Background
Laser-assisted liposuction (LAL) has been used to maximize viable adipocyte yields in lipoaspirates, although optimizing tissue processing methods is still a challenge. A high-quality lipoaspirate has been a key factor for extended graft longevity.
Objective
To assess the viability and potency of stromal vascular fraction (SVF) cells and adipose-derived stem cells (ASCs) in fat samples from lipoaspirates harvested with a novel 1470-nm diode, radial emitting LAL platform. Two processing methods, enzymatic and nonenzymatic, were compared.
Methods
Laser-assisted liposuction lipoaspirates harvested from 10 subjects were examined for cell viability after processing by enzymatic or nonenzymatic methods. Isolated SVF cells were cultured with an ASC-permissive medium to assess their viability and proliferation capacity by cell proliferation assay. Flow cytometric analysis with ASC-specific markers, gene expression levels, and immunofluorescence for ASC transcription factors were also conducted.
Results
Lipoaspirates showed high SVF cell viability of 97% ± 0.02% and 98% ± 0.01%, averaged SVF cell count of 8.7 × 106 ± 3.9 × 106 and 9.4 × 106 ± 4.2 × 106 cells per mL, and averaged ASC count of 1 × 106 ± 2.2 × 105 and 1.2 × 106 ± 5 × 105 cells per mL in nonenzymatic and enzymatic methods, respectively. The ASC-specific markers, gene expression levels, and immunofluorescence for ASC transcription factors confirmed the adipose origin of the cells.
Conclusions
The laser lipoaspirates provide a high yield of viable and potent SVF cells and ASCs through both nonenzymatic and enzymatic processes. Improved purity of the harvested lipoaspirate and high ASC content are expected to result in extended graft longevity. Furthermore, eliminating enzymatic digestion may provide advantages, such as reducing process time, cost, and regulatory constraints.
Key Words: laser liposuction, lipoaspirate, adipose-derived stem cells, stromal vascular fraction, autologous fat transfer
Liposuction is the second most popular aesthetic procedure performed in the United States.1 It is commonly assisted by mechanical suction, also known as suction-assisted liposuction, power-assisted liposuction, ultrasound, or laser energy.2–4 Laser-assisted liposuction (LAL) is a cosmetic, minimally invasive surgical procedure that allows rapid, comfortable postoperative recovery and quick return to daily activities.5 It is considered safe and tolerable and has been associated with a lower touchup rate and fewer complications compared with conventional liposuction techniques.6–13 In addition, LAL significantly minimizes tissue trauma compared with mechanical liposuction.14
One of the main concerns in autologous fat grafting is the unpredictable resorption after transplantation.3 In fact, the rate of resorption over time in the grafted site may range from 20% to 90% of the filled volume.15,16 This could be attributed to the fact that the aspirated fat is poor in quality and has low number of adipose-derived stem cells (ASCs).17 Therefore, optimizing methods of harvesting adipose tissue is of maximal importance18,19 as it can have a significant impact on the yield and viability of isolated cells.3,5,17,20,21
Stromal vascular fraction (SVF) cells are a heterogeneous population of cells including endothelial cells, erythrocytes, fibroblasts, lymphocytes, monocytes/macrophages, and most importantly ASCs.22,23 Adipose-derived stem cells can differentiate into a range of mesenchymal tissues,17,24 and their enrichment in the lipoaspirate has become important in aesthetic surgery recently.25–28
To isolate ASCs from lipoaspirates, disruption of the adipose tissue by enzymatic or nonenzymatic manipulation of lipoaspirates is necessary, followed by centrifugation to obtain the SVF cell pellet.23 Enzymatic digestion is the most commonly used method for SVF isolation, providing high nucleated cells per mL of lipoaspirate.29 However, it is associated with regulatory concerns and limitations. Nonenzymatic methods, although known to produce significantly lower yields of ASCs, are appealing as they are simple, fast, and associated with less regulatory issues.30 Therefore, researchers are exploring nonenzymatic methods of separation using protocols that involve pressure, shear and centrifugal force.30,31
We conducted an open-label, prospective clinical study using a novel 1470-nm radial emitting LAL (Alma Lasers, BeautiFill by LipoLife) to assess the viability and differentiation potential of SVF cells and ASCs extracted from the collected lipoaspirates. Moreover, we compared nonenzymatic and enzymatic processing methods of these lipoaspirates.
MATERIALS AND METHODS
Donors
The study included 10 consecutive subjects (1 man and 9 women) with a mean age of 47.7 ± 12 years and body mass index (BMI) of 27.7 ± 4.4 kg/m2. All subjects provided informed consent for bench processing of their fat aspirate in accordance with the Shamir Medical Center Institutional Review Board, Helsinki approval number 0095-17-ASF. The average volume obtained from the abdomen and thighs was 1844 mL. The maximum aspirated material was 3620 mL, and the minimum was 400 mL (Table 1).
TABLE 1.
Subject Demographics and Procedural Data
| Subject Number | Weight (kg) | BMI | Age | Sex | Liposuction Area | Total Lipoaspirate Volume (mL) |
|---|---|---|---|---|---|---|
| 1 | 72.5 | 29.21 | 44 | Female | Abdomen | 900 |
| 2 | 99 | 30.56 | 22 | Male | Abdomen | 1900 |
| 3 | 51 | 18.73 | 51 | Female | Abdomen | 400 |
| 4 | 81.4 | 30.49 | 55 | Female | Abdomen | 2050 |
| 5 | 68 | 27.94 | 58 | Female | Abdomen | 500 |
| 6 | 93.8 | 35.3 | 47 | Female | Thighs | 3620 |
| 7 | 73 | 26.81 | 45 | Female | Thighs | 1870 |
| 8 | 63.5 | 24.01 | 37 | Female | Thighs | 2500 |
| 9 | 70 | 25.71 | 65 | Female | Thighs | 2500 |
| 10 | 71.8 | 28.44 | 53 | Female | Thighs | 2200 |
| Average | 74.4 | 27.7 | 47.7 | 1844 | ||
| SEM | 4.4 | 1.4 | 3.8 | 316 |
Surgical Procedure
Liposuction with LipoLife (Alma Lasers, BeautiFill by LipoLife) involves the simultaneous action of laser and suction. The LipoLife system consists of a 1470-nm diode laser and LipoFlow system (Alma Lasers, Ltd.), which provides vacuum for liposuction and enables infiltration. The procedures were performed under general anesthesia. Before liposuction, the subjects were prepped with betadine. Tumescent solution (400 mg lidocaine and 1 mg adrenaline per liter saline) was introduced into the treated area using Lipoflow with a ratio of injected liquid (tumescent) to aspirated material of 2:1. For fat aspiration, a 4-mm cannula specially designed with a swivel handle (Alma Lasers, BeautiFill by LipoLife) was used. The 1470-nm, 600-micron, radial emitting laser fiber (Alma Lasers, Ltd.) was maneuvered and positioned at the center of the distal opening of the cannula. The harvested lipoaspirate was collected in a sterile canister and filtered through a canister mesh to separate the adipocytes from the tumescent fluid, blood, cell debris, and free oil.
Adipose Tissue Harvesting
Enzymatic or nonenzymatic manipulation processing began within 1 to 2 hours of harvesting. Lipoaspirate samples (100 mL) were washed extensively 3 times with sterile Dulbecco phosphate-buffered saline (PBS) without calcium and magnesium (Biological Industries, Beit HaEmek, Israel) to dispose of tissue debris and blood residuals. Before the processing methods, small samples of lipoaspirate were taken for further RNA analysis (Fig. 1).
FIGURE 1.
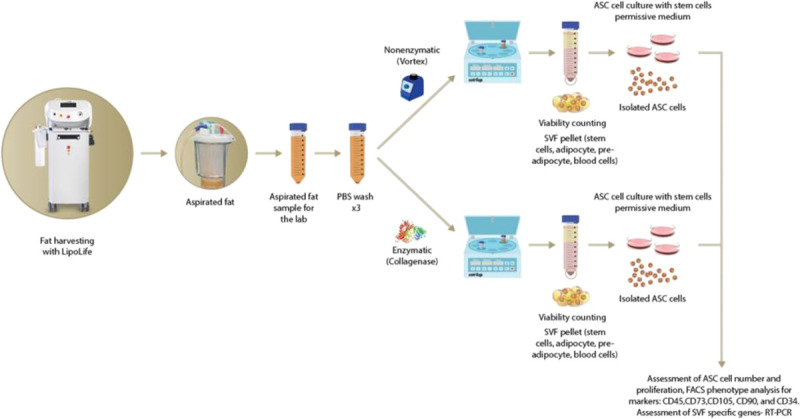
Isolation and characterization of SVF cells and ASCs from laser-assisted lipoaspirate.
Isolation of ASCs
Lipoaspirate processing by enzymatic or nonenzymatic manipulation for isolation of SVF cells.
Enzymatic Preparation
Fifteen milliliters of washed lipoaspirate sample was transferred to a 50-mL tube containing 30 mL of 0.1% collagenase IV (Sigma-Aldrich cat. no. C2139) and 5% BSA in PBS (Biological Industries). The sample was incubated for 45 minutes at 37°C and 5% CO2 at 100 rpm. Stromal vascular fraction was obtained by centrifuging the sample at 1200 rpm for 5 minutes and discarding the collagenase solution without disturbing the SVF cell pellet. This preparation is based on the protocol of Aronowitz et al.30,32
Nonenzymatic Preparation
Fifteen milliliters of washed lipoaspirate sample was transferred to a 50-mL tube containing 30 mL PBS (Biological Industries). The sample was vortexed gently for 6 minutes at 600 rpm to disrupt the tissue and release the cells. Then, the cells were centrifuged at 1600 rpm for 6 minutes to obtain the SVF. The supernatant was removed without disturbing the SVF cell pellet.
The SVF cell pellets containing ASCs were resuspended in MSC NutriStem XF Medium supplemented with MSC NutriStem XF, Male AB Human Serum 2.5%, and 0.1% penicillin/streptomycin (Biological Industries) and seeded on precoated cell culture plates with MSC attachment solution (Biological Industries) and grown for 7 days at 37°C and 5% CO2 before further analysis. The remaining floating cells and debris were aspirated after 72 hours. Using this permissive medium33 resulted in a pure homogenous ASC culture characterized by typical morphology of spindle-shaped cells as described by others27,29,34 (bright-field images in Fig. 3). PromoCell commercial human mesenchymal stem cells (hMSCs) from adipose tissue were defined as a positive control (Biological Industries).
FIGURE 3.
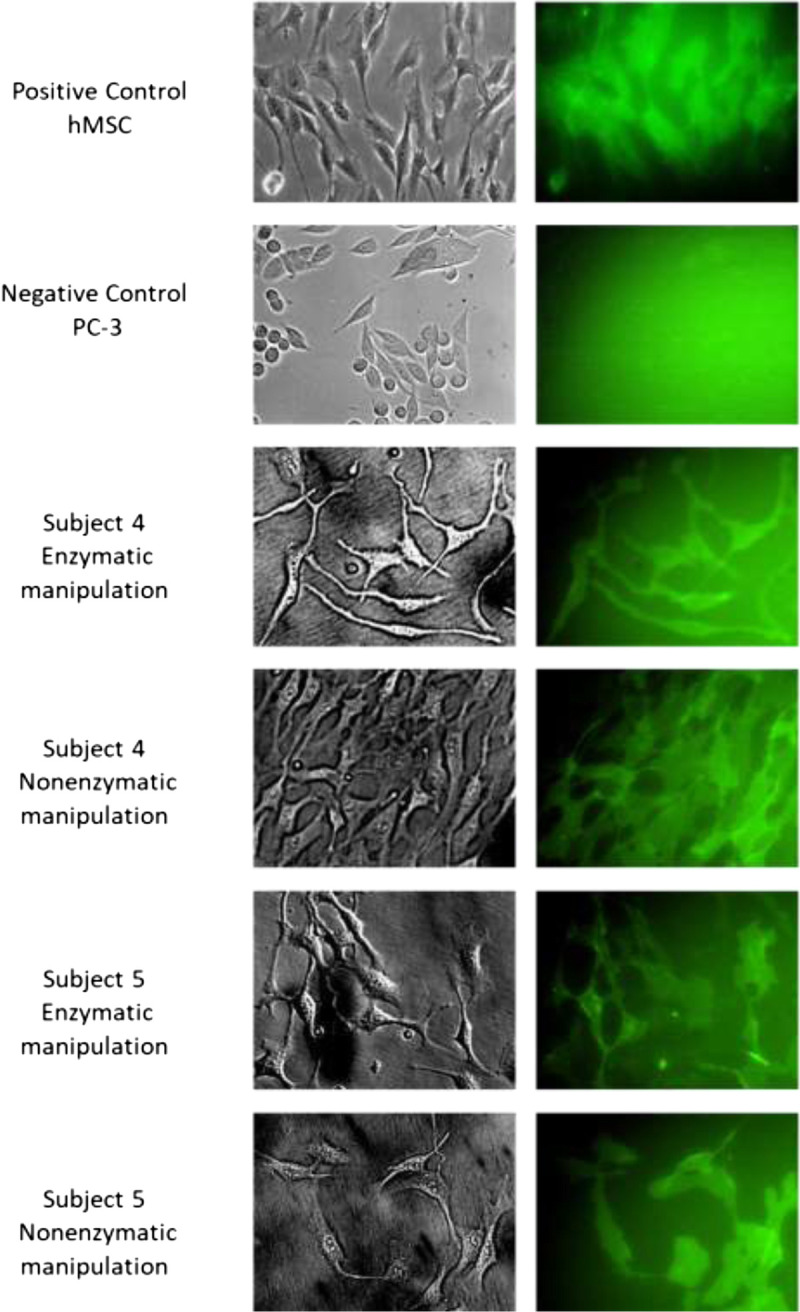
Live Staining of ASCs Derived from LAL (CD90+ Marker). Analysis of live ASCs was performed using a fluorescence microscope. ASCs isolated using enzymatic and nonenzymatic processing methods highly express CD90. Representative data of subjects 4 and 5 are shown.
SVF Cell Viability Count
To determine the number of viable cells present in the SVF cell pellet, the cell suspension was diluted 1:1 with 0.4% trypan blue dye (Sigma-Aldrich). The viable cells were counted with a hemocytometer and calculated to determine the number of viable cells per mL.
Live Staining of ASCs
Cell culture medium was replaced with fresh medium containing the primary antibody CD90 2.5 μg/mL (eBioscience, San Diego, CA). Adipose-derived stem cells were incubated in 5% CO2 at 37°C for 30 minutes. The antibody-containing medium was gently removed, and cells were washed 3 times with PBS (Biological Industries). Fresh cell culture medium was added, and cells were immediately examined by fluorescence microscopy with appropriate filters.
FACS
Samples were analyzed using an 8-color 3-laser FACSCanto II flow cytometer (BD Biosciences, Franklin Lakes, NJ) using a 100-μm nozzle. Forward-sideward-scatter dot plots were used to exclude debris and cell aggregates.
The following fluorochrome-conjugated monoclonal antibodies were used: CD45-eFluor 450, CD90-FITC, CD34-APC, CD105-PE, and CD73-PerCP-eFluor 710. All antibodies were purchased from eBioscience. Data were analyzed using BD FACSDiva software (BD Biosciences).
XTT Cell Proliferation Assay
Adipose-derived stem cells were seeded in 96-well plates (300 cells/well) for cell proliferation assays using a 3-bis-(2-methoxy-4-nitro-5-sulfenyl)-(2H)-tetrazolium-5-carboxanilide (XTT) kit (Biological Industries). The absorbance of the samples was measured using an ELISA reader 680 (Bio-Rad, Hercules, CA) at a wavelength of 450 nm subtracted by 655 nm. All experiments were performed in duplicate.
Immunofluorescence Staining and Microscopic Analysis
Adipose-derived stem cells were plated into 6-well plates with 13-mm-diameter cover glasses. After incubation, the cells were fixed and permeabilized with cold PHEMO buffer, 3.7% formaldehyde, 0.05% glutaraldehyde, and 0.5% Triton X-100 for 10 minutes. Briefly, blocking was performed in 1% BSA and 10% normal donkey serum in PBS. Cells were subsequently incubated with primary anti-NANOG antibody (Abcam, Cambridge, United Kingdom, NNG-811 ab62734) or with anti-OCT4 (Abcam, Cambridge, United Kingdom, EPR17929 ab181557) in primary antibody dilution buffer (Biomeda, Foster City, CA), followed by incubation with Alexa Fluor 594 donkey antimouse or Alexa Fluor 488 donkey antirabbit (Invitrogen, Carlsbad, CA), respectively. Cells were mounted with DAPI fluorescence mounting medium (Golden Bridge International [GBI] Life Science, Inc., Mukilteo, WA), and fluorescence digital images were captured using an Olympus ix81-ZDC microscope.
Quantitative Real-Time PCR Analysis
Total RNA was extracted from cells using a NucleoSpin RNA II kit (Macherey-Nagel, Düren, Germany) following the manufacturer's instructions. One microgram of total RNA was reverse transcribed into cDNA using ProtoScript II First Strand cDNA Synthesis Kit (New England Biolabs, Ipswich, MA). Quantitative real-time PCR analyses were performed to determine the expression of the target genes: vascular endothelial growth factor (VEGF), von-Willebrand factor (v-WF), and CD31. β-actin was selected as a housekeeping gene for mRNA normalization. Specific primers for the genes v-WF and CD31 were designed using the GenScript primer design tool (https://www.genscript.com/tools/real-time-pcr-taqman-primer-design-tool). Primers for VEGF and β-actin were designed as previously described.35 Quantitative real-time PCR was conducted in duplicate sets for each sample using SyGreen Blue Mix Hi-ROX (PCR Biosystems, London, United Kingdom) in a StepOnePlus Real-Time PCR System (Applied Biosystems).
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). Student's t-test was used to calculate P values, and a P value of 0.05 or less was considered statistically significant.
RESULTS
Adipose tissue laser lipoaspiration was performed in all 10 subjects. The average age of the subjects was 47.7 ± 3.8 years, and the average BMI was 27.7 ± 1.4. Liposuction sites included the abdomen and thighs, and the average lipoaspirate volume was 1844 ± 316 mL (Table 1). After liposuction with LipoLife, harvested lipoaspirate was either enzymatically digested (collagenase) or nonenzymatically treated (vortex). Then, fat samples were centrifuged to isolate the SVF. The SVF cell pellet was cultured, and the adherent ASCs were further analyzed to confirm stem cell markers (Fig. 1).
Viability of SVF Cells and ASCs in Lipoaspirates Obtained With Laser
The average SVF cell viability measured with trypan blue was 8.7 × 106 ± 3.9 × 106 and 9.4 × 106 ± 4.2 × 106 cells per mL of lipoaspirate and reached high SVF viability values of 97% ± 0.02% and 98% ± 0.01% in nonenzymatic and enzymatic manipulations, respectively. After 7 days of culture, the isolated ASC average counts were 1 × 106 ± 2.2 × 105 and 1.2 × 106 ± 5 × 105 cells per mL in nonenzymatic and enzymatic manipulations, respectively (Figs. 2A–B).
FIGURE 2.
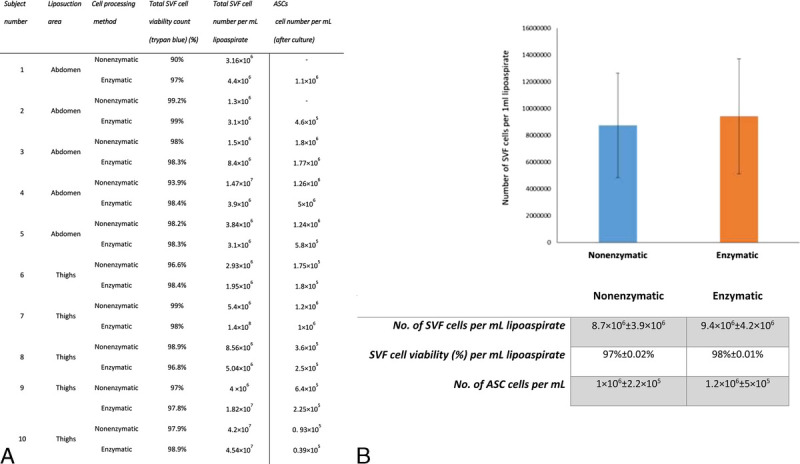
SVF cells and ASCs yield in enzymatic and nonenzymatic processing methods in LAL aspirates. A, SVF viability and ASC count in samples derived from abdominal and thigh laser-assisted lipoaspirates following enzymatic and nonenzymatic processing methods. B, Graphical representation of averaged values of A. Error bars represent SEM from all subjects. No significant differences were found between the 2 groups.
Live Staining of ASCs Derived From LAL
Live staining of ASCs revealed intense and homogenous CD90 staining in ASCs isolated by the 2 processing methods (Fig. 3). This demonstrates that the isolated ASCs expressed the necessary mesenchymal marker, verifying them as stem cells. This marker was missing in the negative control (prostate carcinoma (PC-3) cell line).
Viability of SVF Cells and ASCs in Lipoaspirates Obtained With or Without Laser
Preliminary analysis of the yield obtained in 2 subjects revealed 3.6-fold SVF cells levels in the enzymatic manipulation of lipoaspirates obtained with the laser (2.5 × 107) compared with mechanical liposuction (6.9 × 106). This difference was found to be highly significant (P < 0.001) (Figs. 4A–B). CD90 expression levels in the isolated ASCs were as high as those in the positive control hMSC (Fig. 4C).
FIGURE 4.
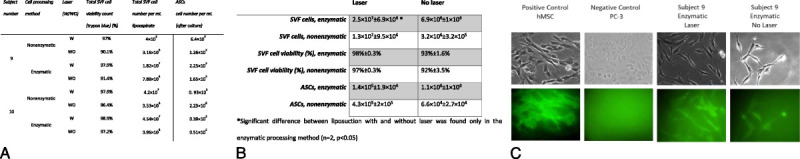
SVF and ASC yield per mL of lipoaspirates harvested from the thighs with or without laser. These are representative results of ASCs isolated from an enzymatically processed laser and nonlaser lipoaspirates of subject 9. A, Table showing SVF viability and ASC count in samples derived from abdominal and thigh lipoaspirates following enzymatic and nonenzymatic processing methods. B, Summary of the data presented in panel A. The corresponding SEM values from 2 individuals are shown. A significant difference (P < 0.001) was found between the laser and mechanical liposuction in the enzymatic processing method. *Significant difference between liposuction with and without laser was found only in the enzymatic processing method (n = 2, P < 0.05). (C) Fluorescent staining of ASCs derived from the enzymatic processing method. Higher expression of CD90 was detected during laser harvesting. hMSC was used as a positive control, and the PC-3 cell line was used as a negative control (microscope magnification, 20×). SVF and ASCs of lipoaspirates obtained from 2 subjects with or without laser liposuction.
FACS of ASCs Derived From Laser-Assisted Aspirates
Stromal vascular fraction cells contained a large number of erythrocytes; therefore, erythrocytes and other debris were excluded from the analysis by gating them out by cell size. Consequently, only nucleated cells were analyzed. Compared with commercial hMSCs, samples of isolated cells contained similar percentages of cells positive markers CD90, CD105, and CD73 and similar percentages for negative markers CD34 and CD45, suggesting that isolated cells from samples contained a large number of ASCs (Figs. 5A–B).
FIGURE 5.
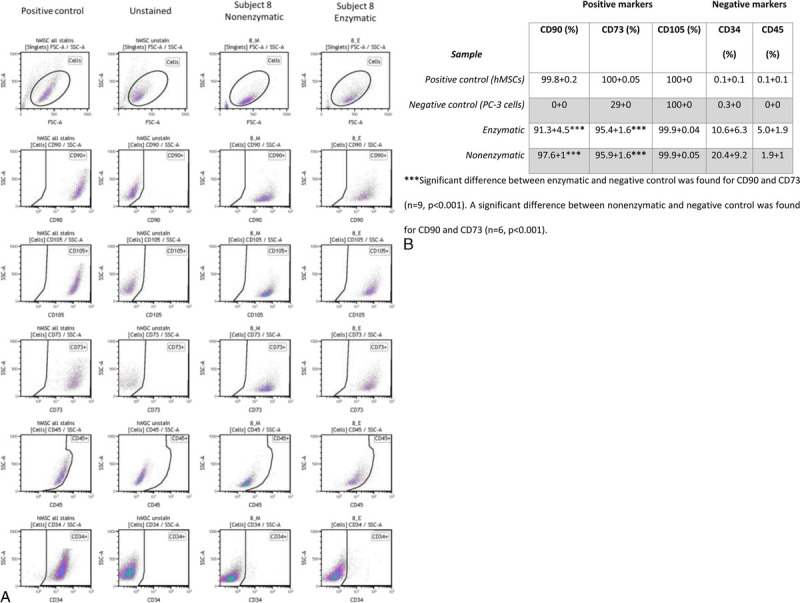
FACS analysis of ASCs. A, Expression of surface stem cell markers, positive (CD90, CD73, and CD105) and negative (CD34 and CD45) detected by flow cytometric analysis of cultured ASCs. Data show a representative set of dot plot from subject 5 compared with the positive control (hMSCs) and negative control (unstained hMSCs) (B, Summary of the immunophenotypic characteristics of ASCs. For the enzymatic processing method, data are representative of the analysis of 9 individuals, and for the nonenzymatic processing method, data are representative of the analysis of 6 individuals compared with the positive control (hMSCs) and negative control (PC-3 cells). ***Significant difference between enzymatic and negative control was found for CD90 and CD73 (n = 9, P < 0.001). A significant difference between nonenzymatic and negative control was found for CD90 and CD73 (n = 6, P < 0.001).
Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction Analysis of Laser Lipoaspirates and ASC mRNA
Quantitative real-time Polymerase Chain Reaction (PCR) was performed to evaluate the gene expression levels of the endothelial cell markers, endothelial growth factor (VEGF-A), CD31, and v-WF. All laser lipoaspirates showed significantly high expression of VEGF-A (**, P < 0.01), CD31 (***, P < 0.001), and v-WF (***, P < 0.001) compared with non–laser-assisted harvested lipoaspirates. All ASC samples showed significantly high expression of endothelial markers CD31 (*, P < 0.05) and v-WF (***, P < 0.001) compared with positive control hMSCs (Figs. 6A–B).
FIGURE 6.
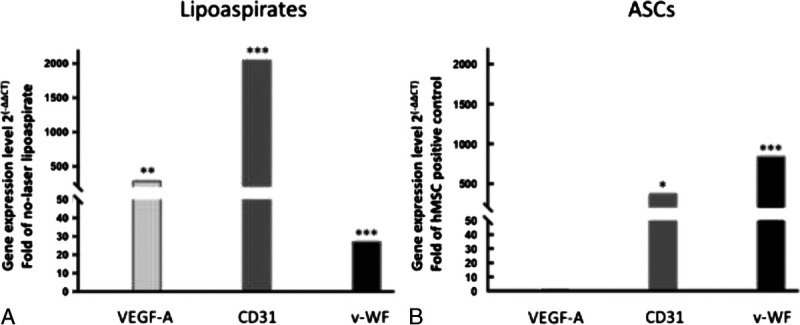
Real-time quantitative analysis of gene expression levels of VEGF-A, CD31, and v-WF in laser lipoaspirates and isolated ASCs. Quantitative real-time PCR analysis was performed in triplicate for each laser lipoaspirate to evaluate the gene expression levels of the endothelial cell markers VEGF-A, CD31, and v-WF. A, Bars represent the average value of gene expression in laser lipoaspirate harvested from 8 subjects. Results were compared to non-laser-assisted harvested lipoaspirates from 2 subjects. All laser lipoaspirates showed significantly high expression of VEGF-A (**, P < 0.01), CD31 (***, P < 0.001), and v-WF (***, P < 0.001) compared to non-laser-assisted harvested lipoaspirates. B, Bars represent the average value of gene expression from 5 ASC samples extracted from laser lipoaspirates that were processed by enzymatic and nonenzymatic methods. Results were compared with those of the commercial positive control hMSCs. All ASC samples showed significantly high expression of endothelial markers CD31 (*, P < 0.05) and v-WF (***, P < 0.001) compared with positive control hMSCs.
Immunofluorescence Staining of Proliferating ASCs
NANOG and OCT4 play key roles in the process of differentiation in MSCs as they are related to self-renewal capacity.24,36 Adipose-derived stem cells from enzymatic and nonenzymatic processing methods were found to similarly express these 2 transcription factors 7 days after seeding (Fig. 7).
FIGURE 7.
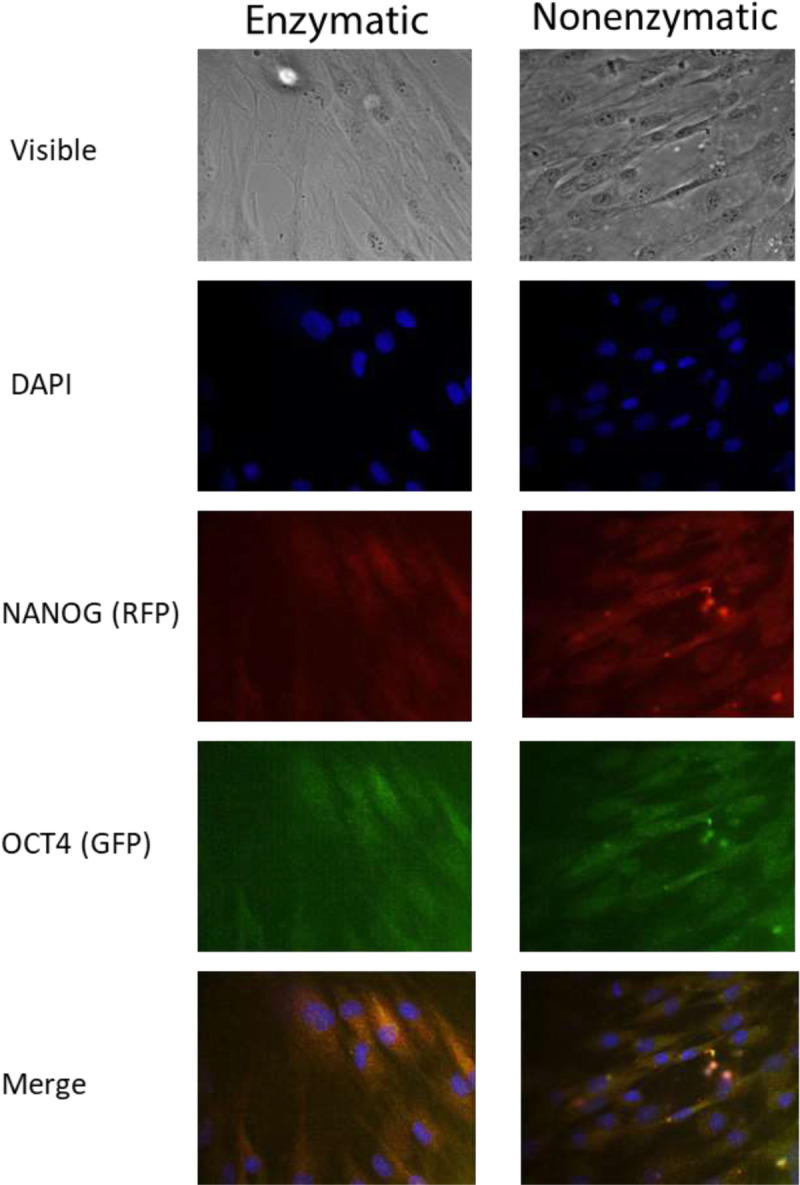
Immunofluorescence expression of OCT4 and NANOG in proliferating ASCs derived from LAL. Expression of ASCs markers in primary cultures. Proliferating ASCs expressed high levels of the transcription factors NANOG (red fluorescence) and OCT4 (green fluorescence). Nuclei are depicted by the blue fluorescence of 4′,6-diamidino-2-phenylindole (DAPI). The representative data shown are of subject 8 (microscope magnification, 20×).
ASCs Proliferation Capacity
The XTT cell proliferation assay was performed after 1, 4, 8, 12, 16, and 20 days (Fig. 8). Two growth curves for the 2 processing methods are presented with higher variability noted in the last 2 time points. In both processing methods, ASCs continue to proliferate on day 12 and reach the highest proliferating rate on day 16, followed by a subsequent decline.
FIGURE 8.
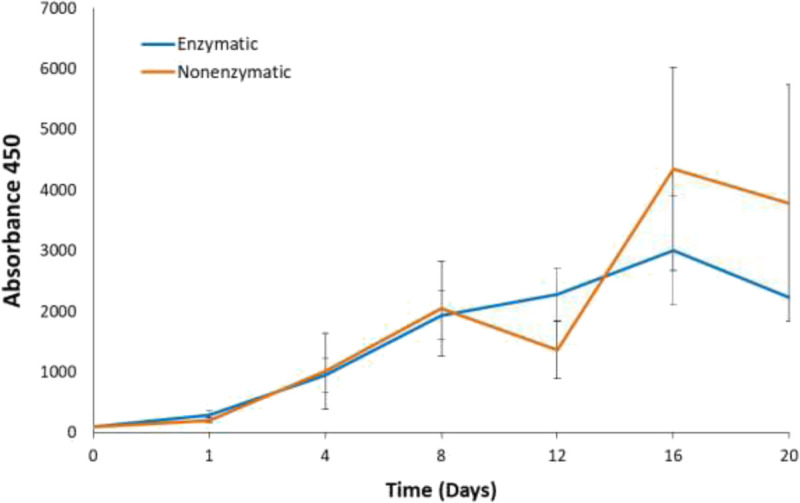
XTT proliferation assay for ASCs derived from LAL. A total of 300 SVF cells, derived from enzymatic or nonenzymatic processing methods, were seeded in a 96-well plate with different incubation periods. No statistical significance was detected between the 2 processing methods. Values are presented as mean ± SEM of 10 lipoaspirates.
DISCUSSION
This study aimed to assess the viability and proliferative potency of SVF and ASCs isolated from harvested laser lipoaspirates. Achieving high cell viability and high cell count in nonenzymatic manipulation is a huge advantage.
Human nonembryonic adult MSCs, including blood, bone marrow, and ASCs, represent important cell resources and hold great promise for cell-based therapies.17 Bone marrow-derived MSCs are considered the main source of MSCs for clinical applications.37–39 In comparison, adipose tissue also contains a large number of MSCs and is easier to isolate.39
Fat tissue consists of mature adipocytes, SVF cells, blood vessels, lymph nodes, and nerves. The SVF contains ASCs, preadipocytes, endothelial cells, pericytes, macrophages, and fibroblasts. Adipose-derived stem cells or multipotent MSCs can proliferate or differentiate into adipocytes.29
The relative ease by which ASCs can be obtained has prompted many studies on their reconstructive potential. Adipose SVF cells can be easily isolated from the lipoaspirate using enzyme digestion or mechanical protocols. Within heterogeneous SVF cells, the subgroup of ASCs is usually isolated through plastic adherence in culture conditions.
Using biological enzymes to disrupt the tissue has raised safety concerns where some countries do not even permit its use for fat grafting.29 In fact, the FDA considers cell populations produced by enzymatic manipulation “more than minimally manipulated” and demands heavy regulation.30 Thus, alternative processing techniques need to be developed. Achieving high cell viability and cell count in nonenzymatic manipulation would definitely be an advantage.29
In this study, we report high yields of SVF cells and ASCs from both enzymatically and nonenzymatically processed lipoaspirates. Other studies have reported lower yields from mechanical processing compared with that from enzymatic methods, probably due to the tightly bound cells in adipose connective tissue, which is not efficiently disrupted by mechanical action alone.30,40 We suggest that our reported similar cell numbers, using both preparation methods, were caused by laser assistance in liposuction even though lasers are known to hinder the quality of isolated adipocytes in laser liposuction.10,41 Furthermore, histology data of the 1470-nm laser liposuction showed no effect on the quality of the adipocytes (data not shown). The lipospirates were gently harvested due to the unique radial design of the fiber, which results in a less aggressive treatment due to the low energy density. This could be also attributed to the fact that the 1470-nm diode laser emits light that is preferentially absorbed by water and collagen,42 rendering it ideal for gentle fat tissue collection.
Strengthening our findings, Levenberg et al14 reported that abdominal fat samples harvested with the LipoLife were more homogenous, demonstrated higher viable adipocyte counts, and contained fewer fibrous and blood contaminants than those collected via mechanical liposuction.
In accordance with the criteria defined by the International Society for Cell Therapy for ASC identification,43 the FACS analysis and live staining showed specific profile markers for ASCs, which were found to be similar to the commercial human positive control ASCs. The controversial CD34 marker was found to be positive in subjects 3 and 4, similar to the positive control ASCs. This marker in ASCs has been the subject of dispute for many years. Its presence depends on the culture conditions, such as seeding density or type of culture medium.26,44
High gene expression levels of VEGF-A, CD31, and v-WF in the ASCs and their high expression of NANOG and OCT4 transcription factors confirmed the cells adipose tissue-derived origin and their pluripotency, consistent with the findings of Domenis et al.45
Another major issue with fat grafting is its absence of reproducibility.16 This could be attributed to variability in fat harvesting and processing methods3 and patient factors. These can include decreased proliferation and differentiation potential of ASCs with age, BMI, diabetes mellitus, and exposure to radiotherapy and tamoxifen.46 Our results show highly reproducible values in terms of the viability percentage and number of cells. The negligible variability observed in 10 different patients, with 2 different body areas and performed with 4 different surgeons, makes our clinical protocol highly reliable.
Comparing our findings to different processing methods is hard due to the use of different methodologies.47–49 Therefore, comparative investigations to other liposuction methods (eg, ultrasound-assisted, power-assisted, and mechanical) are essential. The lack of comparison to other liposuction methods, and the viability of these fat cells once they are grafted into the patient are limitations of the current study. Further studies should use the mechanically isolated fat cells as grafts and follow graft viability and longevity. In addition, future studies should examine a larger cohort with a larger number of males and a wider range of ages and BMIs. Another interesting direction will be to isolate spherical ASCs from LAL and examine their viability. The ASCs used in the study are slightly differentiated stem cells since they are incubated with serum and adhere to the cell culture plate, as compared with the spherical ASCs, which are less differentiated50 and therefore exhibit higher regenerative properties.51
An important consideration going forward is regulatory approval. Although autologous fat grafting, consisting of removing autologous cells from an individual and reimplanting them without intervening processing steps beyond rinsing, cleansing, sizing, or shaping does not require approval, adding cell expansion steps using serum will necessitate regulatory approval. Developing a protocol in which SVF cells and ASCs are isolated and prepared for implantation without adding external factors, will make this procedure easily clinically applicable.
CONCLUSIONS
The quality of harvested SVF cells and ASCs from lipoaspirates is of exceptional importance in the field of fat grafting and reconstruction surgery. It is vital to have a large number of stem cells that are in a state of potency for subsequent transfer.
We present an improved clinical and processing protocol for the harvesting of high-quality SVF cells and ASCs. The 1470-nm radial emitting laser fiber with a specialized cannula that enables simultaneous lasing and suction, thus keeping the adipose tissue intact, with nonenzymatic processing of the harvested lipoaspirates, demonstrated excellent cell count numbers and high-quality adipose stem cells, compared with the enzymatic processing. Clinically, this study shows that LAL extracts viable SVF cells and ASCs, and thus provide a source of cells for fat grafting. Furthermore, achieving high viability levels using LAL and mechanical isolation makes cell isolation simpler and safer, and does not require regulatory approval.
The improved purity of the harvested lipoaspirate and high ASC content are expected to result in extended graft longevity.
Footnotes
Conflicts of interest and sources of funding: None declared.
Contributor Information
Lori Plonski, Email: loriplonski@gmail.com.
Shaked Menashe, Email: Mailtoshaked@gmail.com.
Andre Ofek, Email: ofek@post.bgu.ac.il.
Adaya Rosenthal, Email: adayaar@gmail.com.
Massimiliano Brambilla, Email: massimiliano.brambilla@policlinico.mi.it.
Gary Goldenberg, Email: garygoldenbergmd@gmail.com.
Sahar Haimowitz, Email: haimowitz.sahar@gmail.com.
Lior Heller, Email: heller001@hotmail.com.
REFERENCES
- 1.Statistics P . American Society of Plastic Surgeons 2018 Plastic Surgery Statistics Report. Plastic Surgery. 2017;25. [Google Scholar]
- 2.Zunic B, Peter S. World’s Largest Science, Technology & Medicine Open Access Book Publisher. Vol Chapter 10; 2018. [Google Scholar]
- 3.Fontes T Brandão I Negrão R, et al. Autologous fat grafting: harvesting techniques. Annals of Medicine and Surgery. 2018;36:212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schafer ME Hicok KC Mills DC, et al. Acute adipocyte viability after third-generation ultrasound-assisted liposuction. Aesthet Surg J. 2013;33:698–704. [DOI] [PubMed] [Google Scholar]
- 5.Palumbo P Lombardi F Siragusa G, et al. Methods of isolation, characterization and expansion of human adipose-derived stem cells (ASCs): an overview. Int J Mol Sci. 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leclere FMP Trelles M Moreno-Moraga J, et al. 980-nm laser lipolysis (LAL): about 674 procedures in 359 patients. J Cosmet Laser Ther. 2012;14:67–73. [DOI] [PubMed] [Google Scholar]
- 7.Chia CT, Theodorou SJ. 1,000 consecutive cases of laser-assisted liposuction and suction-assisted lipectomy managed with local anesthesia. Aesthet Plast Surg. 2012;36:795–802. [DOI] [PubMed] [Google Scholar]
- 8.Katz B, Mcbean J. Laser-assisted lipolysis: a report on complications. J Cosmet Laser Ther. 2008;10:231–233. [DOI] [PubMed] [Google Scholar]
- 9.Leclère FM Alcolea JM Vogt PM, et al. Laser-assisted lipolysis for arm contouring in Teimourian grades III and IV: a prospective study involving 22 patients. Plast Surg (Oakv). 2016;24:35–40. [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira-Netto D Montano-Pedroso JC Aidar AL, et al. Laser-assisted liposuction (LAL) versus traditional liposuction: systematic review. Aesthet Plast Surg. 2018;42:376–383. [DOI] [PubMed] [Google Scholar]
- 11.Trelles MA Mordon SR Bonanad E, et al. Laser-assisted lipolysis in the treatment of gynecomastia: a prospective study in 28 patients. Lasers Med Sci. 2013;28:375–382. [DOI] [PubMed] [Google Scholar]
- 12.Rho YK Kim BJ Kim MN, et al. Laser lipolysis with pulsed 1064 nm Nd:YAG laser for the treatment of gynecomastia. Int J Dermatol. 2009;48:1353–1359. [DOI] [PubMed] [Google Scholar]
- 13.Ali YH. Laser-assisted lipolysis burn safety: proposed detailed parameters with assessment of their efficacy and safety. Plastic Reconstr Surg–Global Open. 2018;6:e1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levenberg A, Scheinowitz M, Sharabani-Yosef O. Higher cell viability and enhanced sample quality following laser-assisted liposuction versus mechanical liposuction. J Cosmet, Dermatol Sciences and Applications. 2015;5:238–245. [Google Scholar]
- 15.Doornaert M Colle J De Maere E, et al. Autologous fat grafting: latest insights. Annals of Medicine and Surgery. 2019;37:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma L Wen H Jian X, et al. Cell-assisted lipotransfer in the clinical treatment of facial soft tissue deformity. Can J Plast Surg. 2015;23:199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellei B Migliano E Tedesco M, et al. Maximizing non-enzymatic methods for harvesting adipose-derived stem from lipoaspirate: technical considerations and clinical implications for regenerative surgery. Sci Rep. 2017;7:10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iyyanki T Hubenak J Liu J, et al. Harvesting technique affects adipose-derived stem cell yield. Aesthet Surg J. 2015;35:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabriel A, Champaneria MC, Maxwell GP. Fat grafting and breast reconstruction: tips for ensuring predictability. Gland Surg. 2015;4:232–23243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cucchiani R, Corrales L. The effects of fat harvesting and preparation, air exposure, obesity, and stem cell enrichment on adipocyte viability prior to graft transplantation. Aesthet Surg J. 2016;36:1164–1173. [DOI] [PubMed] [Google Scholar]
- 21.Duscher D Luan A Rennert RC, et al. Suction assisted liposuction does not impair the regenerative potential of adipose derived stem cells. J Transl Med. 2016;14:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberbauer E Steffenhagen C Wurzer C, et al. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: current state of the art. Cell Regen. 2015;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiu CH Tong YW Yeh WL, et al. Self-renewal and differentiation of adipose-derived stem cells (ADSCs) stimulated by multi-axial tensile strain in a pneumatic microdevice. Micromachines (Basel). 2018;9:607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francis A Wang WZ Goldman JJ, et al. Enhancement of viable adipose-derived stem cells in lipoaspirate by buffering tumescent with sodium bicarbonate. Plast Reconstr Surg Glob Open. 2019;7:e2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshimura K Sato K Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthet Plast Surg. 2008;32:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuk PA Zhu M Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sterodimas A De Faria J Nicaretta B, et al. Autologous fat transplantation versus adipose-derived stem cell-enriched lipografts: a study. Aesthet Surg J. 2011;31:682–693. [DOI] [PubMed] [Google Scholar]
- 29.Bourin P Bunnell BA Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aronowitz JA, Lockhart RA, Hakakian CS. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus. 2015;4:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Condé-Green A Kotamarti VS Sherman LS, et al. Shift toward mechanical isolation of adipose-derived stromal vascular fraction: review of upcoming techniques. Plast Reconstr Surg Glob Open. 2016;4:e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aronowitz JA Lockhart RA Hakakian CS, et al. Adipose stromal vascular fraction isolation. Ann Plast Surg. 2016;77:354–362. [DOI] [PubMed] [Google Scholar]
- 33.Cai Z Pan B Jiang H, et al. Chondrogenesis of human adipose-derived stem cells by in vivo co-graft with auricular chondrocytes from microtia. Aesthet Plast Surg. 2015;39:431–439. [DOI] [PubMed] [Google Scholar]
- 34.Gimble JM. Adipose tissue-derived therapeutics. Expert Opin Biol Ther. 2003;3:705–713. [DOI] [PubMed] [Google Scholar]
- 35.Amir S Wang R Matzkin H, et al. MSF-A interacts with hypoxia-inducible factor-1alpha and augments hypoxia-inducible factor transcriptional activation to affect tumorigenicity and angiogenesis. Cancer Res. 2006;66:856–866. [DOI] [PubMed] [Google Scholar]
- 36.Jang Y Koh YG Choi YJ, et al. Characterization of adipose tissue-derived stromal vascular fraction for clinical application to cartilage regeneration. In Vitro Cell Dev Biol Anim. 2014;51:142–150. [DOI] [PubMed] [Google Scholar]
- 37.Strioga M Viswanathan S Darinskas A, et al. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–2752. [DOI] [PubMed] [Google Scholar]
- 38.Ayatollahi M Geramizadeh B Zakerinia M, et al. Human bone marrow-derived mesenchymal stem cell: a source for cell-based therapy. Int J Organ Transplant Med. 2012;3:32–41. [PMC free article] [PubMed] [Google Scholar]
- 39.Busser H De Bruyn C Urbain F, et al. Isolation of adipose-derived stromal cells without enzymatic treatment: expansion, phenotypical, and functional characterization. Stem Cells Dev. 2014;23:2390–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Research and Therapy. 2017;8:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mulholland RS, Paul MD, Chalfoun C. Noninvasive body contouring with radiofrequency, ultrasound, cryolipolysis, and low-level laser therapy. Clin Plast Surg. 2011;38:503–520. [DOI] [PubMed] [Google Scholar]
- 42.Sekar SK Bargigia I Mora AD, et al. Diffuse optical characterization of collagen absorption from 500 to 1700 nm. J Biomed Opt. 2017;22:15006. [DOI] [PubMed] [Google Scholar]
- 43.Dominici M Le Blanc K Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell JB McIntosh K Zvonic S, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. [DOI] [PubMed] [Google Scholar]
- 45.Domenis R Lazzaro L Calabrese S, et al. Adipose tissue derived stem cells: in vitro and in vivo analysis of a standard and three commercially available cell-assisted lipotransfer techniques. Stem Cell Res Ther. 2015;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varghese J Griffin M Mosahebi A, et al. Systematic review of patient factors affecting adipose stem cell viability and function: implications for regenerative therapy. Stem Cell Res Ther. 2017;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Argentati C Morena F Bazzucchi M, et al. Adipose stem cell translational applications: from bench-to-bedside. Int J Mol Sci. 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simonacci F Bertozzi N Grieco MP, et al. Procedure, applications, and outcomes of autologous fat grafting. Ann Med Surg (Lond). 2017;20:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markarian CF Frey GZ Silveira MD, et al. Isolation of adipose-derived stem cells: a comparison among different methods. Biotechnol Lett. 2014;36:693–702. [DOI] [PubMed] [Google Scholar]
- 50.di Stefano AB Grisafi F Castiglia M, et al. Spheroids from adipose-derived stem cells exhibit an miRNA profile of highly undifferentiated cells. J Cell Physiol. 2018;233:8778–8789. [DOI] [PubMed] [Google Scholar]
- 51.di Stefano A Leto Barone A Giammona A, et al. Identification and expansion of adipose stem cells with enhanced bone regeneration properties. Journal of Regenerative Medicine. 2016;2016. [Google Scholar]


