Abstract
A transdermal drug delivery system (TDDS) is generally designed to deliver an active pharmaceutical ingredient (API) through the skin for systemic action. Permeation of an API through the skin is controlled by adjusting drug concentration, formulation composition, and patch design. A bilayer, drug-in-adhesive TDDS design may allow improved modulation of the drug release profile by facilitating varying layer thicknesses and drug spatial distribution across each layer. We hypothesized that the co-release of two fixed-dose APIs from a bilayer TDDS could be controlled by modifying spatial distribution and layer thickness while maintaining the same overall formulation composition. Franz cell diffusion studies demonstrated that three different bilayer patch designs, with different spatial distribution of drug and layer thicknesses, could modulate drug permeation and be compared with a reference single-layer monolith patch design. Compared with the monolith, decreased opioid antagonist permeation while maintaining fentanyl permeation could be achieved using a bilayer design. In addition, modulation of the drug spatial distribution and individual layer thicknesses, control of each drug’s permeation could be independently achieved. Bilayer patch performance did not change over an 8-week period in accelerated stability storage conditions. In conclusion, modifying the patch design of a bilayer TDDS achieves an individualized permeation of each API while maintaining constant patch composition.
Keywords: transdermal drug delivery, bilayer, multi-layer, fixed-dose combination, abuse deterrence
INTRODUCTION
Since the approval of the scopolamine patch by the US Food & Drug Administration (FDA) in 1979, transdermal drug delivery systems have demonstrated an ability to deliver potent, lipophilic, low molecular weight molecules while avoiding the hepatic first-pass effect and potentially decreasing side effects (1,2). Transdermal drug delivery is now considered an established route of administration to improve patient compliance by delivering a steady drug concentration over prolonged periods of time on the order of days to a week (3–5). This study’s objective was to advance current transdermal technology by understanding how bilayer patches may be used to deliver fixed-dose combination drugs at specific therapeutic concentrations.
Over the past few decades, transdermal drug delivery system (TDDS) designs have moved from the conventional reservoir design to the drug-in-adhesive (DIA) matrix design. This DIA design has several advantages including a lightweight and thin presentation providing patients with a more flexible patch while also eliminating the potential for drug leakage (6–8). The DIA’s relatively simple design appearance belies the relatively complex and critical role of the pressure-sensitive adhesive (PSA) that must maintain proper adhesive and cohesive properties while concurrently acting as a carrier for the drug substance (9,10).
Drug release from DIA systems is dependent on several factors including time, drug solubility, and the diffusion coefficient in the solid matrix, among others (11). Formulation of a DIA system involves PSA polymer selection based on the physicochemical properties of the drug and the intended therapeutic application of the system. It has been demonstrated that different PSAs significantly impact drug solubility, diffusion coefficient, and permeation coefficient (12). Fentanyl permeation rate is expected to increase with respect to drug loading within the patch until a concentration above the equilibrium solubility in the matrix is reached (13). The influence that excipients and drug loading have on permeation rate is unique to each drug polymer combination (12,14,15). Excipients can further modify the permeation rate of drugs across the skin (16–18). An example of excipients that promote this increase is chemical permeation enhancers which, in a similar way to drug loading, can increase permeation in a concentration-dependent manner over a certain range of concentrations of the permeation enhancer (13). In some cases, excipients can inhibit permeation or have non-linear effects on skin permeation enhancement (19). The effect of excipient changes on the desired patch performance is further complicated in a fixed-dose combination TDDS where the goal may be to have similar or different permeation rates for each drug in the matrix.
Examples of two commercially relevant approaches applied to the transdermal delivery of fentanyl using different pressure-sensitive adhesives are exemplified by Duragesic® and the Fentanyl Transdermal System (therapeutic equivalent to Duragesic®) that use either an acrylic or a silicone PSA, respectively. Despite differences in patch surface area and PSA, both products release fentanyl at the same dose per hour (20); fentanyl having a slower permeation profile in the acrylic polymer requires a larger surface area to deliver the same dose (12). The physicochemical properties of the drug (e.g., fentanyl) (Fig. 1) and the pressure-sensitive adhesives facilitate the design of different patches to deliver a consistent dose within the narrow therapeutic window of a drug like fentanyl (21). However, with the current opioid epidemic in the USA, there is a need for improvement of the current formulations to prevent or minimize abuse by minimizing the total amount of drug in the formulation, minimizing the residual amount in the patch after use, and incorporation of an opioid antagonist (21–24).
Fig. 1.

Physiochemical considerations for transdermal drug delivery (25) applied to fentanyl
A fixed-dose combination TDDS is a drug delivery system containing predetermined amounts of more than one API. These fixed-dose combination TDDS can be a viable way to formulate transdermal systems to deter or minimize abuse. This can be achieved, for example, by incorporation of opioid antagonists into a fixed-dose system to produce a tamper-sensitive design that only releases the antagonist during situations of manipulation or misuse of the dosage form. Naltrexone (NTX) is an effective opioid receptor antagonist that lacks the model physiochemical properties that fentanyl exhibits (26,27). Despite its transdermal properties (26,27), NTX can achieve 85% blockade of opioid receptors at blood serum concentrations of 2 ng/mL (28). The FDA-approved morphine sulfate capsule Embeda® uses a tamper-sensitive NTX core that if physically abused will release NTX and provide pharmacological competition at the opioid receptor abating euphoria (29). In the same manner, NTX has been synthesized into an abuse-deterrent API (AD-API) through the formation of an NTX salt that promotes tamper-resistant qualities by minimizing its solubility in the formulation while maintaining high solubility in potential conditions of abuse. Minimizing the transdermal permeation of AD-API incorporated into a fentanyl transdermal patch system will prevent unwanted antagonism that could compromise opioid efficacy (30) under proper use and maximizes the amount of naltrexone available for preventing harm when the patch is abused or misused. There is a particular need for this to be achieved while maintaining an effective rate of drug permeation similar to Duragesic® (fentanyl) TDDS.
The effect of formulation composition and the patch geometry has been reported to control drug permeation in DIA systems (31). However, modified permeation from a fixed-dose combination bilayer transdermal system by altering drug spatial distribution and layer thickness has not been shown. Bilayer DIA patches have expanded the potential of transdermal delivery by laminating a secondary DIA layer that contains the same or different compositions of PSA and drug to further control drug release (32). Fentalgon® uses different fentanyl concentrations in its bilayer design to achieve the same permeation as the commercial fentanyl products (33). Alghedon overcomes the lag effect by incorporating 15% of the fentanyl drug load in close contact with the skin while using a rate controlling membrane to separate the second layer containing the remaining 85% of the dose (34). This formulation achieves the same permeation profile but with less total drug in the formulation that could be abused and with lower amounts of drug retained in the patch after use.
Formulation composition and patch size can be modified to deliver multiple APIs (35–40). Using a transdermal gel formulation, Kamil et al. demonstrated that delivery of multiple APIs across the skin is possible (38). Similarly, Heard et al. successfully formulated a transdermal patch to deliver four drugs simultaneously, demonstrating release could be controlled by modification to the formulations matrix or an increase in concentration (39). In the study that provides a basis for the Climara Pro™ product, Harrison et al. modified the permeation of levonorgestrel while delivering estradiol at the same rate by altering patch size and levonorgestrel drug loading (40). In the case of fixed-dose combination of two APIs, altering the formulation composition may affect the release profile of each API to different extents (41). Therefore, this makes formulation optimization difficult in these cases.
Employing the same composition (i.e., same amount of each component of the formulation), but only changing the spatial distribution and layer thickness within a bilayer DIA patch to control multi-drug permeation, has not yet been reported. We hypothesize that the co-release of two fixed-dose APIs can be controlled from a bilayer TDDS by modifying spatial distribution and layer thickness while maintaining the same overall formulation composition. Selecting a bilayer patch design allowed for different spatial distribution of drug within the respective layers, ultimately influencing the individual permeation rates of both APIs (Fig. 2).
Fig. 2.

Graphical representation of the different spatial distribution and layer thicknesses the prototypes used to control multi-drug release
MATERIALS AND METHODS
Materials
Fentanyl base with a defined particle size (D90 of no more than (NMT) 55 μm) was purchased from Johnson Matthey Pharmaceutical Materials, Inc. (West Deptford, NJ). Proprietary AD-API supplied by Cassava Sciences, Inc. (Austin, TX). FDA-approved generic Fentanyl Transdermal System (Mylan Technologies) was purchased from Myoderm (Norristown, PA). Silicone-based pressure-sensitive adhesive, Bio PSA 7–4201, and silicone medical fluid #360, was purchased from Dow Corning (Midland, MI). Ethylene vinyl acetate (EVA) 12% and polyester laminate backing layer (9733, 3M (St. Paul, MN)) and release liner (fluoropolymer coated on polyester film, 1022) were purchased from 3M Company (St. Paul, MN). Strat-M® was purchased from Millipore Sigma (Merck KGaA, Darmstadt, Germany). Human skin was purchased from National Disease Research Interchange (Philadelphia, PA). HPLC grade acetonitrile and methanol were purchased from Fisher Scientific International, Inc. (Pittsburgh, PA). All other chemicals and reagents were of ACS grade or higher.
Establishing Formulation Compatibility and Composition
Preparation of Transdermal Patches
Solid content of the silicone Bio PSA 7–4201 was determined by gravimetric analysis before and after evaporation of the organic solvent, heptane, at 60°C for 4 h. Calculations were then performed and PSA solid content was adjusted in order to maintain the same percent solids between batches when formulating. Transdermal patch composition was determined by weight percent and performed as follows: A specific quantity of dimethicone was added dropwise to a known amount of fentanyl base. Upon addition of heptane, the mixture was stirred until fentanyl was completely dispersed followed by AD-API addition at a 1:1 M ratio with fentanyl, sonication, and stirring magnetically until a uniform dispersion was present (Table I).
Table I.
Formulation Composition of the Dry Cast Components Along with Their Respective Excipient and Drug Ratios. Bilayer Formulations Had a Combined Target Weight Equivalent to that of the Monolayer
| Monolith | Bilayer A | Bilayer B | Bilayer C | ||||
|---|---|---|---|---|---|---|---|
| Drug layer | Layer 1 | Layer 1 | Layer 2 | Layer 1 | Layer 2 | Layer 1 | Layer 2 |
| Target coat weight (mg/cm2) | 10.3 | 2.916 | 7.385 | 5.831 | 4.469 | 6.803 | 3.497 |
| Fen drug load | 100% | 30% | 70% | 60% | 40% | 70% | 30% |
| AD-API drug load | 100% | 0% | 100% | 0% | 100% | 0% | 100% |
| Thickness | 350 μm | 50 μm | 300 μm | 200 μm | 153 μm | 225 μm | 100 μm |
| Fen (w/w) | 4.0% | 4.0% | 3.8% | 4.3% | 3.7% | 4.3% | 3.6% |
| PSA (w/w) | 83.7% | 89.9% | 82% | 88.9% | 77.1% | 88.8% | 73.8% |
| Silicone oil (w/w) | 6.5% | 6.1% | 6.5% | 6.8% | 6.0% | 6.9% | 5.7% |
| PSA:oil | 12.90 | 14.61 | 12.62 | 13.05 | 12.86 | 12.88 | 12.91 |
| Fen:oil | 0.62 | 0.64 | 0.58 | 0.63 | 0.62 | 0.62 | 0.62 |
Casts were made using an EC-200 variable speed drawdown coater by ChemInstruments (Fairfield, OH) equipped with a #20 wire rod. Control of the desired wet cast thickness was achieved by addition of feeler gauge to the casting surface. Formulations were cast onto the release liner, allowed to sit for 5 min, then oven-dried at 60°C for 30 min to ensure solvent removal. The backing layer was applied directly to the dried cast and adhered using an ASTM lamination roller by ChemInstruments. Bilayer patches were manufactured by casting each layer separately on a release liner, laminating the layers together, and removing the release liner that is to be laminated with a backing layer followed by a final lamination with the backing layer. The layer in contact with the skin is defined as layer 1, while the layer in contact with the backing layer is defined as layer 2. Patches were cut to a surface area of either 1.5 cm2 or 6.25 cm2 using a dead-blow mallet and mallet handle dies (Fremont Machining, Fremont, OH).
Differential Scanning Calorimetry
Modulated DSC (DSC Q20, TA Instruments, New Castle, DE) was used to assess the compatibility of the formulation. Using standard aluminum pans, experiments were performed with a modulation temperature amplitude of 0.3°C every 50 s; heating ramp was performed from 35 to 250°C at a rate of 3°C/min with a nitrogen purge of 50 mL/min. Universal Analysis 2000 software was used for data interpretation (TA Instruments, New Castle, DE).
Patch Performance Studies
Skin Preparation
Fresh-frozen dermatomed human skin from the back of a 68-year-old Caucasian male was obtained from the National Disease Research Interchange (NDRI). The skin was frozen in a − 80°C freezer for no longer than 6 months until use.
Human skin was cut with the 1.5 cm2 die and immediately thawed in pH 7.4 phosphate buffer solution (PBS). The Franz cells were purchased from PermeGear (Hellertown, PA) with a receptor volume of 5 mL and area of diffusion of 0.64 cm2. The receptor compartments were filled with PBS (pH 7.4) and temperature adjusted to 37°C. The skin was then placed over the Franz cell, stratum corneum up and dermis down in contact with the receptor compartment. PBS (pH 7.4) was used to fill the donor compartment. An equilibrium time of 1 h was used to achieve a skin surface temperature of 32 + 2°C before electrical resistance measurements were taken. Electrical resistance was measured as previously described by Puri (42) and Martins (43) using a waveform generator model 142 HF VCG by Wavetek (San Diego, CA) at a frequency of 100 Hz. Silver chloride electrode was placed in the donor compartment without touching the skin while a silver electrode was placed in the receptor fluid. A load resistor (0.995 kΩ) was added to the circuit, in series with the skin, and the change in voltage was recorded using a multimeter. Human skin samples were visually inspected and those with an electrical resistance, < 10 kΩ, were discarded (44).
Franz Cell Diffusion and Sample Analysis
Once appropriate electrical resistance was confirmed, the PBS (pH 7.4) was removed from the donor and receptor compartment. The receptor compartment was washed two times with receptor solution (PBS pH 3) and then filled with receptor solution at 37°C. A more acidic receptor solution was chosen rather than physiological conditions due to increased solubility of both components to maintain sink conditions during testing. The donor compartment was removed and the patches were applied to the skin; once applied, the donor compartment was reapplied. The area of the Franz diffusion cell was 0.64 cm2. At each time point (6, 12, 24, 48, and 72 h), 2.2 mL was removed and filtered through a 0.2-μm nylon filter into HPLC vials stored in the refrigerator. After filtration, the Franz cell was immediately refilled with receptor solution. Strat-M® is an artificial membrane that is used to mimic permeation within the skin while minimizing variability and eliminating the need for hydration and preparation steps. Strat-M® was used for the initial development and screening permeation studies whereas the skin was used to confirm the permeation results.
A Thermo Scientific Dionex UltiMate 3000 HPLC system was used to quantify the results from the permeation study (Thermo Scientific, Sunnyvale, CA). The HPLC system contained an Ultimate 3000 Autosampler, injection volume 10 μL, a binary UltiMate 3000 pump, and an UltiMate 3000 RS Variable Wavelength Detector operating at 210 and 233 nm. The aqueous mobile phase contained 0.1% v/v Triethylamine in pH 2.5 potassium phosphate buffer and the organic phase consisted of Acetonitrile. A gradient was run starting with 5% organic phase to 45% organic phase over 10 min, then stepped to 70% organic for 5 min and back to 5% organic phase for the remainder of the 20 min run time. Injections were passed through an Inerstil ODS-2 Guard Column E and an Inerstil ODS-2, C18 Reverse Phase column, 4.6 mm × 150 mm with a 5 μm particle size (Gl Sciences, Tokyo, Japan) at a flow rate of 1 mL/min at 40°C. Chromeleon Version 7.2 software (Thermo Scientific, Sunnyvale, CA) was used for data processing. The HPLC method was found to be linear in quantifying fentanyl concentrations from 0.10 to 51.95 Tg/mL and from 0.03 to 63.60 μg/mL for AD-API. We set 0.25 μg/mL (Signal/noise 26.5) as our detection limit for fentanyl quantitation and 0.5 μg/mL for AD-API (signal/noise 13.8).
Adhesion Studies
A TA-XTplus texture analyzer (Surrey, UK) using 6.25 cm2 transdermal cuts was used for the probe tack, 90° peel adhesion and 90° release liner peel tests. The probe tack test was performed using a trigger force of 60 g, which once detected a 100 g force was applied for 2 s. After this time, the probe was withdrawn at a rate of 5.0 mm/s to a distance of 15 mm above the transdermal system. The resistance to withdrawal is an indication of patch adhesiveness. Both 90° tests used similar conditions to evaluate differences between prototypes. A reproducible force of 1500 g was applied to the patch; after 2 min, the measured force in tension was measured by retracting the clamp at a rate of 5 mm/s over 100 mm.
Stability Study
Stability studies were pursued for one monolayer and one bilayer formulations. Stability conditions were 25°C with 60% relative humidity and 40°C with 75% relative humidity maintained in a Darwin Chambers stability chamber, model no. PH030-AA-DA (Darwin Chambers Company, St. Louis, MO). Stability time points were chosen for 2, 4, and 8 weeks with each time point containing 4 samples for permeation analysis and 3 samples for uniformity evaluation. Immediately after formulation of the time zero batch, stability samples were heat sealed in single-unit aluminum pouches and placed on stability.
A stability-indicating method was used for the stability studies and content uniformity. The extraction method for content uniformity entailed dissolving one TDDS in a mixture of tetrahydrofuran, methanol, and aqueous phase. An aliquot was taken, centrifuged for 5 min at 5000 RPM, and filtered through 0.45-μm PTFE syringe filters. The same HPLC system used for Franz cell permeation quantification was utilized for content uniformity with modifications. The wavelength detector was adjusted to 220 and 290 nm and 50 μL injections were passed through a Waters XBridge BEH Phenyl, C18 reverse phase column, 3 mm × 150 mm with a 3.5 μm particle size (Waters Corporation, Milford, MA) at a flow rate of 0.7 mL/min at room temperature. The aqueous phase used contained 20 mM ammonium bicarbonate buffer adjusted to pH 9.5 and a 60:40 MeOH:ACN organic phase. The gradient ran started with a 10% organic phase to 85% organic phase over 25 min.
RESULTS
Establishing Formulation Compatibility and Composition
Prior to prototype formulation development, the compatibility of the AD-API with the excipient patch components and complete patch matrix was assessed via mDSC. The thermograms presenting the physical stability of the AD-API in these excipients are depicted in Fig. 3. The AD-API melting point remains consistent at 230°C. Prior literature has shown the compatibility between fentanyl and the formulation excipients (12).
Fig. 3.
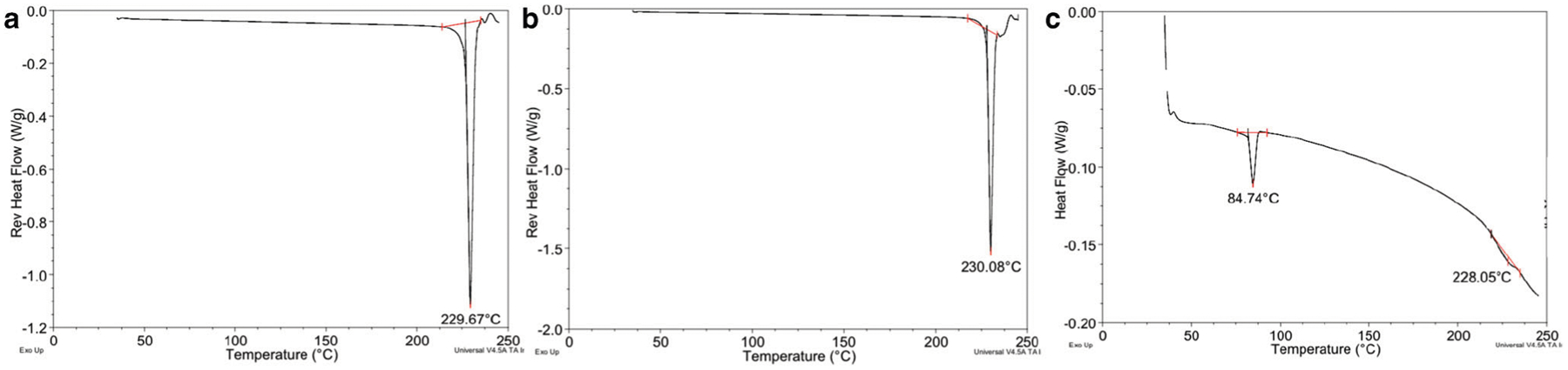
Reverse heat flow mDSC exhibiting unchanged endotherm profile of AD-API. The thermograms are presented as a AD-API and b AD-API and heptane mixture shaken 30 min and dried at room temperature. c Monolayer final formulation dried at 60°C for 30 min. Fentanyl endotherm is represented by the peak at 84°C and AD-API at 228°C
Compatibility was evaluated to determine possible solid-state interactions between the suspended APIs and the formulation matrix. A 1:1 M AD-API:fentanyl solid-state interaction was also evaluated after storage at 40°C for 2 weeks. Analysis from the stability-indicating method detected no change or presence of degradants in the chromatogram (data not shown.)
To eliminate drug migration between layers during storage and preserve fentanyl permeation, all bilayer formulations contained the same weight ratio of silicone oil to both fentanyl and silicone PSA. Different bilayer prototypes were formulated that maintained this composition. The prototypes differed by altering layer thicknesses and drug distribution between layers (Table I).
Patch Design and its Influence on Drug Permeation
A monolithic patch design incorporating both APIs was formulated by optimizing the percent solids of the casting solution and cast thickness to produce a patch that mimics the permeation of fentanyl from the marketed Fentanyl Transdermal System product, referred to as commercial comparator. In order to reflect the fentanyl permeation with both APIs present, an increase in the patch weight (10.3 mg/cm2) compared with the comparator product (10.0 mg/cm2) was required. The cumulative permeation of AD-API from the Franz cell study was determined to be 58 ± 23 μg/cm2 after 72 h for the monolith (Fig. 4).
Fig. 4.
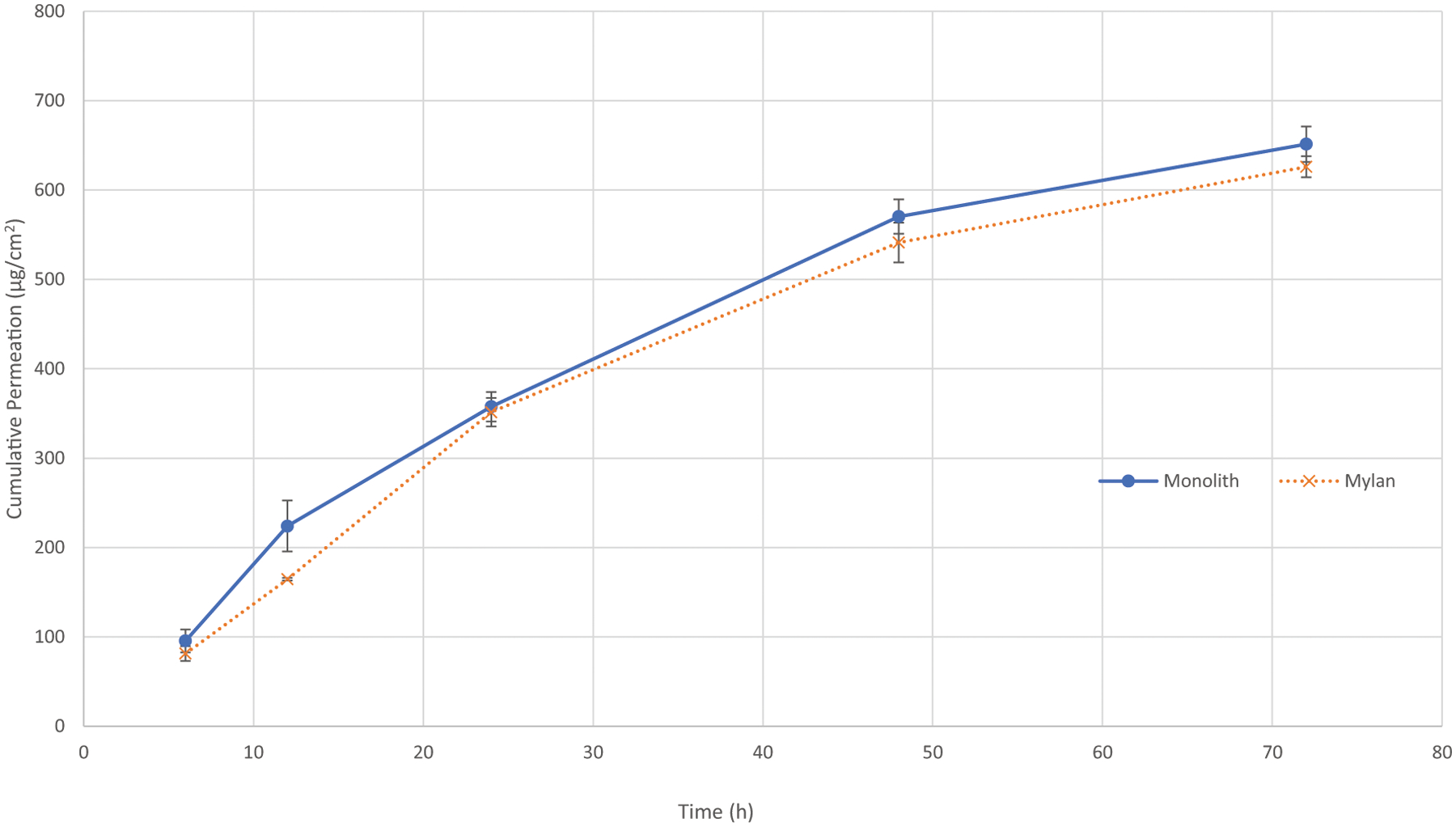
Franz cell diffusion using human skin membrane demonstrated the feasibility of reproducing in vitro fentanyl permeation of the commercial comparator when another hydrophilic API was introduced into the matrix (n = 6). The target coat weight achieving equivalence was 10.3 mg/cm2
From these results, our aim was to maintain the formulation composition in order to preserve the fentanyl permeation profile while minimizing the permeation of AD-API by modifying the patch design. In order to minimize variability of initial permeation experiments, Strat-M, a synthetic membrane that purports to mimic fentanyl skin permeation (45), was used for prototype screening. Using the monolith formulation as the reference, three different bilayer prototypes described in Table I were developed to evaluate the influence of patch design on the permeation profile from our system. Figs. 5 and 6 depict the resulting drug permeation across the synthetic membrane in which we observed an inverse relationship between the permeation profile of fentanyl and AD-API as the layer 1 thickness in the bilayer was increased.
Fig. 5.

Fentanyl permeation was measured across a Strat-M membrane during Franz cell permeation studies. Bilayer patches containing the same total dose but with different drug distributions and layer thicknesses were analyzed and compared with the monolith reference. The error bars represent the standard deviation (n = 4)
Fig. 6.

AD-API permeation was measured across a Strat-M membrane during Franz cell permeation studies. Bilayer patches containing the same total dose but with different drug distributions and layer thicknesses were analyzed and compared with the monolith reference. The error bars represent the standard deviation (n = 4)
Having most closely matched the target fentanyl permeation profile while minimizing AD-API permeation, Bilayer C was further evaluated to determine the influence of patch design on drug permeation using human skin ex vivo. Without modification of the formulation composition, Fig. 7 shows a significant reduction between the permeation of AD-API in the bilayer formulation compared with the monolith. Future studies in relevant species are planned to determine the drug release characteristics of the model system in vivo. Of particular interest will be the circulating concentration of AD-API and evaluating if these amounts would result in a decrease of the analgesic effect of fentanyl.
Fig. 7.

Franz cell permeation using human skin compared multi-drug permeation of the monolith to Bilayer. a Fentanyl permeation results. b AD-API permeation results. The error bars represent the standard deviation (n = 4)
Patch Performance
The effects of patch design modifications on patch performance were first evaluated by adhesion studies using the Fentanyl Transdermal System as the comparator. The monolith, with the additional drug loading compared with the Fentanyl Transdermal System, differed from the reference in all adhesion criteria (probe tack test P < 0.0023, 90° peel adhesion test P < 0.001), whereas the bilayer with the same composition as the monolith only showed a significant difference during the probe test (P < value 0.008) and generally resulted in values closer to the marketed product (Fig. 8).
Fig. 8.

Texture analyzer results of adhesion studies to assess patch performance (n = 4). The analysis is presented as a probe tack test and b 90° peel adhesion test. *P < 0.01, **P < 0.001
The patch design effects on performance were further investigated by determining the ability to release a consistent dose after 8 weeks at accelerated stability conditions. No changes in the amount of fentanyl or AD-API were observed in either of the patches throughout the study. The stability study demonstrated no change in permeation at either stability condition (Figs. 9 and 10). Maintaining permeation over time is of particular importance for the bilayer design due to the propensity of the patch components to migrate towards equilibrium if concentration differences between layers exist.
Fig. 9.

Franz cell permeation evaluation of the stability study to determine homogenous permeation of fentanyl over time. a Monolayer fentanyl permeation via Strat-M membrane for all time points. b Bilayer C fentanyl permeation via Strat-M membrane for all time points. One-way ANOVA P value > 0.05 at 72 h. The error bars represent the standard deviation (n = 4)
Fig. 10.
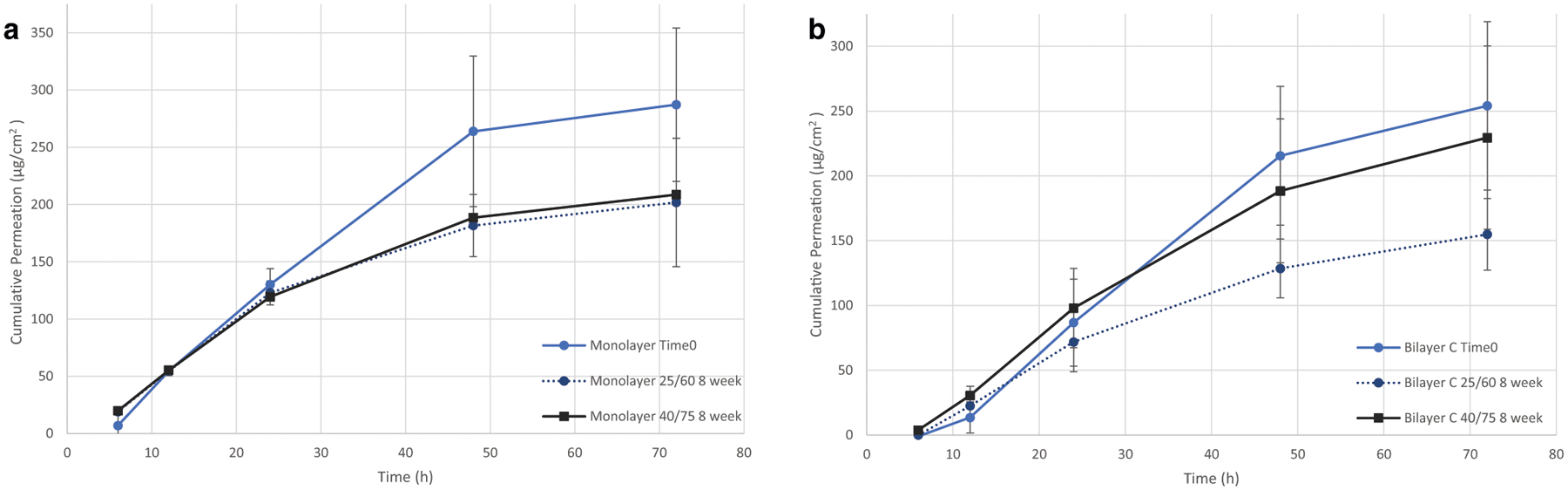
Franz cell permeation evaluation of the stability study to determine homogenous permeation of AD-API over time. a Monolayer AD-API permeation via Strat-M membrane for all time points. b Bilayer C AD-API permeation via Strat-M membrane for all time points. One-way ANOVA P value > 0.05 at 72 h. The error bars represent the standard deviation (n = 4)
DISCUSSION
Altered Patch Design with Maintained Composition Altered Drug Permeation of each API
The most important finding of this study was the ability to control multi-drug permeation by solely modifying the patch design without changing the overall formulation composition. In our experiment, we initially anticipated it would be crucial to preserve formulation composition in order to retain fentanyl permeation while using patch design to minimize AD-API permeation. Interestingly, while bilayer C was able to achieve this, comparing the permeation profiles of the different prototypes demonstrated the ability to modify the permeation of both fentanyl and the AD-API by adjusting thickness and relative drug loading. This phenomenon for a multi-drug bilayer system has not been previously reported in the literature. These findings are supported by findings of other investigators studying the diffusion and release of single-drug formulations from matrix systems. For example, Paul D.R. expanded on the original Higuchi model for diffusion-controlled release of a solute from a single-layer matrix (11) by laminating two matrixes with different spatial loadings of a single solute to illustrate an improved constant rate of release is achieved compared with a single-layer matrix structure (30). Charalambopoulou et al. modeled the advantages of such a bilayer design with a single API. It was determined that spatial variation of an API can significantly influence its release and provide improved performance compared with a monolithic DIA when distributed appropriately (46). Nauman et al. further expanded this idea by creating a model that can predict optimal patch design configuration for a single drug that is distributed in a bilayer based on minimizing the burst effect and maximizing drug depletion to increase patch efficiency (47). Both authors concluded that increased drug loading within the second layer increases controlled release and decreases burst effect. The different bilayer designs followed this trend for fentanyl permeation. Bilayer A contained the least amount of fentanyl in layer one and corresponded with the lowest burst, whereas Bilayer C contained the greatest fentanyl amount in the first layer and was responsible for the highest fentanyl bilayer permeation. Similarly, bio-equivalent fentanyl products Fentalgon and Alghedon use this optimized spatial distribution to maximize patch efficiency (33,34). At this time, no prior literature or marketed products have demonstrated controlled release by modifying the patch design of a bilayer system containing multiple APIs.
The above literature has demonstrated that optimizing patch configuration can better control release of a single drug from a bilayer TDDS. Our results expand on this literature to demonstrate that patch configuration can dictate the co-release of two fixed-dose APIs from a bilayer TDDS. Controlling multi-drug release from a transdermal system has previously been demonstrated by modifying excipient concentration or drug loading in a monolithic system. Harrison et al. demonstrated the ability to maintain release of estradiol and increase levonorgestrel permeation solely by maintaining estradiol drug load and increasing levonorgestrel drug load in a monolith DIA TDDS (40). However, this strategy is only effective when the drug loading requirements do not require concentrations of the drug above the saturation solubility in the patch matrix; once the saturation solubility is achieved, permeation rate cannot be increased by increasing drug load (13). Heard et al. created a monolith matrix system that shows TDDS capability of delivering multiple API; in their study, they successfully delivered 4 different APIs across the skin simultaneously, although optimization of the release of each drug independently was not investigated (39). Multi-drug release can also be controlled by modifying excipient concentrations, considering excipients have different degrees of impact on API; complexities arise when adjusting the formulations composition to modify permeation of a multi-drug system (41). Chen et al. specifically showed the varying degrees of impact permeation enhancers, Azone® and oleic acid, can have on drug release in multi-drug systems (35). Mutalik et al. further demonstrated the ability to control drug release of multi-drug monolithic systems, using different permeation enhancers and altering the patches surface area (37). Kamil et al. attribute the different permeation effects to the diffusion coefficient differences unique to each API within the system which illustrate the interplay between API physicochemical properties and patch matrix components that make this a complex strategy for individually controlling the release of different drugs when even possible (38).
Dimethicone oil has been shown to significantly affect fentanyl permeation (48) while the insoluble AD-API should not be impacted. Therefore, in a two-drug multi-layer system, optimization slightly compromises efficiency in order to achieve the desired permeation of both APIs. Using the monolith patch as the reference, we wanted to match the fentanyl permeation while minimizing the permeation of AD-API. From the Franz cell studies, different bilayer geometries utilizing different spatial drug distribution and thicknesses were able to modify the permeation profile of both APIs. While bilayer A showed the greatest decrease in fentanyl permeation, bilayer B was able to show both a significant decrease in fentanyl and AD-API permeation. Bilayer C’s layer 1 composition is closely approaching that of the monolith thereby exhibiting a comparable permeation profile of fentanyl while also decreasing AD-API permeation. These experiments yielded three possible generic permeation outcomes that can be achieved dependent on patch design: Decrease drug 1 permeation and maintain drug 2 permeation, decrease both drugs’ permeation, and maintain drug 1 permeation while decreasing drug 2 permeation.
In our instance, one API was practically insoluble within the matrix, allowing the entire drug load to be incorporated within the second layer. This design could also be applicable to systems where both drugs experience solubility within the matrix and the goal is not to minimize the permeation but decrease the permeation. To achieve the minimum permeation possible for an API that experiences partial solubility within the matrix, an amount slightly above the equilibrium solubility can be incorporated in the first layer to prevent transport between layers on storage. On the other hand, permeation can be maximized by incorporating an amount slightly above the equilibrium solubility in the second layer to prevent transport between layers. Any distribution between these two bounds would allow for modified permeation from the TDDS.
While maintaining composition, switching from a monolith to a bilayer system for controlled release of a fixed-dose combination is limited if either of the drug’s permeation rate is below their target value. Formulation modification into a bilayer will not increase the flux of a drug but will either maintain the permeation of one drug while decreasing the other slightly or decrease the permeation of both drugs. This is a particular benefit once a monolith formulation has been maximized to achieve its closest permeation possible without achieving its target permeation profile.
CONCLUSION
Adjusting the spatial distribution of APIs and layer thicknesses within a bilayer patch demonstrated that patch design can control the permeation of multiple APIs from a TDDS without adjusting formulation composition. Modification of bilayer patch design in a multi-drug system achieves three generalizable outcomes: Maintain permeation of drug 1 while decreasing permeation of drug 2, decrease permeation of both drug 1 and drug 2, and decrease permeation of drug 1 while maintaining permeation of drug 2. These outcomes can be used to purposely tailor a TDDS to release the desired amount from multi-drug systems. In our example, we maintained the permeation of fentanyl while significantly decreasing the permeation of AD-API to create a novel abuse-deterrent effect in a transdermal patch design.
ACKNOWLEDGMENTS
Research reported in this paper was supported by the National Institute on Drug Abuse (NIDA) of the NIH under project grant R44DA042639 awarded to Cassava Sciences, Inc. We also acknowledge the use of tissues procured through the National Disease Research Interchange (NDRI) with support from NIH grant U42OD11158.
Footnotes
Disclaimer The content is solely the responsibility of the authors of this paper and does not necessarily represent the official views of NIH.
REFERENCES
- 1.Naik A, Kalia YN, Guy RH. Transdermal drug delivery: overcoming the skin’s barrier function. Pharmaceut Sci Tech Today. 2000;3(9):318–26. [DOI] [PubMed] [Google Scholar]
- 2.Potts RO, Guy RH. Predicting skin permeability. Pharm Res. 1992;9(5):663–9. [DOI] [PubMed] [Google Scholar]
- 3.Mutalik S, Udupa N. Glibenclamide transdermal patches: physicochemical, pharmacodynamic, and pharmacokinetic evaluations. J Pharm Sci. 2004;93(6):1577–94. [DOI] [PubMed] [Google Scholar]
- 4.Ravnikar VA. Compliance with hormone therapy. Am J Obstet Gynecol. 1987;156(5):1332–4. [DOI] [PubMed] [Google Scholar]
- 5.Wiseman LR, McTavish D. Transdermal estradiol/norethisterone: a review of its pharmacological properties and clinical use in postmenopausal women. Drugs Aging. 1994;4(3):238–56. [DOI] [PubMed] [Google Scholar]
- 6.Dhiman S, Singh TG, Rehni AK. Transdermal patches: a recent approach to new drug delivery system. Int J Pharm Pharm Sci. 2011;3(5):26–34. [Google Scholar]
- 7.Li L, Fang L, Xu X, Liu Y, Sun Y, He Z. Formulation and biopharmaceutical evaluation of a transdermal patch containing letrozole. Biopharm Drug Dispos. 2010;31(2–3):138–49. [DOI] [PubMed] [Google Scholar]
- 8.Abrams LS, Skee DM, Natarajan J, Wong FA, Anderson GD. Pharmacokinetics of a contraceptive patch (Evra™/Ortho Evra™) containing norelgestromin and ethinyloestradiol at four application sites. Br J Clin Pharmacol. 2002;53(2):141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lobo S, Sachdeva S, Goswami T. Role of pressure-sensitive adhesives in transdermal drug delivery systems. Ther Deliv. 2016;7(1):33–48. [DOI] [PubMed] [Google Scholar]
- 10.Tan HS, Pfister WR. Pressure-sensitive adhesives for transdermal drug delivery systems. Pharmaceut Sci Tech Today. 1999;2(2):60–9. [DOI] [PubMed] [Google Scholar]
- 11.Higuchi T Rate of release of medicaments from ointment bases containing drugs in suspension. J Pharm Sci. 1961;50(10):874–5. [DOI] [PubMed] [Google Scholar]
- 12.Roy SD, Gutierrez M, Flynn GL, Cleary GW. Controlled transdermal delivery of fentanyl: characterizations of pressure-sensitive adhesives for matrix patch design. J Pharm Sci. 1996;85(5):491–5. [DOI] [PubMed] [Google Scholar]
- 13.Mehdizadeh A, Ghahremani M, Rouini M, Toliyat T. Effects of pressure sensitive adhesives and chemical permeation enhancers on the permeability of fentanyl through rat skin. Acta Pharma. 2006;56:219–29. [PubMed] [Google Scholar]
- 14.Naruse M, Ogawara K, Kimura T, Konishi R, Higaki K. Development of transdermal therapeutic formulation of CNS5161, a novel N-methyl-D-aspartate receptor antagonist, by utilizing pressure-sensitive adhesives I. Biol Pharm Bull. 2012;35(3):321–8. [DOI] [PubMed] [Google Scholar]
- 15.Snorradóttir BS, Gudnason PI, Thorsteinsson F, Másson M. Experimental design for optimizing drug release from silicone elastomer matrix and investigation of transdermal drug delivery. Eur J Pharm Sci. 2011;42(5):559–67. [DOI] [PubMed] [Google Scholar]
- 16.Gorukanti SR, Li L, Kim KH. Transdermal delivery of antiparkinsonian agent, benztropine. I. Effect of vehicles on skin permeation. Int J Pharm. 1999;192(2):159–72. [DOI] [PubMed] [Google Scholar]
- 17.Moser K, Kriwet K, Naik A, Kalia YN, Guy RH. Passive skin penetration enhancement and its quantification in vitro. Eur J Pharm Biopharm. 2001;52(2):103–12. [DOI] [PubMed] [Google Scholar]
- 18.Williams AC, Barry BW. Skin absorption enhancers. Crit Rev Ther Drug Carrier Syst. 1992;9(3–4):305–53. [PubMed] [Google Scholar]
- 19.Kaushik D, Batheja P, Kilfoyle B, Rai V, Michniak-Kohn B. Percutaneous permeation modifiers: enhancement versus retardation. Expert Opin Drug Del. 2008;5(5):517–29. [DOI] [PubMed] [Google Scholar]
- 20.Prodduturi S, Sadrieh N, Wokovich AM, Doub WH, Westenberger BJ, Buhse L. Transdermal delivery of fentanyl from matrix and reservoir systems: effect of heat and compromised skin. J Pharm Sci. 2010;99(5):2357–66. [DOI] [PubMed] [Google Scholar]
- 21.Padula C, Pescina S, Nicoli S, Santi P. Generic patches containing fentanyl: in vitro equivalence and abuse deterrent evaluation according to EMA and FDA guidelines. Int J Pharm. 2018;537(1):57–63. [DOI] [PubMed] [Google Scholar]
- 22.Kuczyńska K, Grzonkowski P, Kacprzak Ł, Zawilska JB. Abuse of fentanyl: an emerging problem to face. Forensic Sci Int. 2018;289:207–14. [DOI] [PubMed] [Google Scholar]
- 23.Rudd RA, Aleshire N, Zibbell JE, Matthew GR. Increases in drug and opioid overdose deaths — United States, 2000–2014. Morb Mortal Wkly Rep. 2016;64(50–51):1378–82. [DOI] [PubMed] [Google Scholar]
- 24.Schauer CKMW Shand JAD, Reynolds TM. The fentanyl patch boil-up - a novel method of opioid abuse. Basic Clin Pharmacol Toxicol. 2015;117(5):358–9. [DOI] [PubMed] [Google Scholar]
- 25.Naik A, Kalia YN, Guy RH. Transdermal drug delivery: overcoming the skin’s barrier function. Pharmaceut Sci Tech Today. 2000;3(9):318–26. [DOI] [PubMed] [Google Scholar]
- 26.Hammell DC, Hamad M, Vaddi HK, Crooks PA, Stinchcomb AL. A duplex “Gemini” prodrug of naltrexone for transdermal delivery. J Control Release. 2004;97(2):283–90. [DOI] [PubMed] [Google Scholar]
- 27.Nalluri BN, Milligan C, Chen J, Crooks PA, Stinchcomb AL. In vitro release studies on matrix type transdermal drug delivery systems of naltrexone and its acetyl prodrug. Drug Dev Ind Pharm. 2005;31(9):871–7. [DOI] [PubMed] [Google Scholar]
- 28.Verebey K, Volavka J, Mule SJ, Resnick R. Naltrexone: disposition, metabolism, and effects after acute and chronic dosing. Clin Pharmacol Ther. 1976;20(3):315–28. [DOI] [PubMed] [Google Scholar]
- 29.Inc. KP. Embeda® extended release capsules: joint anesthetic and life support drugs (ALSDAC) and drug safety and risk management (DSaRM) advisory committee briefing document. FDA; 2010. [Google Scholar]
- 30.Passik SD, editor Issues in long-term opioid therapy: unmet needs, risks, and solutions. Mayo Clinic Proceedings; 2009: Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paul DR. Modeling of solute release from laminated matrices. J Membr Sci. 1985;23(2):221–35. [Google Scholar]
- 32.Georgiadis MC, Kostoglou M. On the optimization of drug release from multi-laminated polymer matrix devices. J Control Release. 2001;77(3):273–85. [DOI] [PubMed] [Google Scholar]
- 33.Zecca E, Manzoni A, Centurioni F, Farina A, Bonizzoni E, Seiler D, et al. Pharmacokinetic study between a bilayer matrix fentalyl patch and a monolayer matrix fentanyl patch: single dose administration in healthy volunteers. Br J Clin Pharmacol. 2015;80(1):110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romualdi P, Santi P, Candeletti S. Alghedon Fentanyl Transdermal System. Minerva Med. 2016;108(2):169–75. [DOI] [PubMed] [Google Scholar]
- 35.Chen G-S, Kim D-D, Chien YW. Dual-controlled transdermal delivery of levonorgestrel and estradiol: enhanced permeation and modulated delivery. JCR. 1995;34:129–43. [Google Scholar]
- 36.Cordery SF, Husbands SM, Bailey CP, Guy RH, Delgado-Charro MB. Simultaneous transdermal delivery of buprenorphine hydrochloride and naltrexone hydrochloride by iontophoresis. Mol Pharm. 2019;16(6):2808–16. [DOI] [PubMed] [Google Scholar]
- 37.Mutalik S, Udupa N. Transdermal delivery of glibenclamide and glipizide: in vitro permeation studies through mouse skin. Pharmazie. 2002;57(12):838–41. [PubMed] [Google Scholar]
- 38.Kamil N, Nair AB, Attimarad M. Development of transdermal delivery system of dexamethasone, palonosetron and aprepitant for combination antiemetic therapy. Indian J Pharm Educ Res. 2016;50(3):472–81. [Google Scholar]
- 39.Heard CM, Johnson S, Moss G, Thomas CP. In vitro transdermal delivery of caffeine, theobromine, theophylline and cate-chin from extract of Guarana, Paullinia cupana Int J Pharm 2006;317(1):26–31. [DOI] [PubMed] [Google Scholar]
- 40.Harrison LI, Zurth C, Gunther C, Karara AH, Melikian A, Lipp R. Simultaneous estradiol and levonorgestrel transdermal delivery from a 7-day patch: in vitro and in vivo drug deliveries of three formulations. Drug Dev Ind Pharm. 2007;33(4):373–80. [DOI] [PubMed] [Google Scholar]
- 41.Lee PJ, Langer R, Shastri VP. Novel microemulsion enhancer formulation for simultaneous transdermal delivery of hydrophilic and hydrophobic drugs. Pharm Res. 2003;20(2):264–9. [DOI] [PubMed] [Google Scholar]
- 42.Puri A, Murnane KS, Blough BE, Banga AK. Effects of chemical and physical enhancement techniques on transdermal delivery of 3-fluoroamphetamine hydrochloride. Int J Pharm. 2017;528(1–2):452–62. [DOI] [PubMed] [Google Scholar]
- 43.Martins PP, Estrada AD, Smyth HD. A human skin high-throughput formulation screening method using a model hydrophilic drug. Int J Pharm. 2019;565:557–68. [DOI] [PubMed] [Google Scholar]
- 44.Davies DJ, Ward R, Heylings J. Multi-species assessment of electrical resistance as a skin integrity marker for in vitro percutaneous absorption studies. Toxicol in Vitro. 2004;18(3):351–8. [DOI] [PubMed] [Google Scholar]
- 45.Haq A, Dorrani M, Goodyear B, Joshi V, Michniak-Kohn B. Membrane properties for permeability testing: skin versus synthetic membranes. Int J Pharm. 2018;539(1):58–64. [DOI] [PubMed] [Google Scholar]
- 46.Charalambopoulou GC, Kikkinides ES, Papadokostaki KG, Stubos AK, Papaioannou AT. Numerical and experimental investigation of the diffusional release of a dispersed solute from polymeric multilaminate matrices. J Control Release. 2001;70(3):309–19. [DOI] [PubMed] [Google Scholar]
- 47.Nauman EB, Patel K, Karande P. Design of optimized diffusion-controlled transdermal drug delivery systems. Drug Dev Ind Pharm. 2011;37(1):93–102. [DOI] [PubMed] [Google Scholar]
- 48.Miller KJSA II, VT, US), Govil, Sharad K. (Essex, VT, US), Bhatia Kuljit Singh (Scottsdale, AZ, US), inventor; MYLAN PHARMACEUTICALS INC., assignee. Fentanyl suspension-based silicone adhesive formulations and devices for transdermal delivery of fentanyl. United States. 2007. 11/08/2007. [Google Scholar]


