Ntp toxicology and carcinogenesis studies of 1,3-butadiene (cas no. 106–99-0) in b6c3f1 mice (inhalation studies). National Toxicology Program technical report series. 1993/05/01 ed. p. 1–389. [PubMed]
- NTP. 2011. Toxicology and carcinogenesis studies of 1-bromopropane (cas no. 106-94-5) in f344/n rats and b6c3f1 mice (inhalation studies). National Toxicology Program technical report series. 2011/09/17 ed. p. 1–190. [PubMed]
- NTP. 2018. Ntp research report on national toxicology program approach to genomic dose-response modeling: Research report 5. Durham (NC). [PubMed] [Google Scholar]
- Olsson AY, Feber A, Edwards S, Te Poele R, Giddings I, Merson S, Cooper CS. 2007. Role of e2f3 expression in modulating cellular proliferation rate in human bladder and prostate cancer cells. Oncogene. 26(7):1028–1037. [DOI] [PubMed] [Google Scholar]
- Owen PE, Glaister JR. 1990. Inhalation toxicity and carcinogenicity of 1,3-butadiene in sprague-dawley rats. Environmental health perspectives. 86:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen PE, Glaister JR, Gaunt IF, Pullinger DH. 1987. Inhalation toxicity studies with 1,3-butadiene. 3. Two year toxicity/carcinogenicity study in rats. American Industrial Hygiene Association journal. 48(5):407–413. [DOI] [PubMed] [Google Scholar]
- Özen OuA, Songur A, Sarsılmaz M, Yaman M, Kuş İ. 2003. Changes of zinc, copper, and iron levels in the lung of male rats after subacute (4‐week) and subchronic (13‐week) exposure to formaldehyde. The Journal of Trace Elements in Experimental Medicine: The Official Publication of the International Society for Trace Element Research in Humans. 16(2‐3):67–74. [Google Scholar]
- Paul Friedman K, Gagne M, Loo L-H, Karamertzanis P, Netzeva T, Sobanski T, Franzosa JA, Richard AM, Lougee RR, Gissi A et al. 2019. Utility of in vitro bioactivity as a lower bound estimate of in vivo adverse effect levels and in risk-based prioritization. Toxicological Sciences. 173(1):202–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham LL, Watford SM, Pradeep P, Martin MT, Thomas RS, Judson RS, Setzer RW, Paul Friedman K. 2020. Variability in in vivo studies: Defining the upper limit of performance for predictions of systemic effect levels. Computational Toxicology. 15:100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JR, Svoboda DL, Tandon A, Patel S, Sedykh A, Mav D, Kuo B, Yauk CL, Yang L, Thomas RS et al. 2019. Bmdexpress 2: Enhanced transcriptomic dose-response analysis workflow. Bioinformatics. 35(10):1780–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast JA, Jones RA, Jenkins LJ Jr., Siegel J. 1967. Effects on experimental animals of long-term inhalation of trichloroethylene, carbon tetrachloride, 1,1,1-trichloroethane, dichlorodifluoromethane, and 1,1-dichloroethylene. Toxicol Appl Pharmacol. 10(2):270–289. [DOI] [PubMed] [Google Scholar]
- Ross AJ, Dailey LA, Brighton LE, Devlin RB. 2007. Transcriptional profiling of mucociliary differentiation in human airway epithelial cells. Am J Respir Cell Mol Biol. 37(2):169–185. [DOI] [PubMed] [Google Scholar]
- Roversi G, Pfundt R, Moroni RF, Magnani I, van Reijmersdal S, Pollo B, Straatman H, Larizza L, Schoenmakers EF. 2006. Identification of novel genomic markers related to progression to glioblastoma through genomic profiling of 25 primary glioma cell lines. Oncogene. 25(10):1571–1583. [DOI] [PubMed] [Google Scholar]
- Rusch GM, Clary JJ, Rinehart WE, Bolte HF. 1983. A 26-week inhalation toxicity study with formaldehyde in the monkey, rat, and hamster. Toxicol Appl Pharmacol. 68(3):329–343. [DOI] [PubMed] [Google Scholar]
- Selgrade MK, Gilmour MI. 2010. Suppression of pulmonary host defenses and enhanced susceptibility to respiratory bacterial infection in mice following inhalation exposure to trichloroethylene and chloroform. Journal of immunotoxicology. 7(4):350–356. [DOI] [PubMed] [Google Scholar]
- Sellakumar AR, Snyder CA, Solomon JJ, Albert RE. 1985. Carcinogenicity of formaldehyde and hydrogen chloride in rats. Toxicol Appl Pharmacol. 81(3 Pt 1):401–406. [DOI] [PubMed] [Google Scholar]
- Smyth H, Smyth H Jr, Carpenter C. 1936. The chronic toxicity of carbon tetrachloride: Animal exposures and field studies. Journal of Industrial Hygiene and Toxicology. 18:277–298. [Google Scholar]
- Struski S, Lagarde S, Bories P, Puiseux C, Prade N, Cuccuini W, Pages MP, Bidet A, Gervais C, Lafage-Pochitaloff M et al. Nup98 is rearranged in 3.8% of pediatric aml forming a clinical and molecular homogenous group with a poor prognosis. (1476–5551 (Electronic)). [DOI] [PubMed]
- Sweet-Cordero A, Mukherjee S, Subramanian A, You H, Roix JJ, Ladd-Acosta C, Mesirov J, Golub TR, Jacks T. 2005. An oncogenic kras2 expression signature identified by cross-species gene-expression analysis. Nat Genet. 37(1):48–55. [DOI] [PubMed] [Google Scholar]
- Swenberg JA, Kerns WD, Mitchell RI, Gralla EJ, Pavkov KL. 1980. Induction of squamous cell carcinomas of the rat nasal cavity by inhalation exposure to formaldehyde vapor. Cancer Res. 40(9):3398–3402. [PubMed] [Google Scholar]
- Takeda A, Goolsby C, Yaseen NR. 2006. Nup98-hoxa9 induces long-term proliferation and blocks differentiation of primary human cd34+ hematopoietic cells. Cancer Res. 66(13):6628–6637. [DOI] [PubMed] [Google Scholar]
- Thomas RS, Allen BC, Nong A, Yang L, Bermudez E, Clewell HJ 3rd, Andersen ME. 2007. A method to integrate benchmark dose estimates with genomic data to assess the functional effects of chemical exposure. Toxicol Sci. 98(1):240–248. [DOI] [PubMed] [Google Scholar]
- Thomas RS, Clewell HJ 3rd, Allen BC, Wesselkamper SC, Wang NC, Lambert JC, Hess-Wilson JK, Zhao QJ, Andersen ME. 2011. Application of transcriptional benchmark dose values in quantitative cancer and noncancer risk assessment. Toxicol Sci. 120(1):194–205. [DOI] [PubMed] [Google Scholar]
- Thomas RS, Wesselkamper SC, Wang NC, Zhao QJ, Petersen DD, Lambert JC, Cote I, Yang L, Healy E, Black MB et al. 2013. Temporal concordance between apical and transcriptional points of departure for chemical risk assessment. Toxicol Sci. 134(1):180–194. [DOI] [PubMed] [Google Scholar]
- Tobe M, Kaneko T, Uchida Y, Kamata E, Ogawa Y, Ikeda Y, Saito M. 1985. Studies of the inhalation toxicity of formaldehyde. National Sanitary and Medical Laboratory Service; (Japan: ).1–94. [Google Scholar]
- USEPA. 1991. Iris toxicological review and summary documents for acetaldehyde. [Google Scholar]
- USEPA. 2003. Iris toxicological report of acrolein. In: Agency USEP, editor. [Google Scholar]
- USEPA. 2010a. Iris toxicological report for formaldehyde (inhalation). In: Agency USEP, editor. External Review Draft ed. [Google Scholar]
- USEPA. 2010b. Iris toxicological review of carbon tetrachloride. In: Agency USEP, editor. [Google Scholar]
- USEPA. 2011a. Iris toxicological review of dichloromethane (methylene chloride). In: Agency USEP, editor. Washington D.C. [Google Scholar]
- USEPA. 2011b. Iris toxicological review of trichloroethylene. In: Agency USEP, editor. [Google Scholar]
- USEPA. 2014. Tsca work plan for chemical assessments 2014 update. [Google Scholar]
- USEPA. 2020a. Iris risk evaluation for 1-bromopropane (n-propyl bromide). In: Agency USEP, editor. [Google Scholar]
- USEPA. 2020b. Tsca chemical substance inventory. https://wwwepagov/tsca-inventory.
- Villanueva A, Hoshida Y, Battiston C, Tovar V, Sia D, Alsinet C, Cornella H, Liberzon A, Kobayashi M, Kumada H et al. 2011. Combining clinical, pathology, and gene expression data to predict recurrence of hepatocellular carcinoma. Gastroenterology. 140(5):1501–1512.e1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIL. 2001. An inhalation two-generation reproductive toxicity study of 1-bromopropane in rats. Ashland, OH: WIL Research Laboratories. [Google Scholar]
- Woolhiser MR, Krieger SM, Thomas J, Hotchkiss JA. 2006. Trichloroethylene (tce): Immunotoxicity potential in cd rats following a 4-week vapor inhalation exposure. Midland, MI: Dow Chemical Company. No. 031020. [Google Scholar]
- Yang L, Allen BC, Thomas RS. 2007. Bmdexpress: A software tool for the benchmark dose analyses of genomic data. BMC Genomics. 8:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeakley JM, Shepard PJ, Goyena DE, VanSteenhouse HC, McComb JD, Seligmann BE. 2017. A trichostatin a expression signature identified by tempo-seq targeted whole transcriptome profiling. PloS one. 12(5):e0178302-e0178302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala J, Greenan R, Krantz QT, DeMarini DM, Higuchi M, Gilmour MI, White PA. 2017. Regulating temperature and relative humidity in air-liquid interface in vitro systems eliminates cytotoxicity resulting from control air exposures. Toxicology research. 6(4):448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala J, Ledbetter AD, Morgan DS, Dailey LA, Puckett E, McCullough SD, Higuchi M. 2018. A new cell culture exposure system for studying the toxicity of volatile chemicals at the air-liquid interface. Inhalation toxicology. 30(4–5):169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart A, Woutersen RA, Wilmer JW, Spit BJ, Feron VJ. 1988. Cytotoxic and adaptive effects in rat nasal epithelium after 3-day and 13-week exposure to low concentrations of formaldehyde vapour. Toxicology. 51(1):87–99 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.