Abstract
For the fungus Histoplasma capsulatum, and for other microbial pathogens, iron is an essential nutrient. Iron sequestration in response to infection is a demonstrated host defense mechanism; thus, iron acquisition may be considered an important pathogenic determinant. H. capsulatum is known to secrete Fe(III)-binding hydroxamate siderophores, which is one common microbial process for acquiring iron. Here, we report H. capsulatum ferric reduction activities in whole yeast cells and in both high- and low-molecular-weight fractions of culture supernatants. Each of these activities was induced or derepressed by growth under iron-limiting conditions, a phenomenon often associated with specific iron acquisition mechanisms. The high-molecular-weight culture supernatant activity was enhanced by the addition of reduced glutathione, was proteinase K sensitive and heat labile, and could utilize ferric chloride, ferric citrate, and human holotransferrin as substrates. The low-molecular-weight culture supernatant activity was resistant to proteinase K digestion. These results are consistent with the expression by H. capsulatum of both enzymatic ferric reductase and nonproteinaceous ferric reductant, both of which are regulated by iron availability. Such components could be involved in fungal acquisition of iron from inorganic or organic ferric salts, from H. capsulatum hydroxamate siderophores, or from host Fe(III)-binding proteins, such as transferrin.
Histoplasma capsulatum is the causative agent of histoplasmosis. Although distributed worldwide, H. capsulatum has classically been associated with the U.S. Midwest. Infection, detected by delayed-type hypersensitivity to H. capsulatum antigens, can occur in 90% of the population in areas where it is endemic. H. capsulatum is thermally dimorphic, existing as a mold in soil and as a yeast in the mammalian host; these morphotypes are also observed in laboratory medium at room temperature and 37°C, respectively. Infection occurs through the inhalation of conidia or mycelial fragments. H. capsulatum then converts to yeast and infects pulmonary macrophages, where it replicates in the phagolysosome. Histoplasmosis poses a greater risk to and may be manifested as severe or fatal infection in immunocompromised individuals, such as AIDS or cancer patients, those on immunosuppressive drugs, the elderly, and the very young (12, 38). The identification of factors involved in H. capsulatum survival within the host is important for the development of vaccines or chemotherapeutic agents as well as understanding pathogenic mechanisms.
Iron limitation has been shown to be a specific mechanism of H. capsulatum infection control. H. capsulatum is incapable of growing in mouse macrophages that have been activated with gamma interferon (22), which results in a decrease in numbers of cellular transferrin (Tf) receptors (5). A similar intracellular growth inhibition occurs when mouse or human macrophages have been treated with the Fe(III) chelator deferoxamine (Df) (32, 33). In both cases, iron-laden holo-Tf, but not iron-poor apo-Tf, reverses growth inhibition.
Although iron is required by most organisms, most environmental sources contain the insoluble ferric form. In addition, excessive iron is toxic because iron reacts with oxygen to cause the formation of free radicals. Iron inside the host is complexed to high-affinity binding proteins, such as Tf or ferritin, and is sequestered in response to microbial infection (8, 36, 42). Thus, iron acquisition is an essential function that is generally tightly regulated and is important in the interplay between the host and pathogenic microbe.
One microbial strategy to circumvent the inaccessibility and toxicity of iron is the secretion of siderophores, low-molecular-weight (low-MW) high-affinity Fe(III) chelators, during growth in an iron-limiting environment (19). H. capsulatum has been reported to produce hydroxamate siderophores during mycelial and yeast phase growth in iron-limited media (4, 17). Although studies of H. capsulatum and other pathogens indicate that the host environment may be iron limiting, H. capsulatum siderophore production during infection has not been demonstrated. A system for iron uptake from H. capsulatum siderophores has also not been described.
An additional or alternative strategy is the extracellular reduction of Fe(III) followed by the import of Fe(II) (1). Such a mechanism may be associated with the acquisition of iron from Fe(III)-binding molecules like microbial siderophores or Tf. In this study, we present evidence of iron reduction by H. capsulatum cells and culture supernatants. These Fe(III)-reducing activities are increased in cultures grown under iron-limited conditions. We found that culture supernatants contain both a high-MW reduced glutathione (GSH)-dependent ferric reductase activity and a low-MW ferric reductant activity.
MATERIALS AND METHODS
Fungal strain and growth conditions.
H. capsulatum G217B (ATCC 26032) is a clinical isolate of restriction fragment length polymorphism class 2. Stock cultures were maintained in HMM, which has been previously described and is based on the tissue culture medium F-12, which contains 3 μM FeSO4 (44). For all experiments, G217B was grown in RPMI 1640 medium (Gibco-BRL, Grand Island, N.Y.) without phenol red or bicarbonate but supplemented with 1.8% dextrose, 25 mM HEPES, and penicillin and streptomycin (each at 10 μg/ml) and adjusted to pH 7.5 to produce modified RPMI (mRPMI). This medium contains no added iron, but the occurrence of trace contaminating amounts is highly likely. Cultures were grown in mRPMI containing 0.5 μM Df mesylate (Sigma, St. Louis, Mo.) to obtain a high-MW culture supernatant fraction. Cultures were grown without supplemental Df or FeSO4 to obtain a low-MW culture supernatant fraction. To obtain cultures for iron regulation experiments, 3 ml of log-phase cells in HMM was washed with phosphate-buffered saline (PBS), pelleted, and resuspended in 5 ml of mRPMI containing 10 μM Df. After overnight incubation to reduce intracellular stores of iron, cells were washed, resuspended in PBS, and inoculated at a 1:50 dilution into fresh medium. Typically, mRPMI was supplemented with 5 μM Df for iron-limited conditions or 10 μM FeSO4 (Fisher, Fair Lawn, N.J.) for iron-replete conditions. For growth curve experiments, G217B was partially depleted of intracellular iron, washed with PBS, and diluted 1:66 in mRPMI supplemented with 10 mM NaHCO3, which served as a cofactor for Fe(III) binding by Tf and was included in all cultures for uniformity (29). To measure growth under iron-limited conditions, media were supplemented with 5 or 100 μM Df or 1 μM human apo-Tf (Sigma). To measure growth under iron-replete conditions, media were supplemented with 1 μM human holo-Tf (Sigma), 10 μM FeSO4, or 10 μM FeCl3 (Fisher). Baseline growth in unsupplemented medium was also measured. Glassware was soaked in Contrad 70 (Fisher) and rinsed with distilled water prior to use. Culture turbidity was monitored with a photoelectric colorimeter (Manostat Corp., New York, N.Y.). All cultures were grown at 37°C in 5% CO2.
Sample preparation.
Log-phase cultures were used for all assays. Cells were harvested by centrifugation (1,200 × g, 10 min). Culture supernatants were sterilized through 0.2-μm-pore-size filters, and high-MW fractions were concentrated 60- to 80-fold by ultrafiltration with Ultrafree-15 Biomax polysulfone membranes (Millipore, Bedford, Mass.), which have a nominal MW limit (NMWL) of 5,000. Low-MW flowthrough filtrates were concentrated 10- to 20-fold by lyophilization. Low-MW filtrates obtained with Macrosep polyethersulfone membranes (NMWL, 3,000) (Pall Filtron, Northborough, Mass.) and Ultrafree-CL regenerated cellulose membranes (NMWL, 5,000) (Millipore) were also tested for Fe(III)-reducing activity.
Ferric-reduction assays.
Fe(II) was quantitated with either of the chromogenic Fe(II) chelators bathophenanthrolinedisulfonic acid (BPDS) and Ferrozine (both from Sigma) in a microtiter plate-based assay (7). For all assays, samples were compared to a reference blank containing all reaction mixture components with the same buffer. Blanked absorbance values were compared to a standard curve generated with FeSO4, and the amount of Fe(II) produced was normalized to cell number (whole cells and low-MW culture supernatants) or amount of protein (high-MW culture supernatants) as determined by a protein assay (Bio-Rad, Hercules, Calif.). All assays were performed in triplicate. Fe(II) was quantitated with BPDS in the absence of cofactors. Supernatant fractions (20 μl) were mixed with 50 μl of 2 mM BPDS, and the reaction was started by the addition of 50 μl of 0.2 mM FeCl3. Following a 1-h incubation (30 min for low-MW supernatants) at 37°C on a gyratory platform rocker (Nutator; Becton Dickinson, Sparks, Md.), the A534 of the mixture was measured. To measure cell surface ferric reduction, log-phase cells were harvested by centrifugation, washed with PBS, and resuspended at a concentration of 5 × 109 CFU/ml (one optical density unit at 700 nm = 1 × 108 CFU/ml [data not shown]) in PBS. Assays were performed as described above, with a 1-h incubation, except that volumes were doubled and cells were removed by centrifugation (2,700 × g, 10 min) before the A534 was measured. BPDS is unable to permeate membranes (16), and no significant A534 was detected when BPDS was incubated with cells in the absence of an Fe(III) source (data not shown).
Ferrozine was used in the presence of cofactors because it was less sensitive to nonenzymatic reduction by the reaction mixture components than BPDS (data not shown). Assays were performed similarly to those with BPDS, except with 2 mM Ferrozine. After a 2-h incubation at 37°C on a gyratory platform rocker, the A562 was measured. Reduced GSH was used at concentration of 0.5 mg/ml (1.63 mM). For GSH-dependent ferric reductase assays on cultures grown with 5 μM Df or 10 μM FeSO4, supernatants were concentrated and diafiltered with 3 volumes of 0.2 M sodium phosphate, pH 7.0. Ferric citrate (0.2 mM) (Fisher) and holo-Tf (0.1 mM) were also evaluated as substrates. Flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), NADH, NADPH, and reduced and oxidized glutathione were obtained from Sigma.
Cell extract preparation.
Crude cell extracts were prepared essentially as described previously except with PBS instead of sodium phosphate (44). Following cell disruption with glass beads and centrifugation, the pellet was resuspended in PBS.
Proteinase K digestion.
The protease sensitivities of the Fe(III)-reducing activities of the high- and low-MW culture supernatants were investigated by the addition of approximately 0.4 U of proteinase K attached to agarose beads (Sigma) resuspended in 50 μl of 10 mM Tris-HCl (pH 7.5) to 400 μl of a high- or low-MW culture supernatant and incubation for 24 h at 37°C on a gyratory platform rocker. An equivalent amount of Tris buffer was added to a control sample.
RESULTS
Iron requirement for H. capsulatum growth in vitro.
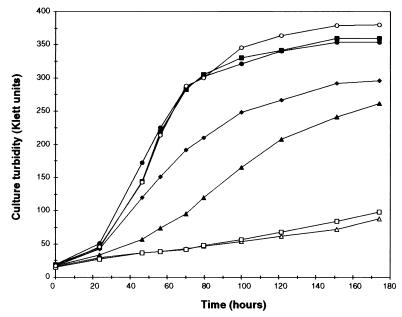
We examined H. capsulatum growth under iron-sufficient conditions with different iron sources and under iron-limited conditions with different chelators (Fig. 1). Baseline growth in mRPMI indicated the presence of contaminating trace concentrations of this essential nutrient. The growth rate and culture turbidity were increased when the medium was supplemented with 10 μM FeSO4, 10 μM FeCl3, or 1 μM holo-Tf. The growth rate was decreased in the presence of 5 μM Df, and very little growth was observed in the presence of 100 μM Df, indicating that this chelator does not serve as an effective iron source for H. capsulatum. Growth was also markedly restricted in the presence of 1 μM apo-Tf.
FIG. 1.
Effect of iron limitation on H. capsulatum growth in vitro. Log-phase cells were partially depleted of intracellular iron, washed with PBS, and inoculated at a 1:66 dilution into the indicated medium. Growth was measured by culture turbidity. Supplements to the basal growth medium are indicated by the following symbols: ⧫, no addition; ▴, 5 μM Df; ▵, 100 μM Df; □, 1 μM apo-Tf; ■, 1 μM holo-Tf; ●, 10 μM FeSO4; and ○, 10 μM FeCl3.
Iron reduction by whole H. capsulatum cells and high- and low-MW supernatant fractions.
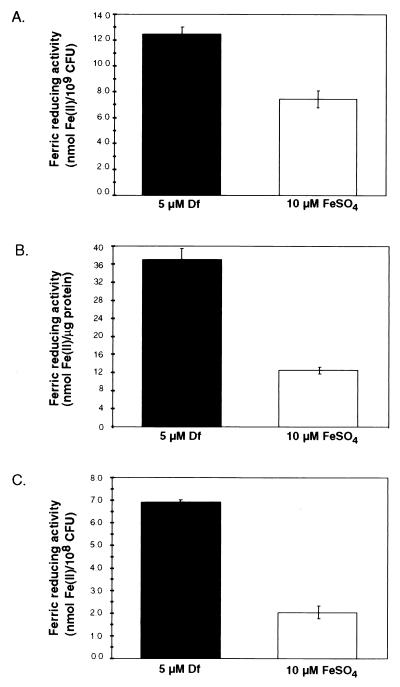
We determined the Fe(III)-reducing activities of washed cells and supernatant fractions separated by ultrafiltration into high- and low-MW components (Fig. 2). When activities were normalized to cell number (whole cells and low-MW supernatants) or amount of protein (high-MW supernatants), higher levels of Fe(III)-reducing activities were observed in cultures that had been grown in the presence of 5 μM Df than in cultures grown with 10 μM FeSO4. Ferric reduction after growth under iron-limited conditions was increased approximately 1.5-fold in whole cells and 3-fold in high- and low-MW culture supernatants. These results are consistent with either the repression of Fe(III)-reducing activities after growth under iron-replete conditions or the derepression or induction of Fe(III)-reducing activities after growth under iron-limited conditions. Low-MW filtrates generated by separation with ultrafiltration devices with polyethersulfone and regenerated cellulose membranes contained levels of Fe(III)-reducing activities similar to those of filtrates generated by separation with Biomax polysulfone membranes (data not shown). This observation is consistent with the filtrate containing a low-MW Fe(III)-reducing component rather than a filterable high-MW compound. Insoluble cell extracts (crude cell wall and cell membrane) contained Fe(III)-reducing activity. Soluble cell extracts (crude cytoplasm) contained Fe(III)-reducing activity, but the levels of this activity were similar in cultures grown with 5 μM Df and 10 μM FeSO4 (data not shown).
FIG. 2.
Fe(III)-reducing activities of whole cells (A) and high-MW (B) and low-MW (C) culture supernatant fractions of cultures grown in the presence of 5 μM Df (iron limited) or 10 μM FeSO4 (iron replete). Ferric reduction was assayed with BPDS as the chromogenic Fe(II) chelator. The averages of triplicate wells from a representative experiment are shown; standard deviations are indicated by bars. Similar results were obtained in three independent experiments.
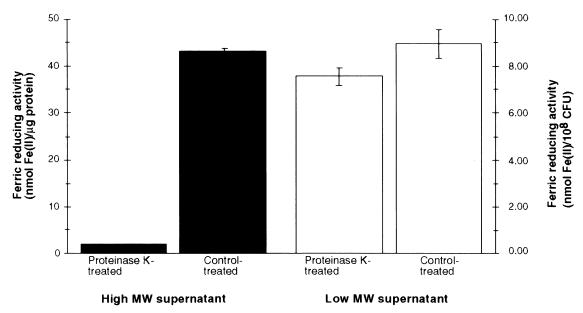
Sensitivities of Fe(III)-reducing activities of high- and low-MW culture supernatants to proteinase K digestion.
Because Fe(III) can be reduced by both enzymes and nonproteinaceous reductants, we examined supernatant ferric reduction following proteinase K treatment (Fig. 3). Following proteinase K digestion, the Fe(III)-reducing activity of the low-MW supernatant was similar to that of a control-treated sample. In contrast, the Fe(III)-reducing activity of the high-MW supernatant was diminished from 43.1 to 1.79 nmol of Fe(II)/μg of protein. No decrease in Fe(III)-reducing activity was observed in soluble cell extracts (crude cytoplasm) following treatment with proteinase K (data not shown).
FIG. 3.
Fe(III)-reducing activities of high- and low-MW supernatant components after treatment with proteinase K. Ferric reduction was assayed with BPDS as the chromogenic Fe(II) chelator. The averages of triplicate wells from a representative experiment are shown; standard deviations are indicated by bars. Similar results were obtained in three independent experiments.
The high-MW culture supernatant contained a reduced-glutathione-dependent ferric reductase.
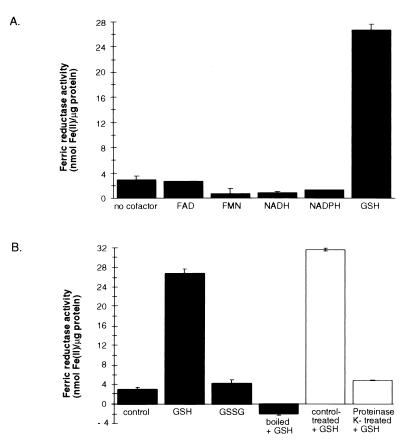
Unlike ferric reductants, ferric reductases require cofactors, so we surveyed a range of compounds reported to serve as electron donors for other ferric reductases for the ability to enhance ferric reduction in this system. Supplementing the reaction mixture with 0.5 mg of GSH per ml (1.63 mM) resulted in an increase of ferric reductase activity from 2.95 to 26.7 nmol of Fe(II)/μg of protein (Fig. 4A). Addition of higher concentrations resulted in excessive nonenzymatic ferric reduction by GSH (data not shown). Activity was not significantly enhanced by the addition of 5 μM FAD, 5 μM FMN, 50 μM NADH, or 50 μM NADPH. There also was no increase after the addition of 100 μM NADH or NADPH or combinations of NADH, NADPH, FAD, and FMN (data not shown). No additional increase in reductase activity was observed when GSH was added with FAD or FMN (data not shown). Ferric reductase activity was increased by the addition of 35 μg of cysteine per ml, but an excessive level of nonenzymatic ferric reduction was detected in the reference blank. Virtually no GSH-dependent ferric reductase activity was observed after the high-MW culture supernatant was boiled for 15 min or digested with proteinase K (Fig. 4B). Ferric reductase activity was not increased when the reaction mixture was supplemented with 0.5 mg of oxidized glutathione (GSSG) per ml (0.82 mM). These results are consistent with high-MW culture supernatant containing a ferric reductase protein that utilizes GSH as an electron donor. These experiments included an inorganic salt, FeCl3, as the Fe(III) substrate. We found that the high-MW GSH-dependent ferric reductase could use ferric citrate and holo-Tf as Fe(III) substrates (data not shown). Assaying for GSH-dependent ferric reductase on iron-limited and iron-replete culture supernatant fractions that had been diafiltered in 0.2 M sodium phosphate (pH 7.0) revealed a 1.4-fold induction under conditions of iron limitation (data not shown). These results are consistent with the extracellular GSH-dependent ferric reductase being regulated in an iron-dependent manner.
FIG. 4.
Effects of electron donors on ferric reductase activities of high-MW supernatants. (A) Concentraitons of 5 μM FAD, 5 μM FMN, 50 μM NADH, 50 μM NADPH, and 1.63 mM (0.5 mg/ml) GSH were evaluated for the capacity to increase ferric reductase activity. (B) The role of GSH as a specific electron donor for an enzymatic ferric reductase was examined. GSH was added to a high-MW supernatant that was left untreated, boiled for 15 min, or digested with proteinase K. The untreated high-MW supernatant was supplemented with 0.82 mM (0.5 mg/ml) oxidized glutathione (GSSG). Ferric reduction was assayed with Ferrozine as the chromogenic Fe(II) chelator. The averages of triplicate wells from a representative experiment are shown; standard deviations are indicated by bars. Similar results were obtained in three independent experiments.
DISCUSSION
Survival of a pathogenic microbe in the environment and in the host requires many factors, some of which facilitate nutrient acquisition. During the mammalian acute-phase response to severe infection, the saturation of Tf is reduced. Additionally, intracellular iron is limited in macrophages in response to infection, e.g., by the reduction of the number of cell surface Tf receptors (42). Thus, microbial mechanisms to acquire iron are adaptive and important for virulence. We have demonstrated H. capsulatum Fe(III)-reducing activities located on the cell surface and in high- and low-MW culture supernatant fractions that were greater after growth under iron-limited conditions than after growth under iron-replete conditions. The culture supernatant contained both a high-MW, proteinase K-sensitive, heat-labile, GSH-dependent ferric reductase and a low-MW, proteinase K-resistant ferric reductant. To our knowledge, this is the first report of iron-repressible Fe(III)-reducing activity in H. capsulatum.
These Fe(III)-reducing agents may be iron acquisition mechanisms that are supplemental or alternative to H. capsulatum siderophores. Induction under conditions of iron-limited growth is often associated with specific iron acquisition mechanisms. These Fe(III)-reducing agents may reduce iron bound to siderophores or host iron-binding proteins. Although an iron import system has not yet been described for H. capsulatum, the reduction of Fe(III) would presumably be followed by the import of soluble Fe(II). Iron import systems in other fungi involve high- and low-affinity transmembrane transporters (2, 20, 21).
Extracellular ferric reduction, which potentially facilitates iron uptake, has been reported for other microbes. Iron-repressible cell surface ferric reductases in Saccharomyces cerevisiae (24), Schizosaccharomyces pombe (39), Candida albicans (30), and Cryptococcus neoformans (34) have been reported. A component exported by C. albicans also reduces iron, but this activity does not appear to be subject to iron-dependent regulation, and the membrane reductase is predominant. The ferric reductase genes FRE1 and FRE2 from S. cerevisiae and frp1+ from S. pombe encode predicted proteins that have homology with human gp91-phox, a subunit of an NADPH- and FAD-dependent oxidase (8, 9, 15, 39). Secreted ferric reductases have been described for Mycobacterium paratuberculosis and Listeria monocytogenes (3, 18). C. neoformans secretes the ferric reductant 3-hydroxyanthraline, and its secretion is increased after growth under iron-limited conditions (34). With the exception of C. albicans, these microbes have not been reported to secrete siderophores, although S. cerevisiae can both bind and reduce ferric siderophores (23, 25). To our knowledge, this is the first report of the production of a cell surface Fe(III)-reducing agent and extracellular ferric reductase and reductant in addition to siderophores by a single microorganism. These mechanisms could be independent of one another or could interact through the reduction of iron chelated to siderophores. Multiple mechanisms of iron acquisition may be necessary for growth in the environment and host.
The extracellular H. capsulatum ferric reductase uses GSH as an electron donor, which to our knowledge has not been previously described for fungal ferric reductases. GSH serves as an electron donor for a Pseudomonas aeruginosa periplasmic and cytoplasmic ferrisiderophore reductase and a Legionella pneumophila periplasmic ferric reductase (6, 37). Macrophages contain glutathione, which is the major thiol antioxidant in mammals (40). Interestingly, production of a membrane-bound cystine reductase and an intracellular glutathione reductase by yeast-phase H. capsulatum has been described (28).
These Fe(III)-reducing agents may facilitate iron acquisition from siderophores or host iron-binding proteins. H. capsulatum can grow in vitro with holo-Tf as an iron source, and the GSH-dependent ferric reductase can use holo-Tf as a substrate. Other ferric reductases have been reported to reduce Fe(III) from siderophores or mammalian iron-binding proteins. HeLa S3 cells have been reported to reduce holo-Tf for iron uptake independent of Tf receptors and endosome acidification (26). L. monocytogenes cultures can reduce Fe(III) bound to Tf, ferritin, and the siderophore 2,3-dihydroxybenzoic acid (10). Intracellular and membrane-bound ferrisiderophore reductases in other microbes have been described (13, 14, 23, 35). If expressed by mold, the yeast-phase H. capsulatum Fe(III)-reducing activities described here could facilitate the acquisition of iron from siderophores secreted by other fungi and bacteria in soil. A similar utilization by bacteria and other fungi has been demonstrated (1, 43).
By facilitating iron uptake, these Fe(III)-reducing agents may be involved in pathogenesis. The requirement of iron for productive H. capsulatum infection has previously been demonstrated. Iron is required for H. capsulatum intracellular growth and is limiting in gamma interferon-activated macrophages (22). Iron limitation by Tf is also responsible for serum-mediated in vitro growth inhibition (41). Mechanisms to acquire iron circumvent these host defenses and thus are important for pathogenesis.
Siderophore production is a classic mechanism associated with iron acquisition, and H. capsulatum has been shown to make hydroxamate siderophores in vitro under iron-limiting conditions, but their production during infection has not been demonstrated. Extracellular siderophore-bound iron would still require a microbial iron acquisition mechanism for the uptake of the complex and/or the release of the bound Fe(III). The components and kinetics of H. capsulatum iron uptake from siderophores have not been determined. The plant-pathogenic fungus Stemphylium botryosum produces the siderophores dimerum acid and coprogen B (27), which are also produced by H. capsulatum (4). The uptake of iron complexed to these siderophores by S. botryosum has been studied, and in the case of coprogen B, it involves the internalization of the ferrisiderophore complex (27).
Newman has suggested that as an alternate iron acquisition mechanism, the acidification of the intracellular environment in which H. capsulatum thrives facilitates iron availability by causing the release of iron bound to Tf (31). However, Eissenberg and Goldman have shown that H. capsulatum can inhibit or reverse host phagolysosomal acidification to some degree (11). Such neutralization would inactivate host hydrolytic enzymes with acid pH optima but would also lessen pH-associated iron release from Tf. Iron-reducing activities, such as those we report here, including cell-associated activity and extracellular high-MW protein reductase and low-MW reductant, could provide a supplemental or alternate iron acquisition mechanism. If H. capsulatum siderophores are produced during infection, iron reduction could provide a means for the release of bound Fe(III). If Tf serves as an important iron source, iron reduction could provide a relatively pH-independent means for the release of bound Fe(III). We are interested in further characterizing H. capsulatum iron acquisition mechanisms, identifying the relevant fungal genes and understanding their regulation, and determining the importance of these processes and components for pathogenicity.
ACKNOWLEDGMENTS
J.P.W. is a Lucille P. Markey Scholar, and this work was supported in part by Scholar Award 94-21 from the Lucille P. Markey Charitable Trust. M.M.T. is a trainee under NIH predoctoral training grant T32 GM07215.
We thank Sara Engstrand for preliminary work and Diane Retallack for critical reading of the manuscript.
REFERENCES
- 1.Askwith C C, de Silva D, Kaplan J. Molecular biology of iron acquisition in Saccharomyces cerevisiae. Mol Microbiol. 1996;20:27–34. doi: 10.1111/j.1365-2958.1996.tb02485.x. [DOI] [PubMed] [Google Scholar]
- 2.Askwith C C, Kaplan J. An oxidase-permease-based iron transport system in Schizosaccharomyces pombe and its expression in Saccharomyces cerevisiae. J Biol Chem. 1997;272:401–405. doi: 10.1074/jbc.272.1.401. [DOI] [PubMed] [Google Scholar]
- 3.Barchini E, Cowart R E. Extracellular iron reductase activity produced by Listeria monocytogenes. Arch Microbiol. 1996;166:51–57. doi: 10.1007/s002030050354. [DOI] [PubMed] [Google Scholar]
- 4.Burt W R. Identification of coprogen B and its breakdown products from Histoplasma capsulatum. Infect Immun. 1982;35:990–996. doi: 10.1128/iai.35.3.990-996.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrd T F, Horwitz M A. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophilaby limiting the availability of iron. J Clin Investig. 1989;83:1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox C D. Iron reductases from Pseudomonas aeruginosa. J Bacteriol. 1980;141:199–204. doi: 10.1128/jb.141.1.199-204.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox C D. Deferration of laboratory media and assays for ferric and ferrous ions. Methods Enzymol. 1994;235:315–329. doi: 10.1016/0076-6879(94)35150-3. [DOI] [PubMed] [Google Scholar]
- 8.Dancis A. Genetic analysis of iron uptake in the yeast Saccharomyces cerevisiae. J Pediatr. 1998;132:S24–S29. doi: 10.1016/s0022-3476(98)70524-4. [DOI] [PubMed] [Google Scholar]
- 9.Dancis A, Roman D G, Anderson G J, Hinnebusch A G, Klausner R D. Ferric reductase of Saccharomyces cerevisiae: molecular characterization, role in iron uptake, and transcriptional control by iron. Proc Natl Acad Sci USA. 1992;89:3869–3873. doi: 10.1073/pnas.89.9.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deneer H G, Boychuk I. Reduction of ferric iron by Listeria monocytogenes and other species of Listeria. Can J Microbiol. 1993;39:480–485. doi: 10.1139/m93-068. [DOI] [PubMed] [Google Scholar]
- 11.Eissenberg L G, Goldman W E. Histoplasmavariation and adaptive strategies for parasitism: new perspectives on histoplasmosis. Clin Microbiol Rev. 1991;4:411–421. doi: 10.1128/cmr.4.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eissenberg L G, Goldman W E. The interplay between Histoplasma capsulatumand its host cells. Bailliere's Clin Infect Dis. 1994;1:265–283. [Google Scholar]
- 13.Ernst J F, Winkelmann G. Enzymatic release of iron from sideramines in fungi: NADH:sideramine oxidoreductase in Neurospora crassa. Biochim Biophys Acta. 1977;500:27–41. doi: 10.1016/0304-4165(77)90043-5. [DOI] [PubMed] [Google Scholar]
- 14.Fischer E, Strehlow B, Hartz D, Braun V. Soluble and membrane-bound ferrisiderophore reductases of Escherichia coliK-12. Arch Microbiol. 1990;153:329–336. doi: 10.1007/BF00249001. [DOI] [PubMed] [Google Scholar]
- 15.Georgatsou E, Alexandraki D. Two distinctly regulated genes are required for ferric reduction, the first step of iron uptake in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:3056–3073. doi: 10.1128/mcb.14.5.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassett R F, Romeo A M, Kosman D J. Regulation of high affinity iron uptake in the yeast Saccharomyces cerevisiae: role of dioxygen and Fe(II) J Biol Chem. 1998;273:7628–7636. doi: 10.1074/jbc.273.13.7628. [DOI] [PubMed] [Google Scholar]
- 17.Holzberg M, Artis W M. Hydroxamate siderophore production by opportunistic and systemic fungal pathogens. Infect Immun. 1983;40:1134–1139. doi: 10.1128/iai.40.3.1134-1139.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Homouth M, Valentin-Weigand P, Rohde M, Gerlach G-F. Identification and characterization of a novel extracellular ferric reductase from Mycobacterium paratuberculosis. Infect Immun. 1998;66:710–716. doi: 10.1128/iai.66.2.710-716.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard D H. Acquisition, transport, and storage of iron in pathogenic fungi. Clin Microbiol Rev. 1999;12:394–404. doi: 10.1128/cmr.12.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobson E S, Goodner A P, Nyhus K J. Ferrous iron uptake in Cryptococcus neoformans. Infect Immun. 1998;66:4169–4175. doi: 10.1128/iai.66.9.4169-4175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan J, O'Halloran T V. Iron metabolism in eukaryotes: Mars and Venus at it again. Science. 1996;271:1510–1512. doi: 10.1126/science.271.5255.1510. [DOI] [PubMed] [Google Scholar]
- 22.Lane T E, Wu-Hsieh B A, Howard D H. Iron limitation and the gamma inteferon-mediated antihistoplasma state of murine macrophages. Infect Immun. 1991;59:2274–2278. doi: 10.1128/iai.59.7.2274-2278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesuisse E, Crichton R R, Labbe P. Iron-reductases in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 1990;1038:256–259. doi: 10.1016/0167-4838(90)90213-y. [DOI] [PubMed] [Google Scholar]
- 24.Lesuisse E, Raguzzi F, Crichton R R. Iron uptake by the yeast Saccharomyces cerevisiae: involvement of a reduction step. J Gen Microbiol. 1987;133:3229–3236. doi: 10.1099/00221287-133-11-3229. [DOI] [PubMed] [Google Scholar]
- 25.Lesuisse E, Simon-Casteras M, Labbe P. Siderophore-mediated iron uptake in Saccharomyces cerevisiae: the SIT1gene encodes a ferrioxamine B permease that belongs to the major facilitator superfamily. Microbiology. 1998;144:3455–3462. doi: 10.1099/00221287-144-12-3455. [DOI] [PubMed] [Google Scholar]
- 26.Low H, Grebing C, Lindgren A, Tally M, Sun I L, Crane F L. Involvement of transferrin in the reduction of iron by the transplasma membrane electron transport system. J Bioenerg Biomembr. 1987;19:535–549. doi: 10.1007/BF00770036. [DOI] [PubMed] [Google Scholar]
- 27.Manulis S, Kashman Y, Barash I. Identification of siderophores and siderophore-mediated uptake of iron in Stemphylium botryosum. Phytochemistry. 1987;26:1317–1320. [Google Scholar]
- 28.Maresca B, Jacobson E, Medoff G, Kobayashi G. Cystine reductase in the dimorphic fungus Histoplasma capsulatum. J Bacteriol. 1978;135:987–992. doi: 10.1128/jb.135.3.987-992.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merck Research Laboratories. The Merck index: an encyclopedia of chemicals, drugs, and biologicals. Whitehouse Station, N.J: Merck & Co., Inc.; 1996. [Google Scholar]
- 30.Morrissey J A, Williams P H, Cashmore A M. Candida albicanshas a cell-associated ferric-reductase activity which is regulated in response to levels of iron and copper. Microbiology. 1996;142:485–492. doi: 10.1099/13500872-142-3-485. [DOI] [PubMed] [Google Scholar]
- 31.Newman S L. Macrophages in host defense against Histoplasma capsulatum. Trends Microbiol. 1999;7:67–71. doi: 10.1016/s0966-842x(98)01431-0. [DOI] [PubMed] [Google Scholar]
- 32.Newman S L, Gootee L, Brunner G, Deepe G S., Jr Chloroquine induces human macrophage killing of Histoplasma capsulatumby limiting the availability of intracellular iron and is therapeutic in a murine model of histoplasmosis. J Clin Investig. 1994;93:1422–1429. doi: 10.1172/JCI117119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman S L, Gootee L, Stroobant V, van der Goot H, Boelaert J R. Inhibition of growth of Histoplasma capsulatumyeast cells in human macrophages by the iron chelator VUF 8514 and comparison of VUF 8514 with deferoxamine. Antimicrob Agents Chemother. 1995;39:1824–1829. doi: 10.1128/aac.39.8.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nyhus K J, Wilborn A T, Jacobson E S. Ferric iron reduction by Cryptococcus neoformans. Infect Immun. 1997;65:434–438. doi: 10.1128/iai.65.2.434-438.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paoletti L C, Blakemore R P. Iron reduction by Aquaspirillum magnetotacticum. Curr Microbiol. 1988;17:339–342. [Google Scholar]
- 36.Payne S M. Iron acquisition in microbial pathogenesis. Trends Microbiol. 1993;1:66–69. doi: 10.1016/0966-842x(93)90036-q. [DOI] [PubMed] [Google Scholar]
- 37.Poch M T, Johnson W. Ferric reductases of Legionella pneumophila. Biometals. 1993;6:107–114. doi: 10.1007/BF00140111. [DOI] [PubMed] [Google Scholar]
- 38.Retallack D M, Woods J P. Molecular epidemiology, pathogenesis, and genetics of the dimorphic fungus Histoplasma capsulatum. Microb Infect. 1999;1:817–825. doi: 10.1016/s1286-4579(99)80084-7. [DOI] [PubMed] [Google Scholar]
- 39.Roman D G, Dancis A, Anderson G J, Klausner R D. The fission yeast ferric reductase gene frp1+ is required for ferric iron uptake and encodes a protein that is homologous to the gp91-phoxsubunit of the human NADPH phagocyte oxidoreductase. Mol Cell Biol. 1993;13:4342–4350. doi: 10.1128/mcb.13.7.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenblat M, Aviram M. Macrophage glutathione content and glutathione peroxidase activity are inversely related to cell-mediated oxidation of LDL: in vitro and in vivostudies. Free Radic Biol Med. 1998;24:305–317. doi: 10.1016/s0891-5849(97)00231-1. [DOI] [PubMed] [Google Scholar]
- 41.Sutcliffe M C, Savage A M, Alford R H. Transferrin-dependent growth inhibition of yeast-phase Histoplasma capsulatumby human serum and lymph. J Infect Dis. 1980;142:209–219. doi: 10.1093/infdis/142.2.209. [DOI] [PubMed] [Google Scholar]
- 42.Weinberg E D. The iron-withholding defense system. ASM News. 1993;59:559–562. [Google Scholar]
- 43.Williams P, Morton D J, Towner K J, Stevenson P, Griffiths E. Utilization of enterobactin and other exogenous iron sources by Haemophilus influenzae, H. parainfluenzae and H. paraphrophilus. J Gen Microbiol. 1990;136:2343–2350. doi: 10.1099/00221287-136-12-2343. [DOI] [PubMed] [Google Scholar]
- 44.Woods J P, Heinecke E L, Goldman W E. Electrotransformation and expression of bacterial genes encoding hygromycin phosphotransferase and β-galactosidase in the pathogenic fungus Histoplasma capsulatum. Infect Immun. 1998;66:1697–1707. doi: 10.1128/iai.66.4.1697-1707.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]