Significance
The pathogenic mechanisms that trigger the most severe complications of COVID-19 are still largely unknown. Widespread formation of microscopic thrombi has been detected in the lungs and other organs, suggesting that coagulation abnormalities are involved in the pathogenic process. This study shows that virtually all patients with severe COVID-19 develop anomalous antibodies that target an endogenous protein, platelet factor 4 (PF4), and that are the hallmark of two life-threatening disorders characterized by thrombosis and thrombocytopenia: heparin-induced thrombocytopenia and vaccine-induced thrombosis with thrombocytopenia. Anti-PF4 antibodies are the hallmark of two life-threatening disorders: heparin-induced thrombocytopenia and vaccine-induced thrombosis with thrombocytopenia. Higher antibody levels were found in patients with the most severe disease and the most conspicuous platelet reductions. These findings suggest that anti-PF4 antibodies may play a role in the severe multiorgan disease manifestations of COVID-19.
Keywords: COVID-19, PF4, anti-PF4 antibodies, microthrombosis, thrombocytopenia
Abstract
Severe COVID-19 is characterized by a prothrombotic state associated with thrombocytopenia, with microvascular thrombosis being almost invariably present in the lung and other organs at postmortem examination. We evaluated the presence of antibodies to platelet factor 4 (PF4)–polyanion complexes using a clinically validated immunoassay in 100 hospitalized patients with COVID-19 with moderate or severe disease (World Health Organization score, 4 to 10), 25 patients with acute COVID-19 visiting the emergency department, and 65 convalescent individuals. Anti-PF4 antibodies were detected in 95 of 100 hospitalized patients with COVID-19 (95.0%) irrespective of prior heparin treatment, with a mean optical density value of 0.871 ± 0.405 SD (range, 0.177 to 2.706). In contrast, patients hospitalized for severe acute respiratory disease unrelated to COVID-19 had markedly lower levels of the antibodies. In a high proportion of patients with COVID-19, levels of all three immunoglobulin (Ig) isotypes tested (IgG, IgM, and IgA) were simultaneously elevated. Antibody levels were higher in male than in female patients and higher in African Americans and Hispanics than in White patients. Anti-PF4 antibody levels were correlated with the maximum disease severity score and with significant reductions in circulating platelet counts during hospitalization. In individuals convalescent from COVID-19, the antibody levels returned to near-normal values. Sera from patients with COVID-19 induced higher levels of platelet activation than did sera from healthy blood donors, but the results were not correlated with the levels of anti-PF4 antibodies. These results demonstrate that the vast majority of patients with severe COVID-19 develop anti-PF4 antibodies, which may play a role in the clinical complications of COVID-19.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of coronavirus disease 2019 (COVID-19), the most devastating pandemic to have plagued the world in more than a century (1). Although effective vaccines have been developed and deployed at an unprecedented pace on a global scale (2–5), morbidity and mortality remain at alarming levels, particularly in areas with limited access or resistance to vaccination. Furthermore, the virus, because of its RNA nature, continues to evolve and generate novel variants that escape from neutralizing antibodies and other immunologic mechanisms of protection elicited by current vaccines (6–8). Thus, a further delineation of the mechanisms of COVID-19 disease remains a high priority, as it may foster the development of increasingly effective therapeutic strategies.
The clinical spectrum of COVID-19 is broad, ranging from an asymptomatic state to severe disease leading to multisystemic involvement and death (9–11). The lung is the most frequently targeted organ, with the development of acute respiratory distress syndrome for which patients may require mechanical ventilation. Among the distinctive features of COVID-19 are vascular changes affecting the lung as well as other organs. Although clinically apparent thrombosis of major vessels can occur in severely ill patients with COVID-19 (12, 13), disseminated microthrombosis affecting multiple organs is an almost invariable finding at postmortem examination, particularly in the lungs, where diffuse platelet microthrombi are associated with alveolar damage (14–16). In addition, mortality in COVID-19 is associated with progressive thrombocytopenia, apparently as a consequence of disseminated platelet activation and consumption rather than of immune-mediated platelet destruction or splenic sequestration (9, 17). Thus, even in the absence of clinically apparent thrombosis, systemic microvascular thrombosis with thrombocytopenia may represent a common pathological mechanism underlying multiple organ failures in fatal COVID-19.
The simultaneous presence of thrombosis and thrombocytopenia is the hallmark of heparin-induced thrombocytopenia (HIT), a dramatic clinical syndrome associated with heparin treatment especially in patients recovering from cardiac or orthopedic surgery (18). The pathogenic mechanism of the HIT syndrome involves the elicitation of autoantibodies that target partially cryptic epitopes in the α-chemokine platelet factor 4 (PF4 or CXCL4), which are fully revealed upon binding to heparin or other polyanionic molecules. Severe thrombosis associated with thrombocytopenia and anti-PF4–polyanion (anti-PF4) antibodies has also recently been reported as a rare complication of adenovirus-vectored anti–SARS-CoV2 vaccines, such as AZD1222 and Ad26.COV2.S, and defined as vaccine-induced thrombosis with thrombocytopenia (VITT) (19–22).
Given the simultaneous occurrence of thrombosis, especially systemic microthrombosis, and thrombocytopenia in patients with severe COVID-19, we investigated the presence of anti-PF4 antibodies in the serum of patients with COVID-19.
Results
Patient Characteristics, Clinical Features, and Laboratory Findings.
A cohort of 100 patients admitted to the Johns Hopkins Hospital for moderate or severe COVID-19 between April 2020 and April 2021 was studied. None of the patients had received COVID-19 vaccination. The cohort was heterogenous with regard to age, sex, ethnic origin, race, and comorbidities (Table 1). Their maximum World Health Organization (WHO) disease severity score during hospitalization varied between 4 and 10 (23). Nearly half the patients (n = 47 of 100; 47.0%) required organ support and were admitted to the intensive care unit (ICU) during hospitalization; 19 of them died of COVID-19 complications (WHO score, 10). In addition, we studied serum or plasma from the following six groups: 1) 25 patients who visited the emergency department (ED) of Johns Hopkins Hospital for symptomatic acute COVID-19. All were discharged during the same day, and their subsequent disease course is unknown; 2) Sixty convalescent patients with COVID-19 who had an established diagnosis of COVID-19 and had been free of symptoms for at least 3 mo at the time of sampling; 30 of them had previously been hospitalized for COVID-19 during the acute stage of the disease; 3) Patients hospitalized with severe respiratory disease (n = 28), 24 of whom had acute influenza; the majority (n = 25) required oxygen ventilation and 9 were admitted either to the ICU (n = 8) or to the intermediate care unit (n = 1); 4) Patients with HIT (n = 29), all of whom had received unfractionated heparin (UFH) for thrombus prevention for more than 6 d following cardiac or orthopedic surgery; 5) Eight patients with VITT, who had received the Ad26.COV2.S vaccine within 21 d of the development of symptoms; and 6) 50 healthy blood donors.
Table 1.
Demographic and clinical characteristics of hospitalized patients with COVID-19
| Parameter | Value |
|---|---|
| Age, mean (range), y | 59.1 (25 to 90) |
| Sex, No. (%) | |
| Male | 54 (54) |
| Female | 46 (46) |
| Race or ethnic group, No. (%) | |
| White | 33 (33) |
| Black | 40 (40) |
| Hispanic | 22 (22) |
| Asian | 2 (2) |
| Missing data | 3 (3) |
| BMI, mean (range) | 32.0 (19 to 65) |
| Maximum disease severity score, No. (%) | |
| <6 | 44 (44) |
| ≥6 | 56 (56) |
| Thrombocytopenia (<150,000/mm3), No. (%) | 48 (48) |
| Comorbidities, No. (%) | |
| Type 1 diabetes | 4 (4) |
| Type 2 diabetes | 48 (48) |
| Asthma | 14 (14) |
| Hypertension, uncomplicated | 56 (56) |
| Hypertension, complicated | 29 (29) |
| Active thrombotic event, No. (%) | |
| Pulmonary embolism | 13 (13) |
| Cardiac ischemia | 13 (13) |
| Other | 7 (7) |
| Admission to ICU, No. (%) | 47 (47) |
High Prevalence of Anti-PF4 Antibodies in Patients with COVID-19.
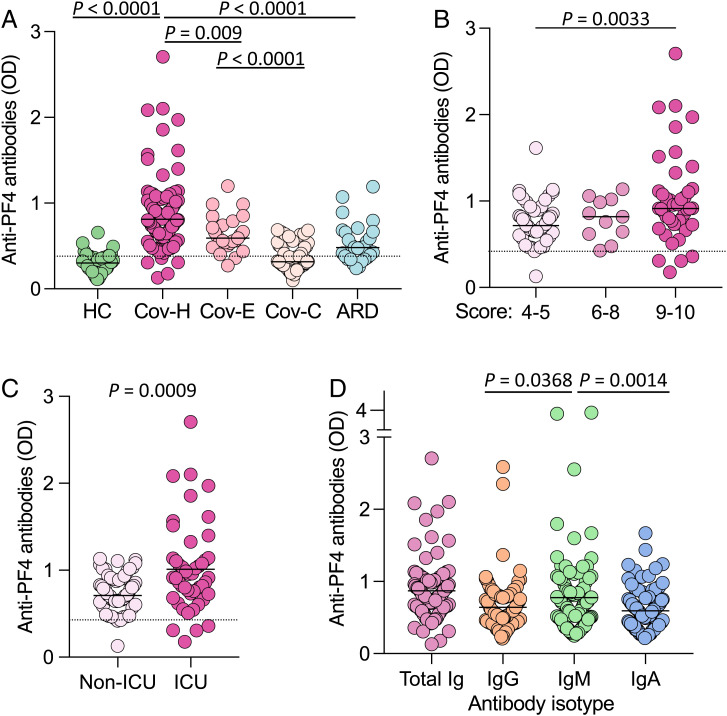
Anti-PF4 antibodies were measured using a clinically validated assay in a single sample from each patient (24), which, in all cases except five, was obtained within 7 d of hospitalization. Sera yielding values over the threshold of assay positivity (0.4 optical density [OD] units) were considered positive. Anti-PF4 antibodies were detected in serum or plasma of the vast majority of hospitalized patients with COVID-19 (n = 95 of 100; 95.0%) with a mean OD value of 0.871 ± 0.405 SD (range, 0.177 to 2.706); in 65 patients (65.0%), the OD value was greater than 0.75, which is a potential threshold for clinical significance (25), and in 28 (28.0%), it was greater than 1.0 (Fig. 1A). The results were specific, as shown by a reduction of the signal by more than 50% upon addition of high-dose heparin (SI Appendix, Fig. S1). The OD level of anti-PF4 antibodies was significantly higher in hospitalized patients with COVID-19 than in patients with acute COVID-19 visiting the ED (mean, 0.614 ± 0.212 SD; P = 0.009) and in convalescent patients with COVID-19 (mean, 0.354 ± 0.148 SD; P < 0.0001). A high rate of anti-PF4 antibody positivity was also detected in patients hospitalized for severe acute respiratory disease (ARD; n = 18 of 28; 64.3%); however, the level was markedly lower than in hospitalized patients with COVID-19 (mean OD value, 0.533 ± 0.235 SD; range, 0.238 to 1.192; P < 0.0001), with only 4 of 28 (14.3%) over the 0.75 OD value and only 2 (7.2%) over 1.0. As expected, both the prevalence and the levels of anti-PF4 antibodies in healthy blood donors were very low (n = 5 of 50 [10.0%]; mean OD value, 0.294 ± 0.110 SD) (Fig. 1A).
Fig. 1.
Anti-PF4 antibodies in patients with COVID-19 and controls. (A) Anti-PF4 antibodies in healthy donor control patients (HC), hospitalized patients with COVID-19 (Cov-H), patients with COVID-19 visiting the ED (Cov-E), convalescent patients with COVID-19 (Cov-C), and patients hospitalized for severe ARD unrelated to COVID-19. The dotted horizontal line shows the conventional threshold for assay positivity (0.4 OD units). (B) Anti–PF4 antibodies in hospitalized patients with COVID-19 according to their clinical score (WHO score, 4 to 10). (C) Anti-PF4 antibodies in hospitalized patients with COVID-19 admitted or not to the ICU. (D) Antibody isotype of anti-PF4 antibodies in hospitalized patients with COVID-19. The IgM- and IgA-specific assays were not standardized for clinical use. Statistical differences were evaluated by unpaired, two-tailed t tests.
Among the 100 hospitalized patients with COVID-19, the level of anti-PF4 positivity was greater in patients with the highest clinical score (i.e., 9 to 10; mean OD value, 1.027 ± 0.519 SD), followed by those with intermediate score (6 to 8; mean, 0.800 ± 0.239 SD) and, last, by patients with the lowest score (4 to 5; mean OD value, 0.736 ± 0.220 SD) (Fig. 1B). In agreement, higher values were detected in patients who were admitted to the ICU than in those who were not admitted (mean OD value, 1.012 ± 0.511 vs. 0.747 ± 0.219 SD; P = 0.0009) (Fig. 1C).
Since immunoglobulin (Ig) G is the isotype of anti-PF4 antibodies more frequently associated with the HIT syndrome (18), we investigated the prevalence of IgG, IgM, and IgA among anti-PF4 antibodies in hospitalized patients with COVID-19. Although anti-PF4 IgG over the threshold of 0.4 OD units was detected in 85 of 100 patients (85%), all three isotypes tested, IgG, IgM, and IgA, were frequently represented (Fig. 1D). IgM was detected at higher levels than both IgG and IgA (mean OD value, 0.775 vs. 0.643 and 0.592, respectively; P = 0.0368 and 0.0014, respectively). However, it is noteworthy that 54 patients (54%) had elevated levels of all three Ig isotypes simultaneously, and 30 (30.0%) had two with a predominance of the IgG/IgM combination. Indeed, the vast majority of patients with anti-PF4 antibodies of any isotype over 1.0 OD units (n = 25 of 28 of total Ig >1.0 OD; n = 9 of 9 of IgG; n = 17 of 22 of IgM; and n = 8 of 9 of IgA) had simultaneous elevations of all three isotypes. These data indicate that in patients with COVID-19, the induction of high-level anti-PF4 antibodies was predominantly multi-isotype.
Anti-PF4 Antibodies and Prior Heparin Treatment.
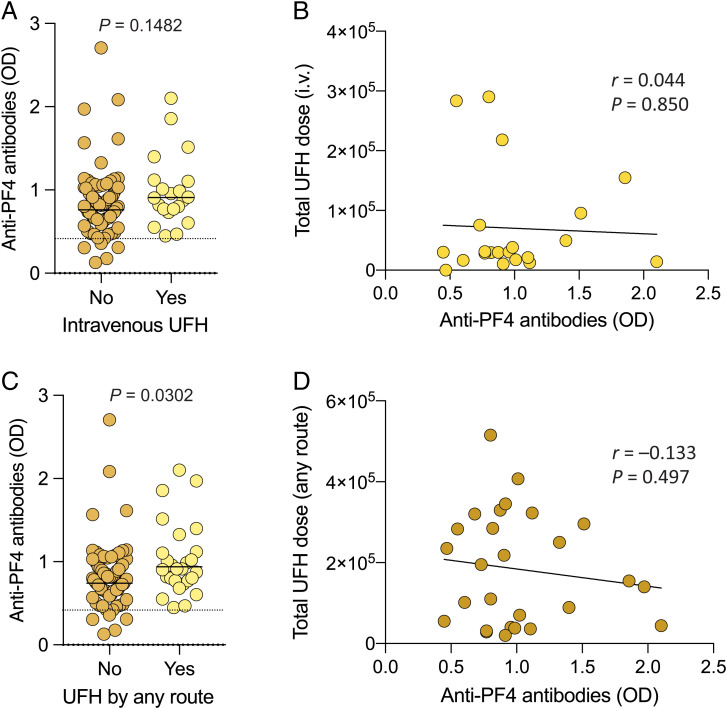
Patients with COVID-19 are often exposed to heparin for treatment or prevention of thrombotic complications. Thus, to investigate whether the presence of anti-PF4 antibodies in hospitalized patients with COVID-19 could be a sign of HIT syndrome development, which is etiologically linked with exposure to heparin (18), we evaluated in detail the time, dose, and type of heparin treatment in these patients. In patients (n = 21) who had received UFH intravenously for at least 6 d prior to the time of sampling [i.e., the heparin treatment associated with the highest risk of HIT development (25)], the antibody levels were not significantly higher than in the other 79 patients (mean OD value, 0.985 ± 0.425 vs. 0.841 ± 0.397 SD) (Fig. 2A). Importantly, the antibody levels did not correlate with the total dose of intravenous UFH received prior to the time of sampling (Fig. 2B). When all the patients treated with UFH by either the intravenous or subcutaneous route (n = 27) were compared with all those untreated, the difference was significant (P = 0.0302), although it should be emphasized that UFH-treated patients included the most severe cases (n = 23 of 27 with a disease score of 9 or 10), and that anti-PF4 antibodies were also detected in 68 of 73 patients (93.1%) who were never treated with UFH, including 38 with OD values greater than 0.75 and 18 greater than 1.0 (Fig. 2C). Indeed, when UFH-treated patients with a clinical score of 9 or 10 were compared with UFH-untreated patients with a clinical score of 9 or 10, the difference was not significant (P = 0.6629). In addition, the antibody levels did not correlate with the total UFH dose received (Fig. 2D). Treatment with any form of heparin, including both UFH and low-molecular-weight heparin that carries a lower risk of HIT development (25), was associated with higher antibody levels (P = 0.0007) and, as expected since heparin prophylaxis is a standard measure in severe COVID-19 cases, was correlated with the disease severity score (r = 0.471; P < 0.0001); nevertheless, 47 of 100 patients (47.0%) developed anti-PF4 antibodies without having received heparin in any form for at least 6 d at the time of sampling (SI Appendix, Fig. S2). Altogether, these results allowed us to rule out that exposure to heparin was a necessary requirement for the development of anti-PF4 antibodies.
Fig. 2.
Anti-PF4 antibodies and prior heparin treatment in patients with COVID-19. (A) Levels of anti-PF4 antibodies in hospitalized patients with COVID-19 divided according to prior treatment with intravenous (i.v.) UFH. (B) Linear correlation between anti-PF4 antibody levels and total UFH dose (units) received by the intravenous route. (C) Levels of anti-PF4 antibodies in hospitalized patients with COVID-19 divided according to prior treatment with UFH by any route. Statistical differences in A and C were evaluated by unpaired, two-tailed t tests. (D) Linear correlation between anti–PF4 antibody levels and total UFH dose (units) received by any route. Statistical associations in B and D were evaluated using linear regression. Pearson’s correlations and P values are shown along with the fitted regression lines.
Clinical Correlates of Anti-PF4 Antibodies.
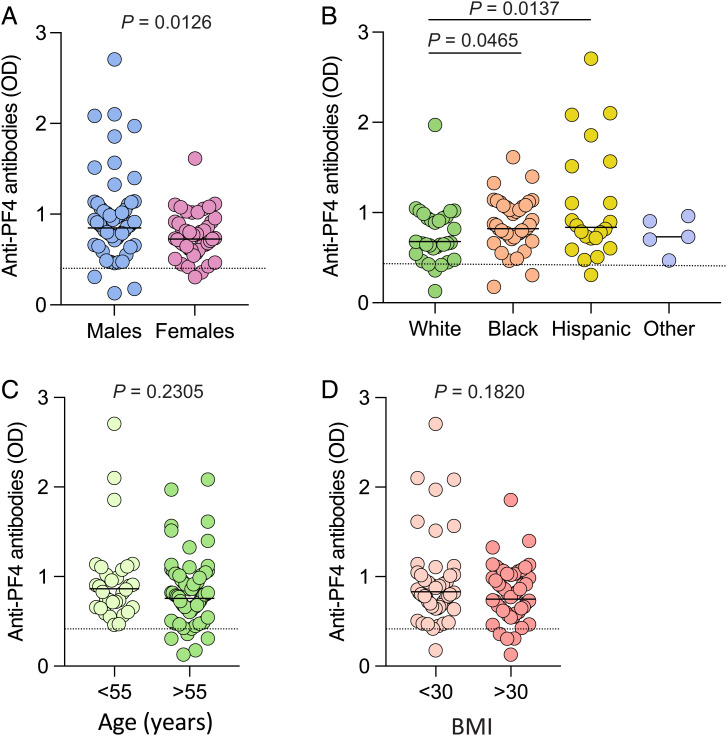
Next, we studied the correlation between anti-PF4 antibodies in hospitalized patients with COVID-19 and various demographic, clinical, and laboratory parameters. Higher antibody levels were detected in male (mean OD value, 0.964 ± 0.487 SD) than in female (mean OD value, 0.763 ± 0.244 SD) patients (Fig. 3A) and in African American patients (mean OD value, 0.876 ± 0.283 SD) and Hispanic patients (mean OD value, 1.079 ± 0.626 SD) compared with White patients (mean OD value, 0.744 ± 0.322 SD) (Fig. 3B), while no association was found with older age (Fig. 3C) and obesity (Fig. 3D). By linear regression analysis, a significant correlation was found between the levels of anti-PF4 antibodies and sex, race, ethnicity, circulating white blood cell counts, platelet reductions, or maximum disease severity score, while no correlation was seen with age; body mass index (BMI); plasma levels of C-reactive protein, D-dimer, ferritin, or lactic dehydrogenase; intravenous heparin treatment; or preexisting comorbidities (Table 2 and SI Appendix, Fig. S3). Also, in this cohort of hospitalized patients, the disease severity score was not correlated with age or sex. A multiple regression analysis was performed to assess the association of anti-PF4 antibodies with the maximum disease severity score after adjusting for age, race, intravenous heparin treatment, and BMI. The levels of anti-PF4 antibodies were independently associated with the severity of the disease (Table 2).
Fig. 3.
Levels of anti-PF4 antibodies according to various demographic and clinical parameters in hospitalized patients with COVID-19. (A–D) Shown are the levels of anti-PF4 antibodies in hospitalized patients with COVID-19 analyzed according to sex (A), ethnic origin (B), age (C), and BMI (D). The statistical differences reported on the top for the indicated groups were evaluated by unpaired, two-tailed t tests; the other comparisons were not statistically significant.
Table 2.
Correlation of anti-PF4 antibodies with demographic, clinical, and laboratory parameters in hospitalized patients with COVID-19
| Regression analyses and parameters | |||
|---|---|---|---|
| Linear regression analysis Age |
Pearson’s correlation −0.1384 |
95% CI −0.33 to 0.06 |
P value 0.1698 |
| Sex | — | — | 0.0126 |
| Race | — | — | 0.0312 |
| Ethnicity | — | — | 0.0059 |
| BMI | −0.0655 | −0.26 to 0.13 | 0.5172 |
| C-reactive protein | 0.1406 | −0.07 to 0.34 | 0.1992 |
| D-dimer | 0.1709 | −0.05 to 0.38 | 0.1296 |
| White blood cells | 0.2491 | 0.04 to 0.44 | 0.0223 |
| Ferritin | −0.0687 | −0.49 to 0.37 | 0.7672 |
| Lactic dehydrogenase | 0.3920 | −0.13 to 0.74 | 0.1332 |
| Platelet reduction | 0.3678 | 0.17 to 0.54 | 0.0004 |
| Intravenous heparin treatment | — | — | 0.1482 |
| Maximum disease severity score | 0.2910 | 0.10 to 0.46 | 0.0033 |
| Multiple regression analysis | Estimate | SE | P value |
| Intercept | 0.7938 | 0.3742 | 0.0366 |
| Age | −0.0034 | 0.0030 | 0.2564 |
| Race | |||
| White | −0.0610 | 0.2833 | 0.8301 |
| Black | 0.0508 | 0.2835 | 0.8581 |
| Other | 0.1798 | 0.2863 | 0.5317 |
| Intravenous heparin treatment | 0.0506 | 0.0938 | 0.5912 |
| BMI | −0.0037 | 0.0049 | 0.4514 |
| Maximum disease severity score | 0.0482 | 0.0197 | 0.0163 |
Anti-PF4 Antibodies and Thrombocytopenia.
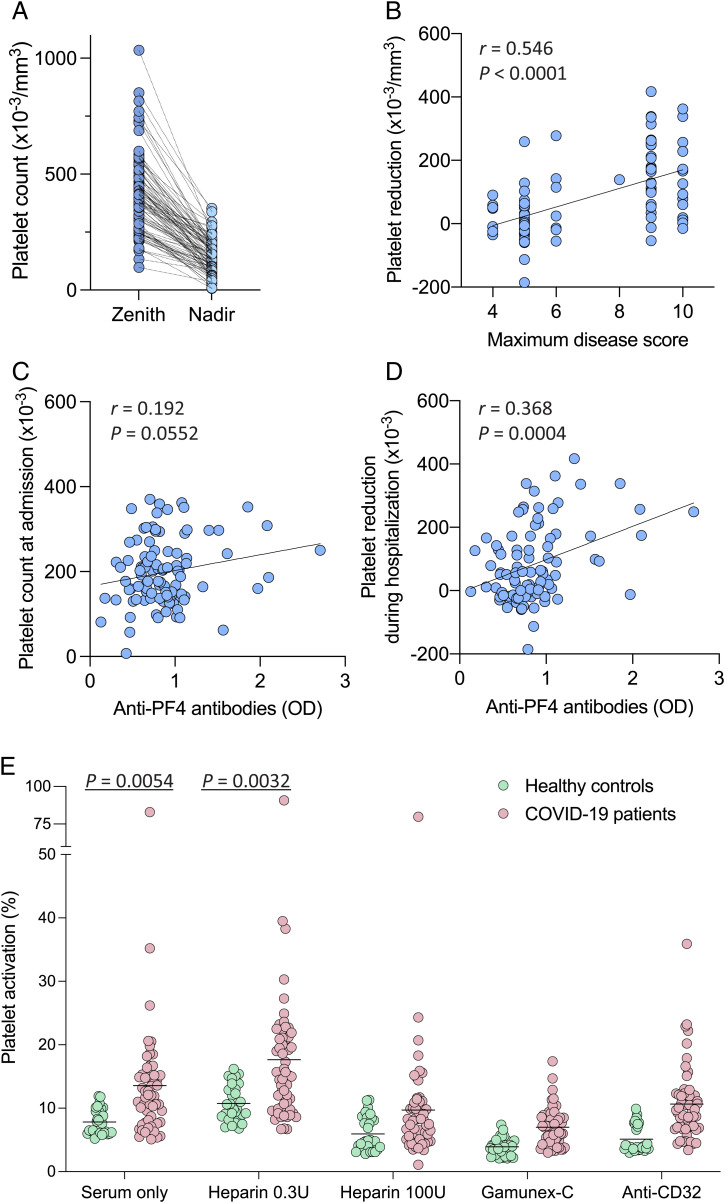
In line with previous reports (9, 17), a high proportion of hospitalized patients with COVID-19 experienced significant reductions in circulating platelet counts during hospitalization (mean loss of platelets from peak value, 260,390 ± 143,073 SD/mm3), with 48 of 100 (48.0%) developing thrombocytopenia as defined by a platelet count below 150,000/mm3 (Table 1 and Fig. 4A). As expected, platelet reductions were correlated with the maximum disease severity score (r = 0.546; P < 0.0001) (Fig. 4B). Although anti-PF4 antibody levels were not correlated with the platelet counts measured at the time of hospital admission (Fig. 4C), they were significantly correlated with the reductions in platelet count during hospitalization (r = 0.368; P = 0.0004) (Fig. 4D), which is consistent with a potential role of these antibodies in the development of thrombocytopenia. Anti-PF4 antibodies were not correlated with clinically apparent thrombotic events, which were diagnosed in 30 of 100 patients (30%) (SI Appendix, Fig. S3). Thus, the lack of correlation with prior heparin treatment and clinical thrombotic events [two of the four parameters of the 4Ts diagnostic scoring system for HIT (26)] allowed us to exclude that, in the vast majority of patients with COVID-19, the presence of anti-PF4 antibodies was due to the development of a classic HIT syndrome.
Fig. 4.
Anti-PF4 antibodies, circulating platelet counts, and ability to induce platelet activation in hospitalized patients with COVID-19 and controls. (A) Maximum (zenith) and minimum (nadir) circulating platelet counts in individual patients with COVID-19 during their hospitalization period (n = 100). The statistical difference was calculated by paired, two-tailed t test. (B) Linear correlation between platelet reductions and maximum disease severity score in patients with COVID-19 during hospitalization. (C) Linear correlation between anti–PF4 antibody levels and platelet counts in patients with COVID-19 at the time of hospital admission. (D) Linear correlation between anti–PF4 antibody levels in patients with COVID-19 and platelet reductions during hospitalization. Platelet reductions were calculated as the difference between the maximum platelet count recorded before the sampling date and the minimum value within a period up to 30 d after the sampling date. Statistical associations in B–D were evaluated using linear regression. Pearson’s correlations and P values are shown along with the fitted regression lines. (E) Platelet activation induced by sera from hospitalized patients with COVID-19 (n = 57) vs. healthy blood donors (n = 30). Platelet activation was measured by surface expression of P-selectin (CD62P). The tests were performed in the presence or absence of low-dose heparin (0.3 U/mL) as a stimulant, or high-dose heparin (100 U/mL), concentrated human Igs (Gamunex-C), or an anti-CD32 blocking antibody as inhibitors. Statistical differences for the indicated comparisons were calculated by unpaired, two-tailed t tests.
Induction of Platelet Activation by Serum of Patients with COVID-19.
To evaluate the potential functional effects of anti-PF4 antibodies, we studied the ability of sera from patients with COVID-19 to induce activation of fresh platelets obtained from healthy donors, using a flow cytometry assay (27). A representative plot for the platelet activation assay is shown in SI Appendix, Fig. S4. To validate the assay, we tested sera from patients with a diagnosis of HIT or VITT syndrome. The majority of HIT serum samples (n = 29) induced activation in a high proportion of donor platelets (mean, 38.2% ± 20.0% vs. 7.8% ± 2.0% in 30 healthy blood donors; P < 0.0001), with 9 of 15 sera samples (60.0%) activating more than 30% of the platelets. As expected, addition of low-dose heparin (0.3 U/mL) enhanced platelet activation (mean, 49.4% ± 25.9% vs. 10.7% ± 2.8% in healthy blood donors; P < 0.0001), whereas high-dose heparin (100 U/mL) or an antibody to the FcγIIa receptor (CD32) had inhibitory effects (SI Appendix, Fig. S5). Sera from patients with VITT (n = 8) induced significantly lower responses compared with HIT sera (mean, 19.1% ± 11.1%) and exhibited an unusual behavior in that low-dose heparin did not enhance but, to the contrary, slightly reduced platelet activation (mean, 15.7% ± 6.9%), in line with the results of a previous study (28) (SI Appendix, Fig. S5).
Well-preserved serum suitable for platelet activation testing was available from 57 of the 100 hospitalized patients with COVID-19. The results showed higher levels of platelet activation in patients with COVID-19 compared with healthy blood donors both in the absence of heparin (mean, 13.6% ± 10.9% vs. 7.8% ± 2.0%; P = 0.0054) and in the presence of low-dose heparin (mean, 17.6% ± 12.2% vs. 10.7% ± 2.8%; P = 0.0033), which were inhibited by concentrated human Igs (Gamunex-C; P < 0.0001) as well as, albeit less effectively, by high-dose heparin (P < 0.0001) and anti–CD32 antibody (P = 0.0129) (Fig. 4E). The levels of platelet activation were significantly lower with COVID-19 sera than with HIT sera (P < 0.0001), with the exception of a single patient with COVID-19 who showed a very high activity (82.9% and 90.7% in the absence and presence of low-dose heparin, respectively), associated with high anti–PF4 antibody levels of all three isotypes (total Ig: 2.101 OD units). This individual may have developed an authentic HIT syndrome, since he had received high doses of both subcutaneous and intravenous UFH for more than 5 d at the time of sampling and suffered from multiple subsegmental pulmonary emboli without acute cor pulmonale, associated with a dramatic drop in platelet count (nadir, 49,000/mm3). The only feature that distinguished this serum from those of classic HIT was its lack of sensitivity to inhibition by high-dose heparin (Fig. 4E). When this single outlier was excluded from the analysis, the levels of platelet activation with or without low-dose heparin were not correlated with the levels of anti-PF4 antibodies (SI Appendix, Fig. S6).
To investigate the possibility that COVID-19 sera might induce platelet aggregation in the absence of robust platelet activation, we measured the ability of serum from selected hospitalized patients with COVID-19 to induce platelet aggregation. The results of the two assays were closely correlated (SI Appendix, Fig. S6). Finally, in a subset of COVID-19 and healthy donor sera samples, we evaluated the effect of the addition of exogenous PF4 to the assay, which was reported to enhance platelet activation in patients with VITT (20). Exogenous PF4 at high dose (50 µg/mL) did increase the level of platelet activation in all the sera tested, but the magnitude of increase was even greater in healthy donors than in patients with COVID-19 (SI Appendix, Fig. S6). Altogether, these results indicate that serum from patients with severe COVID-19 induces higher levels of platelet activation than serum from healthy donors, albeit significantly lower than the majority of HIT and VITT sera. The precise mechanism and the clinical significance of such low-level platelet activation detected in the serum of patients with COVID-19 remain unclear.
Discussion
We report herein that the vast majority of hospitalized patients with COVID-19 develop antibodies against PF4–polyanion complexes analogous to the pathogenic antibodies that are a hallmark of HIT and VITT, two syndromes characterized by thrombosis and thrombocytopenia. Since the levels of anti-PF4 antibodies in patients with COVID-19 correlated with the disease severity score and with decreases in circulating platelet counts, our findings raise the possibility that these antibodies may play a role in the pathogenesis of the clinical complications of COVID-19. In particular, the strong correlation with reductions in platelet counts underscores the potential involvement of anti-PF4 antibodies in the formation of microthrombi, which are almost invariably present at postmortem examination in the lungs and other organs of patients with COVID-19. Of note, the delayed appearance of the severe, life-threatening complications of COVID-19 after the initial acute symptoms is consistent with the time required for the induction of anti-PF4 antibodies, which in HIT and VITT is typically 7 to 10 d after the administration of heparin and adenovirus-vectored vaccines, respectively (18–22). We found a higher prevalence of anti-PF4 antibodies in male than in female patients and in patients of African American or Hispanic ethnic origin compared with other groups, which parallels the greater severity of COVID-19 in these groups (11, 13, 17), while there was no association with age, BMI, or preexisting comorbidities. As seen in the setting of HIT and VITT, anti-PF4 antibodies in patients with COVID-19 were transient, as indicated by the low levels detected in convalescent individuals, including those who were previously hospitalized for COVID-19. We also detected anti-PF4 antibodies over the conventional threshold of positivity (0.4 OD units) in a high proportion of SARS-CoV-2–negative patients hospitalized for severe ARD associated with influenza or other causes; however, the levels were markedly lower than those in patients with COVID-19. It should be emphasized that low levels of anti-PF4 antibodies can occasionally occur even in healthy individuals, as also confirmed in this study. Thus, the high frequency of low-level anti-PF4 antibodies that we detected in patients with non-COVID severe ARD may reflect nonspecific activation of innate immune mechanisms without a direct relevance to disease pathogenesis. This interpretation is in line with the finding that in vitro stimulation of B cells from healthy donors may elicit the production of anti-PF4 antibodies (29). In contrast with influenza, the levels of anti-PF4 antibodies detected in a large fraction of patients with COVID-19 were over the threshold for clinical significance, suggesting a specific mechanism of induction of such antibodies with potential pathological consequences.
Progression of COVID-19 is frequently associated with thrombosis and thrombocytopenia, which are reminiscent of the clinical presentation of the HIT and VITT syndromes. However, there are some important differences between COVID-19 and both heparin-induced and vaccine-induced prothrombotic syndromes. Although clinically apparent thrombosis affecting medium-size or large-size blood vessels may occur in patients with COVID-19, as typically observed in HIT and VITT, the major pathological finding in fatal COVID-19 is a diffuse microvascular thrombosis, which does not present with focal symptoms but restricts the blood supply to parenchymatous organs and thereby may result in multiple organ failures (12–16). In our cohort, thrombotic events were relatively infrequent, with pulmonary embolism recorded in only 12% of the hospitalized patients, and were not correlated with the levels of anti-PF4 antibodies. In addition, although sera from hospitalized patients with COVID-19 showed an increased platelet-activating activity compared with sera from healthy blood donors, the activation levels were markedly lower than in patients with HIT and VITT and did not correlate with anti-PF4 antibody levels. Thus, these findings do not allow one to draw definitive conclusions on the role of anti-PF4 antibodies in the pathogenesis of the vascular complications of COVID-19. Nevertheless, such a role is plausible and should be considered. For example, it is possible that in vitro platelet activation assays may not accurately recapitulate the in vivo complexities that exist in patients with COVID-19, in whom multiple factors could trigger a state of preactivation of circulating platelets. Indeed, the cytokine storm that accompanies severe COVID-19, with widespread activation of innate immune responses and release of multiple soluble inflammatory mediators (30), may lower the in vivo threshold for platelet activation by anti-PF4 antibodies. Interestingly, in this respect, platelets from patients with COVID-19 have been reported to be hyperactivated in vivo, as shown by multiple parameters, including endogenous PF4 depletion and increased PF4 plasma levels; such hyperactivation may be induced, at least in part, by the association of platelets with SARS-CoV-2 RNA (31).
Previous studies have reported the occurrence of anti-PF4 antibodies in patients with COVID-19, but the majority investigated small and heterogenous patient groups and were mainly focused on the possible occurrence of classic HIT syndrome in the course of COVID-19, as a consequence of heparin treatment (32, 33). However, two large surveys reported markedly lower rates of anti–PF4 antibody positivity than those observed in our study (34, 35). The reasons for these different rates of positivity are unclear at present. Among the possible reasons are differences in patient selection criteria, although all studies included a group of severe cases. Possibly more relevant is the fact that previous studies were focused on IgG testing, which is the prevalent isotype detected in the HIT and VITT syndromes, while we documented a multi-isotype anti-PF4 antibody response in patients with severe COVID-19 with a prevalence of IgM, rather than IgG, antibodies. Additional studies will be important to address these discrepancies.
The mechanism at the basis of the generation of anti-PF4 antibodies in patients with COVID-19 is currently unknown. Although many patients in our cohort received heparin for thrombus prevention, our results excluded that prior exposure to heparin was a requirement for the development of anti-PF4 antibodies and indicate that patients with severe COVID-19 do not frequently develop a classic HIT syndrome. Nevertheless, we cannot exclude that heparin treatment, especially UFH administered at high doses, could have further enhanced the levels of anti-PF4 antibodies in some patients. In recent years, it has been recognized that triggers other than heparin, including other polyanionic drugs, systemic infections, and vaccines, can induce anti-PF4 antibodies and even prothrombotic clinical disorders that resemble HIT (36–38). Thus, some inherent virus component or virus-induced endogenous factor may be responsible for the induction of anti-PF4 antibodies. Of interest, we have reported that PF4 is a broad-spectrum inhibitor of HIV-1 through a mechanism dependent on a direct interaction between PF4 and the HIV-1 envelope spike (39). Preliminary work from our laboratory and others (40) indicates that PF4 directly interacts with the SARS-CoV-2 spike protein leading to the formation of ultra-large molecular complexes, which raises the possibility that anti-PF4 antibodies in patients with COVID-19 might be elicited by multimolecular aggregates encompassing PF4 and viral spike proteins, similar to those elicited by heparin (41). Antigenic modifications of PF4 induced upon binding to the viral spike might, in turn, expose cryptic immunogenic epitopes in the chemokine that are recognized by the immune system. We are currently evaluating this hypothesis in a small-animal model. The fact that we observed a multi-isotype anti–PF4 antibody response, with a prevalence of IgM antibodies, is compatible with an innate form of B cell response, as previously suggested (29). The time lag required for the induction of anti-PF4 antibodies after the initial viremic phase of SARS-CoV-2 infection, when a heavy load of spike protein is released and may interact with PF4, is consistent with the delayed onset of severe clinical complications in patients with COVID-19, which are no longer related to sustained viral replication (9–11). Whether the spike proteins of different SARS-CoV-2 variants may interact with PF4 with the same affinity remains at present unknown. It will be interesting to evaluate if infection with variants with reduced pathogenicity compared with the original Wuhan strain is associated with similar rates of anti–PF4 antibody induction.
In conclusion, we have identified a high prevalence of anti-PF4 antibodies which may be involved in the pathophysiology of severe clinical complications of COVID-19. If the role of such antibodies is confirmed, it would have implications for the treatment of patients with COVID-19. Further mechanistic studies will help to validate the role of anti-PF4 antibodies in the pathogenesis of COVID-19 complications and provide guidance for the management of patients severely ill with COVID-19.
Materials and Methods
Patients.
Patients diagnosed with COVID-19 by positive SARS-CoV-2 RNA testing in the Johns Hopkins Healthcare System were enrolled in a prospective consented protocol to investigate research questions specific to the clinical course of COVID-19 (institutional review board [IRB] approval no. 00245545). Demographic information, clinical laboratory test results; International Classification of Diseases, 10th Revision, coded diagnoses (comorbidities); BMI; heparin administration; and other clinical parameters were linked to data for patients with COVID-19 in the study. The study was conducted with 100 hospitalized patients with COVID-19 randomly selected to represent a spectrum of disease severity (score 4 to 10 according to the WHO scoring system) (23), whose serum or plasma was stored in the Johns Hopkins Medicine COVID-19 Related Biospecimen Repository between April 2020 and April 2021. A score of 4 or 5 denotes moderate disease in hospitalized patients with no oxygen therapy or oxygen administered by mask or nasal prongs; a score of 6 to 9 denotes severe disease with oxygen administered at a high-flow rate or treated with mechanical ventilation; a score of 10 denotes death due to COVID-19 complications. We utilized the maximum WHO score recorded during the entire hospitalization period, rather than at a specific time point, because it was the most reliable overall index of disease severity. In addition, we studied 25 patients who visited the ED of the Johns Hopkins Hospital for symptoms of COVID-19 and 60 individuals who were convalescent from COVID-19 (30 of whom had previously been hospitalized for COVID-19) (IRB approval no. 00245545). As controls, we tested sera from 28 hospitalized patients with severe ARD unrelated to COVID-19, 24 of whom had acute influenza, as well as 29 patients with HIT, 8 patients with VITT, and 50 healthy blood donors. Patients with ARD were prospectively consented into an influenza research protocol (IRB approval no. 00168163); the diagnosis of influenza was based on positive clinical laboratory PCR testing. The diagnosis of HIT was based on the following criteria: onset of symptoms 5 to 15 d after the initiation of heparin treatment, presence of clinical thrombosis, thrombocytopenia or a reduction in platelet counts greater than 30% from an initially normal count, exclusion of other causes of thrombocytopenia, and presence of antibodies to PF4. The diagnosis of VITT was based on the following criteria: onset of symptoms 5 to 30 d after adenovirus-vectored vaccination against SARS-CoV-2, presence of thrombosis, thrombocytopenia (platelet count <150,000/mm3), and presence of antibodies to PF4 as assessed by enzyme-linked immunosorbent assay (ELISA). Whole blood was collected in acid citrate dextrose (ACD) or sodium citrate (plasma), or without anticoagulant (serum) using aseptic technique, and processed by centrifugation. The research protocol was approved by the Johns Hopkins Hospital IRB. All participants or their next of kin gave written informed consent to participate in the study.
Immunoassays.
A clinically validated ELISA (PF4 Enhanced, Immucor) with a heparin neutralization step to confirm specificity was used to measure anti–PF4-polyanion antibodies in frozen serum or plasma samples from patients. The assay positivity was defined according to the threshold indicated by the manufacturer (>0.400 OD units). The samples from hospitalized patients with COVID-19 were also tested using a commercial IgG-specific assay (PF4 IgG, Immucor) and an in house-modified, isotype-specific immunoassay for IgM and IgA in which the secondary anti–human Ig antibody provided with the PF4 Enhanced kit was replaced by a µ-specific or α-specific anti–human Ig secondary antibody (Invitrogen). Addition of unfractionated porcine heparin (Hikma Pharmaceuticals) at high dose (100 U/mL) was used to demonstrate specificity.
Platelet Activation Assay.
The ability of patient serum to induce platelet activation was assessed using a flow cytometry assay that reveals surface exposure of P-selectin (CD62P). As a source of platelets, we used freshly isolated platelet-rich ACD plasma from healthy blood donors obtained after low-speed centrifugation at 200 × g. None of the donors had been taking antiplatelet drugs or had been vaccinated in the previous 10 d. Platelets (10 µL) were incubated in a total volume of 50 µL with 10 µL of serum from either healthy donors or patients with COVID-19, HIT, or VITT, in the presence or absence of unfractionated porcine heparin (Hikma Pharmaceuticals) at either low (0.3 U/mL) or high (100 U/mL) concentration, or concentrated human Igs (Gamunex-C, Grifols) at 10 mg/mL, or an anti-FcγIIa receptor (CD32) blocking antibody (clone IV.3, Bio X Cell) at 50 µg/mL. In some experiments, recombinant human PF4 (R&D Systems) was added to the platelet-serum mixtures at the concentration of 50 µg/mL. Selected sera samples from patients and control patients were also tested using a platelet aggregation assay.
Platelet Aggregation Assay.
Platelet aggregation was assessed using a flow cytometry assay. Freshly isolated, platelet-rich ACD plasma was obtained after low-speed centrifugation at 200 × g at room temperature from samples from healthy blood donors. Undiluted and 1:10 phosphate buffered saline–diluted platelets (100 µL) were incubated at 37 °C for 15 min with 5 µL of either Calcein Violet 450 AM Viability Dye (eBioscience) at a concentration of 21 µM or with 5 µL Calcein AM Viability Dye (eBioscience) at a concentration of 1 µM in a total volume of 100 µL. From each stained platelet preparation, 10 µL was mixed and incubated in a total volume of 50 µL with 10 µL of serum from healthy donors or from patients with COVID-19 in the presence or absence of unfractionated porcine heparin (Hikma, Pharmaceuticals) at either low (0.3 U/mL) or high (100 U/mL) concentration, an anti-FcγIIa receptor (CD32) blocking antibody (clone: IV.3, Bio X Cell) at 50 µg/mL, or concentrated human Igs (Gamunex-C, Grifols) at 10 mg/mL for 1 h at room temperature. The reaction was stopped by fixation in 2% paraformaldehyde, and data were collected using a BD LSRFortessa flow cytometer.
Statistical Analysis.
The outcome variable in this study was the level of anti-PF4 antibodies in serum assessed at a single time point during hospitalization. Other variables measured against anti-PF4 antibodies included disease severity score, age, sex, race, ethnicity, BMI, circulating white blood cell counts, circulating platelet counts at admission, platelet count reduction during hospitalization, and plasma levels of D-dimer, C-reactive protein, ferritin, and lactic dehydrogenase. For platelet reduction during hospitalization, we calculated the difference between the maximum counts before the sampling date and the minimum count within 30 d after the sampling date for each patient. The association between anti–PF4 antibody levels and multiple demographic, clinical, and laboratory variables was analyzed using linear regression if the covariate is a continuous variable, ANOVA if the covariate is a categorical variable, and unpaired two-tailed t test if the covariate represents two groups of samples. For each covariate, a null model that only contains an intercept term was compared with an alternative model that contains both an intercept and a linear term for the single covariate. Association was determined by P values from testing nonzero regression coefficient of the covariate term, ANOVA F test, or t test. For each continuous covariate, the Pearson’s correlation between anti-PF4 and the covariate along with its 95% CI derived from Fisher Z-transformation was calculated. Multiple regression analysis was used to evaluate the association between anti-PF4 and maximum disease severity score after adjusting for age, race, BMI, and UFH treatment. In each analysis, patients with missing data were excluded. In regression analyses and t tests, all P values were two-tailed. A P value of less than 0.05 was considered to indicate statistical significance in all analyses.
Supplementary Material
Acknowledgments
We thank Guido Massaccesi and Rey Fernandez (Johns Hopkins University, Baltimore, MD) for their help with the storage and transfer of patient samples, and the donors and personnel of the NIH Blood Bank. The specimens from patients with COVID-19 used for this study were part of the Johns Hopkins Biospecimen Repository, which is made up of the contribution of many patients, their families, research teams, and clinicians. This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, by NIH Grant U54CA260492 (to S.L., H.J., and A.L.C.) and Contract HHSN272201400007C (to R.E.R.), and by the Johns Hopkins COVID-19 Research Response Program.
Footnotes
Reviewers: L.F., Université Laval; and D.H., Columbia University.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2213361119/-/DCSupplemental.
Data, Materials, and Software Availability
All study data and methods are included in the article and/or SI Appendix. Primary data from individual patients cannot be shared to protect confidentiality.
References
- 1.Zhu N., et al. ; China Novel Coronavirus Investigating and Research Team, A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382, 727–733 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson L. A., et al. ; mRNA-1273 Study Group, An mRNA vaccine against SARS-CoV-2—Preliminary report. N. Engl. J. Med. 383, 1920–1931 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folegatti P. M., et al. ; Oxford COVID Vaccine Trial Group, Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396, 467–478 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack F. P., et al. ; C4591001 Clinical Trial Group, Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadoff J., et al. , Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N. Engl. J. Med. 384, 1824–1835 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mascola J. R., Graham B. S., Fauci A. S., SARS-CoV-2 viral variants-tackling a moving target. JAMA 325, 1261–1262 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Corti D., Purcell L. A., Snell G., Veesler D., Tackling COVID-19 with neutralizing monoclonal antibodies. Cell 184, 3086–3108 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karim S. S. A., Karim Q. A., Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 398, 2126–2128 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W. J., et al. ; China Medical Treatment Expert Group for Covid-19, Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C., et al. , Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mir T., et al. , Coronavirus disease 2019 (COVID-19): Multisystem review of pathophysiology. Ann. Med. Surg. (Lond.) 69, 102745 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pancaldi E., et al. , Thrombotic risk in patients with COVID-19. Rev. Cardiovasc. Med. 22, 277–286 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Wichmann D., et al. , Autopsy findings and venous thromboembolism in patients with COVID-19: A prospective cohort study. Ann. Intern. Med. 173, 268–277 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ackermann M., et al. , Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 383, 120–128 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rapkiewicz A. V., et al. , Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine 24, 100434 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sauter J. L., et al. , Insights into pathogenesis of fatal COVID-19 pneumonia from histopathology with immunohistochemical and viral RNA studies. Histopathology 77, 915–925 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bashash D., et al. , The prognostic value of thrombocytopenia in COVID-19 patients; a systematic review and meta-analysis. Arch. Acad. Emerg. Med. 8, e75 (2020). [PMC free article] [PubMed] [Google Scholar]
- 18.Greinacher A., Clinical practice. Heparin-induced thrombocytopenia. N. Engl. J. Med. 373, 252–261 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Schultz N. H., et al. , Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 384, 2124–2130 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greinacher A., et al. , Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 384, 2092–2101 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scully M., et al. , Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 384, 2202–2211 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muir K. L., Kallam A., Koepsell S. A., Gundabolu K., Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N. Engl. J. Med. 384, 1964–1965 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection, A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 20, e192–e197 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warkentin T. E., Sheppard J. I., Moore J. C., Sigouin C. S., Kelton J. G., Quantitative interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. J. Thromb. Haemost. 6, 1304–1312 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Martel N., Lee J., Wells P. S., Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: A meta-analysis. Blood 106, 2710–2715 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Warkentin T. E., Heddle N. M., Laboratory diagnosis of immune heparin-induced thrombocytopenia. Curr. Hematol. Rep. 2, 148–157 (2003). [PubMed] [Google Scholar]
- 27.Tardy-Poncet B., et al. ; on Behalf of the GFHT-HIT Study Group, Functional flow cytometric assay for reliable and convenient heparin-induced thrombocytopenia diagnosis in daily practice. Biomedicines 9, 332 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huynh A., Kelton J. G., Arnold D. M., Daka M., Nazy I., Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature 596, 565–569 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Krauel K., et al. , Further insights into the anti-PF4/heparin IgM immune response. Thromb. Haemost. 115, 752–761 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Fajgenbaum D. C., June C. H., Cytokine storm. N. Engl. J. Med. 383, 2255–2273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaid Y., et al. , Platelets can associate with SARS-Cov-2 RNA and are hyperactivated in COVID-19. Circ. Res. 127, 1404–1418 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Favaloro E. J., Henry B. M., Lippi G., The complicated relationships of heparin-induced thrombocytopenia and platelet factor 4 antibodies with COVID-19. Int. J. Lab. Hematol. 43, 547–558 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brodard J., et al. , COVID-19 patients often show high-titer non-platelet-activating anti-PF4/heparin IgG antibodies. J. Thromb. Haemost. 19, 1294–1298 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueland T., et al. , Anti-PF4/polyanion antibodies in COVID-19 patients are associated with disease severity and pulmonary pathology. Platelets 33, 640–644 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Greinacher A., et al. , Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Blood 138, 2256–2268 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krauel K., et al. , Platelet factor 4 binding to lipid A of Gram-negative bacteria exposes PF4/heparin-like epitopes. Blood 120, 3345–3352 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Warkentin T. E., Greinacher A., Spontaneous HIT syndrome: Knee replacement, infection, and parallels with vaccine-induced immune thrombotic thrombocytopenia. Thromb. Res. 204, 40–51 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Warkentin T. E., Makris M., Jay R. M., Kelton J. G., A spontaneous prothrombotic disorder resembling heparin-induced thrombocytopenia. Am. J. Med. 121, 632–636 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Auerbach D. J., et al. , Identification of the platelet-derived chemokine CXCL4/PF-4 as a broad-spectrum HIV-1 inhibitor. Proc. Natl. Acad. Sci. U.S.A. 109, 9569–9574 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Passariello M., Vetrei C., Amato F., De Lorenzo C., Interactions of spike-RBD of SARS-CoV-2 and platelet factor 4: New insights in the etiopathogenesis of thrombosis. Int. J. Mol. Sci. 22, 8562 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rauova L., et al. , Ultralarge complexes of PF4 and heparin are central to the pathogenesis of heparin-induced thrombocytopenia. Blood 105, 131–138 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data and methods are included in the article and/or SI Appendix. Primary data from individual patients cannot be shared to protect confidentiality.