Background:
We aimed to evaluate the correlation between serum sirtuin 6 (sirt6) level and clinicopathological characteristics and prognosis of gastric cancer (GC) patients.
Methods:
The serum sirt6 levels of subjects (135 cases of GC, 68 cases of atrophic gastritis, 60 cases of healthy controls) were analyzed by enzyme-linked immunosorbent assay. The predictive and prognostic values of sirt6 serum level for GC were determined by performing receiver operating characteristic curve (ROC), Kaplan–Meier analysis, as well as univariate and multivariate Cox regression, respectively.
Results:
GC patients showed lower sirt6 serum levels than that of atrophic gastritis patients and healthy control. Taking the healthy control as a reference, the area under the ROC curve (AUC) of sirt6 serum level for diagnosing GC was 0.955 with a sensitivity of 91.85% and a specificity of 90.0%. Based on ROC analysis using atrophic gastritis as the state variable, serum sirt6 had a high diagnostic efficiency for GC (AUC = 0.754). Serum sirt6 was related to the clinicopathological features (tumor size, Lauren’s classification, tumor node metastasis staging, lymph node metastasis) and overall survival (log-rank χ2 = 12.22, P < .001). The AUC of serum sirt6 predicting death in GC patients was 0.731. At the optimal cutoff value (16.83 ng/mL), the sensitivity and specificity of sirt6 were 59.57% and 79.55%, respectively. Moreover, lower sirt6 level as independent risk factor was revealed to affect prognosis of GC patients (P = .018).
Conclusion:
Serum sirt6 level was positively associated with the tumor stage and metastasis conditions, which could be served as diagnostic and predictive biomarkers in GC.
Keywords: cox regression analyses, diagnosis, gastric cancer, prognosis, serum sirt6
1. Introduction
As the most prevalent malignancy of the digestive system and the third greatest cause of cancer-related fatalities,[1] the prognosis of patients with advanced gastric cancer (GC) is still poor although the overall survival has been significantly improved in the past decades.[2] The misdiagnosed result in the delay in treatment due to the unspecific early symptoms of GC patients, implying the importance of early and accurate diagnosis in improving GC prognosis.[3] Gastroscopy and histological examination are the most commonly used screening methods for gastrointestinal diseases with the disadvantages of high cost, invasiveness and uncomfortableness.[4] Body fluid-based detection is thought to be a good noninvasive option, such as serum biomarkers carcino-embryonic antigen (CEA), carbohydrate antigen (CA) 19-9 and CA72-4, which, however, demonstrated low sensitivity for GC diagnosis.[5] The identification of effective serum biomarkers is of great significance for the diagnosis and prognosis of GC.
Sirtuin 6 (sirt6) a nicotinamide adenine dinucleotide-dependent histone deacetylase[6] has long-chain fatty acylase activity, thus playing an eventful role in cell metabolism, cell cycle, apoptosis and transcriptional regulation.[7] In addition, its dysregulated has been confirmed to be involved in the occurrence of many diseases including tumors.[8] For example, Shen et al[9] found that sirt6 expression in GC tissues was related to Lauren’s classification, and the high expression of sirt6 was unfavorable for overall survival via analyzing the sirtuins expression profiles form GEO datasets (GSE62254 and GSE15459). In addition, Zhou et al[10] proposed that sirt6 in GC tissues is a protective factor for the prognosis of GC via detecting the protein expression by immunohistochemical methods. It is worth noting that the level of serum sirt6 is considered to be an aid in the diagnosis of coronary artery disease.[11] Besides, the level of serum sirt6 is considered as a potential sex-specific aging marker.[12] However, there is still a lack of relevant reports about the preoperative serum sirt6 predicting the prognosis of GC.
2. Objectives
In the current study, the sirt6 serum level was detected by an enzyme-linked immunosorbent assay, and the correlation between its concentration with clinicopathological characteristics and prognosis in GC patients was analyzed, thus providing suitable relevant targets for the clinical diagnosis and prognostic judgment of GC.
3. Materials and Methods
3.1. Clinical data and pathological evaluation
The 135 cases of gastric cancer (GC group) patients admitted to Xi’an international medical center Hospital between June 2015 and June 2016 were enrolled, who all met the inclusion criteria: all patients were diagnosed with GC by gastroscopy and pathological examination; no drugs or chemotherapy were received before surgery. The following were the criteria for exclusion: have a history of gastric surgery; taken the following drugs, including proton pump inhibitors, gastric protectants and other drugs within 2 weeks; have coagulation dysfunction or other diseases. Neoadjuvant and adjuvant chemotherapy were administered in 3 (2.22%) and 82 (60.74%) patients, respectively. A total of 80 patients (59.26%) received fluorouracil-based chemotherapy. A total of 65 patients (48.15%) received adjuvant chemotherapy over 3 cycles. No patient received adjuvant radiotherapy. Besides, patients with atrophic gastritis (atrophic gastritis [AG] group, n = 68) and healthy controls (HC group, n = 60) without stomach diseases or other diseases that affected the results of this study were selected during the same period. Patients with AG were referred for gastric antrum biopsy and upper gastrointestinal endoscopy due to dyspeptic symptoms, and had no history of malignant tumors.
All procedures involving human participants are in compliance with the Declaration of Helsinki (revised in 2013). This study obtained written informed consent from all participants before participating in the study, which also was approved by Xi’an international medical center Hospital.
3.2. Laboratory analysis
Fasting venous blood (5 mL) was centrifuged at 4,000 rpm for 5 minutes to obtain serum (the supernatant), which was stored at −80°C for the measurement of the levels of CEA, carbohydrate antigen 19-9 (CA 19-9), and carbohydrate antigen 72-4 (CA 72-4) using automatic electrochemical luminescence analyzer (Roche Applied Science, Germany), as well as sirt6 levels by human enzyme-linked immunosorbent assay kit in accordance with the kit’s directions (CSB-E17018h, Shunyuan Biotech Co., LTD, Shanghai, China).
3.3. Data collection and follow-up
Patients’ characteristics, including age, gender, metastasis condition, differential, tumor node metastasis (TNM) stage, were recorded. Survival was measured from the time of surgery until follow-up on June 31, 2021. The time from admission to death or the last follow-up was used to calculate overall survival.
3.4. Statistical analysis
For comparisons of data presented as the mean ± standard deviation (SD) between 2 group, Student t-test was performed, and 1-way analysis of variance following with Tukey post hoc test for more than 2 groups. The χ2 or Fisher’s exact tests were used to analyze odds ratios. Pearson correlation analysis was used to assess correlations between sirt6 and other biomarkers. The cutoff value of sirt6 level for diagnosing GC was determined using the receiver operating characteristics (ROC) curve. Kaplan–Meier curves (log-rank test), as well as univariate and multivariate Cox regression analyses were used for survival analysis. P < .05 was considered as statistically significant via SPSS 22.0 (IBM Corp., Armonk, NY).
4. Results
4.1. Basic clinical information for all participants
No significant difference was showed among the GC, AG, and HG groups in terms of age and gender (P > .05; Table 1). Before the subject receives any surgery, chemotherapy or radiotherapy, the serum simple of subject was collected for measuring CEA, CA 19-9, and CA 72-4, which were markedly higher in GC patients and AG patients than healthy controls (P < .05; Table 1), especially in GC patients (P < .05). Moreover, GC patients showed lower sirt6 serum levels than that of AG patients and healthy control (P < .05; Table 1).
Table 1.
Characteristics of study subjects.
| Baseline | HC group (n = 60) | AG group (n = 68) | GC group (n = 135) | P |
|---|---|---|---|---|
| Age (yr) | 64.55 ± 13.32 | 62.56 ± 11.55 | 64.79 ± 12.36 | .464 |
| Gender | .237 | |||
| Female | 33 | 35 | 58 | |
| Male | 27 | 33 | 77 | |
| Sirt6 (ng/mL) | 35.01 ± 7.30 | 25.13 ± 6.84 | 19.09 ± 5.36 | <.001 |
| CEA (ng/mL) | 2.15 ± 1.15 | 3.56 ± 1.50 | 3.89 ± 1.59 | <.001 |
| CA 19-9 (U/mL) | 14.04 ± 4.46 | 14.60 ± 3.94 | 17.81 ± 4.79 | <.001 |
| CA 72-4 (IU/mL) | 5.37 ± 1.43 | 7.11 ± 1.60 | 8.43 ± 2.18 | <.001 |
| CA 125 (IU/mL) | 33.49 ± 14.03 | 36.96 ± 9.49 | 38.42 ± 7.42 | .006 |
| CA 50 (µg/L) | 21.99 ± 4.76 | 22.93 ± 4.96 | 25.44 ± 4.66 | <.001 |
AG = atrophic gastritis, CA 19-9 = carbohydrate antigen 19-9, CA 72-4 = carbohydrate antigen 72-4, CEA = carcino-embryonic antigen, Sirt6 = sirtuin 6.
4.2. The diagnostic value of serum sirt6 for GC
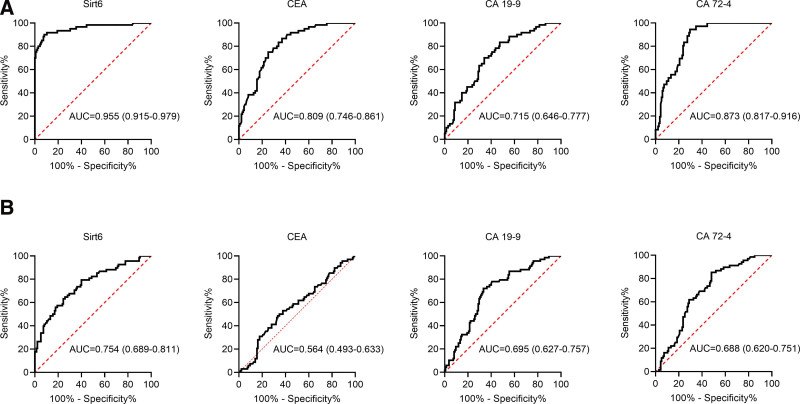
Next, the diagnostic capacities of serum biomarkers in GC were evaluated and compared using ROC curves in GC patients and healthy controls (Fig. 1A). At the optimal cutoff value (26.18 ng/mL), an area under the ROC curve (AUC) of serum sirt6 level was 0.955 with a sensitivity of 91.85% and a specificity of 90.0%, which was higher than that of CEA (0.809), CA 19-9 (0.715) and CA 72-4 (0.873). Furthermore, we created ROC curves in GC and AG patients to assess the performance of serum indicators for GC and AG discrimination (Fig. 1B), and the result also showed higher AUC of serum sirt6 level (0.754) as compared with CEA (0.564), CA 19-9 (0.695) and CA 72-4 (0.688). These findings suggested that serum sirt6 could be a useful diagnostic marker for GC, but its ability to discriminate between GC and AG was limited.
Figure 1.
ROC curve analysis of serum sirt6 to predict and identify GC. (A) The ROC curves of serum sitr6, CEA, CA 19-9, and CA 72-4 were used to diagnose GC in GC patients and health control. (B) The ROC curves of serum sitr6, CEA, CA 19-9, and CA 72-4 were used to identify GC and AG in GC and AG patients. AG = atrophic gastritis, CA 19-9 = carbohydrate antigen 19-9, CA 72-4 = carbohydrate antigen 72-4, CEA = carcino-embryonic antigen, GC = gastric cancer, ROC = receiver operating characteristic curve, Sirt6 = sirtuin 6.
4.3. The correlation between sirt6 serum level and clinicopathological features of GC
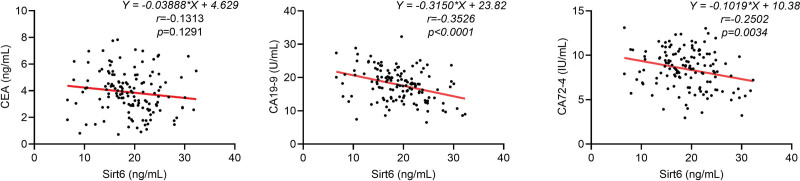
As shown in Table 2, serum sirt6 level was related to tumor size, differentiate grade, lauren classification, depth of invasion, lymph nodes metastasis, distant metastasis and TNM stage of GC (P < .05) rather than age and gender (P > .05). Moreover, Pearson analysis revealed negatively correlation of sirt6 concentration with serum CA 19-9 and CA 72-4 in GC patients (P < .05, Fig. 2).
Table 2.
Relationship between serum sirt6 and clinicopathological characteristics of GC.
| Characteristics | Case No. | Sirt6 (ng/mL) | P |
|---|---|---|---|
| Age (yr) | .087 | ||
| <60 | 47 | 18.58 ± 5.95 | |
| ≥60 | 88 | 19.37 ± 5.04 | |
| Gender | .942 | ||
| Female | 58 | 19.81 ± 5.3 | |
| Male | 77 | 18.55 ± 5.38 | |
| Tumor size (cm) | <.001 | ||
| <4 | 78 | 21.19 ± 4.51 | |
| ≥4 | 57 | 16.23 ± 5.15 | |
| Pathological differention | <.001 | ||
| Well + Moderate | 93 | 20.40 ± 5.17 | |
| Poor | 42 | 16.19 ± 4.65 | |
| Lauren classification | <.001 | ||
| Intestinal | 86 | 20.36 ± 4.97 | |
| Diffuse | 49 | 16.88 ± 5.36 | |
| T classification | <.001 | ||
| T1 | 26 | 24.45 ± 4.07 | |
| T2 | 9 | 24.21 ± 3.12 | |
| T3 | 34 | 19.82 ± 4.95 | |
| T4 | 66 | 15.91 ± 3.70 | |
| Lymph nodes metastasis | <.001 | ||
| Negative | 92 | 20.29 ± 4.83 | |
| Positive | 43 | 16.52 ± 5.60 | |
| Distant metastasis | <.001 | ||
| No | 116 | 19.80 ± 5.12 | |
| Yes | 19 | 14.77 ± 4.86 | |
| TNM stage | .001 | ||
| I | 21 | 24.18 ± 3.29 | |
| II | 31 | 20.49 ± 5.18 | |
| III | 64 | 18.03 ± 4.67 | |
| IV | 19 | 14.77 ± 4.87 |
GC = gastric cancer, Sirt6 = sirtuin 6, TNM = tumor node metastasis.
Figure 2.
Correlation between serum sirt6 and other serum biomarkers in GC patients. Serum sirt6 in GC patients were significantly negatively correlated with CA 19-9 and CA 72-4 (P < .05), but not significantly correlated with CEA (P > .05). CA 19-9 = carbohydrate antigen 19-9, CA 72-4 = carbohydrate antigen 72-4, CEA = carcino-embryonic antigen, GC = gastric cancer, Sirt6 = sirtuin 6.
4.4. Sirt6 serum level was served as a prognostic biomarker for GC
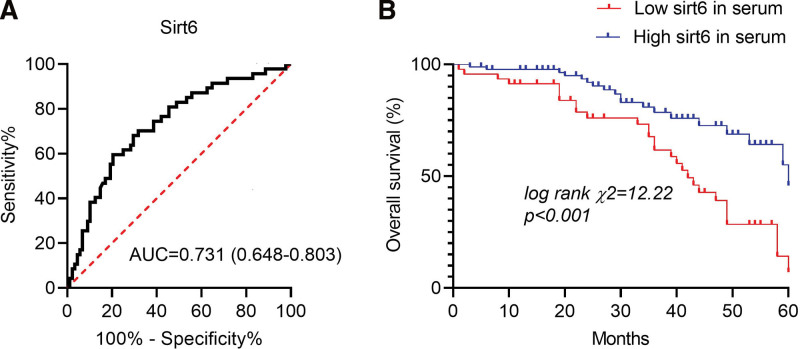
Compared with the survival group, the serum sirt6 level of the death group was significantly lower (20.48 ± 5.11 vs 16.49 ± 4.88, t = 4.386, P < .001). We then applied the ROC curve to determine the most valuable cutoff point of serum sirt6 related to prognosis. We found that the predictive AUC of serum sirt6 for prognosis of GC patients was 0.731 (0.648–0.803) at a cutoff value of 16.83 ng/mL (P < .001, Fig. 3A) with the sensitivity and specificity of sirt6 were 59.57% and 79.55%, respectively. Subsequently, we found the median survival time of GC patients with high sirt6 serum level (>16.83 ng/mL) was 50.66 months, which was shorter in patients (40.27 months) with low sirt6 (≤16.83 ng/mL) via performing Kaplan–Meier curves (log-rank test) (χ2 = 12.22, P < .001; Fig. 3B).
Figure 3.
The relationship between serum sirt6 and prognosis of GC. (A) ROC curve analysis of serum sirt6 predicting the prognosis of GC patients. (B) Kaplan–Meier curves of GC patients based on sirt6 levels in their blood. ROC = receiver operating characteristic curve. Sirt6 = sirtuin 6.
4.5. Cox regression analysis for overall survival
In univariate analyses, lower sirt6 serum level (P = .001), higher CEA levels (P = .013), larger tumor size (P = .025), higher Lauren’s classification (P = .012), poor differentiate (P = .021), lymph nodes metastasis (P = .009), and higher TNM stage (P = .017) were all associated with short overall survival. In multivariate analyses, lower sirt6 serum level (P = .018) and distant metastasis (P = .007) were independent risk factors that affect GC prognosis (Table 3).
Table 3.
Univariate and multivariate Cox regression analyses for overall survival (n = 135).
| Variables | Overall survival | |||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Sirt6 levels (low vs high) | 2.69 | 1.498–4.829 | .001 | 2.030 | 1.106–3.726 | .022 |
| Age (<60 vs ≥60) | 1.329 | 0.718–2.458 | .365 | |||
| Gender (female vs male) | 1.323 | 0.728–2.406 | .358 | |||
| CEA levels (high vs low) | 2.988 | 1.262–7.076 | .013 | 2.882 | 1.202–6.907 | .018 |
| CA 19-9 levels (high vs low) | 1.044 | 0.584–1.867 | .885 | |||
| CA 72-4 levels (high vs low) | 1.726 | 0.856–3.480 | .127 | |||
| Tumor size (<4 cm vs ≥4 cm) | 1.969 | 1.088–3.565 | .025 | |||
| Lauren classification (intestinal vs diffuse) | 2.108 | 1.175–3.782 | .012 | |||
| Pathological differention (well + moderate vs poor) | 1.976 | 1.107–3.527 | .021 | |||
| Lymph nodes metastasis (negative vs positive) | 2.147 | 1.211–3.808 | .009 | |||
| TNM stage (I and II vs III and IV) | 2.024 | 1.135–3.610 | .017 | 2.332 | 1.015–5.354 | .046 |
CA 19-9 = carbohydrate antigen 19-9, CA 72-4 = carbohydrate antigen 72-4, CEA = carcino-embryonic antigen, Sirt6 = sirtuin 6, TNM = tumor node metastasis.
5. Discussion
Sirtuins are a type of protein deacetylase that is activated by NAD + and is involved in stress resistance and metabolic homeostasis.[13,14] Among the seven mammalian sirtuins, sirt6 has been confirmed to be involved in regulating embryonic development, DNA repair, inflammation, cancer and aging.[15,16] In thyroid cancer,[17,18] breast cancer[19] and prostate cancer,[20] the expression of sirt6 is significantly up-regulated, and its inhibitions enhances the chemosensitivity of pancreatic cancer cells.[21] Moreover, sirt6 suppressed the apoptosis of hepatocellular carcinoma cells by regressing the expression of B-cell lymphoma 2 (Bcl-2) related X protein.[22] AG is considered to be an important precancerous lesion of gastric cancer.[23] In our study, the serum sirt6 level of patients with GC was observed to be substantially lower than that of AG patients and healthy controls in the current investigation, being consistent with the results obtained by Zhou et al[10] in GC. Besides, serum sirt6 had a certain diagnostic value for AG and GC versus health controls, which also could distinguish GC versus AG with low accuracy. What is more, serum sirt6 in GC patients was negatively correlated with CA 19-9 and CA 72-4, indicating sirt6 might act as a tumor suppressor in GC, and monitoring serum sirt6 might provide benefits for the clinical diagnosis of GC.
The link between serum sirt6 and clinicopathological features of GC patients was also investigated. The findings revealed that serum sirt6 was linked to tumor size, stage, and metastasis, implying that sirt6 downregulation may boost tumor cell proliferation, migration, and invasion, hence aiding in the progression of GC. It was found in GC cells that sirt6 blocked the activation of JAK2/STAT3 and inhibits the cyclin D1 and Bcl-2 expressions to inhibit tumor cell proliferation and to promote cell apoptosis,[10] and sirt6 also overcomes sorafenib resistance by inhibiting ferroptosis.[24] Besides, sirt6, as a new tumor suppressor, prevents the occurrence of tumors by inhibiting glycolysis[25] and enhancing oxidative phosphorylation.[26] These studies suggest that sirt6 directly acts on tumor cells, or indirectly regulates related signal pathways, as well as cancer metabolism, to participate in the occurrence and progression of GC. However, its exact mechanism in GC needs to be further explored.
Some scholars have confirmed through immunohistochemical analyses that sirt6 has a certain value in predicting the long-term prognosis of a variety of tumors. In hepatocellular carcinoma[27] and non-small cell lung cancer,[28] the upregulation of sirt6 expression is closely related to poor overall survival and disease-free survival, which was also related to drug resistance and a poor prognosis in breast cancer[19] and prostate cancer.[20] In addition, Zhou et al[10] used immunohistochemical analysis and found that the decreased expression of sirt6 was associated with poor prognosis of GC. Recent studies have proposed that noninvasive serological biomarkers for real-time monitoring of targeted prognosis have higher safety and effectiveness.[29,30] The level of sirt6 in the peripheral circulation has also been used to predict coronary artery disease,[11] diabetes,[31] and aging.[12,32] However, no research has been done on the link between serum sirt6 and prognosis in GC patients. We discovered that GC patients with low serum sirt6 had a significantly shorter 5-years overall survival than those with high serum sirt6. Furthermore, low serum sirt6 was found to be an independent risk factor for GC patients’ prognosis. These findings suggested that serum sirt6 could be utilized to predict the prognosis of GC patients.
6. Limitations
There were some limitations in this study that deserve further discussion, including a small sample size and the source of the research items, thus may resulting some selection bias. The prognostic significance of serum sirt6 needs to be verified in a larger cohort. Moreover, the relationship between sirt6 and specific molecular subtypes of GC remains to be explored.
7. Conclusions
To summarize, this was the first study to examine the association between preoperative serum sirt6 and the prognosis of GC patients, as far as we know, which could be employed as a useful serum biomarker for the diagnosis of GC in the current investigation. Additionally, low levels of sirt6 were significantly associated with several clinic features, implying that down-regulation of sirt6 was implicated in the progression of GC. Furthermore, low serum sirt6 levels exhibited a significant prognostic value for GC patients’ poor prognosis, and it was one of the independent factors influencing GC prognosis. The assessment of serum sirt6 has significant implications for the diagnosis, prognosis, and subsequent therapy options for GC.
Author contributions
Conceptualization: Cheng Cao.
Formal analysis: Danyang Li.
Abbreviations:
- AG =
- atrophic gastritis
- AUC =
- area under the ROC curve
- CA 19-9 =
- carbohydrate antigen 19-9
- CA 72-4 =
- carbohydrate antigen 72-4
- CEA =
- carcino-embryonic antigen
- GC =
- gastric cancer
- ROC =
- receiver operating characteristic curve,
- Sirt6
- = sirtuin 6
- TNM =
- tumor node metastasis
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors have no conflicts of interest to disclose.
How to cite this article: Li D, Cao C. The correlation of serum sirt6 with clinical outcome and prognosis in patients with gastric cancer. Medicine 2022;101:47(e31568).
References
- [1].Chen S, Wei Y, Liu H, et al. Analysis of collagen type X alpha 1 (COL10A1) expression and prognostic significance in gastric cancer based on bioinformatics. Bioengineered. 2021;12:127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lordick F, Nilsson M, Leong T. Adjuvant radiotherapy for gastric cancer-end of the road?. Ann Oncol. 2021;32:287–9. [DOI] [PubMed] [Google Scholar]
- [3].Lin Z, Bian H, Chen C, et al. Application of serum pepsinogen and carbohydrate antigen 72-4 (CA72-4) combined with gastrin-17 (G-17) detection in the screening, diagnosis, and evaluation of early gastric cancer. J Gastrointest Oncol. 2021;12:1042–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Association JGC. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen L, Hong J, Hu R, et al. Clinical value of combined detection of serum sTim-3 and pepsinogen for gastric cancer diagnosis. Cancer Manag Res. 2021;13:7759–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Saiyang X, Deng W, Qizhu T. Sirtuin 6: a potential therapeutic target for cardiovascular diseases. Pharmacol Res. 2021;163:105214. [DOI] [PubMed] [Google Scholar]
- [7].Grootaert MOJ, Finigan A, Figg NL, et al. SIRT6 protects smooth muscle cells from senescence and reduces atherosclerosis. Circ Res. 2021;128:474–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu G, Chen H, Liu H, et al. Emerging roles of SIRT6 in human diseases and its modulators. Med Res Rev. 2021;41:1089–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shen X, Li P, Xu Y, et al. Association of sirtuins with clinicopathological parameters and overall survival in gastric cancer. Oncotarget. 2017;8:74359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhou J, Wu A, Yu X, et al. SIRT6 inhibits growth of gastric cancer by inhibiting JAK2/STAT3 pathway. Oncol Rep. 2017;38:1059–66. [DOI] [PubMed] [Google Scholar]
- [11].Yan Z, Wang X, Liu YS, et al. Decreased serum SIRT6 as a novel predictor of coronary artery disease. Eur Rev Med Pharmacol Sci. 2021;25:6660–9. [DOI] [PubMed] [Google Scholar]
- [12].Zhao Y, Bai X, Jia X, et al. Age-related changes of human serum sirtuin6 in adults. BMC Geriatr. 2021;21:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Singh CK, Chhabra G, Ndiaye MA, et al. The role of sirtuins in antioxidant and redox signaling. Antioxid Redox Signal. 2018;28:643–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vargas-Ortiz K, Pérez-Vázquez V, Macías-Cervantes MH. Exercise and sirtuins: a way to mitochondrial health in skeletal muscle. Int J Mol Sci. 2019;20:2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tasselli L, Zheng W, Chua KF. SIRT6: novel mechanisms and links to aging and disease. Trends Endocrinol Metab. 2017;28:168–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chang AR, Ferrer CM, Mostoslavsky R. SIRT6, a mammalian deacylase with multitasking abilities. Physiol Rev. 2020;100:145–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yang Z, Huang R, Wei X, et al. The SIRT6-autophagy-warburg effect axis in papillary thyroid cancer. Front Oncol. 2020;10:1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Qu N, Hu JQ, Liu L, et al. SIRT6 is upregulated and associated with cancer aggressiveness in papillary thyroid cancer via BRAF/ERK/Mcl-1 pathway. Int J Oncol. 2017;50:1683–92. [DOI] [PubMed] [Google Scholar]
- [19].Uzelac B, Krivokuca A, Brankovic-Magic M, et al. Expression of SIRT1, SIRT3 and SIRT6 genes for predicting survival in triple-negative and hormone receptor-positive subtypes of breast cancer. Pathol Oncol Res. 2020;26:2723–31. [DOI] [PubMed] [Google Scholar]
- [20].Fu W, Li H, Fu H, et al. The SIRT3 and SIRT6 promote prostate cancer progression by inhibiting necroptosis-mediated innate immune response. J Immunol Res 2020;2020:8820355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sociali G, Galeno L, Parenti MD, et al. Quinazolinedione SIRT6 inhibitors sensitize cancer cells to chemotherapeutics. Eur J Med Chem. 2015;102:530–9. [DOI] [PubMed] [Google Scholar]
- [22].Ran LK, Chen Y, Zhang ZZ, et al. SIRT6 overexpression potentiates apoptosis evasion in hepatocellular carcinoma via BCL2-associated X protein-dependent apoptotic pathway. Clin Cancer Res. 2016;22:3372–82. [DOI] [PubMed] [Google Scholar]
- [23].Raza M, Bhatt H. Atrophic Gastritis. StatPearls Treasure Island (FL): StatPearls Publishing LLC; 2021. [PubMed] [Google Scholar]
- [24].Cai S, Fu S, Zhang W, et al. SIRT6 silencing overcomes resistance to sorafenib by promoting ferroptosis in gastric cancer. Biochem Biophys Res Commun. 2021;577:58–164. [DOI] [PubMed] [Google Scholar]
- [25].Sebastián C, Zwaans BM, Silberman DM, et al. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell. 2012;151:1185–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Becherini P, Caffa I, Piacente F. SIRT6 enhances oxidative phosphorylation in breast cancer and promotes mammary tumorigenesis in mice. Cancer Metab. 2021;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Han LL, Jia L, Wu F, et al. Sirtuin6 (SIRT6) promotes the EMT of hepatocellular carcinoma by stimulating autophagic degradation of E-Cadherin. Mol Cancer Res. 2019;17:2267–80. [DOI] [PubMed] [Google Scholar]
- [28].Zhu B, Yan Y, Shao B, et al. Downregulation of SIRT6 is associated with poor prognosis in patients with non-small cell lung cancer. J Int Med Res. 2018;46:1517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shi J, Bao X, Liu Z, et al. Serum miR-626 and miR-5100 are promising prognosis predictors for oral squamous cell carcinoma. Theranostics. 2019;9:920–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yang J, Song YC, Dang CX, et al. Serum peptidome profiling in patients with gastric cancer. Clin Exp Med. 2012;12:79–87. [DOI] [PubMed] [Google Scholar]
- [31].Bian C, Gao J, Wang Y, et al. Association of SIRT6 circulating levels with urinary and glycometabolic markers in pre-diabetes and diabetes. Acta Diabetol. 2021;58:1551–62. [DOI] [PubMed] [Google Scholar]
- [32].Hooshmand-Moghadam B, Eskandari M, Golestani F, et al. The effect of 12-week resistance exercise training on serum levels of cellular aging process parameters in elderly men. Exp Gerontol. 2020;141:111090. [DOI] [PubMed] [Google Scholar]