Significance
We interpret the offering of a complete skeleton of a charismatic animal—a captively managed spider monkey—at Plaza of the Columns as the subject of a strategic gift exchange between Teotihuacan and the Maya that reified diplomatic ties between these two major regions of Classic Mesoamerica. We suggest a more multilateral mode of ritual exchange preceded the Teotihuacan state’s eventual ascent to prominence prior to the epigraphically attested militaristic involvement of Teotihuacan in local Maya politics. A multimethod archaeometry study of a spider monkey sacrificed at Teotihuacan provides the earliest evidence of primate captivity and translocation in the Americas over 1,500 y ago.
Keywords: Mesoamerica, primate captivity, animal translocation, archaeometry, gift exchange
Abstract
A multimethod archaeometry study (zooarchaeological, isotopic, ancient DNA, paleobotanical, and radiocarbon dating) of a spider monkey sacrificed in the ceremonial center of Teotihuacan, Mexico (1 to 550 CE) is interpreted as a diplomatic gift exchange with neighboring Maya. Not only does this spider monkey provide the earliest known instance of primate translocation and captivity in Mesoamerica, it helps date incipient modes of interregional diplomacy between two major powers during Early Classic Mesoamerica: Teotihuacan and the Maya. Details of human–primate interaction include age at capture and transport (before ∼3 y of age), captive duration (over 2 y), anthropogenic diet (staple was maize, though secondary resources unique to anthropogenic diet including arrowroot and chili pepper were also found), context of sacrifice (tethered and associated with complete golden eagle and an array of other statecrafts), and general site context (including presence of Maya vessels and Maya-style murals). The timing of the spider monkey’s sacrifice (250 to 300 CE) and its life history suggest a reconsideration of epigraphically attested militaristic involvement of Teotihuacan at certain Maya sites. We propose that a period of more multilateral and fluid ritual exchange with Maya dignitaries preceded the Teotihuacan state’s eventual ascent to prominence.
In 1972, China famously gifted a pair of pandas, Ling-Ling and Hsing-Hsing, to the United States following President Richard Nixon’s state visit to that nation. This diplomatic exchange was the result of a casual dinner conversation wherein the first lady, Pat, expressed her fondness for these charismatic mammals. Ling-Ling and Hsing-Hsing would eventually be seen by over 75 million visitors at their home at the National Zoo in Washington, DC and were highly influential in transforming American perceptions of China (1). Now, as in the past, state-level gift exchange is a social mechanism by which interregional sociopolitics are negotiated and reified (2). In the case of the ancient city of Teotihuacan (1 to 550 CE), despite its role as the metropolitan hub of Early Classic Mesoamerica’s dynamic interconnected landscape, both the timeline and the character of its foreign affairs have been subjects of seven decades of intense debate (3–6). Current consensus about Teotihuacan–Maya relations is largely supported by interpretation of the prolific epigraphic and material culture evidence of Teotihuacan activity discovered throughout the Maya heartland. The relationship can be roughly compared with that of Hellenistic Greece and the Roman Republic, which similarly exhibited extensive interaction and influence despite maintaining cultural and linguistic distinction. Just as it is impossible to study either culture in isolation, and the nature of their exchange contributes to our understanding of Classical Antiquity at large, Teotihuacan–Maya interaction studies informs aspects of each cultural region as well as Classic Mesoamerica more broadly.
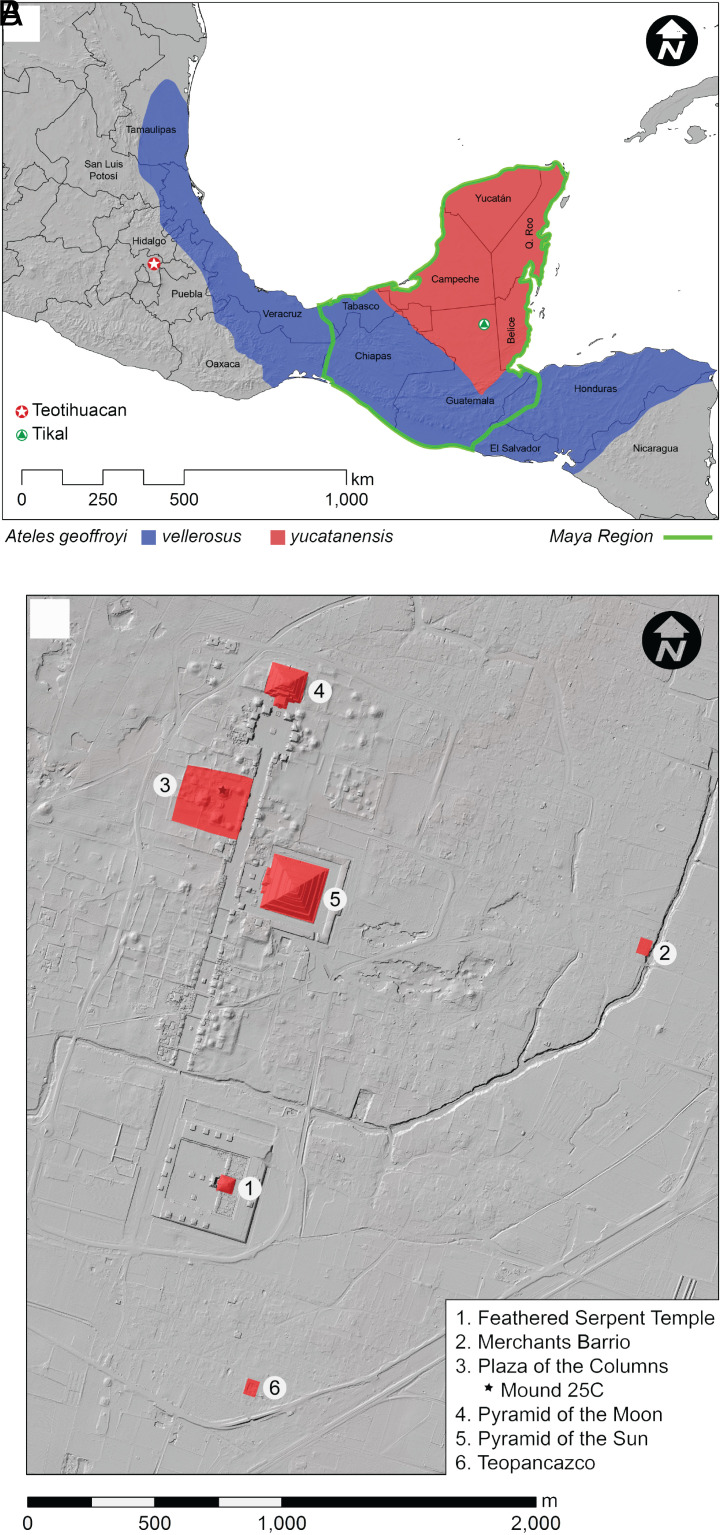
Epigraphic records documenting the arrival of a Teotihuacan emissary to the Maya center of Tikal on January 14th, 378 CE (referred to as the “Entrada”) suggests the Teotihuacan state exercised direct military involvement in local Maya sociopolitics at certain sites (7) (Fig. 1A, green triangle), but what if a more multilateral and fluid mode of ritual-mediated exchange preceded (and possibly even perpetuated) the Teotihuacan state’s eventual ascent to prominence? We present evidence that formal contact with Maya dignitaries was established a century prior to the Entrada via the gifting of a spider monkey (Ateles geoffroy), an exotic curiosity alien to the high elevations of Teotihuacan, which was found sacrificed within a prominent compound in the city’s ceremonial precinct.
Fig. 1.
(A) Distribution of A. geoffroyi vellerosus (blue) and A. geoffroyi yucatanensis (red) and sites/cultural zones mentioned in the text. (B) Lidar map of the core region of Teotihuacan highlighting key archaeological sites mentioned in the text. © Project Plaza of the Columns Complex.
We apply a multimethod archaeometric study (zooarchaeology, isotopes, ancient DNA [aDNA], paleobotany, and radiocarbon dating) to detail a life history of the Teotihuacan spider monkey, whose finding represents the earliest evidence of primate captivity and translocation in the Americas. We consider the longevity and biological/cultural significance of human–primate interactions in Mesoamerica and unravel aspects of primate captivity such as subspecies captured (A. geoffroy cf vellerosus), date and age of capture and translocation (circa 250 to 300 CE, before the primate reached ∼3 y of age), duration of captivity (over 2 y), anthropogenic diet (staple was maize, Zea mays), and potential source population.
Maya–Teotihuacan Interaction and the Project Plaza of the Columns Complex.
Despite its importance, data pertaining to early periods of interaction between Teotihuacan governing individual(s) and those of Maya kingdoms remain sparse, are derived mainly from evidence found outside Teotihuacan, and reveal little to no information about any direct impact the Maya had on Teotihuacan governance. Evidence thus far of Maya presence within Teotihuacan has primarily been restricted to immigrant communities residing within the metropolitan center (8, 9). Recent excavation data from Project Plaza of the Columns Complex (2015 to present) is already radically redefining our perspectives regarding the timing and role of foreign diplomacy as expressed in Teotihuacan’s highest state-level rituals as early as the third to fourth century CE (10, 11). Plaza of the Columns (the western sector of the complex), located roughly between the Sun Pyramid and Moon Pyramid, was an important civic administrative compound (Fig. 1B). Defined by the fourth-, fifth-, and sixth-largest pyramids in the city and extensive plazas engineered to host large public events, the project explores how such public functions perpetuated Teotihuacan state governance.
The remains of an extravagant feast consisting of more than 14,000 ceramic sherds, many of which were from wares of nonlocal origin, dates to the early pulse of Teotihuacan–Maya interaction circa 300 to 350 CE, a period just prior to the sudden florescence of epigraphy announcing Teotihuacan’s military presence in the Maya heartland (Fig. 1A, green outline). The high craftsmanship and exotic design of these serving vessels as well as the exceptional scale of the event where they were used indicate a state-sponsored ritual feast attended by important foreign elites. Such feasts are strategic arenas for alliance building, power negotiations, and social identity construction (12, 13). Fine Maya mural iconography (e.g., mythological creatures, gods, and zoomorphic/anthropomorphic forms) were also discovered at the site. As nonportable art publicly displaying Maya affinities exists in this civic–administrative (possibly even a palatial) complex, Sugiyama and colleagues have argued Maya dignitaries may have been housed, at least temporarily, within a once-decadent chamber with walls replete with royal Maya symbology (10).
The complete skeleton of a spider monkey was found in Offering D4, deep in the fill of Mound 25C, one of three major pyramids (Fig. 1B, star). The dedicatory cache was placed next to an early structure that was destroyed in order to construct the pyramid over it and stratigraphically predates the aforementioned feasting event and mural deposit. Fine greenstone figurines and adornments (some sourced as jade from the Motagua Valley, Guatemala) (14), copious shell/snail artifacts, and a lavish quantity of quality obsidian goods (blades, projectile points, and eccentrics) were distributed throughout the cache (Fig. 2A). A complete golden eagle (Aquila chrysaetos) was sacrificed next to the spider monkey (Fig. 2B). A puma skull (Puma concolor), various rattlesnakes (Crotalus sp., minimum number of indivdiuals = 9), and a few unidentified small birds were also uncovered. As the craftmanship of these artifacts is comparable to dedicatory offerings from the Sun, Moon, and Feathered Serpent Pyramids (15–17), this collection was likewise assembled at the direction of the highest levels of the Teotihuacan state. Flotation samples from Offering D4 yielded maize, nopal (Opuntia sp.), chenopods (Dysphania sp.), amaranth (Amaranthus sp.), bean fragments, chia (Salvia hispanica), and Solanaceae seeds, as well as a range of unidentified seeds and other plant parts (18).
Fig. 2.
(A) Plan view map of Offering D4. Pink: animal remains, green: shell, purple: obsidian, yellow: other. Drawing by N.S. (B) Photograph of eagle (left) and spider monkey (right). (C) Scan of mural fragment of a spider monkey. © Project Plaza of the Columns Complex.
Biological/Ecological Considerations.
The Americas are home to 152 species of monkeys (platyrrhines) (19), and archaeological and ethnographic records abound with iconographic, epigraphic, and ethnohistorical evidence of sustained relationships with many species (20). Mesoamerica’s geographic extent coincides with the territory of modern Mexico, a nation whose accentuated topography and complex geography straddles the contact of two major biogeographic zones (Nearctic realm and Neotropical zone) and boasts an almost peerlessly megadiverse biome (21, 22). As exotic fauna, flora, and other natural resources vastly distinct from the local environment were accessible relatively close by, important interregional trade networks were established since Early Formative periods (ca. 1250 to 900 BCE) in Mesoamerica (23).
There are no extant primate species in the arid highlands of Central Mexico. The habitats of two subspecies, the Mexican spider monkey (A. geoffroyi vellerosus) and the Yucatan spider monkey (A. geoffroyi yucatanesis) coincide with the Mesoamerican region (Fig. 1A). In the wild, spider monkeys primarily subsist on fruits and nuts (55 to 90%) complemented by other parts of the plant (shoots, flowers, seeds, palm hearts, etc.) (24, 25). Their ability to consume an impressive range of seed sizes (up to 27 mm) and use of fusion–fission social patterns with extended home ranges (150 to 350 ha) have contributed to their role as primary seed dispersers for important neotropical plants, particularly palms (Oenocarpus sp.) and other large-seed (>20 mm) species (Iryanthera sp.) (26, 27). Water needs are satisfied by the fruits and plants they consume, though they may also drink directly (28).
Spider monkeys are highly tolerant of human presence and readily adapt to life in zoological gardens as well as exotic pets. Indigenous communities also regularly capture spider monkeys as pets and to attract tourism (28, 29). Spider monkeys are endangered (CITES Appendix I, ref. 30) because they are hunted for their meat, trafficked as exotic pets, and because their natural habitat is increasingly fragmented (31, 32).
Modern captive spider monkeys consume between 200 g to 1 kg (infant) and 2 kg (adult) of plant matter per day, 80% of which is supplied as fruit. Common resources include melon, papaya, beet, tomato, cucumber, apple, pineapple, chard, and celery. Seasonal variations include oranges, zapote, pears, watermelons, and prickly pears (33). Agricultural maize, though uncommon, is likely also consumed during captivity.
Archaeological/Cultural Evidence of Spider Monkeys.
Characterized as playful, capable, and mischievous, these charismatic primates were associated by the Maya with the arts and craftsmanship and comprise a notable motif in Mesoamerican iconography (20, 27, 34). The bipedality, dexterity, curiosity, and sexuality of the species parallel human traits, which may be why both Aztec and Maya origin myths tell of a failed creation whereby humans were turned into monkeys (27, 35, 36). The practice of primate captivity was recorded in the Florentine Codex, a 16th-century colonial source that describes an elaborate ploy in which the warmth of a fire and roasted maize kernels lured the animals, and an explosive stone (cacalotetl) was used to frighten the adults into abandoning their young, who are seized and “later they are raised, tamed” (37).
Despite their abundance in the iconographic record, biological traces of their use in archaeological contexts are relatively scarce. At Teotihuacan, physical evidence of nonhuman primates is mentioned only twice. The first concerns an isolated first upper molar from a howler monkey (Alouatta sp.) found in the Merchants Barrio (38), a primarily Maya residential enclave (9), and the second concerns a portion of a spider monkey’s left arm excavated from Burial 5 of the Moon Pyramid where Maya elite were likely sacrificed (39). In both cases, it seems only a small portion of the complete animal was transported to contexts closely associated with Maya presence.
Elsewhere in Mesoamerica, spider monkey skeletal data are similarly uncommon. The oldest record pertains to a foreign import of an ochre-reddened skull offered in a men’s house floor at the Formative site of San Jose Mogote, Oaxaca (700 to 500 BCE) (40). Even at sites within the spider monkey’s home range, finds are minimal and usually restricted to refuse deposits (20, 41, 42). A remarkable example of a complete spider monkey from Tikal was found within Burial 162, an Early Classic burial of a middle-aged female (hypothesized to be the tomb of the Woman of Tikal, who ruled during the early sixth century) (20, 43–45). It is noteworthy that evidence of a spider monkey burial originates from Tikal, the Maya site where Teotihuacan presence is epigraphically and architecturally evident (7, 46).
State choreographed sacrifice of apex predators (eagles, pumas, jaguars, and wolves) held in captivity recorded at the Moon Pyramid and Sun Pyramid demonstrated that highly symbolic animals participated as key symbols of the Teotihuacan state in public rituals (39, 47). Not only does this assemblage provide comparative zooarchaeological and isotopic baselines, it also strengthens arguments of the prominent role public display of symbolic fauna played in the reification of power.
Results
Radiocarbon/Ceramic Dating.
A phalange of an eagle sacrificed next to the spider monkey (D18-0704, EL-D449, AA112737) and a carbon fragment found in the fill of the offering (M-D3050, AA112734) were analyzed to date the sacrificial event (Table 1). Bayesian statistical model of the two samples as a single phase utilizing OxCal 4.4 INTERCAL 13 calibration curve consistently dates Offering D4 to sometime between 145 and 345 CE (SI Appendix, Fig. S1).
Table 1.
Radiocarbon results and Bayesian statistical model of likely date of Offering D4
Ceramic analysis of the fill matrix of Offering D4 (n = 177) and the general fill of Tunnel 5 (n = 842) are consistent with the radiocarbon results (SI Appendix, Table S1). Ceramics were dominantly composed of Early Tlamimilolpa (200 to 350 CE) (60%) and Miccaotli (150 to 200 CE) (29%) phase sherds with minor inclusions of Tzacuali materials (100 to 150 CE) (6 and 9%, respectively) (48). Given the dominant presence of Early Tlamimilolpa ceramics, and the stratigraphic relationship with Offering D1 feasting deposit (dated to 300 to 350 CE), we assert Offering D4 took place sometime circa 250 to 300 CE, just before the feasting event.
Zooarchaeology.
The spider monkey skeleton was remarkably complete and well-preserved. It was deposited on its left side on the northeastern sector of the cache to the east of a complete golden eagle. Hands bound behind its back and tethered feet indicate en vivo burial, common among human and animal sacrifices at Teotihuacan (16, 39, 47, 49). Given the combination of complete permanent dentition, unfused skeleton, and small size, the specimen was likely a subadult (5 to 8 y). Despite its relatively young age, sexual dimorphic characteristics of the canine and generally gracile skeleton suggests the Teotihuacan specimen was female (see also aDNA). Morphometric characteristics allowed us to tentatively assign the specimen as A. geoffroy cf vellerosus (see SI Appendix, Supplementary Text).
Pathological indicators of captivity noted on other ritually sacrificed animals (healed fractures, infectious disease, and vitamin deficiencies) from Teotihuacan (47, 49) were scarce and limited to oral health. The incisors and premolars of the spider monkey were extensively worn despite its relatively young age (SI Appendix, Fig. S5). The maxillary right first premolar was missing and the alveolar cavity filled.
Light Isotopes (δ13C, δ15N, δ18O).
Isotope analysis of collagen (δ13Ccollagen, δ15N) and carbonate (δ13Ccarbonate, δ18O) samples of Offering D4 included a bone (metapodial) and two teeth (lower-E1 and upper-E2 canine) of a spider monkey, two bones (maxilla and phalanx) and a tooth (premolar) of a puma, and an eagle bone (phalanx) (Table 2). δ15N values indicate trophic level of the consumer (protein intake) (50), while δ13Ccollagen and δ13Ccarbonate provide the relative contribution of plant type consumed: C3 (most trees, shrubs, and temperate grasses, δ13C −26.5‰), C4 (arid to subarid grasses, −12.5‰), and crassulacean acid metabolism (CAM) (epiphytes and xerophytes, δ13C overlap with C4 plants) (51). Importantly, in the Mesoamerican context the dominant domesticated staple, maize, contributes to elevated δ13C values and has been a useful indicator of animal captivity and domestication (47, 52). The δ18O from carbonates is directly related to consumed water, geographic location, and local environment (e.g., humidity). It is thus a useful proxy to map origin and migration patterns of humans and animals (53). Tooth enamel (carbonate) and dentine (carbonate and collagen) are generally indicative of diet and environmental conditions prevalent during tooth formation, whereas bone (carbonate and collagen) continuously turns over. Sampling multiple elements from the same individual therefore allows us to establish its isotopic life history (54, 55). As the lower canine (E1) erupts before the upper canine (E2) isotopic changes during youth can be captured. Only dentine was analyzed for the lower canine (E1).
Table 2.
Descriptive statistics of the Plaza of the Columns isotope data from Mound 25C, Offering D4
| Collagen | Carbonate | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Sample | Element | δ15N | δ13C | C:N | % Yield | δ13C | δ18O (VSMOW) | C/P | IRSF |
| Spider monkey | NS352B, EL-D447 | Bone | 6.4 | −11.9 | 3.2 | 3 | −5.7 | 23.7 | 0.1 | 3.9 |
| Spider monkey | NS352d, EL-D447 | Dentine (E1) | 5.5 | −19.0 | 3.3 | 2 | −10.0 | 26.0 | 0.1 | 3.7 |
| Spider monkey | NS352e, EL-D447 | E1-lower canine | — | — | — | — | −12.9 | 16.6 | 0.1 | 3.4 |
| Spider monkey | NS353e, EL-D447 | E2-upper canine | — | — | — | — | −10.2 | 24.6 | — | — |
| Puma | NS0325, EL-D450 | Bone | F | F | 3.6 | 1 | F | F | 0.1 | 4.7 |
| Puma | NS0326d, EL-D450 | Dentine | F | F | 4.4 | 1 | F | F | 0.1 | 4.4 |
| Puma | NS0326e, EL-D450 | Enamel | — | — | — | — | −12.1 | 28.2 | 0.2 | 3.4 |
| Puma | NS0327, EL-D450 | Bone | 7.7 | −20.0 | 3.3 | 2 | F | F | 0.1 | 4.5 |
| Eagle | NS0328, EL-D450 | Bone | 7.4 | −16.5 | 3.4 | 4 | −6.7 | 25.3 | 0.1 | 4.3 |
Underlined cells and F indicate values of failed diagenesis tests.
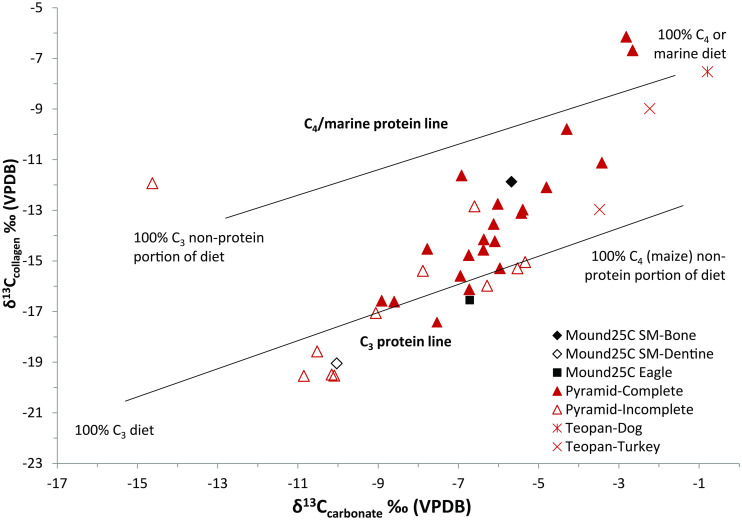
Dedicatory offerings within the Moon Pyramid and Sun Pyramid at Teotihuacan provide isotope data for comparison as many sacrificed carnivores with elevated δ13C indicated consumption of anthropogenic diets composed of increased C4/CAM resources, likely maize (Pyramid-Complete in Table 3 and Fig. 3 and also see Fig. 6) (47). These captive-reared carnivores were distinguished from incomplete prepared animal paraphernalia (pelts, skulls, etc.) that displayed significantly lower δ13C values reflective of a “wilder” diet (Pyramid-Incomplete). Two specimens each of the domesticated dog (Canis familiaris) and turkey (Meleagris gallopavo) excavated from the Teopancazco apartment complex at Teotihuacan provide parameters of a primarily C4-based domestic diet (56). In addition, collagen isotope values were plotted against modern wild specimens of spider monkey (A. geoffroyi, primarily frugivores), howler monkey (Alouatta palliata, varied diet of mixed leaves >50% and fruit), capuchin monkey (Cebus capucinus, omnivores consuming insects and fruit), and muriqui or woolly spider monkey (Brachyteles arachnoides, primarily folivorous) from Costa Rica (Ateles, Alouatta, Cebus) and Brazil (Brachyteles) (57).
Table 3.
Descriptive statistics of comparative archaeological and modern isotope data
| Collagen | Carbonate | |||||||
|---|---|---|---|---|---|---|---|---|
| n | δ13C (mean) | SD | δ15N (mean) | SD | n | δ13C (mean) | SD | |
| Archaeological | ||||||||
| Pyramid-Complete1 | 28 | −13.3 | 2.9 | 8.3 | 1.5 | 29 | −6.2 | 2.5 |
| Pyramid-Incomplete1 | 18 | −15.8 | 2.7 | 7.3 | 1.1 | 43 | −12.0 | 4.1 |
| Teopancazco-Dog2 | 2 | −7.6 | 0.1 | 10.0 | 1.2 | 2 | −1.3 | 0.8 |
| Teopancazco-Turkey2 | 2 | −11.0 | 2.8 | 5.9 | 0.8 | 2 | −2.9 | 0.9 |
| Modern | ||||||||
| Spider monkey3* | 5 | −22.0 | 0.2 | 5.9 | 0.5 | — | — | — |
| Howler monkey3* | 12 | −20.5 | 0.2 | 4.4 | 0.7 | — | — | — |
| Capuchin monkey3* | 4 | −21.7 | 0.2 | 7.9 | 0.1 | — | — | — |
| Muriqui or woolly-spider monkey3* | 7 | −20.6 | 0.2 | 6.5 | 0.4 | — | — | — |
Fig. 3.
(A) Collagen isotope results of Mound 25C (black), modern comparative (blue), and other archaeological (red) comparative data. Error bars on comparative datasets represent two SDs (Table 2). SM: spider monkey, Teopan: Teopancazco. (B) Carbonate isotope results of PPCC samples and comparative archaeological data. Spider monkey samples include B: bone, D: dentine, E1: canine lower, E2: canine upper. 1Sugiyama et al. (47). 2Morales Puente et al. (56).
Fig. 6.
Simple carbon model following Froehle et al. (72) of Mound 25C (black) spider monkey (SM) and other archaeological (red) comparative data.
Collagen isotope results demonstrated gradual shifts in δ13Ccollagen and δ15N values throughout the life of the Mound 25C spider monkey. There was a 7.1‰ δ13Ccollagen enrichment between the spider monkey’s dentine (−19‰) and bone (−11.9‰), representing a shift from values close to modern wild platyrrhine and Pyramid-Incomplete ranges to one that coincides with Pyramid-Incomplete, Pyramid-Complete, and Teopancazco-turkey ranges (Fig. 3A). Offering D4 puma and eagle samples plotted closer to the “wild” diets represented by the Pyramid-Incomplete specimens. The spider monkey’s dentine to bone δ15N value was likewise enriched by 0.9‰. Given preweaning diets typically express 2 to 3‰ higher δ15N and 1 to 2‰ higher δ13Ccollagen values (58), the observed dentine–bone offset is likely caused by other external sources such as dietary and/or environmental shifts.
Carbonate isotope results parallel findings in the collagen samples. A gradual δ13Ccarbonate enrichment is recorded from Mound 25C spider monkey’s E1-enamel (lower canine, −12.9‰), E2-enamel (upper canine), and E1-dentine (−10.2 and −10.0 ‰, respectively), and bone (−5.7‰) samples (Fig. 3B). The eagle, also a sacrificed complete specimen, displayed a δ13Ccarbonate value (−6.7‰) in line with the Pyramid-Complete samples while the prepared skull of the puma had much more depleted values (−12.1‰) typical of Pyramid-Incomplete specimens. Oxygen isotope values were not directly compared across species as body physiology and behavior greatly influence its outcome. Mound 25C spider monkey δ18Ocarbonate values displayed a >7‰ enrichment between E1-enamel (16.6‰) and the other three elements (E2-enamel, dentine, and bone, 23.7 to 26‰).
Heavy Isotopes (87Sr/86Sr).
Strontium isotope ratios (87Sr/86Sr) were measured in the upper canine (E2) of the sacrificed spider monkey to assess the animal’s geographic location at the time of tooth enamel formation since 87Sr/86Sr varies across the landscape according to the type and age of the underlying geology. The individual’s observed 87Sr/86Sr of 0.7051 indicates residence in an area characterized by relatively recent volcanic-derived bedrock and soils (59). Potential source locations in Mesoamerica matching this geology include the highlands of Central Mexico and Guatemala and the Guatemalan Pacific coast (60). The spider monkey’s strontium ratio is similar to values previously reported from soil, fauna, and archaeological humans from Teotihuacan (0.7042 to 0.7055) (61–67). Geographic source areas within the biogeographic range of spider monkeys that can be ruled out based on 87Sr/86Sr include the northern and southern Maya Lowlands (0.707 to 0.709) and the Maya Mountains of Belize (0.710 to 0.720). Other regions of Mesoamerica are more difficult to rule out due to geological heterogeneity (e.g., Metamorphic province of southeastern Maya region) or a lack of 87Sr/86Sr baseline data (e.g., Gulf Coast). However, the spider monkey’s strontium value does not match 87Sr/86Sr from the major Maya site of Copan, which lies with the Metamorphic providence (0.7052 to 0.7072) (68, 69) or major sites sampled in the Gulf Coast region (<0.7043 or 0.7060 to 0.7083) (60).
aDNA.
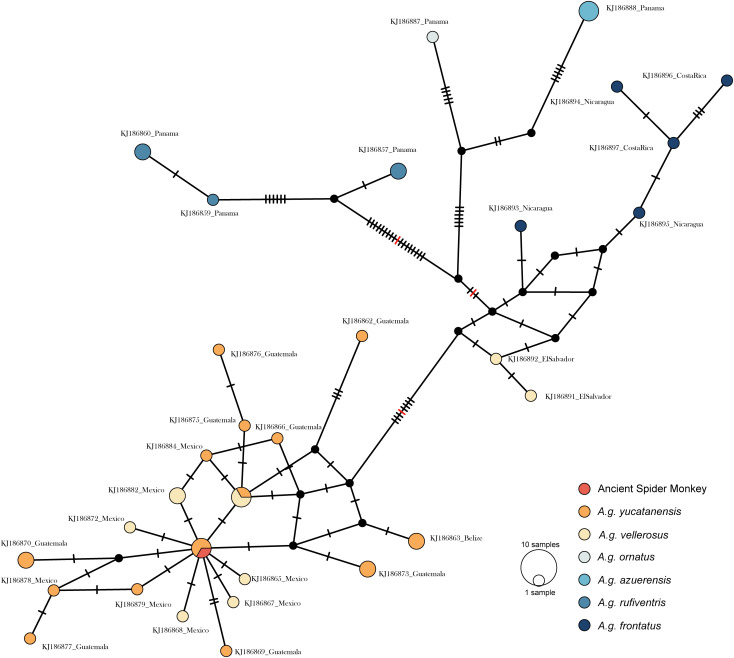
DNA was successfully extracted from a maxillary second left molar in a specialized aDNA laboratory. Double-stranded Illumina libraries were sequenced and aligned to published A. geoffroyi datasets (SI Appendix, Table S4). The resulting sequence data showed strong evidence of DNA degradation expected with aDNA (SI Appendix, Fig. S6). Phylogenetic analysis using comparative mitochondrial DNA sequences identified in GenBank (SI Appendix, Table S5) placed the Mound 25C spider monkey in a poorly resolved branch that includes the Mexican, Guatemalan, and Belizean samples belonging to A. geoffroyi yucatanensis and vellerous (SI Appendix, Fig. S7). A median-joining network also clearly placed the Mound 25C sample with the yucatanensis and vellerous comparative samples (Fig. 4). Genetic sex identification was performed and supported the zooarchaeological assessment that the specimen was female.
Fig. 4.
Median-joining cytochrome b gene network with ancient spider monkey. The ancient spider monkey clusters with A. geoffroyi vellerosus and A. geoffroyi yuctanensis subspecies. Comparative data are from Morales-Jimenez et al. (89). Samples are color-coded by subspecies. Red hashmarks indicated shortened edges.
Paleobotany.
A range of microremains, which includes vegetal matter such as starch grains, phytoliths, and vascular tissue, as well as nonvegetal elements were recovered from the wet (n = 3) and dry (n = 3) (dental calculus) samples collected. The remains found in the wet samples likely represent some of the more recent consumption or breathing in of the spider monkey (even just before death), whereas the dry samples are more likely to have been accumulated through longer periods of time as calculus is hardened dental plaque that will trap particles entering the oral cavity.
A total of 69 starch grains were recovered (not including the two clusters of grains) (SI Appendix, Table S6). Taxa that could be identified include maize (Z. mays; Fig. 5 A and B) and chili peppers (Capsicum sp., Fig. 5 C and D). Evidence of underground storage organs (i.e., tubers, rhizomes) belonging to at least four different taxa was also recovered, including probable arrowroot (Maranta arundinacea, Fig. 5 E and F). Unidentified starch grains belonging to tubers (SI Appendix, Fig. S8 A–D) and other grains (SI Appendix, Fig. S8E) were also present.
Fig. 5.
Microbotanical remains recovered from the teeth of the spider monkey. Starch grains viewed under transmitted and cross-polarized light (A and B, maize; C and D, chili pepper; E and F, arrowroot). Phytoliths (G–I), trichome (J), crystals (K), and softwood tissue (L); white arrow indicates cross-field pitting. Photographs by C.C.
Phytoliths (microscopic silica bodies produced by plants) were also recovered. We recorded only their presence, and not their quantities, and therefore statistical calculations were not possible. The morphotypes as shown in SI Appendix, Table S7 were identified following the terminology recommended by the International Committee for Phytolith Taxonomy (70). Long and short cells (Fig. 5G), bulliform flabellate (Fig. 5H), and acute bulbosus (Fig. 5I) phytoliths indicate the predominant presence of monocots (grasses and sedges) and perhaps some dicots. Other plant remains include trichomes (plant appendages; Fig. 5J and SI Appendix, Fig. S8Q) as well as calcium oxalate crystals (Fig. 5K and SI Appendix, Fig. S8R) and vascular tissues indicating the presence of both hardwoods and softwoods (Fig. 5L and SI Appendix, Fig. S8 S–U). Nonvegetal remains identified include mammal hair fragments as well as fungal spores (see SI Appendix, Supplementary Text).
Discussion
A multimethod archaeometry investigation of the Mound 25C spider monkey captured anthropogenic alterations to the animal’s diet and environment. Both collagen and carbonate δ13C values showed a positive trend: a 7.1‰ δ13Ccollagen enrichment between the dentine and bone, a nearly 3‰ δ13Ccarbonate enrichment between E1-enamel and E2-enamel/E1-dentine, and a subsequent 4.5‰ enrichment with the bone sample. Together, they record a transition from a C3-dominated diet (during lower canine formation) to one that integrates C4/CAM resources (upper canine formation), a trend that continued and intensified throughout the rest of the spider monkey’s life (as reflected in the bone).
Carbon isotope values from mineral bioapatite-carbonate (δ13Ccarbonate) and organic collagen (δ13Ccollagen) phases yield slightly different information (total diet and dietary protein, respectively) (71–74). A simple carbon model plotting both δ13Ccarbonate and δ13Ccollagen on a pair of parallel regression lines then allows us to examine the spider monkey values in relation to experimental animal populations of known C3 or C4 diets that can help infer relative proportions of the contributing sources (72, 73). Fig. 6 demonstrates the spider monkey dentine distributed firmly within the expected “natural” range on the simple carbon model within the distribution of Pyramid-Incomplete specimens with respect to the C3 protein and C3-dominated diet. In contrast, the bone sample’s elevated δ13Ccarbonate and δ13Ccollagen values places this sample closer to the C4 protein line, indicating a mixed C3 and C4/CAM total diet reminiscent of Pyramid-Complete values, argued by the authors to be animals held in captivity. These models confirm both dietary protein and total diet were affected by increasing reliance on C4/CAM resources, likely of anthropogenic origin as the animal habituated to a captive diet.
There is a complementary 0.9‰ enrichment in the δ15N value between the spider monkey’s E1-dentine and bone. While this difference is still within one SD of the wild spider monkey’s dietary range, a parallel shift in δ13Ccarbonate and δ13Ccollagen suggests slightly elevated nitrogen values likewise reflect a distinct captive diet after its lower canine had formed. The elevated δ15N value may originate from the natural raw resource itself, or because cultivation practices often lead to δ15N enrichment (75). Slightly elevated δ15N enrichment among domesticated turkeys has been attributed to greater consumption of other invertebrates also attracted to human settlements (76).
Though we know the eruption sequence for the spider monkey, no references linked tooth formation to a particular developmental age. Based on pertinent literature and other primate eruption sequences, the isotopic shift occurred after the lower canine had formed but while the upper canine was still developing, indicating the shift had to occur prior to about 3 to 3.5 y of age (SI Appendix, Supplementary Text). Given the estimated subadult age (5 to 8 y) of the Mound 25C specimen, we can infer the spider monkey was maintained in captivity for at least 2 y.
Two food resources may be responsible for the heightened C4/CAM values. First, of course, is that quintessentially Mesoamerican staple which has helped identify evidence of captivity among dogs, turkeys, parrots, and even apex predators: maize (47, 76–81). As spider monkeys are primarily frugivorous, we also hypothesize that prickly pear fruit, which was cultivated extensively at Teotihuacan and remains a ubiquitous fruit resource in the region to this day (82), would have made a readily available supplement to maize feeding. Modern maize δ13C values (average δ13C −11.8‰, δ15N 3.1‰), prickly pear fruit (average δ13C −13.0‰, δ15N 3.9‰) and stems (average δ13C −13.7‰, δ15N 9.4‰) reported for samples from Oaxaca and Central Mexico demonstrate comparable values (56, 75) (SI Appendix, Table S3) and are thus isotopically indistinguishable.
Starch and phytolith samples from the spider monkey’s dentition were obtained with the objective of further reconstructing its captive diet. The different vegetal elements indicate the consumption of grass (Poaceae family) and possibly sedge (Cyperaceae family) leaves/culms, maize kernels, chili peppers, and different types of tubers, including arrowroot. Tuber consumption has not, to our knowledge, been observed in wild spider monkeys. Capuchin monkeys have been reported to use tools to dig up roots and tubers, including cassava tuber raids (83). The consistent presence of chili peppers in both wet and dry samples is somewhat surprising, although we know that wild spider monkey diets consist predominantly of fruit (25). Food residues recovered from the animal’s dental samples correspond to consumption of domesticated plants (including maize), a pattern that agrees with an isotopic signature consistent with a wild animal that has spent at least part of its life in captivity. Of particular interest is the presence of arrowroot, a domesticated plant associated with tropical environments. There was no indication of prickly pear, despite the frugivorous tendencies of the animal.
The softwood fibers seemingly indicate the presence of conifer wood, while plant vessel elements suggest mastication of dicot stems or wood. Live and decaying wood consumption has been noted among wild spider monkeys, possibly as a source of minerals (25, 84). We were unable to identify the crystals to their botanical origin, but their presence supports the consumption of diverse plants and different plant parts (organs). These findings are consistent with aforementioned ethnohistorical sources that list, “maize, fruit, meat, … pine nuts, acorns, and also tender shoots of trees” as sources of a captive primate’s diet (37).
It should be noted that some of the elements might not necessarily be related to the diet of the spider monkey (85). The presence of a mammal hair might be explained by an accidental inhalation or environmental contamination. The same can be said for the Alternaria fungus, which is a common pathogen found on plants but is also airborne (86, 87). Notably, the plant vessels originating from either hardwoods (stem or wood) or the softwoods (woods) may not have entered the mouth due to consumption but chewing or gnawing wood (perhaps from the cage) or even from putting a twig inside the mouth. The hypothesis that wooden cages may have confined the spider monkey is suggested based on confirmed cases of postholes delimiting the cages surrounding two pumas and a wolf sacrificed in Burial 2 of the Moon Pyramid (39). The spider monkey’s extensive mesial wear patterns, including the complete loss of its upper premolar, is reminiscent of a puma from Burial 6 with significant incisor wear extending to complete loss of its first and second incisors. The puma’s deteriorated dentition was interpreted as the result of prolonged gnawing on restrictive devices and intensive stress during confinement. Thus, the spider monkey’s captive environment likely led to its compromised oral health, possibly from gnawing on restrictive devices.
δ18Ocarbonate and strontium isotope data can help identify potential regional origin and translocation of the spider monkey. Like the paleodietary data obtained from carbon and nitrogen isotopes, the δ18Ocarbonate data confirm a significant life event sometime between the formation of the lower canine (E1) and the upper canine (E2). A dramatic increase in δ18Ocarbonate value from 16.6‰ (E1-enamel) to 24 to 26‰ (E1-dentine, E2-enamel, and bone) suggests the spider monkey moved from a humid to an arid environment. Unfortunately, we cannot pinpoint the specific locality based on oxygen alone as body size and physiology affect δ18Ocarbonate water to tooth/bone offset, prohibiting us to correlate the results with known archaeological human or local meteoric water sources. This is why we took a dual isotope approach integrating δ18Ocarbonate data with strontium isotope (87Sr/86Sr) results that are more closely related to the geographic characteristics of the bedrock (64).
The strontium isotope value from the upper canine (E2) of 0.7051 could reflect several different regions within Mesoamerica, including the Central Mexican highlands (within and around Teotihuacan), the Guatemalan highlands, and the Guatemalan Pacific coast. The isotope signal also eliminates numerous spider monkey habitats which are geologically inconsistent with its signature, including both the southern and northern Maya lowlands of Belize, northern Guatemala, and Mexico’s Yucatan Peninsula. The Gulf Coast region of Veracruz is also an unlikely match for the observed strontium value.
Considering the major geographic shift suggested by the δ18Ocarbonate data, which took place between the formation of the monkey’s upper and lower canines, the 87Sr/86Sr value in the monkey’s later-forming upper canine most likely reflects the individual’s arrival in Central Mexico rather than its origins from the Guatemala highlands or Pacific coast. A dietary shift observed in the δ13C of the upper and lower canines further supports a transition from wild to captive status and translocation while the individual’s dental tissues were still forming. The strontium isotope value from the upper canine is thus less indicative of the monkey’s birth location than its area of residence 2 to 3 y before death. Additional strontium isotope samples will be obtained to assess birth origin.
Genomic analysis confirmed species identification of A. geoffroyi; however, the few population-level genetic studies of A. geoffroyi have focused on mitochondrial markers (88, 89), limiting the available data for comparative analyses. The two possible subspecies are not monophyletic with the available mitochondrial markers (SI Appendix, Fig. S7) (89); as such, subspecies could only be narrowed down to A. geoffroyi vellerosus or A. geoffroyi yucatanensis with genetic data. Genomic data support zooarchaeological assessment that the spider monkey was female. Future genomic studies of modern A. geoffroyi populations may provide additional resolution into the origins of this spider monkey as well as insight into how human-mediated landscape change over the last 2,000 y impacted the spider monkey genome.
The spider monkey was deposited alongside a golden eagle and several rattlesnakes. Its arms were bound behind its back. Offering D4’s faunal assemblage is consistent with evidence of live sacrifice of symbolically potent animals participating in state rituals observed in Moon and Sun Pyramid dedicatory caches (39, 49). Among the thousands of fragments recovered from the fastidiously demolished Maya-style murals in the Plaza of the Columns was a clear depiction of a spider monkey seated with an elaborate belt decorated with what Karl Taube identified as the the ajaw (king) sign (Fig. 2C). These murals represent some of the finest examples of Classic period Maya-style mural art with regal overtones, undoubtedly the work of renowned artisans (perhaps accompanying Maya dignitaries) who must have visited and perhaps even resided within the metropolis (10).
Ceramic and radiocarbon dating indicates Offering D4 event took place sometime between 250 and 300 CE, making it the earliest context recording Teotihuacan–Maya interaction documented thus far at Plaza of the Columns, followed closely by the feasting deposit with clear Maya-style ceramic vessels (300 to 350 CE) and the destruction of the Maya-style murals (350 to 450 CE) (10). The only other context within Teotihuacan with early evidence of Maya diplomatic ritual exchange pertain to trumpet shells with foreign iconography, including several executed in fine Maya style, in the subterranean tunnel beneath the Feathered Serpent Pyramid, possibly dating to 150 to 250 CE (90). In all these instances, Maya high (likely royal) elite presence is associated with large-scale public rituals directly orchestrated by the Teotihuacan state. That the centerpiece of Offering D4 was an exotic species translocated live from a Maya territory adds to the growing consensus that the influence of Maya dignitaries at this central civic administrative complex was established by this early date. It strengthens the argument that sustained high-level diplomatic interaction with the Maya and other regional cultures powers was a crucial factor in Teotihuacan’s ascent to prominence in Mesoamerica (11).
It is worth noting the Maya-style mural fragments were marked repeatedly with impact fractures and found buried among the plaza fill. These auspicious signs of an iconoclastic act are dated to circa 350 to 450 CE, a timeframe that conspicuously overlaps with the introduction of Entrada scenes in several Maya centers (10). It also coincides with a state-sponsored sacrificial event at the Moon Pyramid of Teotihuacan, where three individuals of apparently Maya royal lineage (based on isotope analysis and the greenstone pendants symbolizing Maya rulership) were buried (350 ± 50 CE) (17). Thus, the iconoclastic termination of Maya royal symbols on the murals at Plaza of the Columns may be an indication of a sudden shift in Teotihuacan–Maya sociopolitics (11, 48).
Conclusion
We assert that exotic and highly valued artifacts may have been exchanged as part of a diplomatic gift protocol that would have carried considerable sociopolitical obligations at Plaza of the Columns (2). There is direct comparative value to document effective use of exotic animals in diplomatic gift exchange in the rise and sustenance of ancient states. The sacrificed spider monkey from Teotihuacan not only confirms that ritual exchange/gifting had been practiced with Maya dignitaries by 250 to 300 CE, prior to the epigraphically attested Entrada event, it is also the earliest documented instance of primate captivity and translocation in the Americas. Parallel evidence from other contexts at the Plaza of the Columns, including the depiction of this exotic captive animal among regal Maya mural art and an elaborate feasting deposit containing fine Maya vessels, suggests sustained state-mitigated ritual exchange, conspicuously displayed in public rituals and architecture, preceded Teotihuacan’s apparent militaristic involvement with some Maya sites.
Multimethod archaeometry is increasingly becoming an essential component of zooarchaeology, enhancing our ability to directly assess short- and long-term patterns in habitat alteration, plant and animal physiology, subsistence strategies, mobility, and anthropogenic impacts on fauna and even reconstruct individual life histories (91). In the case of the Mound 25C spider monkey, we registered that this possible female subadult (5 to 8 y old), likely A. geoffroyi cf vellerosus, was evidently captured just prior to ca. 3 y of age, when the upper canines were still forming in its alveolar. The monkey was shortly introduced to a drastically changed environment and dietary intake. Artificial feeding resulted in elevated degrees of C4/CAM resources in its diet (primarily maize), and other secondary resources atypical of wild diets were also introduced (e.g., arrowroot and chili pepper). Oxygen isotope results indicate that it was around this time that the animal was likely translocated from the humid biome of its native habitat to a comparatively arid biome characteristic of highland Central Mexico. This primate was sustained in captivity for over 2 y before being sacrificed during a state-coordinated consecration ritual at Mound 25C of the Plaza of the Columns Complex in Teotihuacan.
Precedent of mass animal sacrifice of captive carnivores at the Moon Pyramid and Sun Pyramid support that public displays incorporating highly conspicuous and symbolic animals were effective arenas for the reification of power (47, 49). This case study proposes a scenario of a personal gift exchange of an exotic and charismatic mammal between high diplomats of two powerful centers likely shaped public opinion, to the point that a spider monkey was depicted on the walls of a public administrative complex at the heart of Teotihuacan’s ceremonial core. As millions of tourists celebrated the life of Ling-Ling and Hsing-Hsing at the National Zoo, the gift of the spider monkey who likely resided, and thus observed by the public, at the Plaza of the Columns Complex held important sociopolitical implications. It is at least intriguing to contemplate the broad cultural implications and the effectiveness of this captive primate’s status as a cultural nexus between the Maya and Teotihuacan.
Materials and Methods
Excavations.
Excavations of Offering D4 were carried out by the Project Plaza of the Columns Complex in 2018 with permission from the National Institute of Anthropology and History (Oficio 401.1S.3-2018/963). Bone and teeth samples were exported with authorization under documents Oficio 401.3S.16-2018/1911, 401.3S.16-2018/1912, 401.3S.16-2018/1913, and 401.3S.16-2018/1914.
Radiocarbon/Ceramic Dating.
An eagle bone and carbon fragment from Offering D4 was analyzed at the University of Arizona Accelerator Mass Spectrometry Laboratory for radiocarbon analysis and the results modeled in OxCal 4.4 program modeled with the INTCAL 13 calibration curve. Ceramic analysis followed general ceramic phases published by Rattray (92).
Zooarchaeology.
Standard zooarchaeological methods of species, age, sex, and surface modification designations were assessed (93). Detailed descriptions of spider monkey morphometric traits can be consulted in SI Appendix, SupplementaryText and figures.
Light Isotopes.
Collagen and carbonate were extracted from tooth and bone samples using standard methods and examined for post mortem diagenesis using elemental abundances and Fourier transform infrared–attenuated total reflection (FTIR-ATR) spectroscopy, respectively (SI Appendix, SupplementaryText). Briefly, collagen was extracted using an acid–base–acid method. Carbonates were isolated via removal or organic material and potential secondary carbonates. Collagen was considered well-preserved if it fell within prescribed ranges for collagen yield and C:N ratios. Carbonate was considered well-preserved if FTIR-ATR spectroscopy peak height ratios for C/P and IRSF fell within prescribed ranges (94). All isotope data are reported in standard delta notation: δX = [(Rsample – Rstandard)/Rstandard] * 1,000, where X is the heavy isotope of interest (15N, 13C, 18O), R is the isotope ratio (15N/14N, 13C/12C, 18O,16O), internationally accepted standards are air, Vienna Pee Dee Belemnite, and Vienna Standard Mean Ocean Water (N, C, O, respectively), and units are parts per thousand (‰). Errors are ±0.2‰ (1σ).
Heavy Isotopes.
Tooth enamel was cleaned and prepared in a HEPA-filtered class 1000 clean laboratory within the Washington State University Radiogenic Isotope and Geochronology Laboratory (WSU RIGL) (SI Appendix, Supplementary Text). Strontium isotope ratios (87Sr/86Sr) were measured using a ThermoFinnigan Neptune multicollector inductively coupled plasma mass spectrometer housed in the same laboratory facility (WSU RIGL). Instrument accuracy was monitored and confirmed through replicate measurements of the strontium standard NBS-987.
aDNA.
DNA was extracted from a maxillary second left molar and libraries prepared from the extract using published protocols (95). The sample and negatives were dual-indexed and sequenced on a 2 × 150 NextSeq run. Raw reads were quality filtered with AdapterRemoval2 and mapped with bwa using parameters for aDNA to the A. geoffroyi genome (GenBank assembly: GCA_004024785.1) and competitively mapped to publicly available spider monkey mitochondrial sequences (89, 96, 97). A section of the CytB gene was isolated from the competitively mapped alignment and compared to the regional spider monkey dataset (SI Appendix, Supplementary Text) for phylogenetic analysis. Maximum likelihood gene trees with 100 bootstrap replicates were made with both RAxML and in MEGA with partial deletion (98, 99). Median-joining haplotype network was generated in PopART (v: 1.7) (100). Raw sequencing data has been deposited the NCBI Sequence Read Archive under the BioProject accession number PRJNA882120.
Paleobotany.
Dental washes were obtained from three teeth (mandible), and small quantities of dental calculus were obtained from another three teeth (maxilla). The samples were processed in a dedicated laboratory using different chemical products with the aim of separating the microremains from the rest of the sample. The residues were observed using a cross-polarized microscope (100 to 600×) and identified using a reference collection and published materials.
Supplementary Material
Acknowledgments
Project Plaza of the Columns Complex was codirected by N.S., S.S., V. Ortega, W. Fash, and D. Carballo.; N.S. acknowledges the support of Yen-Shin T. Hsu in studying the comparative spider monkey collection at the National Museum of Natural History, Smithsonian Institution. Project members Ariel Texis, Yolanda Peláez C., and Adriana Sanchez assisted in excavation, curation, and photography of the Mound 25C spider monkey from Plaza of the Columns. Esther Aguayo assisted with light isotope laboratory work. N.S. thanks B.H. Smith for her time and support in assessing approximate ages for spider monkey tooth formation and Sandra Koch for examining a microscope photograph of a hair sample. E.T. thanks Kim Sheets, Dr. Chao Zhang, and Dr. Jeff Vervoort for their assistance with strontium isotope sample preparation and analysis. C.C. thanks the phytolith laboratory (ArScAn UMR 7041) and the ArchéoScopie Platform, both located at the MSH Mondes in Nanterre, France. Excavations of Offering D4 at Plaza of the Columns Complex were conducted with support from the NSF (Archaeology BCS 1638525) and the Japan Society for the Promotion of Science (JSPS 25257016, 17H01650, 19H05732, 19H05736, 21H04378). Laboratory work was completed with support of the NSF (Archaeology BCS 1638525), National Endowment for the Humanities (RFW-279331-21), and Smithsonian Museum Conservation Institute Federal and Trust Funds.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2212431119/-/DCSupplemental.
Data, Materials, and Software Availability
Raw sequencing data have been deposited in NCBI Sequence Read Archive (PRJNA882120) (102). All other raw data are reported in the main text or SI Appendix.
References
- 1.Chen W., Case study of panda diplomacy in 1972. Int. J. Soc. Sci. Educ. 4, 54–58 (2021). [Google Scholar]
- 2.Mauss M., The Gift: The Form and Reason for Exchange in Archaic Societies (W.W. Norton, 2000). [Google Scholar]
- 3.Braswell G. E., Ed., The Maya and Teotihuacan: Reinterpreting Early Classic Interaction (University of Texas Press, 2003). [Google Scholar]
- 4.Hirth K. G., Carballo D. M., Arroyo B., Eds., Teotihuacan: The World Beyond the City (Dumbarton Oakes Research Library and Collection, 2020). [Google Scholar]
- 5.Kidder A. V., Jennings J., Shook E., “Exterior relationships as indicated by pottery and other artifacts” in Excavations at Kaminaljuyú, Guatemala, Kidder A. V., Jennings J., Shook E., Eds. (Carnegie Institution of Washington Publication, 1946), pp. 218–240, 255–260. [Google Scholar]
- 6.García-Des Lauriers C., Murakami T., Eds., Teotihuacan and Early Classic Mesoamerica: Multiscalar Perspectives on Power, Identity, and Interregional Relations (University Press of Colorado, 2022). [Google Scholar]
- 7.Stuart D., “‘The arrival of strangers’: Teotihuacan and Tollan in classic Maya history” in Mesoamerica’s Classic Heritage: From Teotihuacan to the Aztecs, Carrasco D., Jones L., Sessions S., Eds. (University Press of Colorado, 2000), pp. 465–514. [Google Scholar]
- 8.Clayton S. C., Interregional relationships in mesoamerica: Interpreting Maya ceramics at Teotihuacan. Lat. Am. Antiq. 16, 427–448 (2005). [Google Scholar]
- 9.Rattray E. C., “Los Barrios Foráneos de Teotihuacan” in Teotihuacan, Nuevos Datos, Nuevas Síntesis, Nuevos Problemas, McClung de Tapia E., Rattray E. C., Eds. (Universidad Nacional Autónoma de México, 1987), pp. 243–273. [Google Scholar]
- 10.Sugiyama N., Fash W. L., Fash B. W., Sugiyama S., “The Maya at Teotihuacan? New insights into Teotihuacan-Maya interactions from plaza of the columns complex” in Teotihuacan: The World Beyond the City, Hirth K. G., Carballo D. M., Arroyo B., Eds. (Dumbarton Oaks Research Library and Collection, 2020), pp. 139–171. [Google Scholar]
- 11.Sugiyama S., Sugiyama N., “Interactions between ancient Teotihuacan and the Maya world” in The Maya World, Hutson S. R., Ardren T., Eds. (Routledge, 2020), pp. 689–708. [Google Scholar]
- 12.Bray T. L., The Archaeology and Politics of Food and Feasting in Early States and Empires (Kluwer Academic/Plenum, 2003). [Google Scholar]
- 13.Dietler M., Hayden B., Eds., Feasts: Archaeological and Ethnographic Perspectives on Food, Politics, and Power (Smithsonian Institution Press, 2001). [Google Scholar]
- 14.Manrique-Ortega M. D., et al. , Material study of green stone artifacts from a Teotihuacan complex. Mater. Manuf. Process. 35, 1431–1445 (2020). [Google Scholar]
- 15.Sugiyama N., Sugiyama S., Alejandro S. G., Inside the sun pyramid at Teotihuacan, Mexico: 2008-2011 excavations and preliminary results. Lat. Am. Antiq. 24, 403–432 (2013). [Google Scholar]
- 16.Sugiyama S., Human Sacrifice, Militarism, and Rulership: Materialization of State Ideology at the Feathered Serpent Pyramid, Teotihuacan (Cambridge University Press, 2005). [Google Scholar]
- 17.Sugiyama S., López Luján L., Dedicatory burial/offering complexes at the Moon Pyramid, Teotihuacan: A preliminary report of 1998-2004 exploration. Anc. Mesoam. 18, 127–146 (2007). [Google Scholar]
- 18.Cagnato C., “Análisis paleobotánicos” in Proyecto Complejo Plaza de Las Columnas, Teotihuacan: Informe Parcial de La Cuarta Temporada 2018, Sugiyama N., Sugiyama S., Ortega Cabrera V., Fash W. L., Eds. (INAH, 2019), pp. 280–294. [Google Scholar]
- 19.Rylands A. B., Mittermeier R. A., Silva J. S. Jr., Neotropical primates: Taxonomy and recently described species and subspecies. Int. Zoo Yearb. 46, 11–24 (2012). [Google Scholar]
- 20.Rice P. M., South K. E., Revisiting monkeys on pots: A contextual consideration of primate imagery on classic lowland Maya pottery. Anc. Mesoam. 26, 275–294 (2015). [Google Scholar]
- 21.Ramamoorthy T. P., Bye R., Lot A., Fa J., Biological Diversity of Mexico: Origins and Distribution (Oxford University Press, 1993). [Google Scholar]
- 22.McClung de Tapia E., Sugiyama N., Conservando la diversidad biocultural de México: El uso de algunas plantas y animales en el pasado y presente. Arqueol. Mex. 19, 20–25 (2012). [Google Scholar]
- 23.Rosenswig R. M., The Beginnings of Mesoamerican Civilization: Inter-Regional Interaction and the Olmec (Cambridge University Press, 2009). [Google Scholar]
- 24.Di Fiore A., Link A., Dew J. L., “Diets of wild spider monkeys” in Spider Monkeys: Behavior, Ecology and Evolution of the Genus Ateles, Campbell C. J., Ed. (Cambridge University Press, 2008), pp. 81–137. [Google Scholar]
- 25.González-Zamora A., et al. , Diet of spider monkeys (Ateles geoffroyi) in Mesoamerica: Current knowledge and future directions. Am. J. Primatol. 71, 8–20 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Dew J. L., “Spider monkeys as seed dispersers” in Spider Monkeys: Behavior, Ecology and Evolution of the Genus Ateles, Campbell C. J., Ed. (Cambridge University Press, 2008), pp. 155–182. [Google Scholar]
- 27.Taube K. A., et al. , The Initial Series Group at Chichen Itza, Yucatan: Archaeological Investigations and Iconographic Interpretations (Precolumbia Mesoweb Press, 2020). [Google Scholar]
- 28.Miles P., Notes on the rearing and development of a hand-reared spider monkey Ateles geoffroyi. Int. Zoo Yearb. 7, 82–85 (1967). [Google Scholar]
- 29.Cormier L. A., Urbani B., “The ethnoprimatology of spider monkey (Ateles spp.): From past to present” in Spider Monkeys: Behavior, Ecology and Evolution of the Genus Ateles, Campbell C. J., Ed. (Cambridge University Press, 2008), pp. 377–403. [Google Scholar]
- 30.CITES, Convention on International Trade in Endangered Species of Wild Fauna and Flora, Appendices. https://cites.org/eng/app/index.php. Accessed 18 April 2022.
- 31.Estrada A., Luecke L., Van Belle S., Barrueta E., Meda M. R., Survey of black howler (Alouatta pigra) and spider (Ateles geoffroyi) monkeys in the Mayan sites of Calakmul and Yaxchilán, Mexico and Tikal, Guatemala. Primates 45, 33–39 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Rylands A. B., Mittermeier R. A., Rodriguez-Luna E., Conservation of neotropical primates: Threatened species and an analysis of primate diversity by country and region. FPR 68, 134–160 (1997). [Google Scholar]
- 33.López Ochoa L., “Manual para manejo de Mono Araña (Ateles geoffroyi) en semicautiverio y cautiverio,” BS thesis, Universidad Veracruzana, Veracruz, Mexico (2013).
- 34.Bruner E., Cucina A., Alouatta, Ateles, and the ancient Mesoamerican cultures. J. Anthropol. Sci. 83, 111–117 (2005). [Google Scholar]
- 35.Aguilera C., Flora y Fauna Mexicana: Mitología y Tradiciones (Editorial Everest Mexicana, S.A., 1985). [Google Scholar]
- 36.Stone A. J., Zender M., Reading Maya Art: A Hieroglyphic Guide to Ancient Maya Painting and Sculpture (Thames & Hudson, 2011). [Google Scholar]
- 37.de Sahagún B., Florentine Codex. Book 11-Earthy Things (The School of American Research and the University of Utah, 1963). [Google Scholar]
- 38.Valadez Azúa R., Rattray E. C., “Restos arqueológicos relacionados con monos mexicanos encontrados en ‘El Barrio de los Comerciantes’ de la antigua Ciudad de Teotihuacan” in Estudios Primatológicos en México, Estrada Medina J. A., Rodríguez Luna E., López Wilchis R., Coates Estrada R., Eds. (Universidad Veracruzana, 1993), pp. 215–229. [Google Scholar]
- 39.Sugiyama N., “Animals and sacred mountains: How ritualized performances materialized state-ideologies at Teotihuacan, Mexico,” Ph.D dissertation, Harvard University, Cambridge, MA (2014).
- 40.Flannery K. V., Excavations at San José Mogote (Museum of Anthropology, University of Michigan, 2005). [Google Scholar]
- 41.Alexander R. T., Hunter J. A., Arata S., Martínez Cervantes R., Scudder K., “Archaeofauna at Isla Cilvituk, Campeche, Mexico: Residential site structure and taphonomy in postclassic Mesoamerica” inThe Archaeology of Mesoamerican Animals, Götz C. M., Emery K. F., Eds. (Lockwood Press, 2013), pp. 281–314. [Google Scholar]
- 42.Pohl M. D., “The privileges of Maya elites: Prehistoric vertebrate fauna from Seibal” in Prehistoric Lowland Maya Environment and Subsistence Economy, Pohl M., Ed. (Peabody Museum of Archaeology and Ethnology, Harvard University, 1985), pp. 133–145. [Google Scholar]
- 43.Moholy-Nagy H., “A preliminary report on the use of vertebrate animals at Tikal, Guatemala” in Anatomía de una Civilización: Aproximaciones Interdisciplinarias a la Cultura Maya, Ciudad Ruz A., Marquinez F., Garcia Campillo J. M., Iglesias Ponce de León Ma. J., Eds. (Sociedad Española de Estudios Mayas, 1998), pp. 115–130. [Google Scholar]
- 44.Moholy-Nagy H., “Vertebrates in tikal burials and caches” in Maya Zooarchaeology: New Directions in Method and Theory, Emery K. F., Ed. (Costen Institute of Archaeology, University of California, 2004), pp. 193–205. [Google Scholar]
- 45.Haviland W. A., “Dower houses and minor centers at Tikal, Guatemala: An investigation into the identification of valid units in settlement hierarchies” inLowland Maya Settlement Patterns, Ashmore W., Ed. (A School of American Research Advanced Seminar Series, School of American Research, ed. 1, 1981), pp. 89–117. [Google Scholar]
- 46.Houston S., et al. , A Teotihuacan complex at the classic Maya City of Tikal, Guatemala. Antiquity 95, E32 (2021). [Google Scholar]
- 47.Sugiyama N., Somerville A. D., Schoeninger M. J., Stable isotopes and zooarchaeology at Teotihuacan, Mexico reveal earliest evidence of wild carnivore management in Mesoamerica. PLoS One 10, e0135635 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sugiyama S., Sugiyama N., “Monumental cityscape and polity at Teotihuacan” in Mesoamerican Archaeology: Theory and Practice, Hendon J. A., Overholtzer L., Joyce R. A., Eds. (Wiley-Blackwell, ed. 2, 2021), pp. 98–128. [Google Scholar]
- 49.Sugiyama N., Pérez G., Rodríguez B., Torres F., Valadez R., “Animals and the state: The role of animals in state-level rituals in Mesoamerica” in Animals and Inequality in the Ancient World, McCarty S. A., Arbuckle B., Eds. (University Press of Colorado, 2014), pp. 11–31. [Google Scholar]
- 50.Ambrose S. H., Effects of diet, climate and physiology on nitrogen isotope abundances in terrestrial foodwebs. J. Archaeol. Sci. 18, 293–317 (1991). [Google Scholar]
- 51.O’Leary M. H., Carbon isotopes in photosynthesis: Fractionation techniques may reveal new aspects of carbon dynamics in plants. Bioscience 38, 328–336 (1988). [Google Scholar]
- 52.Sugiyama N., Martínez-Polanco M. F., France C. A. M., Cooke R. G., Domesticated landscapes of the neotropics: Isotope signatures of human-animal relationships in pre-Columbian panama. J. Anthropol. Archaeol. 59, 101195 (2020). [Google Scholar]
- 53.Hobson K. A., Tracing origins and migration of wildlife using stable isotopes: A review. Oecologia 120, 314–326 (1999). [DOI] [PubMed] [Google Scholar]
- 54.Knudson K. J., Pestle W. J., Torres-Rouff C., Pimentel G., Assessing the life history of an Andean traveller through biogeochemistry: Stable and radiogenic isotope analyses of archaeological human remains from northern Chile. Int. J. Osteoarchaeol. 22, 435–451 (2012). [Google Scholar]
- 55.Sealy J., Armstrong R., Schrire C., Beyond lifetime averages: Tracing life histories through isotopic analysis of different calcified tissues from archaeological human skeletons. Antiquity 69, 290–300 (1995). [Google Scholar]
- 56.Morales Puente P., Cienfuegos Alvarado E., Manzanilla L. R., Otero Trujano F. J., “Estudio de la paleodieta empleando isótopos estables de los elementos carbono, oxígeno y nitrógeno en restos humanos y de fauna encontrados en el Barrio Teotihuacano de Teopancazco” inEstudios Arqueométricos del Centro de Barrio de Teopancazco en Teotihuacan, Manzanilla L. R., Ed. (Instituto de Investigaciones Antropológicas, UNAM, 2012), pp. 347–423. [Google Scholar]
- 57.Schoeninger M. J., Iwaniec U. T., Glander K. E., Stable isotope ratios indicate diet and habitat use in New World monkeys. Am. J. Phys. Anthropol. 103, 69–83 (1997). [DOI] [PubMed] [Google Scholar]
- 58.Tsutaya T., Yoneda M., Reconstruction of breastfeeding and weaning practices using stable isotope and trace element analyses: A review. Am. J. Phys. Anthropol. 156 (suppl. 59), 2–21 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Hodell D. A., Quinn R. L., Brenner M., Kamenov G., Spatial variation of strontium isotopes (87Sr/86Sr) in the Maya region: A tool for tracking ancient human migration. J. Archaeol. Sci. 31, 585–601 (2004). [Google Scholar]
- 60.Price T. D., et al. , Strontium isotopes and the study of human mobility in ancient Mesoamerica. Lat. Am. Antiq. 19, 167–180 (2008). [Google Scholar]
- 61.Buckley G. M., et al. , New perspectives on migration into the tlajinga district of Teotihuacan: A dual-isotope approach. Lat. Am. Antiq. 32, 536–556 (2021). [Google Scholar]
- 62.Pacheco-Forés S. I., Gordon G. W., Knudson K. J., Expanding radiogenic strontium isotope baseline data for central Mexican paleomobility studies. PLoS One 15, e0229687 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Price D., Manzanilla L. R., Middleton W. D., Immigration and the ancient city of Teotihuacan in Mexico: A study using strontium isotope ratios in human bone and teeth. J. Archaeol. Sci. 27, 903–913 (2000). [Google Scholar]
- 64.Price T. D., Spence M. W., Longstaffe F. J., The temple of Quetzalcoatl, Teotihuacan: New data on the origins of the sacrificial victims. Anc. Mesoam. 32, 215–230 (2020). [Google Scholar]
- 65.Solís Pichardo G., et al. , “Migrants in Teopancazco: Evidence from strontium isotopic studies” in Multiethnicity and Migration at Teopancazco: Investigations of a Teotihuacan Neighborhood Center, Manzanilla L. R., Ed. (University Press of Florida, 2017), pp. 143–163. [Google Scholar]
- 66.White C. D., Storey R., Longstaffe F. J., Spence M. W., Immigration, assimilation, and status in the ancient city of Teotihuacan: Stable isotopic evidence from Tlajinga 33. Lat. Am. Antiq. 15, 176–198 (2004). [Google Scholar]
- 67.White C. D., Price D., Longstaffe F. J., Residential histories of the human sacrifices at the moon pyramid, Teotihuacan: Evidence from oxygen and strontium isotopes. Anc. Mesoam. 18, 159–172 (2007). [Google Scholar]
- 68.Price T. D., et al. , Kings and commoners at Copan: Isotopic evidence for origins and movement in the classic Maya period. J. Anthropol. Archaeol. 29, 15–32 (2010). [Google Scholar]
- 69.Price T. D., Nakamura S., Suzuki S., Burton J. H., Tiesler V., New data on maya mobility and enclaves at classic Copan, Honduras. J. Anthropol. Archaeol. 36, 32–47 (2014). [Google Scholar]
- 70.International Committee for Phytolith Taxonomy (ICPT), International Code for Phytolith Nomenclature (ICPN) 2.0. Ann. Bot. 124, 189–199 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ambrose S. H., Norr L., “Experimental evidence for the relationship of the carbon isotope ratios of whole diet and dietary protein to those of bone collagen and carbonate” in Prehistoric Human Bone: Archaeology at the Molecular Level, Lambert J. B., Grupe G., Eds. (Springer-Verlag, 1993), pp. 1–37. [Google Scholar]
- 72.Froehle A. W., Kellner C. M., Schoeninger M. J., FOCUS: Effect of diet and protein source on carbon stable isotope ratios in collagen: Follow up to Warinner and Tuross (2009). J. Archaeol. Sci. 37, 2662–2670 (2010). [Google Scholar]
- 73.Kellner C. M., Schoeninger M. J., A simple carbon isotope model for reconstructing prehistoric human diet. Am. J. Phys. Anthropol. 133, 1112–1127 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Tieszen L. L., Fagre T., “Effect of diet quality and composition on the isotopic composition of respiratory CO2, bone collagen, bioapatite, and soft tissue” in Prehistoric Human Bone: Archaeology at the Molecular Level, Lambert J. B., Grupe G., Eds. (Springer-Verlag, 1993), pp. 121–155. [Google Scholar]
- 75.Warinner C., Robles Garcia N., Tuross N., Maize, beans and the floral isotopic diversity of highland Oaxaca, Mexico. J. Archaeol. Sci. 40, 868–873 (2013). [Google Scholar]
- 76.Thornton E., Emery K. F., Speller C., Ancient Maya turkey husbandry: Testing theories through stable isotope analysis. J. Archaeol. Sci. Rep. 10, 584–595 (2016). [Google Scholar]
- 77.Schwartz C. W., Somerville A. D., Nelson B. A., Knudson K. J., Investigating pre-Hispanic scarlet macaw origins through radiogenic strontium isotope analysis at Paquimé in Chihuahua, Mexico. J. Anthropol. Archaeol. 61, 101256 (2021). [Google Scholar]
- 78.Sharpe A. E., et al. , Earliest isotopic evidence in the Maya region for animal management and long-distance trade at the site of Ceibal, Guatemala. Proc. Natl. Acad. Sci. U.S.A. 115, 3605–3610 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Somerville A., Sugiyama N., Schoeninger M., An Isotopic Investigation of Lagomorph Management and Breeding at Teotihuacan, Mexico (2014).
- 80.A. D. Somerville, N. Sugiyama, L. R. Manzanilla, M. J. Schoeninger, Leporid Management and Specialized Food Production at Teotihuacan: Stable Isotope Data from Cottontail and Jackrabbit Bone Collagen. Archaeol Anthropol Sci. 9, 83–97 (2017). [Google Scholar]
- 81.White C. D., Pohl M. E. D., Schwarcz H. P., Longstaffe F. J., Isotopic evidence for maya patterns of deer and dog use at Preclassic Colha. J. Archaeol. Sci. 28, 89–107 (2001). [Google Scholar]
- 82.McClung de Tapia E., Martínez-Yrizar D., The potential of paleoethnobotanical evidence for the study of Teotihuacan foodways. Archaeol. Anthropol. Sci. 9, 39–50 (2016). [Google Scholar]
- 83.Falótico T., Siqueira J. O., Ottoni E. B., Digging up food: Excavation stone tool use by wild capuchin monkeys. Sci. Rep. 7, 6278 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chaves O. M., Stoner K. E., Ángeles-Campos S., Arroyo-Rodríguez V., Wood consumption by Geoffroyi’s spider monkeys and its role in mineral supplementation. PLoS One 6, e25070 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Radini A., Nikita E., Buckley S., Copeland L., Hardy K., Beyond food: The multiple pathways for inclusion of materials into ancient dental calculus. Am. J. Phys. Anthropol. 162 (suppl. 63), 71–83 (2017). [DOI] [PubMed] [Google Scholar]
- 86.Hardy K., et al. , Diet and environment 1.2 million years ago revealed through analysis of dental calculus from Europe’s oldest hominin at Sima del Elefante, Spain. Naturwissenschaften 104, 2 (2017). [DOI] [PubMed] [Google Scholar]
- 87.Power R. C., Salazar-García D. C., Straus L. G., González Morales M. R., Henry A. G., Microremains from El Mirón Cave human dental calculus suggest a mixed plant–animal subsistence economy during the Magdalenian in Northern Iberia. J. Archaeol. Sci. 60, 39–46 (2015). [Google Scholar]
- 88.Rodríguez-Luna E., Shedden A., Solórzano-García B., “A region-wide review of mesoamerican primates: Prioritizing for conservation” in Primates in Fragments: Complexity and Resilience, Marsh L. K., Chapman C. A., Eds. (Developments in Primatology: Progress and Prospects, Springer, 2013), pp. 47–55. [Google Scholar]
- 89.Morales-Jimenez A. L., Cortés-Ortiz L., Di Fiore A., Phylogenetic relationships of Mesoamerican spider monkeys (Ateles geoffroyi): Molecular evidence suggests the need for a revised taxonomy. Mol. Phylogenet. Evol. 82, 484–494 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Grube N., Gómez Chávez S., “Preliminary iconographic study of the shell trumpets from the Tlalocan project” in Teotihuacan: City of Water, City of Fire, Robb M., Ed. (Fine Arts Museum of San Francisco in association with University of California Press, 2017), p. 248. [Google Scholar]
- 91.Crowley B. E., Stable isotope techniques and applications for primatologists. Int. J. Primatol. 33, 673–701 (2012). [Google Scholar]
- 92.Rattray E. C., Teotihacan: Cerámica, Cronología y Tendencias Culturales (Instituto Nacional de Antropología e Historia, 2001). [Google Scholar]
- 93.Reitz E. J., Wing E. S., Zooarchaeology (Cambridge University Press, 2004). [Google Scholar]
- 94.France C. A. M., Sugiyama N., Aguayo E., Establishing a preservation index for bone, dentin, and enamel bioapatite mineral using ATR-FTIR. J. Archaeol. Sci. Rep. 33, 102551 (2020). [Google Scholar]
- 95.Wellman H. P., et al. , Archaeological mitogenomes illuminate the historical ecology of sea otters (Enhydra lutris) and the viability of reintroduction. Proc. Biol. Sci. 287, 20202343 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schubert M., Lindgreen S., Orlando L., AdapterRemoval v2: Rapid adapter trimming, identification, and read merging. BMC Res. Notes 9, 88 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li H., Durbin R., Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kumar S., Stecher G., Li M., Knyaz C., Tamura K., MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kozlov A. M., Darriba D., Flouri T., Morel B., Stamatakis A., RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35, 4453–4455 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leigh J. W., Bryant D., Popart: Full-feature software for haplotype network construction. Methods Ecol. Evol. 6, 1110–1116 (2015). [Google Scholar]
- 101.O’Connell T. C., Hedges R. E., Healey M. A., Simpson A. H., Isotopic comparison of hair, nail and bone: Modern analyses. J. Archaeol. Sci. 28, 1247–1255 (2001). [Google Scholar]
- 102.N. Sugiyama et al., Earliest Evidence of Primate Captivity and Translocation Supports Gift Diplomacy Between Teotihuacan and the Maya. NCBI BioProject. https://www.ncbi.nlm.nih.gov/bioproject/PRJNA882120/. Deposited 19 September 2022. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequencing data have been deposited in NCBI Sequence Read Archive (PRJNA882120) (102). All other raw data are reported in the main text or SI Appendix.