Abstract
The immune response to Haemophilus ducreyi is mediated in part by T cells infiltrating the site of infection. In this study, we show that H. ducreyi antigen preparations inhibited the proliferation of peripheral blood mononuclear cells and primary human T-cell lines. H. ducreyi also inhibited Jurkat T-cell proliferation and induced apoptosis of Jurkat T cells, confirmed through the detection of DNA degradation and membrane unpacking. The cytotoxic product(s) was present in cell-free culture supernatant and whole-cell preparations of H. ducreyi and was heat labile. H. ducreyi produces two known heat-labile toxins, a hemolysin and a cytolethal distending toxin (CDT). Whole cells and supernatants prepared from a hemolysin-deficient mutant had the same inhibitory and apoptotic effects on Jurkat T cells as did its isogenic parent. Preparations made from an H. ducreyi cdtC mutant were less toxic and induced less apoptosis than the parent. The toxic activity of the cdtC mutant was restored by complementation in trans. CdtC-neutralizing antibodies also inhibited H. ducreyi-induced toxicity and apoptosis. The data suggest that CDT may interfere with T-cell responses to H. ducreyi by induction of apoptosis.
Haemophilus ducreyi causes chancroid, a common genital ulcer disease in developing countries. H. ducreyi and human immunodeficiency virus infections amplify one another, and their epidemiological synergy may have contributed to the human immunodeficiency virus pandemic in Asia and Africa (14, 16, 53). H. ducreyi readily acquires antimicrobial resistance factors, and the options for treatment are few and often expensive (6). Understanding the pathogenesis of and the immune response to H. ducreyi infection may facilitate the development of alternative strategies to control chancroid.
A major component of the immune response to H. ducreyi is a T-cell infiltrate at the site of infection. Naturally occurring ulcers contain an infiltrate that consists predominantly of both CD4 and CD8 cells and macrophages with areas of granulomatous change (17, 23). In the experimental model of infection in human volunteers, the cellular infiltrate at the site of inoculation is similar to that seen in natural infection (31, 46, 47). The cellular infiltrate in experimental infection is accompanied by HLA-DR expression on mononuclear cells and expression of cytokine mRNAs for gamma interferon, tumor necrosis factor alpha, and interleukin-8 (IL-8), consistent with a delayed-type hypersensitivity reaction (31, 46). Although subjects who are experimentally infected for 1 to 2 weeks do not have systemic blastogenic responses to H. ducreyi antigens (3, 46), patients with ulcers have systemic blastogenic responses to H. ducreyi (52) and increased levels of soluble IL-2 receptors in urine and serum (1).
Whether host responses to H. ducreyi confer immunity to subsequent exposure is uncertain. Ducrey was able to maintain serial ulcers in patients by weekly autoinoculation of pus from infected ulcers to the skin of the forearm for up to 15 weeks (26). Experimental infection for 6 to 14 days up to the pustular stage of disease does not provide protective immunity against subsequent challenge (3). In outbreaks of culture-proven chancroid, a few patients have had two to three recurrences (5, 13). Thus, clinical data indicate that infection does not reliably confer protective immunity against subsequent exposure and suggest the possibility that H. ducreyi interferes with the host response.
H. ducreyi produces two known heat-labile toxins that have a cytopathic effect on human cells. The cell-associated hemolysin is related to the Proteus-Serratia pore-forming family of hemolysins and kills human foreskin fibroblasts and keratinocytes in vitro but has no activity against HeLa cells (2, 28–30, 51). However, a hemolysin-deficient mutant caused pustules at the same rate as the parent in the human challenge model of infection (32). The cytolethal distending toxin (CDT), which is encoded by the gene cluster cdtABC, is found in cell-free culture supernatants and induces cell cycle arrest of cultured human epithelial cells, including HeLa cells, in the G2 phase (8, 10, 20–22, 35–37). Whether these H. ducreyi toxins affect human lymphoid cells or cause apoptosis is unclear.
Here we examined the effect of H. ducreyi cells and cell-free culture supernatants of H. ducreyi on proliferation of human T cells in vitro. We also examined whether these preparations induced apoptosis of Jurkat T cells and examined the roles of the hemolysin and CDT in induction of T-cell death.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Actinobacillus actinomycetemcomitans (ATCC 29522) was kindly provided by Dominique Galli of the Indiana University School of Dentistry. Haemophilus parahaemolyticus (ATCC 10014), Haemophilus paraphrohaemolyticus (ATCC 29237), Haemophilus parainfluenzae (ATCC 33392), and Haemophilus parainfluenzae (paraphrophilus) (ATCC 29242) were purchased from the American Type Culture Collection, Manassas, Va. Nontypeable Haemophilus influenzae 1479 and H. ducreyi 35000HP (HP stands for human passaged) and its isogenic hemolysin mutant, 35000HP-RSM1, were described previously (4, 32, 45). H. ducreyi 35000 and its isogenic cdtC mutant, designated 35000.303, were described previously (48). The cdtC mutant complemented with the cdtC gene in trans was designated 35000.303(pJL300-C), while the cdtC mutant transformed with the vector alone was designated 35000.303(pLS88), as described previously (48).
Bacterial strains were maintained on chocolate agar plates or grown in brain heart infusion broth containing 50 μg of hemin per ml, 1% IsoVitaleX, and 5% fetal bovine serum as described elsewhere (15). Where appropriate, H. ducreyi transformants were selected with kanamycin (30 μg/ml).
Preparation of freeze-thawed whole cells (FTWC) and cell-free culture supernatants.
Bacteria were grown to mid-log phase, collected by centrifugation at 10,000 × g, and washed three times with sterile saline. The remaining culture supernatant was filtered through 0.2-μm-pore-size filters. Bacterial pellets were frozen, thawed, suspended in 10 mM HEPES, and adjusted to a final protein concentration of 1 mg/ml. The amount of protein in the preparations was determined by the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, Calif.) with bovine serum albumin as a standard. In some experiments, FTWC were heat treated at temperatures ranging from 56 to 100°C for time points between 2 and 60 min.
Monoclonal antibodies (MAbs) and other reagents.
Recombinant human IL-2 was purchased from Biotest Diagnostics Corp. (Danville, N.J.). Hybridoma culture supernatant fluids containing the H. ducreyi CdtA- and CdtB-specific MAbs 1G8 and 20B2 were described previously (48). The CdtC-neutralizing MAbs 9E9 and 13D9 and their immunoglobulin G1 isotype control 2B7, which does not bind to H. ducreyi, were described elsewhere (8, 9, 48).
Human subjects.
Blood and biopsy samples of pustules were obtained from two subjects who participated in a losB mutant and parent comparison trial (unpublished data). Blood was obtained from one volunteer who had been infected for a second time with H. ducreyi in a reinfection trial 7 months previously (3), from one volunteer who had been infected for 9 days in a major outer membrane protein mutant-parent trial (unpublished data), and from one healthy volunteer with no history of chancroid. Informed consent was obtained from the subjects for participation, in accordance with the human experimentation guidelines of the U.S. Department of Health and Human Services and the Institutional Review Board of Indiana University-Purdue University at Indianapolis.
Isolation of peripheral blood mononuclear cells (PBMCs) and T-cell lines.
PBMCs were enriched from whole blood by density gradient centrifugation and diluted in RPMI 1640 (Gibco-BRL, Gaithersburg, Md.), supplemented with penicillin-streptomycin, l-glutamine (Bio Whittaker, Walkersville, Md.), and 10% heat-inactivated human AB serum (Sigma Chemical Co., St. Louis, Mo.) as described previously (46).
Mononuclear cells were obtained from biopsy samples by mincing of tissue with a scalpel, extrusion through a 70-μm-mesh filter, and density gradient centrifugation as described elsewhere (31). T-cell lines were generated from these mononuclear cells by use of IL-2 (30 U/ml) and a γ-irradiated allogeneic Epstein-Barr virus-transformed B-cell line that had been treated with galactose oxidase and neuraminidase as feeder cells (12). T-cell lines were also established from PBMCs by stimulation with IL-2 (50 U/ml) and phytohemagglutinin (PHA; Sigma) (2 μg/ml). The human acute T-lymphoblastic leukemia cell line, Jurkat (44), was kindly provided by Zacharie Brahmi, Indiana University School of Medicine, Indianapolis. Cells were cultivated in RPMI 1640 medium supplemented as described above at 37°C in a humid atmosphere containing 5% CO2.
Proliferation assay.
PBMCs, primary T-cell lines, or Jurkat T cells were washed extensively and suspended to a density of 4 × 105 cells/ml. Approximately 2 × 104 to 1 × 105 cells were seeded in wells of a 96-well plate with varying amounts of the H. ducreyi preparations in triplicate. For primary T-cell lines, 105 γ-irradiated autologous PBMCs were added as feeder cells. The cells were incubated for 96 h, and [3H]thymidine (0.5 μCi/well; Amersham Pharmacia Biotech, Piscataway, N.J.) was added for the last 8 h of incubation. Cells were harvested with a Filtermate Packard cell harvester (Packard Instrument Co., Inc., Rockville, Md.), and [3H]thymidine uptake was determined with a Direct Beta Counter Matrix 9600 (Packard). Percent inhibition of proliferation was determined as (C − E)/C × 100, where C (control) is counts per minute in the absence of H. ducreyi antigen and E (experimental) is counts per minute in the presence of H. ducreyi antigen. For each value, a standard error was calculated as an approximate estimate of the variance for the ratio of two means (18).
JAM test.
The JAM test measures DNA retained by living cells rather than DNA lost by dying cells (24). Briefly, Jurkat T cells were labeled with [3H]thymidine (5 μCi/ml) for 4 h at 37°C. The cells were pelleted and washed, and 2 × 104 cells/well were cultured with varying amounts of H. ducreyi or medium alone for 18 or 24 h and harvested onto filters as described above. Percent specific DNA loss was calculated as (C − E)/C × 100, where C (control) is DNA retained on the filter in the presence of medium alone and E (experimental) is DNA retained on the filter in the presence of H. ducreyi antigens (24). For each value, a standard error was calculated as an approximate estimate of the variance for the ratio of two means (18).
DNA fragmentation assay.
Jurkat T cells (2 × 106) were incubated with varying amounts of H. ducreyi preparations for 18 h, harvested, suspended in lysis buffer (10 mM Tris-HCl [pH 7.6], 10 mM EDTA, 50 mM NaCl, 1.25% sodium dodecyl sulfate), and incubated with proteinase K (10 μg/ml) at 56°C for 1 h. After addition of 5 M NaCl, the mixture was shaken vigorously and centrifuged at 15,000 × g. DNA was precipitated from the supernatant by adding 2 volumes of 100% ethanol, suspended in ddH2O, and digested with RNase A for 30 min at 37°C. The DNA was electrophoresed on a 1% agarose gel and stained with a 1:10,000 dilution of SYBR Green I (Molecular Probes, Eugene, Oreg.).
Flow cytometry.
To determine the phenotype of T-cell lines obtained from PBMCs, approximately 105 to 106 cells were stained with fluorescent antibodies (Becton Dickinson Immunocytometry Systems, San Jose, Calif.) as described previously (31). Cells were washed, suspended in phosphate-buffered saline, fixed with fluorescence-activated cell sorting lysing solution (Becton Dickinson), and analyzed with a FACScan flow cytometer (Becton Dickinson). A combination of CD3-fluorescein isothiocyanate (FITC)–CD16-phycoerythrin (PE)–CD56-PE and CD3-FITC–CD19-PE and CD4-FITC–CD8-PE fluorescent antibodies was used for these studies. As a control, an isotype-matched antibody was used to detect nonspecific staining.
Jurkat T cells were cultured with varying amounts of H. ducreyi FTWC. To detect apoptotic nuclei, the cells were stained with propidium iodide (PI) solution (PI, 5 μg/ml; saponin, 0.3%; EDTA, 5 mM; RNase, 50 μg/ml) (11). To detect loss of membrane asymmetry, the cells were stained with merocyanine 540 (MC540; Upstate Biotechnology Inc., Lake Placid, N.Y.) (38). Cells were stained in the dark, centrifuged, washed, and suspended in phosphate-buffered saline. Samples were analyzed within 2 h on a FACScan flow cytometer. Data from 104 cells were collected and analyzed with CellQuest software (Becton Dickinson).
Effect of MAbs on inhibition of cell growth and apoptosis.
Bacterial preparations were mixed with varying amounts of hybridoma culture fluid supernatants and incubated for 30 min at 37°C. Jurkat cells were added to this mixture, cultured, and analyzed as described for the proliferation assay.
RESULTS
H. ducreyi inhibits the proliferation of human T-cell lines and PBMCs.
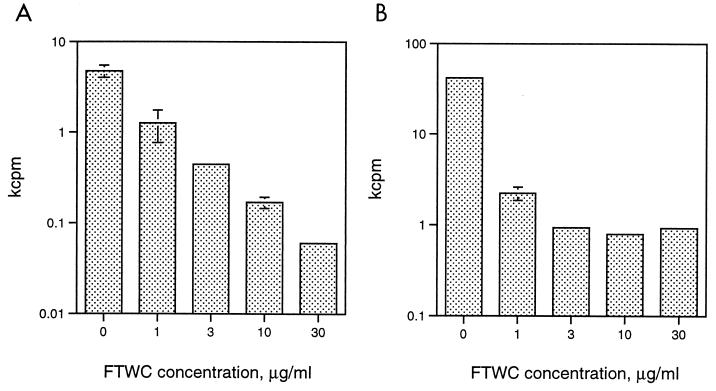
In numerous attempts to isolate H. ducreyi antigen-specific T-cell lines from experimental lesions, we were unable to propagate T cells in the presence of H. ducreyi FTWC, IL-2, and γ-irradiated autologous PBMCs. To examine why primary T cells did not proliferate under these conditions, we expanded human T cells in the absence of antigen and studied their interaction with H. ducreyi. Four volunteers who had been experimentally infected with H. ducreyi and one volunteer who had no history of infection with H. ducreyi supplied clinical specimens for these studies. Punch biopsy samples were obtained from two subjects at sites where pustules had developed 5 and 14 days after inoculation with H. ducreyi 35000HP. Mononuclear cells were isolated from the biopsy samples and expanded in the absence of antigen and in the presence of allogeneic γ-irradiated feeder cells and IL-2. The cells were then stimulated with or without IL-2 in the presence of autologous γ-irradiated feeder cells and different concentrations of H. ducreyi FTWC. The cells did not proliferate in the absence of IL-2 (data not shown). Addition of H. ducreyi FTWC inhibited the IL-2-mediated proliferative response of both primary T-cell lines in a dose-dependent fashion (Fig. 1 and data not shown). H. ducreyi FTWC also inhibited PHA-induced proliferation of PBMCs obtained from a third volunteer who had been infected with H. ducreyi for 9 days (Fig. 1).
FIG. 1.
Effect of H. ducreyi on T-cell proliferation. (A) Cells from a non-antigen-specific line derived from a biopsy sample were cultured with varying concentrations of H. ducreyi FTWC in the presence of autologous PBMCs and IL-2 for 96 h. (B) PBMCs were stimulated with 1 μg of PHA per ml in the presence of varying concentrations of H. ducreyi FTWC. The results are expressed as the means ± standard deviations of [3H]thymidine incorporation from triplicate wells and are representative of experiments done on cell lines or PBMCs obtained from five donors.
T-cell lines were also obtained by expansion of PBMCs with PHA and IL-2 from one naive donor and from a fourth volunteer who had been infected with H. ducreyi 7 months earlier. Fluorescence-activated cell sorting analysis showed that these lines were composed primarily (>98%) of CD4 cells. The IL-2-mediated proliferation of these cell lines in the presence and absence of feeder cells was also inhibited by H. ducreyi FTWC (data not shown), suggesting that FTWC were toxic for primary T cells.
H. ducreyi FTWC inhibit proliferation and induce apoptosis in Jurkat T cells.
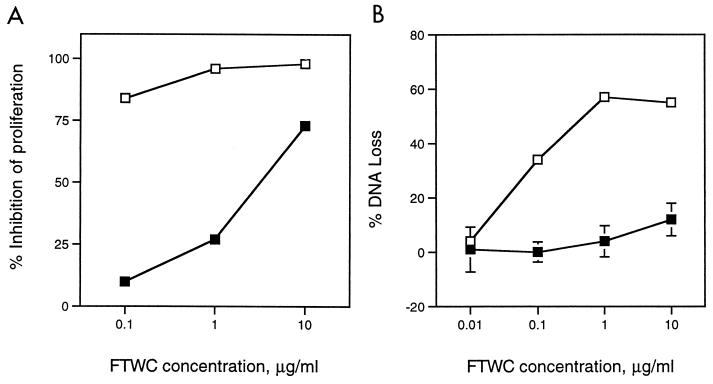
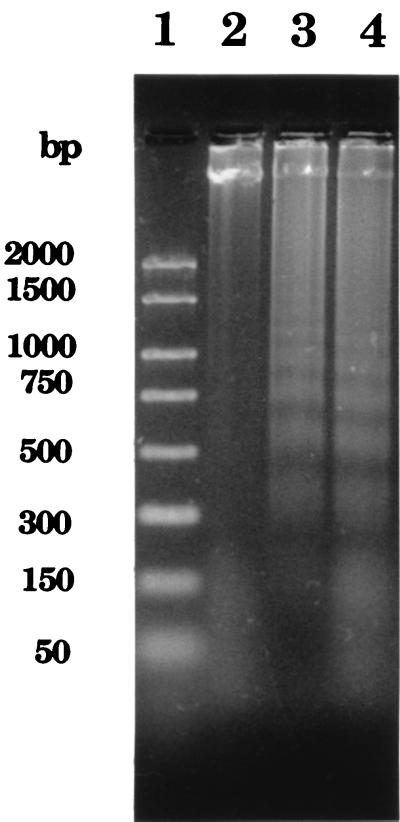
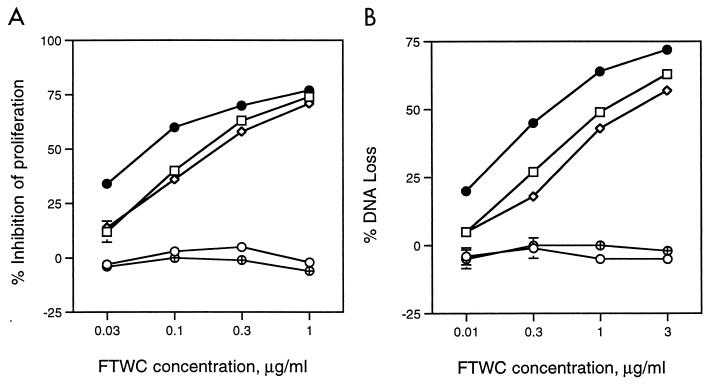
A major goal of our laboratory is to isolate cutaneous T-cell lines from experimental lesions and determine their phenotypes and antigen specificity. To avoid consumption of the limited clinical material obtained from experimentally infected subjects, we used the Jurkat human acute T-lymphoblastic leukemia cell line to study the effect of H. ducreyi on T-cell growth and viability. The spontaneous proliferation of Jurkat T cells as measured by [3H]thymidine incorporation was also inhibited by H. ducreyi FTWC in a dose-dependent fashion (Fig. 2). To examine whether FTWC induced cell death in Jurkat T cells, DNA loss was measured by the JAM test (24). Briefly, the cells were prelabeled with [3H]thymidine and then cultured in the presence of FTWC for 4, 14, 18, and 24 h. In this assay, DNA loss was not detectable at 4 h but was evident by 14 h (data not shown) and occurred in a dose-dependent fashion (Fig. 2). The DNA loss induced by H. ducreyi in Jurkat T cells was accompanied by the appearance of fragmented DNA in a typical oligonucleosomal ladder in agarose gel electrophoresis (Fig. 3). The data suggested that the inhibition of Jurkat T-cell proliferation in the presence of FTWC was in part due to cell death by apoptosis.
FIG. 2.
Effect of H. ducreyi on proliferation and DNA loss of Jurkat T cells. (A) Jurkat T cells were incubated with varying concentrations of FTWC for 24 h and then pulsed with [3H]thymidine. Percent inhibition of proliferation was determined as described in the text. (B) Jurkat T cells were [3H]thymidine labeled, washed, and cultured with FTWC for 18 h. Percent specific DNA loss was calculated as described in the text. FTWC were not treated (open squares) or heat treated for 40 min at 60°C (filled squares). Results are expressed as the means ± standard errors of triplicate wells and are representative of three independent experiments.
FIG. 3.
Effect of H. ducreyi on DNA fragmentation. Samples were electrophoresed on an agarose gel and stained with SYBR Green I. Lane 1, DNA standards; lanes 2 to 4, chromosomal DNA isolated from Jurkat T cells cultured with medium (lane 2) or 1 (lane 3) or 10 (lane 4) μg of FTWC per ml for 18 h.
Flow cytometry analysis of H. ducreyi-induced apoptosis in Jurkat T cells.
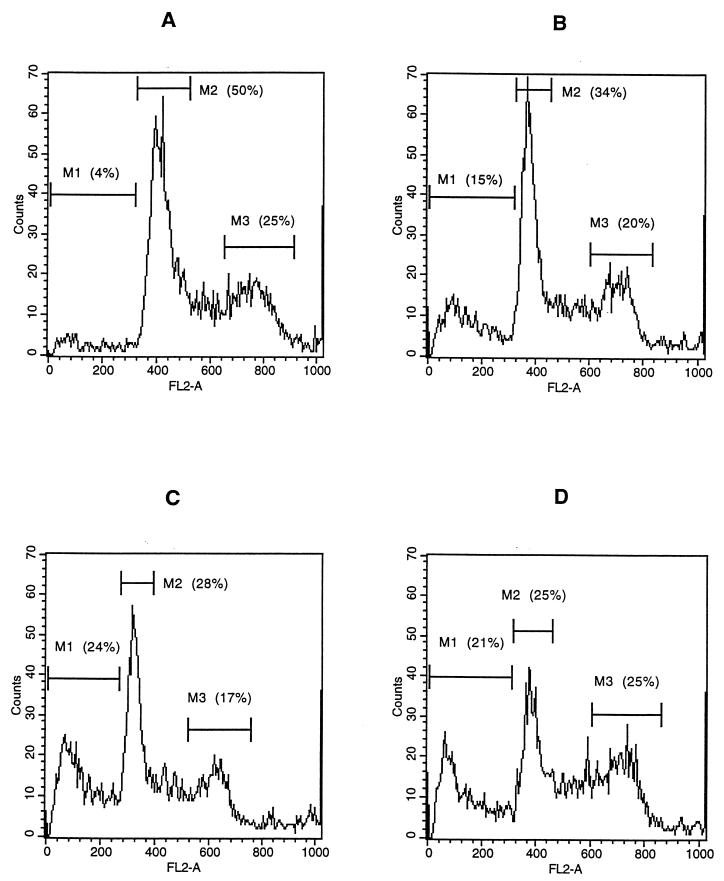
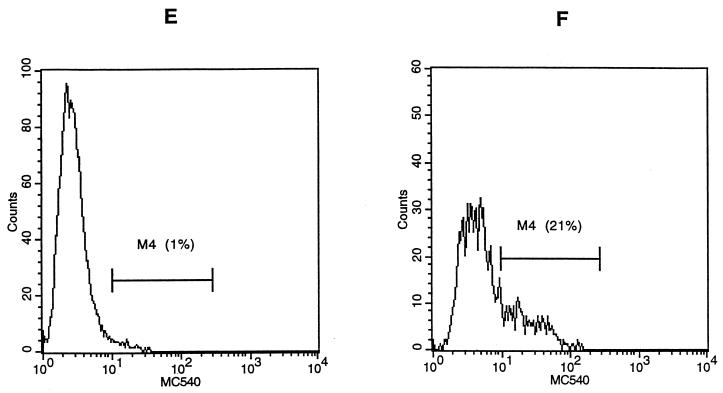
To confirm that the DNA loss induced by H. ducreyi was due to apoptosis, Jurkat T cells were cultured with varying concentrations of H. ducreyi FTWC for 2 to 24 h and stained with PI or MC540. Apoptotic nuclei produce a broad hypodiploid DNA peak, which is easily distinguished from the narrow peak of cells with normal DNA content (11). In contrast to the cells cultured in control medium, large hypodiploid DNA peaks were seen for Jurkat cells as early as 2 h after exposure to 10 μg of FTWC per ml. By 4 to 6 h, up to 25% of the cells had hypodiploid nuclei (Fig. 4). The percentage of cells decreased from 50 to 25% in the G1 phase and remained constant (25%) in the G2/M phase during this period (Fig. 4). The percentage of cells with hypodiploid nuclei was also dependent on the dose of FTWC (compare Fig. 4 to Table 1).
FIG. 4.
Flow cytometric analysis of H. ducreyi-induced apoptosis of Jurkat T cells. The cells were stained with PI after incubation in medium (A) or in medium containing 10 μg of FTWC per ml for 2 (B), 4 (C), or 6 (D) h. Cells were stained with MC540 after incubation in medium (E) or in medium containing 10 μg of FTWC per ml for 24 h (F). M1, cells with hypodiploid nuclei; M2, cells in the G1 phase; M3, cells in the G2/M phase; M4, region of positive MC540 fluorescence. FL2-A, area of DNA fluorescence. The results are representative of at least four independent experiments; approximately 10,000 live cells were analyzed per experiment.
TABLE 1.
Effect of H. ducreyi on Jurkat T cells
| Condition | Description | % Apoptosis at 18 h
for staining with:
|
|
|---|---|---|---|
| PIa | MC540b | ||
| Medium | Control | 9.3 | 1.5 |
| 35000c | Parent | 14.0 | 24.0 |
| 35000HP-RSM1 | Hemolysin mutant | 13.3 | 23.8 |
| 35000.303 | cdtC mutant | 2.4 | 2.2 |
| 35000.303(pLS88) | cdtC mutant and vector | 5.4 | 2.8 |
| 35000.303(pJL300-C) | Complemented cdtC mutant | 14.5 | 30.8 |
Values are representative of four experiments. The FTWC concentration was 1 μg/ml.
Values are representative of five experiments. The FTWC concentration was 10 μg/ml.
35000 and 35000HP gave similar results; for simplicity, only data obtained with 35000 are shown.
MC540 is a lipophilic dye that binds strongly to membranes that have lost their normal phospholipid asymmetry; redistribution of phosphatidylserine loosens phospholipid packing and allows increased binding of MC540 (38). Gating only on cells that had forward and side scatter characteristics of viable cells, increased MC540 staining was easily detected for Jurkat T cells incubated with FTWC (Fig. 4 and Table 1). Thus, exposure of Jurkat T cells to H. ducreyi resulted in morphological alterations such as chromatin condensation and membrane asymmetry that are associated with apoptosis (11, 38).
The toxic effect of H. ducreyi is heat labile and present in cell-free culture supernatants.
Results of time course, dose, and temperature studies indicated that the toxicity of FTWC for Jurkat T cells in concentrations up to 1 μg/ml was nearly eliminated by incubating the FTWC for 2 min at 100°C or for 40 min at 60°C (data not shown and Fig. 2). The proliferation of Jurkat T cells was still suppressed by higher concentrations (10 μg/ml) of FTWC heat treated at 60°C (Fig. 2), suggesting that the preparation also contained an additional heat-stable inhibitor. The proliferation data correlated with the results of the JAM test, in which a lower percentage of DNA loss was detected for cells incubated with heat-treated antigen (Fig. 2).
To test whether the toxic activity was H. ducreyi specific and was cell associated or secreted, FTWC and cell-free culture supernatants were prepared from several members of the family Pasteurellaceae that colonize humans. Only FTWC and supernatants prepared from H. ducreyi and A. actinomycetemcomitans inhibited the growth of Jurkat T cells in a dose-dependent manner (data not shown). Heat treatment at 60°C for 40 min removed the inhibitory activity from both preparations (data not shown), suggesting that the toxic factors produced by the two species were similar.
The toxic effect of H. ducreyi on Jurkat T cells is due to CDT.
H. ducreyi makes two known heat-labile toxins, CDT (8, 10, 20–22, 35–37, 48) and hemolysin (2, 28–30, 51). The former activity is present in cell-free culture supernatants and in cellular preparations; hemolytic activity is cell associated. Some strains of A. actinomycetemcomitans make CDT that is nearly identical to H. ducreyi CDT (25, 41, 49). In Western blots, MAbs that recognize H. ducreyi CdtA, CdtB, and CdtC bound to A. actinomycetemcomitans ATCC 29522 (data not shown).
To analyze whether H. ducreyi CDT was toxic for T cells, we prepared FTWC and supernatants from 35000HP and its isogenic hemolysin mutant 35000HP-RSM1 and from 35000 and its isogenic cdtC mutant, 35000.303. Both culture supernatants (data not shown) and whole cells from 35000, 35000HP, and 35000HP-RSM1 (Fig. 5) inhibited Jurkat T-cell proliferation in a time- and dose-dependent manner and to a similar extent. Supernatants and whole cells made from 35000.303 had no effect on Jurkat T-cell growth (Fig. 5). Supernatants and FTWC prepared from 35000, 35000HP, and 35000HP-RSM1 resulted in DNA loss as measured by the JAM test (Fig. 5), hypodiploid nuclei as measured by PI staining, and membrane unpacking as determined by MC540 staining (Table 1). FTWC prepared from 35000.303 and 35000.303 transformed with the plasmid vector pLS88 did not cause DNA loss, the appearance of hypodiploid nuclei, or increased MC540 staining (Fig. 5 and Table 1). Complementation of 35000.303 with pJL300-C in trans restored the ability of the mutant to inhibit the growth and induce the apoptosis of Jurkat T cells (Fig. 5 and Table 1).
FIG. 5.
Effect of H. ducreyi on proliferation and DNA loss of Jurkat T cells. Panels A and B correspond to those shown in Fig. 2. FTWC were prepared from 35000 (squares), 35000HP-RSM1 (diamonds), 35000.303 (open circles), 35000.303(pLS88) (crosshatched circles), and 35000.303(pJL300-C) (filled circles). Results are expressed as the means ± standard errors of triplicate wells and are representative of three independent experiments.
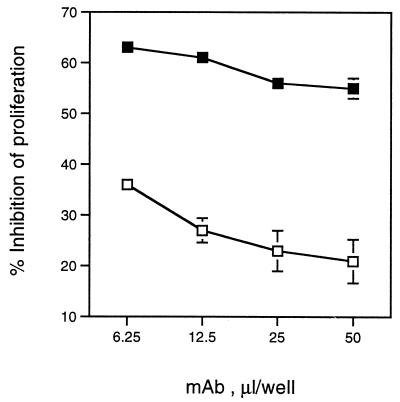
To confirm the role of CDT in inhibition of Jurkat T-cell proliferation, FTWC were incubated with different amounts of H. ducreyi CdtC-directed neutralizing MAbs 9E9 and 13D9 or their isotype control MAb 2B7. Jurkat T cells were added to these solutions and incubated for 24 h. The proliferation of Jurkat T cells was suppressed in the presence of control antibodies by 60% (Fig. 6). H. ducreyi whole cells pretreated with CdtC-neutralizing MAbs were less inhibitory for T cells, and the effect of antibodies was dose dependent (Fig. 6 and data not shown). MAb 13D9 also neutralized the toxic effect of FTWC prepared from A. actinomycetemcomitans ATCC 29522 (data not shown). These data confirm that CDT is responsible for inhibition of T-cell growth.
FIG. 6.
Effect of anti-CdtC MAbs on the ability of H. ducreyi to inhibit proliferation of Jurkat cells. Varying amounts of hybridoma culture supernatants containing the CdtC-neutralizing MAb 9E9 (open squares) or its isotype control (filled squares) were incubated with or without FTWC (3 μg/ml). Jurkat T cells were added to wells and incubated for 24 h. Results are expressed as the percentages of inhibition of [3H]thymidine incorporation in wells without FTWC and the means ± standard errors of triplicate wells and are representative of three independent experiments.
DISCUSSION
The immune response to H. ducreyi is mediated in part by T cells infiltrating the site of infection (17, 23, 31, 46). The T-cell infiltrate occurs within 24 h of infection and is maintained throughout the pustular and ulcerative stages of the disease (17, 23, 31). How H. ducreyi evades the host response is unclear. In this study, we found that H. ducreyi inhibited the proliferation of primary T-cell lines and PBMCs. The toxic activity of H. ducreyi on T-cell growth was in part heat labile, suggesting that H. ducreyi expressed both heat-labile and heat-stable inhibitors of T-cell growth. With Jurkat T cells, inhibition of proliferation was accompanied by induction of apoptosis by several criteria, including membrane unpacking, DNA fragmentation and loss, and the appearance of hypodiploid nuclei (50). The ability of H. ducreyi to induce apoptosis was heat labile, was inhibited by a CdtC-neutralizing MAb, was absent in a cdtC mutant, and was restored by complementation in trans. Taken together, the data suggest that CDT is a major cause of the inhibition of T-cell growth, is the factor responsible for apoptosis of T cells, and may counteract T-cell responses to the organism.
We tested FTWC and cell-free culture supernatants for inhibitory activity. The hemolysin of H. ducreyi is thought to be a pore-forming toxin and is active only in association with live bacteria (28, 29). Since we did not test live 35000.303 for inhibitory activity, we cannot exclude the possibility that the hemolysin may also be toxic for T cells.
H. ducreyi FTWC inhibited the proliferation of primary T-cell lines and PBMCs that had been expanded in the absence of antigen and in the presence of allogeneic feeder cells and IL-2 or mitogen. We subsequently isolated several T-cell lines from biopsy samples of experimental pustules in the presence of heat-treated FTWC, autologous PBMCs, and IL-2 (12a). These lines were composed primarily of CD4 cells and proliferated in response to heat-treated FTWC in a dose-dependent manner but did not proliferate in response to untreated FTWC (data not shown). Thus, H. ducreyi also inhibits the proliferation of antigen-dependent primary T-cell lines. These data imply that measurement of blastogenic responses to H. ducreyi (3) or isolation of antigen-responsive lines should be done with heat-treated antigens or preparations devoid of CDT.
CDT was originally defined by its ability to induce cell swelling and elongation of epithelial cells. Numerous studies done primarily on HeLa cells have shown that CDT induces an irreversible block in the G2 phase of the cell cycle, preventing cells from entering mitosis and eventually resulting in cell death (7, 10, 33, 41, 49, 54). The block in the G2 phase is accompanied by increased levels of tyrosine-phosphorylated cyclin-dependent kinase, cdc2 (cdk1), whose dephosphorylation is required for the formation of an active cdc2-cyclin B complex and entry into mitosis. Cell cycle analysis of CDT-treated epithelial cells has consistently shown an increase in the number of cells with a 4N DNA content. We found that H. ducreyi preparations that contained CDT induced a large decrease in the number of Jurkat T cells in G1, no change in the number of cells in G2/M, and the appearance of hypodiploid nuclei. Thus, the relative proportion of live cells in the G2/M phase increased after exposure to H. ducreyi. We did not test purified CDT or our preparations on epithelial cells. However, the accumulated data raise the possibility that CDT induces both G2/M arrest and apoptosis in lymphocytes and may have somewhat different effects on lymphocytes and epithelial cells.
The CDT of H. ducreyi is encoded by the gene cluster cdtABC (8). The proteins encoded by these genes are highly homologous (91 to 96% identity) to those found in A. actinomycetemcomitans and are also homologous to CDTs found in Escherichia coli, Shigella spp., and Campylobacter spp. (8, 25, 33, 34, 39, 41, 49). For all species, the three gene products have putative signal sequences, and expression of all three gene products is required for CDT activity in cell-free culture supernatants. However, the functions of the individual gene products are unclear. Using a MAb that neutralizes H. ducreyi cytotoxic activity, Lagergard and coworkers purified what was later proven to be CdtC from cell-free culture supernatants (37). Immunoaffinity-purified H. ducreyi CdtC induces cell cycle arrest of epithelial cell lines in the G2 phase (10). MAbs that neutralize the cytotoxic activity of H. ducreyi for epithelial cells (8, 10, 48) and for T cells bind to CdtC. Taken together, the data suggest that expression of CdtC is required for activity of H. ducreyi CDT. CdtC may be the active toxin, may be required to activate CdtA or CdtB, or may act in concert with CdtA and CdtB to promote cytotoxicity.
A. actinomycetemcomitans produces several toxins active on lymphoid cells, including a 110-kDa pore-forming leukotoxin (27, 44), a secreted 80- to 85-kDa toxin that induces apoptosis in B cells (27), and a 14-kDa cytoplasmic protein that inhibits proliferation of T cells (19). A toxin called immunosuppressive factor (ISF) was originally isolated as a 60-kDa protein from sonic extracts of the organism (40, 42, 43). ISF is heat labile and inhibits DNA, RNA, and protein synthesis in activated T lymphocytes. An unusual population of CD4+ CD8+ dual-positive T cells that are arrested in the G2/M phase of the cell cycle is induced when PBMCs are incubated with mitogen and ISF (42). ISF was recently purified by methods similar to those used to isolate the 60-kDa protein (42, 43), and the toxic fraction contained a predominant 35-kDa protein (41). The N-terminal amino acid sequence of the Immobilon-transferred 35-kDa protein is identical to A. actinomycetemcomitans CdtB. Activated lymphocytes are five times more sensitive to the toxic fraction than are HeLa cells, and this preparation induces the appearance of CD4+ CD8+ dual-positive T cells arrested in the G2/M phase of the cell cycle. Although the toxic fraction causes cell elongation and distention of HeLa cells, this effect is not seen for T cells, suggesting different mechanisms of action for the two cell types. The data suggest that A. actinomycetemcomitans CdtB is capable of all biologic properties attributable to ISF and CDT (41). However, polyclonal serum raised to His-tagged recombinant CdtC encoded by A. actinomycetemcomitans neutralizes cytotoxic activity for epithelial cells, while serum raised to A. actinomycetemcomitans CdtB lacks neutralizing activity (49).
Previous studies demonstrated that H. ducreyi CDT has toxic activity for many epithelial cell lines including human keratinocytes (10, 48). Our studies have shown that the spectrum of CDT toxicity also includes human T cells and that CDT induces apoptotic Jurkat T-cell death. Studies that address whether H. ducreyi induces apoptosis of T cells that infiltrate experimental lesions will be facilitated by comparisons between lesions caused by the isogenic cdtC mutant and its parent in human volunteers and are in progress in our laboratories.
ACKNOWLEDGMENTS
This work was supported by grants AI31494, AI27863, AI32011, and MO1RR00750 from the National Institutes of Health.
We thank Zacharie Brahmi, Byron Batteiger, and Iwona Stroynowski for their thoughtful criticism of the manuscript; Kate Fortney for her excellent technical assistance; and Barry Katz for statistical support.
REFERENCES
- 1.Abeck D, Korting H C, Zaba R, Dangor Y, Fehler G, Ballard R C. Soluble interleukin-2 receptors in serum and urine of patients with chancroid and their response to therapy. Int J Sex Transm Dis AIDS. 1990;1:282–284. doi: 10.1177/095646249000100411. [DOI] [PubMed] [Google Scholar]
- 2.Alfa M J, Degagne P, Totten P A. Haemophilus ducreyihemolysin acts as a contact cytotoxin and damages human foreskin fibroblasts in cell culture. Infect Immun. 1996;64:2349–2352. doi: 10.1128/iai.64.6.2349-2352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Tawfiq J A, Palmer K L, Chen C-Y, Haley J C, Katz B P, Hood A F, Spinola S M. Experimental infection of human volunteers with Haemophilus ducreyidoes not confer protection against subsequent challenge. J Infect Dis. 1999;179:1283–1287. doi: 10.1086/314732. [DOI] [PubMed] [Google Scholar]
- 4.Al-Tawfiq J A, Thornton A C, Katz B P, Fortney K R, Todd K D, Hood A F, Spinola S M. Standardization of the experimental model of Haemophilus ducreyiinfection in human subjects. J Infect Dis. 1998;178:1684–1687. doi: 10.1086/314483. [DOI] [PubMed] [Google Scholar]
- 5.Blackmore C A, Limpakarnjanarat K, Rigau-Perez J G, Albritton W L, Greenwood J R. An outbreak of chancroid in Orange County, California: descriptive epidemiology and disease-control measures. J Infect Dis. 1985;151:840–844. doi: 10.1093/infdis/151.5.840. [DOI] [PubMed] [Google Scholar]
- 6.Brunham R C, Ronald A R. Epidemiology of sexually transmitted diseases in developing countries. In: Wasserheit J N, Aral S O, Holmes K K, Hitchcock P J, editors. Research issues in human behavior and sexually transmitted diseases in the AIDS era. Washington, D.C: American Society for Microbiology; 1991. pp. 61–80. [Google Scholar]
- 7.Comayras C, Tasca C, Peres S Y, Ducommun B, Oswald E, De Rycke J. Escherichia coli cytolethal distending toxin blocks the HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infect Immun. 1997;65:5088–5095. doi: 10.1128/iai.65.12.5088-5095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cope L D, Lumbley S, Latimer J L, Klesney-Tait J, Stevens M K, Johnson L S, Purven M, Munson R S, Jr, Lagergard T, Radolf J D, Hansen E J. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cope L D, Yogev R, Muller-Eberhard U, Hansen E J. A gene cluster involved in the utilization of both free heme and heme:hemopexin by Haemophilus influenzaetype b. J Bacteriol. 1995;177:2644–2653. doi: 10.1128/jb.177.10.2644-2653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortes-Bratti X, Chaves-Olarte E, Lagergard T, Thelestam M. The cytolethal distending toxin from the chancroid bacterium Haemophilus ducreyiinduces cell-cycle arrest in the G2 phase. J Clin Investig. 1999;103:107–115. doi: 10.1172/JCI3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darzynkiewicz Z, Bruno S, Del Bino G, Gorczyca W, Hotz M A, Lassota P, Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- 12.Fleischer B. Non-specific propagation of human antigen-dependent T lymphocyte clones. J Immunol Methods. 1988;109:215–219. doi: 10.1016/0022-1759(88)90245-1. [DOI] [PubMed] [Google Scholar]
- 12a.Gelfanova V P, Fortney K R, Al-Tawfiq J A, Palmer K L, Spinola S M. Abstracts of the 99th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1999. Isolation of Haemophilus ducreyi-specific T cell lines from lesion of experimentally infected human subjects, abstr. B/D-251; p. 78. [Google Scholar]
- 13.Hammond G W, Slutchuk M, Scatliff J, Sherman E, Wilt J C, Ronald A R. Epidemiologic, clinical, laboratory, and therapeutic features of an urban outbreak of chancroid in North America. Rev Infect Dis. 1980;2:867–879. doi: 10.1093/clinids/2.6.867. [DOI] [PubMed] [Google Scholar]
- 14.Hayes R J, Schulz K F, Plummer F A. The cofactor effect of genital ulcers on the per-exposure risk of HIV transmission in sub-Saharan Africa. J Trop Med Hyg. 1995;98:1–8. [PubMed] [Google Scholar]
- 15.Hiltke T J, Bauer M E, Klesney-Tait J, Hansen E J, Munson R S, Jr, Spinola S M. Effect of normal and immune sera on Haemophilus ducreyi35000HP and its isogenic MOMP and LOS mutants. Microb Pathog. 1999;26:93–102. doi: 10.1006/mpat.1998.0250. [DOI] [PubMed] [Google Scholar]
- 16.Jessamine P G, Ronald A R. Chancroid and the role of genital ulcer disease in the spread of human retrovirus. Med Clin N Am. 1990;74:1417–1431. doi: 10.1016/s0025-7125(16)30488-6. [DOI] [PubMed] [Google Scholar]
- 17.King R, Gough A, Nasio J, Ndinya-Achola F, Plummer F, Wilkins J. An immunohistochemical analysis of naturally occurring chancroid. J Infect Dis. 1996;174:427–430. doi: 10.1093/infdis/174.2.427. [DOI] [PubMed] [Google Scholar]
- 18.Kish L. Survey sampling. New York, N.Y: John Wiley and Sons, Inc.; 1965. [Google Scholar]
- 19.Kurita-Ochiai T, Ochiai K. Immunosuppressive factor from Actinobacillus actinomycetemcomitansdown regulates cytokine production. Infect Immun. 1996;64:50–54. doi: 10.1128/iai.64.1.50-54.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagergard T. The role of Haemophilus ducreyibacteria, cytotoxin, endotoxin and antibodies in animal models for study of chancroid. Microb Pathog. 1992;13:203–217. doi: 10.1016/0882-4010(92)90021-f. [DOI] [PubMed] [Google Scholar]
- 21.Lagergard T, Purven M. Neutralizing antibodies to Haemophilus ducreyicytotoxin. Infect Immun. 1993;61:1589–1592. doi: 10.1128/iai.61.4.1589-1592.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagergard T, Purven M, Frisk A. Evidence of Haemophilus ducreyiadherence to and cytotoxin destruction of human epithelial cells. Microb Pathog. 1993;14:417–431. doi: 10.1006/mpat.1993.1041. [DOI] [PubMed] [Google Scholar]
- 23.Magro C M, Crowson A N, Alfa M, Nath A, Ronald A, Ndinya-Achola J O, Nasio J. A morphological study of penile chancroid lesions in human immunodeficiency virus (HIV)-positive and -negative African men with a hypothesis concerning the role of chancroid in HIV transmission. Hum Pathol. 1996;27:1066–1070. doi: 10.1016/s0046-8177(96)90285-3. [DOI] [PubMed] [Google Scholar]
- 24.Matzinger P. The JAM test. A simple assay for DNA fragmentation and cell death. J Immunol Methods. 1991;145:185–192. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 25.Mayer M P A, Bueno L C, Hansen E J, Dirienzo J M. Identification of a cytolethal distending toxin gene locus and features of a virulence-associated region in Actinobacillus actinomycetemcomitans. Infect Immun. 1999;67:1227–1237. doi: 10.1128/iai.67.3.1227-1237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morse S A. Chancroid and Haemophilus ducreyi. Clin Microbiol Rev. 1989;2:137–157. doi: 10.1128/cmr.2.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohguchi M, Ishisaki A, Okahashi N, Koide M, Koseki T, Yamato K, Noguchi T, Nishihara T. Actinobacillus actinomycetemcomitans toxin induces both cell cycle arrest in the G2/M phase and apoptosis. Infect Immun. 1998;66:5980–5987. doi: 10.1128/iai.66.12.5980-5987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer K L, Goldman W E, Munson R S., Jr An isogenic hemolysin-deficient mutant of Haemophilus ducreyilacks the ability to produce cytopathic effects on human foreskin fibroblasts. Mol Microbiol. 1996;21:13–19. doi: 10.1046/j.1365-2958.1996.00615.x. [DOI] [PubMed] [Google Scholar]
- 29.Palmer K L, Grass S, Munson R S., Jr Identification of a hemolytic activity elaborated by Haemophilus ducreyi. Infect Immun. 1994;62:3041–3043. doi: 10.1128/iai.62.7.3041-3043.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer K L, Munson R S., Jr Cloning and characterization of the genes encoding the haemolysin of Haemophilus ducreyi. Mol Microbiol. 1995;18:821–830. doi: 10.1111/j.1365-2958.1995.18050821.x. [DOI] [PubMed] [Google Scholar]
- 31.Palmer K L, Schnizlein-Bick C T, Orazi A, John K, Chen C-Y, Hood A F, Spinola S M. The immune response to Haemophilus ducreyiresembles a delayed-type hypersensitivity reaction throughout experimental infection of human subjects. J Infect Dis. 1998;178:1688–1697. doi: 10.1086/314489. [DOI] [PubMed] [Google Scholar]
- 32.Palmer K L, Thornton A C, Fortney K R, Hood A F, Munson R S, Jr, Spinola S M. Evaluation of an isogenic hemolysin-deficient mutant in the human model of Haemophilus ducreyiinfection. J Infect Dis. 1998;178:191–199. doi: 10.1086/515617. [DOI] [PubMed] [Google Scholar]
- 33.Peres S Y, Marches O, Daigle F, Nougayrede J-P, Herault F, Tasca C, DeRycke J, Oswald E. A new cytolethal distending toxin (CDT) from Escherichia coliproducing CNF2 blocks HeLa cell division in G2/M phase. Mol Microbiol. 1997;24:1095–1107. doi: 10.1046/j.1365-2958.1997.4181785.x. [DOI] [PubMed] [Google Scholar]
- 34.Pickett C L, Pesci E C, Cottle D L, Russell G, Erdem A N, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtBgenes. Infect Immun. 1996;64:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purven M, Falsen E, Lagergard T. Cytotoxin production in 100 strains of Haemophilus ducreyifrom different geographic locations. FEMS Microbiol Lett. 1995;129:221–224. doi: 10.1111/j.1574-6968.1995.tb07583.x. [DOI] [PubMed] [Google Scholar]
- 36.Purven M, Lagergard T. Haemophilus ducreyi, a cytotoxin-producing bacterium. Infect Immun. 1992;60:1156–1162. doi: 10.1128/iai.60.3.1156-1162.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purven M, Frisk A, Lonnroth I, Lagergard T. Purification and identification of Haemophilus ducreyicytotoxin by use of a neutralizing monoclonal antibody. Infect Immun. 1997;65:3496–3499. doi: 10.1128/iai.65.8.3496-3499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reid S, Cross R, Snow E C. Combined Hoechst 33342 and merocyanine 540 staining to examine murine B cell cycle stage, viability and apoptosis. J Immunol Methods. 1996;192:43–54. doi: 10.1016/0022-1759(96)00004-x. [DOI] [PubMed] [Google Scholar]
- 39.Scott D A, Kaper J B. Cloning and sequencing of the genes encoding Escherichia colicytolethal distending toxin. Infect Immun. 1994;62:244–251. doi: 10.1128/iai.62.1.244-251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shenker B J, McArthur W P, Tsai C C. Immune suppression induced by Actinobacillus actinomycetemcomitans. I. Effects on human peripheral blood lymphocyte responses to mitogens and antigens. J Immunol. 1982;128:148–154. [PubMed] [Google Scholar]
- 41.Shenker B J, McKay T, Datar S, Miller M, Chowhan R, Demuth D. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2arrest in human T cells. J Immunol. 1999;162:4773–4780. [PubMed] [Google Scholar]
- 42.Shenker B J, Vitale L, King C. Induction of human T cells that coexpress CD4 and CD8 by an immunomodulatory protein produced by Actinobacillus actinomycetemcomitans. Cell Immunol. 1995;164:36–46. doi: 10.1006/cimm.1995.1140. [DOI] [PubMed] [Google Scholar]
- 43.Shenker B J, Vitale L A, Welham D A. Immune suppression induced by Actinobacillus actinomycetemcomitans: effects on immunoglobulin production by human B cells. Infect Immun. 1990;58:3856–3862. doi: 10.1128/iai.58.12.3856-3862.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simpson D L, Berthold P, Taichman N S. Killing of human myelomonocytic leukemia and lymphocytic cell lines by Actinobacillus actinomycetemcomitansleukotoxin. Infect Immun. 1988;56:1162–1166. doi: 10.1128/iai.56.5.1162-1166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spinola S M, Griffiths G E, Bogdan J A, Menegus M A. Characterization of an 18,000-molecular-weight outer membrane protein of Haemophilus ducreyithat contains a conserved surface-exposed epitope. Infect Immun. 1992;60:385–391. doi: 10.1128/iai.60.2.385-391.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spinola S M, Orazi A, Arno J N, Fortney K, Kotylo P, Chen C-Y, Campagnari A A, Hood A F. Haemophilus ducreyielicits a cutaneous infiltrate of CD4 cells during experimental human infection. J Infect Dis. 1996;173:394–402. doi: 10.1093/infdis/173.2.394. [DOI] [PubMed] [Google Scholar]
- 47.Spinola S M, Wild L M, Apicella M A, Gaspari A A, Campagnari A A. Experimental human infection with Haemophilus ducreyi. J Infect Dis. 1994;169:1146–1150. doi: 10.1093/infdis/169.5.1146. [DOI] [PubMed] [Google Scholar]
- 48.Stevens M K, Latimer J L, Lumbley S R, Ward C K, Cope L D, Lagergard T, Hansen E J. Characterization of a Haemophilus ducreyimutant deficient in the production of the cytolethal distending toxin. Infect Immun. 1999;67:3900–3908. doi: 10.1128/iai.67.8.3900-3908.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sugai M, Kawamoto T, Peres S Y, Ueno Y, Komatsuzawa H, Fujiwara T, Kurihara H, Suginaka H, Oswald E. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitansis a cytolethal distending toxin. Infect Immun. 1998;66:5008–5019. doi: 10.1128/iai.66.10.5008-5019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1461. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 51.Totten P, Norn D, Stamm W. Characterization of the hemolytic activity of Haemophilus ducreyi. Infect Immun. 1995;63:4409–4416. doi: 10.1128/iai.63.11.4409-4416.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Laer L, Vingerhoets J, Vanham G, Kestens L, Bwayo J, Otido J, Piot P, Roggen E. In vitro stimulation of peripheral blood mononuclear cells (PBMC) from HIV− and HIV+ chancroid patients by Haemophilus ducreyiantigens. Clin Exp Immunol. 1995;102:243–250. doi: 10.1111/j.1365-2249.1995.tb03772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wasserheit J N. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19:61–77. [PubMed] [Google Scholar]
- 54.Whitehouse C A, Balbo P B, Pesci E C, Cottle D L, Mirabito P M, Pickett C L. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun. 1998;66:1934–1940. doi: 10.1128/iai.66.5.1934-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]