Abstract
Rationale
The role of obesity-associated insulin resistance (IR) in airflow limitation in asthma is uncertain.
Objectives
Using data in the Severe Asthma Research Program 3 (SARP-3), we evaluated relationships between homeostatic measure of IR (HOMA-IR), lung function (cross-sectional and longitudinal analyses), and treatment responses to bronchodilators and corticosteroids.
Methods
HOMA-IR values were categorized as without (<3.0), moderate (3.0–5.0), or severe (>5.0). Lung function included FEV1 and FVC measured before and after treatment with inhaled albuterol and intramuscular triamcinolone acetonide and yearly for 5 years.
Measurements and Main Results
Among 307 participants in SARP-3, 170 (55%) were obese and 140 (46%) had IR. Compared with patients without IR, those with IR had significantly lower values for FEV1 and FVC, and these lower values were not attributable to obesity effects. Compared with patients without IR, those with IR had lower FEV1 responses to β-adrenergic agonists and systemic corticosteroids. The annualized decline in FEV1 was significantly greater in patients with moderate IR (−41 ml/year) and severe IR (−32 ml/year,) than in patients without IR (−13 ml/year, P < 0.001 for both comparisons).
Conclusions
IR is common in asthma and is associated with lower lung function, accelerated loss of lung function, and suboptimal lung function responses to bronchodilator and corticosteroid treatments. Clinical trials in patients with asthma and IR are needed to determine if improving IR might also improve lung function.
Keywords: asthma, obesity, insulin resistance, lung function
At a Glance Commentary
Scientific Knowledge on the Subject
Obesity is a global epidemic. Poor metabolic health in obese patients is associated with dysfunction in multiple organs but the impact of obesity-associated metabolic disease on lung disease is poorly understood. Poor metabolic health in obesity is reflected in dyslipidemia, hyperglycemia, and insulin resistance (IR) with hyperinsulinemia. Insulin resistance is strongly related to asthma risk in adults, and insulin itself may activate airway immune cells and structural cells to mediate airway inflammation and airway narrowing. This prompted us to explore if insulin resistance is associated with impairments in lung function or alters the treatment response to asthma medications. We conducted metabolic phenotyping of participants in the Severe Asthma Research Program 3 (SARP-3), a 5-year longitudinal cohort study. Metabolic phenotyping included comprehensive measures of obesity and obesity-associated metabolic dysfunction, including homeostatic model assessment of insulin resistance (HOMA-IR), which was categorized as without (<3.0), moderate (3.0–5.0), or severe (>5.0) IR. We evaluated whether HOMA-IR is associated with cross-sectional measures of lung function, lung function responses to β-adrenergic agonists and intramuscular triamcinolone (TA), and annualized declines in lung function.
What This Study Adds to the Field
Our cross-sectional analyses determined that obesity and insulin resistance (IR) are common in participants in SARP-3 and that participants with moderate or severe insulin resistance have significantly lower lung function and significantly worse responses to β agonists and TA than those without IR. In longitudinal analyses we found that, compared to participants without IR, those with moderate or severe IR have accelerated loss of lung function. Our findings suggest that IR leads to cross sectional and longitudinal impairments in lung function in asthma and provides rationale to test whether treatments for insulin resistance prevent accelerated loss of lung function in asthma.
Obesity is a significant modifier of disease severity in asthma (1) and putative mechanisms include obesity-associated systemic inflammation occurring in the context of poor metabolic health (2, 3). Poor metabolic health in obesity includes pathologies such as dyslipidemia, hyperglycemia, and insulin resistance (IR). Interestingly, prior research in community-based cohort studies of patients not selected for an asthma diagnosis has found relationships between impaired glucose homeostasis, diabetes, or insulin resistance on lung function. For example, in cross-sectional analyses from the American National Health and Nutrition Examination Survey (NHANES-III) and in the American Atherosclerosis Risk in Communities (ARIC) cohort, a diagnosis of diabetes was associated with a lower FEV1 and lower FVC (4, 5). In addition, in prospective analyses in ARIC, the FVC (but not FEV1) declined faster in diabetics than nondiabetic controls (5). Furthermore, in the Ansan-Ansung (AA) cohort, which was established to study noncommunicable chronic diseases (including diabetes) in South Korea, cross-sectional analyses showed that the FVC was significantly lower in subset with IR, and prospective analyses showed that both FEV1 and FVC declined faster in those with IR (6). Together, these cohort data in patients not selected for lung disease demonstrate that impaired glucose homeostasis and insulin resistance is associated with abnormal lung function and accelerated loss of lung function over time.
The effect of insulin resistance on disease severity and lung function in asthma is poorly understood, and the effect of IR on changes in lung function over time has not been studied to our knowledge. In addition, the effect of IR on lung function responses to β-adrenergic agonists or corticosteroids has not been reported. The Severe Asthma Research Program 3 (SARP-3) is a multiyear longitudinal cohort study of asthma patients in the United States designed to evaluate molecular phenotypes of asthma and how these phenotypes influence lung function and treatment responses to β-adrenergic agonists and corticosteroids. Using data from SARP-3, we set out to determine if IR is associated with impairments in lung function or blunting of treatment responses to asthma medications. Some of the results of this study have been previously presented in the form of an abstract (7).
Methods
Participants
The NHLBI’s SARP-3, now in its seventh year, is a longitudinal cohort study in adults and children with asthma whose mission is to improve the understanding of severe asthma to develop better treatment strategies (8). Participants enrolled in SARP were followed for at least three years to determine how clinical and molecular features develop or change with time. The protocol involves detailed disease characterization at baseline and yearly thereafter. Our previous finding that systemic IL-6 inflammation is common in SARP-3 participants who are obese and that “IL-6-high” asthma is associated with more severe asthma (2, 9) prompted the steering committee to modify the Year 3 characterization procedures to add a broader range of metabolic health measures. The metabolic measures included a metabolic health questionnaire and blood tests for metabolic analytes (insulin, glucose, glycated-hemoglobin [HbA1c], triglycerides, cholesterol and lipoproteins). The protocol was for collection of blood in the fasting state, although blood was collected in the nonfasting state from a subset of participants unable to fast. For the purposes of the research presented here, data were restricted to the 307 adult participants (age >18 years at Year 3) who had blood collected in the fasting state. The clinical features of SARP participants with or without fasting blood measures did not differ significantly (see Table E1 in the online supplement). Complete details of the SARP-3 protocol have been described previously (8), and a summary is presented in the supplemental appendix (Figure E1 and Table E2). To facilitate the research presented here, an additional questionnaire was completed by each participant at Year 3 visit to gather information about their metabolic health. This comprehensive metabolic health questionnaire collected data about history of metabolic disease (e.g., hypertension, diabetes, gout) and medications for these diseases (including medications for diabetes and lipid disorders). All participants signed an informed consent, adherent to the Declaration of Helsinki and approved by the Institutional Review Boards of each center and the NHLBI Data and Safety Monitoring Board.
Blood Measures of Metabolic Dysfunction
Participants fasted for at least 12 hours prior to blood sampling. Glucose, insulin, and lipid panels were analyzed from plasma samples prepared from whole blood using Fisher (Fisher Scientific) lithium heparin tubes (cat# 02–687–97). Whole blood was collected and plasma was prepared for measures of HbA1c using Fisher (Fisher Scientific) plasma-EDTA tubes (cat# 02–689–03). Plasma was stored at each of the clinical sites at −80°C, shipped to Washington University on dry ice, and measurements were performed in batch at the Clinical Laboratory Improvement Amendments certified clinical laboratory at the Washington University School of Medicine, St. Louis, MO (10).
Calculations of Obesity and Metabolic Dysfunction
Classification of obesity, waist circumference, and waist-to-hip ratio (WHR) were determined according to the World Health Organization (WHO) definitions (11). Body mass index (BMI kg/m2) was used to classify participants as normal (<25), pre-obese (25–29.99), obese class 1 (30–34.99), obese class 2 (35–39.99), or obese class 3 (⩾40). Waist circumference was considered increased at >40 inches in men and >35 inches in women and WHR was considered abnormal at >0.9 in men and >0.85 in women (12). The definition of metabolic syndrome was defined per National Cholesterol Education Program guidelines (13).
The homeostatic model assessment of insulin resistance (HOMA-IR) estimates steady-state β-cell function and insulin sensitivity by multiplying the fasting plasma glucose (mg/dl) value by the serum insulin (mIU/ml) value divided by 405 (14). HOMA-IR values <3 were classified as without IR, 3–5 were classified as moderate IR, and >5 were classified as severe IR (14, 15).
Treatment Response to Asthma Medications
Maximum bronchodilator reversibility testing (MBRT) was performed at the baseline visit and each annual in-person visit thereafter. MBRT involved measures of spirometry after up to 720 ug of inhaled albuterol, as previously described (8). The SARP-3 protocol also included a systemic corticosteroid response test (SCRT) which involved an intramuscular injection of triamcinolone acetonide (40 mg) at the baseline visit and repeat characterization (including MBRT) on a visit 2–4 weeks later (16). The change in FEV1% was calculated by the absolute change in FEV1% predicted after and before drug challenge.
Statistical Methods
The data analyzed and presented were largely from the Year 3 characterization visit because the metabolic phenotyping occurred at this visit. Data from other yearly visits, including the baseline visit and through Year 5 visits, are analyzed for longitudinal lung function outcomes and outcomes related to treatment responses (as indicated in the results text). Statistical analyses were performed with the JMP 12 software package (SAS Institute), Stata 15.0 (StataCorp), and R statistical package. P values of less than 0.05 were taken as statistically significant. Nonparametric tests of trend were utilized to compare clinical traits across insulin resistance subgroups. Because our predictor variable (HOMA-IR subgroups) was ordinal, we utilized Cuzick nonparametric tests of trend to determine the association between worse insulin resistance and clinical traits. Data was also analyzed using linear regression with the HOMA-IR as a continuous predictor variable (Table E3). Linear regression models were informed by directed acyclic graphs (DAGs) and minimal adjustment sets required for regression modeling were identified using the web-based “DAGitty” platform (17). The models were developed to accomplish the study objective to determine the independent causal effect of IR on lung function (17). The DAG identified age at study enrollment, asthma exacerbations, BMI, and systemic corticosteroids in the minimal sufficient adjustment set (Figure E2A). Because the association between plasma IL-6 and IR is unknown, we also performed a sensitivity analysis that included adjustments for plasma IL-6, and additional adjustments for race and high-dose inhaled corticosteroids (Figure E2A). None of these additional sensitivity analyses altered our results (supplemental appendix). Multilevel mixed-effects linear regression was utilized to determine the impact of insulin resistance on longitudinal changes of lung function. Random effects were incorporated as random intercepts at the participant level. Because the outcome of interest in these models (lung function) was measured at annual intervals we modeled time as a continuous linear variable to determine the annual change in lung function and this was performed using an interaction term between HOMA-IR subgroups and year of study follow up. As a complementary approach we also fit a point-to-point model. This approach did not force the data into a linear function and instead modeled lung function at each distinct visit. The “margins” command in Stata was used to estimate the marginal means of our outcome variables (FEV1 and FVC) at each of the insulin resistance subgroups over the 5 years of longitudinal follow-up. The contrast command in Stata was used to compare the change in lung function over time between HOMA-IR subgroups which utilizes an ANOVA comparison test (18). In all models, without IR was considered the group of reference. Informed by our DAG we also repeated all mixed effects models controlling for the potential confounding effects of BMI, age at time of study enrollment, systemic corticosteroid exposure, and annual number of asthma exacerbations.
Results
Obesity and Metabolic Impairment Are Common in the SARP-3 Cohort
BMI was increased in the majority of participants (Figure 1A) and the percentage of participants with a BMI greater than 40 (WHO obesity class III) was similar to the percentage of participants with a normal BMI (Figure 1A). Values for waist circumference and waist-to-hip ratio were also increased in the majority of participants (Figures 1B and 1C). Abnormal values for blood insulin and HbA1c were more frequent than abnormal values for fasting blood glucose (Figures 1D–1F). The homeostatic model assessment (HOMA) is used to quantify IR, and HOMA-IR values were abnormal (>3.0) in nearly half the participants (Figure 1G).
Figure 1.
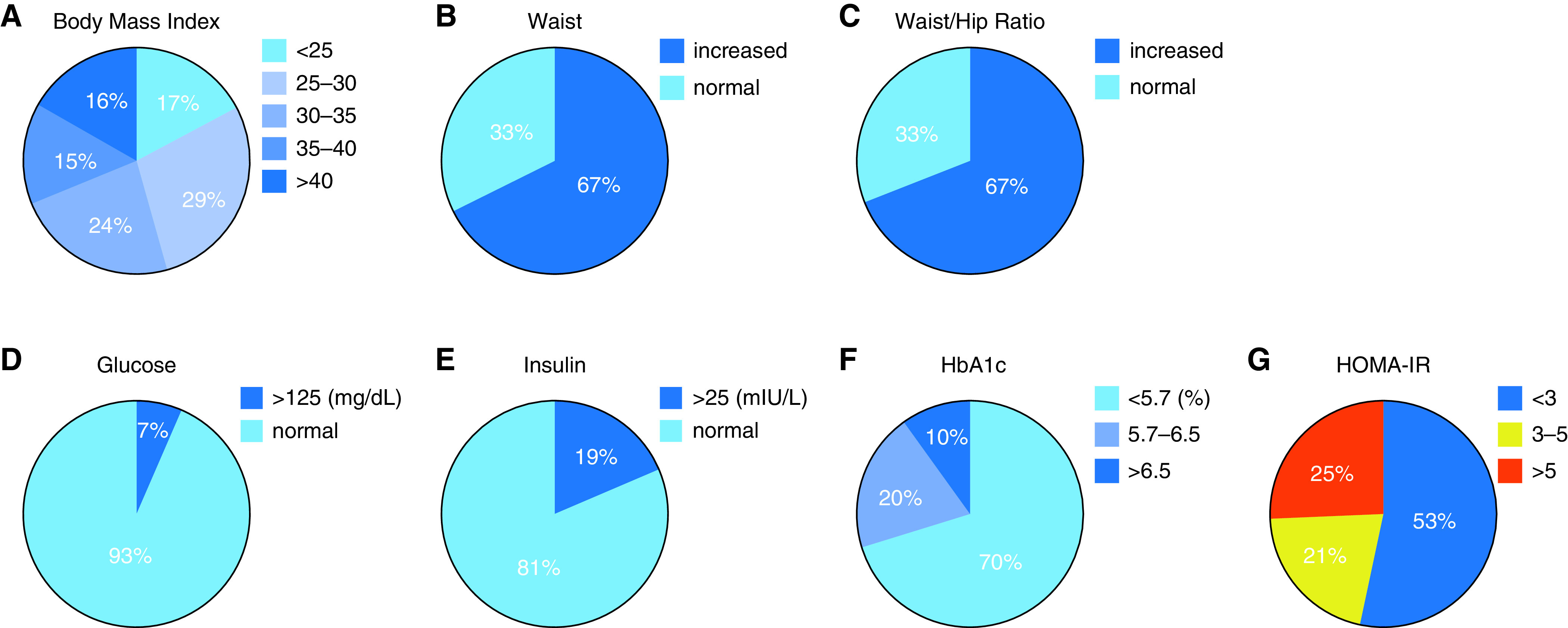
Measures of obesity and insulin resistance in the SARP-3 cohort. (A) Percentages of participants whose body mass index (BMI) (kg/m2) falls within the five World Health Organization categories of obesity. (B) Percentage of participants with increased waist circumference (defined as >40 in for men and >35 in for woman. (C) Percentage of participants with increased waist/hip ratio (defined at >0.9 for men and >0.85 for woman). (D) Percentage of participants with an increased fasting blood glucose (defined as >125 mg/dl). (E) Percentage of participants with an increased fasting blood insulin (defined as >25 mIU/L). (F and E) Percentage of participants with normal glycated-hemoglobin (HbA1c) (<5.7%), increased HbA1c (5.7–6.5%), or diabetes level HbA1c (>6.5%). (G) Percentage of participants with homeostatic model assessment of insulin resistance (HOMA-IR) (molar units) that is normal range (<3), moderately increased (3–5), or severely increased (>5).
Asthma Participants with IR have Systemic Inflammation, Dyslipidemia, and more Severe Asthma
Compared with participants without IR, those with moderate or severe IR were characterized by high BMI, high waist circumference, high waist/hip ratio, high frequency of a diagnosis of hypertension or diabetes, increased frequency of sleep apnea, and a high frequency of metabolic syndrome (Table 1). In addition, participants with moderate or severe IR were characterized by systemic inflammation (high values for blood white blood cells, blood neutrophils, and plasma interleukin-6) and dyslipidemia (high blood triglycerides and low cholesterol high-density lipoproteins) (Table 1). Furthermore, participants with moderate or severe IR were also characterized by lower (worse) scores on the Asthma Control Test (19), and by a higher frequency of severe asthma based on the European Respiratory Society (ERS)/American Thoracic Society (ATS) Task Force definition of severe asthma (20) (while participants with moderate or severe IR experienced more frequent exacerbations, this difference did not reach statistical significance) (Table 1). Finally, participants with IR were characterized by a lower sputum eosinophil percentage (Table 1).
Table 1.
Characteristics of Asthma Participants with Insulin Resistance
| HOMA-IR < 3.0 Without Insulin Resistance (n = 167) | HOMA-IR 3.0–5.0 Moderate Insulin Resistance (n = 63) | HOMA-IR > 5.0 Severe Insulin Resistance (n = 77) | P Value Test for Trend* | |
|---|---|---|---|---|
| Demographics | ||||
| Age, ys | 52 (15) | 52 (14) | 52 (12) | 0.73 |
| Female, n (%) | 120 (72) | 47 (75) | 47 (61) | 0.13 |
| Race, n (%) | 0.12 | |||
| Asian | 6 (4) | 1 (2) | 1 (1) | — |
| Black | 31 (19) | 21 (33) | 25 (33) | — |
| Other | 12 (7) | 4 (6) | 3 (4) | — |
| White | 118 (71) | 37 (59) | 48 (62) | — |
| Clinical Features of Obesity | ||||
| Body mass index (kg/m2) | 29.1 (6.2) | 33.6 (8.7) | 37.7 (6.7) | <0.001 |
| WHO obesity categories, n (%) | <0.001 | |||
| <25 | 44 (26) | 6 (10) | 0 (0) | — |
| 25–30 | 59 (35) | 20 (32) | 8 (10) | — |
| 30–35 | 36 (22) | 16 (25) | 24 (31) | — |
| 35–40 | 19 (11) | 11 (18) | 16 (21) | — |
| >40 | 9 (5) | 10 (16) | 29 (38) | — |
| Waist circumference (in) | 37.0 (5.4) | 41.0 (6.7) | 46.4 (6.3) | <0.001 |
| Waist/hip ratio | 0.88 (0.08) | 0.90 (0.09) | 0.97 (0.10) | <0.001 |
| History of diabetes, n (%) | 10 (6) | 7 (11) | 22 (29) | <0.001 |
| Taking diabetes medications, n (%) | 6 (4) | 4 (6) | 16 (21) | <0.001 |
| History of sleep apnea | 30 (18) | 13 (21) | 30 (39) | <0.001 |
| Diagnosis of hypertension n (%) | 53 (32) | 32 (52) | 42 (54) | <0.001 |
| Metabolic syndrome, n (%) | 10 (6) | 13 (21) | 32 (42) | <0.001 |
| Blood Measures of Metabolic Dysfunction | ||||
| Triglycerides (mmol/L) | 100 (54) | 129 (67) | 155 (94) | <0.001 |
| Cholesterol LDL (mg/dl) | 112 (31) | 119 (36) | 108 (29) | 0.38 |
| Cholesterol HDL (mg/dl) | 65 (19) | 55 (14) | 51 (16) | <0.001 |
| HbA1c (%) | 5.4 (0.7) | 5.5 (0.5) | 6.4 (1.5) | <0.001 |
| Blood WBC cells/L | 6.4 (1.8) | 7.3 (2.4) | 8.4 (3.1) | <0.001 |
| Blood neutrophils cells/ul | 3,808 (1,434) | 4,409 (1,972) | 5,154 (2,710) | <0.001 |
| Plasma IL-6 (pg/ml)† | 2.5 (2.3) | 3.5 (2.3) | 3.9 (2.3) | <0.001 |
| Asthma Characteristics | ||||
| High dose ICS, n (%) | 83 (50) | 38 (58) | 53 (69) | 0.007 |
| Oral daily OCS, n (%) | 17 (10) | 10 (15) | 12 (16) | 0.19 |
| Pre-BD spirometry data | ||||
| FEV1 (% predicted) | 76.4 (20.5) | 70.9 (21.0) | 68.3 (18.1) | 0.001 |
| FVC (% predicted) | 88.3 (17.5) | 81.5 (17.0) | 78.3 (15.4) | <0.001 |
| FEV/FVC ratio | 85.3 (12.1) | 85. 4 (13.2) | 86.2 (11.4) | 0.50 |
| Post-BD spirometry data | ||||
| FEV1 (% predicted) | 86.1 (19.5) | 80.7 (19.1) | 76.1 (17.9) | <0.001 |
| FVC (% predicted) | 94.8 (16.2) | 89.5 (14.6) | 83.9 (14.8) | <0.001 |
| FEV/FVC ratio | 90.4 (11.1) | 89.6 (13.0) | 904 (11.7) | 0.94 |
| Score on ACT§ median (IQR) | 20 (16–22) | 19 (15–22) | 19 (14–21) | 0.006 |
| Asthma exacerbations over 5 yr‡ (IRR) | ref | 1.2 (0.8–1.7) | 1.5 (1.0–2.1) | 0.12 |
| ATS criteria severe asthma, n (%) | 76 (46) | 34 (54) | 51 (66) | 0.003 |
| Blood eosinophils cells/ul | 238 (214) | 252 (265) | 237 (177) | 0.45 |
| Sputum eosinophils% median§ (IQR) | 0.6 (0.1–3.7) | 1 (0.2–5.0) | 0.2 (0.1–0.9) | 0.04 |
| Sputum eosinophil >2%, n (%) | 41 (34) | 16 (37) | 6 (13) | 0.02 |
| Sputum neutrophils% median§ (IQR) | 62 (46–76) | 60 (45–72) | 63 (50–77) | 0.87 |
Definition of abbreviations: ACT = asthma control test; BD = bronchodilator response; HDL = high-density lipoproteins; HOMA-IR = homeostatic model of insulin resistance; ICS = inhaled corticosteroids; IQR = interquartile range; IRR = incident rate ratio; LDL = low-density lipoproteins; WHO = World Health Organization.
Data are presented from the Year 3 visit. Data are reported as mean (SD) unless otherwise indicated.
P values for association based upon Cuzick nonparametric tests for trend.
Plasma IL-6 was measured in 157 participants.
Scores on ACT range from 25 to 5 with lower scores indicating worse asthma control.
Asthma exacerbations defined as a course of oral corticosteroids.
Sputum cell percentages were available on 208 participants.
Relationship between IR, BMI, and Cross-sectional Lung Function Measurements
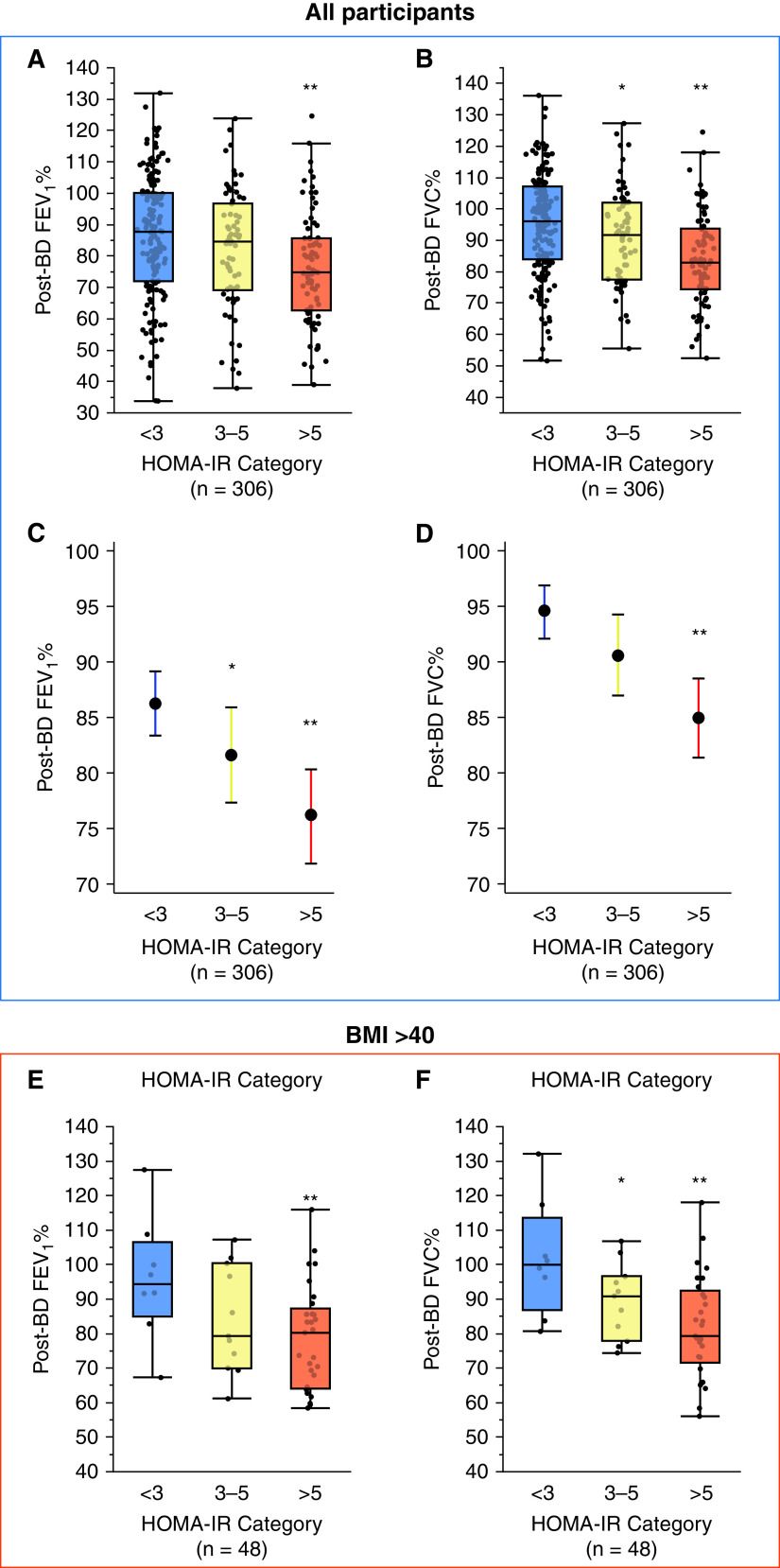
BMI correlated strongly with the HOMA-IR (rho 0.57, P < 0.001), but the relationship was not uniform, and significantly sized subgroups of participants had obesity without IR (21%) or IR without obesity (11%) (Figure E2B). Compared with participants without IR, those with moderate or severe IR were characterized by lower values for FEV1 and FVC, and this was true if either pre-bronchodilator (BD) or post-BD values were used in the analyses (Table 1). The post-BD FEV1 trended lower in participants with moderate IR and was significantly lower in those with severe IR (Figure 2A). The post-BD FVC was significantly lower in participants with moderate and severe IR (Figure 2B). Due to the parallel fall in the FEV1 and FVC, the FEV1/FVC ratio did not differ between participants with moderate IR or severe IR when compared with participants without IR (Table 1). We addressed the possibility that the relationship between IR and low lung function is confounded by the mechanical impact of obesity in two ways. First, we used linear regression to control for BMI (and age). In these analyses we also continued to find that post-BD FEV1 and FVC were lower in participants with IR, especially in those with severe IR (Figures 2C and 2D). Second, we restricted the analysis between HOMA-IR and lung function to participants with morbid obesity (WHO class-III, BMI ⩾40 kg/m2). In this analysis, we found that FEV1 and FVC in morbidly obese participants without IR was normal or nearly so and that morbidly obese participants with IR had lower lung function, and it was especially low in those with severe IR (Figures 2E and 2F).
Figure 2.
Lung function is lower in asthma patients with insulin resistance (IR). (A) The post-bronchodilator (BD) FEV1% is lower in those with severe IR than in those without IR. (B) The post-BD FVC% is significantly lower in participants with moderate IR and severe IR than in those without IR. (C) The covariate controlled post-BD FEV1% predicted is lower in participants with moderate and severe IR than in those without IR. (D) The covariate controlled post-BD FVC% predicted is lower in participants with severe IR than in those without IR. (E). In participants with Class III obesity (n = 48), the post-BD FEV1% trend is lower in participants with severe IR than in participants without IR. (F). In participants with Class III obesity, the post-BD FVC% is significantly lower in participants with moderate and severe IR than in those without IR. *Indicates significantly different from control (participants without IR), P < 0.05; **Indicates significantly different from control (participants without IR), P < 0.01. HOMA-IR = homeostatic model of insulin resistance.
Asthma Participants with Insulin Resistance have Blunted Responses to Albuterol and Systemic Triamcinolone Acetonide (TA)
A feature of the SARP-3 protocol is that it included a maximum bronchodilator reversibility test (MBRT) to albuterol and a systemic corticosteroid response test (SCRT) to intramuscular triamcinolone acetonide (TA). The MBRT was performed at the baseline and Year 3 visits, and the SCRT was performed at the baseline visit only. This allowed us to explore the effect of IR on data generated in the MBRT and SCRTs. Compared with participants without IR, we found that the FEV1 response to albuterol was significantly lower in those with severe IR, and this blunted response was evident in MBRTs performed at both baseline and Year 3 visits (Figure 3A). Compared with participants without IR, we found that the FEV1 response to systemic TA was significantly lower in those with moderate and severe IR (Figure 3B).
Figure 3.
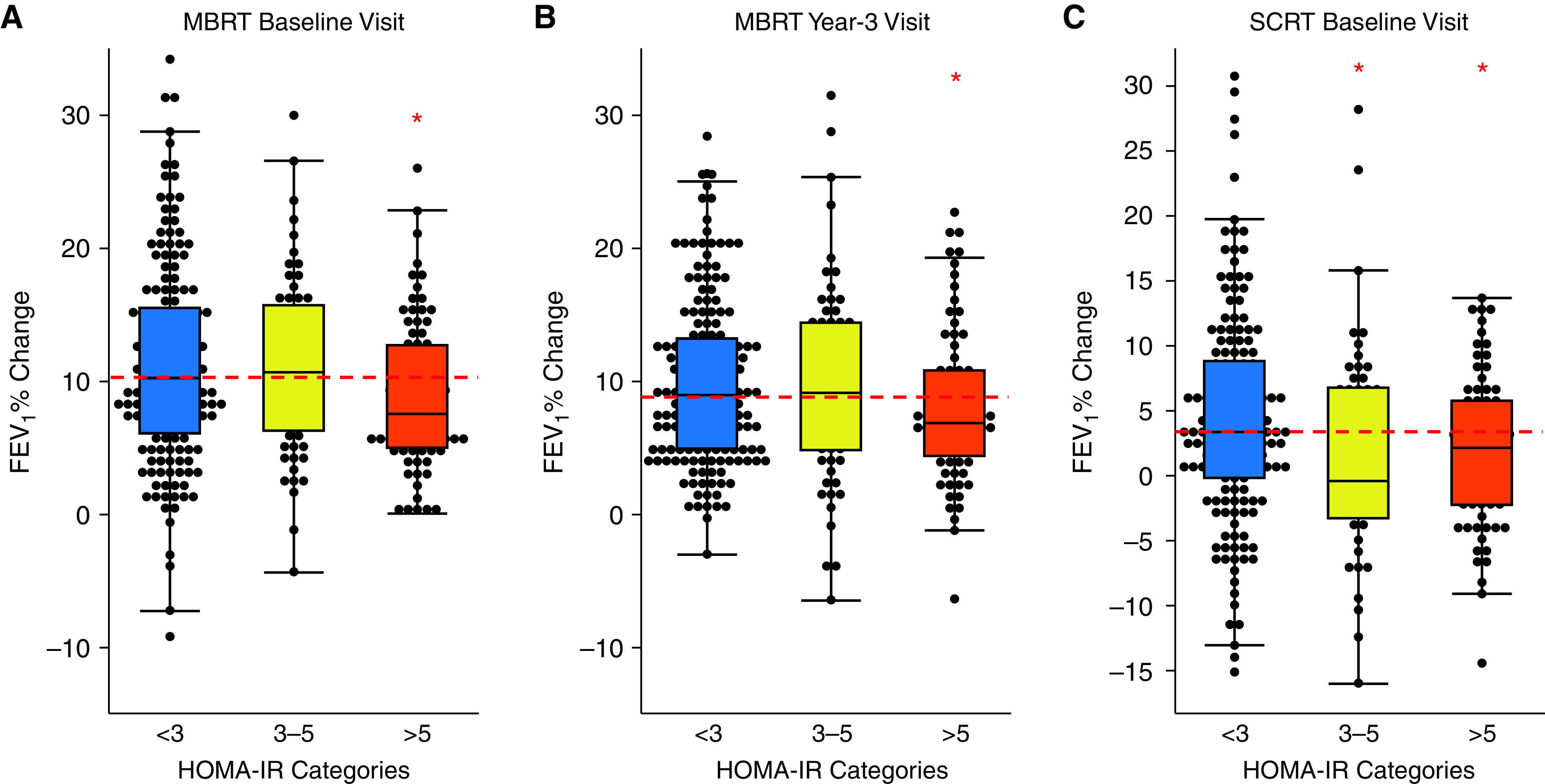
Lung function responses to asthma medications are decreased in asthma patients with insulin resistance (IR). (A) The increase in FEV1 following inhaled albuterol was significantly lower in patients with severe IR at both the baseline and 3-year visits. (B) The increase in FEV1 following intramuscular triamcinolone acetonide (TA) was significantly lower in participants with moderate and severe IR than in those without IR (TA data from the baseline visit). Dashed red line is the median value in participants without IR. *Indicates significant difference from control (participants without IR), P < 0.05. HOMA-IR = homeostatic model of insulin resistance; MBRT = Maximum bronchodilator reversibility testing; SCRT = systemic corticosteroid response test.
Asthma Participants with IR have Accelerated Loss of Lung Function
Using multilevel mixed effects linear regression modeling, we evaluated the annual decline in lung function (ADLF) in participants with and without IR. In these analyses we evaluated FEV1 and FVC measurements and the FEV1% predicted and FVC% predicted from the annual visits starting at baseline and through Year 5. We found that the annual decline in FEV1 and FVC in participants with moderate or severe IR was significantly greater than in those without IR (Table 2, Figures 4A and 4C). The age-corrected FEV1% and FVC% predicted measures demonstrated similar results. Specifically, in participants without IR, the ADLF was similar to the anticipated rate of decline that occurs normally during aging resulting in a minimal change in FEV1% or FVC% predicted values over the 5-year follow-up (Table 2, Figures 4B and 4D). However, in participants with moderate IR or severe IR, the loss of lung function exceeded the loss anticipated with normal lung aging, resulting in declining FEV1% predicted and FVC% predicted values (Table 2). These findings did not change significantly in models that corrected for BMI, age of study enrollment, blood eosinophil cell counts, systemic corticosteroid exposure, and annual number of asthma exacerbations (Table 2, Figures 4B and 4D). Furthermore, this decline in lung function occurred despite a minimal change in BMI over time (Figure 4E). Specifically, the BMI did not change significantly over time in participants without IR, or participants with moderate IR, but increased slightly in participants with severe IR (0.17 kg/m2 per year, P < 0.008) (Figure 4E).
Table 2.
Effect of HOMA-IR on Annual Decline in Lung Function (n = 307)
| Unadjusted |
Adjusted* |
|||
|---|---|---|---|---|
| Effect (95% CI) | P Value | Effect (95% CI) | P Value | |
| Decline in FEV1 ml/yra | ||||
| HOMA-IR < 3 | −12 (−19 to −6) | Ref | −13 (−19 to −6) | Ref |
| HOMA-IR 3–5 | −44 (−56 to −33) | <0.001 | −40 (−52 to −29) | <0.001 |
| HOMA-IR > 5 | −38 (−48 to −27) | <0.001 | −32 (−42 to −23) | 0.001 |
| Decline in FEV1%/yra | ||||
| HOMA-IR < 3 | 0.3 (0.0 to 0.6) | Ref | 0.4 (0.1 to 0.6) | Ref |
| HOMA-IR 3–5 | −0.9 (−1.3 to −0.6) | <0.001 | −0.8 (−1.2 to −0.4) | <0.001 |
| HOMA-IR > 5 | −0.7 (−1.0 to −0.3) | <0.001 | −0.5 (−0.8 to −0.2) | <0.001 |
| Decline in FVC ml/yra | ||||
| HOMA-IR < 3 | −17 (−25 to −9) | Ref | −17 (−25 to −9) | Ref |
| HOMA-IR 3–5 | −47 (−60 to −34) | <0.001 | −43 (−56 to −31) | <0.001 |
| HOMA-IR > 5 | −40 (−51 to −28) | 0.002 | −34 (−45 to −23) | 0.02 |
| Decline in FVC %/yra | ||||
| HOMA-IR < 3 | 0.2 (0.0 to 0.4) | Ref | 0.3 (0.0 to 0.5) | Ref |
| HOMA-IR 3–5 | −0.9 (−1.2 to −0.5) | <0.001 | −0.7 (−1.1 to −0.4) | <0.001 |
| HOMA-IR > 5 | −0·6 (−0.9 to −0.2) | <0.001 | −0.4 (−0.7 to −0.1) | 0.001 |
Definition of abbreviations: CI = confidence interval; HOMA-IR = homeostatic model of insulin resistance.
Adjusted models control for effect of body mass index, age at enrollment, systemic corticosteroid exposure, and annual number of asthma exacerbations.
P values compare the annual change in lung function to the reference group of HOMA-IR < 3 using ANOVA tests with the contrast function in STATA.
Lung function measured after maximum bronchodilator reversibility testing.
Figure 4.
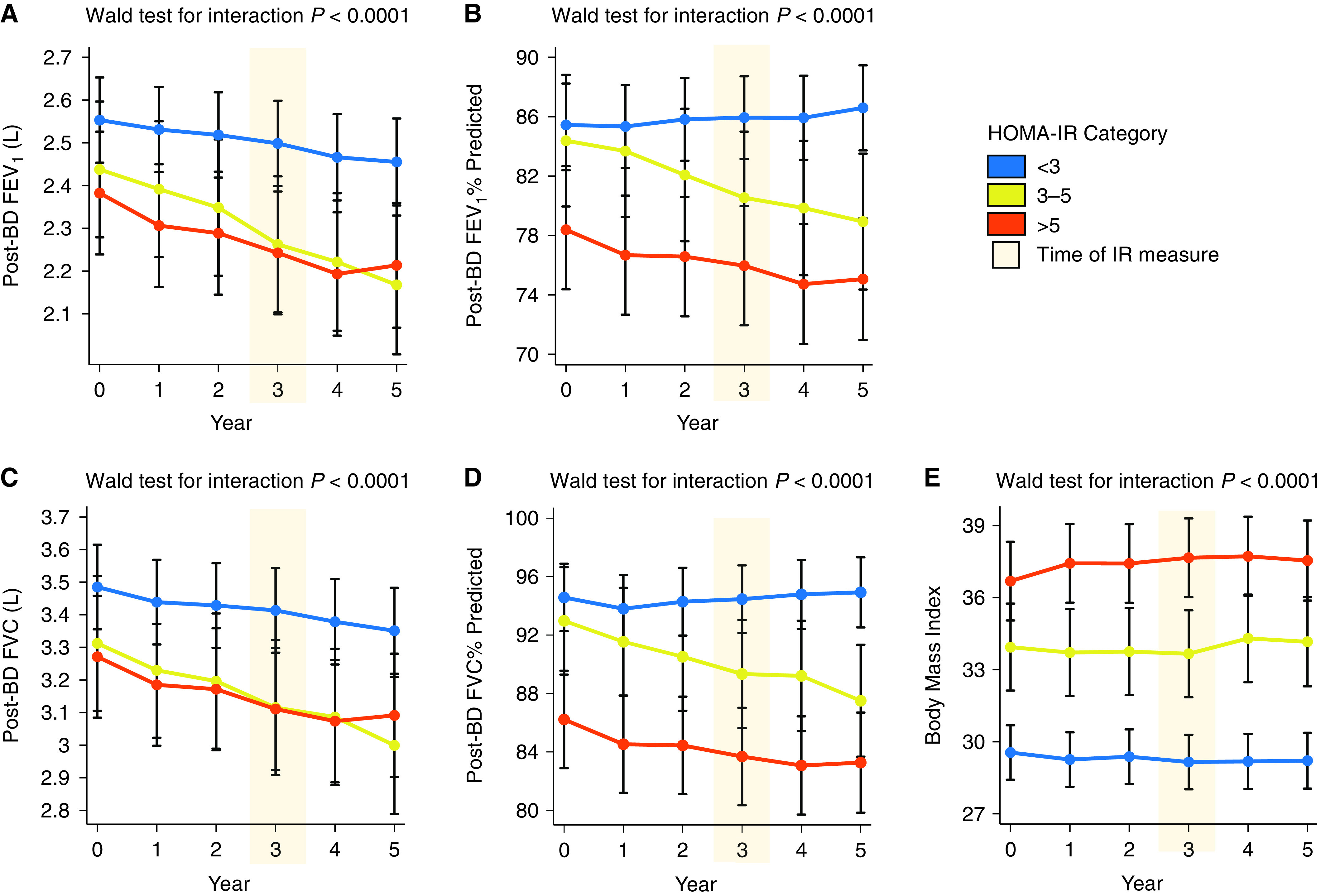
Post-bronchodilator (BD) lung function in the 3 years before and 2 years after metabolic phenotyping. (A) The decline in FEV1 (L) over time is faster in participants with moderate insulin resistance (IR) and severe IR than in participants without IR. (B) The decline in FEV1% predicted is faster in participants with moderate IR and severe IR than in participants without IR. (C) The decline in FVC (L) over time is faster in participants with moderate IR and severe IR than in participants without IR. (D) The decline in FVC % predicted is faster in participants with moderate IR and severe IR than in participants without IR. (E) BMI did not change significantly in participants without IR and in participants with moderate IR, but increased slightly (0.17 kg/m2 per year) in participants with severe IR (P = 0.008). The homeostatic model assessment of insulin resistance (HOMA-IR) was measured at the Year 3 (metabolic phenotyping) visit (shaded orange). Error bars indicated 95% confidence interval for the marginal effect. Wald test for the interaction using the delta method was used to test that the change in lung function over time differed between HOMA-IR subgroups.
Discussion
Obesity is a growing and global epidemic (21), and poor metabolic health in obese patients is associated with heart and brain dysfunction (13, 22). Obesity-associated metabolic disease includes insulin resistance (IR), but the effect of IR on lung health is poorly understood. We found that IR is common in the SARP-3 cohort and that the subset of participants with IR is characterized by more severe airflow limitation in cross-sectional analyses and by accelerated loss of lung function in longitudinal analyses.
The SARP-3 cohort is a valuable resource for studying the effects of obesity and obesity-associated metabolic dysfunction on lung function in asthma. A majority of the cohort are obese, and we show here that nearly half have insulin resistance (IR). The overlap in subgroups with obesity and IR is not complete with about a quarter of the IR subgroup not having obesity and about a third of the obese participants not having IR. A main finding of our study is that cross-sectional analyses at the Year 3 visit shows that FEV1 and FVC values are lower in participants with IR than in those without IR. This association is not explained by obesity in participants with IR, because the association between IR and lower lung function survives correction for BMI in regression models. And in analyses confined to participants with morbid (WHO class III) obesity, the FEV1 and FVC values are much lower in the subgroup with IR than in those without IR. Thus, IR independently associates with low lung function in asthma and body mass effects on chest wall mechanics are unlikely to explain this association.
The longitudinal design of the SARP-3 cohort allowed us to explore how IR affects longitudinal decline in lung function. For these analyses we looked both “backward” from the year 3 measurement of IR to lung function measures at 0, 1, and 2 years and “forward” to lung function measures at Years 4 and 5. In this way, we uncovered that asthma participants with IR had markedly accelerated decline in lung function. Declines in FVC mirrored those in FEV1 so that there was a parallel acceleration in the reduction in FEV1 and FVC in participants with IR with preservation of the FEV/FVC ratio. The decline in FEV1 in patients with IR was approximately 25–30 ml per year greater than in those without IR, an effect significantly larger than the approximately 5 ml per year previously reported in cohort studies in patients that were not selected for an asthma diagnosis (5, 6). Thus, IR and asthma may be additive or interactive morbidities in terms of their effects in lung function over time. Furthermore, by analyzing the FEV1% predicted and FVC% predicted values over time, we demonstrate that participants with IR experience accelerated declines in lung function beyond the decline anticipated with normal aging (23). These findings support an important and underappreciated role of IR as a key risk factor for accelerated lung function decline in asthma. Our study design does not allow us to determine the mechanism of the effect of IR on loss of lung function. IR is a feature of aging, and aging in turn causes premature airway closure which can result in airway injury (24). Thus, one possibility is that IR accelerates aging-related lung pathology. Another possibility is that insulin directly mediates airway dysfunction by activating airway immune cells and structural cells to mediate airway inflammation and airway narrowing (25, 26). Insulin levels are high in IR participants and insulin is a pleotropic hormone with broad-based effects on endothelial cells (27, 28). For example, IR shifts insulin signaling in endothelial cells away from the protective phosphoinositide 3-kinase pathway to the more deleterious mitogen-activated protein kinase pathway that generates the PAI-1 (prothrombotic plasminogen activator inhibitor), among other mediators of thrombosis and vascular occlusive disease (28, 29). In addition, insulin has been shown to directly induce airway fibroblast collagen deposition and can cause airway hyperresponsiveness (26). Our data provide strong rationale to further investigate the effect of insulin on airway cell biology and function.
Our finding that the lung function responses to inhaled albuterol or systemic triamcinolone acetonide were lower in IR participants than in those without IR has important implications for managing asthma participants with IR. For albuterol the lack of effect was more prominent in participants with severe IR, whereas for triamcinolone the lung function response was equally prominent in participants with moderate and severe IR. It is relevant here that the IR participants have a low sputum eosinophil percentage, a marker of airway type 2 inflammation that is known to predict albuterol and triamcinolone responses in this cohort (30, 31). The relative absence of type 2 airway inflammation in asthma participants with IR may help explain our finding that patients with IR were characterized by blunted treatment responses to albuterol and triamcinolone. The limited effect of triamcinolone is important because corticosteroids are the primary controller medicine utilized for asthma therapy. The relative absence of type 2 inflammation in participants with IR make it unlikely that they will be good candidates for therapeutic proteins that target the type 2 pathway. Our work, therefore, uncovers a major unmet therapeutic need for medicines to improve asthma control in patients with IR. One possibility is that treating IR could improve disease control in these patients, but this will require clinical trials.
The HOMA-IR subgroups of 3–5 and >5 reflect a severity scale for degree of insulin resistance, and we found several clinical differences between participants with HOMA-IR values >5 compared with those with values between 3–5. Chief among these was a higher BMI, a higher prevalence of metabolic syndrome, and lower lung function. However, we did not find a difference in the rate of decline in lung function over time between moderate or severe IR participants. These findings suggest that the severity of IR is not a principle factor in driving lung function decline in asthma participants with IR.
In conclusion, IR is common in severe asthma and is associated with decreased lung function, decreased lung function responses to β-adrenergic agonists and corticosteroids, and increases in the rate of decline in lung function over time. The effects of metabolic dysfunction on lung function in asthma are observed independent of the effects of BMI. These results provide rationale to consider clinical trials that test whether treatments for insulin resistance prevent accelerated loss of lung function in asthma (32).
Acknowledgments
National Heart, Lung, and Blood Institute Severe Asthma Research Program-3 members: Michael C. Peters, M.D., Mark Schiebler, M.D., Juan Carlos Cardet, M.D., Mats W. Johansson, Ph.D., Ronald Sorkness, Ph.D., Mark D. DeBoer,M.D., Eugene R. Bleecker, M.D., Deborah A. Meyers, Ph.D., Mario Castro, M.D., Kaharu Sumino, M.D., Serpil C. Erzurum, M.D., Matthew C. Tattersall, D.O., Joe G. Zein, M.D., Annette T. Hastie, Ph.D., Wendy Moore, M.D., Bruce D. Levy, M.D., Elliot Israel, M.D., Melody Duvall, M.D., Brenda R. Phillips, M.S., David T. Mauger, Ph.D., Sally E. Wenzel, M.D., Merritt L. Fajt, M.D., Loren C. Denlinger, M.D., Ph.D., Prescott G. Woodruff, M.D., Nizar N. Jarjour, M.D., and John V. Fahy, M.D.
Footnotes
A complete list of National Heart, Lung, and Blood Institute Severe Asthma Research Program-3 members may be found before the beginning of the References.
Supported by NIH grant K23 HL138303, AstraZeneca, Boehringer Ingelheim, Genentech, and GlaxoSmithKline.
Author Contributions: M.C.P., D.T.M., and J.V.F. are the guarantors of the paper, taking responsibility for the integrity of the work as a whole from inception to published article. M.C.P., M.L.S., J.C.C., M.W.J., R.S., M.D.D., E.R.B., D.A.M., M.C., K.S., S.C.E., M.C.T., J.G.Z., A.T.H., W.M., B.D.L., E.I., M.D.D., B.R.P., D.T.M., S.E.W., M.L.F., S.K.K., L.C.D., P.G.W., N.N.J., and J.V.F. conceived and designed the study. M.C.P. and D.T.M. did the primary analysis and made substantial contributions to the design of the study and interpretation of data. M.C.P. and D.T.M. had access to and verified the underlying data. M.C.P. and J.V.F. prepared the first draft of the manuscript, and all authors revised the draft critically for important intellectual content. All authors gave final approval for the manuscript version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202112-2745OC on June 10, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
for the National Heart, Lung, and Blood Institute Severe Asthma Research Program-3:
Michael C. Peters, Mark Schiebler, Juan Carlos Cardet, Mats W. Johansson, Ronald Sorkness, Mark D. DeBoer, Eugene R. Bleecker, Deborah A. Meyers, Mario Castro, Kaharu Sumino, Serpil C. Erzurum, Matthew C. Tattersall, Joe G. Zein, Annette T. Hastie, Wendy Moore, Bruce D. Levy, Elliot Israel, Melody Duvall, Brenda R. Phillips, David T. Mauger, Sally E. Wenzel, Merritt L. Fajt, Loren C. Denlinger, Prescott G. Woodruff, Nizar N. Jarjour, and John V. Fahy
References
- 1. Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr . 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 2. Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, et al. National Heart, Lung, and Blood Institute Severe Asthma Research Program Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med . 2016;4:574–584. doi: 10.1016/S2213-2600(16)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li X, Hastie AT, Peters MC, Hawkins GA, Phipatanakul W, Li H, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program (SARP) Networks Investigation of the relationship between IL-6 and type 2 biomarkers in patients with severe asthma. J Allergy Clin Immunol . 2020;145:430–433. doi: 10.1016/j.jaci.2019.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McKeever TM, Weston PJ, Hubbard R, Fogarty A. Lung function and glucose metabolism: an analysis of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol . 2005;161:546–556. doi: 10.1093/aje/kwi076. [DOI] [PubMed] [Google Scholar]
- 5. Yeh HC, Punjabi NM, Wang NY, Pankow JS, Duncan BB, Cox CE, et al. Cross-sectional and prospective study of lung function in adults with type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care . 2008;31:741–746. doi: 10.2337/dc07-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim SH, Kim HS, Min HK, Lee SW. Association between insulin resistance and lung function trajectory over 4 years in South Korea: community-based prospective cohort. BMC Pulm Med . 2021;21:110. doi: 10.1186/s12890-021-01478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters MC, Schiebler ML, Bleecker ER, Castro M, Erzurum SC, Hastie A, et al. 2019. pp. A2684–A2684.https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A2684
- 8. Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, et al. Baseline features of the Severe Asthma Research Program (SARP III) Cohort: differences with age. J Allergy Clin Immunol Pract . 2018;6:545–554.e4. doi: 10.1016/j.jaip.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peters MC, Mauger D, Ross KR, Phillips B, Gaston B, Cardet JC, et al. Evidence for exacerbation-prone asthma and predictive biomarkers of exacerbation frequency. Am J Respir Crit Care Med . 2020;202:973–982. doi: 10.1164/rccm.201909-1813OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laboratory Search | CDC 2021https://www.cdc.gov/clia/LabSearch.html.
- 11. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser . 2000;894:i–xii, 1–253. [PubMed] [Google Scholar]
- 12. Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s health: association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Obesity (Silver Spring) . 2007;15:1061–1067. doi: 10.1038/oby.2007.632. [DOI] [PubMed] [Google Scholar]
- 13. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit Pathw Cardiol . 2005;4:198–203. doi: 10.1097/00132577-200512000-00018. [DOI] [PubMed] [Google Scholar]
- 14. Katsuki A, Sumida Y, Gabazza EC, Murashima S, Furuta M, Araki-Sasaki R, et al. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care . 2001;24:362–365. doi: 10.2337/diacare.24.2.362. [DOI] [PubMed] [Google Scholar]
- 15. Qu HQ, Li Q, Rentfro AR, Fisher-Hoch SP, McCormick JB. The definition of insulin resistance using HOMA-IR for Americans of Mexican descent using machine learning. PLoS One . 2011;6:e21041. doi: 10.1371/journal.pone.0021041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Phipatanakul W, Mauger DT, Sorkness RL, Gaffin JM, Holguin F, Woodruff PG, et al. Severe Asthma Research Program. Effects of age and disease severity on systemic corticosteroid responses in asthma. Am J Respir Crit Care Med . 2016;195:1439–1448. doi: 10.1164/rccm.201607-1453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Textor J, Hardt J, Knüppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology . 2011;22:745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- 18.Marginal analysis | Stata. https://www.stata.com/features/overview/marginal-analysis/
- 19. Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol . 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 20. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J . 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 21. Friedrich MJ. Global obesity epidemic worsening. JAMA . 2017;318:603–603. doi: 10.1001/jama.2017.10693. [DOI] [PubMed] [Google Scholar]
- 22. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature . 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 23. Thomas ET, Guppy M, Straus SE, Bell KJL, Glasziou P. Rate of normal lung function decline in ageing adults: a systematic review of prospective cohort studies. BMJ Open . 2019;9:e028150. doi: 10.1136/bmjopen-2018-028150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lalley PM. The aging respiratory system--pulmonary structure, function and neural control. Respir Physiol Neurobiol . 2013;187:199–210. doi: 10.1016/j.resp.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 25. Singh S, Bodas M, Bhatraju NK, Pattnaik B, Gheware A, Parameswaran PK, et al. Hyperinsulinemia adversely affects lung structure and function. Am J Physiol Lung Cell Mol Physiol . 2016;310:L837–L845. doi: 10.1152/ajplung.00091.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park YH, Oh EY, Han H, Yang M, Park HJ, Park KH, et al. Insulin resistance mediates high-fat diet-induced pulmonary fibrosis and airway hyperresponsiveness through the TGF-β1 pathway. Exp Mol Med . 2019;51:1–12. doi: 10.1038/s12276-019-0258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arcaro G, Cretti A, Balzano S, Lechi A, Muggeo M, Bonora E, et al. Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation . 2002;105:576–582. doi: 10.1161/hc0502.103333. [DOI] [PubMed] [Google Scholar]
- 28. Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord . 2013;14:5–12. doi: 10.1007/s11154-012-9229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cesari M, Pahor M, Incalzi RA. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther . 2010;28:e72–e91. doi: 10.1111/j.1755-5922.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med . 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peters MC, Kerr S, Dunican EM, Woodruff PG, Fajt ML, Levy BD, et al. National Heart, Lung and Blood Institute Severe Asthma Research Program 3 Refractory airway type 2 inflammation in a large subgroup of asthmatic patients treated with inhaled corticosteroids. J Allergy Clin Immunol . 2019;143:104–113.e14. doi: 10.1016/j.jaci.2017.12.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Foer D, Beeler PE, Cui J, Karlson EW, Bates DW, Cahill KN. Asthma exacerbations in patients with type 2 diabetes and asthma on glucagon-like peptide-1 receptor agonists. Am J Respir Crit Care Med . 2021;203:831–840. doi: 10.1164/rccm.202004-0993OC. [DOI] [PMC free article] [PubMed] [Google Scholar]