Abstract
The Balloon Analog Risk Task (BART) is increasingly used to assess risk‐taking behavior and brain function. However, the brain networks underlying risk‐taking during the BART and its reliability remain controversial. Here, we combined the activation likelihood estimation (ALE) meta‐analysis with both task‐based and task‐free functional connectivity (FC) analysis to quantitatively synthesize brain networks involved in risk‐taking during the BART, and compared the differences between adults and adolescents studies. Based on 22 pooled publications, the ALE meta‐analysis revealed multiple brain regions in the reward network, salience network, and executive control network underlying risk‐taking during the BART. Compared with adult risk‐taking, adolescent risk‐taking showed greater activation in the insula, putamen, and prefrontal regions. The combination of meta‐analytic connectivity modeling with task‐free FC analysis further confirmed the involvement of the reward, salience, and cognitive control networks in the BART. These findings demonstrate the core brain networks for risk‐taking during the BART and support the utility of the BART for future neuroimaging and developmental research.
Keywords: activation likelihood estimation, age difference, balloon analog risk task, functional connectivity, risk‐taking
A comprehensive meta‐analysis on neuroimaging studies measured by Balloon Analogue Risk Task (BART) was conducted. Adult and adolescent risk‐taking behaviors measured by BART are rooted in distinct brain regions. The core brain networks for risk‐taking during the BART were explored by combining MACM and RSFC. We present the utility of the BART for future neuroimaging and developmental research.
1. INTRODUCTION
The Balloon Analog Risk Task (BART), originally developed by Lejuez et al. (2002), is one of the most widely used paradigms for assessing risk‐taking propensity and behavior. In the BART, Each pumping can either inflate the balloon and increase the monetary reward or lead the balloon to explode and lose all of the monetary rewards for this trial. The average number of pumps for the balloons provides an objective assessment of participants' risk‐taking propensity. The larger number of pumps participants made for each balloon, the greater risk level participants are willing to take. Due to its high ecological validity, the BART was widely used to explore the real‐life risky behavior (Aklin et al., 2005; Lejuez et al., 2002; Lejuez, Aklin, Jones, et al., 2003; Lejuez, Aklin, Zvolensky, et al., 2003; Lejuez et al., 2007; MacPherson et al., 2010), personalities (Mishra & Novakowski, 2016; Parkinson et al., 2012), psychophysiological processes and disorders (Lei et al., 2017). A recent review found that, compared with the Iowa gambling task (IGT), delay discounting task, and other decision tasks, the BART is the most sensitive task to detect alcohol users' risk‐taking behavior (Harmon et al., 2021).
Many previous studies have adopted the BART to explore the neural basis and brain networks of risk‐taking behavior, especially adolescent risk‐taking behavior (e.g., Chiu et al., 2012; Claus & Hutchison, 2012; Hoffmann et al., 2018; McCormick & Telzer, 2017a, 2017b; Pei et al., 2020; Qu, Fuligni, et al., 2015; Telzer et al., 2013a, 2013b). A pioneering work from Rao et al. (2008) combined the BART with functional Magnetic Resonance Imaging (fMRI) and reported that risk‐taking in the BART recruited multiple regions in the mesolimbic‐frontal network. However, neuroimaging research evaluating test–retest reliability of the BART yielded inconsistent results. One study demonstrated that the activation patterns related to the BART showed moderate to high reliability (Li et al., 2020), while another found that the test–retest reliability across different brain regions were not good enough (ICCs of 0–0.8) (Korucuoglu et al., 2020), especially in the insula and anterior cingular cortex (ACC). The BART is also a pioneering behavioral paradigm to identify adolescent risk‐taking behavior (Lejuez, Aklin, Jones, et al., 2003; Lejuez, Aklin, Zvolensky, et al., 2003; Banducci et al., 2015; Braams et al., 2015). Some studies demonstrated that adolescents had higher risk preference than adults (Blankenstein & van Duijvenvoorde, 2019; Van Den Bos & Hertwig, 2017), while some other studies found that risk tolerances were similar between adolescents and adults (Blankenstein et al., 2016). Canning et al. (2021) attributed these debates to differences in study paradigms, which may measure different psychological processes (Bishara et al., 2009). To reevaluate the issues mentioned above, more comprehensive meta‐analyzes research is needed to explore the neural basis and neurodevelopmental differences of risk‐taking during the BART. Here, we conducted a quantitative meta‐analysis using the activation likelihood estimation (ALE) technique to obtain unbiased, objective, and statistically based results (Eickhoff et al., 2012). The ALE meta‐analysis is also able to yield specific coordinates of reported foci that can filter out mislabeling of regions in primary studies (Fusar‐Poli et al., 2011).
The BART demands little learning effort from participants, therefore serves as a better paradigm to resolve debates on neural accounts for risk‐taking differences between adults and adolescents than other tasks (e.g., IGT) (Braams et al., 2015). The neurodevelopmental imbalance model posits that the hyperresponsiveness of the reward system overrides the pubertal‐maturational cognitive control system to ultimately increase adolescent risk‐taking (Casey et al., 2008). By contrast, a new model named the life‐span Wisdom Model holds an alternative view, proposing that the cognitive control system and reward system rise in tandem during adolescent risk‐taking. Accordingly, adolescent risk‐taking behavior could be considered as a form of adaptive exploratory behavior (Romer et al., 2017). The present meta‐study will further conduct a contrast analysis between adult and adolescent groups to ascertain the neural basis underlying their different risk‐taking behavior.
Moreover, the neural networks underlying risk‐taking need to be validated by large‐scale functional connectivity (FC) analyzes. Using synchronized spontaneous signal fluctuation to capture task‐free FC, the resting‐state functional connectivity (RSFC) networks have been shown to play a pivotal role in a range of cognitive and brain functions (Barkhof et al., 2014; Filippini et al., 2012; Smith et al., 2009). The RSFC has also been used to study brain networks related to the BART risk‐taking behavior (Hobkirk et al., 2019; Huo et al., 2020). For example, Hobkirk et al. (2019) found that risk‐taking behavior during the BART was associated with cognitive control network and reward network coupling. Another study demonstrated that the RSFC between the hippocampus cortex and insula, which are the portion of the emotional and motivational control network, was related to the BART active pumps (Huo et al., 2020). However, the results of RSFC may be blunted by its uncontrolled nature. In recent decades, a popular way to implement FC is a data‐driven approach called “meta‐analytic connectivity modeling” (MACM), which was used to robustly determine the connectivity pattern of a given region of interest based on an extensive data set (Eickhoff et al., 2010, 2011). The task‐based MACM can investigate which brain regions have a consistent coactivation tendency across a diverse set of tasks with particular brain regions (Langner & Camilleri, 2021). Besides, a new tendency of FC is to combine RSFC and MACM to provide comprehensive information about the given functional network (Bellucci et al., 2018; Cieslik et al., 2016; Gu et al., 2019; Langner et al., 2018). Moreover, RSFC and MACM are well‐established methods. The common goal of these two techniques is to identify the brain networks interacting with the regions of interest (ROI). The combination of these two FC methods may promote the homogeneity of brain network results and further generalize those FC results (Hardwick et al., 2015). Therefore, the present study also aims to comprehensively explore the neural systems related to BART by combing MACM and RSFC.
To summarize, this study executed a meta‐analysis of fMRI studies using the coordinate‐based ALE technique to identify an unbiased neural substrate of the BART (Turkeltaub et al., 2002). Then, we conducted a contrast analysis between the adolescent group and the adult group to examine the neural substrates accounting for age differences in the BART. Furthermore, we implemented task‐based functional analysis‐MACM and task‐free functional analysis‐RSFC to explore their conjunction and reveal the connectivity patterns of risk‐taking brain regions.
2. MATERIALS AND METHODS
2.1. Primary ALE meta‐analysis
2.1.1. Literature search and selection
The primary risk‐taking meta‐analysis related to active pumps was conducted. The pertinent articles were restricted across systematic online databases, including ISI Web of Science (www.webofknowledge.com), PubMed (www.pubmed.com), ScienceDirect (www.sciencedirect.com). The search keywords included (“Balloon Analog Risk Task” OR “BART”) AND (“fMRI” OR “neuroimaging”). Furthermore, we searched other additional sources, including BrainMap Sleuth (www.brainmap.org); Neurosynth (www.neurosynth.org/); the reference list and citation indices of relevant articles and reviews (Korucuoglu et al., 2020; Li et al., 2020; Mohr et al., 2010; Wu et al., 2021). This comprehensive literature search was implemented on January 13, 2021 according to the PRISMA guidelines (Shamseer et al., 2015). These studies were further considered according to the following criteria: (i) the risk‐taking behavior was measured using the BART; (ii) fMRI was used as the imaging modality; (iii) the study applied whole‐brain analysis instead of the region of interest [ROI] analysis; (iv) the study was published in a peer‐reviewed journal; (v) the brain coordinates were reported in standardized stereotaxic space (Talairach or Montreal Neurological Institute, MNI). For the coordinates reported in Talairach, Brett's algorithm implemented in GingerALE software (version 3.0.2, https://www.brainmap.org/ale/) was used to convert them into MNI space serving to conduct ALE meta‐analysis. With the criteria mentioned above, 22 experiments with 1359 subjects (an average of 62 subjects per experiment) were identified as eligible for meta‐analysis of risk‐taking (Table 1 and Figure 1). It should be noted that the present study pooled the publications for both healthy and clinical populations for primary meta‐analysis under the declaration that at least 20 experiments into ALE analysis are sufficient for moderate effects (Eickhoff et al., 2016). Similarly, the present meta‐analysis included participants from broad age groups (from 8 to 64 years old). The coordinates in the within‐task contrast (e.g., active pumps vs. baseline) and between‐task contrast (e.g., active pumps vs. control task) were also included (for details see Table 1).
TABLE 1.
List of articles included in the risk‐taking meta‐analysis
| Study | Sample size | Age | Contrast | MNI/Talairach | No. of foci |
|---|---|---|---|---|---|
| Adults | |||||
| Claus & Hutchison (2012) | 79 | 21–54 | Mean pumps | MNI | 11 |
| Linear pumps | |||||
| Fukunaga et al. (2012) | 16 | 18–23 | ChooseInflate*P (explode) | MNI | 4 |
| Galván et al. (2013) | 43 | 17–21 | Pumps parametric | MNI | 19 |
| Average pumps | |||||
| Kohno et al. (2015) | 60 | 18–51 | Active pumps > baseline | MNI | 8 |
| Lei et al. (2017) | 37 | 23.1 ± 1.9 | Active pumps > baseline | MNI | 1 |
| Pan et al. (2019) | 35 | 19–40 | Active pumps > baseline | MNI | 9 |
| Qu et al. (2019) | 46 | M = 19.19 | Active pumps > baseline | MNI | 19 |
| M = 19.59 | |||||
| Rao et al. (2008) | 14 | 21–35 | Pumps parametric | MNI | 15 |
| Rao et al. (2010) | 18 | 44–64 | Pumps parametric | MNI | 22 |
| Rao et al. (2018) | 222 | 18–25 | Active pumps > baseline | MNI | 16 |
| Raymond et al. (2020) | 31 | 18–28 | Pumps > control pumps | MNI | 8 |
| Schonberg et al. (2012) | 16 | 21–26 | Pumps parametric | MNI | 12 |
| Average pumps | |||||
| Tisdall et al. (2020) | 116 | 20.4–30.1 | Pumps > control pumps | MNI | 23 |
| Adolescence | |||||
| Chiu et al. (2012) | 19 | 14.3–17 | Pumps > baseline | Talairach | 15 |
| Claus et al. (2018) | 198 | 14–18 | Mean pumps | MNI | 20 |
| Linear pumps | |||||
| Hoffmann et al. (2018) | 75 | 10–14 | Pumps > baseline | MNI | 3 |
| McCormick & Telzer (2017a) | 77 | 8.1–17.7 | Pumps > baseline | MNI | 9 |
| McCormick & Telzer (2017b) | 58 | 13–17 | Pumps after positive feedback | MNI | 34 |
| Pumps after negative feedback | |||||
| Pei et al. (2020) | 83 | 16–17 | Pumps parametric | MNI | 9 |
| Qu et al. (2015) | 22 | 15.4–18.4 | Pumps > baseline | MNI | 18 |
| Telzer et al. (2013a) | 48 | 14–16.5 | Pumps > control pumps | MNI | 13 |
| Telzer et al. (2013b) | 46 | 14–16 | Pumps > control pumps | MNI | 13 |
FIGURE 1.
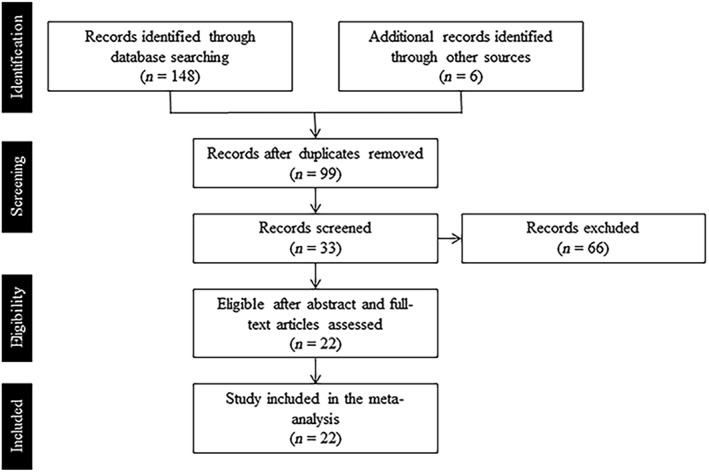
The flow chart of the article related to the BART selection process of the meta‐analysis
2.1.2. ALE analysis
To determine the brain regions across the remained studies, a coordinate‐based meta‐analysis was conducted using the ALE algorithm implemented in MATLAB 2016b (Eickhoff et al., 2009, 2012). This algorithm converses the foci reported in different functional or structural neuroimaging studies with activating foci in standardized space (Turkeltaub et al., 2002). The widths of spatial probability distribution interpreted by foci were based on empirical estimates of spatial uncertainty based on between‐template and between‐subject variability of the functional or structural neuroimaging data (Eickhoff et al., 2009). The ALE algorithm weighted the between‐subject variability based on the sample sizes of studies. The ALE algorithm modeled smaller Gaussian distributions and presupposed a more reliable “true” activation for a larger number of subjects (Eickhoff et al., 2009).
With the maximum probability related to anyone's focus for each voxel, the individual modulated activation maps were created. And then, the ALE map was obtained by calculating the modulated activation maps (Turkeltaub et al., 2012). ALE map could be estimated as a null distribution of random spatial association between included studies by using a nonlinear histogram integration algorithm (Eickhoff et al., 2009). The threshold at a cluster‐level family wise error (FWE) correction at p < .05 with a cluster defining threshold of p < .001 and 10,000 permutations was used to assess the significant P map (Eickhoff et al., 2009; Eklund et al., 2016). In order to get unbiased results, clusters were defined as significant only if they met the criterion: (i) at least two studies contribute same clusters; (ii) the contribution of the most dominant experiment (MDE) on a significant cluster is <50%, and the 2MDEs are <80% (Eickhoff et al., 2016; Bellucci et al., 2018). To obtain experimental contributions, the fraction of the ALE value was computed. The average nonlinear contribution of each experiment to the ALE value can be obtained by computing the ALE value's ratio at the location of the cluster with and without the experiment included (Eickhoff, Laird, et al., 2016).
2.2. Validation analysis
To check the validation of primary active pumps' ALE results, we conducted an additional analysis. Specifically, to confirm that a single experiment did not drive the primary meta‐analysis results, we performed a leave‐one‐experiment‐out (LOEO) analysis. On each fold, we dropped one experiment and conducted the ALE meta‐analysis based on the remaining N‐1 experiments. All ALE maps were also thresholded as primary meta‐analysis using a cluster‐level FWE corrected p < .05 with a cluster‐forming threshold of p < .001 for correcting multiple comparisons.
2.3. Contrast and conjunction analyzes between adults and adolescents
We further carried out contrast and conjunction analyzes to examine the distinct and common neural substrates of risk‐taking behavior between adults and adolescents. The studies in which the participants' ages were older than 18 years old were included as adults whereas the studies in which the participants' ages were younger than 18 years old were included as adolescents (Jones et al., 2003). Based on the age range of participants, the total 22 experiments enrolled in the primary meta‐analysis were divided into the adult group (13 experiments) and the adolescent group (9 experiments). The voxel‐wise difference between adult and adolescent ALE maps was calculated. Then, the difference between each voxel's ALE values was compared. A null distribution of difference ALE value was calculated by repeating 25,000 times of this process. The ALE maps were thresholded posterior probability set at p > 95%. For the conjunction analysis, all the ALE maps were thresholded, and the conjunction map was obtained by simply identifying the intersection between the adult and adolescent ALE results (Eickhoff et al., 2011; Wu et al., 2021).
2.4. Task‐based meta‐analytic connectivity mapping
To further explore the functional role of the brain regions obtained from the primary ALE meta‐analysis results, we conducted MACM analyzes based on the BrainMap database (http://www.brainmap.org/). This new method produces data‐driven FC maps on seed regions, which were defined around the peak coordinates using a 10‐mm radius (Bellucci et al., 2018). For our analysis, studies investigating age difference, experimental design, handedness, and disease were excluded while the focus of ROIs reported by experiments was included. Then, the whole‐brain peak coordinates of these experiments were downloaded for the following ALE meta‐analyses. The independent MACM analyzes were implemented with ACC, left caudate, right putamen, left insula, right insula, right dlPFC as ROIs. The method of the thresholded ALE maps was the same as the primary ALE meta‐analysis (FWE correction at p < .05 and with a cluster‐forming threshold of p < .001). And the corrected ALE maps were converted into Z‐scores for display.
2.5. Task‐free connectivity: RSFC analyzes
To complement task‐based connectivity derived from MACM analyzes, we defined the brain regions obtained from the primary meta‐analysis as seed regions and conducted task‐free connectivity assessed with whole‐brain RSFC. We recruited 74 healthy adults (age range: 21–50 years, 44 male) to obtain the resting‐state fMRI images. All participants had no history of mental illness and were all right‐handed. This study was approved by the Institutional Review Board of the University of Pennsylvania. All participants provided written informed consent, which was in accordance with the Declaration of Helsinki. And participants were compensated for their participation. The seed‐based FC analyzes were conducted with the seed regions in primary ALE. The details depicting the progress of RSFC analysis were provided in the Supporting Information.
2.6. Identification of the neural network in the BART
The key aim of the present studies was to define an extended risk‐taking brain network method by the BART through identifying the neural connectivity with brain regions responsible for active pumps. We evaluated the robust brain regions in task‐based and task‐free FC with the ROIs following the workflow of previous studies (Camilleri et al., 2018). First, the task‐based and task‐free FC maps were obtained from the FC analyzes mentioned above for each seed. Then, we used the minimum statistic to implement conjunction analyzes across MACM and FC connectivity maps for each ROI (Nichols et al., 2005). The consensus FC maps were obtained. These conjunctional maps indicated that the brain regions in these maps were consistently interacting with each ROI across distinct brain states (Hardwick et al., 2015). Then, the extended active pumps network was delineated by the significant overlap between consensus connectivity maps, which needed to show statistically significant MACM and FC connectivity with more than half of the seeds. Additional extended thresholds of 20 voxels and a 5 mm connectivity criterion were implemented to exclude smaller areas of presumably spurious overlap (Camilleri et al., 2018). All anatomical labelings in the current study were defined by the SPM Anatomy toolbox (www.fz-juelich.de/ime/spm_anatomy_toolbox, v.2.2b, Eickhoff et al., 2005, 2007, 2006). MRIcroGL (https://www.mccauslandcenter.sc.edu/mricrogl/home/) and BrainNetviewer (http://www.nitrc.org/projects/bnv/, Xia et al., 2013) were used for brain visualizations.
3. RESULTS
3.1. Primary meta‐analysis results
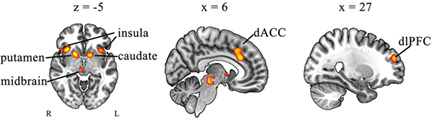
The primary ALE meta‐analysis of 22 included studies that showed seven brain regions of convergence. These significant clusters were located in ACC, bilateral insula, right putamen, left caudate, right dlPFC, and midbrain. The brain maps were displayed in Figure 2. Corresponding MNI coordinates of the significant clusters, MDE, and 2MDEs are provided in Table 2.
FIGURE 2.
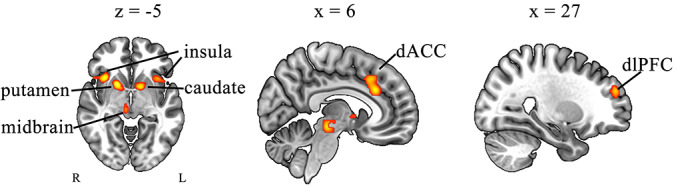
Significant clusters from the primary ALE meta‐analysis (FWE correction at p < .05, with a cluster‐forming threshold of p < .001) for active pumps
TABLE 2.
ALE meta‐analysis results of active pumps
| Brain regions | Anatomical location | BA | MNI coordinates (mm) | Z score | Cluster size (voxels) | Contribution experiments | MDE | 2MDE | ||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||||
| R insula | Insula cortex | 47 | 36 | 20 | 0 | 7.24 | 451 | 18 (81.82%) | 11.25% | 20.57% |
| dACC | Paracingulate gyrus | 24 | 4 | 24 | 34 | 5.44 | 404 | 17 (77.27%) | 10.59% | 20.06% |
| L insula | Insula cortex | 48 | −34 | 20 | 2 | 6.69 | 381 | 17 (77.27%) | 9.95% | 18.45% |
| R dlPFC | Frontal pole | 46 | 32 | 50 | 26 | 6.22 | 260 | 13 (59.09%) | 14.26% | 27.03% |
| R putamen | Right putamen | 48 | 18 | 8 | −4 | 6.23 | 209 | 12 (54.55%) | 12.78% | 25.35% |
| Midbrain | Brain stem | – | 6 | −22 | −12 | 5.30 | 150 | 10 (45.45%) | 16.6% | 31.73% |
| L caudate | Left caudate | 25 | −12 | 8 | −3 | 5.50 | 142 | 9 (40.91%) | 22.13% | 35.85% |
Abbreviations: ACC, anterior cingulate cortex; BA, Brodmann area; dlPFC, dorsolateral prefrontal cortex; L, left; R, right.Cluster‐level FWE correction (p < .05) with a cluster‐forming threshold of p < .001 using 10,000 permutations.
3.2. Validation results
Consistent with the primary ALE results, LOEO analysis also found that the brain regions of bilateral insula, bilateral dlPFC, left caudate, right putamen, and midbrain reached activation maxima (Figure 3). These results demonstrate that the LOEO approach could validate our primary findings.
FIGURE 3.
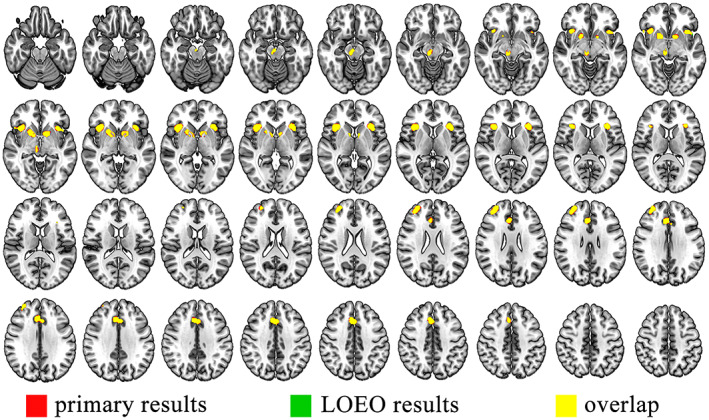
The results of primary active pumps and LOEO analysis (>80% folds)
3.3. Conjunction and contrast of age difference results
The respective ALE results of adults and adolescents were shown in Figure 4. The conjunction analysis found a common brain region activation in the bilateral insula. The contrast analysis demonstrated that the right thalamus/midbrain was more activated in the adult group compared to the adolescent group. And bilateral insula, bilateral putamen, right dlPFC, and left frontal lobe were more activated in the adolescent groups than in the adult group (Figure 4 and Table 3).
FIGURE 4.
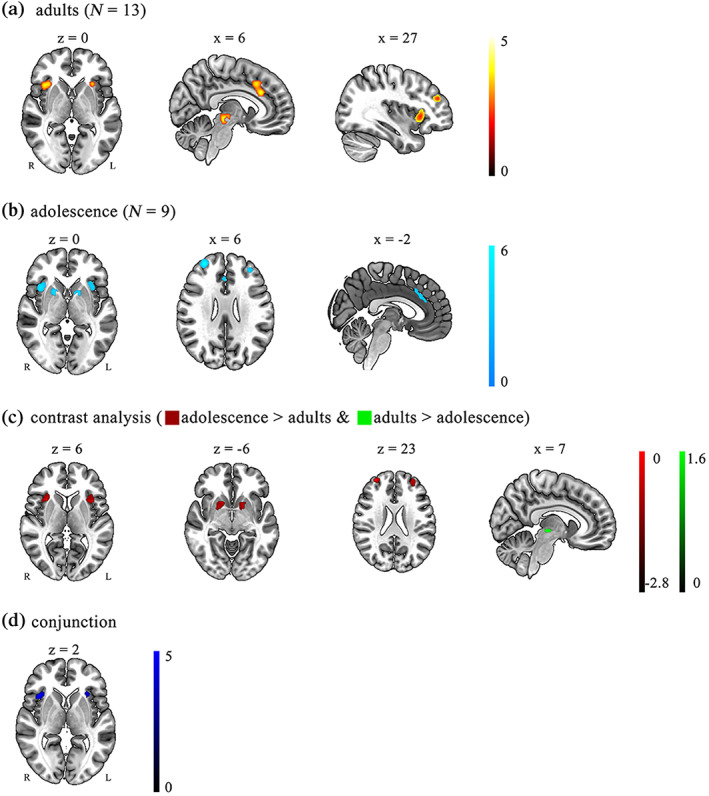
Significant clusters from the risk‐taking for adults group, adolescents groups, and conjunction and contrast between them. (a) Adult risk‐taking; (b) Adolescent risk‐taking; (c) The contrast of adults versus adolescents, red brain regions show higher activation in the adolescent group; green brain regions show higher activation in the adult group; (d) The conjunction of adults and adolescence. All the results were thresholded by FWE correction p < .05 (cluster‐forming threshold of p < .001 using 25,000 permutations)
TABLE 3.
ALE meta‐analysis results of risk‐taking behavior for adults, adolescence, the conjunction and contrast between them
| Cluster size (voxels) | Anatomical location | BA | MNI coordinates (mm) | Peak Z score | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Adults | |||||||
| 1 | 225 | R insula | 46 | 32 | 20 | −4 | 5.03 |
| 2 | 214 | Anterior cingulate gyrus | 32 | 6 | 28 | 28 | 5.00 |
| 3 | 146 | L insula | 47 | −30 | 22 | 2 | 4.46 |
| 4 | 121 | Brain‐stem | – | 6 | −24 | −8 | 5.13 |
| 5 | 92 | R Frontal pole | 10 | 36 | 44 | 26 | 4.70 |
| Adolescence | |||||||
| 1 | 273 | R insula | 13 | 32 | 20 | 6 | 6.11 |
| 2 | 226 | L insula | 13 | −34 | 20 | 6 | 5.87 |
| 3 | 188 | R frontal pole | 10 | 30 | 52 | 26 | 6.62 |
| 4 | 163 | R putamen | – | 18 | 10 | −4 | |
| 5 | 126 | Anterior cingulate gyrus | 24 | −2 | 20 | 38 | 4.87 |
| 6 | 125 | L frontal pole | 10 | −30 | 48 | 20 | |
| 7 | 120 | L putamen | – | −14 | 8 | −4 | |
| Conjunction of adults and adolescence | |||||||
| 1 | 120 | R insula | 13 | 38 | 20 | 2 | 4.95 |
| 2 | 52 | L insula | 13 | −30 | 22 | 2 | 4.26 |
| Adults > adolescence | |||||||
| 1 | 27 | R thalamus/midbrain | – | 6 | −22 | −4 | 1.80 |
| Adolescence > adults | |||||||
| 1 | 155 | R dlPFC | 9 | 28 | 58 | 26 | 3.30 |
| 2 | 120 | L insula | 13 | −38 | 14 | 4 | 3.20 |
| 3 | 112 | R putamen | – | 18 | 12 | −10 | 2.63 |
| 4 | 95 | R insula | 13 | 30 | 16 | 6 | 2.75 |
| 5 | 87 | L putamen | – | −18 | 6 | −4 | 2.66 |
| 6 | 85 | L frontal lobe | 10 | −28 | 48 | 24 | 2.81 |
3.4. FC analysis by MACM
MACM identified consistent task‐based coactivation with the seed regions of primary ALE results. A total of 588 experiments collected from 9051 unique subjects were identified for the ACC, 742 experiments consisting of 11,894 unique subjects were identified for left caudate, and 430 experiments with 6704 unique subjects were identified for right putamen, 524 experiments including 7940 unique subjects were identified for left insula, 752 experiments with 11,613 unique subjects were identified for right insula, and 147 experiments including 2471 unique subjects for right dlPFC. The respective FC pattern of MACM based on the seed regions of primary ALE results were shown in the supplemental materials (Figure S1 and Table S1). To identify the FC patterns corresponding to task fMRI, we used the conjunction analysis between these task‐based functional coupling maps. Coactivation maps for conjunction were significant for left caudate, cingulate gyrus, lateral occipital cortex, supramarginal gyrus, left cerebellum, and planum temporale (Figure 5b1).
FIGURE 5.
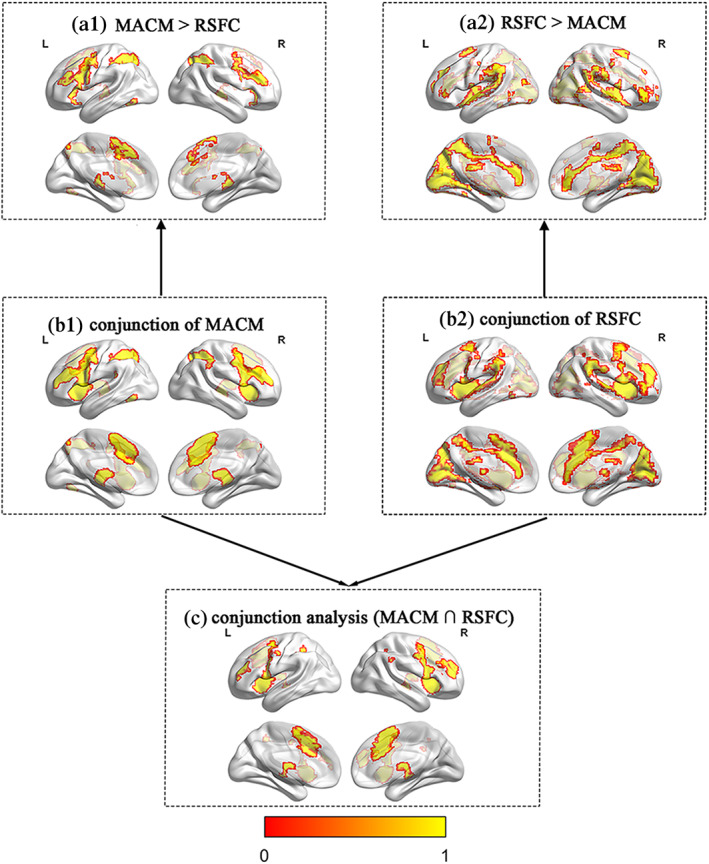
The results of the functional network. (a1) The FC specific for the MACM and for (a2) RSFC. (b1) The conjunction analysis specific for MACM and for (b2) RSFC. (c) The conjunction analysis across MACM and RSFC
3.5. FC analysis by resting‐state functional analysis
The RSFC analyzes revealed the brain regions whose time courses of BOLD signals were associated with the seed regions. The respective FC patterns of RSFC based on ROIs defined by primary ALE results were shown in the supplement (see Figure S2). We also performed a conjunction analysis based on the obtained task‐free functional maps to detect the resting‐state functional coupling. Results demonstrated that the brain patterns coactive with the brain regions of the BART were left thalamus, bilateral cerebellum, paracingulate gyrus, frontal orbital cortex, lateral occipital cortex, as well as posterior cingulate gyrus (Figure 5b2).
3.6. Conjunction across MACM and RSFC analyzes
The present study further conducted a conjunction analysis to probe the common regions identified by the different FC analyzes. Conjunction across MACM and RSFC analysis indicated a shared network comprising frontal orbital cortex, right insula, anterior cingulate gyrus, left frontal pole, right frontal pole, precentral gyrus, left superior parietal lobule, left cerebellum, right supramarginal gyrus, left frontal orbital cortex, and right posterior supramarginal gyrus (Figure 5c).
3.7. Difference between MACM and RSFC analyzes
The contrast of “MACM > RSFC” was shown in Figure 5a1. Results demonstrated that the brain regions showing significantly stronger connectivity in task‐based FC related to the BART included left insula, frontal orbital cortex, right frontal pole, left lateral occipital cortex, right supramarginal gyrus, brain stem, paracingulate gyrus, right frontal orbital cortex/right insula, left cerebellum, and right temporal pole.
The contrast of “RSFC > MACM” illustrated areas featuring stronger task‐free FC related to risk‐taking (Figure 5a2). This pattern was significant in the brain regions of right insula cortex, left hippocampus, left frontal orbital cortex, right frontal pole, left lateral occipital cortex, posterior cingulate gyrus, postcentral gyrus, right fontal pole, right supramarginal gyrus, left lateral occipital cortex, right inferior temporal gyrus, left putamen, left parahippocampal gyrus, left parahippocampal, and bilateral cerebellum.
4. DISCUSSION
The present study quantitatively pooled the existing neuroimaging studies to investigate the critical brain regions involved in the BART. The primary meta‐analysis results showed that the brain regions relevant to the BART were located in dACC, bilateral insula, right putamen, left caudate, right dlPFC, and midbrain. Further, the conjunction analysis of age difference observed that the bilateral insula was the common brain region underlying adult and adolescent risk‐taking. Contrast analysis suggested that the right thalamus/midbrain had more activations in the adult rather than the adolescent group. The brain regions that showed more activation in the adolescent group were bilateral putamen, right dlPFC, left frontal lobe, and bilateral insula. The coactive pattern of MACM and RSFC demonstrated that the BART‐related regions were connected to brain networks involved in reward, salience, and frontal–parietal control network.
4.1. The brain regions involved in the BART
The results of the primary meta‐analysis showed that the brain regions related to active pumps in the BART were bilateral insula, right putamen, left caudate, right dlPFC, dACC, and midbrain. The insula and ACC are the key nodes of the salience network, which play roles in switching and assorting between the default mode network and executive control network (Menon & Uddin, 2010). Right dlPFC is a typical part of the executive control network (Xiong et al., 2021). One study found that when participants' brain regions of dlPFC were disrupted by repetitive transcranial magnetic stimulation, they displayed more risky behavior (Knoch et al., 2006). The executive network integrates information from the salience network to suppress risky decisions (Menon & Uddin, 2010). The putamen and the caudate comprise the striatum (Colich et al., 2017). The striatum and the midbrain are the parts of the brain reward system that play a vital role in reward‐seeking and have been confirmed to influence risk‐taking and impulsive behavior (Parr et al., 2021). The brain regions in the reward system marked an important role in reward expectation and evaluation (Hinvest et al., 2011), pursuing a large potential payoff (Engelmann & Tamir, 2009; Bickel et al., 2012), and tracking decision risk (Suzuki et al., 2016). The dopaminergic projections from the reward system can be released to the salience network (Preuschoff et al., 2008). Previous studies found that the salience network and reward network induced in the BART were correlated with increased risk‐taking behavior (Wagels et al., 2017; Wei et al., 2016). Both the primary ALE and the LOEO validation analysis demonstrated that the risk‐taking measured during the BART robustly recruited the salience, the reward, and the executive control networks. These results are reconciled with previous meta‐analysis studies. Krain et al. (2006) delineated that risk‐decision making engaged brain regions of ACC, orbitofrontal cortex, lateral frontal, caudate, and thalamus. Mohr et al. (2010) implicated insula, thalamus, dmPFC, dlPFC, and parietal cortex in risky decision‐making tasks. Recent meta‐studies also indicated that the reward processing systems participated in risk‐decision making (Poudel et al., 2020; Wu et al., 2021).
4.2. The conjunction and contrast results of the age difference
The present study further investigated common and different neural basis between adult and adolescent risk‐taking behavior. The conjunction analysis observed bilateral insula as the common brain region underlying adult and adolescent risk‐taking behavior measured by the BART. The insula played an important role in monitoring sensory information and inputs to executive control brain regions (Menon & Uddin, 2010). This result indicated that risk‐taking behavior of both adults and adolescents needs to be reconciled by insula.
The contrast analysis revealed that the right thalamus/midbrain showed more activation in the adult group than in the adolescent group. This result was consistent with the existing neuroimaging studies involving age differences. A PET using 6‐[(18)F]FluoroDOPA found a higher midbrain‐lPFC interaction of dopamine synthesis in young adults than in elderly adults (Dreher et al., 2008). According to this PET evidence, the higher midbrain activation in adults versus adolescents is presumably due to the immature coupling in the adolescent brain. This conjecture should be addressed by further studies.
Compared with adults, adolescents exhibited more activations in brain regions of the reward network (bilateral putamen, right thalamus), the cognitive control network (right dlPFC, left frontal lobe), and the salience network (bilateral insula). These results were at odds with the neurodevelopmental imbalance models, which attributed the adolescent risk‐seeking to the consequence of the hyper response of the reward system overriding the slowly developing cognitive control system (Casey et al., 2008). Contradictory to the neurodevelopmental imbalance models, a meta‐study exploring the age difference did not observe risk‐taking differences between early adolescents (11–13 years) and children (5–10 years). A recent study further challenged this model, showing that multivariate brain activity metrics of dlPFC can predict adolescent risk decision tendencies (Moreira et al., 2021). Unlike the neurodevelopment imbalance models, these results demonstrated that adolescent dlPFC did not crucially have fewer effects on risk‐taking behavior than their reward systems. By contrast, our result echoed a new theory named the life span wisdom model, which considers adolescent risk‐taking behavior as a form of adaptive exploratory behavior. According to this model, the cognitive control system and the reward system rise in tandem during adolescent risk‐taking rather than in an imbalanced way (Romer et al., 2017). The results and evidence mentioned above suggest that adolescent risk‐taking behavior induces more cognitive control and reward network than adult risk‐taking. These results related to the age difference facilitate us to clarify some of the proximal mechanisms underlying developmental risk‐taking behavior.
4.3. The brain network of conjunctional FC
We further explored the brain networks that denote brain regions featuring convergent FC with the regions related to risk‐taking during the BART in the task‐based and task‐free state. As the widely used method for conventional FC analysis, the RSFC has uncovered the consensus connectivity networks related to various cognitive processes (Smith et al., 2009; Mennes et al., 2013; Zhang & Raichle, 2010). The recently emerging method of MACM provides a distinct approach to conceiving and quantifying FC throughout various experimental tasks (Eickhoff et al., 2011). These connectivity measures offer different but complementary ways to conceive and quantify inter‐neuronal communication between multiple brain regions. In the current study, we combined these two validated methods and uncovered the robust brain networks underlying the BART related risk taking behavior. The seeds obtained from the primary ALE results showed a coactivation pattern with bilateral fontal pole, bilateral superior parietal lobule, lateral middle frontal gyrus, right posterior supramarginal gyrus, frontal orbital cortex, and anterior cingulate gyrus. This coactive pattern demonstrated that risk‐taking measured during the BART was connected to brain networks involved in reward, salience, frontal–parietal cognitive control network. Our risk‐taking network results deviated slightly from the risk brain network obtained from previous studies. Wu et al. (2021) found that the brain networks related to risk‐processing were reward and salience processing. Our risk‐taking networks had some overlap with their risk‐seeking network. Furthermore, we also found that the risk‐taking measured during the BART recruited the frontal–parietal cognitive control network involved in ambiguous decision‐making. In this vein, the BART might be prone to engage in measuring both risk‐taking and ambiguous decisions.
4.4. BART may gauge both risk‐taking and ambiguous decision
Converging behavioral evidence suggests that the BART may have better reliability and prediction ability for real‐world risky behavior than other one‐shot decision‐making tasks. For instance, Lejuez, Aklin, Jones, et al. (2003) and Lejuez, Aklin, Zvolensky, et al. (2003) found that data from the BART risk‐taking in smokers have more predictive ability for smoking behavior than the data from the Bechara Gambling Task. It is also delineated that the risk‐taking behavior measured by the BART is more reproducible and stable than the Columbia Card Task (Frey et al., 2017). Moreover, although both the BART and the IGT tasks gauge decision‐making under ambiguity, the BART seems to be more sensitive to risky behavior than the IGT (Stout et al., 2005).
Despite the wide utility of the BART in risk‐taking research, whether the BART measures risk or ambiguity is still an ongoing debate. Some researchers believe that the BART cannot straightforwardly measure risk conditions but involve mainly decisions under ambiguity, owing to the unknown probability of exploring (Campbell et al., 2013; Fecteau et al., 2007). However, others think that the BART could capture both risky and ambiguous decisions (Canning et al., 2021; De Groot, 2020; De Groot & Thurik, 2018; Kóbor et al., 2015; Lighthall et al., 2009). The primary ALE results found that the BART recruited the right dlPFC, which plays a pivotal role in ambiguity. This brain region has been revealed to be involved in ambiguous decision‐making (Krain et al., 2006; Poudel et al., 2020; Wu et al., 2021). Blankenstein and van Duijvenvoorde (2019) compared adolescent risky and ambiguous decision‐making, showing that dlPFC tracked the individual differences in the subjective value of an ambiguous decision. The new causal evidence delineated that the anodal stimulation on dlPFC could enhance ambiguity preference but not risky decision‐making (Xiong et al., 2021). Moreover, this result did not resonate with previous meta‐analysis results pertaining to risk decision‐making. Krain et al. (2006) advocated different neural basis of risk and ambiguity for the first time by exploiting the meta‐analysis method and found that ambiguous decision was more dependent on activity in dlPFC. Then, Poudel et al. (2020) conducted a meta‐analysis on the neural basis of risk decision making, ambiguous decision making, and perceptual decision making. Results revealed that risk decision‐making recruited the striatum and ACC, while ambiguous decision‐making induced more activity in the lateral prefrontal cortex and insula than perceptual decision‐making. A more recent study by Wu et al. (2021) made a quantitative comparison between different types of risk and ambiguity based tasks and found that ambiguity specifically engaged dlPFC, inferior parietal lobe, and insula. These results consistently corroborated that dlPFC plays a specific role in ambiguous decision‐making. Therefore, the primary ALE results of the involvement of dlPFC and other brain regions related to risk‐taking suggested that the BART presumably measured both risk and ambiguity. However, the BART is a popular instrument for capturing individual differences for risk‐taking (De Groot, 2020). Some brain regions have been corroborated to be associated with individual differences in risk‐taking behavior (Blankenstein et al., 2018). In this respect, it is noteworthy that individual differences cannot be eliminated from the inconsistent findings mentioned above.
4.5. Limitations and future directions
The present study has several contributions. Unlike the existing meta‐analysis studies related to risk decision‐making (Krain et al., 2006; Mohr et al., 2010; Poudel et al., 2020; Wu et al., 2021), the present study specifically included studies using the BART paradigm, which could exactly explore the neural basis of risk‐taking behavior. The primary ALE analysis and LOEO analysis confirmed that the BART had a high validity in neuroimage studies. Furthermore, the age difference measured during the BART was examined. Then, the present study unraveled the neural systems of the BART by combining RSFC and MACM. It is noteworthy that we found some different results by pooling all articles together and removing the abnormal articles. Also, the left dlPFC showed activations after removing the abnormal groups. This difference might elaborate the BART's sensitivity to distinguish normal and abnormal groups.
Considering the sample size and the fact that the ALE method only incorporates the coordinates of activation, the shortcoming of this method lies in its inability to consider activation magnitude. Other meta‐analysis methods, such as seed‐based D Mapping (SDM) (Albrecht et al., 2019) and image‐based meta‐analysis (Salimi‐Khorshidi et al., 2009), might compensate for the shortcoming of the ALE method. However, some empirical and meta‐studies compared ALE and SDM and found no significant difference between these two methods (Albrecht et al., 2019; Feng et al., 2021). As for image‐based meta‐analysis, this method is based on the statistical parametric maps from the included studies, which are formidable to obtain (Oldham et al., 2018). Future imaging studies are encouraged to upload their statistical map onto neuroimaging databases to probe the aforementioned questions.
Another limitation is that the current meta‐analysis includes those articles that studied both normal and abnormal participants to satisfy the minimum study size required by the ALE method (Eklund et al., 2016). Results from the primary ALE including only the normal group (17 articles, Figure S3) yielded results similar to that including both groups. Moreover, the winning, the losing, and the cash‐out conditions were not investigated in the present analyzes due to the small number of studies about those condition. Future studies should give insight into this contrast to advance a neurobiological understanding of the BART. Moreover, it has been speculated that BART may confound risk and reward (Rao et al., 2008). Further studies are needed to dissociate the neural bases of risk and reward.
5. CONCLUSIONS
In conclusion, the present study systematically identified the brain regions and networks involved in the BART and dissociated the brain regions linked with adult and adolescent risk‐taking. Delineating the neural basis of BART and distinct brain activity related to adult and adolescent risk‐taking in BART may improve the utility of the BART in future neuroimaging and developmental research.
AUTHOR CONTRIBUTIONS
Hengyi Rao conceived the overall project and planned the framework of the study and drafted the original manuscript. Mengmeng Wang conceived the overall project and planned the framework of the study, searched and code the article, executed the data analysis and drafted the original manuscript. Shunmin Zhang searched and code the article, executed the data analysis, and drafted the original manuscript. Simon Eickhoff searched and code the article and executed the data analysis. Tao Suo searched and code the article and executed the data analysis. All other authors reviewed and edited the manuscript for important scientific content and final approval.
FUNDING INFORMATION
This work was supported in part by the National Natural Science Foundation of China (71942003, 71942005, 32100870, and 71772124); Ministry of Education of China (17YJA630097); Shanghai International Studies University Research Projects (2021114002, 2021114003, 2021KFKT012, and 20171140020); Shanghai Post‐doctoral Excellence Program (2020367); China Postdoctoral Science Foundation (2021M692150); the Humanities and Social Science Foundation of Higher Education Institutions in Henan Province, China (2021‐ZZJH‐056); the Science and Technology Innovation Talents Support Foundation of Higher Education Institutions in Henan Province, China (2021‐CX‐045); and the Zhongyuan Top Young Talents Support Foundation in Henan Province, China (K21046Y).
Supporting information
Appendix S1 Supporting Information
Wang, M. , Zhang, S. , Suo, T. , Mao, T. , Wang, F. , Deng, Y. , Eickhoff, S. , Pan, Y. , Jiang, C. , & Rao, H. (2022). Risk‐taking in the human brain: An activation likelihood estimation meta‐analysis of the balloon analog risk task (BART). Human Brain Mapping, 43(18), 5643–5657. 10.1002/hbm.26041
Mengmeng Wang and Shunmin Zhang contributed equally to this study.
Funding information China Postdoctoral Science Foundation, Grant/Award Number: 2021M692150; Ministry of Education of China, Grant/Award Number: 17YJA630097; National Natural Science Foundation of China, Grant/Award Numbers: 71942003, 71942005, 32100870, 71942005; Shanghai International Studies University Research Projects, Grant/Award Number: 20171140020; Shanghai Post‐doctoral Excellence Program, Grant/Award Number: 2020367; The Humanities and Social Science Foundation of Higher Education Institutions in Henan Province, Grant/Award Number: 2021‐ZZJH‐056; The Science and Technology Innovation Talents Support Foundation of Higher Education Institutions in Henan Province, Grant/Award Number: 2021‐CX‐045; Zhongyuan Top Young Talents Support Foundation in Henan Province, Grant/Award Number: K21046Y
Contributor Information
Yu Pan, Email: 13311887777@163.com.
Hengyi Rao, Email: hengyi@gmail.com.
DATA AVAILABILITY STATEMENT
The data and code used in the present study are available from the corresponding author upon request.
REFERENCES
- Aklin, W. M. , Lejuez, C. W. , Zvolensky, M. J. , Kahler, C. W. , & Gwadz, M. (2005). Evaluation of behavioral measures of risk taking propensity with inner city adolescents. Behaviour Research and Therapy, 43(2), 215–228. 10.1016/j.brat.2003.12.007 [DOI] [PubMed] [Google Scholar]
- Albrecht, F. , Bisenius, S. , Neumann, J. , Whitwell, J. , & Schroeter, M. L. (2019). Atrophy in midbrain & cerebral/cerebellar pedunculi is characteristic for progressive supranuclear palsy – A double‐validation whole‐brain meta‐analysis. NeuroImage: Clinical, 22, 101722. 10.1016/j.nicl.2019.101722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banducci, A. N. , Felton, J. W. , Dahne, J. , Ninnemann, A. , & Lejuez, C. W. (2015). Maternal risk taking on the balloon analogue risk task as a prospective predictor of youth alcohol use escalation. Addictive Behaviors, 49, 40–45. 10.1016/j.addbeh.2015.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhof, F. , Haller, S. , & Rombouts, S. A. (2014). Resting‐state functional MR imaging: a new window to the brain. Radiology, 272(1), 29–49. 10.1148/radiol.14132388 [DOI] [PubMed] [Google Scholar]
- Bellucci, G. , Feng, C. , Camilleri, J. , Eickhoff, S. B. , & Krueger, F. (2018). The role of the anterior insula in social norm compliance and enforcement: Evidence from coordinate‐based and functional connectivity meta‐analyses. Neuroscience and Biobehavioral Reviews, 92, 378–389. 10.1016/j.neubiorev.2018.06.024 [DOI] [PubMed] [Google Scholar]
- Bickel, W. K. , Jarmolowicz, D. P. , Mueller, E. T. , Gatchalian, K. M. , & McClure, S. M. (2012). Are executive function and impulsivity antipodes? A conceptual reconstruction with special reference to addiction. Psychopharmacology, 221(3), 361–387. 10.1007/s00213-012-2689-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishara, A. J. , Pleskac, T. J. , Fridberg, D. J. , Yechiam, E. , Lucas, J. , Busemeyer, J. R. , Finn, P. R. , & Stout, J. C. (2009). Similar processes despite divergent behavior in two commonly used measures of risky decision making. Journal of Behavioral Decision Making, 22(4), 435–454. 10.1002/bdm [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenstein, N. E. , & van Duijvenvoorde, A. C. K. (2019). Neural tracking of subjective value under risk and ambiguity in adolescence. Cognitive, Affective, & Behavioral Neuroscience, 19(6), 1364–1378. 10.3758/s13415-019-00749-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenstein, N. E. , Crone, E. A. , van den Bos, W. , & van Duijvenvoorde, A. C. K. (2016). Dealing with uncertainty: Testing risk‐ and ambiguity‐attitude across adolescence. Developmental Neuropsychology, 41(1–2), 77–92. 10.1080/87565641.2016.1158265 [DOI] [PubMed] [Google Scholar]
- Blankenstein, N. E. , Schreuders, E. , Peper, J. S. , Crone, E. A. , & van Duijvenvoorde, A. C. K. (2018). Individual differences in risk‐taking tendencies modulate the neural processing of risky and ambiguous decision‐making in adolescence. NeuroImage, 172, 663–673. 10.1016/j.neuroimage.2018.01.085 [DOI] [PubMed] [Google Scholar]
- Braams, B. R. , van Duijvenvoorde, A. C. K. , Peper, J. S. , & Crone, E. A. (2015). Longitudinal changes in adolescent risk‐taking: A comprehensive study of neural responses to rewards, pubertal development, and risk‐taking behavior. Journal of Neuroscience, 35(18), 7226–7238. 10.1523/JNEUROSCI.4764-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri, J. A. , Müller, V. I. , Fox, P. , Laird, A. R. , Hoffstaedter, F. , Kalenscher, T. , & Eickhoff, S. B. (2018). Definition and characterization of an extended multiple‐demand network. NeuroImage, 165, 138–147. 10.1016/j.neuroimage.2017.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, J. A. , Samartgis, J. R. , & Crowe, S. F. (2013). Impaired decision making on the balloon analogue risk task as a result of long‐term alcohol use. Journal of Clinical and Experimental Neuropsychology, 35(10), 1071–1081. 10.1080/13803395.2013.856382 [DOI] [PubMed] [Google Scholar]
- Canning, J. R. , Schallert, M. R. , & Larimer, M. E. (2021). A systematic review of the balloon analogue risk task (BART) in alcohol research. Alcohol and Alcoholism, 57, 85–103. 10.1093/alcalc/agab004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey, B. J. , Getz, S. , & Galvan, A. (2008). The adolescent brain. Developmental Review, 28(1), 62–77. 10.1016/j.dr.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu, C. Y. P. , Tlustos, S. J. , Walz, N. C. , Holland, S. K. , Eliassen, J. C. , Bernard, L. , & Wade, S. L. (2012). Neural correlates of risky decision making in adolescents with and without traumatic brain injury using the balloon analog risk task. Developmental Neuropsychology, 37(2), 176–183. 10.1080/87565641.2011.632796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik, E. C. , Seidler, I. , Laird, A. R. , Fox, P. T. , & Eickhoff, S. B. (2016). Different involvement of subregions within dorsal premotor and medial frontal cortex for pro‐ and antisaccades. Neuroscience and Biobehavioral Reviews, 68, 256–269. 10.1016/j.neubiorev.2016.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus, E. D. , & Hutchison, K. E. (2012). Neural mechanisms of risk taking and relationships with hazardous drinking. Alcoholism: Clinical and Experimental Research, 36(6), 932–940. 10.1111/j.1530-0277.2011.01694.x [DOI] [PubMed] [Google Scholar]
- Claus, E. D. , Feldstein Ewing, S. W. , Magnan, R. E. , Montanaro, E. , Hutchison, K. E. , & Bryan, A. D. (2018). Neural mechanisms of risky decision making in adolescents reporting frequent alcohol and/or marijuana use. Brain imaging and behavior, 12(2), 564–576. 10.1007/s11682-017-9723-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich, N. L. , Ho, T. C. , Ellwood‐Lowe, M. E. , Foland‐Ross, L. C. , Sacchet, M. D. , LeMoult, J. L. , & Gotlib, I. H. (2017). Like mother like daughter: Putamen activation as a mechanism underlying intergenerational risk for depression. Social Cognitive and Affective Neuroscience, 12(9), 1480–1489. 10.1093/scan/nsx073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot, K. (2020). Burst beliefs – Methodological problems in the balloon analogue risk task and implications for its use. Journal of Trial and Error, 1(1), 43–51. 10.36850/mr1 [DOI] [Google Scholar]
- De Groot, K. , & Thurik, R. (2018). Disentangling risk and uncertainty: When risk‐taking measures are not about risk. Frontiers in Psychology, 9, 1–7. 10.3389/fpsyg.2018.02194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher, J. C. , Meyer‐Lindenberg, A. , Kohn, P. , & Berman, K. F. (2008). Age‐related changes in midbrain dopaminergic regulation of the human reward system. Proceedings of the National Academy of Sciences of the United States of America, 105(39), 15106–15111. 10.1073/pnas.0802127105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Bzdok, D. , Laird, A. R. , Kurth, F. , & Fox, P. T. (2012). Activation likelihood estimation meta‐analysis revisited. NeuroImage, 59(3), 2349–2361. 10.1016/j.neuroimage.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Bzdok, D. , Laird, A. R. , Roski, C. , Caspers, S. , Zilles, K. , & Fox, P. T. (2011). Co‐activation patterns distinguish cortical modules, their connectivity and functional differentiation. NeuroImage, 57(3), 938–949. 10.1016/j.neuroimage.2011.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Heim, S. , Zilles, K. , & Amunts, K. (2006). Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. NeuroImage, 32(2), 570–582. 10.1016/j.neuroimage.2006.04.204 [DOI] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Jbabdi, S. , Caspers, S. , Laird, A. R. , Fox, P. T. , Zilles, K. , & Behrens, T. E. J. (2010). Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. Journal of Neuroscience, 30(18), 6409–6421. 10.1523/JNEUROSCI.5664-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Laird, A. R. , Fox, P. T. , Bzdok, D. , & Hensel, L. (2016). Functional segregation of the human dorsomedial prefrontal cortex. Cerebral Cortex, 26(1), 304–321. 10.1093/cercor/bhu250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Laird, A. R. , Grefkes, C. , Wang, L. E. , Zilles, K. , & Fox, P. T. (2009). Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30(9), 2907–2926. 10.1002/hbm.20718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Nichols, T. E. , Laird, A. R. , Hoffstaedter, F. , Amunts, K. , Fox, P. T. , Bzdok, D. , & Eickhoff, C. R. (2016). Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. NeuroImage, 137, 70–85. 10.1016/j.neuroimage.2016.04.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Paus, T. , Caspers, S. , Grosbras, M. H. , Evans, A. C. , Zilles, K. , & Amunts, K. (2007). Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage, 36(3), 511–521. 10.1016/j.neuroimage.2007.03.060 [DOI] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Stephan, K. E. , Mohlberg, H. , Grefkes, C. , Fink, G. R. , Amunts, K. , & Zilles, K. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage, 25(4), 1325–1335. 10.1016/j.neuroimage.2004.12.034 [DOI] [PubMed] [Google Scholar]
- Eklund, A. , Nichols, T. E. , & Knutsson, H. (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false‐positive rates. Proceedings of the National Academy of Sciences, 113(28), 7900–7905. 10.1073/pnas.1612033113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann, J. B. , & Tamir, D. (2009). Individual differences in risk preference predict neural responses during financial decision‐making. Brain Research, 1290, 28–51. 10.1016/j.brainres.2009.06.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau, S. , Pascual‐Leone, A. , Zald, D. H. , Liguori, P. , Théoret, H. , Boggio, P. S. , & Fregni, F. (2007). Activation of prefrontal cortex by transcranial direct current stimulation reduces appetite for risk during ambiguous decision making. Journal of Neuroscience, 27(23), 6212–6218. 10.1523/JNEUROSCI.0314-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, C. , Eickhoff, S. B. , Li, T. , Wang, L. , Becker, B. , Camilleri, J. A. , Hétu, S. , & Luo, Y. (2021). Common brain networks underlying human social interactions: Evidence from large‐scale neuroimaging meta‐analysis. Neuroscience and Biobehavioral Reviews, 126, 289–303. 10.1016/j.neubiorev.2021.03.025 [DOI] [PubMed] [Google Scholar]
- Filippini, N. , Nickerson, L. D. , Beckmann, C. F. , Ebmeier, K. P. , Frisoni, G. B. , Matthews, P. M. , Smith, S. M. , & Mackay, C. E. (2012). Age‐related adaptations of brain function during a memory task are also present at rest. NeuroImage, 59(4), 3821–3828. 10.1016/j.neuroimage.2011.11.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey, R. , Pedroni, A. , Mata, R. , Rieskamp, J. , & Hertwig, R. (2017). Risk preference shares the psychometric structure of major psychological traits. Science Advances, 3(10), 1–14. 10.1126/sciadv.1701381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga, R. , Brown, J. W. , & Bogg, T. (2012). Decision making in the balloon analogue risk task (BART): Anterior cingulate cortex signals loss aversion but not the infrequency of risky choices. Cognitive, Affective, & Behavioral Neuroscience, 12(3), 479–490. 10.3758/s13415-012-0102-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar‐Poli, P. , Borgwardt, S. , Crescini, A. , Deste, G. , Kempton, M. J. , Lawrie, S. , Mc Guire, P. , & Sacchetti, E. (2011). Neuroanatomy of vulnerability to psychosis: A voxel‐based meta‐analysis. Neuroscience and Biobehavioral Reviews, 35(5), 1175–1185. 10.1016/j.neubiorev.2010.12.005 [DOI] [PubMed] [Google Scholar]
- Galván, A. , Schonberg, T. , Mumford, J. , Kohno, M. , Poldrack, R. A. , & London, E. D. (2013). Greater risk sensitivity of dorsolateral prefrontal cortex in young smokers than in nonsmokers. Psychopharmacology, 229(2), 345–355. 10.1007/s00213-013-3113-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, R. , Huang, W. , Camilleri, J. , Xu, P. , Wei, P. , Eickhoff, S. B. , & Feng, C. (2019). Love is analogous to money in human brain: Coordinate‐based and functional connectivity meta‐analyses of social and monetary reward anticipation. Neuroscience and Biobehavioral Reviews, 100, 108–128. 10.1016/j.neubiorev.2019.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick, R. M. , Lesage, E. , Eickhoff, C. R. , Clos, M. , Fox, P. , & Eickhoff, S. B. (2015). Multimodal connectivity of motor learning‐related dorsal premotor cortex. NeuroImage, 123, 114–128. 10.1016/j.neuroimage.2015.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon, D. A. , Haas, A. L. , & Peterkin, A. (2021). Experimental tasks of behavioral risk taking in alcohol administration studies: A systematic review. Addictive Behaviors, 113, 106678. 10.1016/j.addbeh.2020.106678 [DOI] [PubMed] [Google Scholar]
- Hinvest, N. S. , Elliott, R. , McKie, S. , & Anderson, I. M. (2011). Neural correlates of choice behavior related to impulsivity and venturesomeness. Neuropsychologia, 49(9), 2311–2320. 10.1016/j.neuropsychologia.2011.02.023 [DOI] [PubMed] [Google Scholar]
- Hobkirk, A. L. , Bell, R. P. , Utevsky, A. V. , Huettel, S. , & Meade, C. S. (2019). Reward and executive control network resting‐state functional connectivity is associated with impulsivity during reward‐based decision making for cocaine users. Drug and Alcohol Dependence, 194, 32–39. 10.1016/j.drugalcdep.2018.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, F. , Puetz, V. B. , Viding, E. , Sethi, A. , Palmer, A. , & McCrory, E. J. (2018). Risk‐taking, peer‐influence and child maltreatment: A neurocognitive investigation. Social Cognitive and Affective Neuroscience, 13(1), 124–134. 10.1093/scan/nsx124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo, H. , Zhang, R. , Seger, C. A. , Feng, T. , & Chen, Q. (2020). The effect of trait anxiety on risk‐taking: Functional coupling between right hippocampus and left insula. Psychophysiology, 57(10), 1–10. 10.1111/psyp.13629 [DOI] [PubMed] [Google Scholar]
- Jones, J. S. , Rossman, L. , Wynn, B. N. , Dunnuck, C. , & Schwartz, N. (2003). Comparative analysis of adult versus adolescent sexual assault: Epidemiology and patterns of anogenital injury. Academic Emergency Medicine, 10(8), 872–877. 10.1197/aemj.10.8.872 [DOI] [PubMed] [Google Scholar]
- Knoch, D. , Gianotti, L. R. R. , Pascual‐Leone, A. , Treyer, V. , Regard, M. , Hohmann, M. , & Brugger, P. (2006). Disruption of right prefrontal cortex by low‐frequency repetitive transcranial magnetic stimulation induces risk‐taking behavior. Journal of Neuroscience, 26(24), 6469–6472. 10.1523/JNEUROSCI.0804-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno, M. , Ghahremani, D. G. , Morales, A. M. , Robertson, C. L. , Ishibashi, K. , Morgan, A. T. , Mandelkern, M. A. , & London, E. D. (2015). Risk‐taking behavior: Dopamine D2/D3 receptors, feedback, and frontolimbic activity. Cerebral Cortex, 25(1), 236–245. 10.1093/cercor/bht218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korucuoglu, O. , Harms, M. P. , Astafiev, S. V. , Kennedy, J. T. , Golosheykin, S. , Barch, D. M. , & Anokhin, A. P. (2020). Test‐retest reliability of fMRI‐measured brain activity during decision making under risk. NeuroImage, 214, 116759. 10.1016/j.neuroimage.2020.116759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krain, A. L. , Wilson, A. M. , Arbuckle, R. , Castellanos, F. X. , & Milhama, M. P. (2006). Distinct neural mechanisms of risk and ambiguity: A meta‐analysis of decision‐making. NeuroImage, 32(1), 477–484. 10.1016/j.neuroimage.2006.02.047 [DOI] [PubMed] [Google Scholar]
- Kóbor, A. , Takács, Á. , Janacsek, K. , Németh, D. , Honbolygó, F. , & Csépe, V. (2015). Different strategies underlying uncertain decision making: Higher executive performance is associated with enhanced feedback‐related negativity. Psychophysiology, 52(3), 367–377. 10.1111/psyp.12331 [DOI] [PubMed] [Google Scholar]
- Langner, R. , & Camilleri, J. A. (2021). Meta‐analytic connectivity modelling (MACM): A tool for assessing region‐specific functional connectivity patterns in task‐constrained states. In Diwadkar V. A. & Eickhoff S. B. (Eds.), Brain Network Dysfunction in Neuropsychiatric Illness (pp. 93–104). Springer. 10.1007/978-3-030-59797-9_5 [DOI] [Google Scholar]
- Langner, R. , Leiberg, S. , Hoffstaedter, F. , & Eickhoff, S. B. (2018). Towards a human self‐regulation system: Common and distinct neural signatures of emotional and behavioural control. Neuroscience and Biobehavioral Reviews, 90, 400–410. 10.1016/j.neubiorev.2018.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, Y. , Wang, L. , Chen, P. , Li, Y. , Han, W. , Ge, M. , Yang, L. , Chen, S. , Hu, W. , Wu, X. , & Yang, Z. (2017). Neural correlates of increased risk‐taking propensity in sleep‐deprived people along with a changing risk level. Brain Imaging and Behavior, 11(6), 1910–1921. 10.1007/s11682-016-9658-7 [DOI] [PubMed] [Google Scholar]
- Lejuez, C. W. , Aklin, W. M. , Jones, H. A. , Strong, D. R. , Richards, J. B. , Kahler, C. W. , & Read, J. P. (2003). The balloon analogue risk task (BART) differentiates smokers and nonsmokers. Experimental and Clinical Psychopharmacology, 11(1), 26–33. 10.1037/1064-1297.11.1.26 [DOI] [PubMed] [Google Scholar]
- Lejuez, C. W. , Aklin, W. M. , Zvolensky, M. J. , & Pedulla, C. M. (2003). Evaluation of the balloon analogue risk task (BART) as a predictor of adolescent real‐world risk‐taking behaviours. Journal of Adolescence, 26(4), 475–479. 10.1016/S0140-1971(03)00036-8 [DOI] [PubMed] [Google Scholar]
- Lejuez, C. W. , Aklin, W. , Daughters, S. , Zvolensky, M. , Kahler, C. , & Gwadz, M. (2007). Reliability and validity of the youth version of the balloon analogue risk task (BART‐Y) in the assessment of risk‐taking behavior among inner‐city adolescents. Journal of Clinical Child and Adolescent Psychology, 36(1), 106–111. 10.1080/15374410709336573 [DOI] [PubMed] [Google Scholar]
- Lejuez, C. W. , Richards, J. B. , Read, J. P. , Kahler, C. W. , Ramsey, S. E. , Stuart, G. L. , Strong, D. R. , & Brown, R. A. (2002). Evaluation of a behavioral measure of risk taking: The balloon analogue risk task (BART). Journal of Experimental Psychology: Applied, 8(2), 75–84. 10.1037/1076-898X.8.2.75 [DOI] [PubMed] [Google Scholar]
- Li, X. , Pan, Y. , Fang, Z. , Lei, H. , Zhang, X. , Shi, H. , Ma, N. , Raine, P. , Wetherill, R. , Kim, J. J. , Wan, Y. , & Rao, H. (2020). Test‐retest reliability of brain responses to risk‐taking during the balloon analogue risk task. NeuroImage, 209, 116495. 10.1016/j.neuroimage.2019.116495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthall, N. R. , Mather, M. , & Gorlick, M. A. (2009). Acute stress increases sex differences in risk seeking in the balloon analogue risk task. PLoS One, 4(7), e6002. 10.1371/journal.pone.0006002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson, L. , Magidson, J. F. , Reynolds, E. K. , Kahler, C. W. , & Lejuez, C. W. (2010). Changes in sensation seeking and risk‐taking propensity predict increases in alcohol use among early adolescents. Alcoholism: Clinical and Experimental Research, 34(8), 1400–1408. 10.1111/j.1530-0277.2010.01223.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick, E. M. , & Telzer, E. H. (2017a). Adaptive adolescent flexibility: Neurodevelopment of decision‐making and learning in a risky context. Journal of Cognitive Neuroscience, 29(3), 413–423. 10.1162/jocn [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick, E. M. , & Telzer, E. H. (2017b). Failure to retreat: Blunted sensitivity to negative feedback supports risky behavior in adolescents. NeuroImage, 147, 381–389. 10.1016/j.neuroimage.2016.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes, M. , Kelly, C. , Colcombe, S. , Castellanos, F. X. , & Milham, M. P. (2013). The extrinsic and intrinsic functional architectures of the human brain are not equivalent. Cerebral cortex, 23(1), 223–229. 10.1093/cercor/bhs010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, V. , & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214(5–6), 655–667. 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, S. , & Novakowski, D. (2016). Personal relative deprivation and risk: An examination of individual differences in personality, attitudes, and behavioral outcomes. Personality and Individual Differences, 90, 22–26. 10.1016/j.paid.2015.10.031 [DOI] [Google Scholar]
- Mohr, P. N. C. , Biele, G. , & Heekeren, H. R. (2010). Neural processing of risk. Journal of Neuroscience, 30(19), 6613–6619. 10.1523/JNEUROSCI.0003-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira, J. F. G. , Leal, A. S. M. , Waizman, Y. H. , Saragosa‐Harris, N. , Ninova, E. , & Silvers, J. A. (2021). Revisiting the neural architecture of adolescent decision‐making: Univariate and multivariate evidence for system‐based models. Journal of Neuroscience, 41(28), 6006–6017. 10.1523/JNEUROSCI.3182-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, T. , Brett, M. , Andersson, J. , Wager, T. , & Poline, J. B. (2005). Valid conjunction inference with the minimum statistic. NeuroImage, 25(3), 653–660. 10.1016/j.neuroimage.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Oldham, S. , Murawski, C. , Fornito, A. , Youssef, G. , Yücel, M. , & Lorenzetti, V. (2018). The anticipation and outcome phases of reward and loss processing: A neuroimaging meta‐analysis of the monetary incentive delay task. Human Brain Mapping, 39(8), 3398–3418. 10.1002/hbm.24184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Y. , Lai, F. , Fang, Z. , Xu, S. , Gao, L. , Robertson, D. C. , & Rao, H. (2019). Risk choice and emotional experience: A multi‐level comparison between active and passive decision‐making. Journal of Risk Research, 22(10), 1239–1266. 10.1080/13669877.2018.1459798 [DOI] [Google Scholar]
- Parkinson, B. , Phiri, N. , & Simons, G. (2012). Bursting with anxiety: Adult social referencing in an interpersonal balloon analogue risk task (BART). Emotion, 12(4), 817–826. 10.1037/a0026434 [DOI] [PubMed] [Google Scholar]
- Parr, A. C. , Calabro, F. , Larsen, B. , Tervo‐Clemmens, B. , Elliot, S. , Foran, W. , Olafsson, V. , & Luna, B. (2021). Dopamine‐related striatal neurophysiology is associated with specialization of frontostriatal reward circuitry through adolescence. Progress in Neurobiology, 201, 101997. 10.1016/J.PNEUROBIO.2021.101997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei, R. , Lauharatanahirun, N. , Cascio, C. N. , O'Donnell, M. B. , Shope, J. T. , Simons‐Morton, B. G. , Vettel, J. M. , & Falk, E. B. (2020). Neural processes during adolescent risky decision making are associated with conformity to peer influence. Developmental Cognitive Neuroscience, 44, 100794. 10.1016/j.dcn.2020.100794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudel, R. , Riedel, M. C. , Salo, T. , Flannery, J. S. , Hill‐Bowen, L. D. , Eickhoff, S. B. , Laird, A. R. , & Sutherland, M. T. (2020). Common and distinct brain activity associated with risky and ambiguous decision‐making. Drug and Alcohol Dependence, 209, 107884. 10.1016/j.drugalcdep.2020.107884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoff, K. , Quartz, S. R. , & Bossaerts, P. (2008). Human insula activation reflects risk prediction errors as well as risk. Journal of Neuroscience, 28(11), 2745–2752. 10.1523/JNEUROSCI.4286-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, Y. , Fuligni, A. J. , Galvan, A. , & Telzer, E. H. (2015). Buffering effect of positive parent‐child relationships on adolescent risk taking: A longitudinal neuroimaging investigation. Developmental Cognitive Neuroscience, 15, 26–34. 10.1016/j.dcn.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, Y. , Galvan, A. , Fuligni, A. J. , Lieberman, M. D. , & Telzer, E. H. (2015). Longitudinal changes in prefrontal cortex activation underlie declines in adolescent risk taking. Journal of Neuroscience, 35(32), 11308–11314. 10.1523/JNEUROSCI.1553-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, Y. , Lin, L. C. , & Telzer, E. H. (2019). Culture modulates the neural correlates underlying risky exploration. Frontiers in Human Neuroscience, 13(May), 1–12. 10.3389/fnhum.2019.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, H. , Korczykowski, M. , Pluta, J. , Hoang, A. , & Detre, J. A. (2008). Neural correlates of voluntary and involuntary risk taking in the human brain: An fMRI study of the balloon analog risk task (BART). NeuroImage, 42(2), 902–910. 10.1016/j.neuroimage.2008.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, H. , Mamikonyan, E. , Detre, J. A. , Siderowf, A. D. , Stern, M. B. , Potenza, M. N. , & Weintraub, D. (2010). Decreased ventral striatal activity with impulse control disorders in Parkinson's disease. Movement Disorders, 25(11), 1660–1669. 10.1002/mds.23147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, L. L. , Zhou, Y. , Zheng, D. , Yang, L. Q. , & Li, S. (2018). Genetic contribution to variation in risk taking: A functional MRI twin study of the balloon analogue risk task. Psychological Science, 29(10), 1679–1691. 10.1177/0956797618779961 [DOI] [PubMed] [Google Scholar]
- Raymond, D. R. , Paneto, A. , Yoder, K. K. , O'Donnell, B. F. , Brown, J. W. , Hetrick, W. P. , & Newman, S. D. (2020). Does chronic cannabis use impact risky decision‐making: An examination of fMRI activation and effective connectivity? Frontiers in Psychiatry, 11, 599256. 10.3389/fpsyt.2020.599256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer, D. , Reyna, V. F. , & Satterthwaite, T. D. (2017). Beyond stereotypes of adolescent risk taking: Placing the adolescent brain in developmental context. Developmental Cognitive Neuroscience, 27, 19–34. 10.1016/j.dcn.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi‐Khorshidi, G. , Smith, S. M. , Keltner, J. R. , Wager, T. D. , & Nichols, T. E. (2009). Meta‐analysis of neuroimaging data: A comparison of image‐based and coordinate‐based pooling of studies. NeuroImage, 45(3), 810–823. 10.1016/j.neuroimage.2008.12.039 [DOI] [PubMed] [Google Scholar]
- Schonberg, T. , Fox, C. R. , Mumford, J. A. , Congdon, E. , Trepel, C. , & Poldrack, R. A. (2012). Decreasing ventromedial prefrontal cortex activity during sequential risk‐taking: An FMRI investigation of the balloon analog risk task. Frontiers in Neuroscience, 6, 80. 10.3389/fnins.2012.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseer, L. , Moher, D. , Clarke, M. , Ghersi, D. , Liberati, A. , Petticrew, M. , Shekelle, P. , & Stewart, L. A. (2015). Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015: Elaboration and explanation. BMJ, 349, 1–25. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , Fox, P. T. , Miller, K. L. , Glahn, D. C. , Fox, P. M. , Mackay, C. E. , Filippini, N. , Watkins, K. E. , Toro, R. , Laird, A. R. , & Beckmann, C. F. (2009). Correspondence of the brain’s func‐ tional architecture during activation and rest. Proceedings of the National Academy of Sciences, 106(31), 13040–13045. 10.1073/pnas.0905267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout, J. C. , Rock, S. L. , Campbell, M. C. , Busemeyer, J. R. , & Finn, P. R. (2005). Psychological processes underlying risky decisions in drug abusers. Psychology of Addictive Behaviors, 19(2), 148–157. 10.1037/0893-164X.19.2.148 [DOI] [PubMed] [Google Scholar]
- Suzuki, S. , Jensen, E. L. S. , Bossaerts, P. , & O'Doherty, J. P. (2016). Behavioral contagion during learning about another agent's risk‐preferences acts on the neural representation of decision‐risk. Proceedings of the National Academy of Sciences of the United States of America, 113(14), 3755–3760. 10.1073/pnas.1600092113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer, E. H. , Fuligni, A. J. , Lieberman, M. D. , & Galván, A. (2013a). Meaningful family relationships: Neurocognitive buffers of adolescent risk taking. Journal of Cognitive Neuroscience, 25(3), 374–387. 10.1162/jocn_a_00331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer, E. H. , Fuligni, A. J. , Lieberman, M. D. , & Galván, A. (2013b). The effects of poor quality sleep on brain function and risk taking in adolescence. NeuroImage, 71, 275–283. 10.1016/j.neuroimage.2013.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdall, L. , Frey, R. , Horn, A. , Ostwald, D. , Horvath, L. , Pedroni, A. , Rieskamp, J. , Blankenburg, F. , Hertwig, R. , & Mata, R. (2020). Brain–behavior associations for risk taking depend on the measures used to capture individual differences. Frontiers in Behavioral Neuroscience, 14, 587152. 10.3389/fnbeh.2020.587152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub, P. E. , Eden, G. F. , Jones, K. M. , & Zeffiro, T. A. (2002). Meta‐analysis of the functional neuroanatomy of single‐word reading: Method and validation. NeuroImage, 16(3), 765–780. 10.1006/nimg.2002.1131 [DOI] [PubMed] [Google Scholar]
- Turkeltaub, P. E. , Eickhoff, S. B. , Laird, A. R. , Fox, M. , Wiener, M. , & Fox, P. (2012). Minimizing within‐experiment and within‐group effects in activation likelihood estimation meta‐analyses. Human brain mapping, 33(1), 1–13. 10.1002/hbm.21186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Bos, W. , & Hertwig, R. (2017). Adolescents display distinctive tolerance to ambiguity and to uncertainty during risky decision making. Scientific Reports, 7(1), 1–11. 10.1038/srep40962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagels, L. , Votinov, M. , Radke, S. , Clemens, B. , Montag, C. , Jung, S. , & Habel, U. (2017). Blunted insula activation reflects increased risk and reward seeking as an interaction of testosterone administration and the MAOA polymorphism. Human Brain Mapping, 38(9), 4574–4593. 10.1002/hbm.23685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Z. , Yang, N. , Liu, Y. , Yang, L. , Wang, Y. , Han, L. , Zha, R. , Huang, R. , Zhang, P. , Zhou, Y. , & Zhang, X. (2016). Resting‐state functional connectivity between the dorsal anterior cingulate cortex and thalamus is associated with risky decision‐making in nicotine addicts. Scientific Reports, 6(1), 1–9. 10.1038/srep21778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S. , Sun, S. , Camilleri, J. A. , Eickhoff, S. B. , & Yu, R. (2021). Better the devil you know than the devil you don't: Neural processing of risk and ambiguity. NeuroImage, 236, 118109. 10.1016/j.neuroimage.2021.118109 [DOI] [PubMed] [Google Scholar]
- Xia, M. , Wang, J. , & He, Y. (2013). BrainNet viewer: A network visualization tool for human brain Connectomics. PLoS One, 8(7), e68910. 10.1371/journal.pone.0068910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, G. , She, Z. , Zhao, J. , & Zhang, H. (2021). Transcranial direct current stimulation over the right dorsolateral prefrontal cortex has distinct effects on choices involving risk and ambiguity. Behavioural Brain Research, 400, 113044. 10.1016/j.bbr.2020.113044 [DOI] [PubMed] [Google Scholar]
- Zhang, D. , & Raichle, M. E. (2010). Disease and the brain's dark energy. Nature Reviews Neurology, 6(1), 15–28. 10.1038/nrneurol.2009.198 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
The data and code used in the present study are available from the corresponding author upon request.


