Abstract
Why there are so few gametocytes (the transmission stage of malaria) in the blood of humans infected with Plasmodium spp. is intriguing. This may be due either to reproductive restraint by the parasite or to unidentified gametocyte-specific immune-mediated clearance mechanisms. We propose another mechanism, a cross-stage immunity to Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP-1). This molecule is expressed on the surface of the erythrocyte infected with either trophozoite or early gametocyte parasites. Immunoglobulin G antibodies to PfEMP-1, expressed on both life cycle stages, were measured in residents from an area where malaria is endemic, Papua New Guinea. Anti-PfEMP-1 prevalence increased with age, mirroring the decline in both the prevalence and the density of asexual and transmission stages in erythrocytes. These data led us to propose that immunity to PfEMP-1 may influence malaria transmission by regulation of the production of gametocytes. This regulation may be achieved in two ways: (i) by controlling asexual proliferation and density and (ii) by affecting gametocyte maturation.
Transmission of malaria parasites from the human host to the anopheline vector involves the production of gametocytes. These stages arise after the commitment of asexually dividing erythrocytes (RBCs) to a pathway of sexual development. Plasmodium falciparum gametocytes develop through several morphologically distinct stages, designated I to V, within the host RBC over a period of 10 to 14 days (25). Stage I to IV gametocytes sequester from the peripheral circulation during maturation (22, 42, 47). Stage V gametocytes circulate in the bloodstream and after a further 2 to 3 days become infectious to the mosquito vector.
Observations of the natural history of malaria infection in humans point to two important features of the transmission biology of malaria. First, blood slide surveys have shown that both the prevalence and density of P. falciparum gametocytes decline in an age-specific manner in hosts living in areas of intense malaria transmission (14, 37). This decline may result from the development of naturally acquired immunity to gametocytes, although no age-dependent mechanisms of immune-mediated clearance of gametocytes have yet been identified (for a review, see reference 45). Second, studies of within-host parasite dynamics as well as population surveys have shown that there are far fewer gametocytes in the peripheral blood than the circulating asexual forms known as trophozoites (14, 28, 30, 37, 46). This paucity of transmission stages reflects, in part, the life history of P. falciparum within the human host; exposure to asexual parasites will necessarily be greater because they mature in 2 days, compared to the 8 to 10 days before the sexual stages are found in the peripheral circulation. Commitment to gametocytogenesis occurs only after peak asexual parasitemia is reached (13). Nonetheless, these aspects of the parasite's biology cannot fully explain why there are so few transmission stages.
Taylor and Read (45) have put forward two mechanisms to explain the low prevalence and density of gametocytes relative to those of asexual parasitemias: (i) natural selection favors reproductive restraint such that only low numbers of gametocytes are ever produced, and (ii) a gametocyte-specific immune mechanism(s) acts in the clearance of gametocytes at some stage in their development. We favor a third mechanism, one involving naturally acquired immunity to the variant surface antigen designated P. falciparum erythrocyte membrane protein 1 (PfEMP-1) (27).
PfEMP-1 is expressed on the surface of trophozoite-infected RBCs (32) and mediates adhesion to CD36 and other host adhesion ligands (for a review, see reference 20). This molecule is highly immunogenic (29) and undergoes clonal antigenic variation (5, 41) with variant forms differing in both antigenic and adhesive characteristics (43). By analogy with animal model experiments, the sequential expression of different antigenic variants is believed to mediate the persistence of the parasite within the human host (8). In humans, variant-specific agglutinating antibodies reactive to the surface of the trophozoite-infected RBC have been observed; seroconversion occurs after an acute P. falciparum infection (21, 33). This variant-specific immunity is acquired in an age-dependent manner (12, 21, 24) and is associated with protection from clinical disease (12, 34). It is believed that these agglutinating antibodies are directed against PfEMP-1 (12, 50). It has recently been demonstrated with a PfEMP-1 deletion mutant that this is the case in sera from Papua New Guinea (40). Cytophilic immunoglobulin G (IgG) antibodies have been shown to mediate the recognition of PfEMP-1 (40).
The genes involved in the expression of PfEMP-1 molecules have been identified as a multigene family designated var (4, 43, 44). The differential expression of var genes is associated with the expression of antigenically distinct PfEMP-1 molecules with different adhesive properties (43). Trophozoites and gametocytes share the same repertoire of var genes and express the same PfEMP-1 variants at the surface of the infected RBC (27). The surface expression of PfEMP-1 is restricted to young gametocytes (stages I and IIA). Based on these molecular data, it has been proposed that immunity against PfEMP-1 variants may limit the numbers of asexual parasites with the potential to become gametocytes and prevent the maturation of sexual stages (27).
To determine whether such an immune mechanism exists and whether it could account for age-specific patterns of gametocytemia, an immunoepidemiological study was designed. IgG antibody responses were measured to the surface of trophozoite- and stage I and IIa early gametocyte-infected RBCs of three P. falciparum isolates. Plasma samples from residents aged 2 to 60 years from Madang, Papua New Guinea, where intense year-round transmission of malaria occurs (14), were screened for these responses. Age-specific patterns of immunity are discussed in the context of the potential role of PfEMP-1-specific IgG antibodies in the regulation of the numbers of transmission stages.
MATERIALS AND METHODS
Study population and plasma collection.
The study was conducted in five rural villages situated along the Gogol River basin in Madang Province on the north coast of Papua New Guinea (for details, see reference 17). This area experiences intense year-round transmission of malaria (14). In 1993, a cross-sectional malariometric survey of 555 individuals aged 2 to 60 years was completed. Thick and thin blood smears were made at the time of plasma collection. Thin films were fixed in methanol and then both thick and thin films were stained with 4% Giemsa stain for 20 min, washed, dried, and stored. Both asexual and gametocyte parasitemias were scored by counting the number of parasites per 200 leukocytes (WBCs). A parasite-negative slide was one on which 2,000 WBCs had been viewed and no parasites had been seen. Parasite densities were converted to parasites per microliter of blood by assuming 8,000 WBCs per μl. Plasma was isolated from EDTA-treated blood samples by Histopaque (Sigma, Poole, United Kingdom) separation. Fifty-nine individuals were selected for measurements of antibody recognition to PfEMP-1 from the cross-sectional study population of 555 individuals and divided by age into the following groups (each designation indicates the age range, in years, for that group): 2-to-4, 5-to-9, 10-to-14, 15-to-19, 20-to-30, and 31-to-60. Hyperimmune plasma (HIP) pools were made from samples from 10 immune adults from the cross-sectional study population, and normal human serum (NHS) was made from samples from three non-malaria-exposed Europeans (Oxford BTS, Oxford, United Kingdom). All serum and plasma aliquots were stored at −70°C.
Parasites.
P. falciparum isolates Muz 37 and Muz 106 were collected from children presenting with acute symptomatic malaria at aid posts in Madang during a longitudinal cohort study from 1990 to 1991 (17). Isolate 1776 was collected in 1987 (21). RBCs were washed three times after buffy-coat depletion in RPMI 1640 medium buffered with 25 mM HEPES and supplemented with 25 mM sodium bicarbonate, 2 mM l-glutamine, 300 mM hypoxanthine and 10 μg of gentamicin per ml (Gibco, Paisley, United Kingdom) (RPMI-HEPES); the cells were then cryopreserved in liquid nitrogen for adaptation at WTCEID, Oxford. Adaptation of primary isolates to in vitro culture was performed at WTCEID, Oxford. Parasites were cultured according to the method of Trager and Jenson (48). Cells were grown in RPMI-HEPES supplemented with 10% blood type AB sera (from donors residing in a nonmalarious area) in an atmosphere of 5% CO2, 5% O2, and 90% N2 and subcultured into O-positive RBCs (Oxford BTS), and stabilates were frozen down. In some cases, feeder cells (peritoneal-wash mouse cells) were required for initial parasite growth (106 cells/ml, added at weekly intervals for up to 3 weeks) (adapted from the protocol outlined in reference 49). Isolates Muz 106, Muz 37, and 1776 were collected 2, 3, and 6 years, respectively, prior to the collection of plasma samples. It has previously been shown that on adaptation of new isolates to in vitro culture, there is a high risk of the loss of expression of PfEMP-1 at the infected RBC surface due to a subtelomeric deletion in chromosome 9 (19). Thus, parasites were selected over a C32 amelanotic melanoma cell line (ATCC CRL 1585 C32r) as previously described (40). Binding was done at 3- to 4-week intervals in order to select CD36-binding infected RBCs with an intact chromosome 9 (19), to minimize the number of null cells being assayed for antibody reactivity. Gametocytogenesis was initiated and gametocytes were maintained in culture according to previously published methods (19).
Surface immunofluorescence assay.
The staining protocol for human serum recognition at the surface of trophozoite- and early gametocyte-infected RBCs has previously been described (27, 40). Briefly, trophozoite-infected RBCs were enriched by plasma gel flotation (Fresenius France Pharma, Sevres, France) (38), adjusted to 10 to 15% parasitemia. Early gametocytes were separated by a Percoll gradient (18) and also resuspended to 10 to 15% stage IB and IIA levels. Parasites were incubated with a 1/50 dilution of individual plasma, HIP, and NHS pools. For trophozoite-infected RBCs, antibody detection was made with a rabbit anti-human IgG (Dako, Cambridge, United Kingdom), followed by a fluorescein isothiocyanate-coupled swine anti-rabbit IgG (Dako) containing 50 μg of ethidium bromide per ml (Sigma). Cells were fixed for 1 h with 0.5% paraformaldehyde diluted in human tonicity phosphate-buffered saline–1% bovine serum albumin. A different staining regimen was used for staining human plasma bound to early-gametocyte-infected RBCs; a biotinylated sheep anti-human IgG (Sigma) was followed by fluorescein isothiocyanate-streptavidin (Sigma). This was to avoid the nonspecific binding of goat anti-mouse antibody, used for the detection of internally labelled gametocytes within the RBC, to externally labelled antibodies on the RBC surface. After surface staining, early-gametocyte-infected RBCs were fixed with 2% paraformaldehyde overnight at 4°C and permeabilized with 0.01% Triton X-100 to allow labelling of the early gametocyte within the infected RBC. Staining was done with a gametocyte-specific mouse monoclonal antibody, 2G7 (10), followed by phycoerythrin-conjugated goat anti-mouse IgG (Sigma). Both trophozoite- and gametocyte-infected RBCs were read on an EPICS/XL counter (Coulter Electronics), where 1,000 infected RBCs were counted. Mean fluorescence intensity (MFI) and the percentage of fluorescence-positive infected RBCs (IRBCs) were calculated from the following formula: (IRBCs of test plasma sample − RBCs of test plasma sample) − (IRBCs in NHS − RBCs in NHS).
Data analysis.
Logistic regression analysis was used to look for age trends in parasitemias and antibody responses. The Spearman rank correlation coefficient test was used to compare the density of parasites to host age and to compare individual antibody titers to trophozoite- and early-gametocyte-infected RBCs from the same isolate and to trophozoite- or early-gametocyte-infected RBCs between isolates. The Kruskal-Wallis H test was used to compare data between age groups. SPSS 7.0 for Windows was used for all statistical analyses.
RESULTS
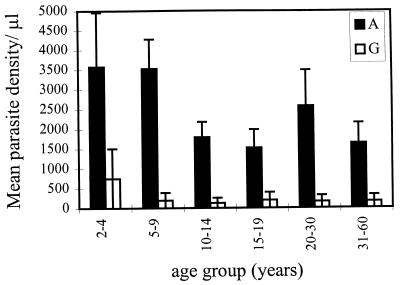
The age-specific density of asexual parasitemia and gametocytemia was measured in a cross-sectional survey of 555 individuals by blood slide positivity. This measure detects both young asexual stages (i.e., ring-infected RBCs) and mature-gametocyte-infected RBCs in the peripheral circulation. As expected from previous studies (37), the density of mature-gametocyte-infected RBCs was lower than that of asexual stages (Fig. 1); the density of gametocytes was an order of magnitude lower in most age classes (Fig. 1). An age-specific decrease in the densities of asexual parasitemia and gametocytemia was observed (Fig. 1). The decrease in asexual parasite density occurred in the 10- to 14-year-old age group such that individuals aged over 10 years had significantly lower parasite densities (<1,800/μl) than those under 10 (>3,000/μl) (χ2 = 18.4; P < 0.001). For gametocyte densities, the drop occurred in the 5- to 9-year-olds such that densities were significantly lower in those over 5 years old (<200/μl) in comparison to 2- to 4-year-olds (>700/μl) (χ2 = 4.7; P < 0.05).
FIG. 1.
Mean age-specific parasite density of asexual stages (A) (solid bars) and gametocyte stages (G) (empty bars) of P. falciparum. Sample sizes for each age group were 17, 55, 62, 30, 22, and 36 for the asexual stages and 5, 9, 9, 3, 1, and 5 for the gametocyte stages. Standard errors are represented as error bars.
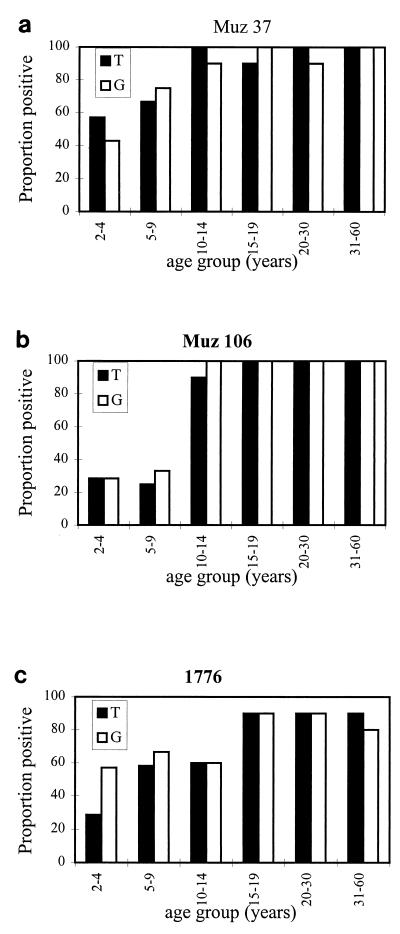
IgG-specific antibodies to the surface of trophozoite- and early-gametocyte-infected RBCs from three Papua New Guinea isolates were measured in 59 individuals by flow cytometric methods. To account for the nonspecific binding of IgG antibodies, normal human serum reactivity was subtracted from test values according to the formula given in Materials and Methods. The age-specific prevalence of IgG antibodies to the surface of both trophozoite- and early-gametocyte-infected RBCs from three isolates is shown in Fig. 2. Age had a significant effect on IgG prevalence to trophozoite- and gametocyte-infected RBCs from Muz 37 and Muz 106 such that near-complete seroconversion was seen in those subjects who were over 10 years old (logistic regression; P < 0.05). For isolate 1776, seroconversion to PfEMP-1 was acquired more slowly with age, reaching maximal prevalence in those subjects over 15 years old (P < 0.05 and P = 0.063 for trophozoite- and gametocyte-infected RBCs, respectively). In the 2- to 4-year-old age group, 2 of 10 individuals were seropositive for trophozoite- but not for gametocyte-infected RBCs of Muz 37. The inverse was true for isolate 1776, such that two sera were positive for gametocyte- but not for trophozoite-infected RBCs. On closer inspection of the data, both plasma samples responsive to Muz 37 and one of those responsive to 1776 were found to be borderline positive and were classed as seronegative. For all seropositive individuals, the MFI of the IgG response was similar across all age groups for all isolates and both life stages; for Muz 37, the range of reactivity for each age class was as follows: the MFI range was 40 to 229, 28 to 232, 60 to 328, 21 to 632, 19 to 257, and 21 to 345 for the 2-to-4, 5-to-9, 10-to-14, 15-to-19, 21-to-30, and 31-to-60 groups, respectively. Ten individual plasma and HIP samples never reacted to the surface of late-gametocyte-infected RBC (26).
FIG. 2.
Age-specific prevalence of antibodies to the surface of trophozoite-infected (T) (solid bars) and gametocyte-infected (G) (empty bars) RBCs for three P. falciparum isolates, Muz 37 (a), Muz 106 (b), and 1776 (c). Each age group contained 10 individuals, except for the 2-to-4 group, with 7, and the 5-to-9 group, with 12.
Other studies have clearly demonstrated the variant specificity of the immune response to PfEMP-1 on trophozoite stage-infected RBCs (21, 33). Using the Spearman rank correlation coefficient test we compared the MFI values of samples from 29 patients to the surface of trophozoite- and early-gametocyte-infected RBCs. Children aged 2 to 14 years were chosen, since those older than 14 years were more likely be positive to all three isolates, as seen from age-prevalence profiles shown in Fig. 2. Antibodies to gametocyte stages from Muz 37, Muz 106, and 1776 did not correlate, as would be expected for variant-specific immunity. Curiously, though, plasma responses to the trophozoite stages of Muz 37 and Muz 106 did correlate (r = 0.58; P < 0.01). This would suggest that only a subpopulation of variants from these two isolates converted to gametocytes, explaining the lack of correlation between antibody responses to gametocyte stages from Muz 37 and Muz 106.
Recent molecular data have demonstrated that asexual stages and gametocytes from one isolate share the same repertoire of var genes and that both stages express PfEMP-1 (27). If PfEMP-1 were the only major immunogenic variant surface antigen on both life-cycle stages, we would expect to see a strong correlation in individual immune responses to both life cycle stages of a single isolate. Using all of the age cohort individuals, we compared plasma reactivity to the surface of trophozoite-infected RBCs and to the surface of early-gametocyte-infected RBCs from the same isolate. The IgG response to trophozoite-infected RBCs correlated significantly (P < 0.01) with that to the homologous early-gametocyte-infected RBCs for each of the three isolates Muz 37, Muz 106, and 1776 (r = 0.549, r = 0.468 and r = 0.371, respectively). Thus, we hypothesize that the major immunogen(s) on the surface of the gametocyte-infected RBC is the same as that on the trophozoite-infected RBC, i.e., PfEMP-1.
DISCUSSION
The immunoepidemiological data presented in this paper provide the first description of an age-dependent naturally acquired immune response to the transmission stages of P. falciparum. Unlike transmission-blocking immunity, this response is functional in the human host rather than in the mosquito vector. The observed IgG antibody response is generated by exposure to PfEMP-1 variants expressed on the surface of RBC infected either with gametocytes or mature asexual stages. This immunity has the potential to regulate the densities of both gametocytes and trophozoites by antibody-dependent cell-mediated cytotoxicity reactions. The magnitude of the asexual parasite population in a human host relative to the gametocyte population indicates that seroconversion to PfEMP-1 variants will occur predominantly as a result of cumulative exposure to trophozoite-infected RBCs. The size of the repertoire of PfEMP-1 variants in the parasite population of any area where disease is endemic is unknown but likely to be large, as there are 50 to 100 var genes per haploid genome (44). The high levels of incidence of malaria infection (9) and the 5 to 10 years of exposure required to seroconvert to any PfEMP-1 variant (21, 24) are consistent with a large repertoire of PfEMP-1 variants in this area of endemicity. The prevalence of these variant-specific responses increased in the age groups (5 to 14 years) where gametocytemia and asexual parasitemia were declining. The decline in gametocytemia compared to that in asexual parasitemia occurred earlier in this data set. Previous analyses of larger data sets from Madang (14, 17) demonstrated that both asexual and gametocyte densities peak in the 1- to 4-year-old subjects and decline with age thereafter. The small size of our data set and the greater variability in asexual parasitemia, compared to the low levels of gametocyte density near the limit of sensitivity, most likely account for the observed differences. This gives weight to the larger analyses performed in the same area of endemicity (14, 17), with the decline of asexual stage and gametocyte densities occurring in the age classes where seroprevalence to PfEMP-1 variants increases, as measured in this study.
Peak seroprevalence to isolate 1776 occurred later, with respect to age, in comparison to Muz 37 and Muz 106. This is consistent with the existence of rare and common variants proposed by Bull et al. (11). The apparent persistence of PfEMP-1 antibodies in the older age classes may be explained by long-lived immunity to PfEMP-1 variants or repeated boosting of PfEMP-1 immunity by low-density infections.
To date, immunity to the surface of the trophozoite-infected RBC is believed to be directed to PfEMP-1 (40, 50). Recently, however, data are emerging about the genes stevor and rif, which may also encode variant surface molecules in asexual blood stages (16). The products of rif (rifin proteins) are expressed on the surfaces of infected RBCs and are phenotypically variable (31). Immune sera, however, failed to immunoprecipate proteins of molecular weight comparable to that of rifin proteins, and antisera raised to these proteins did not recognize the surfaces of infected RBCs (31). Whether these molecules are exposed at the exterior surface of the infected RBCs of both trophozoites and gametocytes and are immunogenic is thus unclear. The correlation between immune responses to trophozoite- and gametocyte-infected RBCs in individuals suggests that gametocytes will express the same variant antigenic repertoire. In a previous study, trophozoite-stage and gametocyte-stage parasites were coagglutinated by hyperimmune plasma (27), supporting the findings of our correlation analysis. Correlations, however, were never 100%, but in culture a clonal population of parasites can express multiple variants, as previously reported in our laboratory, at any one point in time (27), although only one variant is expressed by one individual parasite (15). Both asexual and gametocyte cultures were grown in parallel; the only difference was the addition of fresh RBCs to asexual cultures. Differences could not be due to mixed parasite populations, since microsatellite typing (1) of these isolates revealed that they were mixtures of genetically distinct parasite clones (39a). We cannot rule out the possibility that unknown antigens coordinately expressed with PfEMP-1 may also be recognized by plasma and may explain any differences observed. However, these plasmas do not recognize the surface of trophozoite-infected RBCs from the mutant cell line C10 (39a), which does not express PfEMP-1 at the infected RBC surface.
Observations of the intrahost dynamics of P. falciparum demonstrate that the asexual stages do not commit to sexual development until after a peak density of asexual parasitemia is reached (13). Recent data indicate that commitment is correlated with a decrease in parasite growth rate (20a). This life history strategy could be selected by immunity to PfEMP-1 variants. The asexual parasites expressing PfEMP-1 variants to which there is no pre-existing immunity will replicate successfully and also give rise to early gametocytes expressing the same PfEMP-1 variants (27). During subsequent development (stages IIb to V), PfEMP-1 expression is lost from the surface of the infected RBC, rendering the mature gametocyte safe from anti-PfEMP-1 immunity (27). Thus, the relatively few gametocytes observed within a host may be the consequence of immunity to predominantly PfEMP-1 expression, regulating the proliferation of the asexual population but with the potential to also regulate the maturation of transmission stages.
Variant-specific immunity to PfEMP-1 could also generate the independent transmission dynamics of genetically distinct parasite clones cohabiting the same human host. Such immunity would necessarily generate nonoverlapping infectious periods which would influence parasite mating patterns in the mosquito vector. The observed excess of homozygous infections in mosquitoes that arises from blood meals in human hosts harboring multiple infections from the same study area in Madang, Papua New Guinea (39), may be due to the above-mentioned variant-specific gametocyte dynamics.
Taylor and Read (45) have made a strong argument that the paucity of transmission stages in residents from areas of endemicity is due to reproductive restraint, possibly selected by density-dependent mechanisms directed against sexual stages (i.e., transmission-blocking immunity in the mosquito vector). They have argued that the observation of higher gametocyte densities induced in semi-immune hosts following intensive vector and drug control demonstrates that gametocyte-specific immunity, controlling sexual parasite densities, does not exist. Here, we describe a novel immune response to asexual and gametocyte stages with the potential to regulate populations of both life cycle stages. The ability to induce higher gametocyte densities in semi-immune individuals can occur as a result of the expansion-gametocyte conversion of the subpopulation of trophozoites to which there is no pre-existing variant-specific immunity.
Population studies of other Plasmodium spp. infecting humans have also shown a paucity of transmission stages (for a review, see reference 45). Serological studies demonstrate the existence of variant-specific antigens on the surface of Plasmodium vivax-infected RBCs (36), as well as Plasmodium spp. infecting nonhuman primates (3, 7, 8, 23). It may be that the same variant-specific immune mechanism is operational in P. vivax infection with consequent regulation of transmission.
The view has previously been held that the decline in gametocytemia along with asexual parasitemia is regulated by the immune response to the asexual stages (6, 35). Here, we propose that immunity to PfEMP-1 could regulate both trophozoite and sexual stage densities. The similarity in the slope of the age-specific curve of trophozoite and gametocyte densities from a large data set such as the Garki project (37) supports this opinion. There is some evidence, however, that gametocyte-specific immune responses can regulate gametocytemia independent of asexual-stage-specific immunity (2). A study in Irian Jaya, comparing natives to transmigrants from Java, observed that asexual stage prevalence did not differ significantly between natives and transmigrants but that gametocyte prevalence was lower among Irianese than Javanese subjects; one year on, however, a similar decline in the prevalence of gametocytes was also observed in the Javanese population. In the light of our data showing cross-stage immunity, one would expect that the prevalence of both parasite stages would decline with age in the same way. Recent observations by Bruce (9) may explain this discrepancy. Bruce has shown, from repeated sampling of parasites in children in an area of endemicity in Papua New Guinea, that densities of asexual parasites remain stable regardless of the different numbers of infections between individuals. To explain this, Bruce proposes a mechanism of density-dependent regulation of asexual parasite numbers. If gametocyte production is related to the number of new infections acquired by the host, then density dependence could account for the observed differences in the behavior of asexual and gametocyte prevalence in transmigrants living in Irian Jaya, given that the immunity generating density dependence would be acquired rapidly after 1 year of exposure. Nonetheless, in areas of stable malaria transmission, immunity to PfEMP-1 could contribute to the age-specific patterns of malaria parasitemia and indeed gametocytemia.
ACKNOWLEDGMENTS
This work was supported by the Wellcome Trust, United Kingdom, and by the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (project no. 8904189).
We gratefully acknowledge R. Carter for providing monoclonal antibody 2G7 and C. Donnelly and A. Roddam for statistical advice.
REFERENCES
- 1.Anderson T J C, Su X-Z, Bockarie M, Lagog M, Day K P. Twelve microsatellite markers for characterisation of Plasmodium falciparumfrom finger-prick blood samples. Parasitology. 1999;119:113–125. doi: 10.1017/s0031182099004552. [DOI] [PubMed] [Google Scholar]
- 2.Baird J K, Jones T R, Purnomo, Masbar S, Ratiwayanto S, Leksana B. Evidence for specific suppression of gametocytemia by Plasmodium falciparumin residents of hyperendemic Irian Jaya. Am J Trop Med Hyg. 1991;44:183–190. doi: 10.4269/ajtmh.1991.44.183. [DOI] [PubMed] [Google Scholar]
- 3.Barnwell J W, Howard R J, Coon H G, Miller L H. Splenic requirement for antigenic variation and expression of the variant antigen on the erythrocyte membrane in cloned Plasmodium knowlesimalaria. Infect Immun. 1983;40:985–994. doi: 10.1128/iai.40.3.985-994.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baruch D I, Pasloske B L, Singh H B, Bi X, Ma X C, Feldman M, Taraschi T F, Howard R J. Cloning the P. falciparumgene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 5.Biggs B A, Gooze L, Wycherley K, Wollish W, Southwell B, Leech J H, Brown G V. Antigenic variation in Plasmodium falciparum. Proc Natl Acad Sci USA. 1991;88:9171–9174. doi: 10.1073/pnas.88.20.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop A. Problems concerned with gametogenesis in haemosporidia with particular reference to the genus Plasmodium. Parasitology. 1955;45:163–185. doi: 10.1017/s0031182000027542. [DOI] [PubMed] [Google Scholar]
- 7.Brannan L R, Turner C M R, Phillips R S. Malaria parasites undergo antigenic variation at high rates in vivo. Proc R Soc Lond B Biol Sci. 1994;256:71–75. doi: 10.1098/rspb.1994.0051. [DOI] [PubMed] [Google Scholar]
- 8.Brown K N, Brown I N. Immunity to malaria: antigenic variation in chronic infections of Plasmodium knowlesi. Nature. 1965;208:1286–1288. doi: 10.1038/2081286a0. [DOI] [PubMed] [Google Scholar]
- 9.Bruce M C. D. Phil. thesis. Oxford, United Kingdom: University of Oxford; 1998. [Google Scholar]
- 10.Bruce M C, Alano P, Carter R, Nakamura K-I, Aikawa M, Carter R. Cellular location and temporal expression of the Plasmodium falciparumsexual stage antigen, Pfs16. Mol Biochem Parasitol. 1994;65:11–22. doi: 10.1016/0166-6851(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 11.Bull P C, Lowe B S, Kortok M, Marsh K. Antibody recognition of Plasmodium falciparumerythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect Immun. 1999;67:733–739. doi: 10.1128/iai.67.2.733-739.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bull P C, Lowe B S, Kortok M, Molyneux C S, Newbold C I, Marsh K. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med. 1998;4:358–360. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter R, Graves P M. Gametocytes. In: Wernsdorfer W H, McGregor I, editors. Malaria: principles and practice of malariology. Vol. 1. London, United Kingdom: Churchill Livingstone; 1988. pp. 253–305. [Google Scholar]
- 14.Cattani J A, Tulloch J I, Vrbova H, Jolley D, Gibson F D, Moir J S, Heywood P F, Alpers M P, Stevenson A, Clancy R. The epidemiology of malaria in a population surrounding Madang, Papua New Guinea. Am J Trop Med Hyg. 1986;35:3–15. doi: 10.4269/ajtmh.1986.35.3. [DOI] [PubMed] [Google Scholar]
- 15.Chen Q, Fernandez V, Sundstrom A, Schlichtherle M, Datta S, Hagblom P, Wahlgren M. Developmental selection of var gene expression in Plasmodium falciparum. Nature. 1998;394:392–395. doi: 10.1038/28660. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Q, Cloonan N, Fischer K, Thompson J, Waine G, Lanzer M, Saul A. stevor and rif are Plasmodium falciparummulticopy gene families which potentially encode variant antigens. Mol Biochem Parasitol. 1998;97:161–176. doi: 10.1016/s0166-6851(98)00144-3. [DOI] [PubMed] [Google Scholar]
- 17.Cox M J, Kum D, Tavul L, Narara A, Raiko A, Alpers M, Medley G, Day K P. Dynamics of malaria parasitaemia associated with febrile illness in children from a rural area of Madang, Papua New Guinea. Trans R Soc Trop Med Hyg. 1994;88:191–197. doi: 10.1016/0035-9203(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 18.Day K P, Hayward R E, Smith D, Culvenor J G, Taylor D W, Graves P M. CD36-dependent adhesion and knob expression of the transmission stages of Plasmodium falciparumis stage-specific. Mol Biochem Parasitol. 1998;93:167–177. doi: 10.1016/s0166-6851(98)00040-1. [DOI] [PubMed] [Google Scholar]
- 19.Day K P, Karamalis F, Thompson J, Barnes D A, Peterson C, Brown H, Brown G V, Kemp D J. Genes necessary for expression of a virulence determinant and for a 0.3-megabase region of chromosome 9. Proc Natl Acad Sci USA. 1993;90:8292–8296. doi: 10.1073/pnas.90.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deitsch K W, Wellems T E. Membrane modifications in erythrocytes parasitized by Plasmodium falciparum. Mol Biochem Parasitol. 1996;76:1–10. doi: 10.1016/0166-6851(95)02575-8. [DOI] [PubMed] [Google Scholar]
- 20a.Dyer, M., and K. P. Day. Unpublished observations.
- 21.Forsyth K P, Philip G, Smith T, Kum E, Southwell B, Brown G V. Diversity of antigens expressed on the surface of erythrocytes infected with mature Plasmodium falciparumparasites in Papua New Guinea. Am J Trop Med Hyg. 1989;41:259–265. [PubMed] [Google Scholar]
- 22.Garnham P C C. Observations on Plasmodium falciparumwith special reference to the production of crescents. Kenya East Afr Med J. 1931;8:2–19. [Google Scholar]
- 23.Gilks C F, Walliker D, Newbold C I. Relationships between sequestration, antigenic variation and chronic parasitism in Plasmodium chabaudi chabaudi—a rodent malaria model. Parasite Immunol. 1990;12:45–64. doi: 10.1111/j.1365-3024.1990.tb00935.x. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Trenholme K, Anderson R M, Day K P. Antigenic diversity and the transmission dynamics of Plasmodium falciparum. Science. 1994;263:961–963. doi: 10.1126/science.8310293. [DOI] [PubMed] [Google Scholar]
- 25.Hawking F, Wilson M E, Gammage K. Evidence for cyclic development and short-lived maturity in the gametocytes of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1971;65:547–559. doi: 10.1016/0035-9203(71)90036-8. [DOI] [PubMed] [Google Scholar]
- 26.Hayward R E. D. Phil. thesis. Oxford, United Kingdom: University of Oxford; 1997. [Google Scholar]
- 27.Hayward R E, Tiwari B, Piper K P, Baruch D I, Day K P. Virulence and transmission success of the malarial parasite Plasmodium falciparum. Proc Natl Acad Sci USA. 1999;96:4563–4568. doi: 10.1073/pnas.96.8.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hogh B, Thompson R, Hetzel C, Fleck S L, Kruse N A A, Jones I, Dgedge M, Barreto J, Sinden R E. Specific and nonspecific responses to Plasmodium falciparumblood-stage parasites and observations on the gametocytemia in schoolchildren living in a malaria-endemic area of Mozambique. Am J Trop Med Hyg. 1995;52:50–59. doi: 10.4269/ajtmh.1995.52.50. [DOI] [PubMed] [Google Scholar]
- 29.Howard R J, Barnwell J W, Kao V. Antigenic variation of Plasmodium knowlesimalaria: identification of the variant antigen on infected erythrocytes. Proc Natl Acad Sci USA. 1983;80:4129–4133. doi: 10.1073/pnas.80.13.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeffery G M, Eyles D E. Infectivity to mosquitoes of Plasmodium falciparumas related to gametocyte density and duration of infection. Am J Trop Med Hyg. 1955;4:781–789. doi: 10.4269/ajtmh.1955.4.781. [DOI] [PubMed] [Google Scholar]
- 31.Kyes S A, Rowe J A, Kriek N, Newbold C I. Rifins: a second family of clonally variant proteins expressed on the surface of red cells infected with Plasmodium falciparum. Proc Natl Acad Sci USA. 1999;96:9333–9338. doi: 10.1073/pnas.96.16.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leech J H, Barnwell J W, Miller L H, Howard R J. Identification of a strain specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J Exp Med. 1984;159:1567–1575. doi: 10.1084/jem.159.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsh K, Howard R J. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science. 1986;231:150–153. doi: 10.1126/science.2417315. [DOI] [PubMed] [Google Scholar]
- 34.Marsh K, Otoo L, Hayes R J, Carson D C, Greenwood B M. Antibodies to blood stage antigens of Plasmodium falciparumin rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 35.McGregor I A. Malarial immunity: current trends and prospects. Ann Trop Med Parasitol. 1987;81:647–656. doi: 10.1080/00034983.1987.11812166. [DOI] [PubMed] [Google Scholar]
- 36.Mendis K N, Ihalamulla R I, David P H. Diversity of Plasmodium vivax-induced antigens on the surface of infected human erythrocytes. Am J Trop Med Hyg. 1988;38:42–46. doi: 10.4269/ajtmh.1988.38.42. [DOI] [PubMed] [Google Scholar]
- 37.Molineaux L, Gramiccia G. The Garki project: research on the epidemiology and control of malaria in the Sudan savanna of West Africa. Geneva, Switzerland: World Health Organization; 1980. [Google Scholar]
- 38.Pasvol G, Wilson R J M, Smalley M E, Brown J. Separation of viable schizont-infected red cells of Plasmodium falciparumfrom human blood. Ann Trop Med Parasitol. 1978;72:87–88. doi: 10.1080/00034983.1978.11719283. [DOI] [PubMed] [Google Scholar]
- 39.Paul R E L, Packer M J, Walmsley M, Lagog M, Ranford-Cartwright L C, Paru R, Day K P. Mating patterns in malaria parasite populations of Papua New Guinea. Science. 1995;269:1709–1711. doi: 10.1126/science.7569897. [DOI] [PubMed] [Google Scholar]
- 39a.Piper, K. P. Unpublished data.
- 40.Piper K P, Roberts D J, Day K P. Plasmodium falciparum: analysis of the antibody specificity to the surface of the trophozoite-infected erythrocyte. Exp Parasitol. 1999;91:161–169. doi: 10.1006/expr.1998.4368. [DOI] [PubMed] [Google Scholar]
- 41.Roberts D J, Craig A G, Berendt A R, Pinches R, Nash G, Marsh K, Newbold C I. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 1992;357:689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smalley M E, Abdalla S, Brown J. The distribution of Plasmodium falciparumin the peripheral blood and bone marrow of Gambian children. Trans R Soc Trop Med Hyg. 1980;75:103–105. doi: 10.1016/0035-9203(81)90019-5. [DOI] [PubMed] [Google Scholar]
- 43.Smith J D, Chitnis C E, Craig A G, Roberts D J, Hudson-Taylor D E, Peterson D S, Pinches R, Newbold C I, Miller L H. Switches in expression of Plasmodium falciparum vargenes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su X, Heatwole V M, Wertheimer S P, Guinet F, Herrfeldt J A, Peterson D S, Ravetch J A, Wellems T E. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 45.Taylor L H, Read A F. Why so few transmission stages? Reproductive restraint by malaria parasites. Parasitol Today. 1997;13:135–140. doi: 10.1016/s0169-4758(97)89810-9. [DOI] [PubMed] [Google Scholar]
- 46.Tchuinkam T, Mulder B, Dechering K, Stoffels H, Verhave J P, Cot M, Carnevale P, Meuwissen J H E T, Robert V. Experimental infections of Anopheles gambiae with Plasmodium falciparumof naturally infected gametocyte carriers in Cameroon: factors influencing the infectivity to mosquitoes. Trop Med Parasitol. 1993;44:271–276. [PubMed] [Google Scholar]
- 47.Thomson J G, Robertson A. The structure and development of Plasmodium falciparumgametocytes in the internal organs and peripheral circulation. Trans R Soc Trop Med Hyg. 1935;29:31–40. [Google Scholar]
- 48.Trager W, Jenson J B. Human malaria parasites in continuous culture. Science. 1976;193:674–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 49.Trenholme K, Phillips R S. The use of murine feeder cells in the cultivation of Plasmodium falciparumasexual blood stages. Parasitol Res. 1989;75:518–521. doi: 10.1007/BF00931159. [DOI] [PubMed] [Google Scholar]
- 50.van Schravendijk M R, Rock E P, Marsh K, Ito Y, Aikawa M, Neequaye J, Ofori Adjei D, Rodriguez R, Patarroyo M E, Howard R J. Characterization and localization of Plasmodium falciparumsurface antigens on infected erythrocytes from west African patients. Blood. 1991;78:226–236. [PubMed] [Google Scholar]