To the Editor:
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive disease with high mortality and limited treatment options (1). A potential therapeutic target is the inhibition of active TGF-β (transforming growth factor-β), a critical cytokine in the initiation and maintenance of fibrosis (2). In preclinical studies, partial inhibition of TGF-β using anti-αvβ6 integrin antibodies was effective in blocking murine pulmonary fibrosis without exacerbating inflammation (3–6).
BG00011 (formerly STX-100) is a first-in-class, humanized anti-αvβ6 IgG1 monoclonal antibody that blocks the binding of αvβ6 to the latent form of TGF-β, thereby inhibiting TGF-β activation. Animal studies showed that BAL cells act as biosensors of αvβ6-mediated TGF-β activation (7). pSMAD2, a key mediator of TGF-β activity (8) that correlates with lung fibrosis (9), was selected as an exploratory target biomarker.
We report the results of a phase IIa study that evaluated the safety and tolerability of multiple ascending doses of BG00011 in patients with IPF. The quantification of biomarkers of TGF-β activity in BAL cells was a secondary objective to evaluate biological activity; there were no efficacy objectives. Some of the results of these studies have been previously reported in the form of an abstract (7).
Methods
This randomized, double-blind, placebo-controlled, dose-escalation study (NCT01371305) was conducted from July 16, 2012, through March 31, 2017, at 17 sites in the United States. Patients with IPF with a predicted FVC ⩾ 50% were randomized 3:1 to receive subcutaneous BG00011 (n = 31) or placebo (n = 10) once weekly for 8 weeks. Patients were enrolled into 5 dose cohorts (0.015–3.0 mg/kg, n = 8 each) and followed for an additional 12 weeks. The study was approved by all relevant institutional review boards. Informed consent was obtained from all participants.
Safety evaluations included treatment-emergent adverse events (TEAEs), clinical and laboratory assessments, and pulmonary function testing (PFT). TEAEs of IPF exacerbation were diagnosed by the investigator on the basis of published criteria (10) and were not adjudicated.
BAL cells isolated via bronchoscopy at screening and up to Day 8 after dose were evaluated for changes in TGF-β activity biomarkers (pSMAD2 and seven prespecified genes: ALOX5, ETS1, FN1, OLR1, PAI-1, TGM2, and TREM1). An exposure–response relationship was evaluated on the basis of the BG00011 area under the concentration time curve at steady state (AUCSS) and the percent change from baseline in pSMAD2/tSMAD2.
All analyses were descriptive and exploratory.
Results
Baseline characteristics were similar between the BG00011 and placebo groups (mean age, 69 vs. 72 years; mean percent predicted FVC, 75% vs. 81%). Thirty patients (23 BG00011 and 7 placebo) completed the study (8 doses and follow-up) (Table 1). The 3.0 mg/kg dose cohort was terminated because of the prespecified stopping criteria of a clinically significant decline in respiratory function in four of six patients at Week 16; only two patients in this cohort completed the study.
Table 1.
Summary of Treatment-emergent Adverse Events
| n (%) | BG00011 Dose Cohort |
Overall |
|||||
|---|---|---|---|---|---|---|---|
| 0.015
mg/kg n = 6 |
0.1
mg/kg n = 6 |
0.3
mg/kg n = 6 |
1.0
mg/kg n = 7 |
3.0
mg/kg n = 6 |
BG00011 n = 31 |
Placebo n = 10 |
|
| Completed study (8 doses and follow-up) | 4 (66.7) | 6 (100) | 6 (100) | 5 (71.4) | 2 (33.3)* | 23 (74.2) | 7 (70.0) |
| Any TEAE | 6 (100) | 5 (83.3) | 6 (100) | 4 (57.1) | 6 (100) | 27 (87.1) | 7 (70.0) |
| TEAE occurring in ⩾4 patients | |||||||
| Cough | 1 (16.7) | 0 | 1 (16.7) | 1 (14.3) | 2 (33.3) | 5 (16.1) | 1 (10.0) |
| Dyspnea | 1 (16.7) | 2 (33.3) | 0 | 1 (14.3) | 1 (16.7) | 5 (16.1) | 1 (10.0) |
| IPF exacerbation | 0 | 0 | 0 | 2 (28.6) | 3 (50.0) | 5 (16.1) | 0 |
| Hypoxia | 2 (33.3) | 0 | 0 | 2 (28.6) | 0 | 4 (12.9) | 0 |
| Upper RTI | 2 (33.3) | 0 | 1 (16.7) | 0 | 1 (16.7) | 4 (12.9) | 1 (10.0) |
| Dizziness | 0 | 1 (16.7) | 1 (16.7) | 1 (14.3) | 1 (16.7) | 4 (12.9) | 1 (10.0) |
| Rash | 0 | 2 (33.3) | 2 (33.3) | 0 | 0 | 4 (12.9) | 0 |
| Serious TEAE | 1 (16.7)† | 0 | 0 | 2 (28.6)‡ | 1 (16.7)§ | 4 (12.9) | 0 |
Definition of abbreviations: IPF = idiopathic pulmonary fibrosis; RTI = respiratory tract infection; TEAE = treatment-emergent adverse event.
Adverse events were coded using the Medical Dictionary for Regulatory Activities version 20.0. Safety was assessed in all patients who received at least one dose of the study drug.
The 3.0 mg/kg dose cohort was terminated early because of a clinically significant decline in respiratory function at Week 16 in four of six patients.
One patient experienced two serious TEAEs. A complete atrioventricular block was observed on Day 11, requiring pacemaker placement. The patient restarted treatment and completed dosing. On Day 105, during the follow-up period (approximately 49 days after dosing was completed), the patient experienced a serious adverse event of hypoxemia, which lasted for 2 days and resolved.
One patient had IPF exacerbation on Day 60. Lung transplantation was required, and the patient was ultimately withdrawn from the study. The second patient had type II second-degree atrioventricular block requiring pacemaker implantation, syncope, and jaw fracture on Day 26 and had an IPF exacerbation on Day 38 that resulted in death after 4 days.
Respiratory failure after viral respiratory infection on Day 69, which lasted for 2 days and resolved.
Safety
TEAEs were reported in 27/31 patients (87.1%) who received at least one dose of BG00011 and 7/10 patients (70.0%) who received placebo (Table 1); most were mild or moderate. IPF exacerbation occurred in two patients in the 1.0 mg/kg cohort and three in the 3.0 mg/kg cohort. Eight serious adverse events were reported in four (12.9%) patients treated with BG00011, including one death.
At Week 16 (Day 8 of follow-up), one patient in the 1.0 mg/kg cohort, four patients in the 3.0 mg/kg cohort, and two patients in the placebo group had a clinically meaningful decline from baseline in PFT values (predefined as either total lung capacity ⩾8%, FEV1 ⩾ 12%, FVC ⩾ 12%, and/or diffusing capacity for carbon monoxide adjusted for hemoglobin ⩾15%). Of the four patients in the 3.0 mg/kg cohort, one had a clinically significant decline in total lung capacity without change in spirometry and without symptoms, and three had a clinically significant decline in FVC (one after dosing had been stopped). No clinically meaningful changes in PFT were observed in the 0.015 mg/kg, 0.1 mg/kg, and 0.3 mg/kg dose cohorts.
Biomarkers of TGF-β Activity
A dose-dependent decrease from baseline to last dose in pSMAD2/tSMAD2 ratio was observed in the 0.1, 0.3, 1.0, and 3.0 mg/kg cohorts (mean percentage change of −3.33%, −39.82%, −63.74%, and −76.20%, respectively). A ⩾70% reduction in pSMAD2 concentrations was observed in 1/5, 4/5, and 2/3 patients in the 0.3 mg/kg, 1.0 mg/kg, and 3.0 mg/kg cohorts, respectively. In the 3.0 mg/kg cohort, only three patients underwent BAL, and one did not have analyzable pSMAD2 data. ALOX5, FN1, OLR1, TGM2, and TREM1 also showed dose-dependent decreases in BAL cell expression.
Pharmacokinetics and Exposure–Response
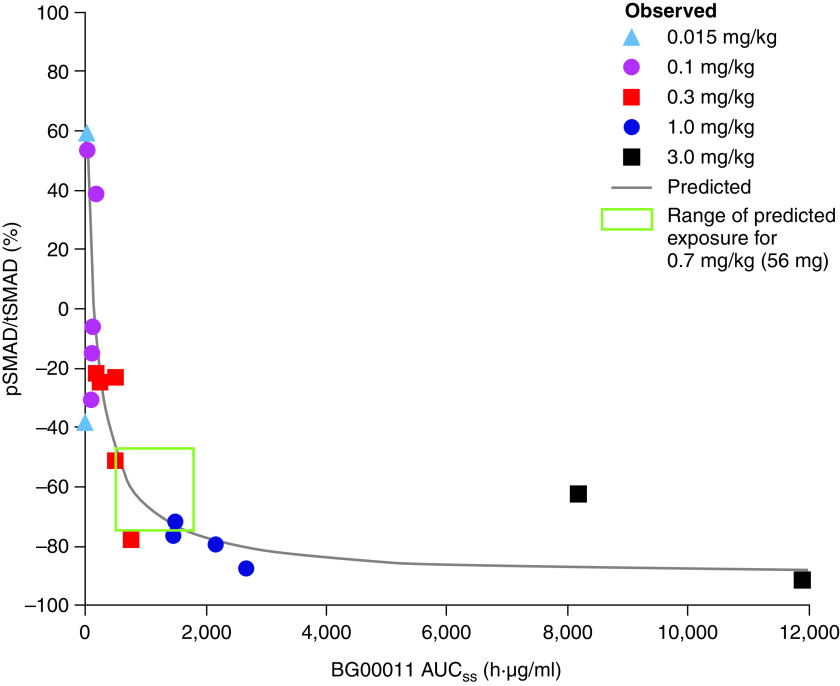
The BG00011 half-life was approximately 6 days, and a steady state was reached after the fifth dose with minimal (∼1.5×) accumulation using weekly dosing. A dose–response was observed between the BG00011 AUCSS after the last dose and the percent change from baseline in pSMAD2/tSMAD2, starting at the 0.3 and 1.0 mg/kg doses (Figure 1).
Figure 1.
Exposure–response analysis: relationship between BG00011 exposure and percent change from baseline in pSMAD2 expression. AUCSS = area under the concentration time curve at a steady state; pSMAD2/tSMAD2 = ratio of phosphorylated SMAD2 to total SMAD2.
Discussion
In this small phase IIa study, once-weekly subcutaneous administration of BG00011, at doses less than 1.0 mg/kg, was generally well tolerated by patients with IPF. However, acute IPF exacerbation occurred among patients at higher doses of BG00011. Furthermore, the study met the predefined stopping criteria at the 3.0 mg/kg dose, identifying a potential risk of decline in respiratory status with higher doses of BG00011.
The results suggest that targeting the αvβ6-integrin with BG00011 inhibits TGF-β activation and may interrupt fibrogenesis. BAL cell analysis showed an inhibitory effect of BG00011 on pSMAD2 expression, starting at the 0.3 mg/kg dose and achieving ⩾70% reduction at 1.0 mg/kg, establishing proof of biological activity. The observed biological response of BG00011 resembled that seen in mice and primates (7).
To guide the selection of a safe and biologically active BG00011 dose for subsequent clinical trials, a pharmacodynamic exposure–response analysis was conducted on the basis of Week 8 AUCSS (Figure 1). A steep dose response was observed between BG00011 exposure and pSMAD2 inhibition up to the 1.0 mg/kg dose, at which point pSMAD2 was reduced by 70%, and efficacy plateaued. A flat dose of 56 mg (∼0.7 mg/kg) was selected for the phase IIb study (NCT03573505) (11) to provide the optimal therapeutic exposure for clinical efficacy on the basis of the exposure–response curve and pharmacodynamics (median predicted 65% [range, ∼40% to ∼75%] pSMAD2 reduction), without achieving median exposures at 1.0 mg/kg. BAL cell analyses provided a novel mechanism to determine the BG00011 dose needed for full target engagement and inhibition of αvβ6-mediated TGF-β activation.
Given the small number of patients and the exploratory nature of this analysis, the results should be interpreted with caution. Larger studies are required to confirm the potential dose–response effect of BG00011 on TGF-β suppression and define clinical efficacy.
Acknowledgments
Acknowledgment
The authors would like to thank the study investigators, Daniel A. Culver, M.D., Mark J. Hamblin, M.D., Jeffrey A. Golden, M.D., Srihari Veeraraghavan, M.D., Richard I. Enelow, M.D., Lisa H. Lancaster, M.D., Hilary J. Goldberg, M.D., Leo C. Ginns, M.D., and Ivan O. Rosas, M.D., as well as the patients who participated in this trial and their families, the study coordinators, study teams, and nurses. The authors also thank the members of the Data and Safety Monitoring Board, Kevin Brown, M.D. (National Jewish Health, Denver, Colorado), Fernando Martinez, M.D. (Weill Cornell Medical Center, New York, New York), and Barry Turnbull, Ph.D. (retired), as well as David Lynch, M.D. (National Jewish Health), for his review of the computed tomography images for idiopathic pulmonary fibrosis diagnosis and participation as an ad hoc member of the Data and Safety Monitoring Board. The authors were assisted in the preparation of the manuscript by Jean Turner, a professional medical writer with Parexel International (Waltham, Massachusetts). Writing support was funded by Biogen (Cambridge, Massachusetts).
Footnotes
Supported by Biogen.
Data Sharing Statement: To request access to data, please visit http://www.biogenclinicaldatarequest.com. The individual participant data collected during the trial, which supports the research proposal, will be available to qualified scientific researchers after anonymization and on approval of the research proposal.
Originally Published in Press as DOI: 10.1164/rccm.202205-0868LE on July 13, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med . 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 2. Tatler AL, Jenkins G. TGF-β activation and lung fibrosis. Proc Am Thorac Soc . 2012;9:130–136. doi: 10.1513/pats.201201-003AW. [DOI] [PubMed] [Google Scholar]
- 3. Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell . 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 4. Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, et al. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med . 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 5. Madala SK, Korfhagen TR, Schmidt S, Davidson C, Edukulla R, Ikegami M, et al. Inhibition of the αvβ6 integrin leads to limited alteration of TGF-α-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol . 2014;306:L726–L735. doi: 10.1152/ajplung.00357.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z, et al. Inhibition of integrin α(v)β6, an activator of latent transforming growth factor-β, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med . 2008;177:82–90. doi: 10.1164/rccm.200706-806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Violette S, Sheppard D, Rosas I, Arjomandi M, Rice T, Gilman M, et al. Identification of biomarkers to monitor the activity of STX-100, a humanized anti-αvβ6 antibody, in a phase 2a trial in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2012;185:A2659. [Google Scholar]
- 8. Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND, et al. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem Biol Interact . 2018;292:76–83. doi: 10.1016/j.cbi.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 9. Prasse A, Binder H, Schupp JC, Kayser G, Bargagli E, Jaeger B, et al. BAL cell gene expression is indicative of outcome and airway basal cell involvement in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2019;199:622–630. doi: 10.1164/rccm.201712-2551OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE, Jr, et al. Idiopathic Pulmonary Fibrosis Clinical Research Network Investigators Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2007;176:636–643. doi: 10.1164/rccm.200703-463PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raghu G, Mouded M, Chambers DC, Martinez FJ, Richeldi L, Lancaster LH, et al. A phase IIb randomized clinical study of an anti-αvβ6 monoclonal antibody in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2022;206:1128–1139. doi: 10.1164/rccm.202112-2824OC. [DOI] [PubMed] [Google Scholar]