SUMMARY
The onset of monophasic sleep, in which napping ceases and sleep consolidates into a single night period, is a key developmental milestone of childhood. Yet to date, there is little consensus regarding the timing of cessation of napping in children. The aim of the current study is to examine global evidence regarding napping patterns in childhood, and, through meta-analysis, describe patterns of napping cessation and duration observed in children aged 0–12 y. A systematic search of all published, original research articles reporting children’s napping patterns, by age, was conducted. The quality of studies was assessed, and meta-analysis of eligible studies undertaken. Risk of bias and heterogeneity of measurement was high. Current evidence indicates that less than 2.5% of children cease napping prior to age 2, while 94% cease napping by age 5. The preschool period (3–5 y; 36–60 mo) represents a particularly dynamic period in napping cessation, with large variation in rates of napping across studies evidencing potential ecological effects. Future studies should focus on understanding of the underlying mechanisms explaining individual variations in napping patterns and the extent to which patterns of napping may represent a marker of child development.
Keywords: Nap, Sleep, Patterns, Transition, Cessation, Children, Infants, Monophasic, Daytime, Diurnal
Introduction
The early years of life are characterised by a dynamic transition in sleep patterns [1]. As children age, sleep transitions from polyphasic sleep, characterised by multiple sleep periods across the 24-h cycle; to biphasic sleep in which napping occurs once per day; and finally monophasic sleep in which habitual napping ceases and all sleep consolidates into a single night episode. The rate of which this transition occurs varies across children and the meaning of this variation is not well understood [2,3]. The purpose of this study is to examine world-wide evidence regarding napping patterns in childhood and, through meta-analysis, describe patterns of napping cessation and duration observed in children aged 0–12 y.
Current research suggests that cessation of habitual napping corresponds to a changes in sleep need, neurocognitive function [20–4] as well as maturation of bio regulatory circadian and homeostatic processes [5–7]. A key interest is the impact of napping on 24-h sleep duration. A recent systematic review [2] reported that while napping in the first two years of life is essential for obtaining sufficient sleep, beyond this age napping is associated with reduced night sleep; thus a redistribution in 24-h sleep timing. Building evidence also suggests that the rate and timing of sleep consolidation and cessation of napping may be a marker of developmental cognitive change. A mix of review, correlational, and quasi-experimental studies describe mixed results of napping for children’s cognition and learning [3,8–13]; a key distinguishing feature being the age and regular napping patterns of the children studied. Benefit for learning and emotional regulation is typically found for younger children, who are less likely to have ceased habitual napping [2,3,11,12]. One study of preschool children (3–5 y) [9], reported a positive effect of napping on learning and memory consolidation, however these effects were only observed for habitual nappers (≥5 d/wk). Correlational studies based on parent reported sleep patterns suggest sleep consolidation (day/night sleep ratio) is a marker of neurocognitive maturity and show that children who cease napping earlier are more advanced on neurocognitive [7] and language assessments [14]. Further, longitudinal analyses report that later sleep consolidation is a risk factor for language delay [15]. These studies direct attention to the changing function of napping across development and suggest that individual cessation patterns may be a marker of clinical significance.
Genetic and ecological factors may influence children’s napping patterns [4,15–17]. Data from behavioural genetics studies, for example, indicate that whilst early napping patterns (prior to 18 mo) are highly heritable [15,17], across time napping trajectories are increasingly explained by shared environmental effects (18mo = 33% variance; 30-mo = 48%; 48-mo = 79%) [16]. There is also evidence of socio-cultural variations in childhood napping patterns [4,18]. For example, in a study of 29,287 infants and toddlers (birth −36 mo) across 17 countries/regions, Mindell and colleagues [19] report small, statistically significant differences in napping frequency and duration across nations. However, this study does not provide data on napping prevalence or age of cessation and does not extend beyond the first three years of life. To date there is little-to-no consensus on timing of napping cessation in children. Yet such knowledge is important in three distinct ways; 1) to inform understanding of sleep development in pediatric populations; 2) to better understand the role of napping in meeting children’s 24-h sleep need and 3) to inform future research to establish reference points for distinguishing typical from atypical sleep behaviours that may signify the need for clinical investigation or intervention.
Methods
A review protocol was developed using the preferred reporting items for systematic reviews and meta-analyses (PRISMA [20,21]) guidelines and prospectively registered via PROSPORO (Registration #: CRD42015020149).
Search strategy
An extensive search for relevant studies published by December 18, 2018 was conducted using the following electronic databases: PubMed, Embase ONLY Embase.com, PsycINFO (via Ebscohost), CINAHL (via Ebscohost), Cochrane, Scopus, Web of Science, ProQuest Dissertation & Theses Global. Searches identified papers that contained the following key words: (“day time sleep” OR “daytime sleep” OR nap* OR “day?sleep” OR “sleep consolidation”) AND (child* OR infant* OR bab*) AND (longitudinal OR cohort OR cross? sectional OR “population stud*”) and were developed in consultation with a liaison librarian. The exact search terms, limiters used and outcomes for each database are provided in Table S1. Reference lists from papers identified by searches were also examined to identify potential papers for inclusion.
Inclusion and exclusion criteria
All published, full-text original research articles published in any language, and employing longitudinal or multiple cross-sectional designs and reporting napping in children aged 0–12 y were reviewed. Studies of children regardless of, gender, geographical location, socioeconomic status, health status or ethnic group were included. Napping was defined as periods of sleep, measured using observational (e.g., actigraphy), parent/carer or self-report (e.g., survey or sleep diary) or physiological (e.g., polysomnography) measurement that occurs during daytime hours. Studies that included napping as part of total or 24-h sleep measurement were included only if the napping component could be reasonably differentiated from night-time sleep or if napping was indicated by a ratio of day-to-night time sleep (an index of sleep consolidation). Consistent with the focus on naturalistic, changes in developing napping patterns by age, a range of study designs were excluded (i.e., single case reports, studies conducted at a single age-point and experimental studies [e.g., drug trials]).
Study selection
A four-step approach to the selection of studies was undertaken. First, two review authors independently examined the title and abstract to determine whether they met the inclusion criteria. Second, full-text versions of relevant articles were obtained, and authors conducted a preliminary screening to assess if they contained information on daytime sleep (using key words “day sleep”, “daytime sleep”, “nap” and “napping”). Third, full texts of identified articles were assessed against the full inclusion criteria, with rationale for inclusion or exclusion recorded. Where full text articles were not available online, the authors were contacted via email to request subsequent full-text publications. Consulting bi-lingual academics translated full-text articles published in languages other than English (N = 18). Any decisions on inclusion, data extraction and risk of bias scoring, was then completed by these authors in consultation with the authorship team. Finally, decisions for inclusion or exclusion were based on consensus amongst study authors. Where multiple studies reported on the same data source, only one study was included in qualitative and quantities analyses, with the corresponding papers indicated.
Quality assessment
The quality of each of the studies was assessed using the quality assessment checklist for prevalence studies [22]. The measure comprises 10 items, assessing both internal (selection and non-response bias) and external (measurement and analysis bias) validity. Studies are rated on each item to generate a total risk of bias score, categorised as low (0–3), moderate (4–6) or high (7–10). As napping patterns may change rapidly, the length of the shortest prevalence period was set between 1 wk and 1 mo. Consistent with reports of validity in sleep diary measurement [23], only diary report studies that included ≥3 d of diary records were rated as low risk for evidence of reliability of measurement. Two authors independently assessed and scored all articles. The initial scoring produced high agreement (ICC = 0.92). Any discrepancies were subsequently discussed to reach consensus. When insufficient information was available to accurately score an article, effort was made to obtain further detail through direct email contact with study authors prior to final assessment.
Meta-analyses
Meta-analyses were conducted to establish prevalence of napping cessation, and changes in duration of napping, by age. To establish estimates of napping cessation, all studies that provided data on the proportion of children not napping at a particular age-point were analysed. For studies that reported napping duration or absence/presence of napping, cessation was based on the proportion of children having “0-min” napping, or “no naps”, respectively, within the measurement period. Napping cessation could not be estimated for studies in which frequency of napping (i.e., “regular” or “usually” naps) was reported, as the converse of this could not be accurately assumed to be zero. The proportion of children ceasing to nap and napping duration was estimated for several age groups (≤12 mo [≤1 y; min possible = 0 – max possible = 12.99], >12.0 to ≤24 mo [>1 y to ≤2 y; 12–24.99], >24.0 to ≤36 mo [>2 y to 3 y; 24–38.99], >36.0 to ≤48 mo [>3 y to ≤4 y; 36–48], >48.0 to ≤60 mo [>4 y to ≤ 5 y; 48–60.99], >60 mo [>5 y; 60–155.99]) using Meta [24] (version 4.9–4), meta for [25] (version 2.0–0), and R [26] (version 3.5.1) packages. Proportions were transformed via the Freeman-Tukey double arcsine method (back transformations are presented) [27]. The Clopper-Pearson method was used to estimate confidence intervals for individual proportions [28]. Meta-analysis of duration included studies where duration averages were only based on children who napped and excluded studies where duration was inclusive of non-napping children or their inclusion could not be reliably inferred.
For meta-analysis of both cessation and duration, random effects models using the restricted maximum likelihood estimator [29] and weighted least squares were used to summarize the studies. The random effect model is presented, to provide an estimate of average napping cessation for the entire population of studies [30]. Weighting was used to account for study and sample size discrepancies [31,32]. Heterogeneity between studies was assessed with Cochran’s Q-test [33] (significant [<0.05] p-value indicates heterogeneity) and associated I2 statistic [34] (0–100%, higher percentage indicates more heterogeneity). Studies identified as having high risk of bias were initially excluded and re-included on the basis of results from sensitivity analysis. R code to replicate, extend and explore the meta-analyses is available in supplementary document 10 and also at Mendeley (https://doi.org/10.17632/c6bkfn9598.1).
Results
Database search and data extraction
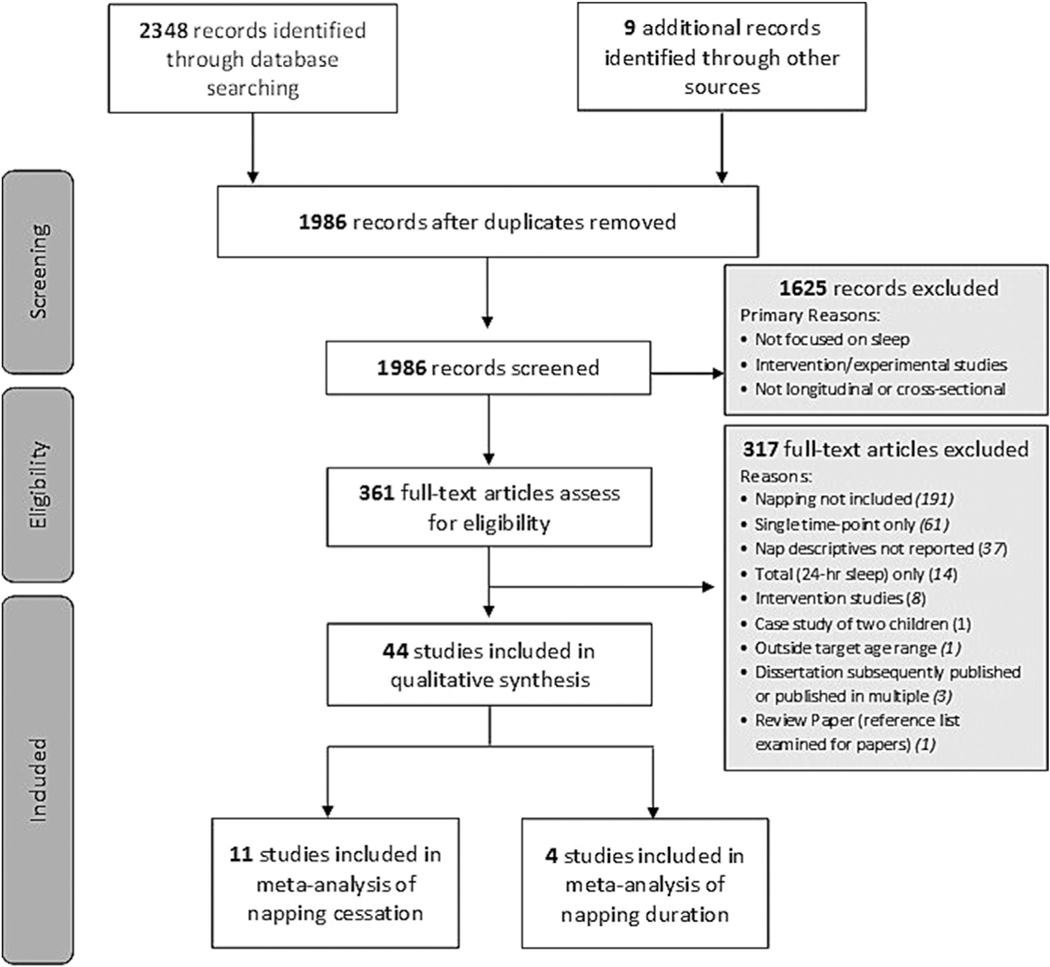
Search results and data extraction for each search stage are presented in Fig. 1. Of the full-text papers reviewed, 343 were published in English, with the remainder published in Chinese (n = 6), German (n = 5), French (n = 4), Japanese (n = 2) and Spanish (n = 1). At the full text level, 321 articles were excluded. Nine articles provided all (n = 4) or some (n = 5) of their napping data in figure form only, which could not be readily extracted. Correspondence with study authors for these papers provided additional extractable data for two studies [35,36]. Studies without any extractable napping data were included in qualitative analyses only. In total, 44 papers met criteria for inclusion (Table 1). Forty-one papers were published in full-text in English, and one each in Chinese, German, and French. Studies included those with longitudinal (n = 24; 55%) and multiple cross-sectional (n = 20; 45%) designs.
Fig. 1.
PRISMA flow diagram.
Table 1.
Sample, recruitment and design characteristics of longitudinal and cross-sectional studies of napping in children (0–12 y).
| Reference | Study type | Country (language) | Sample (N) | Age range (Months) | Sample type | Recruitment setting | Measure# |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Acebo et al 2005 [42] | Cross sectional | USA (English) | 169 | 12–60 | Non-clinical | Commercial survey firm, advertisements, word of mouth | P, Di, Ni |
| Aishworiya et al 2012 [43] | Cross sectional | Singapore (English) | 372 | 24–72 | Non-clinical | Local childcare centres | P, D |
| Armstrong et al 1994 [44] | Cross sectional | Australia (English) | 3269 | 0–38 | Non-clinical | Well-child screenings | P, D |
| BaHamman et al 2006 [45] | Cross sectional | Saudi Arabia (English) | 1012 | 72–156b | Non-clinical | Elementary schools | P, D |
| Basler 1980 [46] | Longitudinal | Switzerland (German) | 320 | 6–60 | Non-clinical | Wave 1954e1979 neonatal units of a hospitald | P,D |
| Bat-Pitault et al 2017 [47] | Longitudinal | France (French) | 133 | 6–24 | Non-clinical | Maternity hospital | D |
| Bell & Zimmerman 2010 [48] | Longitudinal | USA (English) | 1930 | 0–156b | Non-clinical | Households drawn by the census bureau | D |
| Blair et al 2012 [1] | Longitudinal | England (English) | 11,478 | 6–130 | Non-clinical | Expectant mothers | P, D |
| Bordeleau et al 2012 [49] | Longitudinal | Canada (English) | 70 | 12–48 | Non-clinical | Random birth from the Ministry of Health and Social Services | D |
| Bruni et al 2014 [50] | Longitudinal | Italy (English) | 704 | 3–12 | Non-clinical | Family paediatrician routine visits | D, N |
| Cheung et al 2017 [51] | Cross sectional | UK (English) | 715 | 6–36 | Non-clinical | Research databases, study advertisements | D |
| Crosby et al 2005 [18] | Cross sectional | USA (English) | 1043 | 24–96 | Non-clinical | Community sites, word-of-mouth, snowball sampling | P, D |
| Dong et al 2016 [35] | Longitudinal | China (Chinese) | 262 | 1.4–12 | Non-clinical | Local public hospital | D |
| Fernandez et al 2015 [52] | Cross sectional | Spain (English) | 125 | 1–24 | Non-clinical | Healthcare centres | D |
| Galland et al 2016 [39] | Cross sectional | New Zealand (English) | 160 | 6–42 | Non-clinical | Maternity clinic | D, N |
| Geiger et al 2010 [53] | Cross sectional | Switzerland (English) | 60 | 90–134 | Non-clinical | Primary schools | P |
| Ghaem et al 1998 [54] | Cross sectional | Australia (English) | 102 | 1–36 | Clinical (GORD) | Gastroenterology Department of Children’s Hospital | P, D |
| Hanafin et al 2017 [55] | Longitudinal | Ireland (English) | 29,898 | 9–60 | Non-clinical | Random sampling from Child Benefit Register | P, Dg |
| Humphreys et al 2014 [56] | Longitudinal | England (English) | 10,777 | 6–140 | Clinical (ASD)/ Non-clinical |
Expectant mothers (ASD via National Educational Database) | D |
| Iglowstein et al 2003h [36] | Longitudinal | Switzerland (English) | 493 | 6–84c | Non-clinical | Wave 1974–78: neo-natal units, wave 1978–93 uncleard | P, D, N |
| Laberge et al 2001 [57] | Longitudinal | Canada (English) | 1146 | 120–156b | Non-clinical | French language school boards | P |
| Louis et al 1997 [40] | Longitudinal | France (English) | 15 | 6–24 | Non-clinical | Not reported | D |
| Mindell et al 2010 [19] | Cross sectional | Multi-countrye (English) | 29,287 | 0–36 | Non-clinical | Parenting website | Di |
| Mindell et al 2011 [58] | Longitudinal | USA (English) | 117 | 6–18 | Non-clinical | “Recruited from the community” | D |
| Mindell et al 2016 [59] | Cross sectional | USAf (English) | 841 | 0–36 | Non-clinical | iPhone/iPad app for sleep in young children | Di, Ti |
| Murthy et al 2015 [60] | Cross sectional | India (English) | 368 | 12–36 | Non-clinical | Outpatient department, routine medical follow ups and crèches | P, D, N |
| Park et al 2002 [61] | Cross sectional | Japan (English) | 615 | 72–1068b | Non-clinical | Randomly selected companies (parents were workers) | Pi, Di, Ni |
| Price et al 2013 [62] | Longitudinal | Australia (English) | 10,090 | 4–111 | Non-clinical | Australian Medicare Database | D |
| Schwichtenberg et al 2011 j [63] | Longitudinal | USA (English) | 134 | 4–24 | Clinical (pre-term birth) |
Neonatal intensive care units | D, N |
| Seo et al 2010 [64] | Cross sectional | Korea (English) | 3639 | 84–144 | Non-clinical | Random sampling of elementary schools in districts | P, D |
| Smithson et al 2018 [65] | Longitudinal | Canada (English) | 677 | 3–24 | Non-clinical | Recruited during pregnancy (location not specified) | D |
| Sorondo & Reeb-Sutherland 2015 [66] | Longitudinal | USA (English) | 40 | 5–12 | Non-clinical | Local hospital and infant registries | D |
| Taylor et al 2015 [41] | Longitudinal | New Zealand (English) | 194 | 36–84 | Non-clinical | Maternity hospitals | P |
| Taylor et al 2018 [38] | Longitudinal | New Zealand (English) | 380 | 12–60 | Non-clinical | Single Maternity Hospital | D |
| Thorleifsdottir et al 2002 [37] | Longitudinal | Iceland (English) | 668 | 12–240b | Non-clinical | Random sampling from National Register of Iceland | P |
| Tikotzky et al 2015 [67] | Longitudinal | Israel (English) | 57 | 3–6 | Non-clinical | Hospital prenatal courses, prenatal internet forums | D |
| Touchette et al 2013 [16] | Longitudinal | Canada (English) | 983 | 6–48 | Non-clinical | Birth records of the Quebec Statistics Institute | Di |
| Watamura et al 2004 [68] | Cross sectional | Not reported USAa (English) | 83 | 12–36 | Non-clinical | “Indicated interest in research participation at birth” | P, D |
| Weissbluth 1995 [69] | Longitudinal | Not reported USAa (English) | 172 | 6–84 | Non-clinical | Paediatrician’s office | P, D, N |
| Wooding et al 1990 [70] | Cross sectional | New Zealand (English) | 874 | 1–12 | Non-clinical | Child-health community nurses provided lists | Di, N |
| Yang et al 2009 [71] | Cross sectional | China (English) | 1058 | 84–144 | Non-clinical | Rural and urban primary schools | D |
| Young et al 2007 [72] | Longitudinal | Australia (English) | 237 | 0–216b | Clinical (Rett syndrome) |
Australian Rett Syndrome Database | P |
| Yu 2014 [73] | Cross sectional | Hong Kong (English) | 1746 | 36–50 | Non-clinical | Kindergartens | P, D |
| Yu et al 2017 [74] | Cross-sectional | Hong Kong (English) | 1049 | 0–36 | Non-clinical | Mailing lists from marketing firms and parenting websites | D, N |
Note.
Location not reported, assumed from location of study authors;
Data extracted up to 12 y only (144 mo);
Full age range to 16 y (192 mo), napping only measured until 84 mo;
Information from email correspondence with authors, not reported in paper;
China, Hong Kong, India, Indonesia, Korea, Japan, Malaysia, Philippines, Singapore, Taiwan, Thailand, Vietnam; Australia, Canada, New Zealand, United Kingdom, United States of America;
Including Puerto Rico;
Duration reported for one time point only;
Napping data for this study also reported in [75];
Data reported in figure form only;
P = Prevalence, D = Duration, N = Naps per day, T = Timing; ASD = autism spectrum disorder; GORD = gastro-oesophageal reflux disease; USA=United States of America.
Quality assessment
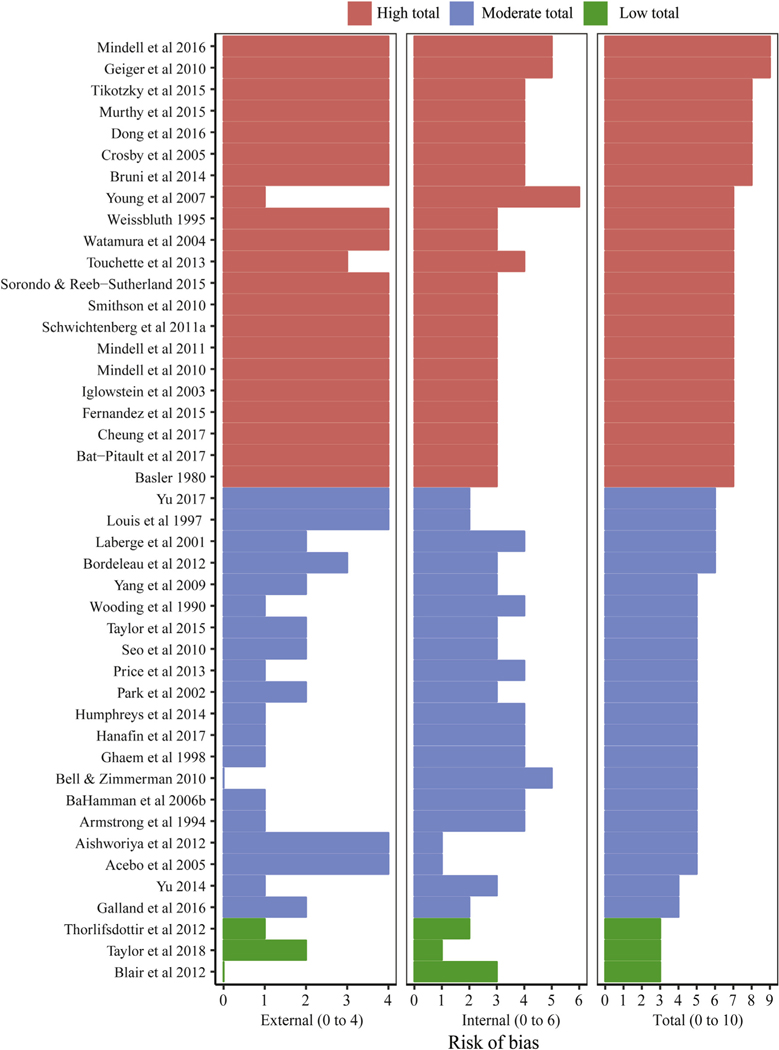
Most studies were rated as having moderate (n = 20, 45.4%) to high (n = 21, 47.7%) risk of bias (Fig. 2). Key sources of external validity biases included risks associated with the use of convenience or purposive sampling or absence of sufficient data to assess the sampling frame. Internal validity biases stemmed from use of subjective, undefined or poorly validated measurement approaches. Only three studies were assessed to have low risk of bias [1,37,38].
Fig. 2.
Risk of bias scores, including internal and external validity, across napping studies. Note. Red = high risk (≥7); Blue = moderate risk (4–6); Green = low risk (≤3). External validity scores range 0–4; internal validity scores range 0–6; total range 0–10. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Sampling and design
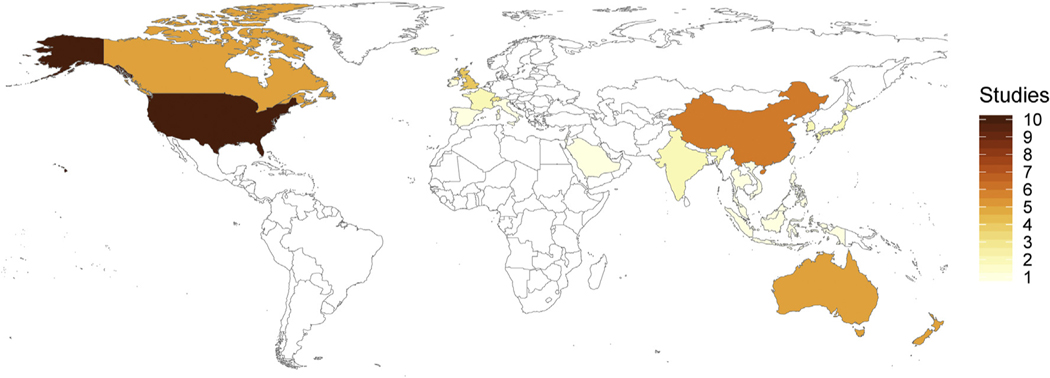
Identified studies were conducted across European (n = 13), North American (n = 15), Asian (n = 22), and Australasian (n = 10) continents (Fig. 3). Two studies did not report the study location, so we assumed they were conducted in the country of the authors. Napping data was primarily derived from parent report, including parent survey and sleep diaries. Only four studies report napping using objective measurement [38–41]. Louis et al. [40] report duration data based on polysomnography measurement of N = 15 children in France across 24-h periods at 3, 6, 9 12 and to 24 mo of age. Three studies, all conducted in New Zealand, report napping data based on accelerometry [38,39,41].
Fig. 3.
Napping studies that met inclusion criteria across geographical region.
Napping prevalence
Napping prevalence rates by age for each study is provided in Table S2. Considerable heterogeneity in measurement of napping across studies was present, thus precluding meta-analysis. Four key approaches to measurement of prevalence were used. Frequency measurement was the most common approach (n = 10), and included reports of 1)‘regular’ [41,43,53] or ‘frequent’ [72] napping that was undefined, 2) napping across multiple frequency descriptors (e.g., ‘never, sometimes, everyday’ [36], or 3) frequency of naps per day [60,70] or week [18]. Only two studies specifically defined regular napping, but varied in definition as that occurring ‘more than once per week’ [57] and ‘at least 3 times per week’ [45]. Presence/Absence of napping (n = 5) included measurement descriptors such as the number of children ‘having daytime sleep’ [54], ‘who slept during the day’ [36] or ‘any naps’ [42], but did not account for daily variation in napping behaviours. Five studies based napping prevalence on measurement of duration of napping, providing prevalence across duration categories (e.g., ‘up to 60 min’ [55]). Only one study reported specifically on ‘napping cessation’ [69]. This study, based on a sample of N = 172 children aged six months to seven years, from the United States of America (USA), focused on parent reports of whether children had ceased (stopped) napping naturally or whether the parent did not allow napping.
Napping cessation
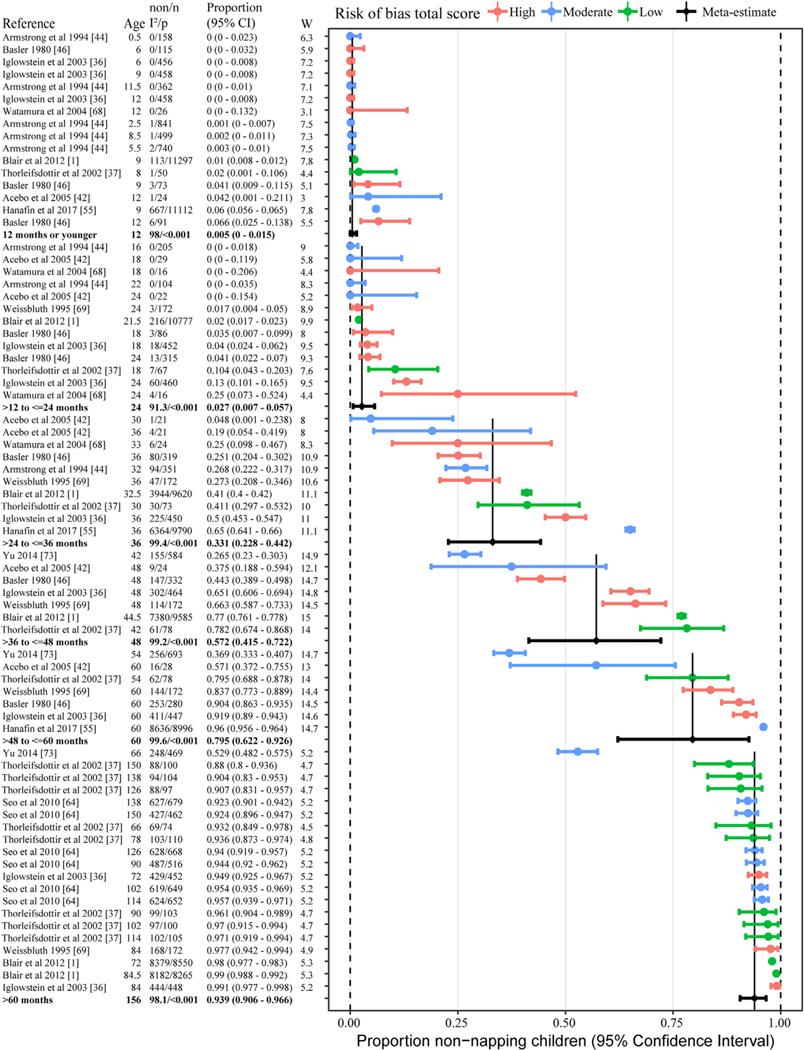
Eleven studies were used to estimate cessation of napping by age [1,36,37,42,44,46,55,64,68,69,73]. These 11 studies provided a total of 73 data-points for meta-analyses (Fig. 4). Results indicate that prior to two years of age, cessation of napping is rare (<2.5%). By the time children are aged three, 33% of children have ceased napping. The largest rates of cessation occur for children across the preschool period, with 57% ceasing to nap by age 36–48 mo and 80% by 48–60 mo. The preschool period also had the largest variation in cessation prevalence rates across studies, with rates ranging from 4.8% to 65% at 36 mo (three years), 26.5%–78.2% at 48 mo (four years) and 36.9%–96% at 60 mo (five years). Beyond 60 mo of age, 94% of children had ceased napping. Exclusion of high risk studies provided slightly lower estimates of cessation (Table S3).
Fig. 4.
Random effects meta-analysis of napping cessation by age groupings. Individual studies are ordered by proportion napping and then median age of sample range within each age grouping. Age = median of age range (months), non = number children not napping, n = number children, I2 = 0–100 indictor of heterogeneity between studies (only for random effect rows), p = p-value for I2 and if less than 0.05 means significant heterogeneity (only for random effect rows), and W = weighting in random effects analysis.
Geographical variations geographical areas
In general, there were not enough studies to make continental comparisons for most age groups (Table S4). However, for children 12–24 mo, European countries tended to have higher cessation rates than Australasia. Additionally, for children 24–36 mo, European countries tended to have higher cessation rates than North America.
Napping duration patterns
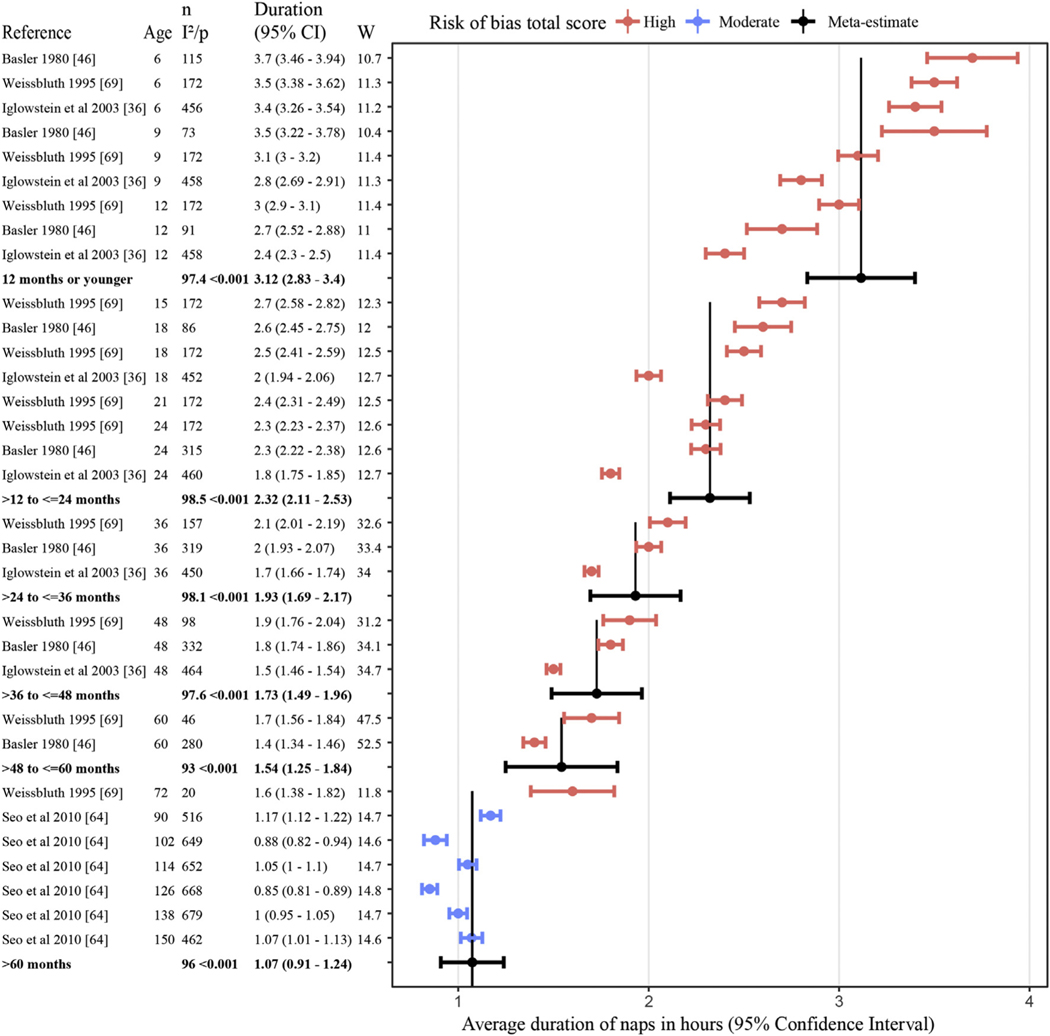
The most commonly reported napping characteristic was nap duration (n = 28 studies, Table S5). Many studies, however, reported mean duration inclusive of children who did not nap (n = 13) or did not clearly define the sample used to calculate duration (n = 9), did not include the standard deviation [42] or used a categorical measure of duration [1] and therefore could not be included in meta-analyses. Estimates were therefore based on a meta-analysis of four eligible studies [36,46,64,69]. On average, the duration of daytime naps for children was 3.1 h in infants below 12 mo, 2.3 h between age 1–2 y,1.9 h between 2 and 3 y,1.7 h between 3 and 4 y, 1.5 h between 4 and 5 y, and 1.1 h for children who napped beyond age five (Fig. 5, Table S6).
Fig. 5.
Random effects meta-analysis of napping duration by age groupings. Individual studies are ordered by napping duration and then median age of sample range within each age grouping. Age = median of age range (months), n = number children, I2 = 0–100 indictor of heterogeneity between studies (only for random effect rows), p = p-value for I2 and if less than 0.05 means significant heterogeneity (only for random effect rows), and W = weighting in random effects analysis.
Napping frequency and timing
Napping frequency was reported by nine studies and showed a reduction in naps per day as children aged (Fig. S7). Studies reporting the proportion of children napping per frequency indicate a dramatic reduction in polyphasic sleep (≥2 naps/day) at 12–18 mo of age [36]. However, polyphasic sleep was still present for a small number (17.1%) of children at 30 mo [60] and was reported up to 72 mo of age (0.84%) [36].
Studies examining changes in weekly distribution of napping were limited. Two studies [18,46], provided data on children’s pattern of napping across the week, with a gradual reduction in the number of days napping per week by age (Table S2). Armstrong et al. [44] also report some evidence of graduated transition to monophasic sleep, reporting children napping every day, most days, some days or never, although, the specific definition of each category were not defined. Yu et al. [73] report less prevalence of napping on weekends, when compared to weekdays.
Only one study [59] reports on the timing of naps by age. This paper analyzes parent report data collected across an 18-mo period via a free digital phone sleep diary application in the USA. Data includes reports for 841 unique children (birth –36 mo) and includes 156,989 recorded sleep periods. Mapping of napping patterns against time of day, show both high individual variation in nap timing, and also distinct changes across age. A bimodal napping pattern emerged from 8 to 12 mo of age with naps clustering around 09:30 and 14:00 h, and from 13 to 34 mo, the timing of naps converged towards the middle of the day and then gradually shift later in the afternoon (mid-point of approximately 14:00).
Napping patterns in clinical populations
Napping patterns in clinical populations were reported by four studies; including gastro-oesophageal reflux disease (GORD) [54], autism spectrum disorder (ASD) [56], pre-term birth [63] and Rett syndrome [72] (Table S8). Clinical studies focused on pre-term birth and autism describe napping patterns that are consistent with non-clinical samples. Infants and children with GORD showed significantly higher rates of napping (63%) compared to population norms (31%) in children beyond 24-mo only, likely reflecting compensation for disrupted night-time sleep [54]. Finally, children with Rett syndrome were reported to have high overall frequency of napping (70% in children 8–12 y) relative to non-clinical population norms and mutation specific variations in napping patterns [72].
Discussion
The aim of this review was to examine global evidence regarding napping patterns in childhood and, through meta-analysis, to describe patterns of napping cessation and duration observed in children aged 0–12 y. In total, 44 papers were identified and reviewed; the majority was of low to moderate quality. The extant literature describes a universal pattern of decline in napping prevalence, duration and frequency across childhood. For most children, this transition is completed within the first five years of life. Three distinct phases in the transition to napping cessation are evident. First, prior to 24 mo of age, napping is common, with rates of cessation less than 2.5%. Beyond this phase, acceleration in cessation rates is evidenced, alongside a notable widening of variation in prevalence across studies and global regions. Finally, beyond five years of age, consistent with school entry in most nations, the majority of children have ceased napping and continuation of napping is uncommon (8%).
Behavioural genetic studies of napping provide data that underpins the three distinct patterns described. These studies suggest that in the first two years of life genetics make a significant contribution to nap patterns, whereas beyond this period there is an increasing shared environment explanation for variations in napping patterns [15–17]. That our sample captures populations from different global regions and cultures, almost certainly increases the variability, reflecting ecological effects. The current data provides initial data to inform future research and efforts to establish reference values for typical napping patterns and those that are atypical. Clinical samples, whilst limited in number, suggest that napping may be associated with disruption of night sleep, as in the case of GORD [54], or specific genetic disorders [72]. However, the extent to which napping in the absence of clinical diagnoses is a marker of disorder warrants further investigation.
Neurophysiological research suggests that the biological drive to nap across the early years of life reflects an interaction between a developing circadian (‘body clock’) timing and a sleep-wake homeostatic (‘sleep drive’ accumulation) processes [5–7]. These homeostatic processes appear to attenuate across childhood, such that sleep pressure accumulates more slowly and thus, developing individuals are able to stay awake for longer periods across the day [5,77]. The changes in both the frequency and duration of napping across age are consistent with the dynamics of this process. For example, the current study showed a 50% reduction in napping duration across the first five years of life. The wide variation in napping cessation timing (between 2 and 5 y for most children) may also be indicative of individual variation in such neurophysiological and cognitive processes [6,7].
Limitation and future directions
Considerable issues of external validity were identified across studies. Few applied sampling frames to achieve population representation. Given that there are evident environmental factors that influence napping patterns [15,16], particularly across the preschool years [16], sampling will likely affect prevalence estimates. Almost all longitudinal and multi-point cross-sectional studies of napping have been based on European and USA populations, with limited studies from the Asia–Pacific region and no studies from African or South American Continents. For this reason, current understanding of napping patterns is restricted to specific geographical and cultural populations and cannot be generalized more broadly. Future studies examining the longitudinal patterns of napping across diverse cultural and geographical contexts are necessary.
The use of heterogeneous, un-validated and poorly defined napping measurements is a key limitation. Problems with measurement included a lack of consensus in the definition of napping, imprecise and ill-defined response categories, lack of objective measurement (i.e., reliance on parent report) and aggregation of data in a form that could not be readily interpreted (e.g., inclusion of non-napping children in duration estimates). Studies of napping patterns using objective measurement were rare (n = 4) and provide a direction for improvement of measurement in future studies. Such measures would allow for greater understanding of the process of change in napping across time, including nap timing, daily variation in napping patterns, associations with 24-h sleep cycles and the possibility of re-emergent napping (e.g., in circumstances of illness or change in care arrangements).
There is also the potential for individual studies to bias the results. Due to overlapping ages and age ranges of children between studies, studies were allocated to groups based on the median value of the possible total age range (as observed age range was sometimes unavailable). These age allocations will affect the meta-analysis results. Readers are encouraged to be aware of each studies possible age range, the associated confidence interval for each study and how a study may differentially affect meta-analysis estimates.
Finally, the current study focused on reported and observed napping patterns in children 0–12 y. Whilst this data can help to inform us what children “do” in regards to napping, it is important to note that this may vary from what children “should do”. There is evidence that napping behaviours are influenced by a number of environment factors, including parent sleep practices [50], child-care attendance [73], social-economic assets [63], parent work patterns [73] and technology use [51] (see Table S7). Sleep behaviours within the population may also change across time [36]. Future studies that account for potential variations in sleep need versus sleep opportunities are a critical next step in informing future establishment of normative reference values for napping in children.
Conclusion
This study examined global evidence to describe napping patterns in children aged 0–12 y. The results suggest three distinct phases of napping development, consistent with changing genetic, biological, and environmental influences on children’s sleep. The current study highlights the need for greater consensus and care in both the measurement and reporting of napping in young children, including greater utilization of objective measurement approaches. Future studies should focus on understanding of the underlying mechanisms explaining individual variations and group trends in napping patterns and the extent to which individual patterns of napping may represent a marker of broader social and cognitive development.
Supplementary Material
Practice points
Napping cessation prevalence rates are useful to:
inform understanding of sleep development in pediatric populations;
better understand the role of napping in meeting children’s 24-h sleep need;
inform future efforts to establish reference points to distinguish typical sleep development, from atypical development that may warrant investigation and/or intervention.
Research agenda
Increased studies of napping patterns in children, applying objective and consistent reporting standards, are warranted.
These studies should include:
measurement of napping across diverse geographic populations;
- extend beyond point-prevalence rates to identify:
- longitudinal patterns of napping transitions, through the application of objective, ambulatory measurement and a focus on intra- and inter-individual variations;
- social and genetic determinants of napping cessation patterns;
- short- and longer-term developmental consequences of timing of napping cessation;
account for potential variations in sleep need, versussleep opportunities; a critical step in establishing normative reference values for napping in children.
Acknowledgements
We would like to thank and acknowledge the contribution of Hannah Gehret who assisted with the initial data screening and translation of the German papers. We would also like to thank Dr Francisco Perales Perez, Dr Naohide Yamamoto and Dr Yangtao Huang who assisted with the translation of review papers. Finally, we gratefully thank contacted authors who provided data, clarifying information and assistance.
Funding source
Dr Staton is also supported by a fellowship from The National Health and Medical Research Council (NHMRC).
Abbreviations:
- ASD
autism spectrum disorder
- GORD
gastro-oesophageal reflux disease
- PRISMA
preferred reporting items for systematic reviews and meta-analyses
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.smrv.2019.101247.
Conflicts of interest None.
References
- [1]. Blair PS, Humphreys JS, Gringras P, Taheri S, Scott N, Emond A, et al. Childhood sleep duration and associated demographic characteristics in an English cohort. Sleep 2012;35(3):353–60 [Internet]. * The most important references are denoted by an asterisk.
- [2]. Thorpe K, Staton S, Sawyer E, Pattinson C, Haden C, Smith S. Napping, development and health from 0 to 5 years: a systematic review. Arch Dis Child 2015;100(7):615–22. * The most important references are denoted by an asterisk.
- [3]. Horvath K, Plunkett K. Spotlight on daytime napping during early childhood. Nat Sci Sleep 2018;10:97–104 [Internet]. * The most important references are denoted by an asterisk.
- 4.Smith SS, Edmed SL, Staton SL, Pattinson CL, Thorpe KJ. Correlates of naptime behaviors in preschool aged children. Nat Sci Sleep 2019;11:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenni OG, LeBourgeois MK. Understanding sleep-wake behavior and sleep disorders in children: the value of a model. Curr Opin Psychiatry 2006;19(3): 282–7 [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akacem LD, Simpkin CT, Carskadon MA, Wright KP, Jenni OG, Achermann P, et al. The timing of the circadian clock and sleep differ between napping and non-napping toddlers. PLoS One 2015;10(4):e0125181 [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurth S, Lassonde JM, Pierpoint LA, Rusterholz T, Jenni OG, McClain IJ, et al. Development of nap neurophysiology: preliminary insights into sleep regulation in early childhood. J Sleep Res 2016;25(6):646–54 [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger SE, Scher A. Naps improve new walkers’ locomotor problem solving. J Exp Child Psychol 2017;162:292–300. [DOI] [PubMed] [Google Scholar]
- 9.Kurdziel L, Duclos K, Spencer RMC. Sleep spindles in midday naps enhance learning in preschool children. Proc Natl Acad Sci 2013;110(43):17267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werchan DM, Gômez RL. Wakefulness (not sleep) promotes generalization of word learning in 2.5-year-old children. Child Dev 2014;85(2):429–36. [DOI] [PubMed] [Google Scholar]
- 11.Miller AL, Seifer R, Crossin R, Lebourgeois MK. Toddler’s self-regulation strategies in a challenge context are nap-dependent. J Sleep Res 2015;24(3):279–87 [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger RH, Miller AL, Steifer R, Cares SR, LeBourgeois MK. Acute sleep restriction effects on emotion responses in 30- to 36-month-old children. J Sleep Res 2012;21(3):235–46 [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam JC, Koriakin TA, Scharf SM, Mason TBA, Mahone EM. Does increased consolidated nighttime sleep facilitate attentional control? A pilot study of nap restriction in preschoolers. J Atten Disord 2019;23(4):333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam JC, Mahone EM, Mason T, Scharf SM. The effects of napping on cognitive function in preschoolers. J Dev Behav Pediatr 2011;32(2):90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dionne G, Touchette E, Forget-Dubois N, Petit D, Tremblay RE, Montplaisir JY, et al. Associations between sleep-wake consolidation and language development in early childhood: a longitudinal twin study. Sleep 2011;34(8): 987–95 [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Touchette E, Dionne G, Forget-Dubois N, Petit D, Perusse D, Falissard B, et al. Genetic and environmental influences on daytime and nighttime sleep duration in early childhood. Pediatrics 2013;131(6):e1874–80. * The most important references are denoted by an asterisk.
- 17.Fisher A, van Jaarsveld CHM, Llewellyn CH, Wardle J. Genetic and environmental influences on infant sleep. Pediatrics 2012;129(6):1091–6. [DOI] [PubMed] [Google Scholar]
- 18.Crosby B, LeBourgeois MK, Harsh J. Racial differences in reported napping and nocturnal sleep in 2- to 8-year-old children. Pediatrics 2005;115(1 Suppl):225–32 [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mindell JA, Sadeh A, Wiegand B, Hwei How T, Goh D. Cross-cultural differences in infant and toddler sleep. Sleep Med 2010;11:274–80 [Internet]. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009. Jul 21;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: elaboration and explanation. BMJ (Online) 2015:349. [DOI] [PubMed] [Google Scholar]
- 22.Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012;65(9):934–9. [DOI] [PubMed] [Google Scholar]
- 23.Thomas KA, Burr RL. Days of diary collection to measure mother and infant sleep. MCN Am J Matern Child Nurs 2009;34(4):256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarzer G. Meta: an R package for meta-analysis. R News 2007;7(3):40–5 [Internet]. [Google Scholar]
- 25.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36(6):1–48. [Google Scholar]
- 26.R Core Team. R. A language and environment for statistical computing [Internet]. Vienna: R Foundation for Statistical Computing; 2018. Available from: https://www.r-project.org. [Google Scholar]
- 27.Freeman MFF, Tukey JWW. Transformations related to the angular and the square root. Ann Math Stat 1950;21(4):607–11 [Internet]. [Google Scholar]
- 28.Newcombe RGG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998;17(8):857–72 [Internet]. [DOI] [PubMed] [Google Scholar]
- 29.Bias Viechtbauer W. and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat 2005. Nov 27;30(3):261–93 [Internet]. [Google Scholar]
- 30.Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychol Methods 1998;3(4):486–504. [Google Scholar]
- 31.Laird N, Fitzmaurice G, Ding X. Comments on “Empirical vs natural weighting in random effects meta-analysis. Stat Med 2010;29(12):1266–81 [Internet]. [DOI] [PubMed] [Google Scholar]
- 32.Schwichtenberg AJ, Shah PE, Poehlmann J. Sleep and attachment in preterm infants. Infant Ment Health J 2013;34(1):37–46 [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- 34.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J 2003;327(7414):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong S-M, Lin Q-M, Zhu L-X, Jiang Y-R, Song Y-J, Sun W-Q, et al. Sleep patterns and sleep-related factors in normal infants: a prospective cohort study. Chinese Ment Heal J 2016;30(10):721–7 [Internet]. [Google Scholar]
- 36.Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics 2003;111(2):302–7 [Internet]. [DOI] [PubMed] [Google Scholar]
- [37]. Thorleifsdottir B, Bjornsson JK, Benediktsdottir B, Gislason T, Kristbjarnarson H. Sleep and sleep habits from childhood to young adulthood over a 10-year period. J Psychosom Res 2002;53(1):529–37 [Internet]. * The most important references are denoted by an asterisk.
- [38]. Taylor RW, Haszard JJ, Meredith-Jones KA, Galland BC, Heath AM, Lawrence J, et al. 24-h movement behaviors from infancy to preschool: cross-sectional and longitudinal relationships with body composition and bone health. Int J Behav Nutr Phys Act 2018;15(118) [Internet]. * The most important references are denoted by an asterisk.
- [39]. Galland B, Meredith-Jones K, Gray A, Sayers R, Lawrence J, Taylor B, et al. Criteria for nap identification in infants and young children using 24-h actigraphy and agreement with parental diary. Sleep Med 2016;19:85–92 [Internet]. * The most important references are denoted by an asterisk.
- [40].Louis J, Cannard C, Bastuji H, Challamel MJ. Sleep ontogenesis revisited: a longitudinal 24-hour home polygraphic study on 15 normal infants during the first two years of life. Sleep 1997;20(5):323–33 [Internet]. [DOI] [PubMed] [Google Scholar]
- [41]. Taylor RW, Williams SM, Farmer VL, Taylor BJ. The stability of sleep patterns in children 3 to 7 years of age. J Pediatr 2015;166(3):697–702 [Internet]. * The most important references are denoted by an asterisk.
- 42.Acebo C, Sadeh A, Seifer R, Tzischinsky O, Hafer A, Carskadon MA. Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year-old children. Sleep 2005;28(12):1568–77 [Internet]. [DOI] [PubMed] [Google Scholar]
- 43. Aishworiya R, Chan P, Kiing J, Chong SC, Laino AG, Tay Sk. Sleep behaviour in a sample of preschool children in Singapore. Ann Acad Med Singapore 2012;41(3):99–104 [Internet]. * The most important references are denoted by an asterisk.
- 44.Armstrong KL, Quinn RA, Dadds MR. The sleep patterns of normal children. Med J Aust 1994;161(3):202–6 [Internet]. [DOI] [PubMed] [Google Scholar]
- 45.BaHammam A, Bin Saeed A, Al-Faris E, Shaikh S. Sleep duration and its correlates in a sample of Saudi elementary school children. Singapore Med J 2006;47(10):875–81 [Internet]. [PubMed] [Google Scholar]
- 46.Basler K, Largo RH, Molinari L . [The development of sleep behavior within the first 5 years of life]. Helv Paediatr Acta 1980;35(3):211–23 [Internet]. [PubMed] [Google Scholar]
- 47.Bat-Pitault F, Da Fonseca D, Flori S, Porcher-Guinet V, Stagnara C, Patural H, et al. Recognition of facial expressions of emotions by 3-year-olds depending on sleep and risk of depression. Encephale 2017;43(5):416–22 [Internet]. [DOI] [PubMed] [Google Scholar]
- 48.Bell JF, Zimmerman FJ. Shortened nighttime sleep duration in early life and subsequent childhood obesity. Arch Pediatr Adolesc Med 2010;164(9):840–5 [Internet]. [DOI] [PubMed] [Google Scholar]
- 49.Bordeleau S, Bernier A, Carrier J. Longitudinal associations between quality of mother-infant interactions and children’s sleep at preschool age. Sleep Med 2012;12:S126 [Internet]. [DOI] [PubMed] [Google Scholar]
- 50.Bruni O, Baumgartner E, Sette S, Ancona M, Caso G, Di Cosimo ME, et al. Longitudinal study of sleep behavior in normal infants during the first year of life. J Clin Sleep Med 2014;10(10):1119–27 [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheung CHM, Bedford R, De Urabain IRS, Karmiloff-Smith A, Smith TJ. Daily touchscreen use in infants and toddlers is associated with reduced sleep and delayed sleep onset. Sci Rep 2017;7(46104) [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernandez Miaja M, Rodriguez Fernandez C, Fernandez Perez ML, Mata Zubillaga D, Miaja Quinones J, Rodriguez Fernandez LM. Amount of sleep and changes in its patterns in children less than two years old. An Pediatría 2015;82(2):89–94 [Internet]. [DOI] [PubMed] [Google Scholar]
- 53.Geiger A, Achermann P, Jenni OG. Association between sleep duration and intelligence scores in healthy children. Dev Psychol 2010;46(4):949–54 [Internet]. [DOI] [PubMed] [Google Scholar]
- 54.Ghaem M, Armstrong KL, Trocki O, Cleghorn GJ, Patrick MK, Shepherd RW. The sleep patterns of infants and young children with gastro-oesophageal reflux. J Paediatr Child Health 1998;34(2):160–3 [Internet]. [DOI] [PubMed] [Google Scholar]
- 55.Hanafin S. Sleep patterns and problems in infants and young children in Ireland. Child Care Health Dev 2017;44(3):470–5 [Internet]. [DOI] [PubMed] [Google Scholar]
- 56.Humphreys JS, Gringras P, Blair P, Scott N, Henderson J, Fleming PJ, et al. Sleep patterns in children with autistic spectrum disorders: a prospective cohort study. Arch Dis Child 2014;99(2):114–8 [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laberge L, Petit D, Simard C, Vitaro F, Tremblay RE, Montplaisir J. Development of sleep patterns in early adolescence. J Sleep Res 2001;10(1):59–67 [Internet]. [DOI] [PubMed] [Google Scholar]
- 58.Mindell JA, DuMond C, Tanenbaum J, Gerdes M, Kulkarni N, Gunn E. Sleep and socio-emotional development in infants. Sleep 2011;34:A285 [Internet]. [Google Scholar]
- [59]. Mindell JA, Leichman ES, Composto J, Lee C, Bhullar B, Walters RM. Development of infant and toddler sleep patterns: real-world data from a mobile application. J Sleep Res 2016;25(5):508–16 [Internet]. * The most important references are denoted by an asterisk.
- 60.Murthy CL, Bharti B, Malhi P, Khadwal A. Sleep habits and sleep problems in healthy preschoolers. Indian J Pediatr 2015;82(7):606–11 [Internet]. [DOI] [PubMed] [Google Scholar]
- 61.Park YM, Matsumoto K, Seo YJ, Kang MJ, Nagashima H. Changes of sleep or waking habits by age and sex in Japanese. Percept Mot Skills 2002;94(3 Pt 2):1199–213 [Internet]. [DOI] [PubMed] [Google Scholar]
- 62.Price A, Brown J, Bittman M, Wake M, Quach J, Hiscock H. How children’s sleep patterns change from 0–9 years: Australian population longitudinal study. Sleep Biol Rhythms 2013;11:73 [Internet]. [DOI] [PubMed] [Google Scholar]
- 63.Schwichtenberg AJ, Anders TF, Vollbrecht M, Poehlmann J. Daytime sleep and parenting interactions in infants born preterm. J Dev Behav Pediatr 2011;32(1):8–17 [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seo WS, Sung HM, Lee JH, Koo BH, Kim MJ, Kim SY, et al. Sleep patterns and their age-related changes in elementary-school children. Sleep Med 2010;11(6):569–75 [Internet]. [DOI] [PubMed] [Google Scholar]
- 65.Smithson L, Baird T, Tamana SK, Lau A, Mariasine J, Chikuma J, et al. Shorter sleep duration is associated with reduced cognitive development at two years of age. Sleep Med 2018;48:131–9 [Internet]. [DOI] [PubMed] [Google Scholar]
- 66.Sorondo BM, Reeb-Sutherland BC. Associations between infant temperament, maternal stress, and infants’ sleep across the first year of life. Infant Behav Dev 2015;39:131–5 [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tikotzky L, Sadeh A, Volkovich E, Manber R, Meiri G, Shahar G. Infant sleep development from 3 to 6 months postpartum: links with maternal sleep and paternal involvement. Monogr Soc Res Child Dev 2015;80(1):107–24 [Internet]. [DOI] [PubMed] [Google Scholar]
- 68.Watamura SE, Donzella B, Kertes DA, Gunnar MR. Developmental changes in baseline cortisol activity in early childhood: relations with napping and effortful control. Dev Psychobiol 2004;45(3):125–33 [Internet]. [DOI] [PubMed] [Google Scholar]
- 69.Weissbluth M. Naps in children: 6 months - 7 years. Sleep 1995;18(2):82–7 [Internet]. [DOI] [PubMed] [Google Scholar]
- 70.Wooding AR, Boyd J, Geddis DC. Sleep patterns of New Zealand infants during the first 12 months of life. J Paediatr Child Health 1990;26(2):85–8 [Internet]. [DOI] [PubMed] [Google Scholar]
- 71.Yang QZ, Bu YQ, Dong SY, Fan SS, Wang LX. A comparison of sleeping problems in school-age children between rural and urban communities in China. J Paediatr Child Health 2009;45(7e8):414–8 [Internet]. [DOI] [PubMed] [Google Scholar]
- 72.Young D, Nagarajan L, de Klerk N, Jacoby P, Ellaway C, Leonard H. Sleep problems in Rett syndrome. Brain Dev 2007;29(10):609–16 [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu X. Childhood sleep/wake patterns: local norms, associations, health outcomes and interventions [Internet], 3707473. Ann Arbor: The Chinese University of Hong Kong; (Hong Kong: ); 2014. [Google Scholar]
- 74.Yu X-T, Sadeh A, Lam HS, Mindell JA, Li AM. Parental behaviors and sleep/ wake patterns of infants and toddlers in Hong Kong, China. World J Pediatr 2017;13(5):496–502 [Internet]. [DOI] [PubMed] [Google Scholar]
- 75.Jenni OG, Iglowstein I, Benz C, Largo RH. Percentile curves for sleep duration in the first 16 years of life. Padiatr Prax 2003;63(3):481–9 [Internet]. [Google Scholar]
- 76.Schwichtenberg AJM. Sleep patterns in preterm and low birthweight infants [Internet], 3328006. Ann Arbor: The University of Wisconsin - Madison; 2008. [Google Scholar]
- [77]. LeBourgeois MK, Rusterholz T, Jenni OG, Carskadon MA, Achermann P. Do the dynamics of sleep homeostatis (process) change across early childhood? Sleep 2012;35:A21. * The most important references are denoted by an asterisk.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.