To the Editor:
A recent study indicated that, in mice, the lung harbors megakaryocytes and is a site of platelet biogenesis (1). The presence of megakaryocytes has also been reported in human lungs (2, 3); however, the characteristics and role of lung megakaryocytes in homeostatic conditions, as well as chronic lung diseases such as idiopathic pulmonary fibrosis (IPF), are not well understood.
We investigated whether megakaryocyte/platelet gene signatures are altered in IPF by analyzing publicly available transcriptome datasets with xCell (4), a novel computational method. We further sought to determine whether platelet count and mean platelet volume (MPV) in peripheral blood, two platelet parameters routinely measured in clinical practice (as a part of complete blood count), are associated with lung transplant-free survival in our own IPF cohort.
Some of the results of these studies have been previously reported in the form of a preprint (medRxiv, [21 June 2022] https://doi.org/10.1101/2022.04.04.22273424).
Methods
We analyzed the following NCBI (National Center for Biotechnology Information) Gene Expression Omnibus (GEO) transcriptome datasets from patients with IPF and healthy control subjects: lung tissue (GSE47460_GPL6480 and GSE47460_GPL14550), BAL cells (GSE70867), and peripheral whole blood (GSE33566 and GSE93606). We generated gene signature enrichment scores for megakaryocytes and platelets with xCell, a computational method that assesses enrichment of individual cell types on the basis of gene expression profile, and compared the scores between patients with IPF and control subjects using an unpaired t test or Mann-Whitney test. In GSE70867 and GSE93606 (in which survival data were available), we compared event-free survival between patients with higher enrichment scores (above the median) and patients with lower enrichment scores (below the median) using a log-rank test.
We also analyzed data from our own Tulane IPF cohort to determine whether platelet count and MPV in peripheral blood are associated with lung transplant-free survival. We performed Cox proportional hazards regression analysis, adjusting for age, sex, forced vital capacity (FVC), and diffusing capacity for carbon monoxide (DlCO). This study was approved by the Tulane University Institutional Review Board. P < 0.05 was considered statistically significant.
Results
In lung tissue, patients with IPF had significantly lower megakaryocyte enrichment scores than control subjects (i.e., IPF lung transcriptome was significantly less enriched with a megakaryocyte gene signature than the control lung transcriptome) (Figure 1A). Platelet scores were not significantly different between IPF lung transcriptome and control lung transcriptome. In BAL cells, patients with IPF had significantly lower platelet scores than control subjects, and IPF patients with lower platelet scores had significantly worse lung transplant-free survival rates than IPF patients with higher scores (log-rank test P < 0.0001; hazard ratio, 2.190; 95% confidence interval [CI], 1.476–3.249) (Figure 1B). In contrast, in peripheral whole blood, patients with IPF had significantly higher megakaryocyte scores than control subjects, and IPF patients with higher megakaryocyte scores had significantly worse disease progression-free survival (i.e., survival without significant [>10%] decline in FVC over 6 months) than IPF patients with lower megakaryocyte scores (log-rank test P = 0.0096; hazard ratio, 2.535; 95% CI, 1.254–5.128) (Figure 1C). Platelet scores were not significantly different between IPF blood transcriptome and control blood transcriptome.
Figure 1.
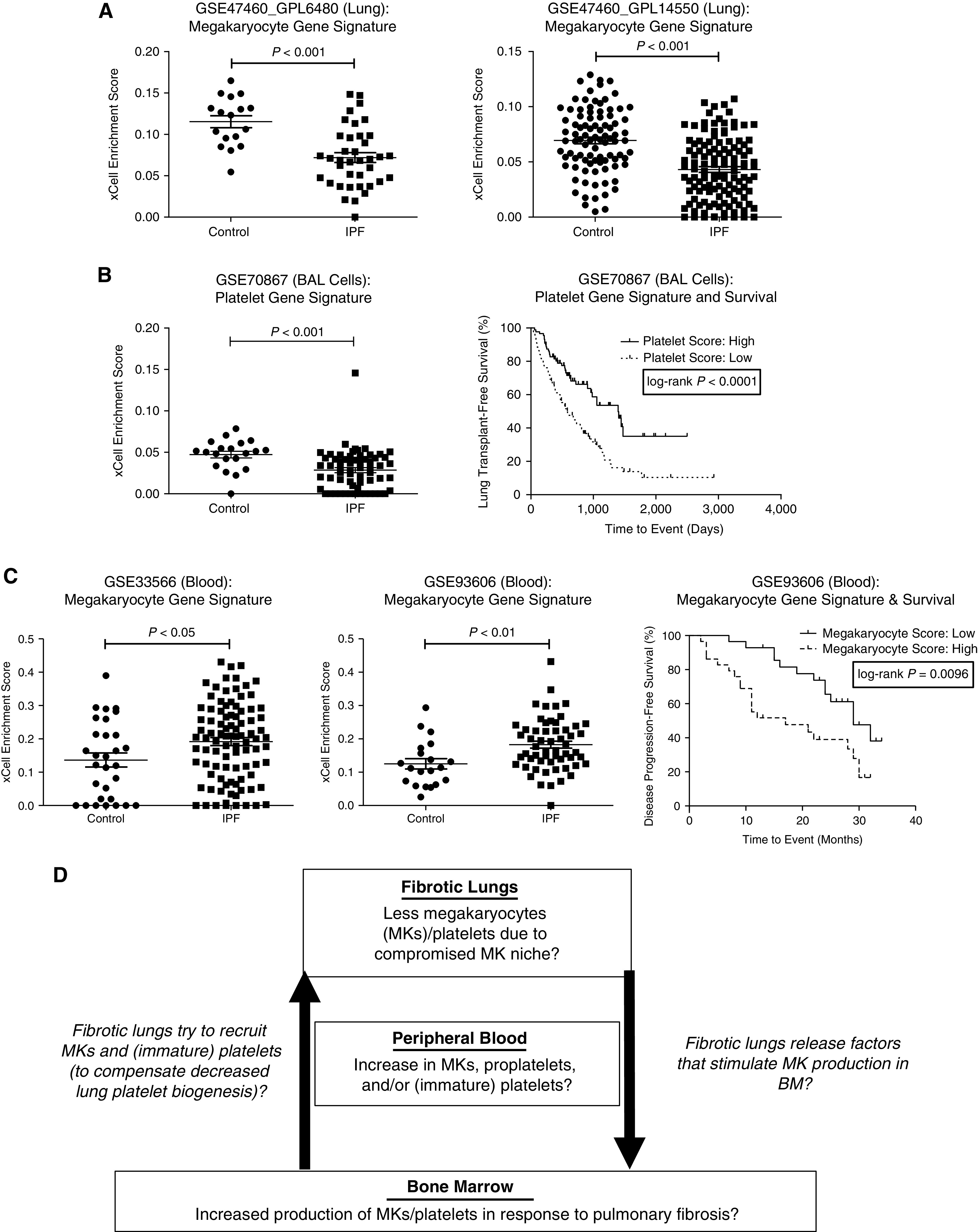
Megakaryocyte/platelet gene signatures were altered in idiopathic pulmonary fibrosis (IPF) in a compartment-specific manner. We analyzed IPF transcriptome datasets available in the NCBI (National Center for Biotechnology Information) Gene Expression Omnibus (GEO). We generated gene signature enrichment scores for megakaryocytes and platelets with xCell (https://xcell.ucsf.edu/), a novel computational method that assesses the enrichment of individual cell types on the basis of gene expression profiles. (A) In lung transcriptome (GSE47460_GPL6480 and GSE47460_GPL14550), patients with IPF had significantly lower megakaryocyte gene signature enrichment scores than control subjects (who underwent surgery for the investigation of a nodule but had no chronic lung disease by computed tomography or pathology). (B) In BAL cell transcriptome (GSE70867), patients with IPF had significantly lower platelet gene signature enrichment scores than control subjects (left: Freiburg cohort only, for which control subjects were available). IPF patients with lower platelet scores (i.e., below the median) had significantly worse lung transplant–free survival than IPF patients with higher platelet scores (i.e., above the median) (log-rank test P < 0.0001; hazard ratio, 2.190; 95% confidence interval [CI], 1.476–3.249) (right: overall cohort [Freiburg, Siena, and Leuven cohort]). (C) In peripheral whole blood transcriptome (GSE33566 and GSE93606), IPF patients had significantly higher megakaryocyte scores than age- and sex-matched healthy control subjects (left, middle). IPF patients with higher megakaryocyte scores (above the median) had worse disease progression–free survival (defined as survival without a significant [>10%] decline in FVC over 6 months) than IPF patients with lower megakaryocyte scores (below the median) (log-rank test P = 0.0096; hazard ratio, 2.535; 95% CI, 1.254–5.128) (right). (D) Working hypothesis (proposed schema): altered megakaryocyte/platelet biogenesis in IPF. BM = bone marrow; MK = megakaryocyte.
In our Tulane IPF cohort (n = 184) (Table 1), univariate analysis revealed that higher MPV (but not higher platelet count) was significantly associated with worse lung transplant-free survival (P = 0.042). More importantly, multivariate analysis revealed that higher MPV (but not higher platelet count) was significantly associated with worse lung transplant-free survival, independent of age, sex, FVC, and DlCO (P = 0.031) (multivariate analysis 1 and 2). Furthermore, higher MPV was significantly associated with worse lung transplant-free survival, independent of age, sex, FVC, DlCO, and platelet count (P = 0.012) (multivariate analysis 3).
Table 1.
Survival Analysis in Our Tulane Idiopathic Pulmonary Fibrosis Cohort
| Clinical Characteristics | |||
|---|---|---|---|
| Age (yr), median (IQR) | 69.6 (64.5–75.7) | ||
| Sex: male, n (%) | 129 (70.1) | ||
| FVC (% predicted), median (IQR) | 69.0 (55.7–81.0) | ||
| DlCO (% predicted), median (IQR) | 45 (37.5–55.5) | ||
| MPV (femtoliters), median (IQR) | 9.90 (8.90–10.6) | ||
| Platelet count (per μl), median (IQR) | 229 (190–264) | ||
| Monocyte count (×109 per L), median (IQR) | 0.670 (0.55–0.84) | ||
| Cox Proportional Hazards Regression Model | |||
|---|---|---|---|
| P value | Hazard Ratio (95% CI) | ||
| Univariate Analysis | Age, yr | 0.006 | 1.037 (1.010–1.064) |
| Sex (male compared with female) | 0.162 | 1.446 (0.863–2.425) | |
| FVC (% predicted) | <0.001 | 0.970 (0.957–0.984) | |
| DlCO (% predicted) | <0.001 | 0.961 (0.944–0.979) | |
| MPV | 0.042 | 1.210 (1.007–1.453) | |
| Platelet count | 0.114 | 1.003 (0.999–1.006) | |
| Monocyte count | 0.502 | 1.435 (0.500–4.116) | |
| Multivariate Analysis 1 | Age, yr | 0.467 | 1.011 (0.982–1.040) |
| Sex (male compared with female) | 0.236 | 1.467 (0.779–2.763) | |
| FVC (% predicted) | 0.031 | 0.982 (0.966–0.998) | |
| DlCO (% predicted) | 0.002 | 0.969 (0.950–0.989) | |
| MPV | 0.031 | 1.235 (1.020–1.496) | |
| Multivariate Analysis 2 | Age, yr | 0.172 | 1.020 (0.992–1.048) |
| Sex (male compared with female) | 0.477 | 1.245 (0.680–2.282) | |
| FVC (% predicted) | 0.007 | 0.980 (0.965–0.994) | |
| DlCO (% predicted) | 0.009 | 0.976 (0.959–0.994) | |
| Platelet count | 0.147 | 1.003 (0.999–1.007) | |
| Multivariate Analysis 3 | Age, yr | 0.454 | 1.011 (0.982–1.040) |
| Sex (male compared with female) | 0.122 | 1.670 (0.872–3.200) | |
| FVC (% predicted) | 0.026 | 0.982 (0.967–0.998) | |
| DlCO (% predicted) | 0.003 | 0.971 (0.952–0.990) | |
| MPV | 0.012 | 1.291 (1.056–1.577) | |
| Platelet count | 0.075 | 1.004 (1.000–1.009) | |
Definition of abbreviations: CI = confidence interval; IQR = interquartile range; MPV = mean platelet volume.
Patients with IPF followed at Tulane Medical Center or University Medical Center New Orleans were enrolled in this study (N = 184). The diagnosis of IPF was made according to the international guideline (10).
Data on age, sex, and platelet count were available from all 184 patients. Data on FVC, DlCO, MPV, and monocyte count were available from 177, 169, 165, and 167 patients, respectively.
We performed a univariate analysis with variables that have been shown to be associated with survival in multiple studies, then performed a multivariate analysis with variables with P < 0.20 in univariate analysis.
Univariate analysis revealed that higher MPV (but not higher platelet count) was significantly associated with worse lung transplant–free survival (P = 0.042; hazard ratio, 1.210; 95% CI, 1.007–1.453).
Multivariate analysis revealed that higher MPV (but not higher platelet count) was significantly associated with worse lung transplant–free survival, independent of age, sex, FVC, and DlCO (P = 0.031; hazard ratio, 1.235; 95% CI, 1.020–1.496) (multivariate analysis 1 and 2). Moreover, higher MPV was significantly associated with worse lung transplant–free survival, independent of age, sex, FVC, DlCO, and platelet count (P = 0.012; hazard ratio, 1.291; 95% CI, 1.056–1.577) (multivariate analysis 3).
Discussion
Our analysis revealed that megakaryocyte/platelet gene signatures were altered in IPF in a compartment-specific manner. On the basis of our findings, one could speculate that IPF lungs harbor fewer (healthy) megakaryocytes because of their altered megakaryocyte niche and that megakaryocytes are actually decreased in the lungs of patients with IPF. So far, published single-cell RNA-sequence analyses have failed to identify a cluster of lung megakaryocytes in fibrotic lung tissue (5, 6). This may be because lung megakaryocytes are too rare or fragile to be isolated without any enrichment or a special protocol optimized for megakaryocytes.
There are a few possible explanations for less enrichment of megakaryocyte gene signature in IPF lung transcriptome and more enrichment of megakaryocyte gene signature in IPF blood transcriptome compared with control counterparts (Figure 1D). One possibility is that decreased megakaryocyte/platelet biogenesis in IPF lungs induces megakaryocyte/platelet biogenesis in the bone marrow. For example, bone marrow may be producing more megakaryocytes and/or releasing more megakaryocytes (or immature platelets) into the circulation in response to factors released by fibrotic lungs. The second possibility is that megakaryocytes cannot remain in the altered niche within IPF lungs and therefore escape into the circulation. The third possibility is that fibrotic lungs are “educating” circulating platelets and modifying their transcriptome, as shown in previous works on “tumor-educated platelets” (7).
There are notable limitations to our study. One major limitation is that in silico prediction/estimation of gene signature enrichment for various types of cells in tissues is still far from perfect. Another limitation is that a megakaryocyte gene signature derived from bone marrow megakaryocytes is unlikely to be fully applicable to lung megakaryocytes because lung megakaryocytes are transcriptionally (and phenotypically) distinct from bone marrow megakaryocytes. For example, lung megakaryocytes have higher expression of major histocompatibility complex class II and other molecules related to antigen presentation compared with bone marrow megakaryocytes (1, 8, 9). Furthermore, the prognostic utility of platelet gene signature in BAL cells, megakaryocyte gene signature in blood, and MPV in blood needs to be confirmed in other validation cohorts. Lastly, our study did not answer the following important questions: 1) Do megakaryocytes and/or platelets contribute to the pathogenesis of IPF?; and 2) Is the human lung a reservoir of megakaryocyte progenitors and a source of platelet biogenesis, as observed in mouse lungs? Further studies are needed to address these questions.
Conclusions
Megakaryocyte/platelet gene signatures are altered and may have prognostic value in IPF. Moreover, MPV, a platelet parameter routinely measured in clinical practice, may be a simple yet novel prognostic biomarker in IPF. Further studies are needed to elucidate megakaryocyte/platelet biogenesis and to validate the prognostic utility of megakaryocyte/platelet gene signatures as well as MPV in IPF.
Footnotes
Supported by the Southern Society for Clinical Investigation and the National Heart, Lung, and Blood Institute (K08HL146979).
Originally Published in Press as DOI: 10.1164/rccm.202206-1195LE on July 19, 2022
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Lefrançais E, Ortiz-Muñoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature . 2017;544:105–109. doi: 10.1038/nature21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine . 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Valdivia-Mazeyra MF, Salas C, Nieves-Alonso JM, Martín-Fragueiro L, Bárcena C, Muñoz-Hernández P, et al. Increased number of pulmonary megakaryocytes in COVID-19 patients with diffuse alveolar damage: an autopsy study with clinical correlation and review of the literature. Virchows Arch . 2021;478:487–496. doi: 10.1007/s00428-020-02926-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aran D, Hu Z, Butte AJ. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol . 2017;18:220. doi: 10.1186/s13059-017-1349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adams TS, Schupp JC, Poli S, Ayaub EA, Neumark N, Ahangari F, et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv . 2020;6:eaba1983. doi: 10.1126/sciadv.aba1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Habermann AC, Gutierrez AJ, Bui LT, Yahn SL, Winters NI, Calvi CL, et al. Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci Adv . 2020;6:eaba1972. doi: 10.1126/sciadv.aba1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sjors GJG, Wurdinger T. Tumor-educated platelets. Blood . 2019;133:2359–2364. doi: 10.1182/blood-2018-12-852830. [DOI] [PubMed] [Google Scholar]
- 8. Pariser DN, Hilt ZT, Ture SK, Blick-Nitko SK, Looney MR, Cleary SJ, et al. Lung megakaryocytes are immune modulatory cells. J Clin Invest . 2021;131:137377. doi: 10.1172/JCI137377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yeung AK, Villacorta-Martin C, Hon S, Rock JR, Murphy GJ. Lung megakaryocytes display distinct transcriptional and phenotypic properties. Blood Adv . 2020;4:6204–6217. doi: 10.1182/bloodadvances.2020002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med . 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]


