Abstract
Covalent linkage of a bacterial polysaccharide to an immunogenic protein greatly enhances the carbohydrate's immunogenicity and induces polysaccharide-specific B-cell memory in vivo. These findings have spurred the development of glycoconjugate vaccines for serious bacterial infections. The specific B-cell–T-cell interactions responsible for recruitment of T-cell help by glycoconjugate vaccines are not well defined. We used mice deficient in molecules critical for stimulatory, cognate B-cell–T-cell interactions to study how T cells improve the immunogenicity of a glycoconjugate vaccine against group B streptococcal disease. Isotype switching to immunoglobulin G (IgG) was abrogated in mice deficient in major histocompatibility complex (MHC) class II antigen (Ag)–T-cell receptor (TCR), B7-CD28, or CD40-CD40L interactions. However, expression of either the B7-1 or the B7-2 molecule on antigen-presenting cells was sufficient for optimal T-cell costimulation. T cells activated by the vaccine also played a pivotal role in determining the magnitude of the IgM response to the polysaccharide. Comparable results were obtained with pathway antagonists. These data suggest that MHC class II Ag-TCR, B7-CD28, and CD40-CD40L interactions are critical for immune responses to glycoconjugate vaccines in vivo.
Despite the availability of effective antibiotics, infections with many encapsulated bacteria (e.g., Neisseria meningitidis, Streptococcus pneumoniae, and Streptococcus agalactiae) remain major health problems throughout the world. The increasing threat of antibiotic resistance among some of these species has accelerated the pace of research into new vaccines to prevent infections. Group B Streptococcus (GBS) is the most common cause of serious infections in newborns and young infants in most of the developed world, with a 5 to 8% case-fatality rate in the United States (2). GBS is usually acquired by perinatal transmission from the genital tract of the mother to her child. Mothers of neonates developing GBS disease generally have very low levels of antibodies to the serotype-specific capsular polysaccharide (CPS) of the organism infecting their children (4). If enough immunoglobulin G (IgG) specific to the CPS of the GBS strain colonizing the mother crosses the placenta (5, 6), her infant will be protected from invasive disease (3). These data support the administration of a CPS-containing glycoconjugate vaccine to women of childbearing age in order to protect neonates and perhaps pregnant women from GBS disease. Clinical trials have shown that the response rate to unconjugated GBS type III (GBSIII) CPS in previously naive adults is approximately 50 to 60% (6, 29). Conjugation of GBSIII CPS to tetanus toxoid (TT) increased the response rate to >90% (29) and significantly enhanced antibody levels in most vaccinees (29). However, the basic cellular mechanisms underlying the enhanced humoral immune responses to bacterial CPSs conjugated to immunogenic carriers are not well defined.
Mice are generally thought to be a good model for studies of antibody responses to bacterial CPSs (59). Immunization of mice with unconjugated CPS fails to induce sustained immunologic memory, and the immune response to CPS has been shown to be thymus independent (9, 19). The antibody response to unconjugated CPS is typically of the IgM isotype, and repeated immunization does not result in increased levels of CPS-specific antibodies (9, 19, 54). Immunization following covalent coupling of CPS to an immunogenic protein (carrier) results in an enhanced immune response with high levels of CPS-specific antibodies, rapid kinetics, and induction of immunologic memory to the CPS component (26, 54, 59). These characteristics indicate the recruitment of T-cell help during the immune response to glycoconjugates (8, 19, 26, 54, 60).
On the basis of research with synthetic hapten-carrier conjugates (10, 17, 30, 38, 50), we hypothesized that the enhanced immunogenicity of bacterial CPSs upon conjugation to carrier protein molecules is a result of specific interactions of the CPS-specific B cell and the T cell, including (i) recruitment of carrier-specific T-cell help by CPS-specific B cells that present carrier-specific T-cell epitopes in the context of class II major histocompatibility complex (MHC) molecules to T-helper cells (signal 1), (ii) T-cell costimulation via the B7-CD28 pathway (signal 2), and (iii) B-cell stimulation via triggering of CD40 through interaction with CD40L on activated T cells. We report here the results of in vivo studies in which mice that were genetically deficient in or immunologically deprived of these receptor-ligand cellular interactions were immunized with a clinically relevant glycoconjugate vaccine against GBS.
MATERIALS AND METHODS
Mice.
Nude mice (BALB/cByJ background), CD3ɛ transgenic (tg) mice (B6.CBA background) (65, 66), and CD40L-deficient mice (B6.129 background) (50) and the appropriate wild-type controls were obtained from the Jackson Laboratory (Bar Harbor, Maine). MHC class II-deficient mice (24) and wild-type controls (C57BL/6) were obtained from Taconic (Germantown, N.Y.). All experiments comparing wild-type, B7-1-, B7-2-, and B7-1/2-deficient mice (57) used 129/SvJ inbred mice. Brigham and Women's Hospital and Harvard Medical School are Association for Assessment and Accreditation of Laboratory Animal Care-accredited institutions, and the mice were cared for in accordance with institutional guidelines.
Cell preparation.
Cell suspensions from thymus, spleen, and lymph nodes were prepared by grinding tissue through a sterile wire mesh, with subsequent filtration through nylon. The cell populations were depleted of erythrocytes by treatment with 0.15 M NH4Cl in 17 mM Tris (pH 7.2) and centrifugation over fetal calf serum. Before purification, T cells were enriched by passage over a nylon wool column. Antigen-presenting cells (APCs) were depleted by antibody-mediated complement lysis with use of complement from irradiated newborn rabbits and a cocktail of monoclonal antibodies (MAbs) to MHC class II (IAb,d,q and IEb). The remaining viable cells were used to replenish the population of CD4+ T cells in MHC class II-deficient mice (24). The administered T-cell preparation was >95% pure, as evaluated by flow cytometric studies after incubation of the cells with antibodies to B- and T-cell markers.
Flow cytometry.
Thymus, spleen, and lymph node cells were stained with a panel of fluorochrome-conjugated antibodies, including fluorescein isothiocyanate-conjugated and phycoerythrin-conjugated anti-CD3, anti-B220, anti-CD4, anti-CD8, and anti-Mac-1 (all purchased from Caltag, South San Francisco, Calif.) as well as anti-Thy1.2, anti-MHC class IIb, and rat isotype controls (all purchased from PharMingen, San Diego, Calif.), according to standard protocols (1). Cells (5 × 103) with the forward and side scatter properties of lymphocytes were analyzed for each sample. Stained cells were analyzed by fluorescence-activated cell sorting (FACS) on a FACScan apparatus (Becton Dickinson, San Jose, Calif.). Data were analyzed with Cell Quest FACS analysis software (Becton Dickinson).
Adoptive cell transfer.
One day prior to the first immunization with the vaccine, MHC class II-deficient mice were provided with helper T cells by adoptive transfer of 0.7 × 108 T cells (a number equivalent to the number of T cells in the spleen) from nonimmunized wild-type mice (C57BL/6).
Immunization protocol.
GBSIII CPS was purified from strain M781 (43), oxidized, and conjugated to monomeric TT as described previously (68). Nude mice, CD3ɛ tg mice, MHC class II-, CD40L-, B7-1-, B7-2-, and B7-1/2-deficient mice, and the appropriate wild-type mice (BALB/cByJ, B6.CBA, C57BL/6, B6.129, and 129/SvJae, respectively) were immunized intraperitoneally (i.p.) on days 0, 21, and 49 with 2 μg of GBSIII CPS either unconjugated or conjugated to TT (both vaccines were adsorbed to alum) (26). Serum samples were obtained 1 day before and 7, 14, 21, 35, 49, 56, 63, and 77 days after primary vaccination.
Reagents.
The rat anti-murine B7-1 MAb (1G10) and anti-murine B7-2 MAb (2D10) (11, 41, 47) (a gift from G. D. Powers) were originally made at Roche Research Center, Nutley, N.J. The hamster anti-murine CD40L MAb (MR1) (42) was supplied by Bioexpress (West Lebanon, N.H.). Rat IgG (Sigma, St. Louis, Mo.) was used as an antibody control for the anti-B7-1 and anti-B7-2 MAbs, and hamster IgG (ICN Pharmaceuticals Inc., Costa Mesa, Calif.) was used as a control for the anti-CD40L MAb. Murine CTLA4-Ig fusion protein (64) and the control murine L6 MAb (15) were provided by R. Peach (Bristol Myers Company, Princeton, N.J.).
In vivo antibody treatment.
129/SvJ mice were immunized once with GBSIII-TT i.p. along with one of these treatment regimens: (i) 200 μg of anti-B7-1, anti-B7-2, both anti-B7-1 and anti-B7-2, or control rat IgG three times a week for 4 weeks; (ii) one 500-μg dose of murine CTLA4-Ig or control L6; or (iii) one 500-μg dose of anti-CD40L or hamster IgG control. Serum samples were taken before and on days 7, 14, 21, and 28 after vaccination.
Measurement of specific serum Igs.
Levels of GBSIII-specific and TT-specific antibody were determined in dilutions of sera by solid-phase enzyme-linked immunosorbent assays (ELISAs). Isotype-specific antibody concentrations were measured on plates coated with GBSIII-human serum albumin or TT and with isotype- and subclass-specific, alkaline phosphatase-conjugated developing reagents, as described previously (25, 26). For specific antibody quantitations, the absorbance of test sera was compared with standard curves generated from separate ELISAs using plates coated with F(ab) fragments of goat anti-mouse IgG and rat anti-mouse IgM and known concentrations of murine IgG and IgM, respectively.
Statistics.
Nonparametric statistics were used, and continuous variables were expressed as medians, using SPSS for Macintosh 6.1.1 (SPSS Inc., Chicago, Ill.) for calculations. The Mann-Whitney U test was used to assess the statistical significance of differences between independent samples. A two-sided P value of <0.05 was considered significant.
RESULTS
Humoral immune response in mice lacking mature T cells immunized with GBS glycoconjugate vaccine.
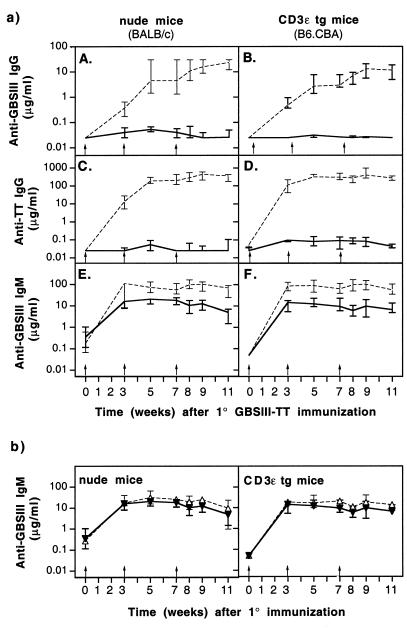
The T-cell dependence of the humoral immune response to the CPS moiety of the GBS glycoconjugate vaccine was examined by immunization of nude mice or CD3ɛ tg mice with GBSIII-TT and testing of their sera for the presence of CPS-specific and carrier-specific Igs. Both nude and CD3ɛ tg mice were profoundly impaired in the ability to switch antibody production to the IgG isotype (Fig. 1a, A and B). Although very low levels of CPS-specific IgG were found after GBSIII-TT immunization in four of the six nude mice, no specific IgG was detectable after immunization of the CD3ɛ tg mice. This result confirms the requirement for T cells in the isotype switch to IgG of the CPS-specific antibodies induced by conjugate vaccines. In these same mice, a similar T-cell dependence was seen for the antibody response to the TT carrier (Fig. 1a, C and D).
FIG. 1.
Polysaccharide- and carrier-specific IgG switch upon immunization with GBSIII-TT conjugate vaccine is abrogated in mice lacking mature T cells. Groups of six mice were immunized three times (arrows) with GBSIII-TT conjugate vaccine. (a) Levels of GBSIII-specific IgG (A and B), carrier-specific IgG (C and D), and CPS-specific IgM (E and F) after GBSIII-TT immunization in nude or CD3ɛ tg mice (———) and the corresponding wild-type mice (–––). (b) Levels of CPS-specific IgM in nude or tg mice immunized with GBSIII-TT (▾) or unconjugated CPS (▵). Lines represent median levels, and error bars indicate ranges.
Immunization of wild-type, CD3ɛ tg, and nude mice with unconjugated GBSIII CPS failed to induce detectable levels of CPS-specific IgG. This result confirmed that conjugation of CPS to a carrier is required for IgG isotype switching. Levels of CPS-specific IgM upon immunization with GBSIII-TT were lower in mice deficient in T cells than in wild-type mice (Fig. 1a, E and F; P < 0.05), a difference suggesting that T cells are involved in the IgM response to conjugated CPS. Furthermore, levels of CPS-specific IgM in CD3ɛ tg and nude mice were not significantly different whether these animals lacking mature T cells were immunized with unconjugated CPS or GBSIII-TT (Fig. 1b).
Humoral immune response of MHC class II-deficient mice immunized with GBS glycoconjugate vaccine.
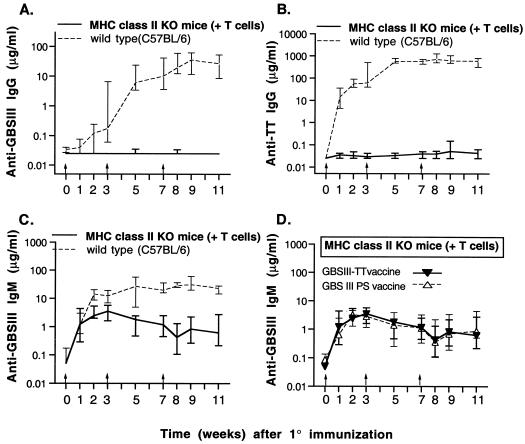
We immunized MHC class II-deficient mice with GBSIII-TT in order to define a role for this complex in initiating T-cell help. Since these knockout (KO) mice also lack mature CD4+ T cells (24), they were reconstituted with 7 × 107 splenic T cells obtained from nonimmune wild-type mice prior to immunization. After immunization with GBSIII-TT, the MHC class II-deficient mice were profoundly impaired in IgG isotype switching for both the CPS-specific and carrier-specific responses (Fig. 2A and B). This impairment demonstrated the essential role of signal 1 (MHC class II antigen–T-cell receptor [Ag-TCR]) in the recruitment of T-cell help by the glycoconjugate vaccine.
FIG. 2.
Polysaccharide- and carrier-specific IgG switch upon immunization with GBSIII-TT conjugate vaccine is abrogated in MHC class II KO mice whose CD4+ T cells have been replenished. Groups of six mice were immunized three times (arrows) with GBSIII-TT conjugate vaccine (A through D) or unconjugated CPS (D). Levels of GBSIII-specific IgG, carrier-specific IgG, and CPS-specific IgM after GBSIII-TT immunization in MHC class II KO mice and wild-type mice are shown in panels A, B, and C, respectively. Levels of CPS-specific IgM in MHC class II KO mice immunized with GBSIII-TT vaccine or unconjugated CPS (GBS III PS vaccine) are shown in panel D. Lines represent median levels, and error bars indicate ranges.
In wild-type mice, levels of CPS-specific IgM antibodies were substantially higher after immunization with GBSIII-TT than those in MHC class II KO mice reconstituted with T cells (Fig. 2C; P < 0.003 2 weeks after immunization), suggesting that T cells recruited by MHC class II Ag-TCR interactions are involved in the type III-specific IgM response. Furthermore, MHC class II-deficient mice reconstituted with T cells mounted an identical CPS-specific IgM response when immunized with conjugated or unconjugated CPS (Fig. 2D).
Humoral immune response of CD40L-deficient mice immunized with GBS glycoconjugate vaccine.
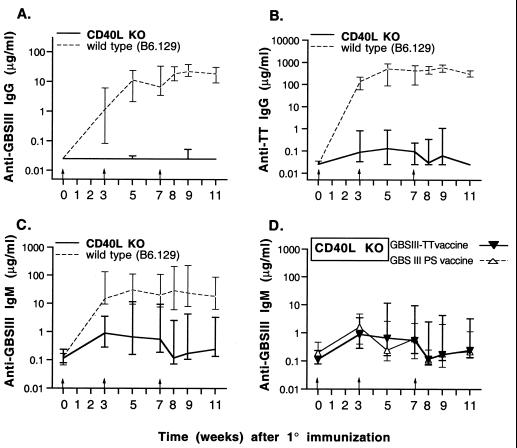
The role of CD40-CD40L (CD154) interactions in antigen-specific T cell-dependent immune responses to glycoconjugate vaccines was investigated by GBSIII-TT immunization of wild-type and CD40L−/− mice (50). There was no detectable CPS-specific isotype switch to IgG in CD40L−/− mice upon immunization with the glycoconjugate vaccine (Fig. 3A). Furthermore, compared with wild-type mice, CD40L−/− mice were profoundly impaired in terms of carrier-specific isotype switch to IgG (Fig. 3B).
FIG. 3.
Polysaccharide- and carrier-specific IgG switch upon immunization with GBSIII-TT conjugate vaccine is abrogated in CD40L KO mice. Groups of six mice were immunized three times (arrows) with GBSIII-TT conjugate vaccine (A through D) or unconjugated CPS (D). Levels of GBSIII-specific IgG, carrier-specific IgG, and CPS-specific IgM after GBSIII-TT immunization in CD40L KO mice and wild-type mice are shown in panels A, B, and C, respectively. Levels of CPS-specific IgM in CD40L KO mice immunized with GBSIII-TT vaccine or unconjugated CPS (GBS III PS vaccine) are shown in panel D. Lines represent median levels, and error bars indicate ranges.
After immunization with GBSIII-TT, levels of CPS-specific IgM were substantially higher in wild-type mice than in CD40L-deficient mice (Fig. 3C; P < 0.03). Moreover, the CD40L-deficient mice mounted an identical CPS-specific IgM response whether or not the CPS was conjugated (Fig. 3D). Therefore, CD40-CD40L interaction is required for both a vigorous CPS-specific IgM response and an isotype switch to IgG induced by the glycoconjugate vaccine.
In vivo blocking of the CD40-CD40L pathway during GBSIII-TT immunization.
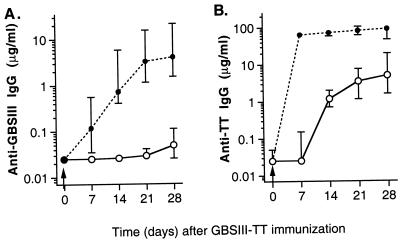
Further elucidation of the role of the CD40-CD40L (CD154) pathway in the immune response to GBSIII-TT was studied in 129/SvJ mice treated with a MAb directed to CD40L (MR1) (42). None of these mice switched to CPS-specific IgG production in the first 21 days after immunization (Fig. 4A). Similarly, there was profound impairment of carrier-specific isotype switching to IgG after immunization with the glycoconjugate vaccine (Fig. 4B). The median levels of TT-specific IgG 14 and 28 days after immunization in mice treated once with MAb to CD40L were 1.6 and 5.5%, respectively, of the level in mice treated with the control hamster antibody.
FIG. 4.
Blocking of CD40-CD40L-mediated B-cell stimulation abrogates CPS- and carrier-specific isotype switching to IgG upon immunization with GBSIII-TT glycoconjugate vaccine (arrows). Groups of four 129/SvJ mice were immunized with GBSIII-TT in the presence of MAb to CD40L (○) or a control antibody (hamster IgG [●]). Levels of GBSIII-specific (A) and TT-specific (B) IgG are shown. Lines represent median levels, and error bars indicate ranges.
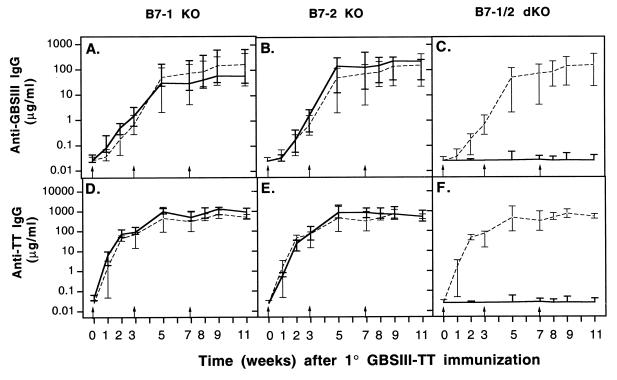
Humoral immune response of mice lacking either B7-1 or B7-2 immunized with GBS glycoconjugate vaccine.
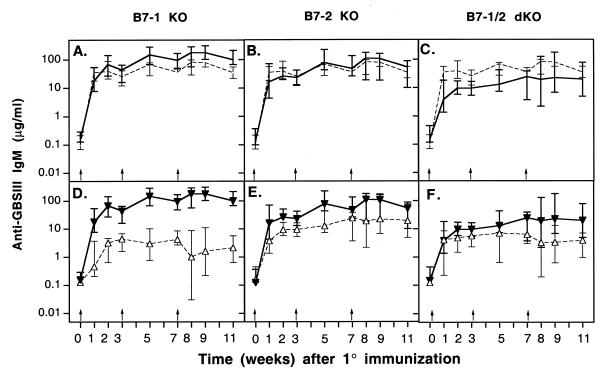
The roles of the B7-1 and B7-2 costimulatory molecules in the humoral immune response to GBSIII-TT were studied in wild-type, B7-1−/−, and B7-2−/− mice. No significant differences were found among these three types of mice in the kinetics of the response or in the levels of CPS-specific IgG (Fig. 5A and B) or carrier-specific IgG (Fig. 5D and E) after primary and secondary immunization. Similarly, the CPS-specific IgM response was identical in the three types of mice (Fig. 6A and B). Therefore, after i.p. immunization with an optimal dose of GBSIII-TT in alum, B7-1 or B7-2 deficiency did not affect the kinetics or the magnitude of the CPS-specific and carrier-specific responses. An interesting finding was that the level of CPS-specific IgM was higher in B7-1- and B7-2-deficient mice after immunization with GBSIII-TT than after immunization with unconjugated CPS (Fig. 6D and E; P < 0.03).
FIG. 5.
Polysaccharide- and carrier-specific IgG switch upon immunization with GBSIII-TT conjugate vaccine is abrogated in B7-1/2 double KO (dKO) mice. Groups of 6 to 10 mice were immunized three times (arrows) with GBSIII-TT conjugate vaccine. Levels of GBSIII-specific (A through C) and carrier-specific (D through F) IgG after GBSIII-TT immunization in B7-1 and/or B7-2 KO mice (———) and wild-type mice (–––) are shown. Lines represent median levels, and error bars indicate ranges.
FIG. 6.
Levels of GBSIII-specific IgM are substantially higher in wild-type mice than in B7-1/2-deficient mice. Groups of 6 to 10 B7-1 and/or B7-2 KO mice were immunized three times (arrows) with GBSIII-TT conjugate vaccine (A through F) or unconjugated CPS (D through F). Levels of GBSIII-specific IgM (A through C) after GBSIII-TT immunization in B7-1 and/or B7-2 KO mice (———) and wild-type mice (–––) are shown. Levels of CPS-specific IgM in B7-1, B7-2, and B7-1/2 KO mice immunized with GBSIII-TT (▾) or unconjugated CPS (▵) are shown in panels D, E, and F, respectively. Lines represent median levels, and error bars indicate ranges.
Humoral responses in mice lacking both B7-1/2 immunized with GBS glycoconjugate vaccine.
The lack of effect of eliminating the genes for either B7-1 or B7-2 on the humoral immune response to GBSIII-TT did not necessarily demonstrate that this axis is unimportant in the response to GBSIII-TT, because it was still not clear whether one costimulatory molecule could compensate for the absence of the other. Therefore, we undertook immunization with GBSIII-TT in B7-1/2−/− mice. These doubly deficient mice did not mount a CPS-specific isotype switch to IgG (Fig. 5C) and exhibited profound impairment in the carrier-specific isotype switch to IgG (Fig. 5F).
After immunization with GBSIII-TT, levels of CPS-specific IgM were substantially higher in wild-type mice than in B7-1/2-deficient mice (Fig. 6C; P < 0.03). The CPS-specific IgM response was similar in B7-1/2-deficient mice whether or not the CPS was conjugated to an immunogenic carrier (Fig. 6F). Therefore, T-cell costimulation through B7/CD28 interaction induced by the glycoconjugate vaccine is required for both a vigorous CPS-specific IgM response and an isotype switch to IgG. In addition, these data also suggest that either molecule can provide the necessary costimulation.
In vivo blocking of the B7/CD28 pathway during immunization with GBS glycoconjugate vaccine.
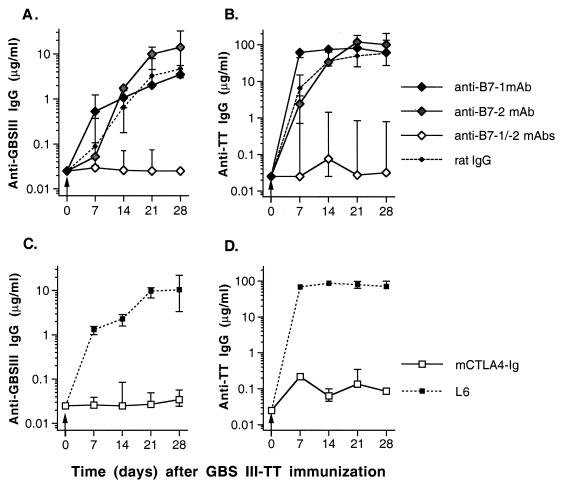
Our in vivo data obtained with B7-1 and B7-2 singly or doubly deficient mice strongly suggest a pivotal role for T-cell costimulation by the glycoconjugate vaccine through the CD28-B7 pathway. To further examine the role for the B7-CD28 costimulatory pathway in the humoral immune response to the glycoconjugate vaccine, we treated 129/SvJ mice with the following MAbs: (i) anti-B7-1 (1G10) (11) and/or anti-B7-2 (2D10) (11) or (ii) murine CTLA4-Ig fusion protein which binds to both B7-1 and B7-2 (49, 53, 64).
Comparable results were obtained in assays using pathway antagonists and B7-deficient mice. These findings support the observation that the presence of either of the B7 molecules is sufficient for costimulation of T cells during recruitment of T-cell help by the glycoconjugate vaccine (Fig. 7A). Results were the same for the carrier-specific IgG response (Fig. 7B). However, mice treated with anti-B7-1 produced higher levels of CPS- and carrier-specific IgG early in the immune response (day 7) than mice treated with anti-B7-2 or control rat IgG (Fig. 7A and B; P < 0.02). The B7/CD28 pathway is pivotal for the induction of isotype switching of CPS-specific antibodies. Mice treated simultaneously with both anti-B7-1 and anti-B7-2 (Fig. 7A) or with murine CTLA4-Ig fusion protein (Fig. 7C) failed to develop detectable CPS-specific IgG in their sera for up to 28 days after immunization, when the experiment was terminated.
FIG. 7.
Blocking of B7/CD28-mediated T-cell costimulation abrogates CPS- and carrier-specific isotype switching to IgG upon immunization with GBSIII-TT glycoconjugate vaccine (arrows). Groups of four 129/SvJ mice were immunized with GBSIII-TT in the presence of MAb to B7-1 and/or B7-2 (A and B) or murine CTLA4-Ig fusion protein (C and D). Levels of GBSIII-specific IgG (A and C) and TT-specific IgG (B and D) are shown. Lines represent median levels, and error bars indicate ranges.
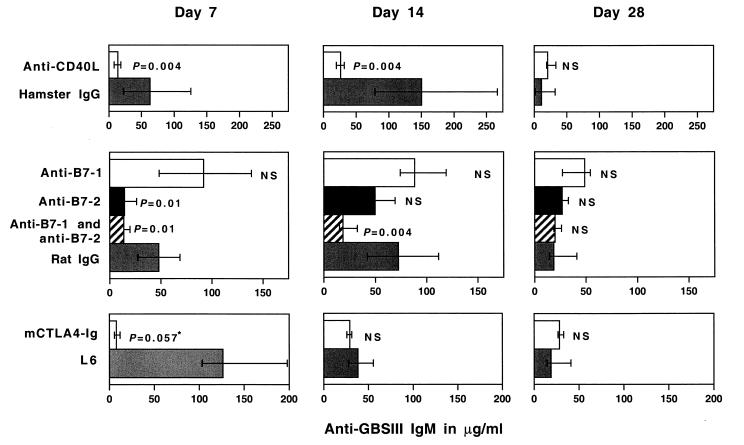
Median levels of CPS-specific IgM after GBSIII-TT immunization of mice treated with anti-B7-1 were not significantly different from levels in mice treated with control rat IgG (Fig. 8). Mice treated with CTLA4-Ig had substantially lower levels of CPS-specific IgM than controls (L6-treated mice) 7 days after immunization but not 14 or 28 days after immunization. Moreover, mice treated continuously with a mixture of anti-B7-1 and anti-B7-2 had substantially reduced levels of CPS-specific IgM on days 7 and 14 after immunization but not on day 28. Treatment with anti-B7-2 also resulted in reduced CPS-specific IgM levels on day 7 after immunization but not at later time points. Even though the numbers of mice in the different treatment groups are low, this finding suggests differences in the kinetics of the IgM response to the glycoconjugate vaccine depending on the B7 costimulatory molecule expressed, indicating an important role of the earliest expressed costimulatory molecule, B7-2, during the initial response to the conjugate vaccine. However, the difference between the GBSIII IgM levels after immunization with unconjugated and conjugated GBSIII PS was more pronounced in B7-1-deficient than in B7-2-deficient mice. Thus, T-cell costimulation through B7-CD28 interaction induced by the glycoconjugate vaccine is required for both a vigorous CPS-specific IgM response and an isotype switch to IgG, and either B7 molecule can provide the necessary costimulation.
FIG. 8.
Levels of CPS-specific IgM are influenced by blocking of the B7-CD28 and CD40-CD40L pathways. Groups of four 129/SvJ mice were immunized with GBSIII-TT conjugate vaccine in the presence of anti-CD40L (top panels), anti-B7-1 and/or B7-2 (middle panels), or murine CTLA4-Ig fusion protein (bottom panels). Levels of GBSIII-specific IgM on days 7, 14, and 28 after immunization are shown. Bars represent median levels, and error bars indicate ranges. ∗, the Mann-Whitney U test could not yield a significant P value (<0.05) because only three serum samples were available for the control group.
DISCUSSION
Antibodies to bacterial CPSs have been shown to protect against infection in humans (4, 46, 52). However, these T-cell-independent antigens are poorly immunogenic in mice (9, 19, 54) and in humans (6, 46, 52, 58), and attempts to prevent infection with encapsulated bacteria by immunization with their polysaccharide capsules have met with only modest success in humans (6, 46, 52, 58). Bacterial CPSs have been coupled to T-cell-dependent antigens in an effort to recruit T-cell help for the CPS, thereby enhancing the humoral immune response and inducing immunologic memory. The widespread use in the United States of one such glycoconjugate vaccine—against Haemophilus influenzae type b—has resulted in the eradication of invasive disease due to this pathogen (52). Candidate vaccines against infections caused by other encapsulated bacteria are currently being tested in clinical trials (29, 46, 58).
Contact-dependent T-cell help plays an obligatory role in humoral immune responses to thymus-dependent protein antigens and synthetic haptens coupled to immunogenic proteins (7, 12, 27, 38, 48). Cognate B-cell–T-cell interactions during the immune responses to protein antigens depend on T-cell costimulation through CD28 (10; reviewed in reference 55) and B-cell stimulation through CD40 (20; reviewed in references 16 and 21) and result in germinal center formation (10, 20) and induction of immunologic memory.
Details of the cognate B-cell–T-cell interactions governing this class of vaccines have not been reported. According to one model for how T cells improve the immunogenicity of conjugate vaccines described by Siber (58), the CPS moiety binds to surface Igs on CPS-specific B cells, with resulting internalization of the conjugate vaccine by receptor-mediated endocytosis. Subsequently, the protein portion of the conjugate is processed into peptides, and the T-cell epitopes are presented—in the context of MHC class II molecules—to TCR on carrier-specific T-helper cells. The carrier specificity of the T-cell help for hapten-carrier and protein-carrier conjugates has been confirmed both in vivo and in vitro (27, 28, 39). Our data confirm an obligatory role for the presentation of T-cell epitopes of the glycoconjugate vaccine to T-helper cells in the context of MHC class II molecules on APCs. Mice that were deficient in MHC class II molecules but whose T-helper cells had been replenished (24, 51) did not mount a CPS-specific isotype switch to IgG upon immunization with GBSIII-TT, whereas wild-type (C57BL/6) mice mounted a significant primary CPS-specific IgG response and upon subsequent immunizations displayed an anamnestic immune response. These results demonstrate that as with protein antigens (40), activation of resting T cells through stimulation of TCR in the context of MHC class II molecules results in induction of contact-dependent T-helper functions that are required in the immune response to glycoconjugate vaccines.
Cognate B-cell–T-cell interactions resulting in enhanced immune responses and induction of immunologic memory are necessary for successful immunization (9). Many prominent B- and T-cell membrane proteins (i.e., CD4, TCR, MHC, LFA-1, ICAM1, B7, CD28, CTLA4, CD40, and CD40L) play a significant role in the formation and stabilization of antigen-specific B-cell–T-cell conjugates (for reviews, see references 13, 14, 33, and 57). CD40-CD40L and B7-CD28 interactions costimulate cell activation and proliferation (13, 45, 57), while B7-CTLA4 interactions exhibit negative regulation of humoral immune responses (61, 62, 67). Our data demonstrate that the major costimulatory pathway involving the ligation of CD28 on T cells with costimulatory B7 molecules on activated B cells is critical for the induction of immunologic memory to CPS and for isotype switching to IgG in the CPS-specific response. After immunization with GBSIII-TT, CPS- and carrier-specific IgG antibodies were not detectable in the B7-1/2 double-KO mice. The results were the same when the B7-CD28 pathway was blocked by treatment of wild-type mice (129/SvJ) with CTLA4-Ig or a mixture of anti-B7-2 and anti-B7-2 during primary immunization. Thus, the class switch to IgG, which depends on the function of T-helper cells through B-cell–T-cell interactions, was profoundly affected. This finding suggests that the observed T-cell help recruited to the CPS by its conjugation to a protein carrier is abrogated by the absence of B7-1 and B7-2.
Our in vivo immunization results are in agreement with the profound deficit in isotype switching and absence of germinal centers observed in B7-1/2-deficient mice upon immunization with the hapten trinitrophenol (TNP) conjugated to keyhole limpet hemocyanin or ovalbumin (10). Similarly, a soluble chimeric fusion protein consisting of human CTLA4 fused to the Fc region of a mouse IgG2a suppressed T-cell-dependent antibody responses to sheep erythrocytes or keyhole limpet hemocyanin and inhibited T-cell clonal expansion after antigen priming (36). In addition, a mixture of antibodies specific for B7-1 and B7-2 completely blocked the primary T-cell responses induced by peptide antigen in complete Freund's adjuvant in vivo (31).
Whether B7-1 and B7-2 are functionally distinct is controversial. The CPS-specific antibody response in mice genetically lacking or immunologically blocked for either B7-1 or B7-2 was similar to that in wild-type mice, a result indicating that the presence of either costimulatory molecule was sufficient for T-cell costimulation through the B7-CD28 pathway. This finding is consistent with the view that B7-1 and B7-2 can function as overlapping ligands during T-cell-dependent isotype switching and germinal center formation (10). Some investigators have observed differences in CD4+ T-cell cytokine profiles in the presence of one versus the other B7 costimulatory molecule (18, 32, 35), while other authors have not confirmed these findings (34). Recent research suggests that the level and/or kinetics of B7 expression may account for the observed cytokine profiles (44, 55).
The engagement of CD40 with CD40L expressed on activated T cells also provides essential B-cell costimulation that results in isotype switching upon stimulation with the glycoconjugate vaccine. Immunization of CD40L−/− mice or injection with anti-CD40L during immunization caused ablation of the T-cell-dependent response to the glycoconjugate vaccine. This result is in accordance with the profound defect in isotype switching and germinal center formation observed in CD40L−/− mice upon immunization with TNP conjugated to a T-cell-dependent antigen (ovalbumin) (30). Furthermore, both IgG and IgM responses to the TI-I form (TNP-lipopolysaccharide) and the TI-II form (TNP-Ficoll) of TNP in CD40L−/− mice were normal (30). Taken together, these results suggest that the CD40-CD40L pathway is essential for T-cell-dependent Ig isotype switching for this glycoconjugate vaccine. This conclusion is in line with the observed failure of B-cell clonal expansion and the significant reduction in T-cell expansion during the primary immune response to protein antigens in the context of treatment with anti-CD40L (20). The failure of T-cell priming in CD40L-deficient mice may also be a direct effect of a lack of B7 induction on CD40-expressing APCs (22, 23, 69).
The role of T cells in IgM responses to bacterial CPSs conjugated to proteins is controversial: some investigators have found substantially higher levels of CPS-specific IgM after CPS conjugation to carriers (8, 37, 63), while others have found minimal or no differences (10, 56). It is interesting that in these studies, levels of CPS-specific IgM were substantially higher when high-molecular-weight CPSs isolated from pathogenic bacteria were used as test antigens (8, 37, 63), whereas minimal or no differences in hapten- or carbohydrate-specific IgM were observed when synthetic haptens or dextran was used (10, 56). We found that immunization of T-cell-sufficient mice with high-molecular-weight GBSIII CPS conjugated to TT resulted in substantially higher levels of CPS-specific IgM than did immunization with unconjugated CPS (Table 1). Taken together, these studies suggest that T cells recruited by glycoconjugate vaccines play a pivotal role in determining the magnitude of the IgM response to CPS isolated from pathogenic bacteria. Furthermore, the functional MHC class II Ag-TCR, CD40-CD40L, and B7-CD28 pathways are critical to the observed increases in CPS-specific IgM.
TABLE 1.
Effect of T-cell help on CPS-specific antibody responses to stimulation with GBSIII-TT glycoconjugate vaccine
| Mouse strain (background strain) | Ratioa
|
|||||
|---|---|---|---|---|---|---|
| GBSIII-specific IgM
|
GBSIII-specific IgG
|
|||||
| After 1° | After 2° | After 3° | After 1° | After 2° | After 3° | |
| Nude (BALB/cByJ) | 0.9 | 0.7 | 0.5 | 1.4 | 0.8 | 0.3 |
| CD3e tg (B6.CBA) | 0.7 | 0.7 | 0.5 | 1 | 0.9 | 0.7 |
| MHC class II KO (C57BL/6) | 1.2 | 1.3 | 1.2 | 1.2 | 1.3 | 0.9 |
| CD40L KO (B6.129) | 0.6 | 2.7 | 0.9 | 1 | 1 | 1 |
| B7-1 KO (129) | 9.8 | 22.2 | 111.5 | 57 | 698 | 2,172 |
| B7-2 KO (129) | 11.5 | 43.2 | 89.3 | 63 | 5,616 | 8,806 |
| B7-1/2 DKO (129) | 1.7 | 3.8 | 6.8 | 1 | 1.2 | 1 |
| Wild type (129/SvJ) | 4.7 | 6.7 | 28.3 | 54 | 2,430 | 1,427 |
Median level of GBSIII-specific antibody induced by GBSIII-TT divided by the level induced by unconjugated CPS. GBSIII-specific antibody levels (nanograms per milliliter) were determined by ELISA in sera obtained on day 21 after the first immunization (1°) and day 14 after the second and third immunizations (2° and 3°). (Groups of 6 to 10 mice were immunized i.p. with three 2-μg doses of CPS adsorbed to alum on days 0, 21, and 49.) For values below the detection limit of the assay (25 ng/ml), a value of 25 ng/ml was used in calculations.
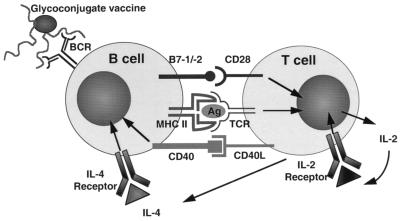
In summary, this study demonstrates an obligatory role for T-helper cells in the immune response to glycoconjugate vaccines in vivo. T-cell help is obtained (i) through presentation of processed antigens in the context of class II MHC molecules on APCs to TCRs, (ii) by the interaction of the costimulatory molecules B7-1 and B7-2 on APCs with CD28 on T-helper cells, and (iii) by stimulation of CD40 on B cells through interaction with CD40L on activated T-helper cells (Fig. 9).
FIG. 9.
Model for the recruitment of T-cell help to polysaccharide-specific B cells after stimulation with a glycoconjugate polysaccharide vaccine. The polysaccharide moiety of the vaccine binds to the B-cell receptor (BCR) of the polysaccharide-specific B cell, and the T-cell epitopes of the processed carrier molecules (Ag) are presented to the T-helper cell in the context of MHC class II, conferring carrier specificity (signal 1). Optimal T-cell activation takes place when the costimulatory molecules B7-1 and/or B7-2 interacts with CD28, providing the necessary costimulation (signal 2) leading to secretion of interleukin-2 (IL-2) and upregulation of the IL-2 receptor on the T cell. The activated T-helper cells upregulate CD40L as well as secrete IL-4, resulting in contact-dependent B-cell activation through CD40 and B-cell activation through signaling through the IL-4 receptor.
ACKNOWLEDGMENTS
We thank Jeanie H. Kwon and Angela C. Tramontano for excellent technical help, Baolin Chang for genotyping of the B7-deficient mice, and Yu Ho for help with T-cell purification.
This work was supported by grants AI 23339 and AI 38310 and contract AI 25152 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. H.-K. Guttormsen is the recipient of an Edward and Amalie Kass fellowship.
REFERENCES
- 1.Anonymous. Immunofluorescence and cell sorting. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1994. pp. 5.3.1–5.4.13. [Google Scholar]
- 2.Baker C J, Edwards M S. Group B streptococcal infections. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 4th ed. Philadelphia, Pa: W. B. Saunders; 1995. pp. 980–1054. [Google Scholar]
- 3.Baker C J, Edwards M S, Kasper D L. Role of antibody to native type III polysaccharide of group B Streptococcusin infant infection. Pediatrics. 1981;68:544–549. [PubMed] [Google Scholar]
- 4.Baker C J, Kasper D L. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976;294:753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- 5.Baker C J, Kasper D L, Tager I B, Paredes A, Alpert S, McCormack W M, Goroff D. Quantitative determination of antibody to capsular polysaccharide in infection with type III strains of group B Streptococcus. J Clin Investig. 1977;59:810–818. doi: 10.1172/JCI108703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker C J, Rench M A, Edwards M S, Carpenter R J, Hays B M, Kasper D L. Immunization of pregnant women with a polysaccharide vaccine of group B streptococcus. N Engl J Med. 1988;319:1180–1185. doi: 10.1056/NEJM198811033191802. [DOI] [PubMed] [Google Scholar]
- 7.Benacerraf B. A hypothesis to relate the specificity of T lymphocytes and the activity of I region-specific Ir genes in macrophages and B lymphocytes. J Immunol. 1978;120:1809–1812. [PubMed] [Google Scholar]
- 8.Beuvery E C, van Rossum F, Nagel J. Comparison of the induction of immunoglobulin M and G antibodies in mice with purified pneumococcal type 3 and meningococcal group C polysaccharides and their protein conjugates. Infect Immun. 1982;37:15–22. doi: 10.1128/iai.37.1.15-22.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishop C T, Jennings H J. Immunology. In: Aspinall G O, editor. The polysaccharides. Vol. 1. New York, N.Y: Academic Press; 1982. pp. 292–328. [Google Scholar]
- 10.Borriello F, Sethna M P, Boyd S D, Schweitzer A N, Tivol E A, Jacoby D, Strom T B, Simpson E M, Freeman G J, Sharpe A H. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Faherty D A, Gault A, Connaughton S E, Powers G D, Godfrey D I, Nabavi N. Monoclonal antibody 2D10 recognizes a novel T cell costimulatory molecule on activated murine B lymphocytes. J Immunol. 1994;152:2105–2114. [PubMed] [Google Scholar]
- 12.Claman H N, Chaperon E A. Immunologic complementation between thymus and marrow cells—a model for the two-cell model of immunocompetence. Transplant Rev. 1969;1:92–119. doi: 10.1111/j.1600-065x.1969.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 13.Clark E A, Lane P J. Regulation of human B-cell activation and adhesion. Annu Rev Immunol. 1991;9:97–127. doi: 10.1146/annurev.iy.09.040191.000525. [DOI] [PubMed] [Google Scholar]
- 14.Clark E A, Ledbetter J A. How B and T cells talk to each other. Nature. 1994;367:425–428. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- 15.Fell H P, Gayle M A, Yelton D, Lipsich L, Schieven G L, Marken J S, Aruffo A, Hellstrom K E, Hellstrom I, Bajorath J. Chimeric L6 anti-tumor antibody. Genomic construction, expression, and characterization of the antigen binding site. J Biol Chem. 1992;267:15552–15558. [PubMed] [Google Scholar]
- 16.Foy T M, Aruffo A, Bajorath J, Buhlmann J E, Noelle R J. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 17.Foy T M, Shepherd D M, Durie F H, Aruffo A, Ledbetter J A, Noelle R J. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. II. Prolonged suppression of the humoral immune response by an antibody to the ligand for CD40, gp39. J Exp Med. 1993;178:1567–1575. doi: 10.1084/jem.178.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman G J, Boussiotis V A, Anumanthan A, Bernstein G M, Ke X Y, Rennert P D, Gray G S, Gribben J G, Nadler L M. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523–532. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 19.Garner C V, Pier G B. Immunologic considerations for the development of conjugate vaccines. In: Cruse J M, Lewis R E J, editors. Conjugate vaccines. Vol. 10. Basel, Switzerland: Karger; 1989. pp. 11–17. [PubMed] [Google Scholar]
- 20.Garside P, Ingulli E, Merica R R, Johnson J G, Noelle R J, Jenkins M K. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 21.Grewal I S, Flavell R A. A central role of CD40 ligand in the regulation of CD4+ T-cell responses. Immunol Today. 1996;17:410–414. doi: 10.1016/0167-5699(96)10030-x. [DOI] [PubMed] [Google Scholar]
- 22.Grewal I S, Foellmer H G, Grewal K D, Xu J, Hardardottir F, Baron J L, Janeway C A J, Flavell R A. Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science. 1996;273:1864–1967. doi: 10.1126/science.273.5283.1864. [DOI] [PubMed] [Google Scholar]
- 23.Grewal I S, Xu J, Flavell R A. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378:617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 24.Grusby M J, Johnson R S, Papaioannou V E, Glimcher L H. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991;253:1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 25.Guttormsen H-K, Baker C J, Edwards M S, Paoletti L C, Kasper D L. Quantitative determination of antibodies to type III group B streptococcal polysaccharide. J Infect Dis. 1996;173:142–150. doi: 10.1093/infdis/173.1.142. [DOI] [PubMed] [Google Scholar]
- 26.Guttormsen H-K, Wetzler L M, Finberg R W, Kasper D L. Immunologic memory induced by a glycoconjugate vaccine in a murine adoptive lymphocyte transfer model. Infect Immun. 1998;66:2026–2032. doi: 10.1128/iai.66.5.2026-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamaoka T, Katz H, Benacerraf B. Hapten-specific IgE antibody responses in mice. II. Cooperative interactions between adoptively transferred T and B lymphocytes in the development of IgE response. J Exp Med. 1973;138:538–556. doi: 10.1084/jem.138.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang Y S, Lim K H, Kim B S. Analysis of T cell reactivities to phosphorylcholine-conjugated hen egg lysozyme in C57BL/6 mice: hapten-conjugate specificity reflects an altered expression of a major carrier epitope. Eur J Immunol. 1991;21:1303–1310. doi: 10.1002/eji.1830210531. [DOI] [PubMed] [Google Scholar]
- 29.Kasper D L, Paoletti L C, Wessels M R, Guttormsen H-K, Carey V J, Jennings H J, Baker C J. Immune response to type III group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J Clin Investig. 1996;98:2308–2314. doi: 10.1172/JCI119042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 31.Kearney E R, Walunas T L, Karr R W, Morton P A, Loh D Y, Bluestone J A, Jenkins M K. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J Immunol. 1995;155:1032–1036. [PubMed] [Google Scholar]
- 32.Kuchroo V K, Das M P, Brown J A, Ranger A M, Zamvil S S, Sobel R A, Weiner H L, Nabavi N, Glimcher L H. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 33.Laman J D, Claassen E, Noelle R J. Immunodeficiency due to a faulty interaction between T cells and B cells. Curr Opin Immunol. 1994;6:636–641. doi: 10.1016/0952-7915(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 34.Lanier L L, O'Fallon S, Somoza C, Phillips J H, Linsley P S, Okumura K, Ito D, Azuma M. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J Immunol. 1995;154:97–105. [PubMed] [Google Scholar]
- 35.Lenschow D J, Ho S C, Sattar H, Rhee L, Gray G, Nabavi N, Herold K C, Bluestone J A. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med. 1995;181:1145–1155. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linsley P S, Wallace P M, Johnson J, Gibson M G, Greene J L, Ledbetter J A, Singh C, Tepper M A. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 37.Lu C-H, Lee C-J, Kind P. Immune responses of young mice to pneumococcal type 9v polysaccharide-tetanus toxoid conjugate. Infect Immun. 1994;62:2754–2760. doi: 10.1128/iai.62.7.2754-2760.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchison N. The carrier effect in the secondary response to hapten-protein conjugates. II. Cellular cooperation. Eur J Immunol. 1971;1:18–25. doi: 10.1002/eji.1830010104. [DOI] [PubMed] [Google Scholar]
- 39.Mitchison N A. The carrier effect in the secondary response to hapten-protein conjugates. I. Measurements of the effect with transferred cells and objections to the local environment hypothesis. Eur J Immunol. 1971;1:10–17. doi: 10.1002/eji.1830010103. [DOI] [PubMed] [Google Scholar]
- 40.Mueller D L, Jenkins M K, Schwartz R H. Clonal expansion versus functional clonal inactivation: a costimulatorysignalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 41.Nabavi N, Freeman G J, Gault A, Godfrey D, Nadler L M, Glimcher L H. Signalling through the MHC class II cytoplasmic domain is required for antigen presentation and induces B7 expression. Nature. 1992;360:266–268. doi: 10.1038/360266a0. [DOI] [PubMed] [Google Scholar]
- 42.Noelle R J, Roy M, Shepherd D M, Stamenkovic I, Ledbetter J A, Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci USA. 1992;89:6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paoletti L C, Ross R A, Johnson K J. Cell growth rate regulates expression of group B Streptococcustype III capsular polysaccharide. Infect Immun. 1996;64:1220–1226. doi: 10.1128/iai.64.4.1220-1226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pape K A, Jenkins M K. A role for inflammatory cytokines in the productive activation of antigen-specific CD4+ T-cells. Agents Actions Suppl. 1998;49:23–31. doi: 10.1007/978-3-0348-8857-8_5. [DOI] [PubMed] [Google Scholar]
- 45.Parker D C. T cell-dependent B cell activation. Annu Rev Immunol. 1993;11:331–360. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 46.Peltola H. Meningococcal vaccines. Current status and future possibilities. Drugs. 1998;55:347–366. doi: 10.2165/00003495-199855030-00003. [DOI] [PubMed] [Google Scholar]
- 47.Powers G D, Faherty D A, Connaughton S E, Biondi D A, Godfrey D I, Gault A, Chen C Y, Nabavi N. Expression and functional analysis of murine B7 delineated by a novel monoclonal antibody. Cell Immunol. 1994;153:298–311. doi: 10.1006/cimm.1994.1030. [DOI] [PubMed] [Google Scholar]
- 48.Raff M C. Role of thymus derived lymphocytes in the secondary humoral immune response in mice. Nature. 1970;1257:226–228. doi: 10.1038/2261257a0. [DOI] [PubMed] [Google Scholar]
- 49.Reiser H, Stadecker M J. Costimulatory B7 molecules in the pathogenesis of infectious and autoimmune diseases. N Engl J Med. 1996;335:1369–1377. doi: 10.1056/NEJM199610313351807. [DOI] [PubMed] [Google Scholar]
- 50.Renshaw B R, Fanslow W C, 3rd, Armitage R J, Campbell K A, Liggitt D, Wright B, Davison B L, Maliszewski C R. Humoral immune response in the CD40 ligand-deficient mice. J Exp Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riberdy J M, Mostaghel E, Doyle C. Disruption of the CD4-major histocompatibility complex class II interaction blocks the development of CD4(+) T cells in vivo. Proc Natl Acad Sci USA. 1998;95:4493–4498. doi: 10.1073/pnas.95.8.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robbins J B, Schneerson R, Anderson P, Smith D H. Prevention of systemic infections, especially meningitis, caused by Haemophilus influenzaetype b. JAMA. 1996;276:1181–1185. doi: 10.1001/jama.276.14.1181. [DOI] [PubMed] [Google Scholar]
- 53.Sayegh M H, Turka L A. The role of T-cell costimulatory activation pathways in transplant rejection. N Engl J Med. 1998;338:1813–1821. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 54.Schneerson R, Barrera O, Sutton A, Robbins J B. Preparation, characterization and immunogenicity of Haemophilus influenzaetype b polysaccharide-protein conjugates. J Exp Med. 1980;152:361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schweitzer A N, Sharpe A H. The complexity of the B7-CD28/CTLA-4 costimulatory pathway. Agents Actions Suppl. 1998;49:33–43. doi: 10.1007/978-3-0348-8857-8_6. [DOI] [PubMed] [Google Scholar]
- 56.Seppala I, Pelkonen J, Makela O. Isotypes of antibodies induced by plain dextran or a dextran-protein conjugate. Eur J Immunol. 1985;15:827–833. doi: 10.1002/eji.1830150816. [DOI] [PubMed] [Google Scholar]
- 57.Sharpe A H. Analysis of lymphocyte costimulation in vivo using transgenic and ‘knockout’ mice. Curr Opin Immunol. 1995;7:389–395. doi: 10.1016/0952-7915(95)80115-4. [DOI] [PubMed] [Google Scholar]
- 58.Siber G R. Pneumococcal disease: prospects for a new generation of vaccines. Science. 1994;265:1385–1387. doi: 10.1126/science.8073278. [DOI] [PubMed] [Google Scholar]
- 59.Stein K E. Glycoconjugate vaccines. What next? Int J Technol Assess Health Care. 1994;10:167–176. doi: 10.1017/s0266462300014094. [DOI] [PubMed] [Google Scholar]
- 60.Stein K E. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J Infect Dis. 1992;165(Suppl. 1):S49–S52. doi: 10.1093/infdis/165-supplement_1-s49. [DOI] [PubMed] [Google Scholar]
- 61.Tivol E A, Borriello F, Schweitzer A N, Lynch W P, Bluestone J A, Sharpe A H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 62.Unkeless J C, Jin J. Inhibitory receptors, ITIM sequences and phosphatases. Curr Opin Immunol. 1997;9:338–343. doi: 10.1016/s0952-7915(97)80079-9. [DOI] [PubMed] [Google Scholar]
- 63.van den Dobbelsteen G P J M, Kroes H, van Rees E P. Characteristics of immune responses to native and protein conjugated pneumococcal polysaccharide type 14. Scand J Immunol. 1995;41:273–280. doi: 10.1111/j.1365-3083.1995.tb03564.x. [DOI] [PubMed] [Google Scholar]
- 64.Wallace P M, Johnson J S, MacMaster J F, Kennedy K A, Gladstone P, Linsley P S. CTLA4Ig treatment ameliorates the lethality of murine graft-versus-host disease across major histocompatibility complex barriers. Transplantation. 1994;58:602–610. doi: 10.1097/00007890-199409150-00013. [DOI] [PubMed] [Google Scholar]
- 65.Wang B, Biron C, She J, Higgins K, Sunshine M-J, Lacy E, Lonberg N, Terhorst C. A block in both early T lymphocyte and natural killer cell development in transgenic mice with high-copy numbers of the human CD3E gene. Proc Natl Acad Sci USA. 1994;91:9402–9406. doi: 10.1073/pnas.91.20.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang B, Levelt C, Salio M, Zheng D, Sancho J, Liu C-P, She J, Huang M, Higgins K, Sunshine M-J, Eichmann K, Lacy E, Lonberg N, Terhorst C. Over-expression of CD3 epsilon transgenes blocks T lymphocyte development. Int Immunol. 1995;7:435–448. doi: 10.1093/intimm/7.3.435. [DOI] [PubMed] [Google Scholar]
- 67.Waterhouse P, Penninger J M, Timms E, Wakeham A, Shahinian A, Lee K P, Thompson C B, Griesser H, Mak T W. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 68.Wessels M R, Paoletti L C, Kasper D L, DiFabio J L, Michon F, Holme K, Jennings H J. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B Streptococcus. J Clin Investig. 1990;86:1428–1433. doi: 10.1172/JCI114858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang Y, Wilson J M. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science. 1996;273:1862–1864. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]