Abstract
In the almost 70 years since the first hints of its existence, the phosphoinositide, phosphatidyl-D-myo-inositol 4,5-bisphosphate has been found to be central in the biological regulation of plasma membrane (PM) function. Here, we provide an overview of the signaling, transport and structural roles the lipid plays at the cell surface in animal cells. These include being substrate for second messenger generation, direct modulation of receptors, control of membrane traffic, regulation of ion channels and transporters, and modulation of the cytoskeleton and cell polarity. We conclude by re-evaluating PI(4,5)P2’s designation as a signaling molecule, instead proposing a cofactor role, enabling PM-selective function for many proteins.
Keywords: lipid rafts, phospholipids, PIP2, PtdIns4, 5P2, signaling
Introduction
Phosphoinositides were first discovered in the 1950s as a class of phospholipid with a staggeringly rapid metabolic turnover [1]. Since then, the most abundant species, PI(4,5)P2, has been associated with a steadily growing list of cellular functions (see the timeline in Figure 1). As we will describe, these functions are legion and sundry; they have been the topic of many recent in-depth reviews [2–7]. However, a combined overview of the full gamut of PI(4,5)P2 function has been lacking in contemporary literature. Our goal here is to provide the reader with a brief but broad summary of PI(4,5)P2 function in cell biology.
Figure 1.
A timeline of several milestones in our understanding of PI(4,5)P2 function.
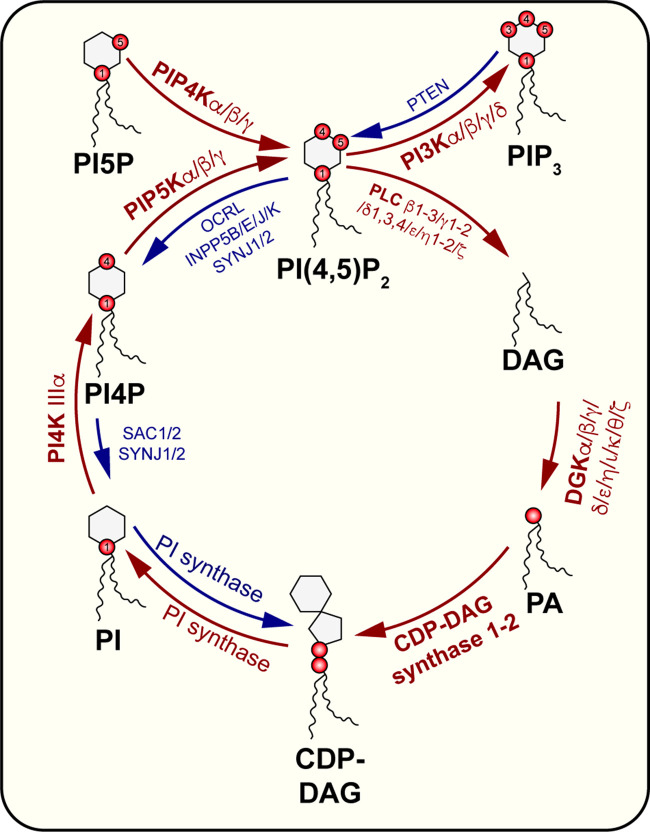
The article will focus on the history and current understanding of PI(4,5)P2 regulation at the plasma membrane (PM). We do not discuss PI(4,5)P2 synthesis and turnover, though this is summarized in Figure 2. Excellent historical perspectives have covered the discovery of PI(4,5)P2 and its metabolism [8,9]. A notable feature of PI(4,5)P2 as a lipid in animal cells is that, like the other phosphoinositides, it seems to be very selectively endowed with sn-1 stearoyl (18:0) and sn-2 arachidonyl (20:4) tails. Intriguingly, despite making a rather large and disordered-domain preferring lipid, recent evidence suggests that the reason for this specific fatty acid profile is to enable ‘metabolic channeling’ of a reserved set of lipid intermediates for phosphoinositide synthesis and recycling [10,11].
Figure 2. Synthesis and turnover of PI(4,5)P2.
The major intermediate lipids species and the enzymes involved are indicated, restricted to those isoforms believed to be directly involved in PM PI(4,5)P2 synthesis.
A confluence of biochemical [12], cytochemical [13,14] and biosensor-based [15,16] approaches have revealed that most PI(4,5)P2, though not all, is found in the inner leaflet of the PM. It is important to note that we are still discovering critical cellular functions that are regulated by smaller, intracellular pools of PI(4,5)P2; recent examples have included the assembly of nuclear signaling complexes [17] and the regulation of peroxisomal fatty acid oxidation [18]. However, as we see in Figure 1, most of the lipid's functions have been associated with the PM. These PMs-selective functions are also those for which we have the most detailed mechanistic knowledge. Therefore, we will focus our discussion exclusively on this membrane.
Before detailing the many PM functions of PI(4,5)P2, it is worth enumerating the molecule in its native cellular environment. It is often remarked in the literature that PI(4,5)P2 is a minor or scarce lipid. We would argue that this depends on perspective. Certainly, PI(4,5)P2 makes up only 1–5% of total inositol lipid in cells [19–21], and the whole family is the least abundant of the phospholipids, accounting for ∼10% of glycerophospholipid [22]. So PI(4,5)P2 is expected to be merely 0.1–0.5% of total glycerophospholipid: scarce indeed. However, it is worth remembering that the confluence of empirical evidence over the last half century has indicated that, unlike most phospholipids, PI(4,5)P2 is heavily enriched in the inner leaflet of the PM. Therefore, the lipid has a much higher mole fraction here, perhaps accounting for up to 5% [23]. So, its local concentration in membranes can be high.
Another consideration is PI(4,5)P2’s abundance compared with the other molecules that it interacts with, often stoichiometrically: the proteins. Let's start by asking how many PI(4,5)P2 there are in the inner leaflet of the PM: assuming that the average leaflet of the bilayer can hold ∼1.5 × 106 phospholipids/µm2 [21,24] and that 60% of that space is not occupied by membrane proteins [21] there is space for a total of ∼900 000 phospholipids/µm2 in the inner leaflet of the PM. Various PI(4,5)P2 densities have been experimentally determined and range from 4000 molecules/µm2 in a neuroblastoma cell line [25] to 34 000 molecules/µm2 in adult rat pinealocytes [21], or 0.4–4 mol%, resulting in an average of ∼20 000 PI(4,5)P2 molecules/µm2 [26], or 2%. Furthermore, estimating for the surface area of the PM at ∼2000 µm2 [27,28], it can be determined that there are anywhere between 8 × 106 and 6.8 × 107 PI(4,5)P2 molecules in the inner leaflet of the PM at any given time. Compare this to the median protein copy number of ∼3 × 104 in typical cultured cells [29,30]. Even the most abundant structural proteins, such as components of the cytoskeleton, are present at ∼ 1 × 106 copies per cell. PI(4,5)P2 outnumbers them all by an order of magnitude, even by the lower estimates of abundance. Put simply, PI(4,5)P2 may be a scarce lipid, but it is an exceptionally abundant regulatory molecule.
Given such a preponderance of PI(4,5)P2 molecules in the PM, it is easy to see how it is able to bind the scores of effector proteins that it regulates. We will focus the majority of this article detailing the core classes of these interacting partners. Furthermore, we subdivide them by the major types of PM function they regulate: signaling, transport and structure.
Signaling: substrate and cofactor
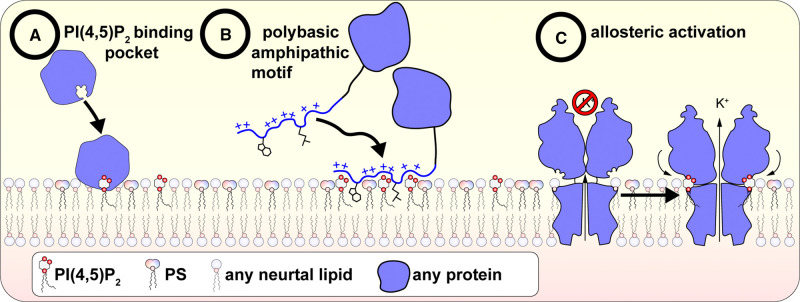
A key function of the PM in metazoans is to relay and amplify signals from impermeant, extracellular hormones and growth factors to the appropriate cellular response machinery. The seminal discovery of PI(4,5)P2 function was the realization that the lipid's hydrolysis by phospholipase C (PLC) in response to hormones stimulated cytosolic calcium entry [31], specifically from intracellular stores [32]. This rapid hydrolysis of PI(4,5)P2 by PLC also explained the unusually rapid turnover of PI(4,5)P2 and its metabolites that had initiated the study of the molecule over two decades earlier. At the time of writing, all of the human PLC isoforms (13 in total) have been identified with physiological functions being assigned [33], and an ever more sophisticated mechanistic knowledge of their regulation is being deduced [34]. The study of PLC activation also gave us the identification of the first highly selective, stoichiometric PI(4,5)P2 binding domain: the pleckstrin homology domain from PLCδ1 [35,36], unveiling a paradigm for PI(4,5)P2-dependent regulation (Figure 3A).
Figure 3. Mechanisms of protein regulation by PI(4,5)P2.
In some ways overtaking the discovery of PLC signaling was the realization that phosphoinositide 3-kinases (PI3Ks) utilize PI(4,5)P2 as a substrate to generate PIP3 [37,38], a critically important second messenger in its own right. The PI3K pathway is now known to activate scores of downstream effector proteins and to play central functions in organismal growth control and immune activation [39]. Consequently, PI3Ks are becoming increasingly important targets for small molecule inhibitors in active clinical use, particularly in cancer [40]. In general, the importance of the PI3K and PLC pathways is now well established, ‘textbook’ knowledge. So, we will not dwell on them here.
Following this foundation as a substrate for second messenger generation, PI(4,5)P2 is now increasingly recognized as an enabling factor in many other signaling pathways at the PM, beyond those employing PLC or PI3K. Some of the first examples came from the large Ras superfamily of small GTPases, which are anchored to membranes by their C-termini. Specific PM targeting is often mediated by a C-terminal prenylation, with an adjacent polybasic stretch of residues that favor the PS-enriched, highly anionic inner leaflet of the PM [41,42]. PI(4,5)P2 is a critical component of this PM anionic lipid mix that allows specific PM targeting by these ubiquitous signaling proteins [43,44]. This non-selective, hydrophobic and electrostatic interaction with the membrane is another fundamental mechanism of lipid regulation (Figure 3B).
Beyond small GTPases, three class A heterotrimeric G-protein-coupled receptors (GPCRs) have been shown to bind to PI(4,5)P2 with high specificity, including adenosine A2A, β1 adrenergic and neurotensin receptor 1 [45]. Furthermore, the PI(4,5)P2 interacting residues of GPCRs were mapped to the cytosolic loops which link specific transmembrane (TM) helices together [45]. Moreover, this interaction with PI(4,5)P2 is responsible for coupling the GPCR to the heterotrimeric Gαsβγ [45]. The binding to PI(4,5)P2 stabilizes the GPCR and stimulates GTP hydrolysis [45]. Further work has shown that PI(4,5)P2 can stabilize the oligomeric state of adenosine A2A, which may lead to multi-output signaling complexes [46].
GPCR signaling can be attenuated in two different ways: (1) receptor molecules can be internalized, thereby slowing or stopping further ligand binding and the resulting cell signaling [47] or (2) the binding to G-proteins can be inhibited [47]. Both of these steps can be accomplished by arrestins [47] Arrestins bind to GPCRs and inhibit their interaction with G-proteins as well as mediate their internalization [47]. Recent work has shown that β-arrestin interacts with neurotensin receptor 1 [48]. Furthermore, this interaction is facilitated by a PI(4,5)P2 specific interaction with the C-lobe of β-arrestin and the TM cytosolic loops [48]. Other work has expanded on this and shown that the β-arrestin-GPCR complex requires PI(4,5)P2 to be fully stabilized [49]. Additionally, the β-arrestin interaction with the PM may actually increase PI(4,5)P2 levels to further facilitate GPCR internalization [50]. Thus, PI(4,5)P2 is involved in both the stabilization of the GPCR-G-protein signaling complex as well as the GPCR-β-arrestin signaling termination complex. In these ways PI(4,5)P2 is absolutely essential to the entire process of GPCR signaling, even when not specifically coupled to PLC or PI3K.
PM transport
Vesicular traffic
PI(4,5)P2 has a key role in recruiting proteins to the PM for the initiation and regulation of both exo- and endocytosis [3,7]. Exocytosis is a highly regulated process that leads to the release of intracellular components through the PM to the extracellular space, and/or the delivery of new membrane [3,7,51]. The three basic steps for exocytosis are vesicular docking, priming and fusion [51]. Docking involves the recruitment of vesicles and their tethering to the inner leaflet of the PM [51]. The exocyst complex of proteins is essential for this tethering process, specifically allowing tethering of the soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) [52,53]. The exocyst complex consists of two independent subcomplexes; subcomplex-1 is made up of Exoc1–4 and subcomplex-2 is made up of Exoc5–8. A majority of the exocyst complex is present on the vesicular surface, and when in proximity to the PM this complex interacts with Exoc1 (Sec3 in yeast) to form the full exocyst complex [52]. Exoc1 interacts with the PM initially due to an interaction with PI(4,5)P2 and a PM anchored SNARE protein [52–55]. After this initial interaction, another exocyst protein, Exoc7 (Exo70 in yeast), is now able to interact with PI(4,5)P2 at the PM as well, stabilizing the interaction of the vesicle with the PM and inducing membrane curvature [52,53,56].
The next step, priming, is responsible for the maturation of PM tethered vesicles [51]. This was in fact the first step to be recognized as PI(4,5)P2-dependent [57]. Munc13 and Ca2+-dependent activator protein for secretion (CAPS) are both required for the transition between docking and priming of vesicles [51]. Specifically, Munc13 and CAPS are involved in the formation and stabilization of the SNARE complex once they are localized with the PM [51,53]. Munc13 is recruited to the PM in a Ca2+-dependent manner, but once at the PM it interacts with and is stabilized by PI(4,5)P2 [51,58]. CAPS contains a PI(4,5)P2 binding PH domain which allows for its recruitment to the PM [58,59].
Finally, fusion allows for the vesicle to merge with the PM to release contents [51]. Components of the SNARE complex include syntaxin-1 and synaptosomal-association protein of 25 kDa (SNAP-25). Syntaxin-1 is a TM protein localized to the PM and containing a juxtamembrane polybasic domain that forms PI(4,5)P2-dependent clusters at sites of membrane fusion [60]. SNAP-25, on the other hand, localizes with the PM due to a palmitoylation motif [61], though a polybasic stretch is required to target the protein to the PM via PI(4,5)P2 for palmitoylation to occur [62]. Because of its myriad requirements, the loss of PI(4,5)P2 at the PM results in dysregulation of exocytosis, hindering this process at all steps [58,63,64].
Opposite of exocytosis, endocytosis is the process of moving cargo from the extracellular surface into the interior of the cell or retrieving PM components. PI(4,5)P2 has been shown to play a key role in clathrin-independent endocytosis [65,66] and phagocytosis [67]. Clathrin-mediated endocytosis (CME) is the best-characterized mechanism of endocytosis with a strict requirement for the lipid [68]. Fundamentally, CME relies on the recognition of cargo proteins on the cytosolic leaflet of the PM by adapter proteins, which then stimulate the assembly of the clathrin coat for vesicle budding. A requirement for PI(4,5)P2 in membrane cargo binding was recognized early on for the major PM adaptor complex, AP-2 [69]. Several additional clathrin adapter proteins have since been recognized as being recruited by PI(4,5)P2, including epsin [70] and AP180 [71]. In addition to being a simple membrane anchor, PI(4,5)P2 also seems to play a pivotal role in the allosteric activation of AP-2 complexes on the membrane [72,73].
In the final stage of CME, the budding clathrin-coated vesicle is separated from the PM via the constriction of the neck due to the action of dynamin [68]. Dynamin is recruited to the PM due its interaction with PI(4,5)P2 [74]. Because of these central roles, loss of PI(4,5)P2 from the PM completely blocks clathrin-mediated endocytosis [63,75–79]. Such a potent role for PI(4,5)P2 in clathrin-coated structure formation is emphasized by the fact that recruitment of PI(4,5)P2 5-OH phosphatases like synaptojanin-1 and OCRL are essential for the uncoating process after vesicles bud [80–82].
Selective permeability of the membrane
The presence of ion channels and transporters in the PM conveys the ability for selective permeability to ions and small molecules, and also confers the capacity for electrical excitability in neural and contractile tissues. The first indications for regulation by PI(4,5)P2 came with the demonstration that both the sodium/calcium exchanger (NCX) and ATP-sensitive potassium channels (KATP) required the lipid to operate in cardiac myocytes [83]. Since then, additional membrane transporters have been shown to require PI(4,5)P2 for assembly and function, including the serotonin [84] and dopamine [85] transporters along with the PM calcium ATPase [86] and the epithelial sodium/proton exchanger, NHE [87].
The number of ion channel classes discovered to be regulated by PI(4,5)P2 has exploded. They include certain members of the voltage-gated (Kv), inwardly rectifying (Kir) and calcium-activated potassium (KCa) channel families, voltage-gated calcium channels, PM-localized transient receptor potential (TRP) channels, epithelial sodium channels (ENaC), cyclic nucleotide-gated channels (CNGs), purinergic-regulated P2X channels and calcium-activated chloride channels [5,88]. The crystal structure of a Kir channel in complex with PI(4,5)P2 revealed a novel paradigm for the lipid's activation of membrane proteins: the acyl chains interact non-selectively with the TM helices of the channel, whereas the inositol headgroup binds selectively to the cytosolic domain, essentially pulling the domain into close contact with the pore helices and partially opening the ion conductance pore [89]. This highlights another fundamental mechanism of PI(4,5)P2 action: allosteric regulation of a protein (Figure 3C). For the most part, channels are activated by PI(4,5)P2 interactions, which have been proposed as a mechanism for restricting their activity to the PM, avoiding leakage during trafficking steps [90]. However, there are also examples of negative regulation of channels by PI(4,5)P2 binding [88], sometimes on the same channel that is also positively regulated by the lipid [91].
Regulation of ion channel activity is also a new paradigm for PLC regulation of PI(4,5)P2 function. Here, rather than generating second messengers, PLC removes PM PI(4,5)P2 to modulate channel activity. This was first described for M-current in neurons. M-current is mediated by Kir, hence is inhibited by PLC-mediated PI(4,5)P2 depletion [92]. Since the M-current ordinarily buffers resting potential, the neurons themselves become more excitable. On the other hand, PI(4,5)P2 hydrolysis (and associated acidification) by PLC directly activates TRP channels in Drosophila photoreceptors [93]. We would underscore, however, that setting aside these specific physiological examples, most instances of PLC activation do not lead to substantial depletion of PI(4,5)P2 (see for example [10]). Most examples in the literature where PLC leads to depletion of PI(4,5)P2 rely on the over-expression of PLC-coupled receptors and/or the application of non-physiological, pharmacologically saturating concentrations of agonist. Even when PI(4,5)P2 depletion does occur, cellular systems possess machinery for its rapid resynthesis. Failure of PI(4,5)P2 resynthesis can be catastrophic. For example, in the Drosophila photoreceptor, failure to regenerate the lipid leads to detachment of the cytoskeleton (discussed in the next section) and degeneration of the photoreceptor cells [94].
Collectively, PI(4,5)P2 synthesis is necessary for maintaining ion and solute homeostasis in a variety of excitable and non-excitable tissues. Indeed, failure to activate NHE after PI(4,5)P2 breakdown by the Salmonella effector SopB is a primary reason why fluid uptake is blocked in the gastrointestinal epithelia during food-born infections, leading to diarrhea and dysentery [95].
Structure and organization of the membrane
Cytoskeleton
The actin cytoskeleton regulates cellular processes ranging from endocytosis to cell motility to cytokinesis, and it is tightly associated with the membrane [2,96]. Control of these dynamic cellular processes by actin relies on the fact that the actin cytoskeleton is itself remarkably dynamic [2,96]. PI(4,5)P2 is a central player in regulating these dynamics.
One of the first proposed roles for PI(4,5)P2 acting as more than a substrate for PLC was its ability to dissociate profilin-G-actin complexes, effectively nucleating actin polymerization on the PM [97]. Furthermore, F-actin severing by another abundant actin regulatory protein, gelsolin, was shown to be blocked by PI(4,5)P2 [98]. Finally, the actin-severing protein cofilin was shown to be inhibited by PI(4,5)P2 [99,100], and so can be released from the membrane after PI(4,5)P2 hydrolysis to sever actin filaments [101]. Therefore, PI(4,5)P2 was established as a pro-actin filament assembly signal. It should be noted, however, that the interaction of factors such as profilin and cofilin with PI(4,5)P2 is very low affinity, with a relatively small fraction of these proteins PM bound at physiological lipid levels [102]
Actin filaments can be capped, at barbed ends, by the capping protein CapZ [103–105] to prevent both actin assembly and disassembly [2,96]. CapZ can be removed from actin filaments due to an interaction with PI(4,5)P2, thus allowing for either PM actin assembly or disassembly [103–105]. Neuronal Wiskot–Aldrich syndrome protein (N-WASP) is recruited to the PM via an electrostatic interaction between the negatively charged inner leaflet, due in part to PI(4,5)P2, and a polybasic domain in N-WASP [106]. This interaction is much tighter than for cofilin or profilin. However, it is still dynamic enough for localization to be driven by dissociation from PI(4,5)P2, rather than by slow lateral diffusion of N-WASP with the lipid [102]. Once localized at the PM, N-WASP activates the actin-related protein 2/3 (Arp2/3) complex [107,108], which then nucleates daughter filaments from existing mother filaments to facilitate the branching of actin [96,109,110].
The actin cytoskeleton is closely linked to the PM by several proteins [2,96]. One such protein family is the ezrin, radixin, moesin (ERM) family of proteins, which like their red blood cell counterpart, band 4.1, all contain a FERM domain. In fact, the PI(4,5)P2-band 4.1 interaction was one of the first functions for intact PI(4,5)P2 to be defined [111]. FERM domains bind to PI(4,5)P2 with high affinity [102,112–115], triggering the unmasking of other binding sites in the ERM proteins which allow them to interact with F-actin or intracellular adhesion molecules (ICAMs) [112–115]. Another protein family linking both actin and microtubules to the PM are septins [2,116]. Septins have been shown to interact with PI(4,5)P2 via a polybasic domain that is separate from their actin/microtubule binding N-terminal domains [116,117]. Spectrins are the last family of proteins which similarly interact with actin and the PM [2,96]. Specifically, β-spectrin contains a PH domain which has been shown in bind PI(4,5)P2, allowing for PM association [118–120].
Cellular adhesion to the extracellular matrix (ECM) is another pivotal function of the cytoskeleton. Focal adhesions (FAs) are made up of a complex network of proteins that link the actin cytoskeleton to the ECM. The physical interaction with the ECM is facilitated by the extracellular portion of integrins [121]. The intracellular portion of integrins then generates the integrin signaling layer [121]. This layer contains integrins, paxillin, focal adhesion kinase (FAK) and talin [121]. Atop this is the force transduction layer, composed of talin, VASP and vinculin [121]. And finally, the actin regulatory layer is made up of VASP and α-actinin, attaching to bundled actin filaments. Each of these layers contains at least one PI(4,5)P2 interacting protein: FAK [121–123], talin [112,113,124–126], vinculin [124,127–130] and α-actinin [127]. PI(4,5)P2 is known to function as either an activator or a stabilizer for each of these proteins in each layer of the FA; thus the lipid seems to be a crucial factor in activating these proteins for FA assembly.
Clearly, PI(4,5)P2 is essential to the integration of a functional cytoskeleton with the PM. Indeed, aberrant PI(4,5)P2 levels can lead to many changes in the organization or regulation of the actin cytoskeleton [131–134]. For example, ectopic accumulation of PI(4,5)P2 on endocytic structures can lead to the polymerization of actin ‘comet tails’ on endosomes, disrupting vesicular traffic in Lowe Syndrome [81,135]. Conversely, loss of PM PI(4,5)P2 leads to the detachment of the actin cytoskeleton and a collapse in membrane tension [136,137].
ER-PM contact sites
Anchoring of the ER to the PM is a requisite for a variety of essential cellular functions, including ER calcium store filling, traffic of lipids to the PM and stress sensing [138]. These functions are mediated by classes of proteins that tether the two membranes, possessing both PM and ER anchoring domains. For PM anchoring, PI(4,5)P2 is a common molecule that serves as the binding partner, mediating the interaction of extended synaptotagmins (E-Syts) [139], oxysterol-binding protein-related proteins (ORPs) 5 and 8 [140,141] and the GRAMD2 but not GRAMD1 proteins [142]. Because of these interactions, PI(4,5)P2 is essential for store-operated calcium entry [143] and the accumulation of PM phosphatidylserine [141].
Cell polarity
PI(4,5)P2 has recently emerged as a key regulator of cell polarity. Particularly, the Par-6 complex is a key determinant of apical–basal polarity across a variety of tissues in animals [4]. Par-6 recruits an atypical protein kinase C (aPKC) to apical domains, which phosphorylates and thereby inhibits apical PM recruitment of basolateral proteins such as Lgl, Numb and Miranda. The specific site of phosphorylation in these proteins is an amphipathic, polybasic domain that targets the proteins to the PI(4,5)P2-rich PM [144,145]. Phosphorylation neutralizes some positive charge in these domains, reducing electrostatic interaction with the membrane. Intriguingly, aPKC itself contains a polybasic, amphipathic pseudosubstrate region that is not phosphorylated. This can similarly target PM PI(4,5)P2, but is not exposed for membrane interaction in the context of full-length aPKC until an allosteric activation mediated by Par-6 at apical domains [146]. Therefore, PI(4,5)P2 appears to be a critical determinant of both apical and basolateral membrane identity through context-dependent interactions with binding partners.
PI(4,5)P2: signal or cofactor for PM function?
Given the many PM PI(4,5)P2-dependent PM functions we have met so far, we can imagine what would happen to a cell unable to synthesize PI(4,5)P2: It will be unable to transmit signals coupled to PLC or PI3K. Many GPCRs coupled to andenylyl cyclases will also not function efficiently, and signaling through a wide range of small GTPases will be blocked as they no longer localize at the PM. The cell will be unable to secrete proteins via the secretory pathway and be deficient in its capacity to undergo all forms of endocytosis. Its actin cytoskeleton will not be tightly integrated with the PM, causing a collapse of membrane tension. It will also be unable to efficiently build contacts with the ECM or neighboring cells. Many key contacts with the ER will not form, compromising the cell's capacity to maintain PM lipid and ER calcium homeostasis. And if it's a polarized cell, it will struggle to maintain polarity determining complexes. Put simply, the cell will be completely kaput.
The genetics seem to support this thought experiment. Loss of PIP5K α and γ genes in mice prevents embryonic development [147]. Loss of just the PIP5Kγ isoform leads to perinatal lethality in both mice and humans [63,148,149], stemming from a drop in PI(4,5)P2 levels in the nervous system [63]. Obviously, PI(4,5)P2 is an essential molecule to support animal life at the cellular level. On the other hand, several diseases are associated with failures to degrade PI(4,5)P2, mainly from loss-of-function mutations in INPP5 phosphatases (Figure 2) [150–155]. While these mutations do not preclude early development in the same way that loss of the kinases does, the phenotypes of these diseases are all devastating and lead to early mortality. So it seems in the case of PI(4,5)P2, there can be too much of a good thing. This also shows us that supporting the PM functions we have explored is more than just assuring that there is enough lipid for proteins to interact with; the levels must be carefully controlled, and accumulations can have adverse phenotypic consequences in their own right.
The shear diversity of PM function regulated by PI(4,5)P2 poses a conundrum in the context of the wide variety of phenotypes associated with aberrant PI(4,5)P2 metabolism: these can effect a range of tissues and cause quite disparate diseases, from problems with kidney filtration to neurodegeneration to muscular dystrophy [150,152–155]. Tissue-specific enrichment of the INPP5s may go a long way to explaining restriction of these diseases to specific organ systems, but the fact that patients survive to adulthood suggests that the phenotype is not driven by the total failure of all PI(4,5)P2-regulated PM function in the affected cell types. This poses a larger question: how is PI(4,5)P2 coupled to individual PM functions in health and disease?
The provenance of our understanding of PI(4,5)P2 function is firmly rooted in cell signaling, with its initial characterization as substrate for PLC and later PI3K signaling (Figure 1). The discovery that the lipid can also modulate actin polymerization led to the idea that PI(4,5)P2 is a second messenger in its own right. It follows that although cells maintain relatively stable PI(4,5)P2 levels, nanoscopic changes in its concentration may modulate individual functions that we describe above; the cacophony of these myriad signals then averages out to the global steady state [156]. Disruption of specific nano-scale pools of PI(4,5)P2 after disruption of individual phosphatases may then explain the restricted phenotypes associate with loss-of-function.
We think that such a view of PI(4,5)P2 as an extremely busy second messenger is unlikely to be correct. Firstly, empirical measurements have shown that many phosphoinositide effectors have dissociation rate constants in the millisecond to second-time scale [102,157,158], so effector-bound lipids are likely to be in rapid dynamic equilibrium with unbound lipids. Secondly, and most crucially, diffusion of lipids like PI(4,5)P2 on the inner leaflet of the membrane is rapid, ranging from 0.1 to 1 µm2/s [157,159–162]. This estimate takes the slowing effect of the cortical membrane cytoskeleton into account; inside cytoskeletal corrals, diffusion is an order of magnitude faster [163,164]. Therefore, even if we grant a ‘nanodomain’ a relatively generous diameter — say r = 100 nm, as found in clathrin-coated structures — at the lower limited of diffusion, it will take ∼80 ms for a PI(4,5)P2 molecule that is released from an effector to diffuse away (from r = √4Dt/π, where D is the diffusion coefficient [165]). Coupled to rapid dynamic exchange, it would be extremely difficult for cells to maintain localized PI(4,5)P2 accumulation on the time scales necessary for the minute-long processes it regulates. Local generation of lipid is likely to ‘spill out’ to neighboring complexes, producing unwanted regulation. Clustering of PI(4,5)P2 can be induced and maintained by electrostatic bridging between the negatively charged phosphates by polybasic peptide stretches in proteins [60,166] or by multivalent cations [167]. This could certainly generate different interaction properties of such clusters, which might favor the binding of polyvalent, non-specific effectors over stoichiometric binders [168]. However, reservation of such nanoscopic lipid clusters for the select physiological function would require either precise tuning of effector affinity to a narrow range of cluster density, or the presence of effector proteins in the cluster that define function through additional protein–protein interactions. In this latter case, note that it would not really be the PI(4,5)P2 that defines the function of the cluster, rather the specific ions or proteins inducing their formation — and thus playing the messenger role. In any case, in terms of PI(4,5)P2 in intact animal cells, several groups have found no evidence of nanoscopic PI(4,5)P2 accumulation [13,169], and others have found sample preparation artifacts that artificially induce them [170,171].
If PI(4,5)P2 is a lousy second messenger, what is it good at? We prefer to think of it in the terms that Hilgemann proposed for ion channels: ‘In terms of cellular homeostasis, differences in the levels of PIP2 in internal compared with surface membranes may help to control the activity of proteins as they progress through the secretory pathway to the surface membrane’ [90]. We would expand this to cover the full gamut of PI(4,5)P2-regulated function: PI(4,5)P2 serves as a necessary cofactor to activate cellular processes selectively at the PM. In other words, it is much more signpost than signal. Under this model, PI(4,5)P2 is a ‘master regulator’ of all PM functions, i.e. it is a common factor required for each. Spatial and temporal regulation of these processes (beyond simple activation by PI(4,5)P2 at the PM) is then driven by the many other, disparate signaling mechanisms that regulate them individually.
If this is the case, why do diseases and experimental manipulations that cause aberrant PI(4,5)P2 levels lead to such diverse phenotypes? Why do they only effect limited subsets of PM function? We think a clue comes from studies of cytoskeletal regulation: the effective PI(4,5)P2 affinity of different actin- binding proteins varies by two orders of magnitude [102]. Under such a paradigm, moderate changes in lipid levels (such as those caused by the loss of an individual INPP5) are unlikely to affect high-affinity processes (in this case, anchoring of cortical actin by ERM proteins), whereas those with low affinities may be drastically altered (e.g. de novo actin polymerization). The extent to which this is true will require a more quantitative analysis of PI(4,5)P2 affinity by its effectors. This is a daunting challenge, given that effectors generally interact with each other and other modulators, generating very high avidity complexes in the cellular milieu. Nonetheless, probing changes in different PI(4,5)P2-regulated processes under conditions of pathological PM PI(4,5)P2 alterations could be transformative; it would illuminate the phenotypic specificity of diseases driven by them — a crucial first step in formulating any new clinical intervention.
In conclusion, there is a vast array of PM function regulated by PI(4,5)P2. We have attempted here to convey the breadth of this function, though we have by no means been exhaustive. A cursory glance of the timeline in Figure 1 shows that the discovery of functions associated with the lipid has been accelerating over the last decade. We would predict that we do not yet have a complete list of PI(4,5)P2-dependent functions. The future is full of surprises.
Perspectives
PI(4,5)P2 is an abundant and crucial regulator of PM function.
Originally discovered as a source of second messengers for the PLC and PI3K signaling pathways. Now, the intact lipid is known to be required for the activation of PM transport, signaling and structural functions in its own right.
Detailed mechanistic studies show how the lipid can recruit proteins to the membrane by stereo-specific or non-specific electrostatic interactions. Additionally, the lipid can induce allosteric activation of binding proteins.
A key question moving forward is how one class of molecule regulates so many parallel processes. Specifically, the extent to which this is controlled by localized PI(4,5)P2 signaling versus activation thresholds of individual processes for a plasma membrane wide PI(4,5)P2 concentration needs to be deduced. This will be crucial to understanding the causes of diseases resulting from aberrant PI(4,5)P2 metabolism.
Acknowledgements
In this relatively brief review of a vast literature, we apologize to the many colleagues whose excellent work we could not include; particularly, to our colleagues doing elegant work in model organisms, since we focused our writing on the mammalian literature. We are grateful to Morgan Ricci, Claire Weckerly and two anonymous reviewers for critical reading of the manuscript and insightful comments, which greatly improved the work.
Abbreviations
- aPKC
atypical protein kinase C
- CAPS
Ca2+-dependent activator protein for secretion
- CME
clathrin-mediated endocytosis
- CNGs
cyclic nucleotide- gated channels
- ECM
extracellular matrix
- ENaC
epithelial sodium channels
- ERM
ezrin, radixin, moesin
- E-Syts
extended synaptotagmins
- FAK
focal adhesion kinase
- Fas
focal adhesions
- GPCRs
G-protein-coupled receptors
- ICAMs
intracellular adhesion molecules
- NCX
sodium/calcium exchanger
- N-WASP
neuronal Wiskot–-Aldrich sSyndrome protein
- ORPs
oxysterol-binding protein-related proteins
- PI3Ks
phosphoinositide 3-kinases
- PLC
phospholipase C
- PM
plasma membrane
- SNAREs
soluble N-ethylmaleimide-sensitive factor attachment protein receptors
- TM
transmembrane
- TRP
transient receptor potential
- Arp2/3
actin- related protein 2/3
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
G.R.V.H. receives funding from the National Institutes of General Medical Sciences (2 R35 GM119412) and R.C.W. from the National Cancer Institute (5F31CA247349).
CRediT Author Contribution
Gerald R. V. Hammond: Writing — review and editing. Rachel C. Wills: Writing — review and editing.
References
- 1.Hokin, L.E. and Hokin, M.R. (1955) Effects of acetylcholine on the turnover of phosphoryl units in individual phospholipids of pancreas slices and brain cortex slices. Biochim. Biophys. Acta 18, 102–110 10.1016/0006-3002(55)90013-5 [DOI] [PubMed] [Google Scholar]
- 2.Saarikangas, J., Zhao, H. and Lappalainen, P. (2010) Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol. Rev. 90, 259–289 10.1152/physrev.00036.2009 [DOI] [PubMed] [Google Scholar]
- 3.Schink, K.O., Tan, K.-W. and Stenmark, H. (2015) Phosphoinositides in control of membrane dynamics. Annu. Rev. Cell Dev. Biol. 32, 1–29 10.1146/annurev-cellbio-111315-125349 [DOI] [PubMed] [Google Scholar]
- 4.Hammond, G.R. and Hong, Y. (2018) Phosphoinositides and membrane targeting in cell polarity. Cold Spring Harb. Perspect. Biol. 10, a027938 10.1101/cshperspect.a027938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickson, E.J. and Hille, B. (2019) Understanding phosphoinositides: rare, dynamic, and essential membrane phospholipids. Biochem. J. 476, 1–23 10.1042/BCJ20180022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammond, G.R.V. and Burke, J.E. (2020) Novel roles of phosphoinositides in signaling, lipid transport, and disease. Curr. Opin. Cell Biol. 63, 57–67 10.1016/j.ceb.2019.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li, S., Ghosh, C., Xing, Y. and Sun, Y. (2020) Phosphatidylinositol 4,5-bisphosphate in the control of membrane trafficking. Int. J. Biol. Sci. 16, 2761–2774 10.7150/ijbs.49665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irvine, R.F. (2003) 20 years of Ins(1,4,5)P3, and 40 years before. Nat. Rev. Mol. Cell Biol. 4, 586–590 10.1038/nrm1152 [DOI] [PubMed] [Google Scholar]
- 9.Balla, T. (2013) Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev. 93, 1019–1137 10.1152/physrev.00028.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barneda, D., Janardan, V., Niewczas, I., Collins, D.M., Cosulich, S., Clark, J.et al. (2022) Acyl chain selection couples the consumption and synthesis of phosphoinositides. EMBO J. 41, e110038 10.15252/embj.2021110038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, Y.J., Sengupta, N., Sohn, M., Mandal, A., Pemberton, J.G., Choi, U.et al. (2022) Metabolic routing maintains the unique fatty acid composition of phosphoinositides. EMBO Rep. 23, e54532 10.15252/embr.202154532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarkes, D. and Rameh, L.E. (2010) A novel HPLC-based approach makes possible the spatial characterization of cellular PtdIns5P and other phosphoinositides. Biochem. J. 428, 375–384 10.1042/bj20100129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watt, S.A., Kular, G., Fleming, I.N., Downes, C.P. and Lucocq, J.M. (2002) Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C δ1. Biochem. J. 363, 657–666 10.1042/bj3630657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammond, G.R.V., Schiavo, G. and Irvine, R.F. (2009) Immunocytochemical techniques reveal multiple, distinct cellular pools of PtdIns4P and PtdIns(4,5)P2. Biochem. J. 422, 23–35 10.1042/bj20090428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Várnai, P. and Balla, T. (1998) Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to Myo-[3H]inositol-labeled phosphoinositide pools. J. Cell Biol. 143, 501–510 10.1083/jcb.143.2.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santagata, S., Boggon, T.J., Baird, C.L., Gomez, C.A., Zhao, J., Shan, W.S.et al. (2001) G-protein signaling through tubby proteins. Science 292, 2041–2050 10.1126/science.1061233 [DOI] [PubMed] [Google Scholar]
- 17.Choi, S., Chen, M., Cryns, V.L. and Anderson, R.A. (2019) A nuclear phosphoinositide kinase complex regulates p53. Nat. Cell Biol. 21, 462–475 10.1038/s41556-019-0297-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravi, A., Palamiuc, L., Loughran, R.M., Triscott, J., Arora, G.K., Kumar, A.et al. (2021) PI5P4Ks drive metabolic homeostasis through peroxisome-mitochondria interplay. Dev. Cell 56, 1661–1676.e10 10.1016/j.devcel.2021.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willars, G.B., Nahorski, S.R. and Challiss, R.A.J. (1998) Differential regulation of muscarinic acetylcholine receptor-sensitive polyphosphoinositide pools and consequences for signaling in human neuroblastoma cells. J. Biol. Chem. 273, 5037–5046 10.1074/jbc.273.9.5037 [DOI] [PubMed] [Google Scholar]
- 20.Anderson, K.E., Kielkowska, A., Durrant, T.N., Juvin, V., Clark, J., Stephens, L.R.et al. (2013) Lysophosphatidylinositol-acyltransferase-1 (LPIAT1) Is required to maintain physiological levels of PtdIns and PtdInsP2 in the mouse. PLoS One 8, e58425 10.1371/journal.pone.0058425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Traynor-Kaplan, A., Kruse, M., Dickson, E.J., Dai, G., Vivas, O., Yu, H.et al. (2017) Fatty-acyl chain profiles of cellular phosphoinositides. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1862, 513–522 10.1016/j.bbalip.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vance, J.E. (2015) Phospholipid synthesis and transport in mammalian cells. Traffic 16, 1–18 10.1111/tra.12230 [DOI] [PubMed] [Google Scholar]
- 23.Zewe, J.P., Miller, A.M., Sangappa, S., Wills, R.C., Goulden, B.D. and Hammond, G.R.V. (2020) Probing the subcellular distribution of phosphatidylinositol reveals a surprising lack at the plasma membrane. J. Cell Biol. 219, e201906127 10.1083/jcb.201906127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrell, J.E. and Huestis, W.H. (1984) Phosphoinositide metabolism and the morphology of human erythrocytes. J. Cell Biol. 98, 1992–1998 10.1083/jcb.98.6.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu, C., Watras, J. and Loew, L.M. (2003) Kinetic analysis of receptor-activated phosphoinositide turnover. J. Cell Biol. 161, 779–791 10.1083/jcb.200301070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hilgemann, D.W. (2007) Local PIP2 signals: when, where, and how? Pflügers Archiv 455, 55–67 10.1007/s00424-007-0280-9 [DOI] [PubMed] [Google Scholar]
- 27.Griffiths, G., Back, R. and Marsh, M. (1989) A quantitative analysis of the endocytic pathway in baby hamster kidney cells. J. Cell Biol. 109, 2703–2720 10.1083/jcb.109.6.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffiths, G., Fuller, S.D., Back, R., Hollinshead, M., Pfeiffer, S. and Simons, K. (1989) The dynamic nature of the Golgi complex. J. Cell Biol. 108, 277–297 10.1083/jcb.108.2.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hein, M.Y., Hubner, N.C., Poser, I., Cox, J., Nagaraj, N., Toyoda, Y.et al. (2015) A human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell 163, 712–723 10.1016/j.cell.2015.09.053 [DOI] [PubMed] [Google Scholar]
- 30.Cho, N.H., Cheveralls, K.C., Brunner, A.-D., Kim, K., Michaelis, A.C., Raghavan, P.et al. (2022) Opencell: endogenous tagging for the cartography of human cellular organization. Science 375, eabi6983 10.1126/science.abi6983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michell, R.H. (1975) Inositol phospholipids and cell surface receptor function. Biochim. Biophys. Acta 415, 81–147 10.1016/0304-4157(75)90017-9 [DOI] [PubMed] [Google Scholar]
- 32.Streb, H., Irvine, R.F., Berridge, M.J. and Schulz, I. (1983) Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 306, 67–69 10.1038/306067a0 [DOI] [PubMed] [Google Scholar]
- 33.Katan, M. and Cockcroft, S. (2020) Phospholipase C families: common themes and versatility in physiology and pathology. Prog. Lipid Res. 80, 101065 10.1016/j.plipres.2020.101065 [DOI] [PubMed] [Google Scholar]
- 34.Muralidharan, K., Camp, M.M.V. and Lyon, A.M. (2021) Structure and regulation of phospholipase Cβ and ε at the membrane. Chem. Phys. Lipids 235, 105050 10.1016/j.chemphyslip.2021.105050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemmon, M.A., Ferguson, K.M., O'Brien, R., Sigler, P.B. and Schlessinger, J. (1995) Specific and high-affinity binding of inositol phosphates to an isolated pleckstrin homology domain. Proc. Natl Acad. Sci. U.S.A. 92, 10472–10476 10.1073/pnas.92.23.10472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferguson, K.M., Lemmon, M.A., Schlessinger, J. and Sigler, P.B. (1995) Structure of the high affinity complex of inositol trisphosphate with a phospholipase C pleckstrin homology domain. Cell 83, 1037–1046 10.1016/0092-8674(95)90219-8 [DOI] [PubMed] [Google Scholar]
- 37.Stephens, L.R., Hughes, K.T. and Irvine, R.F. (1991) Pathway of phosphatidylinositol(3,4,5)-trisphosphate synthesis in activated neutrophils. Nature 351, 33–39 10.1038/351033a0 [DOI] [PubMed] [Google Scholar]
- 38.Hawkins, P.T., Jackson, T.R. and Stephens, L.R. (1992) Platelet-derived growth factor stimulates synthesis of Ptdlns(3,4,5)P3 by activating a Ptdlns(4,5)P2 3-OH kinase. Nature 358, 157–159 10.1038/358157a0 [DOI] [PubMed] [Google Scholar]
- 39.Fruman, D.A., Chiu, H., Hopkins, B.D., Bagrodia, S., Cantley, L.C. and Abraham, R.T. (2017) The PI3K pathway in human disease. Cell 170, 605–635 10.1016/j.cell.2017.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanhaesebroeck, B., Perry, M.W.D., Brown, J.R., André, F. and Okkenhaug, K. (2021) PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov. 20, 741–769 10.1038/s41573-021-00209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeung, T., Terebiznik, M., Yu, L., Silvius, J., Abidi, W.M., Philips, M.et al. (2006) Receptor activation alters inner surface potential during phagocytosis. Science 313, 347–351 10.1126/science.1129551 [DOI] [PubMed] [Google Scholar]
- 42.Yeung, T., Gilbert, G.E., Shi, J., Silvius, J., Kapus, A. and Grinstein, S. (2008) Membrane phosphatidylserine regulates surface charge and protein localization. Science 319, 210–213 10.1126/science.1152066 [DOI] [PubMed] [Google Scholar]
- 43.Heo, W.D., Inoue, T., Park, W.S., Kim, M.L., Park, B.O., Wandless, T.J.et al. (2006) PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science 314, 1458–1461 10.1126/science.1134389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hammond, G.R.V., Fischer, M.J., Anderson, K.E., Holdich, J., Koteci, A., Balla, T.et al. (2012) PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science 337, 727–730 10.1126/science.1222483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yen, H.-Y., Hoi, K.K., Liko, I., Hedger, G., Horrell, M.R., Song, W.et al. (2018) Ptdins(4,5)P2 stabilizes active states of GPCRs and enhances selectivity of G-protein coupling. Nature 559, 423–427 10.1038/s41586-018-0325-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song, W., Duncan, A.L. and Sansom, M.S.P. (2021) Modulation of adenosine A2a receptor oligomerization by receptor activation and PIP2 interactions. Structure 29, 1312–1325.e3 10.1016/j.str.2021.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pierce, K.L. and Lefkowitz, R.J. (2001) Classical and new roles of β-arrestins in the regulation of G-PROTEIN-COUPLED receptors. Nat. Rev. Neurosci. 2, 727–733 10.1038/35094577 [DOI] [PubMed] [Google Scholar]
- 48.Huang, W., Masureel, M., Qianhui, Q., Janetzko, J., Inoue, A., Kato, H.E.et al. (2020) Structure of the neurotensin receptor 1 in complex with β-arrestin 1. Nature 579, 303–308 10.1038/s41586-020-1953-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janetzko, J., Kise, R., Barsi-Rhyne, B., Siepe, D.H., Heydenreich, F.M., Masureel, M.et al. (2022) Membrane phosphoinositides stabilize GPCR-arrestin complexes and provide temporal control of complex assembly and dynamics. Biorxiv 2021.10.09.463790 10.1101/2021.10.09.463790 [DOI] [Google Scholar]
- 50.Jung, S.-R., Jiang, Y., Seo, J.B., Chiu, D.T., Hille, B. and Koh, D.-S. (2021) β-arrestin–dependent PI(4,5)P2 synthesis boosts GPCR endocytosis. Proc. Natl Acad. Sci. U.S.A. 118, e2011023118 10.1073/pnas.2011023118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin, T. (2015) PI(4,5)P2-binding effector proteins for vesicle exocytosis. Biochim. Biophys. Acta 1851, 785–793 10.1016/j.bbalip.2014.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heider, M.R. and Munson, M. (2012) Exorcising the exocyst complex. Traffic 13, 898–907 10.1111/j.1600-0854.2012.01353.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mei, K. and Guo, W. (2018) The exocyst complex. Curr. Biol. 28, R922–R925 10.1016/j.cub.2018.06.042 [DOI] [PubMed] [Google Scholar]
- 54.Maib, H. and Murray, D.H. (2022) A mechanism for exocyst-mediated tethering via Arf6 and PIP5K1C-driven phosphoinositide conversion. Curr. Biol. 32, 2821–2833.e6 10.1016/j.cub.2022.04.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang, X., Orlando, K., He, B., Xi, F., Zhang, J., Zajac, A.et al. (2008) Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J. Cell Biol. 180, 145–158 10.1083/jcb.200704128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He, B., Xi, F., Zhang, X., Zhang, J. and Guo, W. (2007) Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J. 26, 4053–4065 10.1038/sj.emboj.7601834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hay, J.C., Fisette, P.L., Jenkins, G.H., Fukami, K., Takenawa, T., Anderson, R.A.et al. (1995) ATP-dependent inositide phosphorylation required for Ca2+-activated secretion. Nature 374, 173–177 10.1038/374173a0 [DOI] [PubMed] [Google Scholar]
- 58.Kabachinski, G., Yamaga, M., Kielar-Grevstad, D.M., Bruinsma, S. and Martin, T.F.J. (2014) CAPS and Munc13 utilize distinct PIP2-linked mechanisms to promote vesicle exocytosis. Mol. Biol. Cell 25, 508–521 10.1091/mbc.e12-11-0829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Keimpema, L., Kooistra, R., Toonen, R.F. and Verhage, M. (2017) CAPS-1 requires its C2, PH, MHD1 and DCV domains for dense core vesicle exocytosis in mammalian CNS neurons. Sci. Rep. 7, 10817 10.1038/s41598-017-10936-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van den Bogaart, G., Meyenberg, K., Risselada, H.J., Amin, H., Willig, K.I., Hubrich, B.E.et al. (2011) Membrane protein sequestering by ionic protein–lipid interactions. Nature 479, 552–555 10.1038/nature10545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonzalo, S. and Linder, M.E. (1998) SNAP-25 palmitoylation and plasma membrane targeting require a functional secretory pathway. Mol. Biol. Cell 9, 585–597 10.1091/mbc.9.3.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weber, P., Batoulis, H., Rink, K.M., Dahlhoff, S., Pinkwart, K., Söllner, T.H.et al. (2017) Electrostatic anchoring precedes stable membrane attachment of SNAP25/SNAP23 to the plasma membrane. eLife 6, e19394 10.7554/elife.19394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paolo, G.D., Moskowitz, H.S., Gipson, K., Wenk, M.R., Voronov, S., Obayashi, M.et al. (2004) Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature 431, 415–422 10.1038/nature02896 [DOI] [PubMed] [Google Scholar]
- 64.Gong, L.-W., Paolo, G.D., Diaz, E., Cestra, G., Diaz, M.-E., Lindau, M.et al. (2005) Phosphatidylinositol phosphate kinase type Iγ regulates dynamics of large dense-core vesicle fusion. Proc. Natl Acad. Sci. U.S.A. 102, 5204–5209 10.1073/pnas.0501412102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown, F.D., Rozelle, A.L., Yin, H.L., Balla, T. and Donaldson, J.G. (2001) Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J. Cell Biol. 154, 1007–1018 10.1083/jcb.200103107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boucrot, E., Ferreira, A.P.A., Almeida-Souza, L., Debard, S., Vallis, Y., Howard, G.et al. (2015) Endophilin marks and controls a clathrin-independent endocytic pathway. Nature 517, 460–465 10.1038/nature14067 [DOI] [PubMed] [Google Scholar]
- 67.Botelho, R.J., Teruel, M., Dierckman, R., Anderson, R., Wells, A., York, J.D.et al. (2000) Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J. Cell Biol. 151, 1353–1368 10.1083/jcb.151.7.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mettlen, M., Chen, P.-H., Srinivasan, S., Danuser, G. and Schmid, S.L. (2018) Regulation of clathrin-mediated endocytosis. Annu. Rev. Biochem. 87, 871–896 10.1146/annurev-biochem-062917-012644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beck, K.A. and Keen, J.H. (1991) Interaction of phosphoinositide cycle intermediates with the plasma membrane-associated clathrin assembly protein AP-2. J. Biol. Chem. 266, 4442–4447 10.1016/s0021-9258(20)64342-3 [DOI] [PubMed] [Google Scholar]
- 70.Itoh, T., Koshiba, S., Kigawa, T., Kikuchi, A., Yokoyama, S. and Takenawa, T. (2001) Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science 291, 1047–1051 10.1126/science.291.5506.1047 [DOI] [PubMed] [Google Scholar]
- 71.Ford, M.G.J., Pearse, B.M.F., Higgins, M.K., Vallis, Y., Owen, D.J., Gibson, A.et al. (2001) Simultaneous binding of ptdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291, 1051–1055 10.1126/science.291.5506.1051 [DOI] [PubMed] [Google Scholar]
- 72.Kelly, B.T., Graham, S.C., Liska, N., Dannhauser, P.N., Höning, S., Ungewickell, E.J.et al. (2014) AP2 controls clathrin polymerization with a membrane-activated switch. Science 345, 459–463 10.1126/science.1254836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zaccai, N.R., Kadlecova, Z., Dickson, V.K., Korobchevskaya, K., Kamenicky, J., Kovtun, O.et al. (2022) FCHO controls AP2's initiating role in endocytosis through a PtdIns(4,5)P2-dependent switch. Sci. Adv. 8, eabn2018 10.1126/sciadv.abn2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Achiriloaie, M., Barylko, B. and Albanesi, J.P. (1999) Essential role of the dynamin pleckstrin homology domain in receptor-mediated endocytosis. Mol. Cell. Biol. 19, 1410–1415 10.1128/mcb.19.2.1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wenk, M.R. and Camilli, P.D. (2004) Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc. Natl Acad. Sci. U.S.A. 101, 8262–8269 10.1073/pnas.0401874101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Varnai, P., Thyagarajan, B., Rohacs, T. and Balla, T. (2006) Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J. Cell Biol. 175, 377–382 10.1083/jcb.200607116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zoncu, R., Perera, R.M., Sebastian, R., Nakatsu, F., Chen, H., Balla, T.et al. (2007) Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc. Natl Acad. Sci. U.S.A. 104, 3793–3798 10.1073/pnas.0611733104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abe, N., Inoue, T., Galvez, T., Klein, L. and Meyer, T. (2008) Dissecting the role of PtdIns(4,5)P2 in endocytosis and recycling of the transferrin receptor. J. Cell Sci. 121, 1488–1494 10.1242/jcs.020792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jost, M., Simpson, F., Kavran, J.M., Lemmon, M.A. and Schmid, S.L. (1998) Phosphatidylinositol-4,5-bisphosphate is required for endocytic coated vesicle formation. Curr. Biol. 8, 1399–1404 10.1016/s0960-9822(98)00022-0 [DOI] [PubMed] [Google Scholar]
- 80.Cremona, O., Paolo, G.D., Wenk, M.R., Lüthi, A., Kim, W.T., Takei, K.et al. (1999) Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell 99, 179–188 10.1016/s0092-8674(00)81649-9 [DOI] [PubMed] [Google Scholar]
- 81.Nández, R., Balkin, D.M., Messa, M., Liang, L., Paradise, S., Czapla, H.et al. (2014) A role of OCRL in clathrin-coated pit dynamics and uncoating revealed by studies of Lowe syndrome cells. eLife 3, e02975 10.7554/elife.02975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.He, K., Marsland, III, R., Upadhyayula, S., Song, E., Dang, S., Capraro, B.R.et al. (2017) Dynamics of phosphoinositide conversion in clathrin-mediated endocytic traffic. Nature 552, 410–414 10.1038/nature25146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hilgemann, D.W. and Ball, R. (1996) Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science 273, 956–959 10.1126/science.273.5277.956 [DOI] [PubMed] [Google Scholar]
- 84.Anderluh, A., Hofmaier, T., Klotzsch, E., Kudlacek, O., Stockner, T., Sitte, H.H.et al. (2017) Direct PIP2 binding mediates stable oligomer formation of the serotonin transporter. Nat. Commun. 8, 14089 10.1038/ncomms14089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hamilton, P.J., Belovich, A.N., Khelashvili, G., Saunders, C., Erreger, K., Javitch, J.A.et al. (2014) PIP2 regulates psychostimulant behaviors through its interaction with a membrane protein. Nat. Chem. Biol. 10, 582–589 10.1038/nchembio.1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bandara, S., Malmersjö, S. and Meyer, T. (2013) Regulators of calcium homeostasis identified by inference of kinetic model parameters from live single cells perturbed by siRNA. Sci. Signal. 6, ra56 10.1126/scisignal.2003649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aharonovitz, O., Zaun, H.C., Balla, T., York, J.D., Orlowski, J. and Grinstein, S. (2000) Intracellular Ph regulation by Na+/H+ exchange requires phosphatidylinositol 4,5-bisphosphate. J. Cell Biol. 150, 213–224 10.1083/jcb.150.1.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hille, B., Dickson, E.J., Kruse, M., Vivas, O. and Suh, B.-C. (2015) Phosphoinositides regulate ion channels. Biochim. Biophys. Acta 1851, 844–856 10.1016/j.bbalip.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hansen, S.B., Tao, X. and MacKinnon, R. (2011) Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature 477, 495–498 10.1038/nature10370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hilgemann, D.W., Feng, S. and Nasuhoglu, C. (2001) The complex and intriguing lives of PIP2 with Ion channels and transporters. Sci. STKE 2001, re19 10.1126/stke.2001.111.re19 [DOI] [PubMed] [Google Scholar]
- 91.Lukacs, V., Yudin, Y., Hammond, G.R., Sharma, E., Fukami, K. and Rohacs, T. (2013) Distinctive changes in plasma membrane phosphoinositides underlie differential regulation of TRPV1 in nociceptive neurons. J. Neurosci. 33, 11451–11463 10.1523/jneurosci.5637-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suh, B.-C. and Hille, B. (2002) Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron 35, 507–520 10.1016/s0896-6273(02)00790-0 [DOI] [PubMed] [Google Scholar]
- 93.Huang, J., Liu, C.-H., Hughes, S.A., Postma, M., Schwiening, C.J. and Hardie, R.C. (2010) Activation of TRP channels by protons and phosphoinositide depletion in drosophila photoreceptors. Curr. Biol. 20, 189–197 10.1016/j.cub.2009.12.019 [DOI] [PubMed] [Google Scholar]
- 94.Sengupta, S., Barber, T.R., Xia, H., Ready, D.F. and Hardie, R.C. (2013) Depletion of PtdIns(4,5)P2 underlies retinal degeneration in Drosophila trp mutants. J. Cell Sci. 126, 1247–1259 10.1242/jcs.120592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mason, D., Mallo, G.V., Terebiznik, M.R., Payrastre, B., Finlay, B.B., Brumell, J.H.et al. (2007) Alteration of epithelial structure and function associated with PtdIns(4,5)P2 degradation by a bacterial phosphatase. J. Gen. Physiol. 129, 267–283 10.1085/jgp.200609656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bear, J.E., Krause, M. and Gertler, F.B. (2001) Regulating cellular actin assembly. Curr. Opin. Cell Biol. 13, 158–166 10.1016/s0955-0674(00)00193-9 [DOI] [PubMed] [Google Scholar]
- 97.Lassing, I. and Lindberg, U. (1985) Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature 314, 472–474 10.1038/314472a0 [DOI] [PubMed] [Google Scholar]
- 98.Janmey, P.A. and Stossel, T.P. (1987) Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature 325, 362–364 10.1038/325362a0 [DOI] [PubMed] [Google Scholar]
- 99.Gorbatyuk, V.Y., Nosworthy, N.J., Robson, S.A., Bains, N.P.S., Maciejewski, M.W., dos Remedios, C.G.et al. (2006) Mapping the phosphoinositide-binding site on chick cofilin explains how PIP2 regulates the cofilin-actin interaction. Mol. Cell 24, 511–522 10.1016/j.molcel.2006.10.007 [DOI] [PubMed] [Google Scholar]
- 100.Zhao, H., Hakala, M. and Lappalainen, P. (2010) ADF/cofilin binds phosphoinositides in a multivalent manner to act as a PIP2-density sensor. Biophys. J. 98, 2327–2336 10.1016/j.bpj.2010.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van Rheenen, J., Song, X., van Roosmalen, W., Cammer, M., Chen, X., DesMarais, V.et al. (2007) EGF-induced PIP2 hydrolysis releases and activates cofilin locally in carcinoma cells. J. Cell Biol. 179, 1247–1259 10.1083/jcb.200706206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Senju, Y., Kalimeri, M., Koskela, E.V., Somerharju, P., Zhao, H., Vattulainen, I.et al. (2017) Mechanistic principles underlying regulation of the actin cytoskeleton by phosphoinositides. Proc. Natl Acad. Sci. U.S.A. 114, E8977–E8986 10.1073/pnas.1705032114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heiss, S.G. and Cooper, J.A. (1991) Regulation of CapZ, an actin capping protein of chicken muscle, by anionic phospholipids. Biochemistry 30, 8753–8758 10.1021/bi00100a006 [DOI] [PubMed] [Google Scholar]
- 104.Solís, C. and Russell, B. (2019) Capz integrates several signaling pathways in response to mechanical stiffness. J. Gen. Physiol. 151, 660–669 10.1085/jgp.201812199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hartman, T.J., Martin, J.L., Solaro, R.J., Samarel, A.M. and Russell, B. (2009) Capz dynamics are altered by endothelin-1 and phenylephrine via PIP2- and PKC-dependent mechanisms. Am. J. Physiol. Cell Physiol. 296, C1034–C1039 10.1152/ajpcell.00544.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Papayannopoulos, V., Co, C., Prehoda, K.E., Snapper, S., Taunton, J. and Lim, W.A. (2005) A polybasic motif allows N-WASP to act as a sensor of PIP2 density. Mol. Cell 17, 181–191 10.1016/j.molcel.2004.11.054 [DOI] [PubMed] [Google Scholar]
- 107.Higgs, H.N. and Pollard, T.D. (2000) Activation by Cdc42 and Pip2 of Wiskott–Aldrich syndrome protein (Wasp) stimulates actin nucleation by Arp2/3 complex. J. Cell Biol. 150, 1311–1320 10.1083/jcb.150.6.1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Higgs, H. and Pollard, T. (2001) Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem. 70, 649–676 10.1146/annurev.biochem.70.1.649 [DOI] [PubMed] [Google Scholar]
- 109.Helgeson, L.A. and Nolen, B.J. (2013) Mechanism of synergistic activation of Arp2/3 complex by cortactin and N-WASP. eLife 2, e00884 10.7554/elife.00884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smith, B.A., Padrick, S.B., Doolittle, L.K., Daugherty-Clarke, K., Corrêa, I.R., Xu, M.-Q.et al. (2013) Three-color single molecule imaging shows WASP detachment from Arp2/3 complex triggers actin filament branch formation. eLife 2, e01008 10.7554/elife.01008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anderson, R.A. and Marchesi, V.T. (1985) Regulation of the association of membrane skeletal protein 4.1 with glycophorin by a polyphosphoinositide. Nature 318, 295–298 10.1038/318295a0 [DOI] [PubMed] [Google Scholar]
- 112.Moore, D.T., Nygren, P., Jo, H., Boesze-Battaglia, K., Bennett, J.S. and DeGrado, W.F. (2012) Affinity of talin-1 for the β3-integrin cytosolic domain is modulated by its phospholipid bilayer environment. Proc. Natl Acad. Sci. U.S.A 109, 793–798 10.1073/pnas.1117220108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Song, X., Yang, J., Hirbawi, J., Ye, S., Perera, H.D., Goksoy, E.et al. (2012) A novel membrane-dependent on/off switch mechanism of talin FERM domain at sites of cell adhesion. Cell Res. 22, 1533–1545 10.1038/cr.2012.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hao, J.-J., Liu, Y., Kruhlak, M., Debell, K.E., Rellahan, B.L. and Shaw, S. (2009) Phospholipase C-mediated hydrolysis of PIP2 releases ERM proteins from lymphocyte membrane. J. Cell Biol. 184, 451–462 10.1083/jcb.200807047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hamada, K., Shimizu, T., Matsui, T., Tsukita, S., Tsukita, S. and Hakoshima, T. (2000) Structural basis of the membrane-targeting and unmasking mechanisms of the radixin FERM domain. EMBO J. 19, 4449–4462 10.1093/emboj/19.17.4449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Valadares, N.F., d' Muniz Pereira, H., Araujo, A.P.U. and Garratt, R.C. (2017) Septin structure and filament assembly. Biophys. Rev. 9, 481–500 10.1007/s12551-017-0320-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bertin, A., McMurray, M.A., Thai, L., Garcia, G., Votin, V., Grob, P.et al. (2010) Phosphatidylinositol-4,5- bisphosphate promotes budding yeast septin filament assembly and organization. J. Mol. Biol. 404, 711–731 10.1016/j.jmb.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Machnicka, B., Czogalla, A., Hryniewicz-Jankowska, A., Bogusławska, D.M., Grochowalska, R., Heger, E.et al. (2014) Spectrins: a structural platform for stabilization and activation of membrane channels, receptors and transporters. Biochim. Biophys. Acta 1838, 620–634 10.1016/j.bbamem.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 119.Zhang, P., Talluri, S., Deng, H., Branton, D. and Wagner, G. (1995) Solution structure of the pleckstrin homology domain of Drosophila β-spectrin. Structure 3, 1185–1195 10.1016/s0969-2126(01)00254-4 [DOI] [PubMed] [Google Scholar]
- 120.Liem, R.K.H. (2016) Cytoskeletal integrators: the spectrin superfamily. Cold Spring Harb. Perspect. Biol. 8, a018259 10.1101/cshperspect.a018259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kanchanawong, P., Shtengel, G., Pasapera, A.M., Ramko, E.B., Davidson, M.W., Hess, H.F.et al. (2010) Nanoscale architecture of integrin-based cell adhesions. Nature 468, 580–584 10.1038/nature09621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goñi, G.M., Epifano, C., Boskovic, J., Camacho-Artacho, M., Zhou, J., Bronowska, A.et al. (2014) Phosphatidylinositol 4,5-bisphosphate triggers activation of focal adhesion kinase by inducing clustering and conformational changes. Proc. Natl Acad. Sci. U.S.A. 111, E3177–E3186 10.1073/pnas.1317022111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cai, X., Lietha, D., Ceccarelli, D.F., Karginov, A.V., Rajfur, Z., Jacobson, K.et al. (2008) Spatial and temporal regulation of focal adhesion kinase activity in living cells. Mol. Cell. Biol. 28, 201–214 10.1128/mcb.01324-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gilmore, A.P. and Burridge, K. (1996) Regulation of vinculin binding to talin and actin by phosphatidyl-inositol-4-5-bisphosphate. Nature 381, 531–535 10.1038/381531a0 [DOI] [PubMed] [Google Scholar]
- 125.Orłowski, A., Kukkurainen, S., Pöyry, A., Rissanen, S., Vattulainen, I., Hytönen, V.P.et al. (2015) PIP2 and talin join forces to activate integrin. J. Phys. Chem. B 119, 12381–12389 10.1021/acs.jpcb.5b06457 [DOI] [PubMed] [Google Scholar]
- 126.Chinthalapudi, K., Rangarajan, E.S. and Izard, T. (2018) The interaction of talin with the cell membrane is essential for integrin activation and focal adhesion formation. Proc. Natl Acad. Sci. U.S.A. 115, 10339–10344 10.1073/pnas.1806275115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fukami, K., Endo, T., Imamura, M. and Takenawa, T. (1994) Alpha-actinin and vinculin are PIP2-binding proteins involved in signaling by tyrosine kinase. J. Biol. Chem. 269, 1518–1522 10.1016/S0021-9258(17)42287-3 [DOI] [PubMed] [Google Scholar]
- 128.Thompson, P.M., Ramachandran, S., Case, L.B., Tolbert, C.E., Tandon, A., Pershad, M.et al. (2017) A structural model for vinculin insertion into PIP2-containing membranes and the effect of insertion on vinculin activation and localization. Structure 25, 264–275 10.1016/j.str.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Izard, T. and Brown, D.T. (2016) Mechanisms and functions of vinculin interactions with phospholipids at cell adhesion sites. J. Biol. Chem. 291, 2548–2555 10.1074/jbc.R115.686493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chinthalapudi, K., Rangarajan, E.S., Patil, D.N., George, E.M., Brown, D.T. and Izard, T. (2014) Lipid binding promotes oligomerization and focal adhesion activity of vinculin. J. Cell Biol. 207, 643–656 10.1083/jcb.201404128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kunz, J., Wilson, M.P., Kisseleva, M., Hurley, J.H., Majerus, P.W. and Anderson, R.A. (2000) The activation loop of phosphatidylinositol phosphate kinases determines signaling specificity. Mol. Cell 5, 1–11 10.1016/s1097-2765(00)80398-6 [DOI] [PubMed] [Google Scholar]
- 132.Kunz, J., Fuelling, A., Kolbe, L. and Anderson, R.A. (2002) Stereo-specific substrate recognition by phosphatidylinositol phosphate kinases is swapped by changing a single amino acid residue. J. Biol. Chem. 277, 5611–5619 10.1074/jbc.M110775200 [DOI] [PubMed] [Google Scholar]
- 133.Ishihara, H., Shibasaki, Y., Kizuki, N., Wada, T., Yazaki, Y., Asano, T.et al. (1998) Type I phosphatidylinositol-4-phosphate 5-kinases. Cloning of the third isoform and deletion/substitution analysis of members of this novel lipid kinase family. J. Biol. Chem. 273, 8741–8748 10.1074/jbc.273.15.8741 [DOI] [PubMed] [Google Scholar]
- 134.Yamaguchi, H., Yoshida, S., Muroi, E., Kawamura, M., Kouchi, Z., Nakamura, Y.et al. (2010) Phosphatidylinositol 4,5-bisphosphate and PIP5-kinase Iα are required for invadopodia formation in human breast cancer cells. Cancer Sci. 101, 1632–1638 10.1111/j.1349-7006.2010.01574.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vicinanza, M., Campli, A.D., Polishchuk, E., Santoro, M., Tullio, G.D., Godi, A.et al. (2011) OCRL controls trafficking through early endosomes via PtdIns4,5P2-dependent regulation of endosomal actin. EMBO J. 30, 4970–4985 10.1038/emboj.2011.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Raucher, D., Stauffer, T., Chen, W., Shen, K., Guo, S., York, J.D.et al. (2000) Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton–plasma membrane adhesion. Cell 100, 221–228 10.1016/s0092-8674(00)81560-3 [DOI] [PubMed] [Google Scholar]
- 137.Terebiznik, M.R., Vieira, O.V., Marcus, S.L., Slade, A., Yip, C.M., Trimble, W.S.et al. (2002) Elimination of host cell PtdIns(4,5)P2 by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat. Cell Biol. 4, 766–773 10.1038/ncb854 [DOI] [PubMed] [Google Scholar]
- 138.Prinz, W.A., Toulmay, A. and Balla, T. (2020) The functional universe of membrane contact sites. Nat. Rev. Mol. Cell Biol. 21, 7–24 10.1038/s41580-019-0180-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Giordano, F., Saheki, Y., Idevall-Hagren, O., Colombo, S.F., Pirruccello, M., Milosevic, I.et al. (2013) PI(4,5)P2-dependent and Ca2+-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell 153, 1494–1509 10.1016/j.cell.2013.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ghai, R., Du, X., Wang, H., Dong, J., Ferguson, C., Brown, A.J.et al. (2017) ORP5 and ORP8 bind phosphatidylinositol-4, 5-biphosphate (PtdIns(4,5)P2) and regulate its level at the plasma membrane. Nat. Commun. 8, 757 10.1038/s41467-017-00861-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sohn, M., Korzeniowski, M., Zewe, J.P., Wills, R.C., Hammond, G.R.V., Humpolickova, J.et al. (2018) PI(4,5)P2 controls plasma membrane PI4P and PS levels via ORP5/8 recruitment to ER–PM contact sites. J. Cell Biol. 217, 1797–1813 10.1083/jcb.201710095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Besprozvannaya, M., Dickson, E., Li, H., Ginburg, K.S., Bers, D.M., Auwerx, J.et al. (2018) GRAM domain proteins specialize functionally distinct ER-PM contact sites in human cells. eLife 7, e31019 10.7554/elife.31019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Walsh, C.M., Chvanov, M., Haynes, L.P., Petersen, O.H., Tepikin, A.V. and Burgoyne, R.D. (2010) Role of phosphoinositides in STIM1 dynamics and store-operated calcium entry. Biochem. J. 425, 159–168 10.1042/bj20090884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dong, W., Zhang, X., Liu, W., Chen, Y., Huang, J., Austin, E.et al. (2015) A conserved polybasic domain mediates plasma membrane targeting of Lgl and its regulation by hypoxiaMolecular mechanisms of Lgl membrane targeting. J. Cell Biol. 211, 273–286 10.1083/jcb.201503067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bailey, M.J. and Prehoda, K.E. (2015) Establishment of par-polarized cortical domains via phosphoregulated membrane motifs. Dev. Cell 35, 199–210 10.1016/j.devcel.2015.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dong, W., Lu, J., Zhang, X., Wu, Y., Lettieri, K., Hammond, G.R.et al. (2020) A polybasic domain in aPKC mediates Par6-dependent control of membrane targeting and kinase activity. J. Cell Biol. 219, e201903031 10.1083/jcb.201903031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Volpicelli-Daley, L.A., Lucast, L., Gong, L.-W., Liu, L., Sasaki, J., Sasaki, T.et al. (2010) Phosphatidylinositol-4-phosphate 5-kinases and phosphatidylinositol 4,5-bisphosphate synthesis in the brain. J. Biol. Chem. 285, 28708–28714 10.1074/jbc.m110.132191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Narkis, G., Ofir, R., Landau, D., Manor, E., Volokita, M., Hershkowitz, R.et al. (2007) Lethal contractural syndrome type 3 (LCCS3) is caused by a mutation in PIP5K1C, which encodes PIPKIγ of the phophatidylinsitol pathway. Am. J. Hum. Genet. 81, 530–539 10.1086/520771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Legate, K.R., Montag, D., Böttcher, R.T., Takahashi, S. and Fässler, R. (2012) Comparative phenotypic analysis of the two major splice isoforms of phosphatidylinositol phosphate kinase type Iγ in vivo. J. Cell Sci. 125, 5636–5646 10.1242/jcs.102145 [DOI] [PubMed] [Google Scholar]
- 150.Zhang, X., Jefferson, A.B., Auethavekiat, V. and Majerus, P.W. (1995) The protein deficient in Lowe syndrome is a phosphatidylinositol-4,5-bisphosphate 5-phosphatase. Proc. Natl Acad. Sci. U.S.A. 92, 4853–4856 10.1073/pnas.92.11.4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bielas, S.L., Silhavy, J.L., Brancati, F., Kisseleva, M.V., Al-Gazali, L., Sztriha, L.et al. (2009) Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat. Genet. 41, 1032–1036 10.1038/ng.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Osborn, D.P.S., Pond, H.L., Mazaheri, N., Dejardin, J., Munn, C.J., Mushref, K.et al. (2017) Mutations in INPP5K cause a form of congenital muscular dystrophy overlapping Marinesco-Sjögren syndrome and dystroglycanopathy. Am. J. Hum. Genet. 100, 537–545 10.1016/j.ajhg.2017.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wiessner, M., Roos, A., Munn, C.J., Viswanathan, R., Whyte, T., Cox, D.et al. (2017) Mutations in INPP5K, encoding a phosphoinositide 5-phosphatase, cause congenital muscular dystrophy with cataracts and mild cognitive impairment. Am. J. Hum. Genet. 100, 523–536 10.1016/j.ajhg.2017.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Krebs, C.E., Karkheiran, S., Powell, J.C., Cao, M., Makarov, V., Darvish, H.et al. (2013) The Sac1 domain of SYNJ1 identified mutated in a family with early-onset progressive parkinsonism with generalized seizures. Hum. Mutat. 34, 1200–1207 10.1002/humu.22372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hardies, K., Cai, Y., Jardel, C., Jansen, A.C., Cao, M., May, P.et al. (2016) Loss of SYNJ1 dual phosphatase activity leads to early onset refractory seizures and progressive neurological decline. Brain 139, 2420–2430 10.1093/brain/aww180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hammond, G.R.V. (2016) Does PtdIns(4,5)P2 concentrate so it can multi-task? Biochem. Soc. Trans. 44, 228–233 10.1042/bst20150211 [DOI] [PubMed] [Google Scholar]
- 157.Hammond, G.R.V., Sim, Y., Lagnado, L. and Irvine, R.F. (2009) Reversible binding and rapid diffusion of proteins in complex with inositol lipids serves to coordinate free movement with spatial information. J. Cell Biol. 184, 297–308 10.1083/jcb.200809073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Halaszovich, C.R., Schreiber, D.N. and Oliver, D. (2009) Ci-VSP is a depolarization-activated phosphatidylinositol-4,5-bisphosphate and phosphatidylinositol-3,4,5-trisphosphate 5′-phosphatase. J. Biol. Chem. 284, 2106–2113 10.1074/jbc.m803543200 [DOI] [PubMed] [Google Scholar]
- 159.Mashanov, G.I. and Molloy, J.E. (2007) Automatic detection of single fluorophores in live cells. Biophys. J. 92, 2199–2211 10.1529/biophysj.106.081117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yaradanakul, A. and Hilgemann, D.W. (2007) Unrestricted diffusion of exogenous and endogenous PIP2 in baby hamster kidney and Chinese hamster ovary cell plasmalemma. J. Membr. Biol. 220, 53–67 10.1007/s00232-007-9074-4 [DOI] [PubMed] [Google Scholar]
- 161.Golebiewska, U., Nyako, M., Woturski, W., Zaitseva, I. and McLaughlin, S. (2008) Diffusion coefficient of fluorescent phosphatidylinositol 4,5-bisphosphate in the plasma membrane of cells. Mol. Biol. Cell 19, 1663–1669 10.1091/mbc.e07-12-1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pacheco, J., Cassidy, A.C., Zewe, J.P., Wills, R.C. and Hammond, G.R.V. (2022) Free diffusion of PI(4,5)P2 in the plasma membrane in the presence of high density effector protein complexes. Biorxiv 2022.01.07.475414 10.1101/2022.01.07.475414 [DOI] [Google Scholar]
- 163.Andrade, D.M., Clausen, M.P., Keller, J., Mueller, V., Wu, C., Bear, J.E.et al. (2015) Cortical actin networks induce spatio-temporal confinement of phospholipids in the plasma membrane – a minimally invasive investigation by STED-FCS. Sci. Rep. 5, 11454 10.1038/srep11454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fujiwara, T.K., Iwasawa, K., Kalay, Z., Tsunoyama, T.A., Watanabe, Y., Umemura, Y.M.et al. (2016) Confined diffusion of transmembrane proteins and lipids induced by the same actin meshwork lining the plasma membrane. Mol. Biol. Cell 27, 1101–1119 10.1091/mbc.e15-04-0186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Teruel, M.N. and Meyer, T. (2000) Translocation and reversible localization of signaling proteins A dynamic future for signal transduction. Cell 103, 181–184 10.1016/s0092-8674(00)00109-4 [DOI] [PubMed] [Google Scholar]
- 166.Golebiewska, U., Gambhir, A., Hangyás-Mihályné, G., Zaitseva, I., Rädler, J. and McLaughlin, S. (2006) Membrane-bound basic peptides sequester multivalent (PIP2), but not monovalent (PS), acidic lipids. Biophys. J. 91, 588–599 10.1529/biophysj.106.081562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wen, Y., Vogt, V.M. and Feigenson, G.W. (2018) Multivalent cation-bridged PI(4,5)P2 clusters form at very low concentrations. Biophys. J. 114, 2630–2639 10.1016/j.bpj.2018.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wen, Y., Vogt, V.M. and Feigenson, G.W. (2021) PI(4,5)P2 clustering and its impact on biological functions. Annu. Rev. Biochem. 90, 1–27 10.1146/annurev-biochem-070920-094827 [DOI] [PubMed] [Google Scholar]
- 169.Ji, C., Zhang, Y., Xu, P., Xu, T. and Lou, X. (2015) Nanoscale landscape of phosphoinositides revealed by specific pleckstrin homology (PH) domains using single-molecule superresolution imaging in the plasma membrane. J. Biol. Chem. 290, 26978–26993 10.1074/jbc.m115.663013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.van Rheenen, J., Achame, E.M., Janssen, H., Calafat, J. and Jalink, K. (2005) PIP2 signaling in lipid domains: a critical re-evaluation. EMBO J. 24, 1664–1673 10.1038/sj.emboj.7600655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Omar-Hmeadi, M., Gandasi, N.R. and Barg, S. (2018) Ptdins(4,5)P2 is not required for secretory granule docking. Traffic 19, 436–445 10.1111/tra.12562 [DOI] [PubMed] [Google Scholar]