Abstract
Background:
Trichomoniasis commonly affects women of childbearing age and has been linked to several adverse birth outcomes.
Objective:
To elucidate the association between trichomoniasis in pregnant women and adverse birth outcomes, including preterm delivery, pre-labour rupture of membranes, and low birth weight.
Search Strategy:
MEDLINE, EMBASE, and ClinicalTrials.gov were systematically searched in December 2020 without time or language restrictions.
Selection Criteria:
Original research studies were included if they assessed at least one of the specified adverse birth outcomes in pregnant women with laboratory-diagnosed trichomoniasis.
Data Collection and Analysis:
Estimates from included articles were either extracted or calculated and then pooled to produce a combined estimate of the association of trichomoniasis with each adverse birth outcome using the random effects model. Heterogeneity was assessed using the I2 statistic and Cochran’s Q test.
Main Results:
Literature search produced 1,658 publications after removal of duplicates (n=770), with 5 additional publications identified by hand search. After screening titles and abstracts for relevance, full text of 84 studies was reviewed and 19 met inclusion criteria for meta-analysis. Significant associations were found between trichomoniasis and preterm delivery (OR=1.27; 95% CI, 1.08–1.50), pre-labour rupture of membranes (OR=1.87; 95% CI, 1.53–2.29), and low birth weight (OR=2.12; 95% CI, 1.15–3.91).
Conclusions:
Trichomoniasis in pregnant women is associated with preterm delivery, pre-labour rupture of membranes, and low birth weight. Rigorous studies are needed to determine the impact of universal trichomoniasis screening and treatment during pregnancy on reducing perinatal morbidity.
Keywords: Adverse Birth Outcomes, Pregnancy, Trichomoniasis
INTRODUCTION
Trichomoniasis is the most common non-viral sexually transmitted infection (STI) worldwide.1 Approximately 156 million new cases occur worldwide annually among women and men aged 15–49 years.1 Given the burden of trichomoniasis experienced by women of child bearing age, the impact of this infection in pregnancy, particularly with regard to adverse birth outcomes (ABOs), is important to consider.
Preterm delivery (PTD) occurred in 10% of all U.S. live births in 2018 and can be complicated by respiratory distress and death in infants.2 Low birth weight (LBW) occurred in 8% of U.S. births in 2017 and contributes to infant mortality.3,4 Pre-labour rupture of membranes (PROM) complicates approximately 8% of U.S. term pregnancies, with preterm PROM (PPROM) occurring approximately 2–3% of the time.5
Several prospective observational cohort studies in the 1980s investigated relationships between ABOs and trichomoniasis in pregnant women, noting associations between infection and PTD, LBW, and PROM.6–8 This literature influenced clinical practice, leading some providers to screen asymptomatic pregnant women for trichomoniasis in the absence of guidelines recommending this practice.9,10 However, in 2001, one study described increased risk of PTD in asymptomatic pregnant women receiving metronidazole (MTZ) for trichomoniasis, compared to placebo.11 This study raised concerns about trichomoniasis screening in pregnant women,12 leading current guidelines to only recommend testing for symptomatic pregnant women.13 Given that 70%−85% of infected women are asymptomatic, and untreated infections can last 3 months or longer,14 a better understanding of the perinatal effects of untreated trichomoniasis in pregnant women is essential.15,16
In 2014, Silver et al. performed a systematic review and meta-analysis investigating the relationship between ABOs (PTD, PROM, infant mortality, and LBW or small for gestational age [SGA] infants) and trichomoniasis in pregnant women.17 This analysis demonstrated PTD to be more likely in pregnant women with trichomoniasis than in uninfected women (relative risk=1.42; 95% confidence interval [CI], 1.15–1.75). The authors also found significant increases in the risk of PPROM and SGA among infected women, but only in two studies per outcome. They could not calculate the relative risk for LBW and PROM due to excessive heterogeneity among studies assessing those outcomes. Since Silver et al. was published, there has been more research conducted regarding the relationship between ABOs and trichomoniasis.18–27
Objectives
The objective of this systematic review and meta-analysis was to better understand and solidify known associations between ABOs and trichomoniasis, specifically PTD, PROM (including PPROM), and LBW.
METHODS
Protocol
This review was performed and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) protocols.28,29 This study had no patient involvement as it was based completely on data from literature review and was therefore exempt from University of Alabama at Birmingham (UAB) Institutional Review Board approval. This study was submitted for PROSPERO registration and published on their website on January 7, 2021 (registration number CDR42021224985).
Eligibility criteria
Types of studies
Randomised studies and observational studies, including longitudinal cohort, case-control, and retrospective designs, conducted in any country were considered for inclusion without language restrictions. Case reports, case series, and other systematic reviews and meta-analyses were excluded. Articles presenting secondary analyses of already included studies were excluded.
Types of participants
The participants were pregnant women. Outcomes were measured for live newborn infants. No exclusion criteria were applied in terms of participants, including women who had HIV or other STIs.
Types of diagnostics and interventions
To be included, Trichomonas vaginalis had to be diagnosed by wet mount microscopy, culture, nucleic acid amplification testing (NAAT), rapid trichomonas testing (e.g. OSOM®), or seen incidentally on Papanicolaou smear. Studies solely reporting trichomoniasis diagnosis by billing code or patient self-report were excluded. Studies including diagnosis and/or treatment for bacterial vaginosis (BV) and/or other STIs during pregnancy were not excluded.
Types of outcomes
Studies were included if they assessed at least one of the three specified ABOs–PTD, PROM, or LBW–in pregnant women with trichomoniasis. PTD is defined as delivery of a live infant (i.e. stillbirths excluded) prior to 37 weeks gestation.30 PROM is defined as rupture of membranes before the onset of labour. Studies specifically reporting PPROM, or rupture of membranes prior to 37 weeks gestation, were included within this outcome.31 LBW was defined as an infant weighing <2500 grams at birth.32,33
Information Sources
We performed exhaustive searches of the electronic databases MEDLINE (using PubMed), EMBASE, and ClinicalTrials.gov in December 2020. Reference lists of relevant publications were also reviewed to search for additional studies not captured by our search terms. This included review of all studies in Silver et al.17
Search strategy
Appendix S1 presents an overview of the search strategy used in all three databases. This search was created using database-specific combination search queries created by author J.D, an experienced health sciences librarian. Articles with publication dates until December 2020 were included in the final search.
Study selection
Authors O.V. and J.W. independently executed the search strategy. Records obtained from the databases were entered into an Excel spreadsheet and manually de-duplicated. These reviewers then screened the titles and abstracts of all remaining studies and removed those which were not relevant to the study objectives or did not meet inclusion criteria. Remaining records were assessed by full-text analysis independently by O.V. and J.W. to ensure satisfaction of inclusion criteria. Studies were selected through consensus by the two reviewers, and discrepancies were adjudicated by author C.M. Reviewers were not blinded. Non-English studies were reviewed by author P.B. if they were in French, German, or Spanish or translated by Google Translate if they were in another language.
Data collection process and data items
For each study meeting inclusion criteria, authors O.V. and A.J. independently extracted the following information and organised it on an Excel spreadsheet: study author(s), title, year(s) of data collection and publication, setting, study design, sample size, study population demographics (e.g. race, age, socioeconomic status), T. vaginalis testing method and prevalence, ABO(s), and corresponding measures of association and precision for outcomes. If additional data were needed, attempts were made to contact corresponding authors of eligible articles.
Risk of bias in individual studies
The methodological quality of each study was independently reviewed by J.W. and O.V. Cohort and case-controlled studies were assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS).34 This scale uses a checklist to assess study quality by evaluating three areas: selection, comparability, and exposure (for case-controls) or outcome (for cohorts). Based on study performance in these areas, a score of “good”, “fair”, or “poor” quality is assigned. Randomised controlled trials (RCTs) were assessed using the revised Cochrane risk of bias tool for randomised trials (RoB2).35 Like the NOS, the RoB2 employs a checklist to assess each study, but instead of grading quality, it assess for potential risks of bias. Based on checklist results, studies are determined to have low or high risk of bias. Quality assessment scores for the included studies are presented on Table S1.
Methods of analysis
Measures of association (odds ratio [OR], risk ratio [RR], hazard ratio [HR]) and precision (standard error, 95% CI) were extracted from included studies or calculated using reported raw data, if provided. When raw data included a cell with a value of zero, 0.5 was added to all cells to calculate a measure of association. Both crude and adjusted measures were extracted. In the event both were provided, the adjusted effect estimate was used for analysis. Given our rare outcomes, RRs were assumed to be approximate yet conservative estimates of the ORs.36,37 In studies where testing for T. vaginalis was performed at multiple points during pregnancy, data were collapsed across time points where data were provided. When data were not provided, results from one time point were selected.
For each outcome, data were pooled to produce a combined estimate of the association with trichomoniasis. ORs and 95% CIs are reported. The DerSimonian and Laird’s random effects model was used for analysis. The presence of heterogeneity between studies was assessed with Cochran’s Q test and magnitude of heterogeneity was assessed with the I2 statistic.38,39 Heterogeneity was declared when the Q-test p<0.10 and I2>0.50. Publication bias was assessed visually with funnel plots and Begg and Egger tests where p<0.05.40,41
Leave-one-out sensitivity analysis was conducted to examine the impact of individual studies on the pooled estimate for each outcome. Due to the importance of several known confounders, a second sensitivity analysis excluded studies that did not control for two confounders selected a priori: infection with BV and other STIs during pregnancy. Analysis was conducted using STATA 12 software.42
RESULTS
Study selection
Searches in MEDLINE/PubMed (n=1,136), EMBASE (n=1,289), and ClinicalTrials.gov (n=3) identified 2,428 publications. After excluding duplicates (n=770), 1,658 articles remained. Five additional articles were identified via hand search of reference lists and other sources, yielding 1,663 articles. Of these, 1,579 were excluded based on title and/or abstract. The full text of the remaining 84 studies was reviewed and 65 studies were excluded, leaving 19 studies meeting inclusion criteria for meta-analysis.6–8,18,21,22,24–27,43–51 These data are presented as a PRISMA flow diagram in Figure S1 and the list of articles excluded based on full-text review, along with reasons for exclusion, is shown in Table S2.
Study characteristics
Characteristics of the included 19 studies are provided in Table S3. Study designs included prospective cohort studies (n=9)6–8,25,46–48,51, case-control studies (n=6)18,21,26,27,43,44,50, a case-cohort study (n=1)22, a retrospective population-based study (n=1)45, a secondary analysis of an RCT (n=1)24, and a quasi-experimental design study (n=1)49. Included studies were conducted between 1965 and 2015 and published between 1974 and 2019. In total 94,335 pregnant women were included, with study sizes ranging from 115 to 60,296 participants. Our meta-analysis included 4 studies utilizing T. vaginalis NAAT testing18,24–26, with the remaining studies using wet mount microscopy or culture.6–8,21,22,27,43–51 Of the 19 studies, 11 reported on PTD, 8 reported on PROM, and 7 reported on LBW.
Quality assessment and risk of bias within studies
Among case-control, cohort, and quasi-experimental studies included in our analysis, 10 were determined to be good quality,6–8,18,26,27,45,46,48,51 1 was fair quality,43 and 7 were poor quality per NOS assessment.21,22,25,44,47,49,50 Moodley et al. was found to have a low risk of bias based on the RoB2 assessment tool.24 Potential sources of confounding for each study are shown in Table S1. One important area of potential confounding was how each study accounted for BV and/or STI co-infection, as these may also impact perinatal morbidity.52–54 Only 6 studies adjusted for the presence of co-infection with BV and/or other STIs in their analysis of the relationship between ABOs and trichomoniasis. Another important consideration in studies investigating ABOs is the manner by which the estimated gestational age (EGA) is determined. Among the studies included in this meta-analysis, 9 did not report how EGA was determined.6,8,22,25–27,47,50,51 Those that did report this used either the mother’s last menstrual period (LMP), ultrasound, or a combination of the two (Table S1).
Synthesis of results
Eleven studies (n=84,336) reported on PTD.7,18,22,24,43–46,48,49,51 PTD was defined as delivery at <37 weeks gestation in 8 studies 7,18,22,24,43,46,49,51 and <35 weeks in 1 study.48 Another study defined PTD as delivery between 20 and 37 weeks gestation.44 One study distinguished between very preterm delivery (delivery at <32 completed weeks gestation) and moderately preterm delivery (delivery between 32 and 36 weeks gestation).45 Of note, 7 studies reporting on PTD explicitly excluded stillborn infants with regard to this outcome7,18,24,43,45,46,49 with the other 4 not commenting on vital status.22,44,48,51 Pregnant women with trichomoniasis had 1.27 times the odds of PTD compared to uninfected women (OR=1.27; 95% CI, 1.08–1.50) (Figure 1). Heterogeneity among studies was low (Cochran’s Q p=0.24; I2=22%).
Figure 1.
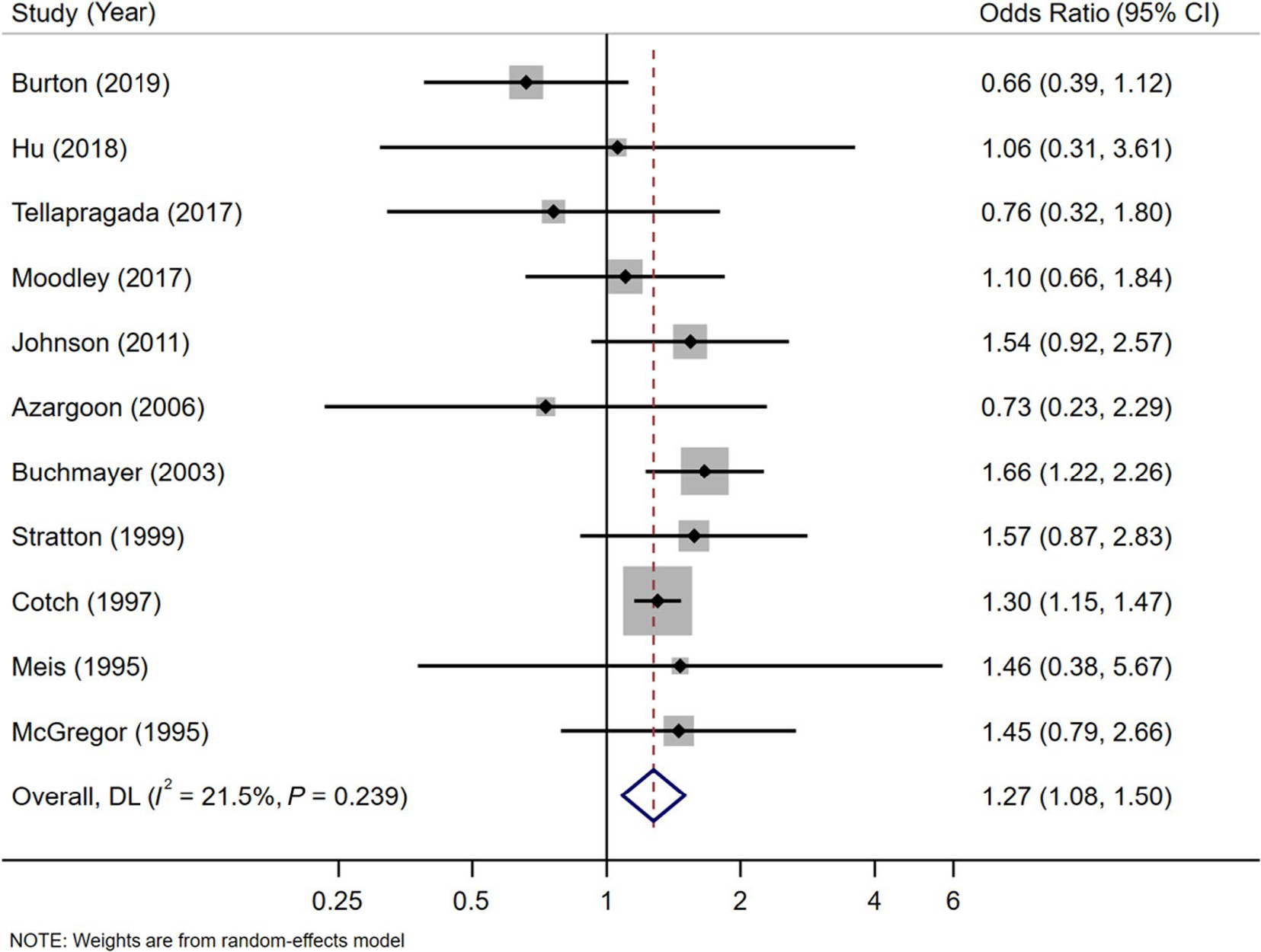
Meta-analysis of the effect of trichomoniasisduring pregnancy on preterm delivery using a random effects model. Solid black diamonds indicate the odds ratio while the solid horizontal lines indicate the 95% CI. The size of the grey box around the black diamond indicates weight of study in the analysis. The diamond with a white centreindicates the pooled odds ratio estimate, with 95% CI represented by the diamond width.
Eight studies (n=11,411) reported on the association of trichomoniasis and PROM.6,25–27,44,47,50,51 One study included patient self-report of fluid draining from the vagina26, while the others confirmed the presence of this outcome by speculum exam revealing pooling of fluid in the posterior vaginal fornix with or without positive nitrazine or fern tests on vaginal fluid. Two of these studies reported PPROM.6,27 Pregnant women with trichomoniasis had 1.87 times the odds of PROM compared to uninfected women (OR=1.87; 95% CI, 1.53–2.29) (Figure 2). Heterogeneity among studies was low (Cochran’s Q test p=0.28; I2=19%).
Figure 2.
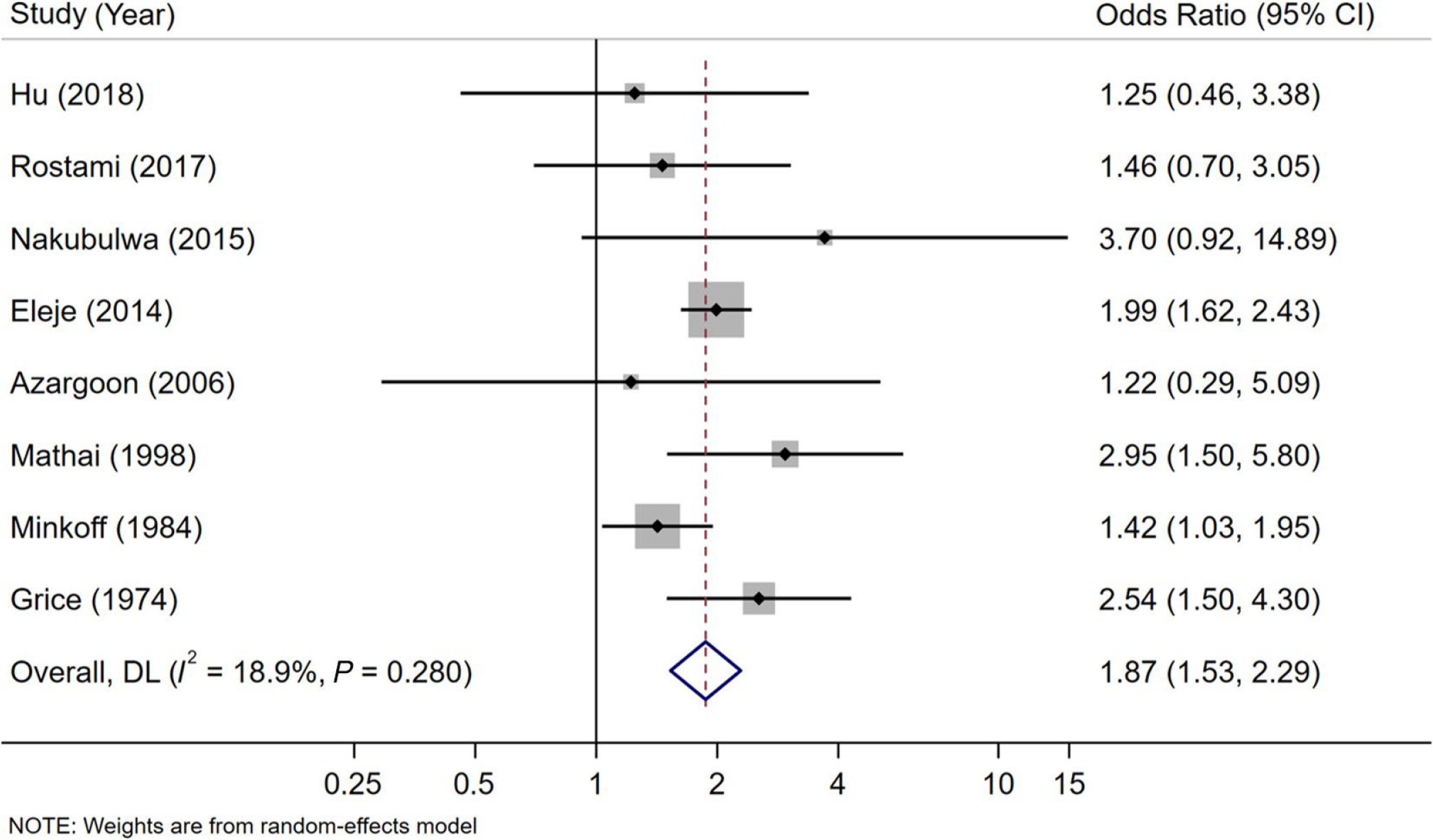
Meta-analysis of the effect of trichomoniasisduring pregnancy on prelabourrupture of membranes using a random effects model. Solid black diamonds indicate the odds ratio while the solid horizontal lines indicate the 95% CI. The size of the grey box around the black diamond indicates weight of study in the analysis. The diamond with a white centreindicates the pooled odds ratio estimate, with 95% CI represented by the diamond width.
Seven studies (n=17,401) reported on LBW with high heterogeneity (Cochran’s Q p<0.001; I2=89%).7,8,21,24,25,43,51 Of note, one study by Stratton et al. included aggregated data for both LBW and SGA and original numbers were unable to be obtained, so results for LBW and SGA were not included in the analysis.46 All studies reporting this outcome defined LBW as a live infant born weighing <2500 grams. The random effects model resulted in a significant association between trichomoniasis and LBW (OR=2.12; 95% CI, 1.15–3.91) (Figure 3).
Figure 3.

Meta-analysis of the effect of trichomoniasisduring pregnancy and low birthweight using a random effects model. Solid black diamonds indicate the odds ratio while the solid horizontal lines indicate the 95% CI. The size of the grey box around the black diamond indicates weight of study in the analysis. The diamond with a white centreindicates the pooled odds ratio estimate, with 95% CI represented by the diamond width.
Leave-one-out sensitivity analysis for PTD revealed that no individual study greatly changed the magnitude of the pooled estimate when excluded (Table S4). However, when the Cotch et al. study7 was excluded, the pooled estimate did become non-significant (OR=1.23; 95% CI 0.97–1.55). Similarly, for PROM, the leave-one-out analysis revealed that excluding any one study did not greatly change the pooled estimate or produce non-significant results. For LBW, removing one study at a time did produce a wider range of pooled estimates. When Rostami et al.25 was removed, the pooled estimate decreased in magnitude and became non-significant (OR=1.91; 95% CI, 0.99–3.69). When the Kamal et al. study21 was excluded, the pooled OR estimate decreased to 1.55, but remained significant (OR=1.55; 95% CI, 1.12–2.13). With a large sample size (n=13,816), the Cotch et al. study7 had a point estimate of similar magnitude to the Johnson et al. and Moodley et al. studies;24,43 when excluded, the pooled OR estimate increased (OR=2.43; 95% CI, 1.34–4.41).
The sensitivity analysis excluding studies that did not adjust for the two potential confounders (i.e. co-infection with BV and STIs during pregnancy) was not completed. While six studies controlled for at least one of these confounders6,7,24,26,43,48, only one controlled for both.7
There was no evidence of publication bias for any outcome, though PROM and LBW had few studies included in the pooled analysis (Figure S2). The wide CI of the estimate of LBW in the study by Hu et al.51 does set the estimate apart from the others, but it still falls within the funnel.
DISCUSSION
Main findings
T. vaginalis is a curable STI that impacts an estimated 25 million pregnant women globally each year.55 While the association between ABOs and trichomoniasis has been described in the literature, additional data over the past seven years have allowed for a more robust evaluation of this relationship compared to prior studies.17 We have conducted an updated systematic review and meta-analysis on this topic, which corroborates the findings of Silver et al. regarding the strong association between trichomoniasis and PTD17 and also identifies more powerful relationships between trichomoniasis and PROM, as well as LBW.
PTD was the ABO with the largest number of studies included in our meta-analysis. Across the eleven studies including PTD, women with trichomoniasis had 1.27 times the odds of experiencing PTD compared to uninfected women. Women with trichomoniasis had 1.87 times the odds of experiencing PROM (including PPROM) compared to uninfected women. This association was not assessed by Silver et al.17 Their analysis was able to establish a relationship between trichomoniasis and PPROM (RR=1.41; 95% CI, 1.10–1.82), but this only incorporated 2 studies.7,44 They were unable to assess the relationship between trichomoniasis and PROM given the high level of heterogeneity among their included studies. In contrast, our analysis captured both PPROM and PROM in a total of eight studies and we established a significant relationship between trichomoniasis and this combined variable. Our analysis strengthens the evidence that pregnant women with trichomoniasis are at higher risk of experiencing PROM than those without trichomoniasis.
Based on our analysis of seven studies, there was a significant association between trichomoniasis and LBW. Heterogeneity among these studies was high (I2=89%). Silver et al. did not conduct a pooled analysis for LBW across 4 studies due to significant heterogeneity.17 However, they did conduct a pooled analysis of two studies for SGA and found a significant, positive association.17 The results of our meta-analysis regarding LBW emphasize the need for further studies investigating this association.
The physiologic mechanisms linking trichomoniasis and ABOs are not well understood. One theory is that PTD and PROM in pregnant women with trichomoniasis are related to the post-infection maternal innate immune inflammatory response, which involves elevated cervical interleukin-8 (IL-8) and vaginal defensin levels.56,57 These cytokines are markers of neutrophil activation, which has been associated with PTD and PPROM.58 Cervical IL-8, in particular, is thought to trigger cervical ripening and dilatation, further supporting its potential role in ABOs.59,60 Some evidence suggests that the immune-inflammatory reaction in pregnant women infected with T. vaginalis extends beyond the reproductive tract; one study has described increased serum C-reactive protein levels in these women.56 The pathophysiology behind LBW/SGA in infected pregnant women is not well understood. It has been hypothesised that intrauterine inflammation associated with trichomoniasis interferes with placental circulation17, however additional studies are needed.
Strengths and limitations
There are several strengths of this study. A larger number of studies are included compared to the Silver et al. meta-analysis (19 versus 11).17 In addition, one significant limitation noted by Silver et al. was the use of suboptimal diagnostic techniques (primarily wet mount microscopy) in many of their included studies.17 None of their studies reported T. vaginalis NAAT testing data. Given that the sensitivity of wet mount microscopy is low (44–68%)61, the authors were concerned for under-detection of T. vaginalis.17 Four studies in our review used the more sensitive and specific T. vaginalis NAATs18,24–26, leading to more robust, precise, and compelling results.
Despite these strengths, there are limitations of our study. Despite a rigorous, systematic approach to reviewing the literature, it is possible that appropriate articles were missed. Second, while studies involving PTD and PROM were minimally heterogeneous in our meta-analysis, marked heterogeneity was observed in studies reporting LBW. The leave-one-out sensitivity analysis demonstrated no single study exerted a large influence over the pooled estimate for PTD and PROM but did result in a wider estimate range for LBW. Although a relationship was found between trichomoniasis and LBW in our analysis, further research should be conducted to further clarify this association.
Another limitation was the lack of detail in how data were collected and outcomes were measured in individual studies. Precision in the reporting of EGA is essential for properly identifying ABOs and many included studies (n=9) did not report which estimation methods was used. There were shortcomings related to ABO definitions for all outcomes, particularly with regard to their temporal relationship with the onset of labour, making it difficult to draw definitive conclusions regarding the impact of trichomoniasis on perinatal morbidity.
Another limitation was the lack of data regarding co-infection with BV and/or other STIs during pregnancy among many included studies. While several individual studies adjusted for the presence of BV and/or other STIs in their analyses,6,7,24,26,43,48 many did not.8,18,21,22,27,44–46,49,50 Among studies reporting both unadjusted and adjusted results, adjusting for co-infections did not routinely shift point estimates toward or away from the null, though other variables were also included in adjustments. Additionally, it is difficult to establish the role that co-infection with BV and/or other STIs plays in the relationship between trichomoniasis and ABOs. It is possible that co-infection confounds the effect of T. vaginalis on perinatal morbidity, but BV and/or other STIs could also be part of the causal pathway between trichomoniasis and ABOs, in which case adjusting by these factors is inappropriate.36 BV and/or other STIs have known associations with ABOs, particularly PTD.52–54 It is unclear what impact co-infection has on the frequency or severity of ABOs in pregnant women with trichomoniasis. Therefore, our results must be viewed in the context of this limitation, particularly that T. vaginalis may not be solely responsible for the ABO effects found.
Interpretation
These results have important clinical implications. As mentioned previously, there are currently no recommendations to screen asymptomatic women for trichomoniasis, regardless of pregnancy status.13 While the treatment of trichomoniasis with MTZ is considered to be safe in all stages of pregnancy62, one study suggested an increased risk of PTD in women with asymptomatic trichomoniasis who received MTZ compared to those who did not.11 Despite this single study suggesting such an association, our meta-analysis augments the evidence of the impact of trichomoniasis has on ABOs.
CONCLUSION
In conclusion, trichomoniasis may be a major source of female reproductive morbidity given its association with PTD, PROM, and LBW. Rigorous studies are needed to determine the impact that universal trichomoniasis screening and treatment during pregnancy may have on reducing the incidence of ABOs.
Supplementary Material
Acknowledgments:
The authors would like to acknowledge Dr. Tanika Kelly for her assistance with the methodology for this study. This study was presented as an oral presentation during the session “STI Epidemiology in Women and Infants” at the 2020 Centers for Disease Control STD Prevention Conference (virtual) on September 23, 2020 as well as a poster presentation at the UAB Department of Medicine Trainee Research Symposium in March 2020, where it received the J. Claude Bennett Award for Excellence in Research by an Associate Fellow (awarded to Olivia Van Gerwen, MD, MPH).
Funding:
Olivia T. Van Gerwen, MD, MPH is currently supported by grant T32 HS013852 from the Agency of Healthcare Research and Quality.
Footnotes
Disclosure of interests
Christina A. Muzny, MD, MSPH is a consultant for Lupin Pharmaceuticals, BioFire Diagnostics, and Cepheid. She has also received honoraria from PhagoMed, Abbott Molecular, Roche Diagnostics, and Becton Dickinson. All other authors declare no conflicts of interest.
References
- 1.Rowley J, Vander Hoorn S, Korenromp E, Low N, Unemo M, Aub-Raddad LJ, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ 2019;97:548–62p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamilton BE, Martin JA, Ostermak MJK, Rossen LM. Births: Provisional Data for 2018. Natl Vital Stat Rapid Release 2019;007. [Google Scholar]
- 3.Martin JA, Hamilton EB, Osterman MJK, Driscoll AK, Drake P. Births: Final Data for 2017. Natl Vital Stat Rep 2018;67(8):1–10. [PubMed] [Google Scholar]
- 4.Heron M Deaths: Leading Causes for 2015. Natl Vital Stat Rep 2017;66(5):1–74. [PubMed] [Google Scholar]
- 5.Prelabor Rupture of Membranes: ACOG Practice Bulletin, Number 217. Obstet Gynecol 2020;135(3):e80–e97. [DOI] [PubMed] [Google Scholar]
- 6.Minkoff H, Grunebaum AN, Schwarz RH, Feldman J, Cummings M, Crombleholme W, et al. Risk factors for prematurity and premature rupture of membranes: A prospective study of the vaginal flora in pregnancy. Am J Obstet Gynecol 1984;150(8):965–72. [DOI] [PubMed] [Google Scholar]
- 7.Cotch M PI, Nugent R. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex Transm Dis 1997;24(6):353–60. [DOI] [PubMed] [Google Scholar]
- 8.Hardy PH, Hardy JB, Nell EE, Graham DA, Spence MR, Rosenbaum RC. Prevalence of six sexually transmitted disease agents among pregnant inner-city adolescents and pregnancy outcome. Lancet 1984;2(8398):333–7. [DOI] [PubMed] [Google Scholar]
- 9.Guidelines for the Management of Sexually Transmitted Infections: World Health Organization; 2003. Available at https://www.who.int/hiv/pub/sti/en/STIGuidelines2003.pdf. Accessed November 1, 2019. [Google Scholar]
- 10.Saurina GR, McCormack WM. Trichomoniasis in Pregnancy. Sex Transm Dis 1997;24(6):361–2. [DOI] [PubMed] [Google Scholar]
- 11.Klebanoff MA, Carey JC, Hauth JC, Hillier SL, Nugent RP, Thom EA, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med 2001;345(7):487–93. [DOI] [PubMed] [Google Scholar]
- 12.Okun N, Gronau KA, Hannah ME. Antibiotics for bacterial vaginosis or Trichomonas vaginalis in pregnancy: a systematic review. Obstet Gynecol 2005;105(4):857–68. [DOI] [PubMed] [Google Scholar]
- 13.Workowski KA. Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines. Clin Infect Dis 2015;61 Suppl 8:S759–62. [DOI] [PubMed] [Google Scholar]
- 14.Van Der Pol B, Williams JA, Orr DP, Batteiger BE, Fortenberry JD. Prevalence, incidence, natural history, and response to treatment of Trichomonas vaginalis infection among adolescent women. J Infect Dis 2005;192(12):2039–44. [DOI] [PubMed] [Google Scholar]
- 15.Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The Prevalence of Trichomonas vaginalis Infection among Reproductive-Age Women in the United States, 2001–2004. Clin Infect Dis 2007;45(10):1319–26. [DOI] [PubMed] [Google Scholar]
- 16.Peterman TA, Tian LH, Metcalf CA, Malotte CK, Paul SM, Douglas JM, et al. Persistent, Undetected Trichomonas vaginalis Infections? Clin Infect Dis 2009;48(2):259–60. [DOI] [PubMed] [Google Scholar]
- 17.Silver BJ, Guy RJ, Kaldor JM, Jamil MS, Rumbold AR. Trichomonas vaginalis as a cause of perinatal morbidity: a systematic review and meta-analysis. Sex Transm Dis 2014;41(6):369–76. [DOI] [PubMed] [Google Scholar]
- 18.Burton AE, Thomas S. Sexually transmitted infections and preterm birth among Indigenous women of the Northern Territory, Australia: A case-control study. Aust N Z J Obstet Gynaecol 2019;59(1):147–53. [DOI] [PubMed] [Google Scholar]
- 19.Warr AJ, Pintye J, Kinuthia J, Drake AL, Unger JA, McClelland RS, et al. Sexually transmitted infections during pregnancy and subsequent risk of stillbirth and infant mortality in Kenya: a prospective study. Sex Transm Infect 2019;95(1):60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schonfeld A, Feldt T, Tufa TB, Orth HM, Fuchs A, Musfun MG, et al. Prevalence and impact of sexually transmitted infections in pregnant women in central Ethiopia. Int J STD AIDS 2018;29(3):251–8. [DOI] [PubMed] [Google Scholar]
- 21.Kamal AM, Ahmed AK, Mowafy NME, Shawki HE, Sanad AS, Hassan EE. Incidence of Antenatal Trichomoniasis and Evaluation of Its Role as a Cause of Preterm Birth in Pregnant Women Referring to Minia University Hospital, Egypt. Iran J Parasitol 2018;13(1):58–66. [PMC free article] [PubMed] [Google Scholar]
- 22.Tellapragada C, Eshwara VK, Bhat P, Kamath A, Aletty S, Mukhopadhyay C. Screening of vulvovaginal infections during pregnancy in resource constrained settings: Implications on preterm delivery. J Infect Public Health 2017;10(4):431–7. [DOI] [PubMed] [Google Scholar]
- 23.Hosny A, El-Khayat W, Kashef MT, Fakhry MN. Association between preterm labor and genitourinary tract infections caused by Trichomonas vaginalis, Mycoplasma hominis, Gram-negative bacilli, and coryneforms. J Chin Med Assoc 2017;80(9):575–81. [DOI] [PubMed] [Google Scholar]
- 24.Moodley D, Sartorius B, Madurai S, Chetty V, Maman S. Pregnancy Outcomes in Association with STDs including genital HSV-2 shedding in a South African Cohort Study. Sex Transm Infect 2017;93(7):460–6. [DOI] [PubMed] [Google Scholar]
- 25.Nateghi Rostami M, Hossein Rashidi B, Habibi A, Nazari R, Dolati M. Genital infections and reproductive complications associated with Trichomonas vaginalis, Neisseria gonorrhoeae, and Streptococcus agalactiae in women of Qom, central Iran. Int J Reprod Biomed 2017;15(6):357–66. [PMC free article] [PubMed] [Google Scholar]
- 26.Nakubulwa S, Kaye DK, Bwanga F, Tumwesigye NM, Mirembe FM. Genital infections and risk of premature rupture of membranes in Mulago Hospital, Uganda: a case control study. BMC Research Notes 2015;8:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eleje GU, Adinma JI, Ugwuanyi DC, Ikechebelu JI. Genital tract microbial isolate in women with preterm pre-labour rupture of membranes in resource-constrained community setting. J Obstet Gynecol 2014;35:465–8. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tatzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta Analyses: the PRISMA statement. PLos Med 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283(15):2008–12. [DOI] [PubMed] [Google Scholar]
- 30.WHO: recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14, 1976. Acta Obstet Gynecol Scand 1977;56:247–53. [PubMed] [Google Scholar]
- 31.Middleton P, Shepherd E, Flenady V, McBain RD, Crowther CA. Planned early birth versus expectant management (waiting) for prelabour rupture of membranes at term (37 weeks or more). Cochrane Database Syst Rev 2017;1(1):CD005302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saari TN. Immunization of preterm and low birth weight infants. American Academy of Pediatrics Committee on Infectious Diseases. Pediatrics 2003;112(1 Pt 1):193–8. [DOI] [PubMed] [Google Scholar]
- 33.Schlaudecker EP, Munoz FM, Bardají A, Boghossian NS, Khalil A, Mousa H et al. Small for gestational age: Case definition & guidelines for data collection, analysis, and presentation of maternal immunisation safety data. Vaccine 2017;35(48 Pt A):6518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stang A Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- 35.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 36.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 37.Cummings P The relative merits of risk ratios and odds ratios. Arch Pediatr Adolesc Med 2009;163(5):438–45. [DOI] [PubMed] [Google Scholar]
- 38.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2006;11(2):193–206. [DOI] [PubMed] [Google Scholar]
- 39.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 40.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 42.StataCorp. 2011. Stata Statistical Software: Release 12. College Station TSL. [Google Scholar]
- 43.Johnson HL, Ghanem KG, Zenilman JM, Erbelding EJ. Sexually transmitted infections and adverse pregnancy outcomes among women attending inner city public sexually transmitted diseases clinics. Sex Transm Dis 2011;38(3):167–71. [DOI] [PubMed] [Google Scholar]
- 44.Azargoon A, Darvishzadeh S. Association of Bacterial Vaginosis, Trichomonas vaginalis, and vaginal acidity with outcome of pregancny. Arch Iran Med 2006;9(3):213–7. [PubMed] [Google Scholar]
- 45.Buchmayer S, Sparen P, Cnattingius S. Signs of infection in Pap smears and risk of adverse pregnancy outcome. Paediatr Perinat Epidemiol 2003;17(4):340–6. [DOI] [PubMed] [Google Scholar]
- 46.Stratton P, Tuomala RE, Abboud R, Rodriguez E, Rich K, Pitt J, et al. Obstetric and newborn outcomes in a cohort of HIV-infected pregnant women: a report of the women and infants transmission study. J Acquir Immune Defic Syndr Hum Retrovirol 1999;20(2):179–86. [DOI] [PubMed] [Google Scholar]
- 47.Mathai E, Muthaiah A, Mathai M, Jasper P. Prevalence and effects of trichomoniasis in pregnancy. Natl Med J India 1998;11(3):151. [PubMed] [Google Scholar]
- 48.Meis PJ, Goldenberg RL, Mercer B, Moawad A, Das A, McNellis D, et al. The preterm prediction study: significance of vaginal infections. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 1995;173(4):1231–5. [DOI] [PubMed] [Google Scholar]
- 49.McGregor JA, French JI, Parker R, Draper D, Patterson E, Jones W, et al. Prevention of premature birth by screening and treatment for common genital tract infections: results of a prospective controlled evaluation. Am J Obstet Gynecol 1995;173(1):157–67. [DOI] [PubMed] [Google Scholar]
- 50.Grice A Vaginal Infection Causing Spontaneous Rupture of the Membranes and Premature Delivery. Aust N Z J Obstet Gynaecol 1974;14:156–8. [Google Scholar]
- 51.Hu CY, Li FL, Hua XG, Jiang W, Zhang XJ. Longitudinal trajectory of vulvovaginal candidiasis, trichomoniasis, and bacterial vaginosis during pregnancy as well as the impact on pregnancy outcomes: a preliminary study. J Matern Fetal Neonatal Med 2019;32:3612–7. [DOI] [PubMed] [Google Scholar]
- 52.Lokken EM, Mandaliya K, Srinivasan S, Richardson BA, Kinuthia J, Lannon S, et al. Impact of preconception vaginal microbiota on women’s risk of spontaneous preterm birth: protocol for a prospective case-cohort study. BMJ Open 2020;10(2):e035186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olson-Chen C, Balaram K, Hackney DN. Chlamydia trachomatis and Adverse Pregnancy Outcomes: Meta-analysis of Patients With and Without Infection. Matern Child Health J 2018;22(6):812–21. [DOI] [PubMed] [Google Scholar]
- 54.Vallely LM, Egli-Gany D, Pomat W, Se Homer C, Guy R, Wand H, et al. Adverse pregnancy and neonatal outcomes associated with Neisseria gonorrhoeae, Mycoplasma genitalium, M. hominis, Ureaplasma urealyticum and U. parvum: a systematic review and meta-analysis protocol. BMJ Open 2018;8(11):e024175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker G Interventions for Trichomoniasis in Pregnancy: RHL Commentary Geneva, Switzerland: The WHO Reproductive Health Library; 2004. [Google Scholar]
- 56.Simhan HN, Anderson BL, Krohn MA, Heine RP, Martinez de Tejada B, Landers DV, et al. Host immune consequences of asymptomatic Trichomonas vaginalis infection in pregnancy. Am J Obstet Gynecol 2007;196(1):59.e1–.e5. [DOI] [PubMed] [Google Scholar]
- 57.Mielczarek E, Blaszkowska J. Trichomonas vaginalis: pathogenicity and potential role in human reproductive failure. Infection 2016;44(4):447–58. [DOI] [PubMed] [Google Scholar]
- 58.Balu RB, Savitz DA, Ananth CV, Hartmann KE, et al. Bacterial vaginosis, vaginal fluid neutrophil defensins, and preterm birth. Obstet Gynecol 2003;101(5 Pt 1):862–8. [DOI] [PubMed] [Google Scholar]
- 59.Fichorova RN. Impact of T. vaginalis infection on innate immune responses and reproductive outcome. J Reprod Immunol 2009;83(1–2):185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka Y, Narahara H, Takai N, Yoshimatsu J, Anai T, Miyakawa I. Interleukin-1β and interleukin-8 in cervicovaginal fluid during pregnancy. Am J Obstet Gynecol 1998;179(3 Pt 1):644–9. [DOI] [PubMed] [Google Scholar]
- 61.Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mann JR, McDermott S, Zhou L, Barnes TL, Hardin J. Treatment of trichomoniasis in pregnancy and preterm birth: an observational study. J Womens Health (Larchmt) 2009;18:493–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


