ABSTRACT
Mango (Mangifera indica L.) is one of the most popular tropical fruits in the world owing to its rich taste, flavor, color, production volume and diverse end usage. Conventional mango breeding practices are unable to withstand the demand for improved varieties as it is time consuming and requires heavy investment. However, problems associated with traditional plant breeding can be curtailed through genetic transformation. Nevertheless, major limitation of transgenic development has been its recalcitrant nature toward tissue culture practices involving latent microbial infection, phenol exudation, etc. This opens wide scope for tissue culture-independent in planta transformation approaches These strategies have proved to be easy to execute and cost effective in producing large number of transformants. One such apical meristem targeted in planta approach was successfully exploited to demonstrate its utility in transforming a tree species. Mango variety Amrapali was transformed with two visual marker gene vectors GFP::hptII in pCAMBIA1302 and GUS::nptII in pCAMBIA2301 individually, to demonstrate its amenability. Preliminary confirmations identified 65.0% of GFP and 57.14% of GUS plants to be transformed. Further, molecular characterization of these primary transformants demonstrated transgene integration at genomic and transcript level in some of the plants. This established protocol holds good for functional gene validation and knock in/out studies and aid in mango improvement programs.
KEYWORDS: Agrobacterium tumefaciens, Amrapali, apical meristem targeted in planta transformation, GFP, GUS, Mangifera indica, transgenics
1. Introduction
Mango (Mangifera indica L.) belonging to family Anacardiaceae is an allopolyploid (2 n = 40) with medium genome size (~439 Mbp). It is the most widely grown fruit crop in India and acclaimed as “King of fruits.” India is one of the largest grower and exporter of mango, yielding foreign earnings of 39.6 million US dollars (https://www.statista.com/).1 In India, mango is being cultivated in an area of 2315 thousand hectares with annual production of 20899 thousand metric tonnes (https://nhb.gov.in/).2
Mango cultivation deals with biennial bearing habit, large tree size, susceptibility to major diseases (mango malformation, anthracnose, powdery mildew, bacterial black spot); pests (mango hopper, mealy bug, fruit fly, stone weevil); short-post-harvest life and physiological disorders (spongy tissue, jelly stone) being the major constraints.3,4 Conventional breeding of woody perennial fruit crops such as mango is difficult due to their long juvenile phase, existence of self-incompatibility, high degree of cross-pollination, low fruit set, high fruit drop, development of single seed per fruit, polyembryony, allopolyploid nature, highly heterozygous genetic background and lack of information about inheritance pattern of important quantitative traits.4,5 Moreover, improving popular mango cultivars by introducing genes from other wild species through interspecific hybridization has also been inadequate due to cross incompatibility barriers.6 Genetic transformation facilitates the introduction of a desired gene into the plant genome to overcome problems associated with traditional plant breeding.7
Mango micropropagation has not achieved much economic success than compared to other horticultural crops. This is due to several challenges that are associated with mango in vitro culture, including latent microbial infection, phenol exudation, culture medium discoloration, explant browning, in vitro recalcitrance of tissues either singly or in combination imperil the entire tissue culture attempts.4
Non-availability of in vitro regeneration protocols is mainly due to the basic barriers which involve excessive phenolic exudation post excision of explants (activation of oxidative enzyme system), explant browning (necrosis), culture media discolorations, deep-seated microbial contamination; slow and sporadic in vitro response of mango to tissue culture.8 Few studies have demonstrated genetic transformation of mango using Agrobacterium tumefaciens6,9–11 and gene gun12 with varying levels of success. Further, in vitro regeneration is genotype-dependent, time resilant and prone to somaclonal variations.13,14 Thus, to overcome the concerns associated with difficult-to-regenerate crops, the need of in planta approaches have begun to gain importance.15,16
Tissue culture-independent in planta transformation has been demonstrated in many crops such as Brassica rapa,17 B. napus,18 B. campestris,19 Arabidopsis thaliana,20 Medicago truncatula,21 Raphanus sativus,22 Solanum lycopersicum,23 Glycine max,24 Melilotus alba,25 Zea mays,26 Oryza sativa,27 Citrus maxima28 and Passiflora edulis.29 Several in planta transformation strategies have been developed using different tissues, i.e., seed, epicotyl, shoot apical meristem, flower, fruit etc.30
The advantages of in planta approaches are that they are cost effective, easy to execute and can produce a large number of transformants in a short period of time. Several reports have confirmed high transformation efficiencies in different crops.31 Among several in planta transformation techniques, apical meristem mediated transformation targets T-DNA to the growing shoot apical meristematic regions in vitro and allows the development of plants ex vitro. The methodology has been unequivocally proved in different crops like field bean,32 groundnut,33 capsicum,34 chili,35 pigeon pea,36,37 flax38 and cotton.39,40
Genetic transformation of mango holds significant potential, which can give leads in solving the problem of flowering, alternate bearing habit, development of parthenocarpy varieties and tolerance to different biotic and abiotic stresses. Furthermore, there is no information available on in planta transformation of mango. In the present study, our team has developed a successful strategy for transforming mango with the apical meristem-targeted in planta genetic transformation protocol. This strategy is expected to provide an alternate approach over tissue culture mediated transformation to develop genetically modified mango genotypes, which can hasten and shorten the varietal improvement programs.
2. Materials and Methods
2.1. Plant Material and Binary Vectors Used for Transformation
In the present study, mango variety Amrapali, was used for the development of transformants. Seeds were surface sterilized with Ridomil Gold® (Syngenta Basal, Switzerland), seed coat was removed to facilitate germination and sown in plastic bags containing sterile potting media (cocopeat, vermiculite and perlite, 3:1:1). These bags were maintained under controlled conditions (26 ± 1°C; RH 65–75%; 16/8 h photoperiod of 57 µmole m−2 s−1) till transformation. Two-week-old seedlings were used as explants for Agrobacterium infection.
Agrobacterium tumefaciens strain EHA105 harboring binary vectors pCAMBIA1302 carrying GFP (Green Fluorescent Protein) gene and hptII as antibiotic gene; pCAMBIA2301 carrying GUS (β-glucuronidase) marker gene which contains a 5’ extension of modified castor bean catalase intron (190 bp) to facilitate expression in plants but not in bacteria and nptII selectable marker gene were used for transformation of mango seedlings individually (Fig. 1a, b).
Figure 1.
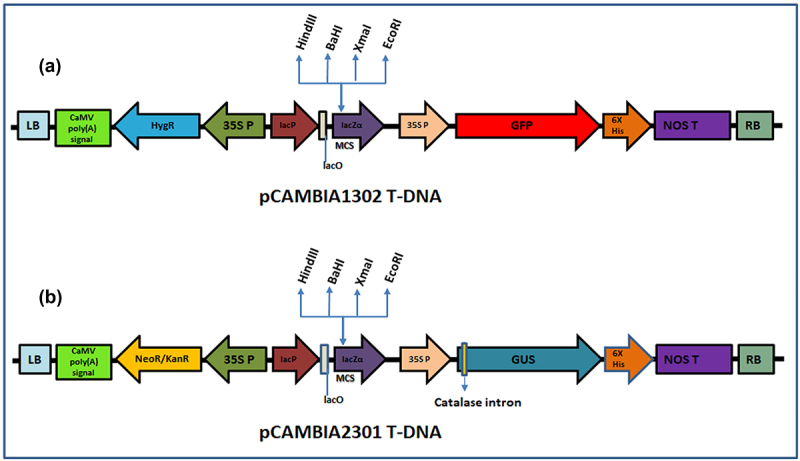
T-DNA of pCAMBIA1302 and pCAMBIA2301 vectors used for transformation. (a) T-DNA region of pCAMBIA1302 harboring GFP reporter and hygromycin resistance genes (b) T-DNA region of pCAMBIA2301 harboring GUS reporter and kanamycin resistance genes. LB; left border, CaMV poly (A) signal; cauliflower mosaic virus polyadenylation signal, HygR; Resistance to hygromycin; NeoR/KanR; Resistance to Kanamycin, 35SP; 35S promoter, lacP; lac promoter, lacO; lac operon, MCS; multiple cloning sites, NOS T; Nopaline synthase terminator, RB; right border.
2.2. Development of Transgenics through an Apical meristem-targeted in planta Transformation Strategy
Axenic culture of Agrobacterium harboring 35S::GFP and 35S::hptII in pCAMBIA1302 and 35S:: GUS and 35S:: nptII in pCAMBIA2301 from freshly streaked culture plate was inoculated into 5 ml LB medium (pH 7.0) containing 50 mg/L kanamycin, 10 mg/L rifampicin and incubated overnight at 28°C. The 5 ml starter culture on the next day was transferred to 200 ml of LB broth suplemeneted with antibiotics, which was later inoculated into 1 L of Winans’ AB minimal medium (pH 5.2)41 and incubated for 18 h at 28°C; 220 rpm. Two-week-old mango seedlings with emerging plumules were punctured 15–20 times with an insulin syringe at the apical meristem and incubated in AB minimal medium previously supplemented with crushed mature tobacco leaf extract42 maintained at 28°C; 50 rpm for 5 h. The plants were later allowed to grow under controlled conditions until they recovered from injury and resumed their growth (Figs. 2 and 3).
Figure 2.

Strategy of tissue culture-independent in planta transformation in Mango (Mangifera indica L.). (a) Seeds without seed coat sown in potting mixture allowed to germinate; (b) Hook shaped plumule emerging out of seed indicating its growth; (c) Stage of seedling suitable for pricking; (d) Pricking of the emerging plumule with an insulin needle; (e) Agrobacterium-mediated transformation of seedlings; (f) Planting of transformed seedlings onto polybag and recovery of primary transformants; (g) Recovered plants in growth chamber (h) Plants transferred to glasshouse; and (i) established plants.
Figure 3.
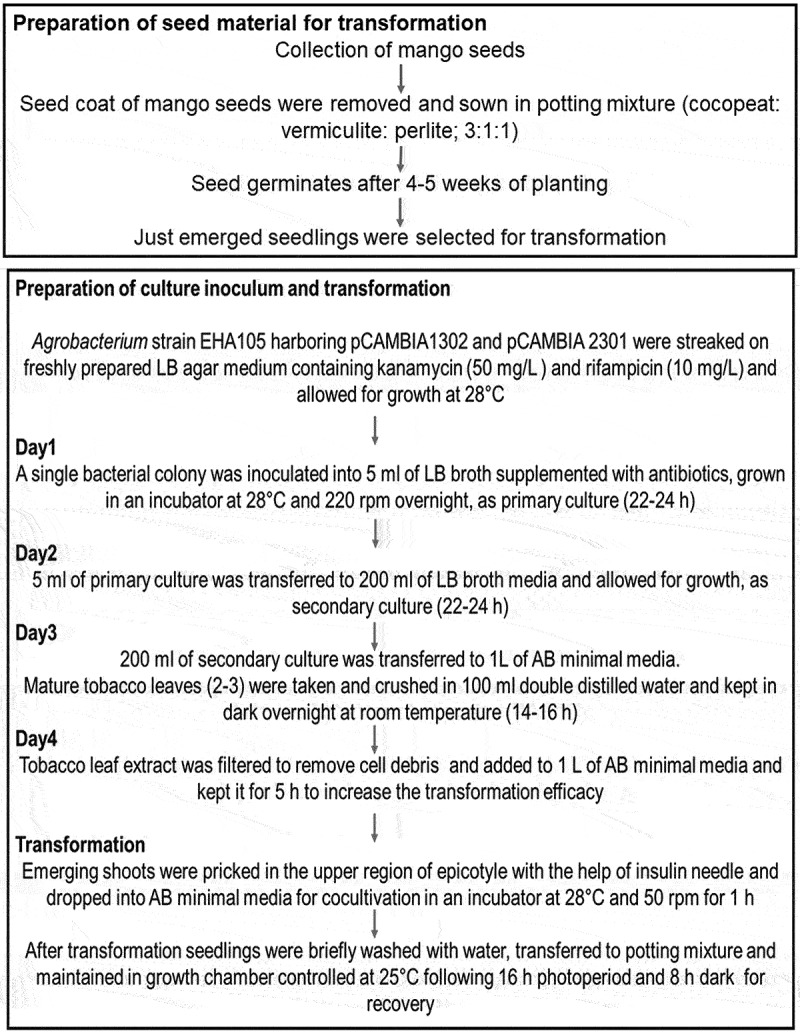
Schematic workflow of different steps involving in planta transformation in Mango.
2.3. Identification of Putative Transformants
2.3.1. GFP Expression
Seedlings transformed with pCAMBIA1302 were preliminarily confirmed using a fluorescence microscope with a 488 nm excitation wavelength. GFP expression in primary transformants, 36 h post infection was observed under a fluorescence microscope (ZEISS SteREO Discovery V20 microscope, Oberkochen, Germany). An excitation wavelength of 488 nm and 505–530 band-path filter (which permits visualization of GFP by blue light) to separate GFP and a 560 long-pass filter to determine chlorophyll fluorescence were used.16
23.2. GUS Histochemical Analysis
Seedlings transformed with pCAMBIA2301 were initially confirmed by GUS histochemical analyses. Excised tissues (leaf and stem) after 76 h of Agrobacterium infection were incubated overnight in GUS assay buffer (0.1 M phosphate buffer, pH 7.0, 2 mM X-Gluc, 5 mM each of potassium ferricyanide and potassium ferrocyanide and 0.1% Triton X100) at 37°C in water bath. Chlorophyll present in the tissues were later destained using 75% ethanol (v/v).43 GUS expression at cellular level was observed using binocular microscope (Olympus, CX33, Shinjuku, Tokyo, Japan).44
2.4. Molecular Analyses of Transgenic Plants
2.4.1. DNA Isolation
The leaves of transgenic and wild-type mango plants were crushed in liquid nitrogen to isolate genomic DNA using a CTAB (Cetyl Trimethyl Ammonium Bromide) method44 with minor modifications (added 1% PVP w/v for removal of phenols). For purification of DNA, 2 µl RNase A (10 mg/ml) was added per 200 µl of crude DNA solution and incubated for 1 h at 37°C, then treated with an equal volume of phenol: chloroform: isoamyl alcohol (25:24:1) and then precipitated with ethanol. The concentration and quality of DNA was estimated using NanoDrop™ (Thermo Scientific, Waltham, Massachusetts, USA) at 260 nm and by electrophoresis on 0.8% agarose gel.
2.4.2. Polymerase Chain Reaction (PCR) Analyses
The presence of transgenes and Agrobacterium specific VirD1 gene in the genome of putative mango transformants was assessed through PCR. The PCR reaction mixture (25 µl) containing 1 U Taq DNA polymerase (GeNie, Bengaluru, Karnataka, India), 1X assay buffer (10 mM pH 9.0 Tris–HCl, 50 mM KCl, 1.5 mM MgCl2, 0.01% gelatin), 2.5 µM of each dNTP, 0.5 µl of each forward and reverse primer (Table 1) at a final concentration of 10 pM and 100 ng of template DNA was used to amplify the transgenes. PCR amplification was carried out in a thermal cycler (Applied Biosystems® Veriti® 96-Well Fast Thermal Cycler, Waltham, Massachusetts, USA) programmed with a hot start of initial denaturation at 95°C for 5 min followed by 35 cycles of denaturation at 95°C for 1 min, annealing at 65°C for GFP, 55°C for hptII & GUS, 61°C for VirD1 and 58°C for nptII for 1 min, extension at 72°C for 1 min, final extension at 72°C for 10 min. Amplified gene products of size 571 bp (GFP), 700 bp (hptII), 438 bp (VirD1) 750 bp (nptII) and 1 kb (GUS) were visualized by gel electrophoresis.
Table 1.
List of primers used in the study.
| Primer ID | Primer sequence (5′-3′) | Amplicon Size (bp) |
|---|---|---|
| Primers used for PCR amplification | ||
| GFP FP | TGGGCACAAATTTTCTGTCAGTGGA | 571 bp |
| GFP RP | ATGCCATGTGTAATCCCAGCAGCT | |
| hptII FP | GCTCGATACAAGCCAACCAC | 700 bp |
| hptII RP | CGAAAAGTTCGACAGCGTCTC | |
| GUS FP | TTA TGC GGG CAA CGT CTG GTAT | 1 kb |
| GUS RP | TGA CAA AAA CCA CCC AAG CGT | |
| NptII FP | CCGGAATTCATGATTGAACAA | 750 bp |
| NptII RP | CCCAAGCTTCAGAAGAACTC | |
| VirD1FP | ATGTCGCAAGGCAGTAAGCCA | 438 bp |
| VirD1 RP | GGAGTCTTTCAGCATGGAGCAA | |
| Primers used for sqRT-PCR | ||
| sqRT-PCR GFP FP | TCCACACAATCTGCCCTTTC | 124 bp |
| sqRT-PCR GFP RP | CTATACAAAGCTAGCCACCACC | |
| sqRT-PCR hptII FP | GTCAGGCTCTCGCTAAACTC | 130 bp |
| sqRT-PCR hptII RP | ATGTCCTGCGGGTAAATAGC | |
| sqRT-PCR GUS FP | ACCTCGCATTACCCTTACGCTG | 122 bp |
| sqRT-PCR GUS RP | CCCGCTTCGAAACCAATG | |
| sqRT-PCR NptII FP | ATTGCACGCAGGTTCTCC | 67 bp |
| sqRT-PCR NptII RP | TGTCTGTTGTGCCCAGTCA | |
| sqRT-PCR MiACT1 FP | GTTTCCCAGTATTGTGGGTAGG | 134 bp |
| sqRT-PCR MiACT1 RP | AGATCTTTTCCATATCATCCCAGTT | |
2.4.3. Genomic Southern Analysis
For identification of T-DNA copy number in transgenic plants developed using both the binary vectors, 10 µg of genomic DNA from transgenic and wild-type plants was digested with HindIII (NEB high fidelity, New England Biolabs, Ipswich, Massachusetts, USA) overnight and separated on 0.8% agarose gel in 1X TAE buffer at constant voltage of 40 V. Restricted fragments were transferred onto a positively charged nylon membrane (Amersham™ Hybond™-N+) by capillary movement using 20× SSC and the membrane was later UV cross-linked. The membrane was hybridized with a DIG labeled 571 bp GFP and 750 bp nptII gene fragment for their corresponding transgenic plants. The blot was further processed with washing, blocking, and development as per manufacturer’s instructions (Roche Holding AG, Basel, Switzerland). The membranes were exposed to X-ray film for 1 h in dark and later observed for hybridization signal.
2.5. Analysis of Transgenic Plants for Transcript Accumulation by sqRT-PCR
Total RNA was isolated from transgenic and wild-type mango plants using a total RNA isolation kit (SpectrumTM, Sigma Aldrich, St. Louis, MO, United States). Further, the isolated RNA was quantified using Nanodrop™ 3300 (ThermoFisher Scientifc, Carlsbad Waltham, Massachusetts, USA) and transcribed to cDNA using SuperScript® (VILOTM, Invitrogen, Carlsbad, CA, USA). sqRT-PCR was performed with 100 ng of diluted cDNA as a template.
M. indica actin 1 (MiACT1) was used as an internal control gene.45 PCR reaction mixture of 25 µL consisting of 2.5 µL 10× Taq buffer, 10 pM each of forward and reverse primer, 2.5 µM dNTPs, and 1 U of Taq DNA polymerase (Bangalore Genei, Bengaluru, India); 1 µL of diluted cDNA was made up to a final volume of 25 µL with nuclease-free water (Invitrogen, Waltham, Massachusetts, USA) in a thermocycler (Applied Biosystems® Veriti® 96-Well Fast Thermal Cycler, Waltham, Massachusetts, USA). PCR program consisted of an initial denaturation step at 95°C for 4 min followed by 30 cycles of denaturation at 95°C for 30s, annealing at 58°C for 30s for MiACT1 (134 bp), nptII (67 bp), GUS (111 bp), GFP (124 bp) and hptII (130 bp) gene RT-primers (Table 1) and extension at 72°C for 30s. The final extension was carried out at 72°C for 7 min to amplify specific gene products. “Blank” was devoid of cDNA, wild type contained 1 µL of cDNA of wild type, and positive control contained 25 ng of pCAMBIA2301/ pCAMBIA1302. The amplified gene products were analyzed on a 2.0% agarose gel.
3. Results and Discussion
Genetic transformation involves introduction of foreign genes to modify horticultural traits in perennial plants without changing their phenotype. Though mango genetic transformation has great potential,46,47,48,11 it is negatively affected by the non-availability of regeneration protocols and recalcitrance to tissue culture. Despite several attempts made by researchers to regenerate mango using leaf49,50 and shoots explants,51,52 it was proved inefficient and established mango in the category of hard to deal with tissue culture-based approaches. Unavailability of an efficient regeneration protocol makes transformation more difficult in mango. However, in planta transformation strategies provide an alternative to evade all the steps involved in tissue culture.15,16 The current study provides evidences for the development of transgenics in mango by an apical meristem targeted in planta transformation protocol.
3.1. Apical meristem-targeted in Planta Transformation
Two-week-old mango seedlings were subjected to transformation with pCAMBIA1302 and pCAMBIA2301 (Fig. 1a-b). The emerging shoots with brown-green coleoptiles were pricked in the upper region of the epicotyl, near the apical meristem with an insulin syringe (Fig. 2a-d). The punctured seedlings were co-cultivated with Agrobacterium strains (pCAMBIA1302/ pCAMBIA2301) in AB minimal medium containing wounded tobacco leaf (mature, yellowish-green) extract to increase the virulence of Agrobacterium (Fig. 2e). Pricked seeds were incubated on a rotatory shaker at 28οC with 50 rpm for 2 h. After infection, seeds were thoroughly washed with autoclaved double distilled water and transferred to polybags containing sterilized growing media (cocopeat, vermiculite and perlite, 3:1:1 ratio) and maintained under diffused light initially and later transferred to direct light in growth chambers maintained at 25°C with 16 h light and 8 h dark photoperiod (fig. 2f). Diffused light has positive effect on the shoot regeneration and dark incubation was always found useful in regeneration and transformation experiments.28,29,53,54 Seedlings took 4–5 weeks for recovery during which, they were regularly irrigated with sterile-double distilled water and Hoagland solution (Fig. 2g). Recovered plants were later transferred to transgenic glasshouse as per regulatory guidelines (Fig. 2h, i). The overview of apical meristem targeted in planta transformation has been provided in a flow chart (Fig. 3).
Tissue culture mediated regeneration and transformation have been proven to be disadvantageous in several plant species due to low transformation efficiencies, genotype dependence and are time consuming.55–57 However, the in planta transformation approach does not demand any sterile growth conditions, phyto-hormones, is less time-consuming, needs low-cost inputs and also genotype independent.
3.2. Preliminary Screening of Transformants
Detection of visible scorable markers like GUS and GFP provide an early signal of transformability and/ or successful transformation of infected tissues.38 Therefore, in this study, GFP expression and GUS histochemical analysis were performed in their specific transformants to identify primary transformants.
As Agrobacterium infection is a random event, GFP expression analysis in the pricked apical meristematic regions (Fig. 4a) identified GFP expression in some transformed seedlings (Fig. 4b iii–v) indicating that few of the infected seedlings were found to be positive toward the transformation strategy. Some transgenic plants lacked expression in the pricked regions indicating the chances of transformation as random (Fig. 4b ii). Further, GFP expression was absent in wild type seedlings (Fig. 4b i). Microscopic observation under UV illumination revealed that out of 40 seedlings taken for visualization, 26 seedlings displayed the presence of green fluorescent protein (GFP) and were selected as primary transformants (T0 plants) of which, 24 plants were transferred in transgenic glasshouse. This precisely demonstrates a preliminary confirmation of 65.0% of the seedlings having GFP expression and putatively transgenic (Table 2).
Figure 4.
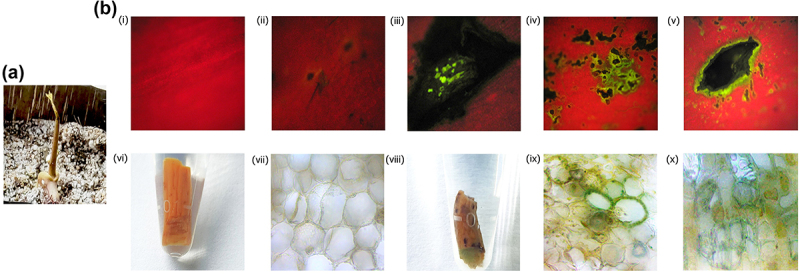
GFP expression and GUS histochemical analysis of primary transformants in (a) Recovered primary transformant exhibiting pricked sites (b) (i) Absence of GFP expression in wild-type seedlings, (ii) Pricked regions not exhibiting GFP fluorescence upon infection (iii–v) GFP expression identified in the pricked region in primary transformants of Mango. Absence of GUS expression in wild type (vi) shoot region (vii) cellular level. GUS expression (viii) in the shoot region (ix–x) at cellular level of primary transformants.
Table 2.
Preliminary confirmation and percentage transformability of Mango using in planta transformation strategy.
| Recovery |
Preliminary confirmation |
Molecular characterization |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No of seeds taken for transformation |
No of plants Recovered | Percentage | No of plants | No of positive plants | Percentage | PCR |
|||
| No of plants | No of positive plants | Percentage | Percentage chimeric plants produced | ||||||
| GFP- 70 | 55 | 78.57 | 40 | 26 | 65.0 | 24 | 13 | 54.16 | 18.57 |
| GUS- 40 | 31 | 77.50 | 7 | 4 | 57.14 | 12 | 08 | 66.67 | 20.0 |
Another batch of plants where GUS gene was used as scorable marker identified primary transformants which demonstrated the presence of GUS expression in the pricked regions. Out of 30 primary transformants, GUS histochemical assay was performed using 7 plants and remaining plants were allowed to grow normally. It was found that out of 7 plants, 4 showed GUS expression at the pricked regions (Fig. 4b, viii). However, wild type plants did not show any color in stem and its dissections as they lacked the GUS transgene (Fig. 4b vi, vii). Presence of GUS expression at cellular level was further confirmed by observing the GUS-stained tissue sections under microscope (Fig. 4b ix, x). Since the binary vector used for transformation had a GUS gene with a catalase intron, the histochemical analysis provided evidences for the integration of the T-DNA into the genome of mango plants. Nearly 57% of the plants showed GUS expression. Based on post pricking recovery and initial evidences of transformability in mango, the remaining 15 seedlings were transferred to the transgenic glasshouse and were allowed for growth.
A successful genetic transformation system seeks an appropriate visualization marker gene for the identification of transgenic plants.58 In our experiment, GFP and GUS were used as screenable marker genes and similar studies have been reported in mango,6 pummelo,28 and passion fruit.29
3.3. Recovery of Primary Transformants
Recovery of plants after genetic transformation and their establishment is essential for the success of the protocol. After transferring plants to glasshouse, 24 of 26 GFP and 12 of 15 GUS plants could survive. These plants were transferred to pots filled with soil, sand and farm yard manure 2:1:1 (FYM) and allowed to grow in glasshouse. Plants were continuously irrigated and supplemented with Hoagland’s solution at regular intervals
3.4. Molecular Analyses for Transgene Integration in Mango
The purview of the study considering the perennial nature of mango (long juvenile phase of 6–8 years), deals with the demonstration of T-DNA integration in the T0 generation, despite the fact that the primary transformants produced through in planta transformation are chimeric. Similar kind of molecular characterization in the chimeric plants of other perennial tree species have been reported in pummelo,28 and passion fruit.29
In order to confirm the presence of transgenes in the transgenic mango plants, genomic DNA was isolated from primary transformants and wild-type mango plants. PCR analysis was carried out using GFP and hptII; GUS and nptII gene-specific primers in their respective transgenic plants. Thirteen out of 24 plants showed the presence of 700 bp hptII and 570 bp GFP gene fragments (Fig. 5 Ai–ii, Bi–ii). Further, no amplification was found in wild type (WT) plants. The absence of Agrobacterium contamination in GFP transgenic plants were confirmed using PCR analysis of VirD1 gene (Fig. 5c). This unequivocally demonstrated that the GFP expression in the transgenic plants was due to the integration of the transgene and not due to the persisting bacteria.
Figure 5.
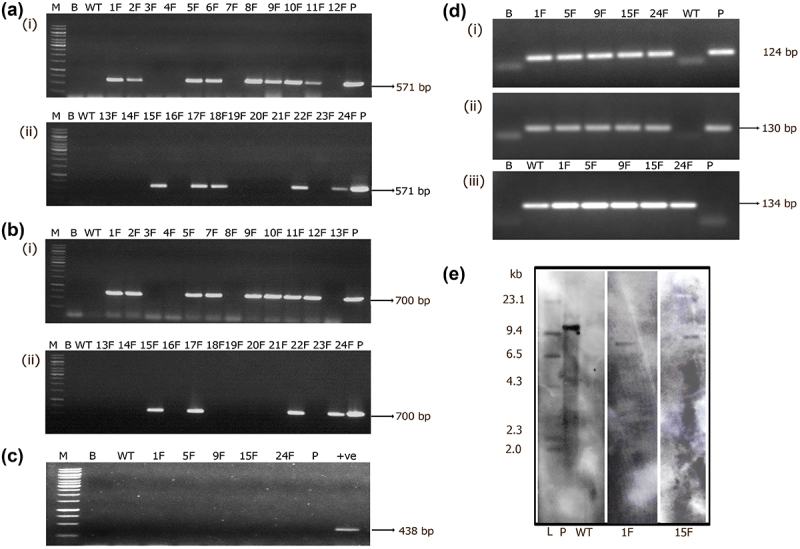
Molecular analysis of primary transformants harboring pCAMBIA1302 GFP::hptII. (a. i–ii; b. i–ii) PCR analysis for the amplification of GFP gene (571 bp) and hptII (700 bp) gene fragments. Lane M- 1Kb marker, Lane B- water blank, Lane WT- wild-type DNA, Lane P- plasmid (25 ng). Lanes 1 F-12 F and 13 F-24 F primary transformants. (c.) PCR analysis for the amplification of 438 bp Agrobactrium-specific VirD1 gene in transgenic Mango plants. Lane M- 1 Kb marker (Thermo scientific), Lane B- water blank, Lane WT- wild type, Lanes 1 F, 5 F, 9 F, 15 F, 24 F- transgenic plants, Lane P- binary vector, +ve is DNA from Agrobacterium strain EHA 105 (d) sqRT-PCR analyses for the assessment of transgene transcripts. (i) 124 bp GFP, (ii) 130 bp hptII and (iii) 134 bp MiACT1. Lane B- water blank, Lane WT- wild type, Lanes 1 F, 5 F, 9 F, 15 F, 24 F- transgenic plants, Lane P- binary vector. (e) Genomic Southern analysis of transgenic plants probed with DIG-labeled 571 bp GFP gene fragment, Lane L- Lambda HindIII DNA digest, Lane WT- untransformed wild type, Lanes 1 F, 15 F- transgenic Mango, P- linearized plasmid of pCAMBIA1302 10 pg.
Transgenic plants that tested positive by PCR were further assessed at transcription level and expression of transgene was confirmed by semi-quantitative RT-PCR analysis. Five transgenic plants and positive control showed the amplification of 124 and 130 bp GFP and hptII gene transcript fragments in the respective transgenic plants. However, amplification was absent in wild type (WT) plants (Fig. 5d i–ii). An internal control (MiACT1) authenticated amplification of 134 bp fragment in both transgenic and wild-type plants (Fig. 5d, iii). Finally, transgenic mango plants were assessed to determine the copy number of the transgene. Two transgenic mango plants (1 F and 15 F) were found to have a single copy of the transgene integrated in the genome and the absence of hybridization signal in the wild type providing proof for transgene integration (Fig. 5e).
Out of 12 plants tested for GUS and nptII genes, 8 plants showed PCR amplification of 1 kb GUS gene and 750 bp nptII gene fragments indicating the presence of transgenes in the primary transformants (Fig. 6a i-ii). Five GUS positive PCR plants were further tested for transgene accumulation that have shown amplification of 122 bp (GUS) and 67 bp (nptII) fragments verifying the presence of transcripts in transgenic plants and their absence in wild-type plants (Fig. 6b i-ii). Amplification of MiACT11 internal gene fragment in both transgenic and wild-type plants by sqRT-PCR further authenticated the results (Fig. 6b iii). Furthermore, transgene integration by Southern blotting identified single copy integration of the transgene in one transgenic plant (1 U) and the hybridization signal was absent in the wild-type plant DNA precisely confirming the transgenic nature of the plant (Fig. 6c).
Figure 6.
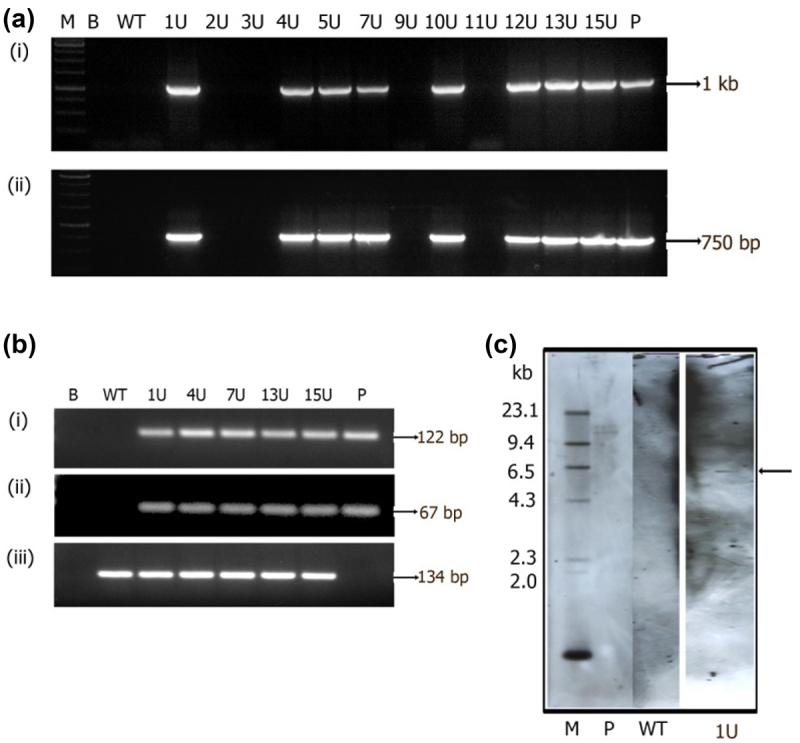
Molecular analysis of primary transformants harboring pCAMBIA 2301 GUS::nptII. (a) PCR analysis for the amplification of (i) GUS gene (1000 bp) and (ii) nptII (750 bp) fragments. Lane M- 1 kb marker, Lane B- water blank, Lane WT- wild-type DNA, Lanes 1 U-12 U- are primary transformants, Lane P- plasmid (25 ng). (b) sqRT-PCR amplified products on 1.5% w/v agarose gel of (i) 122 bp GUS, (ii) 67bp nptII and (iii) 134 bp MiACT1. Lane B- water blank, Lane WT- wild type, Lanes 1 U, 4 U, 7 U, 13 U, 15 U- transgenic plants, Lane P- binary vector. (c) Genomic Southern analysis of transgenic plants probed with DIG-labeled 750 bp nptII gene fragment, Lane L- Lambda HindIII DNA digest, Lane WT- wild type, Lane 1 U- transgenic Mango, Lane P- linearized plasmid of pCAMBIA2301 (10 pg).
Several researchers have tried to develop transformation protocols for mango using different methodologies.9–12,46,59,60 However, in all the previous studies they were unable to unequivocally demonstrate transformability, more so the transgene integration by genomic Southern analysis. This study demonstrated gene introgression in mango genome using shoot apical meristem-targeted in planta transformation and the associated molecular analyses. Tree crops have always been found difficult to improve by conventional breeding tools, biotechnology complement the conventional breeding and amends the mango improvement programs48. Transgenic technology holds several promises and can open ways in tackling a multitude of problems in mango
4. Conclusion
This study demonstrates the amenability of mango to apical meristem targeted in planta transformation protocol in a genotype independent manner. This report is the first successful demonstration of transgenic mango development using in planta transformation, which can assure it to be a significant contribution toward advancement in the area of mango biotechnology.
Acknowledgments
KP thanks and acknowledges ICAR-IARI, Senior Research Fellowship during PhD program. Authors thank Dr. N Srinivas (Late), Division of Plant pathology, ICAR-IARI, New Delhi for providing microscope facility. The authors also thank Mr. Rakesh Kumar Pandey (Late), PhD Research Scholar, Division of Fruits & Horticultural Technology, ICAR-IARI, New Delhi for the assistance during the experiment.
Funding Statement
The author(s) are thankful for funds recieved from ICAR- Indian Agricultural Research Institute, New Delhi, India.
Disclosure Statement
The authors declare no conflict of interest. All the authors have read and approved the final draft of the manuscript.
CRediT Authorship Contribution Statement
Kuldeep Pandey and Kesiraju Karthik developed the transgenic plants and identified putative transformants. Kuldeep Pandey performed molecular analyses. Rohini Sreevathsa and Manish Srivastav hypothesized the study. Kesiraju Karthik, Rohini Sreevathsa designed the experiments. Kuldeep Pandey wrote the manuscript. Kesiraju Karthik, Rohini Sreevathsa, Sanjay Kumar Singh, Vinod and Manish Srivastav critically edited the manuscript and finalized the manuscript. Manish Srivastav was responsible for fund acquisition.
References
- 1.Anonymous . Export value of fresh mangoes from India FY 2016-2021 published by statista research department (Hamburg: Statista; https://www.statista.com/). 2022. Mar 17, 2022. [Google Scholar]
- 2.Anonymous (2021). National horticulture board, ministry of agriculture government of India. 3rd Advance Estimate. Accessed April 12, 2021. http://www.nhb.gov.in/
- 3.Iyer CPA, Degani C.. Classical breeding and genetics. In: Litz RE, editor. The mango-botany, production and Uses, Wallingford Oxon: CAB International. 1997. p. 49–68. [Google Scholar]
- 4.Krishna H, Singh SK. Biotechnological advances in Mango (Mangifera indica L.) and their future implication in crop improvement a review. Biot. Adv. 2007;25:223–43. doi: 10.1016/j.biotechadv.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Ramachandra S, Srivastav M, Singh SK, Mahato AK, Singh N, Arumugam N, Singh NK. New genomic markers for marker assisted breeding in Mango (Mangifera indica L.). The J. of Horti. Sci, and Biotec. 2021;96:624–33. doi: 10.1080/14620316.2021.1906760. [DOI] [Google Scholar]
- 6.Samanta S, Ravindra MB, Dinesh MR, Anand L, Mythili JB. In vitro regeneration and transient gene expression in Mango cv. ‘Vellaikolumban.’ The J. Hort. Sci. Biotech. 2007;82:275–82. doi: 10.1080/14620316.2007.11512229. [DOI] [Google Scholar]
- 7.Niazian M, Noori SS, Galuszka P, Mortazavian SMM. Tissue culture-based Agrobacterium-mediated and in planta transformation methods. Soil and Water Res. 2017;53:133–43. doi: 10.17221/177/2016-CJGPB. [DOI] [Google Scholar]
- 8.Krishna H, Sairam RK, Singh SK, Patel VB, Sharma RR, Grover M, Sachdev A. Mango explant browning: effect of ontogenic age, mycorrhization and pre-treatments. Sci. Horti. 2008;118:132–38. doi: 10.1016/j.scienta.2008.05.040. [DOI] [Google Scholar]
- 9.Litz RE, Mathews H, Bharathan N, Narayan KR, 1990. Transformation of somatic embryos of Mango. Proceedings of the VII International Congress on Plant Tissue and Cell Culture, Amsterdam, 67. Abstract. [Google Scholar]
- 10.Mathews H, Litz RE, Wilde DH, Wetzstein HY. Genetic transformation of Mango. Acta. Hortic. 1993;341:93–97. [Google Scholar]
- 11.Mathews H, Litz RE, Wilde DH, Merkela S, Wetzstein HY. Stable integration and expression of bita-glucuronidase and NPT-II genes in Mango somatic embryo. Vitro Cell Dev Biol Plant. 1992;28:172–78. doi: 10.1007/BF02823312. [DOI] [Google Scholar]
- 12.Cruz-Hernandez A, Town L, Cavallaro A, Botella IR. Transient and stable transformation in Mango by particle bombardment. Acta Hortic. 2000;509:237–42. doi: 10.17660/ActaHortic.2000.509.24. [DOI] [Google Scholar]
- 13.Hamada H, Linghu Q, Nagira Y, Miki R, Taoka N, Imai R. An in planta biolistic method for stable wheat transformation. Sci. Rep. 2017;7:1–8. doi: 10.1038/s41598-017-11936-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaeppler SM, Kaeppler HF, Rhee Y. Epigenetic aspects of somaclonal variation in plants. Plant Gene Silencing. 2000:59–68. doi: 10.1007/978-94-011-4183-3_4. [DOI] [PubMed] [Google Scholar]
- 15.Kesiraju K, Sreevathsa R. Apical meristem-targeted in planta transformation strategy: an overview on its utility in crop improvement. Agri. Res. Technol. Open Access J. 2017;8:555734. doi: 10.19080/ARTOAJ.2017.08.555734. [DOI] [Google Scholar]
- 16.Kesiraju K, Mishra P, Bajpai A, Sharma M, Rao U, Sreevathsa R. Agrobacterium tumefaciens-mediated in planta transformation strategy for development of transgenics in cotton (Gossypium hirsutum L.) with GFP as a visual marker. Physiol. Mol. Biol. Plants. 2020;26:2319–27. doi: 10.1007/s12298-020-00887-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tague BW, Mantis J. In planta Agrobacterium-mediated transformation by vacuum infiltration. In Arabidopsis protocols. Humana Press. 2006:215–23. doi: 10.1385/1-59745-003-0:215. [DOI] [PubMed] [Google Scholar]
- 18.Wang WC, Menon G, Hansen G. Development of a novel Agrobacterium-mediated transformation method to recover transgenic Brassica napus plants. Plant Cell Rep. 2003;22:274–81. doi: 10.1007/s00299-003-0691-9. [DOI] [PubMed] [Google Scholar]
- 19.Chen G, Zeng F, Wang J, Ye X, Zhu S, Yuan L, Wang C. Transgenic Wucai (Brassica campestris L.) produced via Agrobacterium-mediated anther transformation in planta. Plant Cell Rep. 2019;38:577–86. doi: 10.1007/s00299-019-02387-0. [DOI] [PubMed] [Google Scholar]
- 20.Das P, Joshi NC. Minor modifications in obtainable Arabidopsis floral dip method enhances transformation efficiency and production of homozygous transgenic lines harboring a single copy of transgene. Adv. Biosci. Biotechnol. 2011;2:59. doi: 10.4236/abb.2011.22010. [DOI] [Google Scholar]
- 21.Trieu AT, Burleigh SH, Kardailsky IV, Maldonado‐Mendoza IE, Versaw WK, Blaylock LA, Harrison MJ. Transformation of Medicago truncatula via infiltration of seedlings or flowering plants with Agrobacterium. The Plant J. 2000;22:531–41. doi: 10.1046/j.1365-313x.2000.00757.x. [DOI] [PubMed] [Google Scholar]
- 22.Curtis IS, Nam HG. Transgenic radish (Raphanus sativus L. longipinnatus Bailey) by floral-dip method–plant development and surfactant are important in optimizing transformation efficiency. Trans. Res. 2001;10:363–71. doi: 10.1023/A:1016600517293. [DOI] [PubMed] [Google Scholar]
- 23.Shah SH, Ali S, Jan SA, Ali GM. Piercing and incubation method of in planta transformation producing stable transgenic plants by overexpressing DREB1A gene in tomato (Solanum lycopersicum Mill.). Plant Cell, Tissue and Organ Cult. 2015;120:1139–57. doi: 10.1007/s11240-014-0670-6. [DOI] [Google Scholar]
- 24.Li J, Todd TC, Trick HN. Rapid in planta evaluation of root expressed transgenes in chimeric soybean plants. Plant Cell Rep. 2009;29:113–23. doi: 10.1007/s00299-009-0803-2. [DOI] [PubMed] [Google Scholar]
- 25.Hirsch AM, Lee A, Deng W, Tucker SC. An open-flower mutant of Melilotus alba: potential for floral-dip transformation of a papilionoid legume with a short life cycle? Am. J. Bot. 2010;97:395–404. doi: 10.3732/ajb.0900152. [DOI] [PubMed] [Google Scholar]
- 26.Mu G, Chang N, Xiang K, Sheng Y, Zhang Z, Pan G. Genetic transformation of maize female inflorescence following floral dip method mediated by Agrobacterium. Biotechnol. Ther. 2012;11:178–83. doi: 10.3923/biotech.2012.178.183. [DOI] [Google Scholar]
- 27.Ratanasut K, Rod-In W, Sujipuli K. In planta Agrobacterium-mediated transformation of rice. Rice Sci. 2017;24:181–86. doi: 10.1016/j.rsci.2016.11.001. [DOI] [Google Scholar]
- 28.Zhang YY, Zhang DM, Zhong Y, Chang XJ, Hu ML, Cheng CZ. A simple and efficient in planta transformation method for pommelo (Citrus maxima) using Agrobacterium tumefaciens. Sci. Horti. 2017;214:174–79. doi: 10.1016/j.scienta.2016.11.033. [DOI] [Google Scholar]
- 29.Rizwan HM, Yang Q, Yousef AF, Zhang X, Sharif Y, Kaijie J, Chen F. Establishment of a novel and efficient Agrobacterium-mediated in planta transformation system for passion fruit (Passiflora edulis). Plants. 2021;10:2459. doi: 10.3390/plants10112459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaganath B, Subramanyam K, Mayavan S, Karthik S, Elayaraja D, Udayakumar R, Ganapathi A. An efficient in planta transformation of Jatropha curcas (L.) and multiplication of transformed plants through in vivo grafting. Protoplasma. 2014;251:591–601. doi: 10.1007/s00709-013-0558-z. [DOI] [PubMed] [Google Scholar]
- 31.Saifi SK, Passricha N, Tuteja R, Kharb P, Tuteja N. In planta transformation: a smart way of crop improvement. In: Advancement in crop improvement techniques, Narendra Tuteja, Renu Tuteja, Nishat Passricha, Shabnam K. Saifi . Duxford, CB22 4QH, United Kingdom: Woodhead Publishing; 2020. p. 351–62. [Google Scholar]
- 32.Keshamma E, Sreevathsa R, Kumar AM, Reddy KN, Manjulatha M, Shanmugam NB, Kumar ARV, Udayakumar M. Agrobacterium-mediated in planta transformation of field bean (lablab purpureus l.) and recovery of stable transgenic plants expressing the cry1AcF Gene. Plant Mol. Biol. Rep. 2012;30:67–78. doi: 10.1007/s11105-011-0312-7. [DOI] [Google Scholar]
- 33.Keshavareddy G, Rohini S, Ramu SV, Sundaresha S, Kumar ARV, Kumar PA, Udayakumar M. Transgenics in groundnut (Arachis hypogaea L.) expressing cry1AcF gene for resistance to Spodoptera litura (F.). Physio. Mol. Biol. Plants. 2013;19:343–52. doi: 10.1007/s12298-013-0182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar AM, Reddy KN, Manjulatha M, Arellano ES, Sreevathsa R. A rapid, novel and high throughput identification of putative bell pepper transformants generated through in planta transformation approach. Sci. Horti. 2011;129:898–903. doi: 10.1016/j.scienta.2014.02.034. [DOI] [Google Scholar]
- 35.Shivakumara TN, Sreevathsa R, Dash PK, Sheshshayee MS, Papolu PK. Over expression of Pea DNA Helicase 45 (PDH45) imparts tolerance to multiple abiotic stresses in chili (Capsicum annuum L.). Sci Rep. 2017:7. doi: 10.1038/s41598-017-02589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramkumar N, Rathinam M, Singh S, Kesiraju K, Muniyandi V, Singh NK, Dash PKSR. Assessment of Pigeonpea (Cajanus cajan L.) transgenics expressing Bt ICPs, Cry2Aa and Cry1AcF under nethouse containment implicated an effective control against herbivory by Helicoverpa armigera (Hübner). Pest Manag. Sci. 2020;76:1902–11. doi: 10.1002/ps.5722. [DOI] [PubMed] [Google Scholar]
- 37.Singh S, Kumar NR, Maniraj R, Lakshmikanth R, Rao KYS, Muralimohan N. Expression of Cry2Aa, a Bacillus thuringiensis insecticidal protein in transgenic pigeon pea confers resistance to gram pod borer, Helicoverpa armigera. Sci. Rep. 2018;8:8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kesiraju K, Tyagi S, Mukherjee S, Rai R, Singh NK, Sreevathsa R, Dash PK. An apical meristem-targeted in planta transformation method for the development of transgenics in flax (Linum usitatissimum): optimization and Validation. Front. Plant Sci. 2021;11:562056. doi: 10.3389/fpls.2020.562056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karthik K, Nandiganti M, Thangaraj A, Singh S, Mishra P, Sharma MS, K N, Dash PK, Sreevathsa R. Transgenic cotton (Gossypium hirsutum L.) to combat weed vagaries: utility of an apical meristem-targeted in planta transformation strategy to introgress a modified CP4-EPSPS gene for glyphosate tolerance. Front. Plant Sci. 2020;11:768. doi: 10.3389/fpls.2020.00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karthik K, Negi J, Rathinam M, Saini N, Sreevathsa R. Exploitation of novel Bt ICPs for the management of Helicoverpa armigera (Hübner) in cotton (Gossypium hirsutum L.): a transgenic approach. Front. in Micro. 2021;12:794. doi: 10.3389/fmicb.2021.661212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winans SC, Kerstetter RA, Nester EW. Transcriptional regulation of the virA and virG genes of Agrobacterium tumefaciens. J Bacteriol. 1988;170:4047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rohini VK, Rao S. Transformation of peanut (Arachis hypogea L.) A non-tissue culture-based approach for generating transgenic plants. Plant Sci. 2000;150:41–49. doi: 10.1016/s0168-9452(99)00160-0. [DOI] [Google Scholar]
- 43.Keshamma E, Rohini S, Rao K, Madhusudhan B, Kumar MU. Tissue culture-independent in planta transformation strategy: an Agrobacterium tumefaciens-mediated gene transfer method to overcome recalcitrance in cotton (Gossypium hirsutum L.). J. Cotton Sci. 2008;12:264–72. doi:ISSN 1523-6919. [Google Scholar]
- 44.Murray M and Thompson W. (1980). Rapid isolation of high molecular weight plant DNA. Nucl Acids Res, 8(19), 4321–4326. 10.1093/nar/8.19.4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo C, He XH, Hu C, Ying H, Shi-Jin O. Molecular cloning and expression analysis of four actin genes (MiACT) from Mango. Biol Plant. 2013;57:238–44. doi: 10.1007/s10535-012-0278-9. [DOI] [Google Scholar]
- 46.Gomez-Lim MA, Litz RE. Genetic transformation of perennial tropical fruits. In Vitro Cell Dev. Biol. -Plant. 2004;40:442–49. doi: 10.1079/IVP2004547. [DOI] [Google Scholar]
- 47.Cruz-Hernandez A and Gomez-Lim M. (1995). Alternative oxidase from mango (Mangifera indica, L.) is differentially regulated during fruit ripening. Planta, 197(4), 10.1007/BF00191562 [DOI] [PubMed] [Google Scholar]
- 48.Mishra, Manish, Seal , Shubhendu, Chandra, Ramesh. 1 January Mango Advances in Horticultural Biotechnology Transformation System. Singh, H.P., Parthasarathy, V.A., Babu, Nirmal K.. Vol. 5 I (New Delhi: Westville Publishing House; ) 2011 [Google Scholar]
- 49.Raghuvanshi SS, Srivastava A. Plant regeneration of Mangifera indica using liquid shaker to reduce phenolic exudation. Plant Cell Tissue Organ Cult.41. 1995:83–85. doi: 10.1007/BF00124092. [DOI] [Google Scholar]
- 50.Singh SK, Sharma HC, Singh SP, Kishore PBK. Callus induction and root initiation from explants of zygotic embryos in Mango hybrids. Plant Tissue Cult. Biotech. Emerging Trends. Proceeding of Sym. 1991;1:129–33. [Google Scholar]
- 51.Sharma RR, Singh SK. Etiolation reduces phenolic content and polyphenol oxidase activity at the pre-culture and in vitro exudation of phenols from Mango explants. Trop. Agric. 2002;79:94–99. [Google Scholar]
- 52.Thomas P, Ravindra MB. Shoot tip culture of Mango, influence of media, genotype, explant factors, season, and decontamination treatments on phenolic exudation, explant survival and axenic culture establishment. J. Horti. Sci. 1997;72:713–22. doi: 10.1080/14620316.1997.11515563. [DOI] [Google Scholar]
- 53.Ara H, Jaiswal U, Jaiswal VS. Germination and plantlet regeneration from encapsulated somatic embryos of Mango (Mangifera indica L.). Plant Cell Rep. 1999;19:166–70. doi: 10.1007/s002990050728. [DOI] [PubMed] [Google Scholar]
- 54.Litz RE, Mathews H, Moon PA, Pliego-Alfaro F, Yurgalevitch C, Dewald SG. Somatic embryos of Mango (Mangifera indica L.). In: Redenbaugh K (boca raton: CRC press; ), editor. Synseeds applications of synthetic seeds to crop improvement. 1993. p. 409–25. [Google Scholar]
- 55.Da Silva ML, Passos PPDL, Marcelino-Guimarães AB, Rossi FC, B AA, Krause W, Otoni WC. Novel and efficient transformation of wild passion fruit (Passiflora cincinnata Mast.) using sonication-assisted Agrobacterium-mediated transformation.Vitro Cell Dev. Biol. -Plant. 2021;57:380–86. doi: 10.1007/s11627-020-10134-4. [DOI] [Google Scholar]
- 56.Eibl R, Meier P, Stutz I, Schildberger D, Hühn T, Eibl D. Plant cell culture technology in the cosmetics and food industries: current state and future trends. Applied Microbiology and Biotechnology. 2018;102(20):8661–75. doi: 10.1007/s00253-018-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Espinosa-Leal CA, Puente-Garza CA, García-Lara S. In vitro plant tissue culture: means for production of biological active compounds. Planta. 2018;248:1–18. doi: 10.1007/s00425-018-2910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramessar K, Peremarti A, Gómez-Galera S, Naqvi S, Moralejo M, Munoz P, Christou P. Biosafety and risk assessment framework for selectable marker genes in transgenic crop plants: a case of the science not supporting the politics. Trans. Res. 2007;16:261–80. doi: 10.1007/s11248-007-9083-1. [DOI] [PubMed] [Google Scholar]
- 59.Cruz-Hernandez A, Gomez-Lim MA, Litz RE. Transformation of Mango somatic embryos. Acta Hortic. 1997;455:287–91. doi: 10.17660/ActaHortic.1997.455.38. [DOI] [Google Scholar]
- 60.Mathews H, Litz RE. Kanamycin sensitivity of Mango somatic embryos. HortScience. 1990;25:965–66. doi: 10.21273/HORTSCI.25.8.965. [DOI] [Google Scholar]


