Significance
The incidence of type 1 and type 2 diabetes is increasing worldwide. Senescence in pancreatic beta cells is a major contributor to diabetes development. New treatments for cellular senescence are needed to prevent diabetes and its comorbidities. Available senolytics kill senescent cells and reduce cell mass. Here, we discovered that the endogenous lipids palmitic acid hydroxy stearic acids (PAHSAs) prevent and reverse beta cell senescence in autoimmune diabetic mice, clonal beta cells, and human pancreatic islets exposed to metabolic stressors. PAHSAs act by inducing expression of negative regulators of p53 such as Mdm2 and by enhancing protective pathways such as glutathione metabolism and DNA repair. Therefore, PAHSAs have the therapeutic potential to modulate senescence to prevent and reverse diabetes.
Keywords: diabetes, pancreatic islets, cellular senescence, metabolic stress, lipids
Abstract
Senescence in pancreatic beta cells plays a major role in beta cell dysfunction, which leads to impaired glucose homeostasis and diabetes. Therefore, prevention of beta cell senescence could reduce the risk of diabetes. Treatment of nonobese diabetic (NOD) mice, a model of type 1 autoimmune diabetes (T1D), with palmitic acid hydroxy stearic acids (PAHSAs), a novel class of endogenous lipids with antidiabetic and antiinflammatory effects, delays the onset and reduces the incidence of T1D from 82% with vehicle treatment to 35% with PAHSAs. Here, we show that a major mechanism by which PAHSAs protect islets of the NOD mice is by directly preventing and reversing the initial steps of metabolic stress–induced senescence. In vitro PAHSAs increased Mdm2 expression, which decreases the stability of p53, a key inducer of senescence-related genes. In addition, PAHSAs enhanced expression of protective genes, such as those regulating DNA repair and glutathione metabolism and promoting autophagy. We demonstrate the translational relevance by showing that PAHSAs prevent and reverse early stages of senescence in metabolically stressed human islets by the same Mdm2 mechanism. Thus, a major mechanism for the dramatic effect of PAHSAs in reducing the incidence of type 1 diabetes in NOD mice is decreasing cellular senescence; PAHSAs may have a similar benefit in humans.
A major pathogenic factor in both type 1 and type 2 diabetes is pancreatic beta cell dysfunction. In type 1 diabetes, autoimmune attack on beta cells causes cytokine-induced stress (1), endoplasmic reticulum stress (2), and other forms of metabolic stress that result in beta cell destruction. In type 2 diabetes, stresses such as chronic inflammation, glucolipotoxicity, oxidative stress, mitochondrial dysfunction, and persistent endoplasmic reticulum (ER) stress, impair beta cell function and insulin secretion (3–5). A major contributing factor to the development of both type 1 (6) and type 2 (7) diabetes is the appearance of a population of senescent and dysfunctional beta cells. Cellular senescence is a stress response in which cells can no longer respond to proliferative signals but remain metabolically active and secrete cytokines and chemokines that constitute the “senescence-associated secretory phenotype”, known as SASP (8–11). The SASP recruits immune cells locally to clear senescent cells but can also lead to dysfunction and the entry of neighboring cells into senescence. Therefore, there is a need to discover treatments that will prevent and reverse the dysfunctional beta cell phenotype and the activation of senescence pathways in order to blunt the progression to diabetes or even reverse it.
Changes in lipid metabolism are associated with cellular senescence and can contribute to organismal aging (12–15). Certain lipids, such as omega-3 fatty acids, reduce the incidence of type 1 diabetes in mouse models (16), although no information is available about their effects on beta cell senescence. In addition to being fundamental elements of cellular membranes and a key energy source for cellular metabolism via beta oxidation, lipids play important roles as signaling molecules that modify cellular responses (15). In 2014, we identified a class of previously unknown signaling lipids named branched fatty acid esters of hydroxy fatty acids (FAHFAs). A subfamily of FAHFAs known as palmitic acid esters of hydroxy stearic acids (PAHSAs) exert antidiabetic and antiinflammatory effects that improve glucose homeostasis and lower inflammation in mouse models of both type 1 and type 2 diabetes (17–19). We found that PAHSA treatment reduced ER stress and delayed the onset and markedly reduced type 1 diabetes incidence in nonobese diabetic (NOD) mice (18). In addition, in insulin-resistant mice on a high-fat diet, PAHSAs improved insulin sensitivity and glucose tolerance and reduced adipose tissue inflammation (17–19). In human islets, PAHSAs augmented glucose-stimulated insulin secretion (17–19) and restored normal pulsatility of insulin secretion in islets from people with type 2 diabetes (20). While some of the protective effects are likely to result from alterations in immune responses, the presence of protective effects of PAHSAs in a beta cell line and in human islets ex vivo indicates direct effects on beta cells independent of immune modulation.
Therefore, in this study, we sought to determine the mechanisms underlying the beneficial effects of PAHSAs on beta cells. We first performed RNA-sequencing (RNAseq) analysis on islets from control and PAHSA-treated NOD mice. Dispersed isolated islets were sorted by fluorescence-activated cell sorting (FACS) to remove immune cells, and the analysis was performed on the nonimmune cells in the islets. We found that genes involved in pathways related to senescence, SASP, DNA repair, and glutathione (GSH) metabolism were highly modified by PAHSAs. While there are no universal markers for cellular senescence and the senescent phenotypes vary by cell type, we found that PAHSAs prevented up-regulation of classic senescence-related genes, such as p21Cip1 and p16Ink4a, and enhanced expression of protective genes, such as DNA repair and GSH metabolism–related genes, in islets of NOD mice. PAHSAs also efficiently preserved nuclear high mobility group box 1 (HMGB1) in beta cells in NOD mice; nuclear exclusion of HMGB1 is a key marker for senescence (21).
Next, we demonstrated that PAHSAs directly modulate the initiation of senescence in pancreatic beta cells in three models of metabolic stress known to induce senescence (glucose toxicity, ER stress, and cytokines) using a beta cell line MIN6. Using these stress models, we identified Mdm2 as a key target of PAHSAs. Mdm2 is an oncoprotein that decreases the stability of p53 (22), a tumor suppressor that can induce cell death or promote entry into senescence. PAHSAs induce Mdm2 expression and suppress p53 in NOD islets. We demonstrate similar PAHSA effects in human islets subjected to metabolic stress with a conserved effect on the Mdm2–p53 pathway. Thus, PAHSAs constitute an effective treatment to reduce cellular stress and initiation of metabolic stress–induced cell senescence in a mouse model of type 1 diabetes and in human islets.
Results
PAHSA Treatment of NOD Mice Preserved the Beta Cell Phenotype and Protected against Senescence.
We performed bulk RNAseq in islets from female NOD mice treated for 6 wk with a combination of two different biologically active PAHSA isomers, 5- and 9-PAHSA, or vehicle starting at 4 wks of age. We previously reported that treatment with PAHSAs exerts effects on immune cell populations (18, 19, 23). Therefore, to avoid confounding results on gene expression, the isolated islets were dispersed and sorted by FACS (6) to remove the immune cells usually found in NOD islets. We chose a 6-wk treatment period because typically, in our animal facility, NOD mice do not develop diabetes until 13 wks of age or later. At 10 wks of age, a significant amount of insulitis was present, and the total number of CD45+ cells/g pancreas was similar in vehicle- and PAHSA-treated NOD mice (18). At 15 wks of age, the total number of CD45+ cells had not changed in PAHSA-treated NOD mice, but it increased 2.5-fold in the vehicle-treated mice. We wanted to define the islet transcriptome before mice developed hyperglycemia to avoid its effects on beta cell gene expression. All mice were normoglycemic at the time the islets were isolated (Fig. 1A).
Fig. 1.
PAHSA treatment of NOD mice preserves beta cell phenotype and protects beta cells from senescence. (A) NOD mice were treated with either PAHSAs (5- and 9-PAHSA) or vehicle by oral gavage for 6 wks from 4 wks of age until 10 wks of age before hyperglycemia occurred. (B–D) RNAseq data analysis from NOD mouse islets revealed PAHSA effects on the preservation of the expression of genes responsible for maintenance of islet identity and hormones (B), down-regulation of disallowed genes (C), and apoptosis (D). (E) Quantification of TUNEL assay in the beta cells of pancreas sections from NOD mice treated with a combination of 5- and 9-PAHSA or vehicle for 6 wk (n = 5 to 7; *P < 0.05 by two-tailed Student’s t test). (F–H) PAHSA treatment of NOD mice also prevented up-regulation of aging-related (F), senescence-related (G), and SASP-related (H) genes. (I and J). Genes involved in DNA repair (I) and GSH metabolism (J) were maintained with PAHSA treatment. (K) Immunohistochemistry and quantification of pancreas sections from the same NOD mice as in A showing P21Cip1 and P16Ink4a (green), insulin (red), and DAPI (blue). Quantification of p21Cip1 or p16Ink4 was also performed in alpha cells; n = 5 to 7 mice/group. (Scale bars, 50 μm.) Data are shown as means ± SEM; *P < 0.001 versus vehicle p21cip1 or p16ink4. Data were analyzed by two-tailed Student’s t test. (L and M) Immunohistochemistry and quantification of pancreas sections from prehyperglycemic NOD mice (11 wk of age) and hyperglycemic (for vehicle-treated mice) NOD mice (15 wks of age) treated for 6 or 11 wks with either PAHSAs or vehicle. (L) Nuclear HMGB1 (green), insulin (magenta), and DAPI images are shown as well as quantification of HMGB1 at 11 wks of age in alpha cells. (M) γH2Ax (green), insulin (magenta), and DAPI images are shown as well as image quantification; n = 4 to 6 (HMGB1) mice/group. (Scale bar, 50 μm.) Data are shown as means ± SEM (*P < 0.001 versus vehicle HMGB1 [L] or γH2Ax [M]) and were analyzed by two-tailed Student’s t test.
The principal component analysis (PCA) of the RNAseq data showed that one sample in each group was an outlier (SI Appendix, Fig. S1A). Development of diabetes in NOD mice is heterogeneous; some mice become hyperglycemic early, others late, and some not at all. Although none of the mice used in the RNAseq study were hyperglycemic at the time that they were killed, it is common to find NOD mice that develop other illnesses, which could explain the biology behind these two outliers (24). After removing these two outlier samples, there was distinct clustering of genes between the vehicle- and PAHSA-treated NOD mice (SI Appendix, Fig. S1B). The expression of 1,169 genes was statistically different, with 461 up-regulated and 708 down-regulated genes identified in PAHSA-treated versus vehicle-treated mice. Among the most down-regulated genes were major components of the SASP, including interleukin-6 (Il6) and Filip-1l (SI Appendix, Fig. S1C). The beta cell identity marker urocortin 3 (Ucn-3), which is exclusively expressed in murine beta cells (25), and the DNA repair factor MutY DNA glycosylase (Mutyh; induced 84.6-fold) were among the top up-regulated genes (SI Appendix, Fig. S1C). Pathway analysis showed that PAHSA treatment of NOD mice up-regulated expression of beta cell identity (Fig. 1B), GSH metabolism, DNA repair, and cell cycle (i.e., Cbx2) genes and down-regulated senescence genes (i.e., Mcl1), cytokines, and genes involved in ER stress, apoptosis (i.e., Casp4) (Fig. 1D), or cancer (SI Appendix, Figs. S1 D and E and S2 A–D).
Further analysis indicated that PAHSAs preserved islet cell identity by complementary mechanisms. PAHSAs up-regulated expression of multiple genes associated with mature and functional beta cells (Fig. 1B and SI Appendix, Figs. S1 D and E and S2A) as well as genes important for other islet cell types, such as the alpha cell gene glucagon (Glc) (Fig. 1B and SI Appendix, Fig. S2 A and E). PAHSAs also preserved beta cell identity by preventing the up-regulation of “disallowed” genes, for example, Ldha (lactate dehydrogenase), Oat (ornithine aminotransferase), and the solute carrier transporter Scl16a2, which are normally repressed or expressed at very low levels in beta cells (26) but are induced in NOD mouse beta cells (Fig. 1C and SI Appendix, Fig. S2 A and E). This up-regulation of disallowed genes, often referred to as dedifferentiation, leads to dysfunctional cells and reduced functional beta cell mass (27). The majority of genes regulated by transcription factors that promote dedifferentiation (28), such as Foxo1, Arx, or Pbx1, were down-regulated by PAHSAs (SI Appendix, Fig. S3A). However, the majority of genes controlled by transcription factors influencing the beta cell phenotype, such as NFIA, RFX3, or Med2A, were up-regulated by PAHSAs (SI Appendix, Fig. S3B).
Down-regulation of apoptosis (Fig. 1 D and E) is consistent with our previous data that apoptosis was reduced in islets from PAHSA-treated compared to vehicle-treated NOD mice and in PAHSA-treated clonal beta cells (18). In our RNAseq analysis, PAHSAs down-regulated some genes related to the Bcl2 pathway, such as Bnip3 and Bnip3l (Fig. 1D), which can induce autophagy and apoptosis or necrosis following mitochondrial membrane permeabilization (29). In addition, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining in pancreas sections from NOD mice from the same age and treatments as those from the RNAseq experiments demonstrated that the percentage of apoptotic beta cells was decreased ∼60% in PAHSA-treated NOD mice compared to vehicle-treated mice (Fig. 1E). Overall, these data show that at the gene level, PAHSAs preserved the mature and functional beta cell phenotype in NOD mice and reduced apoptosis in the islet endocrine cell population.
Additional genes related to aging and to cellular senescence (9) displayed down-regulation by PAHSA treatment (Fig. 1 F and G and SI Appendix, Fig. S2B). Genes commonly up-regulated either in cell aging, such as Ncor2 (29) and Eps8l1, or in senescent cells, such as prosurvival/antiapoptotic Bcl2 family genes Mcl-1 and Bcl2l1 (6, 7), were decreased in islets of PAHSA-treated NOD mice (Fig. 1 F–G). JunB (30), an upstream regulator of p16Ink4, an effector of cellular senescence, was also decreased with PAHSAs. Several SASP factor genes were also down-regulated with treatment (Fig. 1H and SI Appendix, Fig. S2 B and E). Il6 was the most down-regulated gene, decreasing 93.2-fold in the PAHSA-treated mice, (SI Appendix, Fig. S2C). Igfbp5 (Fig. 1G), IL11 and IL34 (31), Ccl2 and Cxcl14 (Fig. 1H), and SASP transcription factor STAT3 (SI Appendix, Fig. S3D) were also down-regulated.
PAHSAs also up-regulated several cell protective pathways, such as autophagy (Sqstm1) (Fig. 1F), DNA damage response, and DNA repair, that are commonly altered in senescence (32, 33) (Fig. 1I). These data suggest that PAHSAs enhance DNA repair in damaged islet cells. The up-regulation of genes involved in GSH metabolism, which protects against reactive oxygen species (ROS) and glucose toxicity (34), with PAHSA treatment (Fig. 1J and SI Appendix, Fig. S2 D and E) suggests that this may be another protective pathway by which PAHSAs preserve the beta cell phenotype in NOD mice. Overall, these results suggest that under the metabolic stress conditions present in pancreatic islets of NOD mice, PAHSAs exert their beneficial effects by preventing increased expression of genes involved in triggering and promoting senescence and by increasing the expression of genes related to DNA repair and GSH metabolism, thus reducing oxidative stress and progression to senescence.
Protein Levels of Hallmark Genes for Beta Cell Senescence Are Regulated by PAHSA Treatment in NOD Mice.
We did not find differences in expression of the drivers of cellular senescence (9) p21Cip1 or p16Ink4a in the bulk RNAseq in islets from NOD mice, but we found effects on the protein level by immunofluorescence staining. In pancreas from similarly treated mice (before development of hyperglycemia), fewer beta cells stained for nuclear P21Cip1 (22% with vehicle treatment compared to 5% with PAHSA treatment; Fig. 1K). Additionally, P16Ink4a immunostaining was three-fold more intense in beta cells in vehicle-treated NOD mice than in PAHSA-treated mice (Fig. 1K). Exclusion of nuclear HMGB1 protein is an established marker of senescent cells (21, 31). In normoglycemic 11-wk–old NOD mice, only 16% of the beta cells had nuclear HMGB1, whereas with 6 wks of PAHSA treatment, 70% of the beta cells maintained nuclear HMGB1, further indicating that PAHSAs prevented beta cell senescence. This difference persisted in NOD mice at 15 wks of age (11 wks of treatment) when the vehicle-treated, but not the PAHSA-treated, mice had become hyperglycemic (Fig. 1L). Because unrepaired DNA damage induces cellular senescence, we performed immunostaining for the sensitive molecular marker of DNA damage γH2Ax (35). In normoglycemic mice, the percentage of γH2Ax-positive beta cells was 37% in vehicle-treated mice versus 16% in PAHSA-treated mice (Fig. 1M). By 15 wk of age (11 wk of vehicle or PAHSA treatment), the percentage of beta cells positive for γH2Ax was higher than in the younger animals but was still significantly lower in PAHSA-treated mice (22% of beta cells) than in the now hyperglycemic vehicle-treated mice (56% of beta cells; Fig. 1M). At both ages, PAHSA treatment prevented DNA damage in beta cells, contributing to maintenance of the healthy and functional beta cell population.
These results demonstrate that PAHSA treatment in NOD mice has beneficial effects on key molecules that regulate senescence in the islet and effectively prevents DNA damage, one of the main triggers of cellular senescence, which was present in beta cells of NOD mice even before the onset of hyperglycemia. Recently it has been reported that different cell types in isolated mouse islets respond in a similar manner to cytokine exposure (36). Therefore, we investigated the presence of senescence in alpha cells, the second major cell type in pancreatic islets, in the same pancreatic sections of 11-wk–old vehicle-treated NOD mice. Our data show that alpha cells also became senescent (Fig. 1 K and L). However, the frequency of senescent alpha cells was much lower than beta cells for all the markers examined: 22% of beta cells and 5% of the alpha cells for p21Cip1, higher intensity staining for p16ink4 in beta cells (33 pixels/μm2) than in alpha cells (16 pixels/μm2), and 84% of the beta cell population lost nuclear HMGB1 protein compared to only 11% of the alpha cell population (Fig. 1 K and L, respectively). Therefore, beta cells appear to be more affected by senescence than alpha cells in vehicle-treated NOD mice. Nonetheless, PAHSAs also reduced the expression of senescent markers in alpha cells (Fig. 1 K and L). In PAHSA-treated NOD mice, only 1.4% of the total alpha cell population coexpressed p21cip1 compared to 3.7% in vehicle-treated mice. Similarly, intensity of p16ink4 staining in alpha cells from PAHSA-treated mice was reduced (3.5 pixels/μm2) compared to that observed in vehicle-treated mice (16.5 pixels/μm2), and loss of nuclear HMGB1 was seen only in 0.8% of the alpha cell population in PAHSA-treated mice compared to 11% in vehicle-treated mice. These results show that senescence occurs in other islet endocrine cell types which has not been reported previously, and support the notion that PAHSAs have beneficial effects in many different cell types (17–19, 23, 37).
PAHSAs Prevented Senescence and Loss of Functional Beta Cell Phenotype during Exposure to Different Metabolic Stress Conditions.
Induction of cellular senescence and the consequent activation of a specific transcriptome program is heterogenous and depends on multiple variables, including cell type, the source of the metabolic stress, and its duration (38, 39). To follow-up on the RNAseq data and test whether PAHSAs directly protect the pancreatic beta cell, we exposed the clonal beta cell line MIN6 in vitro to well-defined metabolic stressors commonly observed in diabetes and known to induce senescence—high glucose (Fig. 2A), cytokine-induced stress (Fig. 2B), and ER stress (induced with thapsigargin; Fig. 2C). When MIN6 cells were treated with these stressors for different lengths of times, we found that expression of senescence driver genes p21Cip1 (Fig. 2 D–F) and p16Ink4a (Fig. 2 G–I) increased. Expression of p21Cip1 was up-regulated 3-fold in response to cytokines (Fig. 2E) and 1.5-fold with thapsigargin (Fig. 2F). Cotreatment with PAHSAs prevented this increase. Both, cytokines (Fig. 2H) and ER stress (Fig. 2I) increased p16Ink4a expression, which was not prevented by PAHSAs. The different dynamics between these two cyclin dependent kinases inhibitors might be due to the fact that p21Cip1 tends to be expressed earlier after exposure to stress, whereas p16Ink4a expression is observed at later time points (38, 40). Because the cell analysis was performed very early after exposure to the stressor, PAHSAs may require different lengths of time for each metabolic stress condition to prevent increased p16Ink4a, as we observed in later experiments (SI Appendix, Fig. S5). With high glucose, there was a trend for p21Cip and p16Ink4a expression to be up-regulated and for PAHSAs to decrease this, although the variability precluded statistical significance (Fig. 2 D and G, respectively). Expression of the major SASP factor Il6 was increased in response to all three metabolic stress conditions (∼3-fold with high-glucose, 2.5-fold with cytokines, and 1.7-fold with ER stress), and cotreatment with PAHSAs prevented its up-regulation (Fig. 2 J–L). Additionally, expression of the DNA damage marker H2Ax was increased in all three metabolic stress conditions, which was prevented by PAHSAs (Fig. 2 P–R).
Fig. 2.
PAHSAs prevent and reverse the effects of metabolic stress in beta cells in vitro. (A–C) Schemas of studies to prevent the initiation of senescence in MIN6 cells under different metabolic stress conditions: glucotoxicity (A), cytokine stress (B), and ER stress induced by thapsigargin (C). (D–R) PAHSA treatment prevented some changes in gene expression in MIN6 cells exposed to metabolic stress conditions: high glucose (D, G, J, M, and P) cytokine stress (E, H, K, N, and Q) or ER stress (F, I, L, O, and R). Expression of senescence-related genes p21Cip1 (D–F), p16Ink4a (G–I), and IL-6 (J–L), beta cell maturity marker Ucn3 (M–O), and DNA damage gene H2Ax (P–R) is shown; n = 4 independent experiments. Data are shown as means ± SEM (*P < 0.05, **P < 0.005, and ***P < 0.001 versus control) and were analyzed by one-way ANOVA with Bonferroni multiple comparisons test; Cyto, cytokines; Thap, thapsigargin. (S–U) Schema for reversal studies in MIN6 cells under different metabolic stress conditions: glucotoxicity (S), cytokine stress (T), and ER stress induced by thapsigargin (U). The stressors were present until the end of the experiment. (V) HMGB1 immunohistochemistry showing a reversal experiment in MIN6 cells. Separate channels are shown in SI Appendix, Fig. S5. Quantification (W–Y) of loss of nuclear HMGB1 in MIN6 cells under three different metabolic stress conditions: glucotoxicity (W), cytokine stress (X), and ER stress induced by thapsigargin (Y) (Scale bars, 100 μm, left three columns; Scale bars, 32 μm, right three columns). Data are shown as means ± SEM (**P < 0.005 and ***P < 0.001 versus control) and were analyzed by one-way ANOVA with a Bonferroni multiple comparisons test.
We next studied the impact of metabolic stress conditions on markers of the mature and functional beta cell phenotype. Ucn-3 expression was reduced by 60% in high glucose (Fig. 2M), and by 85% with cytokines (Fig. 2N) or thapsigargin (Fig. 2O) treatment. Cotreatment with PAHSAs prevented the decreased Ucn-3 expression under high glucose and thapsigargin conditions but had no benefit with cytokine-induced stress (Fig. 2N). With a longer time (48 h) of PAHSA treatment, Ucn-3 expression increased but not to control levels (SI Appendix, Fig. S4A). PDX1 activates genes essential for beta cell identity (41); PDX1 expression was ∼50% reduced under all three metabolic stress conditions (SI Appendix, Fig. S4 B–D), and PAHSA treatment prevented this decrease.
Because FOXO1 represses Pdx1 expression, and nuclear localization of FOXO1 promotes nuclear export of PDX1 and a consequent decrease in insulin synthesis (42), PDX1 protein was evaluated by immunofluorescence. Nuclear FOXO1 was observed under the same metabolic stress conditions as in Fig. 2 A–C in vehicle-treated cells, while PAHSA treatment prevented nuclear FOXO1 localization (SI Appendix, Fig. S4E), consistent with the preserved Pdx1 gene expression shown above (SI Appendix, Fig. S4 B–D).
We next investigated whether PAHSAs could reverse the initial cellular changes induced by metabolic stressors that could drive beta cell senescence (SI Appendix, Fig. S5 A–C). MIN6 cells were exposed for 5 d to glucose toxicity, and PAHSAs were added during the last 48 h (SI Appendix, Fig. S5A). Expression of p21Cip1, IL6, Serpine1, and Ucn3 was up-regulated with high glucose and was completely reversed with PAHSAs (SI Appendix, Fig. S5D). Similarly, PAHSAs reversed the early effects of cytokine-induced stress and ER stress (SI Appendix, Fig. S5 B and C). p21Cip1, p16Ink4a, and IL6 expression were increased with cytokine exposure and thapsigargin, and PAHSAs reversed this induction (SI Appendix, Fig. S5D). These results are consistent with the changing pattern of expression of SASP factors with different stressors and time (38). A summary of other genes for which PAHSAs reversed stress-induced expression changes is shown in SI Appendix, Tables S1–S3.
We evaluated loss of HMGB1 protein nuclear localization in MIN6 cells subjected to the three metabolic stress conditions because this is a marker of senescence (21) (Fig. 2 S–U). Under all stress conditions, nuclear exclusion of HMGB1 increased to 30 to 40% compared to 2 to 5% under control conditions, while PAHSAs overwhelmingly restored nuclear HMGB1 localization (Fig. 2 V–Y and SI Appendix, Fig. S5 E–G).
Overall, in MIN6 cells, the three metabolic stress conditions present in type 1 diabetes (hyperglycemia, cytokines, and ER stress) increased markers of DNA damage and decreased markers of mature and functional beta cells. Most of these changes were prevented or reversed with PAHSAs (Fig. 2 and SI Appendix, Figs. S4 and S5 and Tables S1–S3).
PAHSAs Targeted Upstream Effectors of Senescence, DNA Repair, and GSH Metabolism to Protect Beta Cells under Metabolic Stress Conditions.
P53 is a major initiator of senescence (Fig. 3A) (43–45), and many factors that negatively regulate p53 were up-regulated by PAHSAs in the RNAseq data. Under nonstress conditions, the main negative regulator of p53 is MDM2 (22, 46–48), which binds to and promotes the translocation of P53 out of the nucleus for degradation (49–51). Also, YY1 (Ying Yang 1), Cbx2 (Chromobox 2), and Kap1/Trim28 (Fig. 3B) interact with Mdm2 and are negative regulators of p53 (43–45, 52–54). In addition, the accumulation of Sqstm1 (autophagy related) indirectly regulates p53 by controlling the NRF2-mediated induction of Mdm2 (55).
Fig. 3.
Mechanism by which PAHSAs protect beta cells under metabolic stress conditions. (A) Proposed model for the mechanism by which PAHSAs reduce stress-induced senescence in beta cells: reduction of p53 by preserving or increasing expression of its negative regulator Mdm2. (B) PAHSA treatment preserved the expression of genes related to Mdm2 and p53 regulation in NOD mouse pancreatic islets (6 wk of treatment, 10 wk of age). (C) PAHSAs reversed cytokine stress–induced changes of senescence and SASP-related gene expression in MIN6 cells transfected with siRNA for Mdm2 (gray bars) or control siRNA (white bars); n = 4 independent experiments, each with three replicates per condition. Data show means ± SEM; *P < 0.05 versus DMSO and PAHSA siRNA control; #P < 0.05 versus cytokines siRNA control; &P < 0.05 different from all other conditions; $P < 0.05 different from all conditions except DMSO and PAHSA controls; †P < 0.05 different from both DMSO and PAHSA conditions. (D) PAHSAs reversed ER stress–induced senescence and SASP-related gene expression in MIN6 cells transfected with siRNA for Mdm2 (gray bars) or control siRNA (white bars). Data show means ± SEM; *P < 0.05 versus DMSO and PAHSA siRNA control; #P < 0.05 different from DMSO and PAHSA controls and thapsigargin siRNA control; &P < 0.05 different from all other conditions; $P < 0.05 different from all except DMSO and PAHSA controls; @P < 0.05 different from all except thapsigargin siRNA control; †P < 0.05 different from both DMSO and PAHSA conditions. Data in C and D were analyzed by one-way ANOVA with Bonferroni multiple comparisons test. (E and F) Western blotting analysis of p53, phospho-p53Ser15, MDM2, YY1, and vinculin in MIN6 cells with reversal by PAHSAs of cytokine-stress (E) and ER stress (F) induced by thapsigargin. Cells were transfected with either siRNA for Mdm2 or control siRNA (n = 3 replicates per condition). Data are representative of three different Western blots. (G) Quantification of the data in E showing means ± SEM; $P < 0.05 different from all except DMSO siRNA control conditions; *P < 0.05 versus DMSO siRNA control and cytokines 48 h + PAHSAs 24 h siRNA control; †P < 0.05 versus all conditions; @P < 0.05 versus all conditions except DMSO siRNA Mdm2. (H) Quantification of F; &P < 0.05 versus all conditions; #P < 0.05 versus DMSO siRNA control conditions and thapsigargin + PAHSAs 3 h siRNA control; @P < 0.05 versus all conditions except DMSO siRNA for Mdm2. Data were analyzed by one-way ANOVA with a Bonferroni multiple comparisons test. (I) PAHSAs preserved and reversed GSH protein levels, which decreased with cytokine exposure in MIN6 cells. Quantification of total GSH, GSSH, and GSH (reduced GSH). White bars show controls containing DMSO, and gray bars show PAHSAs (dissolved in DMSO). Data are shown as means ± SEM; *P < 0.05 versus all conditions; #P < 0.05 versus cytokines without PAHSAs. Data were analyzed by one-way ANOVA with a Bonferroni multiple comparisons test; Cyto, cytokines, Thap, Thapsigargin. (J) Model illustrating the mechanism by which PAHSAs exert their beneficial effects on pancreatic beta cells under metabolic stress conditions. PAHSAs act through Mdm2 and its cofactors to destabilize p53. PAHSAs also alter DNA repair mechanisms and autophagy to preserve the beta cell population. In addition, PAHSAs regulate p53 through the transcription factors E2F1 and E2F6. The effect of PAHSAs on p53 results in decreased expression of downstream genes related to senescence, SASP, and apoptosis. In addition, PAHSAs increase the expression of genes related to GSH metabolism to protect the beta cell from metabolic stress.
To test whether PAHSAs regulated p53, we knocked down Mdm2 (to prevent p53 degradation) in MIN6 cells and exposed them to cytokines, as previously described (Fig. 2T), and ER stress (Fig. 2U and SI Appendix, Fig. S5 B and C). Knockdown of Mdm2 resulted in 60% and 85% decreases in its expression in the presence of cytokines (Fig. 3C) or thapsigargin (Fig. 3D), respectively. Forty-eight hours of continuous cytokine exposure further reduced Mdm2 expression (Fig. 3C). Addition of PAHSAs for the last 24 h increased Mdm2 expression in control small interfering RNA (siRNA)-transfected cells (Fig. 3C, white bars) but not in cells with Mdm2 knockdown (Fig. 3C, gray bars). Similarly, with thapsigargin-induced ER stress, PAHSAs increased expression of Mdm2 in control siRNA–infected cells but had no effect in Mdm2 siRNA–transfected cells (Fig. 3D, gray bars). Mdm2 knockdown resulted in increased P53 expression at 24 h and 48 h of cytokine exposure (SI Appendix, Fig. S6A) and after 9 h of ER stress induction (SI Appendix, Fig. S6B), validating the physiological relevance of these models.
In these studies with cytokines and thapsigargin, cells transduced with control siRNA had increased expression of senescence-related genes and decreased Ucn3 expression, all of which PAHSA treatment reversed (Fig. 3 C and D and SI Appendix, Fig. S6 A and B, white bars). Importantly, PAHSAs alone in the absence of metabolic stressors did not affect the expression of senescence-related genes; that is, in the absence of stressors, the expression in cells treated with PAHSAs was similar to that in cells treated with vehicle (Fig. 3 C and D and SI Appendix, Fig. S6 A and B). Other senescence-related genes, such as p53 and statifin (Sfn), a downstream target of P53 and inducer of cell cycle arrest (56), were increased in response to cytokines and thapsigargin in MIN6 cells with control siRNA and decreased toward dimethyl sulfoxide (DMSO) control levels with PAHSA treatment (SI Appendix, Fig. S6 A and B, white bars). PAHSAs decreased Sfn expression under ER stress. PAHSAs did not alter expression of p21Cip1, p16Ink4a, IL6 (Fig. 3 C and D, gray bars), p53, Mutyh, Chop, or Ucn3 (SI Appendix, Fig. S6 A and B, gray bars) in cells transfected with Mdm2 siRNA and exposed to cytokines or ER stress conditions. PAHSAs also had some beneficial effects in Mdm2-knockdown cells after ER stress (SI Appendix, Fig. S6B). This indicates that additional pathways that do not involve Mdm2 may also mediate the effects of PAHSAs on some genes.
We measured protein levels of p53 and MDM2 in response to cytokines and ER stress with Mdm2 knockdown. Total P53 and phosphorylation of P53Ser15, which is crucial for its stabilization and to promote cell cycle arrest (57, 58), were increased in cells with Mdm2 knockdown compared to in control siRNA–treated cells, indicating potential progression to senescence (Fig. 3 E–H). PAHSAs had no effect on phosphorylated or total p53 in control siRNA cells in the absence of stressors. P53Ser15 levels increased under both metabolic stress conditions when Mdm2 was knocked down, and no further increase was observed with stressors (Fig. 3 E and F). PAHSA treatment reversed the increase in total P53 and P53Ser15 only in the control siRNA cells, indicating that MDM2 mediates these PAHSA effects. PAHSAs also reduced the ratio of P53Ser15 to total P53 with cytokine stress and ER stress in control siRNA cells but not in MDM2-knockdown cells (Fig. 3 G and H). Both stress conditions reduced MDM2 and YY1 protein levels. PAHSAs maintained MDM2 protein levels and increased YY1 levels in control siRNA cells but not in MDM2-knockdown cells (Fig. 3 E–H). These data indicate that maintaining MDM2 levels is necessary for the PAHSA effects on P53. To summarize, cytokine and ER stress decreased Mdm2 expression, and PAHSAs reversed this effect, which would destabilize P53 and thus halt progression to cellular senescence.
We also investigated whether PAHSAs affected genes related to GSH metabolism in MIN6 cells, a pathway that protects against oxidative stress and maintains redox homeostasis. Cytokines decreased total GSH and oxidized GSH (GSSH) and reduced GSH protein content; PAHSA treatment reversed these changes (Fig. 3I). This effect is likely to contribute to the beneficial effects of PAHSAs on maintaining healthy beta cells.
Fig. 3J shows a model for the mechanism by which PAHSAs exert their beneficial effects directly on beta cells. This is based on the RNAseq data in islets from NOD mice and the mechanistic experiments in MIN6 cells. PAHSAs decrease P53 stability by increasing expression of its negative regulators Mdm2, Trim28 (Kap1), and YY1 (44–48) (Fig. 3J). Also, PAHSA treatment increased Cbx2 expression (Fig. 3B); decreased Cbx2 expression induces senescence (43). Cbx2 inhibits Arf, which regulates p53 stability in part by inhibiting Trim28 (Kap1)–Mdm2 binding (48). Trim28 and other transcription factors involved in regulating cell cycle, E2F1 and E2F6 (47, 48, 59, 60), were up-regulated with PAHSA treatment in NOD islets (Figs. 1F). E2F1 impairs insulin secretion and affects beta cell identity (61). Further protection is conferred by effects of PAHSAs to reduce DNA damage by enhancing expression of regulators of DNA repair, autophagy, and GSH metabolism. In addition, PAHSAs decreased expression of Makp11 (p38), which is highly up-regulated upon DNA damage (62) and phosphorylates p53 on Ser15, inducing cell cycle arrest and promoting IL-6 expression/release and STAT3 phosphorylation (63, 64). MDM2 may mediate some of these PAHSA effects on senescence in addition to DNA repair since MDM2 can also regulate the redox state and GSH metabolism (65).
PAHSAs Prevented and Reversed Cellular Senescence Induced by Metabolic Stress in Human Islets.
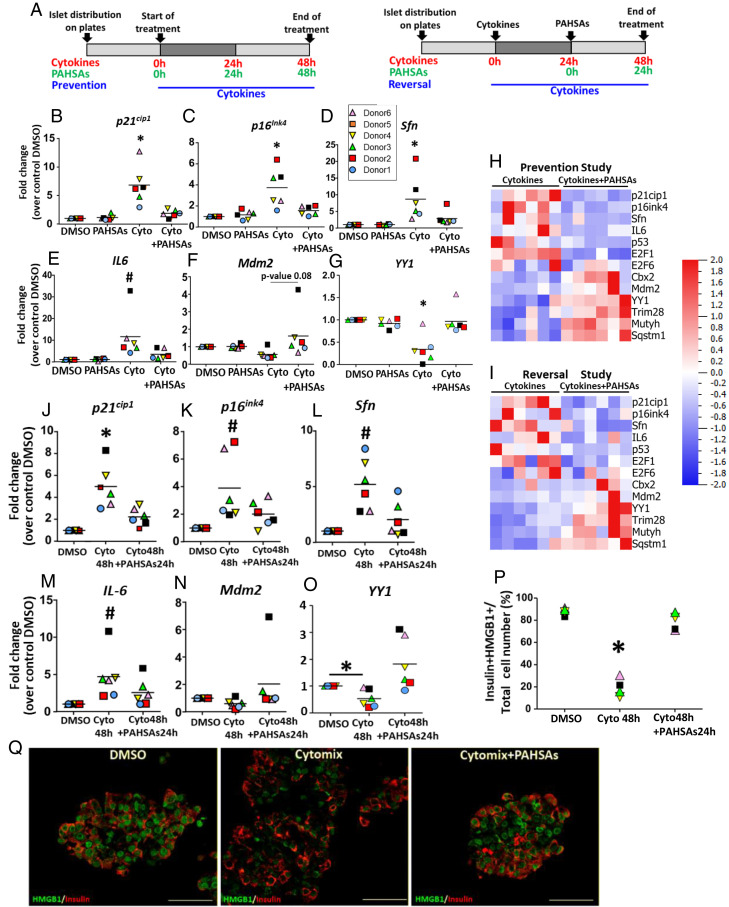
We next determined whether PAHSAs similarly modulated the initiation of senescence in human islets exposed to cytokines (Fig. 4A). We used islets from six nondiabetic donors aged 24 to 39 y since the percentage of senescent beta cells increases with age (7) (SI Appendix, Table S4). Cytokine treatment increased expression of P21Cip1 (Fig. 4B), P16Ink4a (Fig. 4C), SFN (Fig. 4D), and IL6 (Fig. 4E), and PAHSA treatment prevented these increases in islets from all of the human donors (Fig. 4H and SI Appendix, Fig. S7A). Mdm2 (Fig. 4F) and YY1 (Fig. 4G) expression was reduced with cytokine treatment and was restored by PAHSAs in islets from all donors. We found that PAHSAs prevented the decreased expression of Mdm2 cofactors (TRIM28, YY1, and CBX2), the DNA repair gene MUTYH, and autophagy-related gene SQSTM1. These results suggest that in human islets, the mechanism of action of PAHSAs is similar to that in murine MIN6 pancreatic beta cells.
Fig. 4.
PAHSAs prevent and reverse the initiation of senescence induced by metabolic stress in human islets. (A) Schemas of prevention and reversal experiments of PAHSA treatment during cytokine-induced stress in human islets from six different donors. (B–G) PAHSAs prevented up-regulated expression of senescence and SASP genes islets from all human donors (n = 6). Data are shown as means ± SEM; *P < 0.05 versus all conditions; #P < 0.05 versus control DMSO. Data were analyzed by one-way ANOVA with a Tukey’s multiple comparisons test. (H) Heat map of PCR-determined expression of genes related to the mechanism by which PAHSAs prevent senescence in beta cells represented in I. Data are from human islets exposed to cytokines in the prevention study. (I) Heat maps of PCR-determined expression of genes related to the mechanism by which PAHSAs reverse senescence in beta cells represented in Fig. 3J. Data are from human islets exposed to cytokines in the reversal study. (J–O) PAHSAs reversed the decreased expression of senescence and SASP-related genes to levels similar to control conditions. PAHSAs maintained or increased MDM2 and YY1 expression. Data are shown as means ± SEM; *P < 0.05 versus all conditions; #P < 0.05 versus control DMSO. Data were analyzed by one-way ANOVA with a Tukey’s multiple comparisons test. (P and Q) Quantification (P) and representative pictures (Q) of nuclear HMGB1 in beta cells from human islets. Cytomix (Cyto, combination of recombinant human IL1β, Tnfα and IFNγ. (Scale bar, 50 μm.) Data are shown as means ± SEM; *P < 0.05 versus all conditions. Data were analyzed by one-way ANOVA with a Tukey’s multiple comparisons test.
PAHSA treatment of human islets not only prevented, but also reversed, the increased expression of these senescence markers induced by cytokines (Fig. 4 I–O and SI Appendix, Fig. S7B). Similar to reversal studies in MIN6 cells, Mdm2 expression decreased with cytokine stress in human islets and was restored or even increased beyond control levels by PAHSAs (Fig. 4N). In addition, changes in gene expression in response to PAHSAs in human islets (Fig. 4 I, N, and O and SI Appendix, Fig. S7B) were consistent with the mechanism suggested by RNAseq and confirmed in MIN6 cells.
Interestingly, the only gene that we tested for which PAHSAs appeared to have different effects in the human islets compared to NOD mouse islets was E2F1. In islets from NOD mice treated with PAHSAs, E2F1 expression was increased compared to in vehicle-treated mice (Fig. 1F), but in human islets, PAHSAs decreased E2F1 expression (SI Appendix, Fig. S7B). The function of this transcription factor is controversial since it has been implicated in both proliferation and prosenescence (59, 66, 67).
PCA of the genes analyzed in stressed human islets (SI Appendix, Fig. S8) showed that islets treated with PAHSAs and cytokines had clearly distinct gene expression from those treated only with cytokines in both prevention and reversal studies. In human islets treated with cytokines and PAHSAs, 70% of beta cells had nuclear localization of HMGB1 protein compared to 20% with cytokines alone (Fig. 4 P and Q), consistent with PAHSA effects in the NOD islets and in MIN6 cells.
In conclusion, in human islets as in mouse beta cells, PAHSA treatment prevented and reversed the initiation of senescence induced by cytokine stress, which is the main metabolic stress that contributes to the pathophysiology of type 1 diabetes. The high percentage of beta cells with nuclear localization of HMGB1 protein supports the reversal potential of PAHSAs, which would protect human beta cells from permanent senescence after exposure to stressors.
Discussion
In humans and mice, senescent pancreatic beta cells contribute to the development and progression of type 1 and type 2 diabetes. Senotherapeutics have been proposed as a new approach for delaying or blunting the progression of diabetes. Senolytic drugs kill senescent cells (68) by overcoming their resistance to apoptosis, while senomorphic agents reduce the secretion of SASPs (69, 70) without killing the cells. Senolysis results in improved glucose homeostasis with potential deleterious effects on beta cell mass (6, 7). Our previous observation that PAHSAs delayed/prevented diabetes in NOD mice and reduced apoptosis and necrosis in beta cells in vitro when beta cells were exposed to cytokines (18) suggested that PAHSAs act through other mechanisms to maintain functional beta cell mass. Here, we show that PAHSA treatment reduced the frequency of apoptosis (TUNEL staining) in islets of NOD mice even before the onset of hyperglycemia and the expression of senescence markers and SASPs in NOD islets and in beta cells that were exposed to metabolic stress conditions that are found in type 1 and type 2 diabetes. In addition, we found that PAHSA treatment in NOD mice and in MIN6 beta cells and human pancreatic islets exposed to metabolic stressors in vitro preserved the nuclear localization of HMGB1 in the beta cells. Nuclear exclusion of HMGB1 has been described as one of the initial signs of cellular senescence (21). PAHSAs modulate the induction of cellular senescence by regulating p53 through multiple mechanisms that preserve and/or restore senescent beta cells to their previous healthy state (Fig. 3J). Enhanced expression of genes involved in DNA repair and autophagy by PAHSAs may also contribute to their beneficial effects. Overall, our data indicate that PAHSAs act as modulators of cellular senescence and might therefore be an effective approach to blunt or prevent the progression to diabetes.
We made the important observation that alpha cells also became senescent and that PAHSAs have beneficial effects to reduce senescence in alpha cells too. This finding is important since activation of senescence pathways contributes to release of SASP components that will affect neighboring cells and spread senescence. Previous work showed that PAHSAs affect immune cells (18, 19, 23, 37), so some of the beneficial effects of PAHSAs on beta cells in the NOD mice could also be indirect via the immune cells and alpha cells. However, we show that PAHSAs do have direct effects on beta cells using MIN6 cells and human islets. Thus, the beneficial effect of PAHSAs in the NOD mice are due to 1) direct effects on the islet cells and 2) indirect effects on the islet cells by attenuation of the infiltrating immune cells. These effects are not mutually exclusive.
Our experiments in MIN6 cells exposed to metabolic stressors that contribute to the development of diabetes demonstrated the functional significance of the RNAseq data. PAHSAs both prevented and reversed early changes induced by high glucose, cytokines, and ER stress that could lead to senescence. PAHSA treatment also prevented and reversed the beta cell aberrant morphology, reduced expression of senescence-related genes, and restored expression of genes characteristic of the functional beta cell phenotype, such as Ucn-3 and Pdx1. While we found that PAHSA treatment of MIN6 cells under all three metabolic stress conditions reversed the early expression of senescence genes, such as p21Cip1and SASP factors IL6 or Serpine1, to levels similar to control conditions, expression of p16Ink4and Ucn3 were changed only under some of the stress conditions. Thus, the benefit of the PAHSAs may vary with the stage in the progression of senescence, the inducers of stress, and the timing of exposure to the stress or to PAHSAs. Our data showing that PAHSAs can reverse the effects of metabolic stress in human islets exposed to metabolic stressors are consistent with previous microfluidic data showing that PAHSA treatment of islets from people with type 2 diabetes increased glucose-stimulated insulin secretion and restored the normal oscillatory dynamics of insulin secretion (20).
Our data showing that PAHSAs preserved or enhanced protective pathways, such as DNA repair and GSH metabolism, provide additional mechanisms by which PAHSAs protect pancreatic beta cells. Consistent with the up-regulation of DNA repair genes, genes related to apoptosis, such as Casp9, were reduced with PAHSA treatment, suggesting that PAHSAs actively contribute to repair damaged cells, resulting in avoidance of the excessive apoptosis usually observed in NOD mice (18, 71) and preserving beta cell mass. PAHSA treatment reduced expression of Bcl2l1 (known as Bcl-xl), an antiapoptotic gene that maintains cell cycle arrest that is a feature of cellular senescence (6, 72) and also impairs mitochondrial signaling for insulin secretion (73). The beneficial effect of PAHSAs on GSH metabolism is important since GSH prevents oxidative stress that contributes to aging and senescence in many cell types (74, 75) including beta cells. GSH metabolism regulates ROS content and mitochondria function in beta cells (76, 77), and reduced GSH accelerates cellular senescence in fibroblasts and adipocytes (78).
Mechanistically, our data indicate that regulation of the p53 pathway underlies the effects of PAHSAs. p53 is critical for the senescence process; it can induce either cell cycle arrest or apoptosis. Stabilization of p53 under stress conditions results in cell cycle arrest, and p53 degradation is necessary to overcome this arrest (78). Mdm2, the central regulator of p53, exerts its effects by inactivating p53 transcriptional activity, reducing its nuclear localization and protein stability and promoting its degradation (79, 80). However, under stress conditions, Mdm2 expression is reduced, which blocks p53 degradation and promotes either apoptosis or entrance into cellular senescence (81, 82). Activation of p53 or inhibition of Mdm2 impairs glucose-stimulated insulin secretion by decreasing expression of pyruvate carboxylase, leading to glucose intolerance in mice (82). PAHSA treatment prevented and reversed the down-regulation of Mdm2 or increased it after cells were exposed to metabolic stress. Knockdown of Mdm2 in cells exposed to metabolic stressors stabilized p53 as expected, which would result in cell cycle arrest and progression to senescence. Mdm2 knockdown prevented PAHSAs from reducing senescence markers (Fig. 3J), suggesting that repression of p53 through induction of Mdm2 is necessary for the beneficial effects of PAHSAs on senescence.
Besides suppressing p53 expression to prevent cell cycle arrest and senescence, PAHSA treatment may promote removal of damage in cells by increasing autophagy, which would be another mechanism to prevent senescence (83). PAHSA treatment increased the expression of Sqstm1/p62, which encodes a multifunctional protein that promotes autophagy, decreases inflammation, and prevents oxidative stress (50, 51). It is involved in the regulation of GSH production, mitochondrial integrity, and reduction of mitochondrial ROS, and regulates senescence by activating Nrf2-mediated induction of Mdm2 (84). A recent preprint indicated that PAHSAs promote autophagy in SH-SY5Y cells, a neuroblastoma cell line (85).
Inhibitors of Mdm2 are well described as an effective therapy against cancer by blocking proliferation of cancer cells (81, 86, 87). Therefore, there could be a concern that inducing Mdm2 expression might promote tumorigenesis. However, we previously showed that PAHSA treatment of NOD mice did not have tumorigenic effects in the pancreas or any other organ (18). Yet the question of the effects of PAHSA treatment in aged mice or mice in early-cancerous stages needs further study.
Our results have high translational potential for diabetes in humans since we demonstrated that PAHSAs also blocked initiators of senescence induced by metabolic stress in human islets. The increased expression of negative regulators of p53 (Mdm2, YY1, Kap1, and Cbx2) after PAHSA treatment in the human islets suggests that the mechanisms downregulating p53 and thus reducing senescence may be the same as in mouse islets (Fig. 3J). The possibility of using PAHSAs to prevent or reverse the initiation of senescence in humans will depend on safety and efficacy of prolonged treatment. In mice, the safety of prolonged PAHSA treatment (up to 6.5 mo) was demonstrated by histological and functional assays (18, 88). In addition, PAHSA treatment not only prevents type 1 diabetes in NOD mice but also restores hepatic and systemic insulin sensitivity in insulin-resistant C57BL/6 mice on a high-fat diet (17, 37). In humans, serum and adipose PAHSA levels positively correlate with insulin sensitivity (19). Exercise training increases adipose and serum PAHSA levels in elderly women (89) and may predict VO2 max (90). Therefore, PAHSA treatment in humans to prevent or reverse the initiation of cellular senescence in beta cells is likely to be safe, and clinical trials are warranted.
In summary, we demonstrated that the beneficial effects of PAHSA treatment on both mouse and human pancreatic beta cells are mediated by preventing and reversing initiation of stress-induced beta cell senescence, and we identified the mechanism. PAHSAs induce the expression of Mdm2 and its related transcription factors (i.e., Cbx1, YY1, and Kap1) to down-regulate p53. Additionally, enhanced expression of genes involved in DNA repair and autophagy by PAHSAs may contribute to their beneficial effects on beta cells. Thus, PAHSAs potentially represent modulators of cellular senescence that exert their beneficial effects without loss of cells.
Materials and Methods
Animals.
NOD female mice were considered hyperglycemic with two consecutive glucose measurements >250 mg/dL following ad libitum feeding.
Cell Lines.
MIN6 cells were used for the induction of beta cell dysfunction, senescence, and transfection experiments.
Human Islets.
Human islets from nondiabetic donors younger than 40 y old were obtained from Prodo Laboratories, Inc.
See additional details in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Chris Cahill and Brooke Sullivan of the Joslin Advanced Microscopy Core for processing and immunofluorescence staining of human islets and Jennifer Hollister-Lock of the Joslin Islet Isolation Core for the isolation of NOD mouse islets. We thank Dr. Dionicio Siegel at the University of California, San Diego, for synthesizing the PAHSA lipids. We thank Dr. Lay-Hong Ang and Aniket Gad for their expertise in immunostaining and image processing in the confocal imaging core at Beth Israel Deaconess Medical Center. We also thank the Dr. Jonathan M. Dreyfuss and Dr. Hui Pan from the Joslin Diabetes Center's Bioinformatics & Biostatistics Core, and Angela Wood from the Flow Cytometry Core. This work was supported by grants from the Deutsche Forschungsgemeinschaft Fellowship (FE1719/1-1 to M.F.R.d.C. and GA2416/1-1 to R.G.-M.), NIH grants R01 DK43051 and R01 DK106210, a grant from the JPB Foundation (B.B.K.), NIH DK110390 (S.B.-W.), P30 DK036836 from the Joslin Diabetes Research Center, the Diabetes Research and Wellness Foundation (S.B.-W.), NIH K01 DK118041, Joslin Pilot & Feasibility Grant P30 DK046200 (I.S.), and NIH K01 DK114162 (J.L.).
Footnotes
Reviewers: J.C., Medical College of Wisconsin; and J.K., Mayo Clinic Minnesota.
Competing interest statement: I.S. and B.B.K. are inventors on patents related to the FAHFAs.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2206923119/-/DCSupplemental.
Data, Materials, and Software Availability
RNAseq data have been deposited in GEO repository with accession number GSE216051 (91). All other study data are included in the article and/or SI Appendix.
References
- 1.Atkinson M. A., et al. , How does type 1 diabetes develop?: The notion of homicide or β-cell suicide revisited. Diabetes 60, 1370–1379 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cnop M., Toivonen S., Igoillo-Esteve M., Salpea P., Endoplasmic reticulum stress and eIF2α phosphorylation: The Achilles heel of pancreatic β cells. Mol. Metab. 6, 1024–1039 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donath M. Y., et al. , Mechanisms of beta-cell death in type 2 diabetes. Diabetes 54 (suppl. 2), S108–S113 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Hudish L. I., Reusch J. E., Sussel L., β cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J. Clin. Invest. 129, 4001–4008 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weir G. C., Bonner-Weir S., Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 53 (suppl. 3), S16–S21 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Thompson P. J., et al. , Targeted elimination of senescent beta cells prevents type 1 diabetes. Cell Metab. 29, 1045–1060 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Aguayo-Mazzucato C., et al. , Acceleration of β cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab. 30, 129–142 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campisi J., d’Adda di Fagagna F., Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 8, 729–740 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Gorgoulis V., et al. , Cellular senescence: Defining a path forward. Cell 179, 813–827 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Rodier F., et al. , Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 11, 973–979 (2009). Correction in: Nat. Cell Biol. 11, 1272 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tchkonia T., Zhu Y., van Deursen J., Campisi J., Kirkland J. L., Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. J. Clin. Invest. 123, 966–972 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung K. W., Advances in understanding of the role of lipid metabolism in aging. Cells 10, 880 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X., et al. , Impaired lipid metabolism by age-dependent DNA methylation alterations accelerates aging. Proc. Natl. Acad. Sci. U.S.A. 117, 4328–4336 (2020). Retraction in: Proc. Natl. Acad. Sci. U.S.A. 117, 8660 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Millner A., Atilla-Gokcumen G. E., Lipid players of cellular senescence. Metabolites 10, 339 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mutlu A. S., Duffy J., Wang M. C., Lipid metabolism and lipid signals in aging and longevity. Dev. Cell 56, 1394–1407 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bi X., et al. , ω-3 polyunsaturated fatty acids ameliorate type 1 diabetes and autoimmunity. J. Clin. Invest. 127, 1757–1771 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Syed I., et al. , Palmitic acid hydroxystearic acids activate GPR40, which is involved in their beneficial effects on glucose homeostasis. Cell Metab. 27, 419–427 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Syed I., et al. , PAHSAs attenuate immune responses and promote β cell survival in autoimmune diabetic mice. J. Clin. Invest. 129, 3717–3731 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yore M. M., et al. , Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 159, 318–332 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bandak B., Yi L., Roper M. G., Microfluidic-enabled quantitative measurements of insulin release dynamics from single islets of Langerhans in response to 5-palmitic acid hydroxy stearic acid. Lab Chip 18, 2873–2882 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davalos A. R., et al. , p53-dependent release of alarmin HMGB1 is a central mediator of senescent phenotypes. J. Cell Biol. 201, 613–629 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chène P., Inhibiting the p53-MDM2 interaction: An important target for cancer therapy. Nat. Rev. Cancer 3, 102–109 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Lee J., et al. , Branched fatty acid esters of hydroxy fatty acids (FAHFAs) protect against colitis by regulating gut innate and adaptive immune responses. J. Biol. Chem. 291, 22207–22217 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearson J. A., Wong F. S., Wen L., The importance of the non obese diabetic (NOD) mouse model in autoimmune diabetes. J. Autoimmun. 66, 76–88 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blum B., et al. , Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat. Biotechnol. 30, 261–264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemaire K., Thorrez L., Schuit F., Disallowed and allowed gene expression: Two faces of mature islet beta cells. Annu. Rev. Nutr. 36, 45–71 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Weir G. C., Bonner-Weir S., Islet β cell mass in diabetes and how it relates to function, birth, and death. Ann. N. Y. Acad. Sci. 1281, 92–105 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talchai C., Xuan S., Lin H. V., Sussel L., Accili D., Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 150, 1223–1234 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J., Ney P. A., Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 16, 939–946 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konishi N., et al. , Function of JunB in transient amplifying cell senescence and progression of human prostate cancer. Clin. Cancer Res. 14, 4408–4416 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Sofiadis K., et al. , HMGB1 coordinates SASP-related chromatin folding and RNA homeostasis on the path to senescence. Mol. Syst. Biol. 17, e9760 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collin G., Huna A., Warnier M., Flaman J. M., Bernard D., Transcriptional repression of DNA repair genes is a hallmark and a cause of cellular senescence. Cell Death Dis. 9, 259 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kairupan C., Scott R. J., Base excision repair and the role of MUTYH. Hered. Cancer Clin. Pract. 5, 199–209 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka Y., Tran P. O., Harmon J., Robertson R. P., A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc. Natl. Acad. Sci. U.S.A. 99, 12363–12368 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siddiqui M. S., François M., Fenech M. F., Leifert W. R., Persistent γH2AX: A promising molecular marker of DNA damage and aging. Mutat. Res. Rev. Mutat. Res. 766, 1–19 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Stancill J. S., Kasmani M. Y., Khatun A., Cui W., Corbett J. A., Single-cell RNA sequencing of mouse islets exposed to proinflammatory cytokines. Life Sci. Alliance 4, e202000949 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou P., et al. , PAHSAs enhance hepatic and systemic insulin sensitivity through direct and indirect mechanisms. J. Clin. Invest. 129, 4138–4150 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Cecco M., et al. , L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 566, 73–78 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernandez-Segura A., et al. , Unmasking transcriptional heterogeneity in senescent cells. Curr. Biol. 27, 2652–2660 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Midha A., et al. , Unique human and mouse β-cell senescence-associated secretory phenotype (SASP) reveal conserved signaling pathways and heterogeneous factors. Diabetes 70, 1098–1116 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spaeth J. M., et al. , Defining a novel role for the Pdx1 transcription factor in islet β-cell maturation and proliferation during weaning. Diabetes 66, 2830–2839 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitamura T., et al. , The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J. Clin. Invest. 110, 1839–1847 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baumann C., Zhang X., De La Fuente R., Loss of CBX2 induces genome instability and senescence-associated chromosomal rearrangements. J. Cell Biol. 219, e201910149 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J., Zhang C., Wang X., Hu W., Feng Z., Tumor suppressor p53 cross-talks with TRIM family proteins. Genes Dis. 8, 463–474 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sui G., et al. , Yin Yang 1 is a negative regulator of p53. Cell 117, 859–872 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Grönroos E., Terentiev A. A., Punga T., Ericsson J., YY1 inhibits the activation of the p53 tumor suppressor in response to genotoxic stress. Proc. Natl. Acad. Sci. U.S.A. 101, 12165–12170 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inoue K., Fry E. A., Frazier D. P., Transcription factors that interact with p53 and Mdm2. Int. J. Cancer 138, 1577–1585 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang C., et al. , MDM2 interaction with nuclear corepressor KAP1 contributes to p53 inactivation. EMBO J. 24, 3279–3290 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyd S. D., Tsai K. Y., Jacks T., An intact HDM2 RING-finger domain is required for nuclear exclusion of p53. Nat. Cell Biol. 2, 563–568 (2000). [DOI] [PubMed] [Google Scholar]
- 50.Chibaya L., Karim B., Zhang H., Jones S. N., Mdm2 phosphorylation by Akt regulates the p53 response to oxidative stress to promote cell proliferation and tumorigenesis. Proc. Natl. Acad. Sci. U.S.A. 118, e2003193118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu X., Bayle J. H., Olson D., Levine A. J., The p53-Mdm-2 autoregulatory feedback loop. Genes Dev. 7 (7A), 1126–1132 (1993). [DOI] [PubMed] [Google Scholar]
- 52.Bitto A., et al. , P62/SQSTM1 at the interface of aging, autophagy, and disease. Age (Dordr.) 36, 9626 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hewitt G., et al. , SQSTM1/p62 mediates crosstalk between autophagy and the UPS in DNA repair. Autophagy 12, 1917–1930 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mrakovcic M., Fröhlich L. F., p53-mediated molecular control of autophagy in tumor cells. Biomolecules 8, 14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Todoric J., et al. , Stress-activated NRF2-MDM2 cascade controls neoplastic progression in pancreas. Cancer Cell 32, 824–839 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schultz J., Ibrahim S. M., Vera J., Kunz M., 14-3-3sigma gene silencing during melanoma progression and its role in cell cycle control and cellular senescence. Mol. Cancer 8, 53 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shieh S. Y., Ikeda M., Taya Y., Prives C., DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91, 325–334 (1997). [DOI] [PubMed] [Google Scholar]
- 58.Zhao H., Traganos F., Darzynkiewicz Z., Phosphorylation of p53 on Ser15 during cell cycle caused by Topo I and Topo II inhibitors in relation to ATM and Chk2 activation. Cell Cycle 7, 3048–3055 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dimri G. P., Itahana K., Acosta M., Campisi J., Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14(ARF) tumor suppressor. Mol. Cell. Biol. 20, 273–285 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan H., et al. , Key roles for E2F1 in signaling p53-dependent apoptosis and in cell division within developing tumors. Mol. Cell 2, 283–292 (1998). [DOI] [PubMed] [Google Scholar]
- 61.Fajas L., et al. , Impaired pancreatic growth, beta cell mass, and beta cell function in E2F1−/− mice. J. Clin. Invest. 113, 1288–1295 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borisova M. E., et al. , p38-MK2 signaling axis regulates RNA metabolism after UV-light-induced DNA damage. Nat. Commun. 9, 1017 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bulavin D. V., et al. , Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 18, 6845–6854 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zauberman A., Zipori D., Krupsky M., Ben-Levy R., Stress activated protein kinase p38 is involved in IL-6 induced transcriptional activation of STAT3. Oncogene 18, 3886–3893 (1999). [DOI] [PubMed] [Google Scholar]
- 65.Riscal R., et al. , Chromatin-bound MDM2 regulates serine metabolism and redox homeostasis independently of p53. Mol. Cell 62, 890–902 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Itahana K., Dimri G., Campisi J., Regulation of cellular senescence by p53. Eur. J. Biochem. 268, 2784–2791 (2001). [DOI] [PubMed] [Google Scholar]
- 67.Laine A., Westermarck J., Molecular pathways: Harnessing E2F1 regulation for prosenescence therapy in p53-defective cancer cells. Clin. Cancer Res. 20, 3644–3650 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Xie Q., et al. , E2F transcription factor 1 regulates cellular and organismal senescence by inhibiting Forkhead box O transcription factors. J. Biol. Chem. 289, 34205–34213 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.von Kobbe C., Targeting senescent cells: Approaches, opportunities, challenges. Aging (Albany NY) 11, 12844–12861 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim E. C., Kim J. R., Senotherapeutics: Emerging strategy for healthy aging and age-related disease. BMB Rep. 52, 47–55 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu M., et al. , JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl. Acad. Sci. U.S.A. 112, E6301–E6310 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Augstein P., et al. , Beta-cell apoptosis in an accelerated model of autoimmune diabetes. Mol. Med. 4, 495–501 (1998). [PMC free article] [PubMed] [Google Scholar]
- 73.Yosef R., et al. , Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 7, 11190 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou Y. P., et al. , Overexpression of Bcl-xL in beta-cells prevents cell death but impairs mitochondrial signal for insulin secretion. Am. J. Physiol. Endocrinol. Metab. 278, E340–E351 (2000). [DOI] [PubMed] [Google Scholar]
- 75.Simioni C., et al. , Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 9, 17181–17198 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun Y., Zheng Y., Wang C., Liu Y., Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 9, 753 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Newsholme P., Keane K. N., Carlessi R., Cruzat V., Oxidative stress pathways in pancreatic β-cells and insulin-sensitive cells and tissues: Importance to cell metabolism, function, and dysfunction. Am. J. Physiol. Cell Physiol. 317, C420–C433 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Pi J., et al. , Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 56, 1783–1791 (2007). [DOI] [PubMed] [Google Scholar]
- 79.Liao N., et al. , Antioxidants inhibit cell senescence and preserve stemness of adipose tissue-derived stem cells by reducing ROS generation during long-term in vitro expansion. Stem Cell Res. Ther. 10, 306 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kubbutat M. H., Jones S. N., Vousden K. H., Regulation of p53 stability by Mdm2. Nature 387, 299–303 (1997). [DOI] [PubMed] [Google Scholar]
- 81.Moll U. M., Petrenko O., The MDM2-p53 interaction. Mol. Cancer Res. 1, 1001–1008 (2003). [PubMed] [Google Scholar]
- 82.Wiley C. D., et al. , Small-molecule MDM2 antagonists attenuate the senescence-associated secretory phenotype. Sci. Rep. 8, 2410 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu D., Prives C., Relevance of the p53-MDM2 axis to aging. Cell Death Differ. 25, 169–179 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rajendran P., et al. , Autophagy and senescence: A new insight in selected human diseases. J. Cell. Physiol. 234, 21485–21492 (2019). [DOI] [PubMed] [Google Scholar]
- 85.Yu Z. Y., et al. , S-9-PAHSA regulates glycolipid metabolism by enhancing autophagy and upregulating PI3K/AKT pathway. Res Sq [Preprint] (2022). 10.21203/rs.3.rs-1618637/v1 [DOI]
- 86.Konopleva M., et al. , MDM2 inhibition: An important step forward in cancer therapy. Leukemia 34, 2858–2874 (2020). [DOI] [PubMed] [Google Scholar]
- 87.Vassilev L. T., MDM2 inhibitors for cancer therapy. Trends Mol. Med. 13, 23–31 (2007). [DOI] [PubMed] [Google Scholar]
- 88.Syed I., et al. , Methodological issues in studying PAHSA biology: Masking PAHSA effects. Cell Metab. 28, 543–546 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brezinova M., et al. , Exercise training induces insulin-sensitizing PAHSAs in adipose tissue of elderly women. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1865, 158576 (2020). [DOI] [PubMed] [Google Scholar]
- 90.Nelson A. B., et al. , Acute aerobic exercise reveals that FAHFAs distinguish the metabolomes of overweight and normal-weight runners. JCI Insight 7, e158037 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.M. F. R. de Celis et al., PAHSAs reduce cellular senescence and protect pancreatic beta cells from metabolic stress through regulation of Mdm2/p53. NCBI: GEO. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE216051. Deposited 3 November 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNAseq data have been deposited in GEO repository with accession number GSE216051 (91). All other study data are included in the article and/or SI Appendix.