Abstract
Apoptosis and necroptosis regulate many aspects of organismal biology and are involved in various human diseases. TNF is well known to induce both of these forms of cell death and the underlying mechanisms have been elaborately described. However, cells can also engage apoptosis and necroptosis through TNF-independent mechanisms, involving, for example, activation of the pattern recognition receptors Toll-like receptor (TLR)-3 and -4, or zDNA-binding protein 1 (ZBP1). In this context, cell death signaling depends on the presence of receptor-interacting serine/threonine protein kinase 3 (RIPK3). Whereas RIPK3 is required for TNF-induced necroptosis, it mediates both apoptosis and necroptosis upon TLR3/4 and ZBP1 engagement. Here, we review the intricate mechanisms by which TNF-independent cell death is regulated by RIPK3.
Keywords: apoptosis, cell death, immunology, inflammation, necroptosis, RIPK3
Introduction
Regulated cell death is a biological process with essential functions in organismal development, tissue homeostasis, and immune defense. Regulated cell death comes in many forms [1], the best-known being apoptosis, a non-lytic type of cell death. Lytic cell death, known as necrosis, was believed to be unregulated, often resulting from an insurmountable cellular insult. However, over the last two decades it has become clear that necrosis can also be regulated [2], and several forms of regulated necrosis have been described [1]. These include necroptosis [3], ferroptosis [4], NETosis [5], parthanatos [6], pyroptosis [7], and cuproptosis [8], which are all molecularly regulated by distinct intracellular mechanisms.
Necroptosis is a form of regulated necrosis that is mediated by the kinase Receptor Interacting serine/threonine-Protein Kinase-3 (RIPK3) which phosphorylates and thereby activates the pseudokinase Mixed Lineage Kinase-Like (MLKL) to execute cell death [9–16]. Originally, necroptosis was identified to be a consequence of tumor necrosis factor receptor (TNFR)-1 signaling [17,18]. Engagement of the TNFR1 by TNF leads to the formation of a large TNFR1-associated signaling complex (complex I) that functions to induce gene expression, mainly by activating nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling [19]. One component of this complex is RIPK1 [20], a protein that contains an N-terminal kinase domain (KD) required to mediate TNFR1-induced necroptosis, an intermediate domain that is involved in NF-κB signaling and harbors a RIP homotypic interaction motif (RHIM) that allows for the interaction with other RHIM-containing proteins [21], and a C-terminal death domain (DD) which facilitates its binding to TNFR1 [22]. RIPK1 is ubiquitylated by Cellular Inhibitors of Apoptosis Proteins (cIAPs) that are also present in the TNFR1-induced signaling complex [23], resulting in the formation of a complex that leads to NF-κB signaling [24–26]. However, when RIPK1 is deubiquitylated, i.e. as a result of dysfunctional cIAPs [24,27] it releases from the complex and can engage two forms of cell death, apoptosis [23,28] and necroptosis [17,18]. The DD of RIPK1 can associate with the DD of FAS Associated Death Domain (FADD) (complex IIa), which leads to the recruitment of the serine protease Caspase-8, the initiator caspase of extrinsic apoptosis [23,29]. Humans express the Caspase-8 paralogue Caspase-10, which can also interact with FADD upon activation of the death receptors Fas (CD95) or tumor-necrosis factor related apoptosis-inducing ligand (TRAIL) receptor and TNFR1 [30–32], although both enzymes may also have distinct functions [33]. However, since Caspase-10 is absent from rodents it has not been a major focus of investigation.
RIPK1 can also associate with the necroptosis-mediating protein RIPK3 [9,10,15]. Apart from the presence of a C-terminal DD in RIPK1, RIPK3 and RIPK1 are structurally similar, and both contain a kinase domain and RHIM. Homotypic RHIM:RHIM interactions between these proteins leads to auto-phosphorylation and activation of RIPK3 (complex IIb) [9,34]. RIPK3 subsequently oligomerizes into amyloid-like structures [34,35] and recruits and phosphorylates MLKL [13,14]. Activated MLKL then undergoes a conformational change, oligomerizes and localizes to membranes (including the plasma membrane), where it mediates membrane rupture and cell death known as necroptosis [11,12,14,36,37].
TNFR1-induced cell death is strictly regulated. TNFR1 ligation primarily induces NF-κB signaling, which leads to the expression of survival proteins including cellular FLICE (FADD-like IL-1β-converting enzyme)-Inhibitory Protein (cFLIP), an enzymatically inactive Caspase-8-like protein that regulates cell death [38]. Humans express three cFLIP isoforms, long (cFLIPL) and two short isoforms named cFLIP short (cFLIPS) and cFLIP raji (cFLIPR), whereas rodents only express cFLIPL and cFLIPR [39]. All isoforms contain two death effector domains (DED), which mediate binding to Caspase-8 via DED interactions. Heterodimer formation of Caspase-8 and either cFLIP isoform results in the inhibition of apoptosis since Caspase-8 is unable to form fully matured dimers that are able to cleave (and thereby activate) apoptosis effector proteins [40,41]. However, cFLIPL-Caspase-8 heterodimers retain proteolytic activity, although substrate specificity between Caspase-8 homodimers and cFLIPL-Caspase-8 heterodimers appear to slightly differ [42]. Upon formation of complex IIa, Caspase-8 can cleave RIPK1 [43–47] and RIPK3 [48] to prevent necroptosis [49]. Caspase-8 can also cleave CYLD to prevent RIPK1 de-ubiquitylation and cell death [50]. Inhibition of Caspase-8 activity, pharmacologically [51] or upon infection by certain pathogens [52], therefore promotes TNFR1-induced necroptosis. Mice and humans with mutations in the Caspase-8 cleavage site in RIPK1 display embryonic lethality (mice) [44,46] or severe neonatal inflammation (humans) [53]. Engagement of TNFR1 thus primarily results in the activation of (pro-survival) gene expression pathways, but under certain circumstances can result in apoptotic or necroptotic cell death.
The mechanisms of TNFR1-induced apoptosis and necroptosis have been well described (Figure 1). However, these cell death pathways can also be engaged by other, TNF-independent signaling cascades (Figure 1). Engagement of the adapter TIR-domain-containing adapter-inducing interferon-β (TRIF) by Toll-like receptor (TLR) 3 and 4, or the induction of the intracellular receptor zDNA-binding protein-1 (ZBP1, also known as DAI and DLM-1) in response to interferons can result in apoptosis and necroptosis, both of which depend on the presence of RIPK3. In this review, we focus on TNF-independent apoptotic and necroptotic cell death mechanisms in which RIPK3 plays a central role.
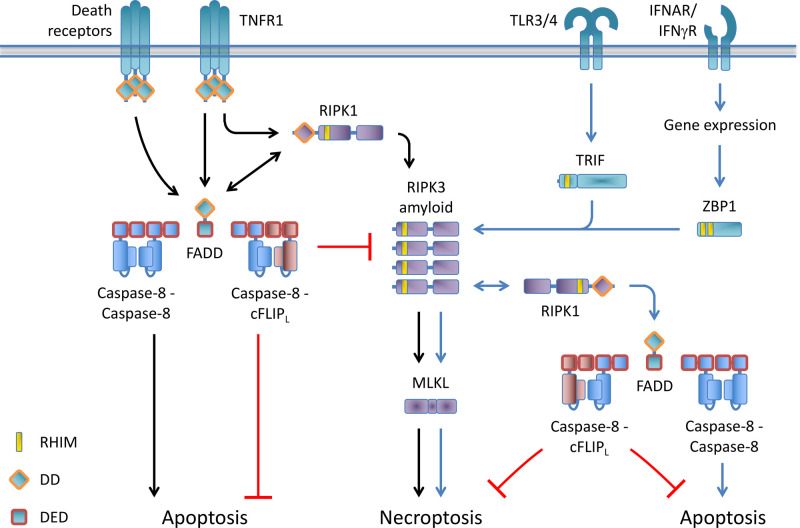
Figure 1. Pathways leading to extrinsic apoptosis and necroptosis.
Upon engagement of death receptors such as TRAIL-R, CD95, and TNFR1, FADD recruits caspase-8 and cFLIP to regulate apoptosis. Caspase-8 homodimerization mediates apoptosis, whereas caspase-8 — cFLIP heterodimerization impairs apoptosis. TNFR1 engagement also results in RIPK1-mediated cell death. RIPK1 can engage with FADD to regulate apoptosis and RIPK3 to mediate necroptosis. RIPK1 — RIPK3 activation is controlled by the FADD — caspase-8 — cFLIP complex. Activation of TRIF-dependent TLRs or ZBP1 mediates cell death through association with RIPK3 and RIPK1. RIPK3 recruits MLKL to mediate necroptosis, which is controlled by the RIPK1 — FADD — caspase-8 — cFLIP complex. RIPK3 can mediate apoptotic signals to FADD — caspase-8. Type I and type II interferons induce the gene expression of ZBP1. Black arrows indicate death receptor-mediated pathways. Blue arrows indicate non-death receptor-mediated pathways.
RIPK3 can mediate death independently of RIPK1
TNFR1-induced necroptosis requires RIPK1 to engage RIPK3 (see above). Although resistant to TNF-induced necroptosis, ripk1-deficient cells show hyper-susceptibility to cell death induced by Poly(IC) (TLR3 ligand) or type I and type II interferons [54]. Moreover, these cells can undergo RIPK3-dependent cell death upon treatment with lipopolysaccharide (LPS, TLR4 agonist) [55], oxidized LDL (which is recognized by TLR4) [56], or upon certain viral infections [57,58]. In this setting, cell death is dependent on the presence of RIPK3 and mainly activates necroptosis. Cell death thus can ensue upon non-TNF-mediated activation of RIPK3. RIPK1, enigmatically, promotes TNF-induced necroptosis but prevents death when necroptosis is induced via alternate mechanisms [22] (Figure 1).
Ripk1-deficient mice die within a few days after birth [59]. Co-ablation of ripk3 partially extends the life of ripk1−/− mice to ∼1 week, and these animals show apoptotic lesions in their intestinal tissue [54]. Co-ablation of fadd or casp8 does not prevent the perinatal lethality of ripk1−/− mice. However, the combinatory ablation of FADD-Caspase-8-mediated apoptosis and RIPK3-MLKL-mediated necroptosis prevents organismal death, and the resulting animals reach adulthood [54,60]. Although RIPK1 kinase-deficient (ripk1K45A) mice are viable, mice in which the RHIM of RIPK1 is rendered dysfunctional (ripk1mRHIM) die in a similar fashion to ripk1−/− mice, and display signs of necroptosis in various tissues [61,62]. Unlike ripk1−/− mice, however, early lethality of ripk1mRHIM mice does not depend on Caspase-8-mediated apoptosis and can be prevented by ablation of ripk3, mlkl, or by introducing kinase inactivating or RHIM mutations in ripk3 (ripk3D161N or ripk3mRHIM, respectively) [61,62]. These genetic data indicate that newborn mice encounter signals that activate receptors which are able to induce apoptosis and necroptosis mediated by FADD-Caspase-8 and RIPK3-MLKL, respectively, and are inhibited by RIPK1 in a RHIM-dependent manner.
The presence of RIPK1 thus suffices to confer protection from Caspase-8-mediated apoptosis in early life. Ripk1−/− MEFs show a reduction in cellular levels of cFLIP upon TNF treatment, whereas ripk1mRHIM MEFs retain cFLIP levels [61]. Although not formally demonstrated, the suggested presence of cFLIP may explain why apoptosis is not observed in ripk1mRHIMripk3−/− mice. These data indicate that TNF-induced Caspase-8-mediated apoptosis is partially responsible for the lethality of newborn ripk1−/− mice. However, ablation of tnfr1 delays the lethality of ripk1−/− pups to ∼2 weeks, which indicates that mechanisms other than those induced by TNF activate RIPK3 in vivo. Co-ablation of tnfr1 from ripk1−/−ripk3−/− mice significantly extends life with onset of death at several months [54]. These triple-deficient mice show signs of tissues undergoing apoptosis, which is in line with the observation that ablation of casp8 or fadd prevents death of ripk1, ripk3 double-deficient mice. It is plausible that in ripk1, ripk3, tnfr1 triple-deficient mice, the FADD-Caspase-8 signaling cascade is activated by other death receptors such as Fas or TRAIL-R, although this has not been formally demonstrated.
The RIPK3 necrosome
RIPK3 activation leads to the formation of a complex called the ‘necrosome’, a macromolecular amyloid-like structure that functions as a platform for MLKL activation. It forms upon the engagement of the RHIM of RIPK3 with other RHIM-containing proteins. RHIMs consist of a conserved tetrad sequence I(V) Q I(V) G that is flanked by hydrophobic sequences [63]. TNFR1-activated RIPK1 induces the formation of a RHIM-mediated RIPK1–RIPK3 hetero-amyloid [21], which forms the basis for the necrosome. The structure of the RIPK1–RIPK3 hetero-amyloid has been elucidated by several groups [63–67] and consists of a pair of parallel β sheets that come together in an antiparallel fashion [64], where the RHIMs of human RIPK1 (IQIG) and RIPK3 (VQVG) alternately stack to create the hydrophobic core of the hetero-amyloid [65]. This leads to the recruitment of additional RIPK3 molecules and growth of the amyloid structure. Whether hetero-amyloids of RIPK3 and non-RIPK1 RHIM-containing proteins form a similar structure remains to be determined.
Mutating residues at the C-terminal flank of the RHIM domain in human RIPK3 (N464 and M468, but not N464 alone) to aspartates does not affect RIPK3 amyloid formation but prevents RIPK3 auto-phosphorylation, MLKL phosphorylation and necroptosis [64,67]. However, RHIM-mutant RIPK3 (VQVG → AAA), which is unable to form amyloids, retains the ability to phosphorylate MLKL but cannot induce MLKL auto-oligomerization, suggesting that the RIPK3 amyloid is essential for MLKL oligomerization but not for MLKL phosphorylation [66]. Thus, RHIM engagement induces RIPK3 to assemble into amyloid-like structures, providing a platform for the recruitment, phosphorylation and oligomerization of MLKL.
Only four human proteins, and several viral proteins [52], are known to contain functional RHIMs: RIPK1 [21], RIPK3 [21], TRIF [68,69], and ZBP1 [70]. TRIF is an adaptor protein that mediates immunological responses to invading pathogens upon ligation of TLR3 and -4, whereas ZBP1 is an intracellular receptor that recognizes Z-form nucleic acids of endogenous and pathogenic origin and is induced by interferon signaling.
TLR3/4-induced TRIF-mediated cell death
TLR signaling is dependent on the recruitment of the adapter proteins Myeloid differentiation primary response 88 (MyD88) and/or TRIF to the activated TLR. Except for TLR3, all TLRs signal via MyD88. TLR3 signaling solely relies on TRIF, whereas TLR4 signaling can be mediated by both MyD88 and TRIF [71], with the latter additionally requiring the adaptor protein TRAM [72]. MyD88 does not contain a RHIM and cannot engage cell death directly. However, ligation of TLR2, TLR5 or TLR9 can lead to necroptosis via an indirect mechanism where MyD88-mediated NF-κB and MAPK pathway activation results in the expression of TNF which, in turn, can induce TNFR1-mediated cell death [73].
TRIF can mediate cell death upon TLR3 or -4 activation (Figure 1). It contains a RHIM through which it can interact with RIPK3 and RIPK1 [68,69]. Ligation of TLR3 or -4 can induce necroptosis in bone marrow-derived macrophages (BMDM) [55,73], fibroblasts [54,73], and microglia [74] when Caspase-8 is inhibited. RIPK1 and Caspase-8 inhibit necroptosis in this setting [54,73], indicating that the RIPK1-FADD-Caspase-8-cFLIP complex (see above) attenuates necroptosis engaged by the activation of TRIF-dependent TLRs. The resulting TRIF–RIPK3–RIPK1–FADD-Caspase-8 complex can mediate apoptosis when the kinase function of RIPK3, and thereby necroptosis, is inhibited (see below) [75]. Thus, cell can undergo TRIF–RIPK3-mediated cell death upon sensing of pathogenic ligands by TLR3 and TLR4.
TRIF-mediated signaling leads to the activation of IRF3 to induce type I interferons [76], interferon-stimulated genes, and Erk1/2, cFos, and NF-κB signaling [69,77,78], which contribute to LPS-induced inflammatory responses in vivo [78]. Moreover, TRIF is required for the activation of type I interferon responses induced by the intracellular DDX1–DDX21–DHX36 RNA helicase dsRNA sensor complex in dendritic cells [79], and for signaling by STimulator of INterferon Genes (STING) [80]. As such, TRIF may play a role in the activation of cell death by interferons (discussed below).
Interferons and cell death
Type I and type II interferons (IFNs) can induce cell death under conditions where caspases are inhibited or when ripk1, fadd, or casp8 are deleted. This death is mediated by RIPK3 [54,81] and MLKL [60] and inhibited by the RIPK1-FADD-Caspase-8 complex [54]. These observations indicate that interferon-induced signaling can lead to RIPK3-mediated death.
However, the proteins that mediate the interferon signaling cascades do not contain RHIMs, and it was long unclear how interferons could induce necroptosis. It was therefore surmised that IFN signaling leads to the expression of genes involved in necroptosis. IFN induces expression of the RNA-responsive protein kinase R (PKR), which was suggested to engage with RIPK1 to induce the necrosome and execute necroptosis [82]. However, this finding is contradicted by studies showing that PKR is not involved [81,83,84], leaving a role for PKR in interferon-induced necroptosis obscure.
Evidence that IFNs induce necroptosis through the expression of downstream genes comes from studies showing that IFN-β-induced necroptosis requires STAT1, STAT2, and IRF9 [81] and IFN-β- and IFN-γ-induced necroptosis requires JAK1/STAT1 signaling [83,84]. Type I IFN signaling induces the formation of the ST2/IRF9 complex (consisting of a STAT2 homodimer and IRF9) and the Interferon Stimulated Gene Factor 3 (ISGF3) complex (consisting of a STAT1–STAT2 heterodimer and IRF9), which binds to interferon-sensitive response elements (ISRE) and regulates expression of downstream genes [85]. Type II IFN signaling induces STAT1 homodimers that engage with Gamma interferon Activation Site (GAS) elements to regulate the expression of downstream Interferon Stimulated Genes (ISGs) [85]. Interferons therefore were deemed likely to induce the expression of an ISG containing a RHIM. Indeed, both type I and type II interferons induce the expression of the interferon responsive gene ZBP1 [83,86,87], a cytosolic receptor that contains two RHIMs. ZBP1-deficient cells are completely protected from IFN-induced necroptosis [83,84], indicating that IFNs up-regulate the ISG ZBP1 to induce RIPK3-mediated cell death.
ZBP1-induced cell death
ZBP1 is a pattern recognition receptor that detects Z-form nucleic acids of endogenous or viral origin [88–94]. It contains two N-terminal tandem Zα domains to recognize nucleic acids [90], followed by two putative RHIM through which it can interact with RIPK3 and RIPK1 [70,87,95], and a C-terminal domain that can recruit TBK1 and IRF3 to induce IFN-β expression and activation of the type I interferon pathway [96].
Although the exact origins of the endogenous ligands that activate ZBP1 remain obscure, deletion or inhibition of the only other Zα domain-containing protein adenosine deaminase acting on RNA 1 (ADAR1) leads to the accumulation of Z-form RNA elements (Z-RNAs) and activation of ZBP1, implying that ZBP1 is activated by endogenous Z-RNAs that are otherwise repressed by ADAR1 [97,98].
ZBP1 can recognize cellular infection by several viral families, including orthomyxoviruses (influenza A virus (IAV) and influenza B virus) [93,99], herpesviruses (murine cytomegalovirus, herpes simplex virus 1 (HSV-1)) [100–102], poxviruses (vaccinia virus) [103], flaviviruses (Zika virus) [104], and β-coronaviruses such as SARS-CoV-2 [105]. Activation of ZBP1, i.e. upon infection with murine cytomegalovirus (MCMV) [102] or IAV [93,99], leads to the recruitment of RIPK3 via RHIM interactions which mediates cell death (Figure 1).
ZBP1 is essential for the control of IAV infections. When the ability of ZBP1 to induce cell death is impaired, viral titers may reach levels that are insurmountable for animals to survive. Indeed, both zbp1- and ripk3-deficient mice are hypersusceptible to lethal IAV infection [93]. Interestingly, however, mice in which necroptosis is ablated by deletion of mlkl can control IAV infection. Similarly, mice in which Caspase-8-mediated apoptosis is impaired (by a Caspase-8 mutation that renders Caspase-8 unable to auto-process and does not engage apoptosis but remains able to inhibit necroptosis) can also overcome infection with IAV. However, mice in which both apoptosis and necroptosis are ablated (i.e. casp8 and mlkl double deficient mice are not able to control rising viral titers and succumb to infection [57,93,106]. This indicates that activated ZBP1 can induce apoptosis as well as necroptosis. Indeed, also in settings of HSV-1 infection ZBP1 can induce both modes of cell death [101].
RIPK1 was thought to bridge the signaling from RIPK3 to FADD-Caspase-8. However, in ripk1-deficient cells, induction of ZBP1 by Interferons leads to RIPK3-dependent MLKL-mediated necroptosis and can also result in RIPK3-dependent Caspase-8-mediated apoptosis [83], indicating that RIPK3 can signal to Caspase-8 in absence of the RIPK1-FADD interaction, although the underlying mechanisms remain to be elucidated.
Canonically, ZBP1 activation is mediated by its Zα domains. However, heat stress induces the heat shock transcription factor 1 (HSF1) to express ZBP1, which is then activated to induce RIPK3-dependent death in a manner that does not depend on its nucleic acid sensing ability [107]. Thus, ZBP1 can induce apoptosis and necroptosis in response to nucleic acid sensing as well as other, yet to be defined, mechanisms. Cell death depends on RHIM:RHIM domain interactions between ZBP1 and RIPK3, and do not require the presence of other (RHIM-containing) adapter protein such as TRIF [58], or RIPK1 [58,83].
ZBP1 was suggested to constitutively bind RIPK1 through RHIM interactions [87]. In settings where TLR4 is activated in combination with the inhibition of TGFβ-activated kinase 1 (TAK1), e.g. during infection with Yersinia spp. (see below), TRIF is activated and recruits ZBP1–RIPK1, which in turn leads to the recruitment of FADD-Caspase-8, and cells subsequently die by apoptosis and/or pyroptosis (see below) [87]. Ripk3−/− cells also die in this setting [87], indicating that RIPK3 may not be absolutely required for TRIF–ZBP1-induced death.
Thus, several signaling cascades have been described through which ZBP1 activation can lead to cell death. The precise mechanisms that determine the outcome of ZBP1-induced, RIPK3-mediated and RIPK3-independent death remain incompletely understood however and may depend on yet to be identified interactors or post-translational modifications (detailed below).
TRIF and ZBP1 promote RIPK3 activation in vivo
Ablation of ripk3 or mlkl prevents the early lethality of ripk1mRHIM mice (see above) [61,62]. But which receptors induce this RIPK3-mediated lethality? Ablation of trif does not prevent lethality of ripk1mRHIM pups, but ablating zbp1 in ripk1mRHIM mice renders animals that are viable and live full lives [61], indicating that ZBP1 engages RIPK3 to trigger necroptosis in ripk1mRHIM mice.
The lethality of ripk1−/− pups is prevented by the combined deletion of casp8 and ripk3, but not either one alone (see above). ZBP1 plays only a partial role in mediating pathology in ripk1−/−casp8−/− pups, since co-ablation of zbp1 delays death to ∼3 weeks after birth [83]. Concomitantly, abrogation of the interferon pathways delays death of ripk1−/−tnfr1−/− pups, with animals now living up to one month [83]. TRIF also seems to mediate pathology, with ripk1−/−tnfr1−/− pups living significantly longer when trif is co-ablated [83]. These data indicate that both the TRIF- and IFN-ZBP1 pathways are activated in vivo to mediate RIPK3 activation. Indeed, co-ablation of trif and zbp1 from ripk1−/−casp8−/− mice yield animals that are similar to ripk1−/−casp8−/−ripk3−/−mice [61]. Thus, TRIF and ZBP1 promote RIPK3 activation when RIPK1 is absent in vivo. These observations support the idea that RIPK1, TRIF, and ZBP1 may represent the only molecules that function to activate RIPK3, at least in the setting where Caspase-8 activity is compromised in vivo.
The kinase function of RIPK3 regulates cell death
RIPK3 deficiency is fully compatible with life, and several vertebrate species thrive whilst having lost ripk3 over the course of evolution [52]. Mice do express RIPK3 and experimental ablation of the gene does not affect development or spontaneous induction of abnormalities throughout life [108].
The catalytic activity of RIPK3, however, can be vital to developing embryos and seems to influence death outcome (Figure 2). Catalytically inactive ripk3D161N mice die mid gestation due to mal-development of the yolk-sac [75,109]. Of note, the ripk3D161N mutation is unlike other mutations that render the kinase function of RIPK3 inactive and catalytically inactive ripk3K51A mice are viable [75]. Moreover, RIPK3 D161N does not act as a dominant negative mutant, since ripk3D161N/+ mice are viable [109]. The mid gestational death of ripk3D161N mice is similar to the time of death of casp8−/− or fadd-deficient mice, which die by TNFR1-induced necroptosis of the yolk-sac vasculature [110–112]. The vascular endothelial cells of ripk3D161N yolk-sacs contain cleaved caspase-3, indicative of apoptosis [109]. This apoptosis is mediated by Caspase-8, and ablation of casp8−/− from ripk3D161N/D161N mice results in mice that reach adulthood and develop a phenotype similar to casp8−/−ripk3−/− mice [49,109]. However, how Caspase-8 is activated in ripk3D161N mice remains unclear and ablation of tnfr1, mlkl, trif, zbp1, cyld, dr3, or cflar does not prevent death of ripk3D161N mice [109]. Death does depend on RIPK1, which, when ablated, delays death from E11.5 to a few days after birth [109], similar to the lethality observed in ripk1−/− mice. Whether the combination of trif and zbp1 or other, unidentified factors relay death signals through RIPK1 to Caspase-8 remains to be determined.
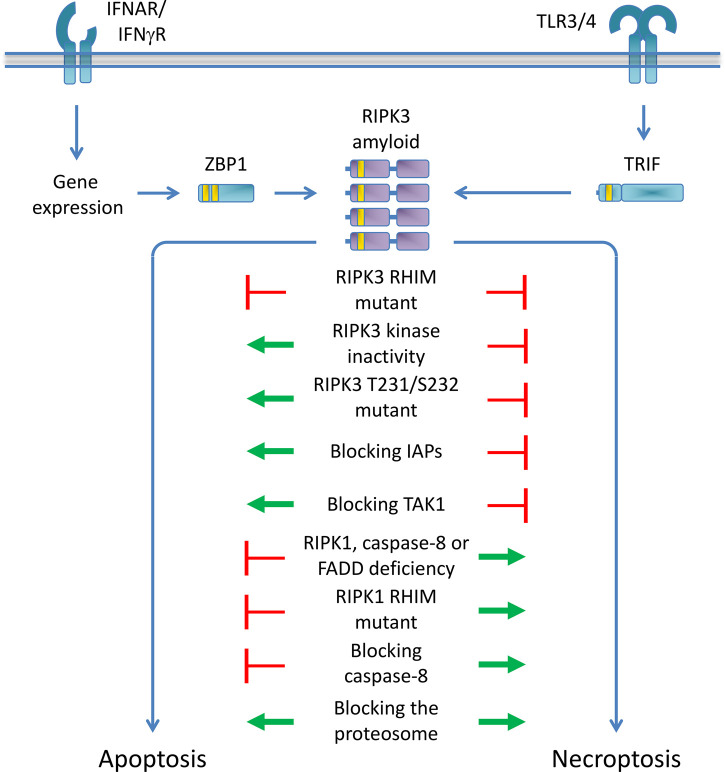
Figure 2. Regulation of non-TNF directed RIPK3-mediated death signals.
Activated RIPK3 is able to induce both apoptosis and necroptosis. Disruption of the RIPK3 RHIM impairs both apoptosis and necroptosis. RIPK3 kinase inactivity or failure to phosphorylate RIPK3 T231/S232 impairs necroptosis but promotes apoptosis, a process that is furthermore regulated by cIAPs and TAK1. RIPK1, FADD, or caspase-8 deficiency, blocking the catalytic activity of caspase-8, or disruption of the RIPK1 RHIM promotes necroptosis and impairs apoptosis, although apoptosis can ensue under certain conditions in absence of RIPK1. Blocking the proteosome primarily promotes necroptosis, although apoptosis can also ensue.
The precise mechanisms by which the kinase activity of RIPK3 functions in biology remains enigmatic. Blocking the RIPK3 kinase activity with the inhibitors GSK'843 or GSK'872 in vitro abrogates necroptosis and can induce the association of RIPK3 with RIPK1, FADD, Caspase-8, and cFLIPL to mediate activation of caspase-3 and apoptosis, suggesting that the kinase activity of RIPK3, in part, determines cell death outcome [75,109]. But why would signals that induce apoptosis in catalytically inactive RIPK3 cells not cause necroptosis in wild-type RIPK3 cells? This may be due to the presence of a RIPK1-FADD-Caspase-8-cFLIPL complex that blocks necroptosis. Why then does this complex not engage apoptosis in wild-type RIPK3 cells, or in cells where MLKL is absent? It has been suggested that the kinase and RHIM domains of RIPK3 collaborate to keep a conformation that controls association with apoptotic and necroptotic machineries [113], but unambiguous data is lacking.
One possible explanation may be that other proteins influence the ‘decisions’ of RIPK3. To be able to mediate necroptosis, RIPK3 must be correctly folded and is facilitated by the molecular co-chaperones heat shock protein 90 (HSP90) and Cell Division Cycle 37 (CDC37) [114]. Cells in which HSP90-CDC37 levels are high can undergo RIPK3-mediated necroptosis, whereas cells in which levels are low RIPK3 predominantly induces apoptosis [115]. In such HSP90-CDC37 low cells, two conserved serine/threonine residues (S164/T165 in human) in the kinase loop of RIPK3 are phosphorylated, possibly by RIPK3 itself, resulting in the abrogation of its kinase activity whilst potentiating the ability to recruit the RIPK1-FADD-Caspase-8 complex [115]. RIPK3 forms puncta in the cytosol upon necroptotic stimuli [10] but forms distinctive high-order structures in HSP90-CDC37 low cells [115]. The ‘decision’ of RIPK3 to relay a death signal to apoptosis or necroptosis therefore seems to depend on the structure of the signaling complex, which may be regulated by yet to be appreciated interactors.
Although the kinase activity of RIPK3 partly determines whether it induces apoptosis or necroptosis, the mechanisms by which RIPK3 controls cell death outcomes may be more subtle. RIPK3 engagement may also lead to either apoptosis or necroptosis in settings where the kinase activity is not impaired. Influenza A virus-derived Z-RNA activates ZBP1 (see above) in the nucleus of infected cells, which induces RIPK3-mediated death to control the infection [99]. IAV-infected individual cells die by either apoptosis or necroptosis [57,93,106], indicating that cells in which RIPK3 kinase activity is retained are able to undergo RIPK3-mediated apoptosis. It will be important to further elucidate how these intricate signaling cascades are regulated to fully understand the biology of RIPK3-mediated cell death.
Post-translational modifications that influence apoptosis and necroptosis
RIPK-mediated cell death is strictly controlled by post-translational modifications, including ubiquitylation, phosphorylation, nitrosylation, PARylation, O-GlcNAcylation and acetylation.
RIPK3 can be S-nitrosylated at C119 by nitric oxide generated by neuronal nitric oxide synthase, mediated by N-Methyl-D-aspartic acid (NMDA) receptor, which affects neuronal damage during cerebral ischemia-reperfusion [116]. Upon TNF stimulation, poly-ADP-ribosylation (PARylation) of RIPK3 at C360 by tankyrase-1 leads to the recruitment of the E3 ligase RNF46 to complex II, resulting in the ubiquitylation and proteasomal degradation of complex II and inhibition of death [117]. O-linked β-N-acetylglucosamination (O-GlcNAcylation) of RIPK3 at T467 by O-GlcNAc transferase inhibits RIPK3s interaction with RIPK1 and other RIPK3 molecules, and necroptosis [118], whereas inhibition of sirtuins enhances RIPK1 acetylation at lysine residues K115, K625, K627, K642 and K648, and induces Caspase-8-mediated apoptosis engaged by the death receptor TRAIL-R [119].
Phosphorylation also plays a significant role in cell death or survival [34]. Auto-phosphorylation of RIPK1 and RIPK3 mediates necroptosis, but other kinases and phosphatases also affect RIPK-dependent cell death. For instance, the phosphatase Ppm1b dephosphorylates the T231/S232 residues of murine RIPK3 required for necroptosis [120] (Figure 2). TNF-mediated RIPK1-dependent cell death is inhibited by MK2, which phosphorylates RIPK1 at S321, thereby disrupting its ability to interact with FADD-Caspase-8 [121,122]. IKKα and IKKβ both directly phosphorylate RIPK1 at S25, inhibiting its kinase activity [123]. The kinase TAK1 functions upstream of the IKKs and help control whether TNFR1 ligation leads to gene expression or cell death. TAK1 kinase activity protects from TNF-induced RIPK1-dependent cell death [124–128]. Inhibition of TAK1, by the virulence factor Yersinia outer protein J (YopJ) expressed by Yersinia species bacteria or the pharmacological TAK1 inhibitor 5Z-7-Oxozeaenol (5z7), prevents the IKKα/β-mediated phosphorylation of RIPK1 at S25 and results in TNF-induced RIPK1-mediated cell death [123]. TAK1 activity is controlled by TAK1-binding protein 2 (TAB2) [129] and protein phosphatase 6 catalytic subunit (PPP6C) [130] and in their absence TAK1 is hyperactivated. However, where PPP6C deficiency prevents TNF-induced RIPK1-mediated necroptosis [130], abrogation of TAB2 leads to an exacerbation of TNF-induced necroptosis mediated by RIPK3 [127]. Moreover, deletion of TAB2 sensitizes PPP6C-deficient cells to necroptosis [130]. This indicates that TAB2 restricts necroptosis in a currently unappreciated manner.
TAK1 also regulates TLR4-mediated cell death (Figure 2). During Yersinia infection, YopJ inhibits TAK1 [131,132] whereas accompanying LPS triggers TLR4, resulting in a mix of apoptosis and pyroptosis in cell pools [133]. Treating cells with LPS and 5z7 mimics these effects [134]. When TAK1 is inhibited, LPS induces CD14-mediated TLR4 internalization, leading to the recruitment of TRAM-TRIF and a pre-formed ZBP1–RIPK1 complex. This induces RIPK1 phosphorylation and the recruitment of FADD-Caspase-8, resulting in Caspase-8 activation and cell death [87]. Death occurs in absence of RIPK3 or MLKL but is completely dependent on Caspase-8, which mediates cleavage (activation) of the apoptosis effector proteins caspase-3 and caspase-7, and the pyroptosis effector proteins gasdermin D (GSDMD) and gasdermin E (GSDME) [87,133,134]. The activity of TAK1 thus seems to control TLR4-induced signaling complex formation, the requirement for RIPK3 in the TLR4-induced death complex, and the mode of cell death this complex engages. The precise mechanisms remain unclear, but it will be interesting to elucidate which proteins are targeted by TAK1 to regulate RIPK3-dependent or -independent death. Moreover, it will be interesting to evaluate whether TAB2 also plays a role in restricting non-TNF-induced necroptosis.
Ubiquitylation also plays a significant role in cell death or survival. cIAP1/2-mediated ubiquitylation of RIPK1 keeps RIPK1 in the TNFR1 complex and impairs cell death [27,28]. The deubiquitylating enzymes Cylindromatosis (CYLD) also regulates RIPK1 ubiquitylation, but after RIPK1s release from the TNFR1 complex [135]. RIPK3 is also ubiquitylated upon TNFR engagement. K63-linked ubiquitylation of RIPK3 Lys-5, regulated by the deubiquitylase A20, is required for TNF-induced necroptosis [136]. cIAP1/2 and XIAP regulates the function of RIPK3 upon TLR4 engagement [137]. In the presence of these E3 ligases, LPS induces TRIF-cIAP-dependent ubiquitylation of RIPK3 and MLKL, allowing necroptosis to ensue (Figure 2). However, in the absence of cIAP1/2 and XIAP, LPS triggers RIPK3-mediated activation of Caspase-8, which results in apoptosis or activation of the NLRP3 inflammasome which does not depend on RIPK3s kinase activity or the presence of MLKL [137]. Thus, the ubiquitylation status of RIPK3 helps determine cell death outcome upon ligation of TLR4.
The ubiquitin-proteosome system regulates RIPK3-mediated necroptosis and can induce necroptosis without the activation of receptors. Blocking the proteosome leads to the accumulation of K48-linked ubiquitylated RIPK3 (at K264) and induces necroptosis (Figure 2). Death is dependent on an intact RHIM in RIPK3, suggesting that RIPK3 accumulation suffices to induce necroptosis. Death is not dependent on the presence of cIAPs and the E3 ligase responsible for the K48-linked ubiquitylation of RIPK3 K264 remains to be determined [138]. Another recently identified mechanism that can induce necroptosis without activating receptors is increase in intracellular pH [139], induced when the Na+/H+ exchanger SLC9A1 mediates a more basic intracellular pH in response to osmotic stress. This pH change activates RIPK3 and induces necroptosis, however without the requirement for its RHIM domain or RIPK1 [139]. This finding underscores that the exact mechanisms and settings by which RIPK3 mediates necroptosis remain incompletely understood.
Concluding remarks
RIPK3-dependent cell death can be engaged by three currently known mechanisms, TNFR1, TRIF-dependent TLRs, and ZBP1, and can lead to apoptosis, necroptosis, and pyroptosis. Although the mechanisms by which RIPK3 is engaged and mediates cell death are becoming increasingly clear, recent insights indicate that many regulatory aspects of RIPK3-dependent cell death remain to be understood. Elucidating how these intricate signaling mechanisms work to control cellular demise will not only be important to fully understand the biology of RIPK3-mediated cell death but may also provide avenues for novel therapeutic strategies to treat the various human diseases that involve RIPK3.
Abbreviations
- ADAR1
adenosine deaminase acting on RNA 1
- CDC37
Cell Division Cycle 37
- CYLD
Cylindromatosis
- DD
death domain
- DED
death effector domains
- FADD
FAS Associated Death Domain
- HSP90
heat shock protein 90
- HSV-1
herpes simplex virus 1
- IAV
influenza A virus
- IFNs
interferons
- LPS
lipopolysaccharide
- MLKL
Mixed Lineage Kinase-Like
- PKR
protein kinase R
- PPP6C
protein phosphatase 6 catalytic subunit
- RHIM
RIP homotypic interaction motif
- RIPK3
receptor-interacting serine/threonine protein kinase 3
- TAB2
TAK1-binding protein 2
- TLR
Toll-like receptor
- TNFR
tumor necrosis factor receptor
- TRAIL
tumor-necrosis factor related apoptosis-inducing ligand
- ZBP1
zDNA-binding protein 1
Competing Interests
The authors declare that there are no competing interests associated with this manuscript. D.R.G. consults for Inzen Pharnaceuticals, Ventus Pharmaceuticals, and Boehringer-Ingleheim.
Funding
This work was supported by ALSAC (SJCRH), and by the U.S. National Cancer Institute grant R35 CA231620 and NIH grant AI44828 to D.R.G and by the NIH Cancer Center Support Grant P30 CA021765. B.T. is supported by King's College London.
CRediT Author Contribution
Bart Tummers: Conceptualization, Writing — original draft, Writing — review and editing. Douglas Green: Supervision, Funding acquisition, Writing — review and editing.
References
- 1.Galluzzi, L., Vitale, I., Aaronson, S.A., Abrams, J.M., Adam, D., Agostinis, P.et al. (2018) Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 25, 486–541 10.1038/s41418-017-0012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laster, S.M., Wood, J.G. and Gooding, L.R. (1988) Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J. Immunol. 141, 2629–2634 [PubMed] [Google Scholar]
- 3.Weinlich, R., Oberst, A., Beere, H.M. and Green, D.R. (2017) Necroptosis in development, inflammation and disease. Nat. Rev. Mol. Cell Biol. 18, 127–136 10.1038/nrm.2016.149 [DOI] [PubMed] [Google Scholar]
- 4.Jiang, X., Stockwell, B.R. and Conrad, M. (2021) Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266–282 10.1038/s41580-020-00324-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papayannopoulos, V. (2018) Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 18, 134–147 10.1038/nri.2017.105 [DOI] [PubMed] [Google Scholar]
- 6.David, K.K., Andrabi, S.A., Dawson, T.M. and Dawson, V.L. (2009) Parthanatos, a messenger of death. Front. Biosci. (Landmark Ed) 14, 1116–1128 10.2741/3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergsbaken, T., Fink, S.L. and Cookson, B.T. (2009) Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7, 99–109 10.1038/nrmicro2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsvetkov, P., Coy, S., Petrova, B., Dreishpoon, M., Verma, A., Abdusamad, M.et al. (2022) Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 375, 1254–1261 10.1126/science.abf0529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho, Y.S., Challa, S., Moquin, D., Genga, R., Ray, T.D., Guildford, M.et al. (2009) Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 10.1016/j.cell.2009.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He, S., Wang, L., Miao, L., Wang, T., Du, F., Zhao, L.et al. (2009) Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137, 1100–1111 10.1016/j.cell.2009.05.021 [DOI] [PubMed] [Google Scholar]
- 11.Linkermann, A. and Green, D.R. (2014) Necroptosis. N. Engl. J. Med. 370, 455–465 10.1056/NEJMra1310050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy, J.M., Czabotar, P.E., Hildebrand, J.M., Lucet, I.S., Zhang, J.G., Alvarez-Diaz, S.et al. (2013) The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39, 443–453 10.1016/j.immuni.2013.06.018 [DOI] [PubMed] [Google Scholar]
- 13.Sun, L., Wang, H., Wang, Z., He, S., Chen, S., Liao, D.et al. (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 10.1016/j.cell.2011.11.031 [DOI] [PubMed] [Google Scholar]
- 14.Wang, H., Sun, L., Su, L., Rizo, J., Liu, L., Wang, L.F.et al. (2014) Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 54, 133–146 10.1016/j.molcel.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 15.Zhang, D.W., Shao, J., Lin, J., Zhang, N., Lu, B.J., Lin, S.C.et al. (2009) RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 325, 332–336 10.1126/science.1172308 [DOI] [PubMed] [Google Scholar]
- 16.Zhao, J., Jitkaew, S., Cai, Z., Choksi, S., Li, Q., Luo, J.et al. (2012) Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc. Natl Acad. Sci. U.S.A. 109, 5322–5327 10.1073/pnas.1200012109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Degterev, A., Hitomi, J., Germscheid, M., Ch'en, I.L., Korkina, O., Teng, X.et al. (2008) Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 4, 313–321 10.1038/nchembio.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degterev, A., Huang, Z., Boyce, M., Li, Y., Jagtap, P., Mizushima, N.et al. (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1, 112–119 10.1038/nchembio711 [DOI] [PubMed] [Google Scholar]
- 19.Hayden, M.S. and Ghosh, S. (2014) Regulation of NF-kappaB by TNF family cytokines. Semin. Immunol. 26, 253–266 10.1016/j.smim.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu, H., Huang, J., Shu, H.B., Baichwal, V. and Goeddel, D.V. (1996) TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4, 387–396 10.1016/S1074-7613(00)80252-6 [DOI] [PubMed] [Google Scholar]
- 21.Sun, X., Yin, J., Starovasnik, M.A., Fairbrother, W.J. and Dixit, V.M. (2002) Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J. Biol. Chem. 277, 9505–9511 10.1074/jbc.M109488200 [DOI] [PubMed] [Google Scholar]
- 22.Weinlich, R. and Green, D.R. (2014) The two faces of receptor interacting protein kinase-1. Mol. Cell 56, 469–480 10.1016/j.molcel.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Micheau, O. and Tschopp, J. (2003) Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 10.1016/S0092-8674(03)00521-X [DOI] [PubMed] [Google Scholar]
- 24.Bertrand, M.J., Milutinovic, S., Dickson, K.M., Ho, W.C., Boudreault, A., Durkin, J.et al. (2008) cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 30, 689–700 10.1016/j.molcel.2008.05.014 [DOI] [PubMed] [Google Scholar]
- 25.Dynek, J.N., Goncharov, T., Dueber, E.C., Fedorova, A.V., Izrael-Tomasevic, A., Phu, L.et al. (2010) . c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling. EMBO J. 29, 4198–4209 10.1038/emboj.2010.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varfolomeev, E., Goncharov, T., Fedorova, A.V., Dynek, J.N., Zobel, K., Deshayes, K.et al. (2008) . c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J. Biol. Chem. 283, 24295–9 10.1074/jbc.C800128200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vince, J.E., Wong, W.W., Khan, N., Feltham, R., Chau, D., Ahmed, A.U.et al. (2007) IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell 131, 682–693 10.1016/j.cell.2007.10.037 [DOI] [PubMed] [Google Scholar]
- 28.Wang, L., Du, F. and Wang, X. (2008) TNF-alpha induces two distinct caspase-8 activation pathways. Cell 133, 693–703 10.1016/j.cell.2008.03.036 [DOI] [PubMed] [Google Scholar]
- 29.Tummers, B. and Green, D.R. (2017) Caspase-8: regulating life and death. Immunol. Rev. 277, 76–89 10.1111/imr.12541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kischkel, F.C., Lawrence, D.A., Tinel, A., LeBlanc, H., Virmani, A., Schow, P.et al. (2001) Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J. Biol. Chem. 276, 46639–46646 10.1074/jbc.M105102200 [DOI] [PubMed] [Google Scholar]
- 31.Sprick, M.R., Rieser, E., Stahl, H., Grosse-Wilde, A., Weigand, M.A. and Walczak, H. (2002) Caspase-10 is recruited to and activated at the native TRAIL and CD95 death-inducing signalling complexes in a FADD-dependent manner but can not functionally substitute caspase-8. EMBO J. 21, 4520–4530 10.1093/emboj/cdf441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, J., Chun, H.J., Wong, W., Spencer, D.M. and Lenardo, M.J. (2001) Caspase-10 is an initiator caspase in death receptor signaling. Proc. Natl Acad. Sci. U.S.A. 98, 13884–8 10.1073/pnas.241358198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wachmann, K., Pop, C., van Raam, B.J., Drag, M., Mace, P.D., Snipas, S.J.et al. (2010) Activation and specificity of human caspase-10. Biochemistry 49, 8307–8315 10.1021/bi100968m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McQuade, T., Cho, Y. and Chan, F.K. (2013) Positive and negative phosphorylation regulates RIP1- and RIP3-induced programmed necrosis. Biochem. J. 456, 409–415 10.1042/BJ20130860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen, W., Zhou, Z., Li, L., Zhong, C.Q., Zheng, X., Wu, X.et al. (2013) Diverse sequence determinants control human and mouse receptor interacting protein 3 (RIP3) and mixed lineage kinase domain-like (MLKL) interaction in necroptotic signaling. J. Biol. Chem. 288, 16247–16261 10.1074/jbc.M112.435545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai, Z., Jitkaew, S., Zhao, J., Chiang, H.C., Choksi, S., Liu, J.et al. (2014) Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat. Cell Biol. 16, 55–65 10.1038/ncb2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen, X., Li, W., Ren, J., Huang, D., He, W.T., Song, Y.et al. (2014) Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 24, 105–121 10.1038/cr.2013.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuchiya, Y., Nakabayashi, O. and Nakano, H. (2015) FLIP the switch: Regulation of apoptosis and necroptosis by cFLIP. Int. J. Mol. Sci. 16, 30321–30341 10.3390/ijms161226232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ueffing, N., Keil, E., Freund, C., Kuhne, R., Schulze-Osthoff, K. and Schmitz, I. (2008) Mutational analyses of c-FLIPR, the only murine short FLIP isoform, reveal requirements for DISC recruitment. Cell Death Differ. 15, 773–782 10.1038/sj.cdd.4402314 [DOI] [PubMed] [Google Scholar]
- 40.Rasper, D.M., Vaillancourt, J.P., Hadano, S., Houtzager, V.M., Seiden, I., Keen, S.L.et al. (1998) Cell death attenuation by ‘Usurpin’, a mammalian DED-caspase homologue that precludes caspase-8 recruitment and activation by the CD-95 (Fas, APO-1) receptor complex. Cell Death Differ. 5, 271–288 10.1038/sj.cdd.4400370 [DOI] [PubMed] [Google Scholar]
- 41.Scaffidi, C., Schmitz, I., Krammer, P.H. and Peter, M.E. (1999) The role of c-FLIP in modulation of CD95-induced apoptosis. J. Biol. Chem. 274, 1541–1548 10.1074/jbc.274.3.1541 [DOI] [PubMed] [Google Scholar]
- 42.Pop, C., Oberst, A., Drag, M., Van Raam, B.J., Riedl, S.J., Green, D.R.et al. (2011) FLIP(l) induces caspase 8 activity in the absence of interdomain caspase 8 cleavage and alters substrate specificity. Biochem. J. 433, 447–457 10.1042/BJ20101738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feoktistova, M., Geserick, P., Kellert, B., Dimitrova, D.P., Langlais, C., Hupe, M.et al. (2011) cIAPs block ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell 43, 449–463 10.1016/j.molcel.2011.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lalaoui, N., Boyden, S.E., Oda, H., Wood, G.M., Stone, D.L., Chau, D.et al. (2020) Mutations that prevent caspase cleavage of RIPK1 cause autoinflammatory disease. Nature 577, 103–108 10.1038/s41586-019-1828-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin, Y., Devin, A., Rodriguez, Y. and Liu, Z.G. (1999) Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 13, 2514–2526 10.1101/gad.13.19.2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newton, K., Wickliffe, K.E., Dugger, D.L., Maltzman, A., Roose-Girma, M., Dohse, M.et al. (2019) Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature 574, 428–431 10.1038/s41586-019-1548-x [DOI] [PubMed] [Google Scholar]
- 47.Zhang, X., Dowling, J.P. and Zhang, J. (2019) RIPK1 can mediate apoptosis in addition to necroptosis during embryonic development. Cell Death Dis. 10, 245 10.1038/s41419-019-1490-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feng, S., Yang, Y., Mei, Y., Ma, L., Zhu, D.E., Hoti, N.et al. (2007) Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 19, 2056–2067 10.1016/j.cellsig.2007.05.016 [DOI] [PubMed] [Google Scholar]
- 49.Oberst, A., Dillon, C.P., Weinlich, R., McCormick, L.L., Fitzgerald, P., Pop, C.et al. (2011) Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 10.1038/nature09852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Donnell, M.A., Perez-Jimenez, E., Oberst, A., Ng, A., Massoumi, R., Xavier, R.et al. (2011) Caspase 8 inhibits programmed necrosis by processing CYLD. Nat. Cell Biol. 13, 1437–1442 10.1038/ncb2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vercammen, D., Beyaert, R., Denecker, G., Goossens, V., Van Loo, G., Declercq, W.et al. (1998) Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 187, 1477–1485 10.1084/jem.187.9.1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tummers, B. and Green, D.R. (2022) The evolution of regulated cell death pathways in animals and their evasion by pathogens. Physiol. Rev. 102, 411–454 10.1152/physrev.00002.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tao, P., Sun, J., Wu, Z., Wang, S., Wang, J., Li, W.et al. (2020) A dominant autoinflammatory disease caused by non-cleavable variants of RIPK1. Nature 577, 109–114 10.1038/s41586-019-1830-y [DOI] [PubMed] [Google Scholar]
- 54.Dillon, C.P., Weinlich, R., Rodriguez, D.A., Cripps, J.G., Quarato, G., Gurung, P.et al. (2014) RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell 157, 1189–1202 10.1016/j.cell.2014.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He, S., Liang, Y., Shao, F. and Wang, X. (2011) Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc. Natl Acad. Sci. U.S.A. 108, 20054–20059 10.1073/pnas.1116302108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karunakaran, D., Geoffrion, M., Wei, L., Gan, W., Richards, L., Shangari, P.et al. (2016) Targeting macrophage necroptosis for therapeutic and diagnostic interventions in atherosclerosis. Sci. Adv. 2, e1600224 10.1126/sciadv.1600224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nogusa, S., Thapa, R.J., Dillon, C.P., Liedmann, S., Oguin, III, T.H., Ingram, J.P.et al. (2016) RIPK3 activates parallel pathways of MLKL-driven necroptosis and FADD-mediated apoptosis to protect against Influenza A virus. Cell Host Microbe 20, 13–24 10.1016/j.chom.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Upton, J.W., Kaiser, W.J. and Mocarski, E.S. (2010) Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7, 302–313 10.1016/j.chom.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelliher, M.A., Grimm, S., Ishida, Y., Kuo, F., Stanger, B.Z. and Leder, P. (1998) The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity 8, 297–303 10.1016/S1074-7613(00)80535-X [DOI] [PubMed] [Google Scholar]
- 60.Alvarez-Diaz, S., Dillon, C.P., Lalaoui, N., Tanzer, M.C., Rodriguez, D.A., Lin, A.et al. (2016) The pseudokinase MLKL and the kinase RIPK3 have distinct roles in autoimmune disease caused by loss of death-receptor-induced apoptosis. Immunity 45, 513–526 10.1016/j.immuni.2016.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Newton, K., Wickliffe, K.E., Maltzman, A., Dugger, D.L., Strasser, A., Pham, V.C.et al. (2016) RIPK1 inhibits ZBP1-driven necroptosis during development. Nature 540, 129–133 10.1038/nature20559 [DOI] [PubMed] [Google Scholar]
- 62.Lin, J., Kumari, S., Kim, C., Van, T.M., Wachsmuth, L., Polykratis, A.et al. (2016) RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature 540, 124–128 10.1038/nature20558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu, X.L., Hu, H., Dong, X.Q., Zhang, J., Wang, J., Schwieters, C.D.et al. (2021) The amyloid structure of mouse RIPK3 (receptor interacting protein kinase 3) in cell necroptosis. Nat. Commun. 12, 1627 10.1038/s41467-021-21881-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li, J., McQuade, T., Siemer, A.B., Napetschnig, J., Moriwaki, K., Hsiao, Y.S.et al. (2012) The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150, 339–350 10.1016/j.cell.2012.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mompean, M., Li, W., Li, J., Laage, S., Siemer, A.B., Bozkurt, G.et al. (2018) The structure of the necrosome RIPK1-RIPK3 core, a human hetero-amyloid signaling complex. Cell 173, 1244–53.e10 10.1016/j.cell.2018.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen, X., Zhu, R., Zhong, J., Ying, Y., Wang, W., Cao, Y.et al. (2022) Mosaic composition of RIP1-RIP3 signalling hub and its role in regulating cell death. Nat. Cell Biol. 24, 471–482 10.1038/s41556-022-00854-7 [DOI] [PubMed] [Google Scholar]
- 67.Hu, H., Wu, X., Wu, G., Nan, N., Zhang, J., Zhu, X.et al. (2021) RIP3-mediated necroptosis is regulated by inter-filament assembly of RIP homotypic interaction motif. Cell Death Differ. 28, 251–266 10.1038/s41418-020-0598-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaiser, W.J. and Offermann, M.K. (2005) Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J. Immunol. 174, 4942–4952 10.4049/jimmunol.174.8.4942 [DOI] [PubMed] [Google Scholar]
- 69.Meylan, E., Burns, K., Hofmann, K., Blancheteau, V., Martinon, F., Kelliher, M.et al. (2004) RIP1 is an essential mediator of toll-like receptor 3-induced NF-kappa B activation. Nat. Immunol. 5, 503–507 10.1038/ni1061 [DOI] [PubMed] [Google Scholar]
- 70.Kaiser, W.J., Upton, J.W. and Mocarski, E.S. (2008) Receptor-interacting protein homotypic interaction motif-dependent control of NF-kappa B activation via the DNA-dependent activator of IFN regulatory factors. J. Immunol. 181, 6427–6434 10.4049/jimmunol.181.9.6427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamamoto, M., Sato, S., Hemmi, H., Hoshino, K., Kaisho, T., Sanjo, H.et al. (2003) Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301, 640–643 10.1126/science.1087262 [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto, M., Sato, S., Hemmi, H., Uematsu, S., Hoshino, K., Kaisho, T.et al. (2003) TRAM is specifically involved in the toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat. Immunol. 4, 1144–1150 10.1038/ni986 [DOI] [PubMed] [Google Scholar]
- 73.Kaiser, W.J., Sridharan, H., Huang, C., Mandal, P., Upton, J.W., Gough, P.J.et al. (2013) Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J. Biol. Chem. 288, 31268–31279 10.1074/jbc.M113.462341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim, S.J. and Li, J. (2013) Caspase blockade induces RIP3-mediated programmed necrosis in Toll-like receptor-activated microglia. Cell Death Dis. 4, e716 10.1038/cddis.2013.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mandal, P., Berger, S.B., Pillay, S., Moriwaki, K., Huang, C., Guo, H.et al. (2014) RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol. Cell 56, 481–495 10.1016/j.molcel.2014.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamamoto, M., Sato, S., Mori, K., Hoshino, K., Takeuchi, O., Takeda, K.et al. (2002) Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J. Immunol. 169, 6668–6672 10.4049/jimmunol.169.12.6668 [DOI] [PubMed] [Google Scholar]
- 77.Cusson-Hermance, N., Khurana, S., Lee, T.H., Fitzgerald, K.A. and Kelliher, M.A. (2005) Rip1 mediates the Trif-dependent toll-like receptor 3- and 4-induced NF-{kappa}B activation but does not contribute to interferon regulatory factor 3 activation. J. Biol. Chem. 280, 36560–6 10.1074/jbc.M506831200 [DOI] [PubMed] [Google Scholar]
- 78.Najjar, M., Saleh, D., Zelic, M., Nogusa, S., Shah, S., Tai, A.et al. (2016) RIPK1 and RIPK3 kinases promote cell-Death-Independent inflammation by Toll-like receptor 4. Immunity 45, 46–59 10.1016/j.immuni.2016.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang, Z., Kim, T., Bao, M., Facchinetti, V., Jung, S.Y., Ghaffari, A.A.et al. (2011) DDX1, DDX21, and DHX36 helicases form a complex with the adaptor molecule TRIF to sense dsRNA in dendritic cells. Immunity 34, 866–878 10.1016/j.immuni.2011.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang, X., Majumdar, T., Kessler, P., Ozhegov, E., Zhang, Y., Chattopadhyay, S.et al. (2016) STING requires the adaptor TRIF to trigger innate immune responses to microbial infection. Cell Host Microbe 20, 329–341 10.1016/j.chom.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McComb, S., Cessford, E., Alturki, N.A., Joseph, J., Shutinoski, B., Startek, J.B.et al. (2014) Type-I interferon signaling through ISGF3 complex is required for sustained Rip3 activation and necroptosis in macrophages. Proc. Natl Acad. Sci. U.S.A. 111, E3206–E3213 10.1073/pnas.1407068111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thapa, R.J., Nogusa, S., Chen, P., Maki, J.L., Lerro, A., Andrake, M.et al. (2013) Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc. Natl Acad. Sci. U.S.A. 110, E3109–E3118 10.1073/pnas.1301218110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ingram, J.P., Thapa, R.J., Fisher, A., Tummers, B., Zhang, T., Yin, C.et al. (2019) ZBP1/DAI drives RIPK3-Mediated cell death induced by IFNs in the absence of RIPK1. J. Immunol. 203, 1348–1355 10.4049/jimmunol.1900216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang, D., Liang, Y., Zhao, S., Ding, Y., Zhuang, Q., Shi, Q.et al. (2020) ZBP1 mediates interferon-induced necroptosis. Cell. Mol. Immunol. 17, 356–368 10.1038/s41423-019-0237-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Platanias, L.C. (2005) Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5, 375–386 10.1038/nri1604 [DOI] [PubMed] [Google Scholar]
- 86.Fu, Y., Comella, N., Tognazzi, K., Brown, L.F., Dvorak, H.F. and Kocher, O. (1999) Cloning of DLM-1, a novel gene that is up-regulated in activated macrophages, using RNA differential display. Gene 240, 157–163 10.1016/S0378-1119(99)00419-9 [DOI] [PubMed] [Google Scholar]
- 87.Muendlein, H.I., Connolly, W.M., Magri, Z., Smirnova, I., Ilyukha, V., Gautam, A.et al. (2021) ZBP1 promotes LPS-induced cell death and IL-1beta release via RHIM-mediated interactions with RIPK1. Nat. Commun. 12, 86 10.1038/s41467-020-20357-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiao, H., Wachsmuth, L., Kumari, S., Schwarzer, R., Lin, J., Eren, R.O.et al. (2020) Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation. Nature 580, 391–395 10.1038/s41586-020-2129-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Devos, M., Tanghe, G., Gilbert, B., Dierick, E., Verheirstraeten, M., Nemegeer, J.et al. (2020) Sensing of endogenous nucleic acids by ZBP1 induces keratinocyte necroptosis and skin inflammation. J. Exp. Med. 217, e20191913 10.1084/jem.20191913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ha, S.C., Kim, D., Hwang, H.Y., Rich, A., Kim, Y.G. and Kim, K.K. (2008) The crystal structure of the second Z-DNA binding domain of human DAI (ZBP1) in complex with Z-DNA reveals an unusual binding mode to Z-DNA. Proc. Natl Acad. Sci. U.S.A. 105, 20671–6 10.1073/pnas.0810463106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schwartz, T., Behlke, J., Lowenhaupt, K., Heinemann, U. and Rich, A. (2001) Structure of the DLM-1-Z-DNA complex reveals a conserved family of Z-DNA-binding proteins. Nat. Struct. Biol. 8, 761–765 10.1038/nsb0901-761 [DOI] [PubMed] [Google Scholar]
- 92.Maelfait, J., Liverpool, L., Bridgeman, A., Ragan, K.B., Upton, J.W. and Rehwinkel, J. (2017) Sensing of viral and endogenous RNA by ZBP1/DAI induces necroptosis. EMBO J. 36, 2529–2543 10.15252/embj.201796476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thapa, R.J., Ingram, J.P., Ragan, K.B., Nogusa, S., Boyd, D.F., Benitez, A.A.et al. (2016) DAI senses influenza A virus genomic RNA and activates RIPK3-Dependent cell death. Cell Host Microbe 20, 674–681 10.1016/j.chom.2016.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sridharan, H., Ragan, K.B., Guo, H., Gilley, R.P., Landsteiner, V.J., Kaiser, W.J.et al. (2017) Murine cytomegalovirus IE3-dependent transcription is required for DAI/ZBP1-mediated necroptosis. EMBO Rep. 18, 1429–1441 10.15252/embr.201743947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rebsamen, M., Heinz, L.X., Meylan, E., Michallet, M.C., Schroder, K., Hofmann, K.et al. (2009) DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB. EMBO Rep. 10, 916–922 10.1038/embor.2009.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takaoka, A., Wang, Z., Choi, M.K., Yanai, H., Negishi, H., Ban, T.et al. (2007) DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448, 501–505 10.1038/nature06013 [DOI] [PubMed] [Google Scholar]
- 97.Hubbard, N.W., Ames, J.M., Maurano, M., Chu, L.H., Somfleth, K.Y., Gokhale, N.S.et al. (2022) ADAR1 mutation causes ZBP1-dependent immunopathology. Nature 607, 769–775 10.1038/s41586-022-04896-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang, T., Yin, C., Fedorov, A., Qiao, L., Bao, H., Beknazarov, N.et al. (2022) ADAR1 masks the cancer immunotherapeutic promise of ZBP1-driven necroptosis. Nature 606, 594–602 10.1038/s41586-022-04753-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang, T., Yin, C., Boyd, D.F., Quarato, G., Ingram, J.P., Shubina, M.et al. (2020) Influenza virus Z-RNAs induce ZBP1-Mediated Necroptosis. Cell 180, 1115–29.e13 10.1016/j.cell.2020.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo, H., Gilley, R.P., Fisher, A., Lane, R., Landsteiner, V.J., Ragan, K.B.et al. (2018) Species-independent contribution of ZBP1/DAI/DLM-1-triggered necroptosis in host defense against HSV1. Cell Death Dis. 9, 816 10.1038/s41419-018-0868-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jeffries, A.M., Suptela, A.J. and Marriott, I. (2022) Z-DNA binding protein 1 mediates necroptotic and apoptotic cell death pathways in murine astrocytes following herpes simplex virus-1 infection. J. Neuroinflammation 19, 109 10.1186/s12974-022-02469-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Upton, J.W., Kaiser, W.J. and Mocarski, E.S. (2012) DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 11, 290–297 10.1016/j.chom.2012.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koehler, H., Cotsmire, S., Zhang, T., Balachandran, S., Upton, J.W., Langland, J.et al. (2021) Vaccinia virus E3 prevents sensing of Z-RNA to block ZBP1-dependent necroptosis. Cell Host Microbe 29, 1266–76.e5 10.1016/j.chom.2021.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Daniels, B.P., Kofman, S.B., Smith, J.R., Norris, G.T., Snyder, A.G., Kolb, J.P.et al. (2019) The nucleotide sensor ZBP1 and kinase RIPK3 induce the enzyme IRG1 to promote an antiviral metabolic state in neurons. Immunity 50, 64–76.e4 10.1016/j.immuni.2018.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karki, R., Lee, S., Mall, R., Pandian, N., Wang, Y., Sharma, B.R.et al. (2022) ZBP1-dependent inflammatory cell death, PANoptosis, and cytokine storm disrupt IFN therapeutic efficacy during coronavirus infection. Sci. Immunol. 7, eabo6294 10.1126/sciimmunol.abo6294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shubina, M., Tummers, B., Boyd, D.F., Zhang, T., Yin, C., Gautam, A.et al. (2020) Necroptosis restricts influenza A virus as a stand-alone cell death mechanism. J. Exp. Med. 217, e20191259 10.1084/jem.20191259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yuan, F., Cai, J., Wu, J., Tang, Y., Zhao, K., Liang, F.et al. (2022) Z-DNA binding protein 1 promotes heatstroke-induced cell death. Science 376, 609–615 10.1126/science.abg5251 [DOI] [PubMed] [Google Scholar]
- 108.Newton, K., Sun, X. and Dixit, V.M. (2004) Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and toll-like receptors 2 and 4. Mol. Cell. Biol. 24, 1464–1469 10.1128/MCB.24.4.1464-1469.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Newton, K., Dugger, D.L., Wickliffe, K.E., Kapoor, N., de Almagro, M.C., Vucic, D.et al. (2014) Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 343, 1357–1360 10.1126/science.1249361 [DOI] [PubMed] [Google Scholar]
- 110.Varfolomeev, E.E., Schuchmann, M., Luria, V., Chiannilkulchai, N., Beckmann, J.S., Mett, I.L.et al. (1998) Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9, 267–276 10.1016/S1074-7613(00)80609-3 [DOI] [PubMed] [Google Scholar]
- 111.Zhang, J., Cado, D., Chen, A., Kabra, N.H. and Winoto, A. (1998) Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 392, 296–300 10.1038/32681 [DOI] [PubMed] [Google Scholar]
- 112.Yeh, W.C., de la Pompa, J.L., McCurrach, M.E., Shu, H.B., Elia, A.J., Shahinian, A.et al. (1998) FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 279, 1954–1958 10.1126/science.279.5358.1954 [DOI] [PubMed] [Google Scholar]
- 113.Zhang, J. and Chan, F.K. (2014) Cell biology. RIPK3 takes another deadly turn. Science 343, 1322–1323 10.1126/science.1252526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li, D., Xu, T., Cao, Y., Wang, H., Li, L., Chen, S.et al. (2015) A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. Proc. Natl Acad. Sci. U.S.A. 112, 5017–5022 10.1073/pnas.1505244112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li, D., Chen, J., Guo, J., Li, L., Cai, G., Chen, S.et al. (2021) A phosphorylation of RIPK3 kinase initiates an intracellular apoptotic pathway that promotes prostaglandin2alpha-induced corpus luteum regression. eLife 10, e67409 10.7554/eLife.67409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miao, W., Qu, Z., Shi, K., Zhang, D., Zong, Y., Zhang, G.et al. (2015) RIP3 S-nitrosylation contributes to cerebral ischemic neuronal injury. Brain Res. 1627, 165–176 10.1016/j.brainres.2015.08.020 [DOI] [PubMed] [Google Scholar]
- 117.Liu, L., Sandow, J.J., Leslie Pedrioli, D.M., Samson, A.L., Silke, N., Kratina, T.et al. (2022) Tankyrase-mediated ADP-ribosylation is a regulator of TNF-induced death. Sci. Adv. 8, eabh2332 10.1126/sciadv.abh2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li, X., Gong, W., Wang, H., Li, T., Attri, K.S., Lewis, R.E.et al. (2019) O-GlcNAc transferase suppresses inflammation and necroptosis by targeting receptor-interacting serine/Threonine-Protein kinase 3. Immunity 50, 1115 10.1016/j.immuni.2019.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carafa, V., Nebbioso, A., Cuomo, F., Rotili, D., Cobellis, G., Bontempo, P.et al. (2018) RIP1-HAT1-SIRT complex identification and targeting in treatment and prevention of cancer. Clin. Cancer Res. 24, 2886–2900 10.1158/1078-0432.CCR-17-3081 [DOI] [PubMed] [Google Scholar]
- 120.Chen, W., Wu, J., Li, L., Zhang, Z., Ren, J., Liang, Y.et al. (2015) Ppm1b negatively regulates necroptosis through dephosphorylating Rip3. Nat. Cell Biol. 17, 434–444 10.1038/ncb3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dondelinger, Y., Delanghe, T., Rojas-Rivera, D., Priem, D., Delvaeye, T., Bruggeman, I.et al. (2017) MK2 phosphorylation of RIPK1 regulates TNF-mediated cell death. Nat. Cell Biol. 19, 1237–1247 10.1038/ncb3608 [DOI] [PubMed] [Google Scholar]
- 122.Jaco, I., Annibaldi, A., Lalaoui, N., Wilson, R., Tenev, T., Laurien, L.et al. (2017) MK2 phosphorylates RIPK1 to prevent TNF-Induced cell death. Mol. Cell 66, 698–710.e5 10.1016/j.molcel.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dondelinger, Y., Delanghe, T., Priem, D., Wynosky-Dolfi, M.A., Sorobetea, D., Rojas-Rivera, D.et al. (2019) Serine 25 phosphorylation inhibits RIPK1 kinase-dependent cell death in models of infection and inflammation. Nat. Commun. 10, 1729 10.1038/s41467-019-09690-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dondelinger, Y., Aguileta, M.A., Goossens, V., Dubuisson, C., Grootjans, S., Dejardin, E.et al. (2013) RIPK3 contributes to TNFR1-mediated RIPK1 kinase-dependent apoptosis in conditions of cIAP1/2 depletion or TAK1 kinase inhibition. Cell Death Differ. 20, 1381–1392 10.1038/cdd.2013.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Arslan, S.C. and Scheidereit, C. (2011) The prevalence of TNFalpha-induced necrosis over apoptosis is determined by TAK1-RIP1 interplay. PLoS ONE 6, e26069 10.1371/journal.pone.0026069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vanlangenakker, N., Vanden Berghe, T., Bogaert, P., Laukens, B., Zobel, K., Deshayes, K.et al. (2011) cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ. 18, 656–665 10.1038/cdd.2010.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morioka, S., Broglie, P., Omori, E., Ikeda, Y., Takaesu, G., Matsumoto, K.et al. (2014) TAK1 kinase switches cell fate from apoptosis to necrosis following TNF stimulation. J. Cell Biol. 204, 607–623 10.1083/jcb.201305070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lamothe, B., Lai, Y., Xie, M., Schneider, M.D. and Darnay, B.G. (2013) TAK1 is essential for osteoclast differentiation and is an important modulator of cell death by apoptosis and necroptosis. Mol. Cell. Biol. 33, 582–595 10.1128/MCB.01225-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Broglie, P., Matsumoto, K., Akira, S., Brautigan, D.L. and Ninomiya-Tsuji, J. (2010) Transforming growth factor beta-activated kinase 1 (TAK1) kinase adaptor, TAK1-binding protein 2, plays dual roles in TAK1 signaling by recruiting both an activator and an inhibitor of TAK1 kinase in tumor necrosis factor signaling pathway. J. Biol. Chem. 285, 2333–2339 10.1074/jbc.M109.090522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zou, Y., Zheng, Q., Jiang, B., Liu, Y., Xu, Y., Ma, L.et al. (2022) Deficiency of PPP6C protects TNF-induced necroptosis through activation of TAK1. Cell Death Dis. 13, 618 10.1038/s41419-022-05076-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meinzer, U., Barreau, F., Esmiol-Welterlin, S., Jung, C., Villard, C., Leger, T.et al. (2012) Yersinia pseudotuberculosis effector yopJ subverts the Nod2/RICK/TAK1 pathway and activates caspase-1 to induce intestinal barrier dysfunction. Cell Host Microbe 11, 337–351 10.1016/j.chom.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 132.Paquette, N., Conlon, J., Sweet, C., Rus, F., Wilson, L., Pereira, A.et al. (2012) Serine/threonine acetylation of TGFbeta-activated kinase (TAK1) by yersinia pestis yopJ inhibits innate immune signaling. Proc. Natl Acad. Sci. U.S.A. 109, 12710–5 10.1073/pnas.1008203109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Orning, P., Weng, D., Starheim, K., Ratner, D., Best, Z., Lee, B.et al. (2018) Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 362, 1064–1069 10.1126/science.aau2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sarhan, J., Liu, B.C., Muendlein, H.I., Li, P., Nilson, R., Tang, A.Y.et al. (2018) Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during yersinia infection. Proc. Natl Acad. Sci. U.S.A. 115, E10888–E10E97 10.1073/pnas.1809548115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Moquin, D.M., McQuade, T. and Chan, F.K. (2013) CYLD deubiquitinates RIP1 in the TNFalpha-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS ONE 8, e76841 10.1371/journal.pone.0076841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Onizawa, M., Oshima, S., Schulze-Topphoff, U., Oses-Prieto, J.A., Lu, T., Tavares, R.et al. (2015) The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis. Nat. Immunol. 16, 618–627 10.1038/ni.3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lawlor, K.E., Khan, N., Mildenhall, A., Gerlic, M., Croker, B.A., D'Cruz, A.A.et al. (2015) RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat. Commun. 6, 6282 10.1038/ncomms7282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Moriwaki, K. and Chan, F.K. (2016) Regulation of RIPK3- and RHIM-dependent Necroptosis by the Proteasome. J Biol Chem. 291, 5948–5959 10.1074/jbc.M115.700997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang, W., Fan, W., Guo, J. and Wang, X. (2022) Osmotic stress activates RIPK3/MLKL-mediated necroptosis by increasing cytosolic pH through a plasma membrane Na+/H+ exchanger. Sci. Signal. 15, eabn5881 10.1126/scisignal.abn5881 [DOI] [PubMed] [Google Scholar]