Abstract
Background
An effective dengue vaccine should ideally induce broadly neutralizing antibody (nAb) responses against all 4 dengue virus (DENV) serotypes.
Methods
We characterized the specificity and breadth of the nAb response to TAK-003, a live-attenuated tetravalent dengue vaccine, in serum samples from phase 2 and 3 clinical trials.
Results
Microneutralization tests using postvaccination serum showed comparable neutralization against diverse DENV-1−4 genotypes. Reporter virus particle neutralization assays after depletion of anti-DENV-2 nAbs demonstrated that the nAb response to DENV-1, -3, and -4 comprises both type-specific (TS) and cross-reactive (CR) nAbs.
Conclusions
Therefore, TAK-003 induces broad tetravalent TS and CR nAb responses.
Keywords: dengue vaccine, dengue virus, neutralizing antibody response, TAK-003, type-specific
We characterized the specificity and breadth of neutralizing antibody (nAb) response to a live-attenuated tetravalent dengue vaccine candidate and found that the nAb response comprised both type-specific and cross-reactive nAbs, with comparable neutralization of diverse dengue genotypes.
Dengue virus (DENV), a mosquito-borne flavivirus, has 4 antigenically distinct serotypes, DENV-1−4 [1], and multiple genotypes within each serotype. Infection can lead to dengue fever, a generally self-limiting yet debilitating acute febrile disease, which can progress to dengue hemorrhagic fever/dengue shock syndrome and death [1]. Neutralizing antibody (nAb) responses to dengue are considered important for protection and are characterized as type-specific (TS), directed against only the infecting serotype, or cross-reactive (CR), directed against more than 1 serotype.
Studies of immunity to natural infection support a role (1) for TS nAbs in protection against infection with the same DENV serotype and (2) for potent CR nAbs in protection against diverse DENV serotypes [2–4]. It is accepted that, after primary dengue infection, potent TS antibodies are elicited against infecting serotype, whereas short-lived CR nAbs are elicited against other serotypes [3, 5]. However, a more recent study demonstrated that CR nAbs can persist and increase over time [6].
Takeda’s live-attenuated tetravalent dengue vaccine (TDV), TAK-003, comprises an attenuated DENV-2 and 3 recombinant viruses with structural premembrane and viral envelope (E) proteins of DENV-1, -3, and -4 cloned into the attenuated DENV-2 backbone [7]. During the phase 3 TIDES study conducted in >20 000 children in dengue-endemic countries, the primary endpoint was achieved with an overall vaccine efficacy of 80.2% against virologically confirmed dengue and 95.4% against hospitalized dengue from any dengue serotype [7]. Vaccine efficacy varied by serotype, and exploratory analysis suggested a lack of efficacy against DENV-3 in baseline seronegative participants, warranting continued monitoring over longer term [7]. TAK-003 elicited tetravalent nAb seropositivity in both baseline seronegative and seropositive participants, with highest titers against DENV-2 [7]. In this study, we characterized nAb responses to TAK-003 for breadth in a subset of baseline-seronegative and seropositive vaccine recipients from a phase 2 study and for serotype specificity in a subset of baseline-seronegative participants from phase 3 clinical studies.
METHODS
Serum Samples
Serum samples were collected during randomized, double-blind, placebo-controlled clinical trials (Supplementary Methods) in accordance with the Edinburgh revision of the Declaration of Helsinki, International Conference on Harmonisation and Good Clinical Practice guidelines, and applicable national and local regulations and requirements. Study protocols were approved by the local internal review boards, and written informed assent or consent was obtained from all participants or their legal guardians.
DEN-205 (ClinicalTrials.gov Identifier NCT02425098) compared immune responses in Singaporean adults to single doses of 2 early formulations of TAK-003, termed high-dose (HD)-TDV or TDV [8]. DEN-205 samples included antisera from individuals who were baseline-seronegative (n = 10) or seropositive (n = 10) to DENV, before vaccination with HD-TDV (n = 18) or TDV (n = 2). DEN-301 (ClinicalTrials.gov Identifier NCT02747927) evaluated efficacy of 2 doses of TAK-003 against symptomatic dengue fever due to DENV-1–4 in participants aged 4–16 years in the Philippines, Thailand, Sri Lanka, Colombia, Panama, Brazil, Dominican Republic, and Nicaragua [7]. DEN-304 (ClinicalTrials.gov Identifier NCT03423173) was a manufacturing consistency study of 2 doses of TAK-003 in baseline-seronegative DEN-301 (n = 46) and DEN-304 (n = 25) adults aged 18–60 years in the United States (VT, PJW, LMT, MR, IE, EH, IL, AB, and DW, 2022, unpublished observations).
Dengue Virus-2 Depletion
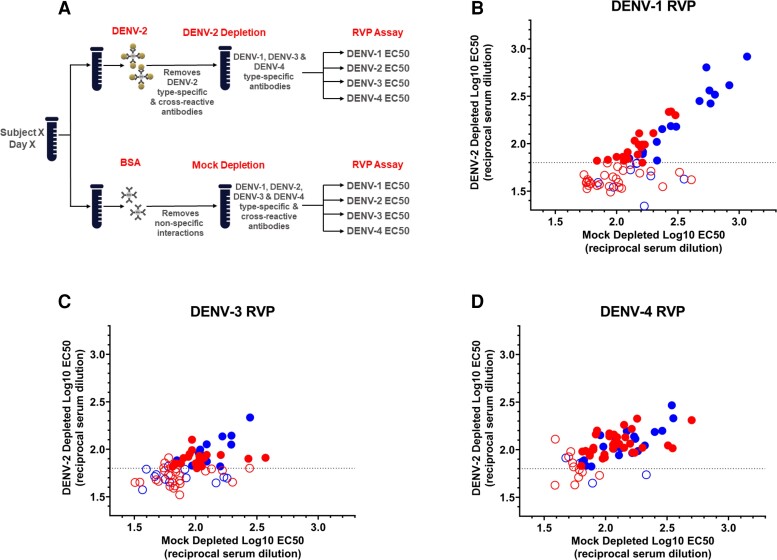
Tosyl-activated Dynabeads coupled with pan-flavivirus 4G2 monoclonal antibody were bound with either live DENV-2 (strain 16681) or bovine serum albumin ([BSA] control), blocked with BSA to prevent nonspecific interactions, and then incubated with heat-inactivated vaccine-recipient sera to deplete DENV-2 TS and CR nAbs (Supplementary Methods, Figure 1A).
Figure 1.
Dengue virus (DENV)-2 antibody depletion and specificity of neutralizing antibody (nAb) response to TAK-003. (A) Schematic of DENV-2 antibody depletion, and log reporter virus particle (RVP) half-maximal effective concentration (EC50) titers for (B) DENV-1, (C) DENV-3, and (D) DENV-4 after DENV-2 antibody depletion. Sera from baseline-seronegative individuals vaccinated with TAK-003 (n = 71) collected 30 days after second vaccination from studies DEN-301 (red dots) and DEN-304 (blue dots) were depleted with mock antigen (x-axis) and DENV-2 antigen (y-axis) bound to magnetic beads, and their neutralization titers were measured by dengue RVP assay. Log10 EC50 represents the dilution factor required to neutralize 50% of RVPs. Samples where depletion of anti-DENV-2 antibodies leads to complete loss of detectable nAbs against DENV-1, 3 and/or 4 (seen as log10 EC50 < limit of detection (LOD) and represented by the open circles) contain a high proportion of cross-reactive antibodies. Samples where DENV-1, -3 and/or -4 titer remain > LOD postdepletion with DENV-2 (represented with closed circles) contain type-specific antibodies. HD, high dose.
Depletion was repeated 6 times. Neutralizing antibodies were then quantitated using a reporter virus particle (RVP) neutralization assay. Reduction of DENV-2 titers postdepletion indicated that DENV-2 TS and CR nAbs had been removed, whereas residual titers to DENV-1, -3, and/or -4 indicated the presence of TS nAbs to these serotypes.
Dengue Virus Reporter Virus Particle Neutralization Assay
To quantitate nAbs against DENV-1, -3, and -4 after DENV-2 depletion, a neutralization assay was conducted using luciferase-expressing dengue RVPs and Raji B cells expressing a flavivirus attachment factor (Supplementary Methods). The RVP assay was selected for higher throughput and lower sample volume requirement compared with microneutralization tests (MNTs). The RVPs, which retain the antigenic determinants of wild-type virions, including quaternary epitopes, were incubated with serial dilutions of DENV-2-depleted vaccine-recipient sera to allow for steady-state binding. Raji DC-SIGN cells were infected with these immune complexes, and the luciferase readout was measured 72 hours postinfection.
Results are reported as half-maximal effective concentration (EC50), the reciprocal dilution of serum required to neutralize 50% of the input RVP, for each serotype after mock or DENV-2 depletion. Results of RVP and MNT generally correlate, but absolute titer values are different. Serotype specificity was defined as RVP neutralization titer ≥ limit of detection after DENV-2 depletion, for each serotype.
Dengue Virus Microneutralization Test and Breadth of Neutralizing Antibody Response
Immunogenicity to DENV serotypes was measured by MNT (Supplementary Methods) [9]. Titers were defined as the dilution resulting in a 50% reduction in plaques (NT50).
Breadth of DENV nAb responses was assessed in serum samples collected from baseline-seronegative and baseline-seropositive DEN-205 study participants on day 30 postvaccination. The NT50 antibody titer was determined for each of the parental vaccine strains (TDV-1–4) and 2 additional strains for each of DENV-1–4 comprising a panel of genetically diverse DENV strains representative of genotypes isolated in Asia and Latin America from 1956 to 2007 (Supplementary Table 1). P values were calculated by ordinary one-way analysis of variance with Sidak’s method for multiple comparisons.
RESULTS
Specificity of Neutralizing Antibody Response to TAK-003 Vaccination in Seronegative Individuals
In studies DEN-301 and DEN-304, TAK-003 elicited tetravalent nAbs against DENV-1–4, with geometric mean titers highest against DENV-2, followed by DENV-1, and lowest against DENV-3 and DENV-4 [7] (VT, PJW, LMT, MR, IE, EH, IL, AB, and DW, 2022, unpublished observations). Because previous studies demonstrated the presence of DENV-2 TS nAbs in postvaccination samples [10], this study assessed the presence of TS nAbs against DENV-1, -3, and -4. Dengue virus-2 TS and CR nAbs were depleted using live DENV-2 antigen (Figure 1A) [11]. Depletion with DENV-2 antigen effectively removed CR nAbs and TS nAbs against DENV-2, in most samples (Supplementary Figure 1, Supplementary Table 4).
Frequency of DENV-1, DENV-3, and DENV-4 TS nAbs was determined using RVP assay (Figure 1B–D, Supplementary Table 4). The extensive sample handling required for the depletion process can reduce assay sensitivity (CRD and MZS, 2019, investigators’ observations). Therefore, samples were defined as eligible for determination of the presence of TS nAbs if detectable nAbs remained after mock BSA depletion. Samples with undetectable titers after mock depletion were omitted from TS analyses (Supplementary Tables 2 and 4).
In samples from baseline-seronegative children and adolescents in dengue-endemic areas from study DEN-301, DENV-1 TS nAb were observed in 18 of 38 (47%) of eligible samples, DENV-3 TS nAbs in 20 of 35 (57%) of eligible samples, and DENV-4 TS nAbs in 34 of 36 (94%) of eligible samples (Figure 1B–D, Supplementary Table 2).
In samples from baseline-seronegative adults in nonendemic areas from study DEN-304, DENV-1 TS nAbs were detected in 17 of 25 (68%) eligible samples, DENV-3 TS nAbs in 13 of 18 (72%) eligible samples, and DENV-4 TS nAbs in 21 of 23 (91%) eligible samples (Figure 1B–D, Supplementary Table 2).
In summary, postdepletion with DENV-2, nAbs to TAK-003 included both TS and CR nAbs to DENV-1, DENV-3, and DENV-4 in sera of the majority of children, adolescents, and adults. We were surprised to find that, despite lower nAb titers against DENV-4, the frequency of TS nAb was higher against DENV-4 compared with DENV-1 or DENV-3 (Supplementary Table 2).
TAK-003-Elicited Neutralizing Antibody Response to Diverse Genotypes
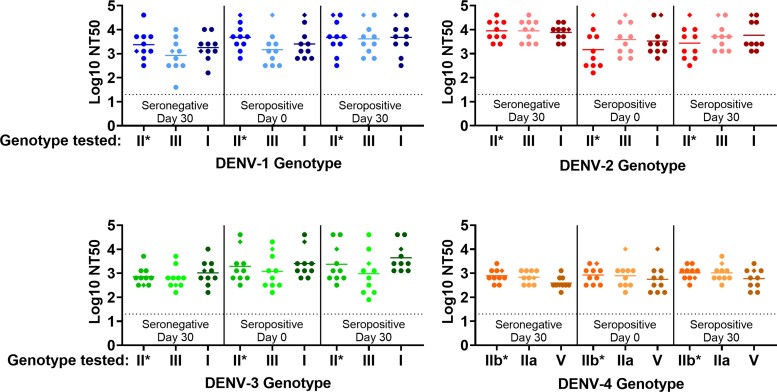
To determine the breadth of nAb responses, nAb titers in samples from study DEN-205 [8] were measured by MNT against the 4 parental DENV strains and 2 strains representing additional genotypes of each DENV serotype (Figure 2, Supplementary Table 3). Sera collected from baseline-seropositive and seronegative vaccine recipients neutralized all genotypes and serotypes tested. Neutralization titers against genotypically and geographically diverse nonvaccine genotypes were similar to titers against the vaccine-matched strains.
Figure 2.
TAK-003-Elicited neutralizing antibody (nAb) microneutralization test (MNT) titers against homologous tetravalent dengue vaccine (TDV) strains and diverse dengue virus (DENV)-1–4 genotypes in postvaccination serum. DEN-205 antisera from baseline-seronegative (n = 10) and seropositive (n = 10) individuals vaccinated with a single dose of high-dose TDV (n = 18; ● symbols) or TDV (n = 2; ◆ symbols) collected prevaccination and 30 days postvaccination were tested in a MNT against a panel of genetically diverse DENV strains representative of historical (1956–2007) genotypes isolated in Asia and Latin America (Supplementary Table 1). Titers were defined as the serum dilution resulting in a 50% reduction in plaque number compared with virus only control (NT50). Data were truncated at MNT limit of detection (log10 NT50 = 1.3). P values were calculated by ordinary one-way analysis of variance with Sidak’s method for multiple comparisons. Neutralizing titers against nonvaccine genotypes were compared with the homologous vaccine strain within each serum group (naive day 30, preimmune day 0, preimmune day 30) and between serum groups (naive day 30 vs preimmune day 30, preimmune day 0 vs preimmune day 30). No significant differences in NT50 were observed in any of the comparisons. *, Homologous (parental) TDV-1, -2, -3, or -4 strain.
DISCUSSION
Depletion analysis demonstrates that 2 doses of TAK-003 elicits both CR and TS nAbs to DENV-1, -3, and -4 in baseline-seronegative individuals. The TS nAbs to DENV-1, -3, and -4 were generally detected in the majority of post-DENV-2 nAb-depleted samples from vaccinated participants living in dengue-endemic and nonendemic areas. The nonquantitative nature of the depletion assay method complicated assessment of statistical significance of trends in the magnitude of TS nAbs in individual samples. Previous studies investigating TS nAbs after vaccination with earlier TAK-003 formulations demonstrated presence of DENV-2 and DENV-1 TS nAbs and DENV-3 TS binding Abs [11]. Another study observed DENV-2 TS nAbs but lower frequencies of DENV-1, DENV-3, and DENV-4 TS nAbs [10]. Differences in vaccine formulation, regimen, assay methods, and the natural variability in frequency of TS nAbs among individuals might account for variable results among studies.
Studies of nAb responses to natural DENV infection support a role (1) for TS nAbs in protecting against repeat infection with the homologous DENV serotype and (2) for CR nAbs in protection against diverse DENV serotypes of different genotypes [2, 4]. In this study, TS nAbs for DENV-1 and -3 were detected in a similar percentage of study participants. However, in phase 3, the efficacy of TAK-003 in baseline-seronegative vaccinees was higher against DENV-1 than DENV-3 [12], demonstrating that efficacy in baseline-seronegative individuals is not solely driven by the presence of TS nAbs to a particular serotype and likely involves additional immune responses elicited by tetravalent vaccination, such as immunity to DENV nonstructural proteins. Dengue virus-4 TS nAbs have been associated with protection against DENV-4 after vaccination with another DENV vaccine, which, unlike TAK-003, does not contain DENV nonstructural proteins [13]. The antibody repertoire after vaccination with TAK-003 may differ from the repertoire after primary or secondary natural DENV infection, as suggested by the observation that TAK-003 vaccination elicits tetravalent TS memory B cells (MBCs), unlike natural infections, which elicit TS MBCs only to the infecting serotype(s) [14]. We did not assess kinetics of TS and CR nAbs; however, the frequency of MBCs producing TS and CR nAbs after TAK-003 vaccination is stable over time [12].
Magnitude of predepletion DENV-2 nAb titers does not determine the efficiency of DENV-2 nAb depletion from postvaccination serum samples (Supplementary Table 4). Moreover, agnitude of nAb does not appear to determine presence of TS nAbs. Consistent with data from a phase 2 study with an early TAK-003 formulation [10], TAK-003 elicits a high frequency of anti-DENV-4 TS nAbs, despite relatively low titers of anti-DENV-4 nAbs. This observation is consistent with the frequency of DENV-4 TS MBCs after TAK-003 vaccination [14]. Further studies are required to determine whether DENV-4 TS nAbs correlate with protection. These results demonstrate that the magnitude of nAb responses to a particular DENV serotype does not necessarily determine the presence of TS nAbs. It will be important to understand DENV serotype-specific immunodominance hierarchy of TS and CR epitopes and their contribution to antibody repertoire and protection.
Although DENV does not undergo antigenic shift and drift to the same extent as some other viruses, antigenic variation in the E protein can influence sensitivity to neutralization by nAbs [15]. In addition, DENV nAb responses focused narrowly on individual TS epitopes may lead to virus immune escape via a sieve effect [15], highlighting the importance of studying broadly cross-reactive nAb responses. In both seronegative and seropositive participants, nAbs elicited by a single dose of TAK-003 neutralized diverse genotypes of DENV-1–4 comparably to the corresponding parental vaccine strains. Future cross-neutralization studies of contemporary circulating DENV strains with larger numbers of sera will contribute to understanding the potential for TAK-003 efficacy globally.
CONCLUSIONS
Although nAbs are considered necessary for protection, the role of TS and/or CR nAb in protection has not been defined. Detailed immune response analyses in the context of DENV vaccine phase 3 clinical studies, where baseline serostatus, vaccination history, and infection timing are known, represent an opportunity to identify specific immune response signatures that correlate with protection. In conclusion, vaccination with TAK-003 elicits nAbs against a broad range of DENV-1–4 genotypes in baseline-seronegative and baseline-seropositive vaccine recipients. In baseline-seronegative TAK-003 recipients, the nAb response to DENV-1–4 is a combination of TS and CR nAbs. The tetravalent, broad, and persistent nAb response elicited by TAK-003 may contribute to the overall efficacy against symptomatic dengue due to any dengue serotype and to vaccine effectiveness against diverse genotypic strains of DENV circulating worldwide.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Christina R DeMaso, Takeda Pharmaceuticals U.S.A., Vaccines Business Unit, Cambridge, Massachusetts, USA.
Lovkesh Karwal, Takeda Pharmaceuticals U.S.A., Vaccines Business Unit, Cambridge, Massachusetts, USA.
Melissa Zahralban-Steele, Takeda Pharmaceuticals U.S.A., Vaccines Business Unit, Cambridge, Massachusetts, USA.
David Dominguez, Takeda Pharmaceuticals U.S.A., Vaccines Business Unit, Cambridge, Massachusetts, USA.
Zhang-Li Springer, Takeda Pharmaceuticals U.S.A., Vaccines Business Unit, Cambridge, Massachusetts, USA.
Maima Kaiser, Takeda Pharmaceuticals U.S.A., Vaccines Business Unit, Cambridge, Massachusetts, USA.
Sunil Palani, Takeda Pharmaceuticals U.S.A., Vaccines Business Unit, Cambridge, Massachusetts, USA.
Tim Rindfleisch, Takeda Pharmaceuticals U.S.A., Vaccines Business Unit, Cambridge, Massachusetts, USA.
Kelly Bohning, Takeda Pharmaceuticals U.S.A., Vaccines Business Unit, Cambridge, Massachusetts, USA.
Greg Hather, Takeda Pharmaceuticals U.S.A., Vaccines Business Unit, Cambridge, Massachusetts, USA.
Subash Das, Takeda Pharmaceuticals U.S.A., Vaccines Business Unit, Cambridge, Massachusetts, USA.
Mayuri Sharma, Takeda Pharmaceuticals U.S.A., Vaccines Business Unit, Cambridge, Massachusetts, USA.
Hansi J Dean, Takeda Pharmaceuticals U.S.A., Vaccines Business Unit, Cambridge, Massachusetts, USA.
Notes
Acknowledgments. We acknowledge Ivo Sonderegger, Anabel Immoos, Vianney Tricou, Shibadas Biswal, Astrid Borkowski, and Derek Wallace for collaboration in provision of clinical trial samples and helpful discussions and Claire Huang, Division of Vector Borne Diseases, Centers for Disease Control and Prevention, for providing the DENV-1-4 strains used in breadth of neutralization studies. Medical writing assistance with preparation of the manuscript was provided by Samantha Santangelo, PhD, of Santangelo Consulting LLC, sponsored by Takeda.
Financial support. This work was funded by Takeda Pharmaceuticals U.S.A.
References
- 1. Guzman MG, Harris E. Dengue. Lancet 2015; 385:453–65. [DOI] [PubMed] [Google Scholar]
- 2. Corbett KS, Katzelnick L, Tissera H, Amerasinghe A, de Silva AD, de Silva AM. Preexisting neutralizing antibody responses distinguish clinically inapparent and apparent dengue virus infections in a Sri Lankan pediatric cohort. J Infect Dis 2015; 211:590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gibbons RV, Kalanarooj S, Jarman RG, et al. . Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am J Trop Med Hyg 2007; 77:910–3. [PubMed] [Google Scholar]
- 4. Olkowski S, Forshey BM, Morrison AC, et al. . Reduced risk of disease during postsecondary dengue virus infections. J Infect Dis 2013; 208:1026–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Montoya M, Gresh L, Mercado JC, et al. . Symptomatic versus inapparent outcome in repeat dengue virus infections is influenced by the time interval between infections and study year. PLoS Negl Trop Dis 2013; 7:e2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Katzelnick LC, Zambrana JV, Elizondo D, et al. . Dengue and Zika virus infections in children elicit cross-reactive protective and enhancing antibodies that persist long term. Sci Transl Med 2021; 13:eabg9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Biswal S, Reynales H, Saez-Llorens X, et al. . Efficacy of a tetravalent dengue vaccine in healthy children and adolescents. N Eng J Med 2019; 381:2009–19. [DOI] [PubMed] [Google Scholar]
- 8. Tricou V, Low JG, Oh HM, et al. . Safety and immunogenicity of a single dose of a tetravalent dengue vaccine with two different serotype-2 potencies in adults in Singapore: a phase 2, double-blind, randomised, controlled trial. Vaccine 2020; 38:1513–9. [DOI] [PubMed] [Google Scholar]
- 9. Osorio JE, Velez ID, Thomson C, et al. . Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: a randomised, placebo-controlled, phase 1 study. Lancet Infect Dis 2014; 14:830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. White LJ, Young EF, Stoops MJ, et al. . Defining levels of dengue virus serotype-specific neutralizing antibodies induced by a live attenuated tetravalent dengue vaccine (TAK-003). PLoS Negl Trop Dis 2021; 15:e0009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Swanstrom JA, Henein S, Plante JA, et al. . Analyzing the human serum antibody responses to a live attenuated tetravalent dengue vaccine candidate. J Infect Dis 2018; 217:1932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. López-Medina E, Biswal S, Saez-Llorens X, et al. . Efficacy of a dengue vaccine candidate (TAK-003) in healthy children and adolescents two years after vaccination. J Infect Dis 2020; 225:1521–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Henein S, Adams C, Bonaparte M, et al. . Dengue vaccine breakthrough infections reveal properties of neutralizing antibodies linked to protection. J Clin Invest 2021; 131:e147066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Michlmayr D, Andrade P, Nascimento EJM, et al. . Characterization of the type-specific and cross-reactive B cell responses elicited by a live-attenuated tetravalent dengue vaccine. J Infect Dis 2020; 223:247–57. [DOI] [PubMed] [Google Scholar]
- 15. Martinez DR, Metz SW, Baric RS. Dengue vaccines: the promise and pitfalls of antibody-mediated protection. Cell Host Microbe 2021; 29:13–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.