Abstract
Antiserum raised against intimin from enterohemorrhagic Escherichia coli (EHEC) O157:H7 strain 86-24 has been shown previously by our laboratory to inhibit adherence of this strain to HEp-2 cells. In the present study, we sought to identify the region(s) of intimin important for the effect of anti-intimin antisera on EHEC adherence and to determine whether antisera raised against intimin from an O157:H7 strain could reduce adherence of other strains. Compared to preimmune serum controls, polyclonal sera raised against the histidine-tagged intimin protein RIHisEae (intiminO157) or against His-tagged C-terminal fragments of intimin from strain 86-24 reduced adherence of this strain. Furthermore, an antibody fraction purified from the anti-RIHisEae serum that contained antibodies to the C-terminal third of intimin, the putative receptor-binding domain, also reduced adherence of strain 86-24, but a purified fraction containing antibodies to the N-terminal two-thirds of intimin did not inhibit adherence. The polyclonal anti-intiminO157 serum raised against RIHisEae inhibited, to different degrees, the adherence of another O157:H7 strain, an EHEC O55:H7 strain, one of two independent EHEC O111:NM isolates tested, and one of two EHEC O26:H11 strains tested. Adherence of the other O26:H11 and O111:NM strains and an EPEC O127:H6 strain was not reduced. Finally, immunoblot analysis indicated a correlation between the antigenic divergence in the C-terminal third of intimins from different strains and the capacity of anti-intiminO157 antiserum to reduce adherence of heterologous strains. Taken together, these data suggest that intiminO157 could be used as an immunogen to elicit adherence-blocking antibodies against O157:H7 strains and closely-related EHEC.
Infection of humans with enterohemorrhagic Escherichia coli (EHEC), such as the prototype E. coli O157:H7, can lead to diarrhea, hemorrhagic colitis, and, in approximately 5 to 15% of infected children, the hemolytic-uremic syndrome (HUS) (reviewed recently in references 23, 50, 51, and 53). EHEC is a subset of Shiga toxin-producing E. coli (STEC) that is characterized by Shiga toxin production, the presence of a 90-kb plasmid, and the capacity to produce attaching and effacing (A/E) lesions on epithelial cells in culture and in the intestines of experimentally inoculated animals (37). A/E histopathology results from intimate attachment of the bacteria to epithelial cells, effacement of the microvilli, and rearrangement of the host cell actin cytoskeleton (33, 49). The factors responsible for this attachment and associated events in the host cell are encoded in a pathogenicity island, the locus of enterocyte effacement, and include the outer membrane protein intimin, encoded by the eae gene, and a variety of secreted proteins (reviewed in reference 50).
Most STEC outbreaks have been caused by strains of the O157:H7 serotype, and in many countries, including the United States, the O157:H7 serotype is the most common cause of human disease (5, 24, 48, 50, 60, 62). However, non-O157:H7 strains are also clinically important; indeed, in some countries non-O157:H7 serotypes are isolated more frequently than O157:H7 strains (9, 24, 41, 56, 60, 62). Among the non-O157 STEC strains associated with human disease, many, although not all, carry the eae gene (67).
Adherence of EHEC O157:H7 to human epithelial cells in vitro and colonization of experimentally infected animals require the function of the adhesin intimin, an outer membrane protein of approximately 94 to 97 kDa encoded by the eae gene (16, 46, 64). The eae gene was originally identified as essential for A/E lesion formation by enteropathogenic E. coli (EPEC), a related diarrheal human pathogen that forms A/E lesions but does not produce Shiga toxins (30, 37). The importance of intimin for full virulence of EPEC was demonstrated in a study of infection by this organism of volunteers (14); the severity of Shiga toxin-mediated HUS has precluded human experimental challenge with EHEC. The sequences of intimin proteins from different strains of EPEC and EHEC and from several animal pathogens show a pattern of strong conservation in the central and N-terminal portions and more divergence in the C-terminal region (1–3, 31, 45). The C-terminal region of intimin has been shown to be critical for interaction with the human cell (13, 18, 19, 27, 32, 40)).
Intimin is immunogenic in humans. Anti-intimin antibodies have been detected in milk and colostrum, in sera from individuals with EPEC infection, and in sera from HUS patients infected with STEC (29, 39, 43, 44, 47, 50, 65). The development of a multivalent anti-EHEC vaccine that would include Shiga toxoids and intimin has been proposed by our laboratory and others (7, 12, 31). The inclusion of intimin in such a vaccine is based on the hypothesis that a sufficient titer of anti-intimin antibodies may reduce or inhibit colonization. Since intimin is an outer membrane protein, regions of the protein may be accessible for antibody binding to interfere with colonization. Intimin has also been proposed as a vaccine candidate for EPEC (reviewed in reference 31).
Our laboratory has previously reported that sera from mice immunized with intimin could block adherence of EHEC O157:H7 strain 86-24 to HEp-2 cells compared with a preimmune serum control (47). Other studies have shown reduced adherence of EPEC to tissue culture cells in the presence of immunoglobulin fractions from human milk and colostrum; the reactivity of these fractions to an unidentified outer membrane protein of the same molecular weight as intimin suggested that anti-intimin antibodies might contribute to the blocking effect (8, 11). In the present report, we sought to (i) confirm our hypothesis that the reduction in adherence of EHEC seen in the presence of anti-intimin polyclonal serum is specifically due to anti-intimin antibodies, (ii) define the region in intimin that is important for the effects on adherence, and (iii) assess whether antisera raised against intimin from an O157:H7 strain could inhibit adherence of intimin-expressing strains of different serotypes.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Clinical isolates of E. coli are described in Table 1. Strain M15[pREP4], obtained from Qiagen, Inc. (Chatsworth, Calif.), was used for expression and purification of the histidine-tagged intimin protein termed RIHisEae (47). Because the E. coli SlyD protein is known to copurify with histidine-tagged fusion proteins on metal affinity chromatography (57, 69), we used a slyD::kan mutant host strain for overexpression and purification of the other His-tagged intimin protein fragments. We obtained strain RY2844, which contains the slyD::kan mutation, from W. D. Roof and R. Young. To construct a host strain with improved expression of the intimin fragments, we transduced the slyD::kan mutation into strain UT5600 (17), and then we introduced into this strain an F′ carrying a lacIq repressor gene by conjugation with strain XL1-Blue (6). The resulting strain, L172, yielded sufficient expression of all intimin fragments tested.
TABLE 1.
EHEC and EPEC strains used in this study
| Strain | Serotype (EHEC or EPEC) | Intimin subtypea | Yr of isolation or date received at CDCb (reference), source |
|---|---|---|---|
| 86-24 | O157:H7 (EHEC) | γ | 1986 (25), P. Tarr |
| 86-24eaeΔ10 | O157:H7 (EHEC) | (46), M. McKee | |
| EDL933 | O157:H7 (EHEC) | γ | 1982 (55, 66), K. Wachsmuth |
| H19 | O26:H11 (EHEC) | β | 1967 (61), W. Smith |
| 97-3250 | O26:H11 (EHEC) | β | 1997, CDC, N. Strockbine |
| 85-3007 | O111:NM (EHEC) | NT | 1985, CDC, N. Strockbine |
| 95-3208 | O111:NM (EHEC) | NT | 1995, CDC, N. Strockbine |
| 97-3256 | O55:H7 (EHEC)c | γ | 1997, CDC, N. Strockbine |
| E2348/69 | O127:H6 (EPEC) | α | 1969 (38, 63), A. Jerse |
Intimins from different organisms have been classified into several different subtypes based on antigenic and sequence divergences in the C-terminal domain of the protein (1, 45). The designations of strain E2348/69 intimin as α and strain EDL933 intimin as γ were published previously (1, 45). Intimin from strain 86-24 was classified as γ based on sequence identity with the intimin from strain EDL933. We have listed the intimin from strain H19 as β because the published sequence encoding the C-terminal region of this protein is identical to the published sequences of β intimins from two other strains (3, 45). Determination of the intimin subtypes for strains 97-3250, 85-3007, 95-3208, and 97-3256 was accomplished by PCR analysis; in the assay, the two O111:NM strains were demonstrated to have the eae gene but the sequence did not fall into α, β, or γ subtypes (54a). Thus, the intimin subtypes of the O111:NM strains are listed here as not typeable (NT).
All EHEC strains are isolates from patients with diarrhea and/or HUS except for strain 86-24eaeΔ10, which is a derivative of strain 86-24 with an eae in-frame deletion mutation constructed in our laboratory (46), and strain EDL933, which was isolated from contaminated meat associated with an outbreak (55, 66). All strains carry the eae gene except the deletion mutant 86-24eaeΔ10 (3, 30, 46, 62a; 70). CDC, Centers for Disease Control and Prevention.
Plasmids encoding fragments of intimin tagged with six histidines at the N termini were constructed by standard procedures (4). For each construct, a fragment of the eae gene was cloned by PCR with plasmid pEB310 as the template. Plasmid pEB310 carries the entire eae gene from EHEC O157:H7 strain 86-24 (46). Primers included sequences for restriction endonuclease sites. The PCR fragments were digested and cloned into the BamHI and KpnI sites of the histidine fusion vectors pQE30 and pQE31 obtained from Qiagen, Inc. Inserts were confirmed by sequencing. Plasmids pMW101, pMW102, and pMW103 encode the N-terminal third (His-IntN1/3), middle third (His-IntMid1/3), and C-terminal third (His-IntC1/3) of intimin, respectively. Primers used were as follows: pMW101, GTACGGATCCGAATTCATTTGCAAATGGTG (forward) and GTACGGTACCTGATCAATGAAGACGTTATAG (reverse); pMW102, GTACGGATCCTGATCAGGATTTTTCTGGTG (forward) and GTACGGTACCTGATCAAAAAATATAACCGC (reverse); pMW103, GTACGGATCCTGATCAAACCAAGGCCAGCATTAC (forward) and GTACGGTACCTTATTCTACACAAACCGCATAG (reverse). Plasmid pMW104 encodes the N-terminal two-thirds of intimin (His-IntN2/3). To construct pMW104, the forward primer was the same as that used to make pMW101 and the reverse primer was the same as that used to prepare pMW102. Plasmid pLG145b encodes the C-terminal half of intimin (His-IntC1/2). To construct pLG145b, the forward primer used was GCTTGGATCCGAACACAGTACGCAGAAGATTCAG and the reverse primer was the same as that used to design pMW103. Plasmid pEB313, which encodes RIHisEae, was described previously (47).
Sequence analysis.
Sequencing was performed with the ABI PRISM Dye Terminator cycle sequencing kit (Perkin-Elmer, Foster City, Calif.). Gels were run by the Biomedical Instrumentation Center at the Uniformed Services University of the Health Sciences. Sequence comparisons were done with the Wisconsin Sequence Analysis package from the Genetics Computer Group, Inc.
His-tagged intimin protein purification.
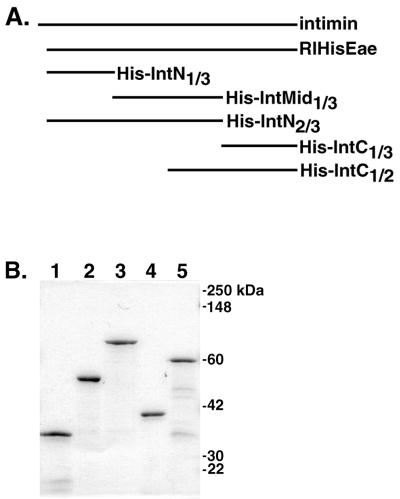
His-tagged intimin protein RIHisEae and His-tagged intimin protein fragments were induced in late-log-phase cultures with 1 to 2 mM IPTG (isopropyl-β-d-thiogalactopyranoside). The His-tagged proteins were then purified over nickel affinity columns essentially as described previously (47), except that after elution from the columns, the proteins suspended in elution buffer were dialyzed sequentially against 6, 4, and 2 M urea buffers and finally against phosphate-buffered saline (PBS). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of His-tagged intimin protein fragments is shown in Fig. 1.
FIG. 1.
(A) His-tagged intimin constructs used in this study. Full-length intimin contains 934 amino acids. The RIHisEae, His-IntN1/3, and His-IntN2/3 proteins lack the N-terminal 35 amino acids of the native protein (47). Each of the intimin fragments has a six-histidine tag at the amino terminus. The construction of the plasmids encoding these proteins is described in Materials and Methods; the plasmid encoding RIHisEae was described previously (47). (B) Coomassie-stained SDS-PAGE gel of His-tagged intimin fragments designed for this study after purification by nickel affinity chromatography. Lane 1, His-IntN1/3; lane 2, His-IntMid1/3; lane 3, His-IntN2/3; lane 4, His-IntC1/3; lane 5, His-IntC1/2. The stained gel was scanned with a Hewlett-Packard ScanJet 4C scanner, and the figure was labeled with Adobe Photoshop version 5.0.2 for Macintosh.
Production of antisera.
For preparation of antisera against RIHisEae, protein was sent to Duncroft, Inc. (Lovettsville, Va.), where a goat was immunized with the material emulsified in Freund's adjuvant. For preparation of antisera against the C-terminal half of intimin, protein was sent to Cocalico Biologicals, Inc. (Reamstown, Pa.), where a rabbit was immunized with the material emulsified in Freund's adjuvant. For preparation of antisera against the C-terminal third of intimin, we immunized four mice with the protein and TiterMax (CytRx Corp., Norcross, Ga.) as an adjuvant. For all animals, prebleeds were obtained before immunization.
Affinity purification of antibodies against different fragments of intimin.
Antibodies specific to different regions of the intimin protein were purified from the goat anti-RIHisEae serum. This serum contained antibodies that recognized N-terminal, central, and C-terminal portions of intimin, as observed by reactivity on an immunoblot with the various intimin protein fragments (data not shown). Affinity columns were prepared. Each column contained a different His-tagged intimin protein fragment coupled to Affi-Gel immunoaffinity support resin (Bio-Rad, Hercules, Calif.) according to instructions from the manufacturer. The antigen columns were prewashed with the buffer to be used for antibody elution, 100 mM glycine (pH 2.5), to remove any contaminants before passage of the serum over the columns, and the columns were then neutralized with Tris-HCl (pH 7.5). Passage of the sera over the immunoaffinity columns and elution of the affinity-purified antibodies were done according to protocols described by Harlow and Lane (26) and the Bio-Rad instructions.
The anti-His-IntN2/3 antibody preparation was concentrated by ultrafiltration with a Microsep 30K concentrator (Pall Filtron Corp., Northborough, Mass.) to bring it to a concentration similar to that of the anti-C-terminal antibody fraction. Both antibody preparations were dialyzed against tissue culture grade PBS (BioWhittaker, Walkersville, Md.) for use in the HEp-2 cell adherence assay. The concentration of the anti-His-IntN2/3 antibody preparation, as determined by the method of Bradford (Bio-Rad protein assay reagent) with bovine serum albumin as the standard, was 0.24 mg/ml; the concentration of the anti-His-IntC1/3 preparation was 0.27 mg/ml. The antibody preparations were also analyzed by SDS-PAGE to confirm that they had similar concentrations (data not shown). To assess blocking capacity in adherence assays (described below), equal microgram quantities of each antibody fraction were compared; PBS was added to the anti-His-IntC1/3 antibody samples so that equal volumes of the anti-N- and anti-C-terminal antibodies were mixed with bacteria.
Adherence assays.
Adherence of EHEC to HEp-2 (human laryngeal epithelial) cells was assessed essentially as described previously (10, 47), with slight modifications. For the assays, 6 × 104 HEp-2 cells were seeded per well into eight-well plastic chamber slides (Nalge Nunc International, Naperville, Ill.) and grown for 20 to 24 h to form subconfluent (approximately 80% confluency) monolayers. Freshly grown single bacterial colonies were inoculated into 0.5-ml Luria broth cultures and grown overnight statically. Preimmune sera and sera from immunized animals were heated to 56°C for 30 min to inactivate complement prior to the assay. Equal volumes of preimmune and postimmunization sera were compared for each strain. An equal volume of PBS buffer was added to an additional well for comparison. Bacteria and serum (or PBS) were preincubated in 300 μl of adherence medium (Eagle minimum essential medium [BioWhittaker], 1% mannose, 0.4% sodium bicarbonate) at 37°C for 35 min, and tubes were mixed gently three times during this period. HEp-2 cell monolayers were washed twice with Hanks balanced salt solution (with Mg2+ and Ca2+), the preincubated solutions of bacteria were added to the cells, and HEp-2 cells and bacteria were incubated for 3 h at 37°C. The wells were washed once with Dulbecco's PBS (with Mg2+ and Ca2+), and fresh medium containing antisera (or PBS buffer) was added for a second 3-h incubation. The monolayers were then washed six times with PBS. Cells were fixed and stained with the Hema-3 staining solution kit according to instructions of the manufacturer (Biochemical Sciences, Inc., Swedesboro, N.J.).
To assess the effect of the purified antibodies on the adherence assay, several minor modifications were made to optimize the above procedure. First, fetal bovine serum, preheated to inactivate complement factors, was added to the adherence medium to a 0.5% final concentration. Second, the bacteria were preincubated in adherence medium for 18 to 20 min before addition of antibodies. An additional well that contained PBS without antibodies was included as a control.
To evaluate the degree of bacterial adherence in each assay, slides were first scored by blinded microscopic observation, i.e., without a key as to which wells contained which antiserum or PBS. Following this, to quantitate the adherence of the bacteria, eight (or more) randomly selected fields from each chamber slide well that contained either preimmune serum or postimmunization serum were photographed and the bacteria and HEp-2 cells were counted. Quantitation was done by this method because, as previously reported, visual observation of the bacteria in situ on the HEp-2 cells was a more sensitive method for detection of differences between strains than viable counts of bacteria eluted from the wells (47). Since this may be due to excess bacteria that were observed to be nonspecifically adherent to the plastic along the very edges of the wells, we did not include fields at the very edges of wells for quantitation. For each independent assay, the total number of HEp-2 cells counted in each set of eight photographs for a serum-containing well was at least 100. For each assay done with affinity-purified antibodies, photographs of 11 or 12 random fields for each well were taken; at least 160 HEp-2 cells per antibody or buffer-alone sample were counted, and at least 3,500 bacteria were counted for the anti-His-IntN2/3 samples. Statistical analysis of the data was performed as described in Tables 2 and 3.
TABLE 2.
Evidence that antibodies to the C-terminal region of intimin reduce adherence of EHEC O157:H7 strain 86-24
| Intimin fragment | Sample antibody (animal source) | Reduced adherence in the presence of the antibody samplea |
|---|---|---|
| IntiminO157 | Polyclonal serum (goat) | + |
| His-IntC1/2 | Polyclonal serum (rabbit) | + |
| His-IntC1/3 | Polyclonal serum (mice) | +b |
| His-IntN2/3 | Purified antibody fraction (goat) | − |
| His-IntC1/3 | Purified antibody fraction (goat) | +c |
+, reduced adherence observed; −, no inhibition of adherence observed. The average reduction in adherence in the presence of anti-intiminO157 polyclonal serum relative to preimmune serum (from three assays) was 8.1-fold; bacterial counts are presented in Table 3 in the context of other experiments. For the other polyclonal sera, bacterial counts were not determined; relative levels of adherence compared to preimmune sera were assessed microscopically.
Serum from three of the four immunized mice reduced adherence of strain 86-24.
The level of adherence in the presence of antibodies to the C-terminal third of intimin (anti-His-IntC1/3) was an average of 4.4-fold lower than that in the presence of antibodies to the N-terminal region (anti-His-IntN2/3). To determine the average fold reduction, three independent assays were performed; for each assay, bacteria and HEp-2 cells were counted in 11 or 12 microscope fields per sample. Each field contained at least 10 HEp-2 cells, although not every HEp-2 cell contained adherent bacteria. Thus, the following data for the three assays are presented as the average numbers of bacteria (± two times the standard errors of the means) per 10 HEp-2 cells (anti-His-IntN2/3/anti-His-IntC1/3): 230 ± 24/101 ± 21, 222 ± 36/43 ± 13; 255 ± 30/45 ± 9. In each experiment, the difference between adherence levels in the presence of the two antibody fractions was significant (Student's unpaired t test; P < 0.001).
TABLE 3.
Adherence of strains of different serotypes in the presence of polyclonal serum that contains antibodies against intiminO157
| Straina | Serotype | Assay | Avg
no. of adherent bacteria ± 2SEM/10 HEp-2 cells in the presence
ofb:
|
Avg fold reduction in adherence by anti-intiminO157 serum (range)c | |
|---|---|---|---|---|---|
| Preimmune serum | Anti-intimin serum | ||||
| 86-24 | O157:H7 | I | 106 ± 25 | 10 ± 5 | 8.1 (4.7–10.9) |
| II | 50 ± 13 | 6 ± 2 | |||
| III | 158 ± 26 | 34 ± 10 | |||
| EDL933 | O157:H7 | I | 263 ± 66 | 30 ± 10 | 5.7 (3.7–8.9) |
| II | 195 ± 25 | 43 ± 12 | |||
| III | 220 ± 39 | 60 ± 21 | |||
| 97-3256 | O55:H7 | I | 109 ± 22 | 11 ± 6 | 10.3 (4.9–16.4) |
| II | 187 ± 49 | 11 ± 4 | |||
| III | 136 ± 34 | 28 ± 10 | |||
| 85-3007 | O111:NM | I | 57 ± 19 | 16 ± 12 | 3.0 (1.7–3.7) |
| II | 29 ± 9 | 17 ± 13 | |||
| III | 61 ± 19 | 17 ± 5 | |||
| H19 | O26:H11 | I | 58 ± 13 | 25 ± 7 | 1.9 (1.6–2.3) |
| II | 67 ± 17 | 37 ± 13 | |||
| III | 65 ± 15 | 41 ± 16 | |||
The numbers of bacteria added to the assay mixtures with respect to strain 86-24 (n = 1.2 × 107) were as follows: EDL933, n/2; 97-3256, n/15; 85-3007, n; H19, n/5. Serum dilutions were 1:140 for strains 86-24 and EDL933 and 1:90 for strains 97-3256, 85-3007, and H19.
Three independent assays were performed. For each assay, bacteria and HEp-2 cells were counted in 8 to 14 microscope fields per sample. The total number of HEp-2 cells counted in the set of fields observed for each sample was at least 100. The difference between the levels of adherence of the O157:H7 and O55:H7 strains in the presence of preimmune serum and polyclonal anti-intimin serum was significant, as calculated by Student's unpaired t test; P < 0.001 for each assay. For O111:NM strain 85-3007, the difference between the sera in assay II was not significant (P > 0.05); the P values for assays I and III were <0.01 and <0.001, respectively. For O26:H11 strain H19, the P values were <0.001, <0.02, and <0.05, respectively, for assays I to III.
The fold reduction in adherence in the presence of anti-intimin antiserum was calculated with respect to the preimmune serum control and was computed before rounding the numbers for each sample. The average fold reductions and ranges for the three assays are presented. For strains 95-3208, 97-3250, and E2348/69 there was no anti-intiminO157-specific reduction in adherence.
Immunoblot analysis.
Whole-cell bacterial lysates were prepared as follows. Bacteria were grown to log phase in Luria broth, harvested by centrifugation, and resuspended in 1/10 volume of medium. Sample buffer that contained SDS was added immediately, and the solution was heated for 4 min at 100°C and loaded onto polyacrylamide-SDS gels. Amounts of the lysates loaded were normalized to the optical densities at 600 nm of the log-phase cultures.
Proteins separated by SDS-PAGE were blotted onto nitrocellulose and visualized on the blot with Ponceau S stain (Sigma, St. Louis, Mo.) to ensure even loading and transfer across all lanes. The nitrocellulose membranes were blocked with 5% nonfat dried milk in PBS, washed with Tris-buffered saline (pH 7.6)–0.2% Tween (TBST), and incubated for 2 h with primary antibody diluted in TBST. The primary antibodies were used at the following dilutions: preimmune and goat anti-intimin serum, 1:2,700; goat anti-His-IntC1/3, 1:167; goat anti-His-IntN2/3, 1:222. Membranes were washed in TBST and incubated for 1 h in TBST plus 5% milk with horseradish peroxidase-conjugated secondary antibody (Boehringer Mannheim). The signal was visualized by chemiluminescence with a detection kit from Amersham (Piscataway, N.J.).
RESULTS
Polyclonal antisera raised against intimin from strain 86-24 reduced the adherence of this strain to HEp-2 cells.
We examined the adherence of strain 86-24 to HEp-2 cells in the presence of antiserum raised in a goat against histidine-tagged intimin protein RIHisEae. The RIHisEae protein is a histidine-tagged version of the entire intimin protein from strain 86-24 minus the N-terminal 35 amino acids, which are thought to be part of a cleaved N-terminal signal sequence (22, 47). Since we also tested this antiserum for its effects on the adherence of strains of other serotypes (listed in Table 1) in later experiments (below), we hereafter refer to it as anti-intiminO157. The presence of goat preimmune serum reduced adherence of strain 86-24 compared to that of controls without serum, but to a lesser extent than an equal volume of the postimmunization serum (Table 2). Our laboratory had previously reported a similar result with antisera raised in mice (47). For the present study, we thus limited the amount of sera added to levels that still permitted good adherence of 86-24 in the presence of preimmune serum to facilitate quantitation of the effect of the anti-intiminO157 antiserum on adherence. The number of bacteria per HEp-2 cell was reduced an average of 8.1-fold in the presence of the anti-intiminO157 antiserum, compared with an equal volume of preimmune serum (Table 2). At the dilutions used in the assays, neither the preimmune nor the immune serum samples affected bacterial growth or viability. These sera also did not agglutinate the bacteria (data not shown).
Polyclonal sera raised against C-terminal fragments of intimin reduced adherence of strain 86-24.
As one way to investigate the importance of the C-terminal region of intimin in the adherence-reducing capacity of anti-intimin antisera, we immunized a rabbit with the His-tagged C-terminal half fragment (His-IntC1/2) and four mice with the His-tagged C-terminal one-third fragment (His-IntC1/3). Intimin fragments are shown in Fig. 1. As assessed by microscopic observation, serum samples from the rabbit immunized with His-IntC1/2 reduced adherence of 86-24 to a greater extent than did the preimmune serum (Table 2). The antisera from three of the four immunized mice also reduced adherence of 86-24 to a greater extent than did the preimmune sera from the respective mice, although to different degrees (Table 2). No difference between pre- and postimmunization sera from one of the mice was observed; this result is addressed in Discussion.
An affinity-purified antibody fraction containing antibodies to the C-terminal third of intimin reduced adherence of strain 86-24 to HEp-2 cells.
Since serum contains other factors in addition to antibodies, we sought to test our hypothesis that the reduction in adherence in the presence of anti-intiminO157 antisera was due to the presence of anti-intimin antibodies. Additionally, we sought to investigate by another method the importance of antibodies to the C-terminal region of intimin, suggested by our results above, for the effect on adherence. We thus purified two antibody fractions from the goat anti-intiminO157 antiserum for use in the adherence assays: an antibody fraction that contained antibodies that recognized the C-terminal third of intimin, His-IntC1/3, and another fraction that contained antibodies that recognized the N-terminal two-thirds of intimin, His-IntN2/3. Since the antibodies were stored in a saline-containing buffer, as recommended by Harlow and Lane (26), we chose to limit the volume of antibody preparations added to the adherence assay mixture because we subsequently observed that adherence could also be affected by excess salt in the assay mixture, at amounts approximately two to three times what we added in these experiments (data not shown). It is potentially relevant to the latter finding that we repeated the experiment on which the published observation (18) that purified EHEC intimin protein can bind to HEp-2 cells was based (data not shown), and observed that this intimin-cell interaction could be disrupted by the addition of similar amounts of excess salt (59). The amounts of antibody tested in the bacterial adherence assays for this study were limited to a range of 0.8 to 4.0 μg.
The anti-His-IntC1/3 antibody fraction reduced the adherence of strain 86-24 to HEp-2 cells compared to that of control samples that received an equal volume of PBS buffer alone, but the antibody fraction that contained antibodies to the N-terminal two-thirds portion of intimin did not block adherence at all at any amount tried. While 0.8 μg of the anti-His-IntC1/3 antibody fraction did not show a consistent effect on adherence, the 2.4- and 4-μg amounts both showed reproducible effects, with the effect of 4 μg about twice that of 2.4 μg. Data for 4 μg of the anti-His-IntC1/3 antibody fraction are presented in Table 2; in three independent experiments, performed on different days, the anti-His-IntC1/3 antibody fraction reduced adherence of strain 86-24 to HEp-2 cells by an average of 4.4-fold compared to adherence levels seen in the presence of the anti-His-IntN2/3 antibodies. The results observed with the fraction of antibodies containing reactivity to the C-terminal third of intimin indicate that anti-intimin antibodies can reduce adherence to HEp-2 cells and that epitopes in the C-terminal third of intimin are critical for this effect.
Immunoblot analysis with whole-cell lysates of E. coli strains demonstrated the presence of reactivity in these antibody fractions to the 97-kDa intimin protein in the pathogenic E. coli examined. The immunoblots revealed additional reactivity to one other band, of approximately 15 kDa, that was present in both clinical and nonpathogenic laboratory strains. However, for reasons discussed below, we think that the antibodies to the C-terminal domain of intimin caused the reduction in adherence of strain 86-24.
Polyclonal antisera raised against intimin from O157:H7 strain 86-24 reduced the HEp-2 cell adherence of another O157:H7 strain and that of some, but not all, strains of other serotypes.
Next, we sought to determine whether antisera raised against intimin from an O157:H7 strain could reduce adherence of EHEC strains of other serotypes. For these experiments, we chose to analyze (i) an additional strain of the O157:H7 serotype to complement our studies on 86-24; (ii) an O55:H7 strain, since this serotype is closely related to O157:H7 strains (54, 67, 68); and (iii) strains from the O26 and O111 serogroups, since these are the most frequent non-O157:H7 isolates from hemorrhagic colitis and HUS patients (58). We analyzed two independently isolated O26:H11 strains and two independently isolated O111:NM strains. Additionally, we tested one EPEC strain, O127:H6 strain E2348/69. All of the strains were isolated either from human patients or from contaminated food associated with an outbreak and are described in Table 1. Since some of these strains, especially the recent isolates, had not been characterized with respect to A/E lesion formation, we first tested all of the strains in HEp-2 cell assays for the capacity to rearrange cellular actin, a hallmark of the A/E lesion and thus of intimin function. We used the standard fluorescence actin staining (FAS) test (33); as expected, all of these eae+ strains were FAS positive (data not shown).
Trial assays were done to assess the adherence capacity of each eae+ strain. Most of the strains displayed a greater level of adherence to HEp-2 cells than did strain 86-24 (data not shown). For more convenient comparison with strain 86-24, cultures of these other strains were diluted prior to the assays, as noted in Table 3. Additionally, we further titrated down the number of bacteria in an attempt to optimize the adherence-blocking effects for strains that did not show a strong effect initially. To observe a reduction of adherence by the goat anti-intiminO157 serum, some strains also required the addition of more serum than was necessary to see an effect on strain 86-24. Data are presented in Table 3 for three independent assays performed on different days.
Addition of anti-intiminO157 antisera to the HEp-2 cell assay mixture reduced adherence of some of the strains compared with their adherence in the presence of preimmune serum; despite some day-to-day variation in the assay, the greatest fold reductions were seen with the two O157:H7 strains and the EHEC O55:H7 strain (Table 3). Somewhat lesser effects, on average, were seen for strain 85-3007 (O111:NM) and for strain H19 (O26:H11). The reduction in adherence seen with these strains was readily apparent by microscopic observation even before bacterial counts were assessed. However, there was no specific anti-intiminO157 serum effect on adherence of strains 95-3208 (EHEC O111:NM), 97-3250 (EHEC O26:H11), and E2348/69 (EPEC O127:H6), as assessed by microscopic observation, in at least two independent assays in which different amounts of sera and bacteria were tested. Thus, adherent bacteria were not counted for these strains.
We also tested our rabbit anti-His-IntC1/2 polyclonal antisera for the capacity to reduce adherence of the strains for which an effect was seen with the anti-intiminO157 serum, as described above. These included O157:H7 strains 86-24 and EDL933, O55:H7 strain 97-3256, O111:NM strain 85-3007, and O26:H11 strain H19. In support of the above results, the rabbit polyclonal serum reduced adherence of these strains compared to the preimmune serum control, as assessed by microscopic observation; the effects on adherence were most readily observed with the two O157:H7 strains, 86-24 and EDL933 (data not shown).
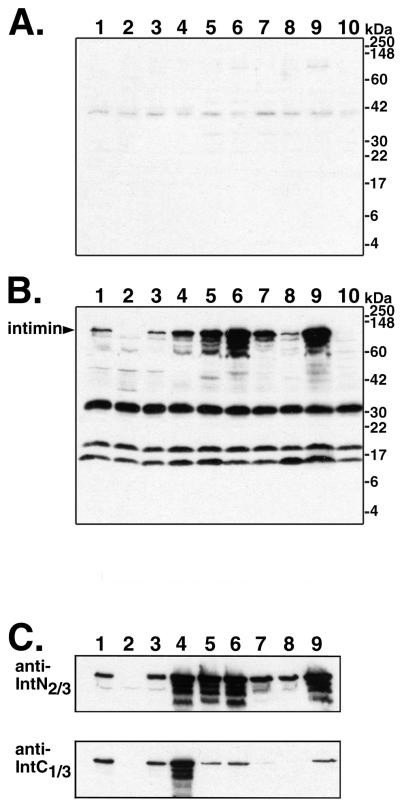
Immunoblot analysis of cross-reactivity of the goat anti-intiminO157 serum with strains of different serotypes; observation of antigenic divergence in the C-terminal third of intimin among the strains.
We assessed the cross-reactivity of our goat antiserum raised against intiminO157 to whole-cell lysates of the different E. coli strains. Data are presented in Fig. 2. The preimmune serum showed relatively little reactivity with E. coli proteins from all of the strains (Fig. 2A). Four major species were detected with the crude polyclonal anti-intimin serum (Fig. 2B). The 97-kDa band is intimin, as demonstrated by a comparison between 86-24 and its eae mutant derivative, 86-24eaeΔ10 (Fig. 2B, lane 2). Strain 86-24eaeΔ10 produced a truncated intimin product of approximately 68 kDa that is visible as a faint band on some blots (46). The anti-intiminO157 polyclonal serum recognized the 97-kDa intimin band in lysates from all of the wild-type pathogenic E. coli strains used in this study (Fig. 2B, lanes 1 and 3 to 9). It is not clear what biological significance there may be to the observation of different amounts of intimin produced by the different strains. The three other major bands detected with the polyclonal serum, of approximately 30, 18, and 15 kDa, are addressed below.
FIG. 2.
Immunoblot analysis of intimin from whole-cell lysates of different E. coli strains with antibodies raised in a goat against intimin from O157:H7 strain 86-24. The strains analyzed were as follows (lanes 1 to 8 are EHEC strains): lane 1, 86-24 (O157:H7); lane 2, 86-24eaeΔ10; lane 3, EDL933 (O157:H7); lane 4, 97-3256 (O55:H7); lane 5, 85-3007 (O111:NM); lane 6, H19 (O26:H11); lane 7, 97-3250 (O26:H11); lane 8, 95-3208 (O111:NM); lane 9, EPEC strain E2348/69 (O127:H6); lane 10, nonpathogenic E. coli K12 strain UT5600. (A) Reactivity of preimmune serum. (B) Reactivity of polyclonal serum raised against intiminO157. For blots A and B, whole-cell lysates were run on 15% polyacrylamide–SDS gels for this figure to display all of the reactive bands. Identical serum dilutions were used for the preimmune and anti-intimin antisera, and blots A and B were exposed to film simultaneously for chemiluminescent detection. (C) Reactivity of purified antibodies against the N-terminal two-thirds (top) and C-terminal third (bottom) of intimin. Bacterial lysates were run on 10% polyacrylamide–SDS gels for the blots shown here. Analysis of higher-percentage gels showed additional reactivity to a 15-kDa band in all lanes (data not shown). All immunoblots were scanned with a Hewlett-Packard ScanJet 4C scanner, and the images of the four blots were arranged for the figure and labeled with Adobe Photoshop version 5.0.2 for Macintosh.
Among the strains of different serotypes, we observed more cross-reactivity with the purified antibodies to the N-terminal two-thirds of intimin than with the anti-His-IntC1/3 antibodies (Fig. 2C). Since equal amounts of lysates for all strains were loaded, the relative degrees of cross-reactivity to the different parts of intimin could be assessed by comparing the signals on the two blots. The O157:H7 strain EDL933 showed a signal similar to that of 86-24 in both the anti-His-IntN2/3 and anti-His-IntC1/3 blots (lane 3). The O55:H7 strain reproducibly displayed a somewhat stronger signal than 86-24 with both the anti-His-IntN2/3 antibodies and the anti-His-IntC1/3 antibodies (lane 4). Compared with strain 86-24, the other strains also presented intimin bands of similar or greater intensity with the antibodies to the N terminus. In contrast to the results observed for the O157:H7 and O55:H7 strains, however, the intensities of the intimin signals detected in the O26:H11, O111:NM, and O127:H6 strains with the antibodies to the C terminus did not correspond to those seen with the antibodies to the N terminus: for each of these strains, the band detected by the antibodies to the C terminus was relatively less intense than the band detected with the antibodies to the N terminus (Fig. 2C, lanes 5–9). These data suggest that epitopes recognized by the antibodies to the C-terminal third of intimin are conserved among the O157:H7 and O55:H7 strains but are significantly more divergent in the other strains.
Immunoblot analysis with the affinity-purified antibody preparations demonstrated the absence of the 30- and 18-kDa bands that were observed with the crude serum; thus, reactivity to these proteins did not contribute to the reduction in adherence of strain 86-24 in the presence of the purified anti-His-IntC1/3 preparation. As noted above, there was reactivity to the 15-kDa band in our purified antibody preparations (data not shown). The His-IntC1/3 antibody preparation contained somewhat stronger reactivity to this band than the His-IntN2/3 antibody preparation. Since this protein was present in all strains examined, including the nonadherent eae mutant derivative of 86-24 and nonpathogenic laboratory E. coli strains (Fig. 2B), it does not function as an adherence factor in the HEp-2 cell assay. Unlike the significant antigenic divergence observed in the C-terminal third of intimin among different strains, the intensities of the 15-kDa bands in all strains, including nonpathogenic laboratory E. coli were similar, as assessed by immunoblotting with polyclonal serum and with the affinity-purified antibody fractions (Fig. 2B and data not shown). Thus, the adherence-reducing capacity of the polyclonal antiserum for the strains of different serotypes correlated only with the pattern of antigenic cross-reactivity in the C-terminal domain of intimin.
DISCUSSION
In this study, we examined the adherence to HEp-2 cells of O157:H7 strain 86-24 and pathogenic eae+ E. coli of other serotypes in the presence of antisera that contained antibodies to intiminO157. We observed a reduction in adherence of O157:H7 strain 86-24 in the presence of crude polyclonal anti-intimin antiserum and in the presence of a purified antibody fraction that contained antibodies to the C-terminal third of intimin. The adherence of some, but not all, of the other EHEC strains tested was also reduced in the presence of the polyclonal anti-intiminO157 antiserum compared with that in the presence of the preimmune serum. Immunoblot analysis revealed antigenic divergence in the C-terminal domains of the intimins from these strains. Although the serum recognized several other E. coli proteins in addition to intimin, these other proteins were not specific to pathogenic E. coli. Furthermore, the fold reduction in adherence in the presence of serum containing anti-intiminO157 antibodies was greatest for strains that expressed an intimin protein that was very similar or identical in the C-terminal domain to intimin from strain 86-24. Conversely, by immunoblot analysis, the bands detected for each of the O26, O111, and O127:H6 strains with antibodies to the C terminus were much less intense than the bands detected with the antibodies to the N terminus. This pattern of differential immunoreactivity between the two domains indicated more divergence in the C terminus and correlated with lesser effects (or no effect) of the anti-intiminO157 sera on adherence of these strains. The C-terminal cross-reactivity detected in these strains by immunoblotting may represent some epitopes in the C terminus that are not critical for adherence or that are not accessible to antibody binding when the protein is in the bacterial outer membrane.
There are data from several laboratories showing the importance of the C-terminal third of intimin for the interaction of EPEC and EHEC with host epithelial cells (13, 18, 19, 27, 32, 40). Thus, the decreased EHEC adherence that we observed in the presence of antibodies to the C-terminal third of intimin may be due to the blocking of functional regions of this domain of intimin, preventing interaction either between intimin and the translocated intimin receptor protein Tir or between intimin and a host cell protein. It is of interest that host cell invasion by a different pathogen, the Yersinia species, can be blocked by antibodies to the Yersinia protein invasin, which shows some homology to the intimin proteins (28, 30, 35, 36, 70). While we did not observe decreased adherence in the presence of antibodies to the N-terminal two-thirds of intimin, our data do not rule out the possibility that a high titer of antibodies specific to a particular epitope within this region of the protein might functionally or sterically reduce adherence.
The relative effects of our anti-intiminO157 serum on adherence of strains of different serotypes correlate with published sequence divergence in the C-terminal domain of intimin and with evolutionary relationships among strains of these serotypes. The trends we observed showed the greater fold reductions of adherence of the two O157:H7 strains and the O55:H7 strain and lesser effects with the other strains. Sequence analysis showed 100% amino acid identity in the regions comprising the C-terminal thirds of the 86-24 and EDL933 intimin proteins (21, 45, 46, 70). Although the eae gene from the EHEC O55:H7 strain examined in this study has not yet been cloned, the eae genes from two other O55:H7 strains have been sequenced (42, 45) and show approximately 99% identity in the C-terminal region to the intimin from strain 86-24. Among the other strains we tested, sequence information is available for the eae genes of O26:H11 strain H19 and EPEC O127:H6 strain E2348/69 (3, 30). These sequences show 59 and 55% amino acid identity, respectively, to the 86-24 sequence in the C-terminal thirds of the proteins. Additionally, consistent with our results, recent studies have identified different subtypes of intimin C-terminal domains based on antigenic variation and PCR analysis (1, 45). As noted in Table 1, the intimins from the O157:H7 and O55:H7 strains used in the present study were of the γ subtype; the intimins from the other strains fell into different subtypes.
Our laboratory and others have proposed intimin as a candidate for an anticolonization vaccine strategy for EHEC (7, 12, 31). Such a vaccine could theoretically be used in humans or in cattle, since cattle are the primary reservoir for STEC that infects humans. Recently, a strategy employing antibodies to colonization factor antigens to protect against oral challenge with another pathogen, enterotoxigenic E. coli, was shown to be successful in a volunteer study (20). A different approach toward a vaccine for EHEC currently under investigation by other researchers is the use of O157:H7 lipopolysaccharide as a candidate antigen to elicit antibodies that may lyse the bacteria in the presence of complement or sterically block adherence (34, 52).
Circumstantial evidence that is consistent with the hypothesis that an immune response to intimin in humans might confer protection against infection with eae+ E. coli exists. As noted above, intimin is immunogenic in humans (29, 39, 43, 44, 47, 50, 65). Additionally, in one study of volunteers experimentally infected with EPEC, 1 of the 10 volunteers did not develop diarrhea; this was the only individual who had prechallenge serum antibodies to a 94-kDa protein later shown to be intimin (39, 50). Another volunteer study that involved challenge with EPEC and subsequent rechallenge did not observe a correlation between antibody titer to intimin and severity of disease upon rechallenge (15). In that model of infection, however, the antibody response to intimin may not have been sufficient. We note in this context that one of the four mice immunized for this study with the fragment comprising the C-terminal third of intimin did not show an adherence-blocking response. Thus, optimization of the immunization protocol might be necessary to elicit a more uniform immune response. We additionally immunized one animal with RIHisEae protein that had been excised from an SDS-PAGE gel, and we found that the serum obtained did not inhibit adherence of 86-24 (data not shown). This result may hint that conformational epitopes, disrupted in this case by SDS, play a role in eliciting a blocking response. Since the anti-His-IntC1/3 antibody-containing fraction from the polyclonal serum was purified on an immunoaffinity column that had been prewashed with acidic buffer, we infer that at least some relevant epitopes of intimin may be stable to exposure to acidic conditions, a property that would be useful in an oral vaccine.
Our data suggest that serum raised against intiminO157 may reduce colonization of O157:H7 and closely related strains but that a higher titer of anti-intimin antibodies may be required to block colonization of strains that display greater adherence capacity due to as yet unidentified regulatory or structural factors. We observed a general correlation between the antigenic similarity in the C-terminal domain of intimin and the capacity of antiserum containing antibodies to intiminO157 to reduce adherence of heterologous strains. Thus, sequence divergence at the C-terminal domain may preclude development of a broadly cross-protective vaccine with intiminO157 as the antigen. While O157:H7 is the most frequent cause of STEC-mediated disease in many countries, one way to encompass more of the non-O157 eae-producing strains in a single vaccine might be to consider a multivalent vaccine with intimin fragments from several major clinically relevant serotypes.
ACKNOWLEDGMENTS
This work was supported by grant AI20148-16 from the National Institutes of Health and by grant 97-35201-4578 from the U.S. Department of Agriculture.
We are grateful to N. Strockbine for providing us with strains 97-3250, 97-3256, 85-3007, and 95-3208 and for helpful discussion. We thank W. D. Roof and R. Young for the generous gift of strain RY2844. We are grateful to T. S. Whittam and S. Plock for the determination of the intimin subtypes of strains 97-3250, 97-3256, 85-3007, and 95-3208 and for communication of these unpublished results. We thank Angela Melton-Celsa for helpful discussion and Clare Schmitt and James Sinclair for critical reading of the manuscript.
REFERENCES
- 1.Adu-Bobie J, Frankel G, Bain C, Goncalves A G, Trabulsi L R, Douce G, Knutton S, Dougan G. Detection of intimins α, β, γ, and δ, four intimin derivatives expressed by attaching and effacing microbial pathogens. J Clin Microbiol. 1998;36:662–668. doi: 10.1128/jcm.36.3.662-668.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adu-Bobie J, Trabulsi L R, Carneiro-Sampaio M M S, Dougan G, Frankel G. Identification of immunodominant regions within the C-terminal cell binding domain of intimin α and intimin β from enteropathogenic Escherichia coli. Infect Immun. 1998;66:5643–5649. doi: 10.1128/iai.66.12.5643-5649.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agin T S, Wolf M K. Identification of a family of intimins common to Escherichia colicausing attaching-effacing lesions in rabbits, humans, and swine. Infect Immun. 1997;65:320–326. doi: 10.1128/iai.65.1.320-326.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley Interscience; 1989. [Google Scholar]
- 5.Boyce T G, Swerdlow D L, Griffin P M. Escherichia coliO157:H7 and the hemolytic-uremic syndrome. N Engl J Med. 1995;333:364–368. doi: 10.1056/NEJM199508103330608. [DOI] [PubMed] [Google Scholar]
- 6.Bullock W O, Fernandez J M, Short J M. XL1-Blue: a high efficiency plasmid transforming recA Escherichia colistrain with beta-galactosidase selection. BioTechniques. 1987;5:376–378. [Google Scholar]
- 7.Butterton J R, Ryan E T, Acheson D W K, Calderwood S B. Coexpression of the B subunit of Shiga toxin 1 and EaeA from enterohemorrhagic Escherichia coli in Vibrio choleraevaccine strains. Infect Immun. 1997;65:2127–2135. doi: 10.1128/iai.65.6.2127-2135.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Câmara L M, Carbonare S B, Scaletsky I C A, Monteiro da Silva M L, Carneiro-Sampaio M M S. Inhibition of enteropathogenic Escherichia coli (EPEC) adherence to HeLa cells by human colostrum. Detection of specific sIgA related to EPEC outer-membrane proteins. In: Mestecky J, Russell M W, Jackson S, Michalek S M, Tlaskalová-Hogenová H, Sterzi J, editors. Advances in mucosal immunology. New York, N.Y: Plenum Press; 1995. pp. 673–676. [DOI] [PubMed] [Google Scholar]
- 9.Caprioli A, Tozzi A E. Epidemiology of Shiga toxin-producing Escherichia coli infections in continental Europe. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C: American Society for Microbiology; 1998. pp. 38–48. [Google Scholar]
- 10.Cravioto A, Gross R J, Scotland S M, Rowe B. An adhesive factor found in strains of E. colibelonging to the traditional infantile enteropathogenic serotypes. Curr Microbiol. 1979;3:95–99. [Google Scholar]
- 11.Cravioto A, Tello A, Villafán H, Ruiz J, del Vedovo S, Neeser J. Inhibition of localized adhesion of enteropathogenic Escherichia colito HEp-2 cells by immunoglobulin and oligosaccharide fractions of human colostrum and breast milk. J Infect Dis. 1991;163:1247–1255. doi: 10.1093/infdis/163.6.1247. [DOI] [PubMed] [Google Scholar]
- 12.Dean-Nystrom E A, Bosworth B T, Moon H W, O'Brien A D. Escherichia coliO157:H7 requires intimin for enteropathogenicity in calves. Infect Immun. 1998;66:4560–4563. doi: 10.1128/iai.66.9.4560-4563.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeVinney R, Stein M, Reinscheid D, Abe A, Ruschkowski S, Finlay B B. Enterohemorrhagic Escherichia coliO157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated. Infect Immun. 1999;67:2389–2398. doi: 10.1128/iai.67.5.2389-2398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnenberg M S, Tacket C O, James S P, Losonsky G, Nataro J P, Wasserman S S, Kaper J B, Levine M M. Role of the eaeA gene in experimental enteropathogenic Escherichia coliinfection. J Clin Investig. 1993;92:1412–1417. doi: 10.1172/JCI116717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnenberg M S, Tacket C O, Losonsky G, Frankel G, Nataro J P, Dougan G, Levine M M. Effect of prior experimental human enteropathogenic Escherichia coliinfection on illness following homologous and heterologous rechallenge. Infect Immun. 1998;66:52–58. doi: 10.1128/iai.66.1.52-58.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donnenberg M S, Tzipori S, McKee M L, O'Brien A D, Alroy J, Kaper J B. The role of the eae gene of enterohemorrhagic Escherichia coliin intimate attachment in vitro and in a porcine model. J Clin Investig. 1993;92:1418–1424. doi: 10.1172/JCI116718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elish M E, Pierce J R, Earhart C F. Biochemical analysis of spontaneous fepA mutants of Escherichia coli. J Gen Microbiol. 1988;134:1355–1364. doi: 10.1099/00221287-134-5-1355. [DOI] [PubMed] [Google Scholar]
- 18.Frankel G, Candy D C A, Everest P, Dougan G. Characterization of the C-terminal domains of intimin-like proteins of enteropathogenic and enterohemorrhagic Escherichia coli, Citrobacter freundii, and Hafnia alvei. Infect Immun. 1994;62:1835–1842. doi: 10.1128/iai.62.5.1835-1842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frankel G, Philips A D, Novakova M, Batchelor M, Hicks S, Dougan G. Generation of Escherichia coliintimin derivatives with differing biological activities using site-directed mutagenesis of the intimin C-terminus domain. Mol Microbiol. 1998;29:559–570. doi: 10.1046/j.1365-2958.1998.00950.x. [DOI] [PubMed] [Google Scholar]
- 20.Freedman D J, Tacket C O, Delehanty A, Maneval D R, Nataro J, Crabb J H. Milk immunoglobulin with specific activity against purified colonization factor antigens can protect against oral challenge with enterotoxigenic Escherichia coli. J Infect Dis. 1998;177:662–667. doi: 10.1086/514227. [DOI] [PubMed] [Google Scholar]
- 21.Gansheroff, L. J., N. Roup, M. L. McKee, and A. D. O'Brien. 1999. Unpublished data.
- 22.Gómez-Duarte O G, Kaper J B. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect Immun. 1995;63:1767–1776. doi: 10.1128/iai.63.5.1767-1776.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin P M. Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press, Ltd.; 1995. pp. 739–761. [Google Scholar]
- 24.Griffin P M. Epidemiology of Shiga toxin-producing Escherichia coli infections in humans in the United States. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C: American Society for Microbiology; 1998. pp. 15–22. [Google Scholar]
- 25.Griffin P M, Ostroff S M, Tauxe R V, Greene K D, Wells J G, Lewis J H, Blake P A. Illnesses associated with Escherichia coliO157:H7 infections. Ann Intern Med. 1988;109:705–712. doi: 10.7326/0003-4819-109-9-705. [DOI] [PubMed] [Google Scholar]
- 26.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 27.Hartland E L, Batchelor M, Delahay R M, Hale C, Matthews S, Dougan G, Knutton S, Connerton I, Frankel G. Binding of intimin from enteropathogenic Escherichia colito Tir and to host cells. Mol Microbiol. 1999;32:151–158. doi: 10.1046/j.1365-2958.1999.01338.x. [DOI] [PubMed] [Google Scholar]
- 28.Isberg R R, Voorhis D L, Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987;50:769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- 29.Jerse A E, Kaper J B. The eae gene of enteropathogenic Escherichia coliencodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect Immun. 1991;59:4302–4309. doi: 10.1128/iai.59.12.4302-4309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jerse A E, Yu J, Tall B D, Kaper J B. A genetic locus of enteropathogenic Escherichia colinecessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaper J B, Gansheroff L J, Wachtel M R, O'Brien A D. Intimin-mediated adherence of Shiga toxin-producing Escherichia coli and attaching-and-effacing pathogens. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C: American Society for Microbiology; 1998. pp. 148–156. [Google Scholar]
- 32.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli(EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 33.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konadu E Y, Parke J C, Jr, Tran H T, Bryla D A, Robbins J B, Szu S C. Investigational vaccine for Escherichia coli O157: phase 1 study of O157 O-specific polysaccharide-Pseudomonas aeruginosarecombinant exoprotein A conjugates in adults. J Infect Dis. 1998;177:383–387. doi: 10.1086/514203. [DOI] [PubMed] [Google Scholar]
- 35.Leong J M, Fournier R S, Isberg R R. Identification of the integrin binding domain of the Yersinia pseudotuberculosisinvasin protein. EMBO J. 1990;9:1979–1989. doi: 10.1002/j.1460-2075.1990.tb08326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leong J M, Fournier R S, Isberg R R. Mapping and topographic localization of epitopes of the Yersinia pseudotuberculosisinvasin protein. Infect Immun. 1991;59:3424–3433. doi: 10.1128/iai.59.10.3424-3433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine M M. Escherichia colithat cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987;155:377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- 38.Levine M M, Bergquist E J, Nalin D R, Waterman D H, Hornick R B, Young C R, Sotman S, Rowe B. Escherichia colistrains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet. 1978;i:1119–1122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- 39.Levine M M, Nataro J P, Karch H, Baldini M M, Kaper J B, Black R E, Clements M L, O'Brien A D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coliis dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985;152:550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- 40.Liu H, Magoun L, Leong J M. β1-chain integrins are not essential for intimin-mediated host cell attachment and enteropathogenic Escherichia coli-induced actin condensation. Infect Immun. 1999;67:2045–2049. doi: 10.1128/iai.67.4.2045-2049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.López E L, Contrini M M, De Rosa M F. Epidemiology of Shiga toxin-producing Escherichia coli in South America. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C: American Society for Microbiology; 1998. pp. 30–37. [Google Scholar]
- 42.Louie M, De Azavedo J, Clarke R, Borczyk A, Lior H, Richter M, Brunton J. Sequence heterogeneity of the eae gene and detection of verotoxin-producing Escherichia coliusing serotype-specific primers. Epidemiol Infect. 1994;112:449–461. doi: 10.1017/s0950268800051153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loureiro I, Frankel G, Adu-Bobie J, Dougan G, Trabulsi L R, Carneiro-Sampaio M M S. Human colostrum contains IgA antibodies reactive to enteropathogenic Escherichia colivirulence-associated proteins: intimin, BfpA, EspA, and EspB. J Pediatr Gastroenterol Nutr. 1998;27:166–171. doi: 10.1097/00005176-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Martinez M B, Taddei C R, Ruiz-Tagle A, Trabulsi L R, Girón J A. Antibody response of children with enteropathogenic Escherichia coliinfection to the bundle-forming pilus and locus of enterocyte effacement-encoded virulence determinants. J Infect Dis. 1999;179:269–274. doi: 10.1086/314549. [DOI] [PubMed] [Google Scholar]
- 45.McGraw E A, Li J, Selander R K, Whittam T S. Molecular evolution and mosaic structure of α, β, and γ intimins of pathogenic Escherichia coli. Mol Biol Evol. 1999;16:12–22. doi: 10.1093/oxfordjournals.molbev.a026032. [DOI] [PubMed] [Google Scholar]
- 46.McKee M L, Melton-Celsa A R, Moxley R A, Francis D H, O'Brien A D. Enterohemorrhagic Escherichia coliO157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect Immun. 1995;63:3739–3744. doi: 10.1128/iai.63.9.3739-3744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKee M L, O'Brien A D. Truncated enterohemorrhagic Escherichia coli(EHEC) O157:H7 intimin (EaeA) fusion proteins promote adherence of EHEC strains to HEp-2 cells. Infect Immun. 1996;64:2225–2233. doi: 10.1128/iai.64.6.2225-2233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michino H, Araki K, Minami S, Nakayama T, Ejima Y, Hiroe K, Tanaka H, Fujita N, Usami S, Yonekawa M, Sadamoto K, Takaya S, Sakai N. Recent outbreaks of infections caused by Escherichia coli O157:H7 in Japan. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C: American Society for Microbiology; 1998. pp. 73–81. [Google Scholar]
- 49.Moon H W, Whipp S C, Argenzio R A, Levine M M, Giannella R A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coliin pig and rabbit intestines. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Brien A D, Kaper J B. Shiga toxin-producing Escherichia coli: yesterday, today, and tomorrow. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C: American Society for Microbiology; 1998. pp. 1–11. [Google Scholar]
- 52.Paton A W, Voss E, Manning P A, Paton J C. Antibodies to lipopolysaccharide block adherence of Shiga toxin-producing Escherichia colito human intestinal epithelial (Henle 407) cells. Microb Pathog. 1998;24:57–63. doi: 10.1006/mpat.1997.0172. [DOI] [PubMed] [Google Scholar]
- 53.Paton J C, Paton A W. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coliinfections. Clin Microbiol Rev. 1998;11:450–479. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perna N T, Mayhew G F, Pósfai G, Elliott S, Donnenberg M S, Kaper J B, Blattner F R. Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coliO157:H7. Infect Immun. 1998;66:3810–3817. doi: 10.1128/iai.66.8.3810-3817.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54a.Plock, S., and T. S. Whittam. Personal communication.
- 55.Riley L W, Remis R S, Helgerson S D, McGee H B, Wells J G, Davis B R, Hebert R J, Olcott E S, Johnson L M, Hargett N T, Blake P A, Cohen M L. Hemorrhagic colitis associated with a rare Escherichia coliserotype. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 56.Robins-Browne R M, Elliott E, Desmarchelier P. Shiga toxin-producing Escherichia coli in Australia. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C: American Society for Microbiology; 1998. pp. 66–72. [Google Scholar]
- 57.Roof W D, Horne S M, Young K D, Young R. slyD, a host gene required for φX174 lysis, is related to the FK506-binding protein family of peptidyl-prolyl cis-trans-isomerases. J Biol Chem. 1994;269:2902–2910. [PubMed] [Google Scholar]
- 58.Schmidt H, Geitz C, Tarr P I, Frosch M, Karch H. Non-O157:H7 pathogenic Shiga toxin-producing Escherichia coli: phenotypic and genetic profiling of virulence traits and evidence for clonality. J Infect Dis. 1999;179:115–123. doi: 10.1086/314537. [DOI] [PubMed] [Google Scholar]
- 59.Sinclair, J. F., and A. D. O'Brien. 1999. Unpublished data.
- 60.Smith H R, Rowe B, Adak G K, Reilly W J. Shiga toxin (verocytotoxin)-producing Escherichia coli in the United Kingdom. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C: American Society for Microbiology; 1998. pp. 49–58. [Google Scholar]
- 61.Smith H W, Linggood M A. The transmissible nature of enterotoxin production in a human enteropathogenic strain of Escherichia coli. J Med Microbiol. 1971;4:301–305. doi: 10.1099/00222615-4-3-301. [DOI] [PubMed] [Google Scholar]
- 62.Spika J S, Khakhria R, Michel P, Milley D, Wilson J, Waters J. Shiga toxin-producing Escherichia coli infections in Canada. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C: American Society for Microbiology; 1998. pp. 23–29. [Google Scholar]
- 62a.Strockbine, N. Personal communication.
- 63.Taylor J. Infectious infantile enteritis, yesterday and today. Proc R Soc Med. 1970;63:1297–1301. doi: 10.1177/003591577006301233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tzipori S, Gunzer F, Donnenberg M S, de Montigny L, Kaper J B, Donohue-Rolfe A. The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coliinfection. Infect Immun. 1995;63:3621–3627. doi: 10.1128/iai.63.9.3621-3627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Voss E, Paton A W, Manning P A, Paton J C. Molecular analysis of Shiga toxigenic Escherichia coli O111:H−proteins which react with sera from patients with hemolytic-uremic syndrome. Infect Immun. 1998;66:1467–1472. doi: 10.1128/iai.66.4.1467-1472.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wells J G, Davis B R, Wachsmuth I K, Riley L W, Remis R S, Sokolow R, Morris G K. Laboratory investigation of hemorrhagic colitis outbreaks associated with a rare Escherichia coliserotype. J Clin Microbiol. 1983;18:512–520. doi: 10.1128/jcm.18.3.512-520.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whittam T S. Evolution of Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C: American Society for Microbiology; 1998. pp. 195–209. [Google Scholar]
- 68.Whittam T S, Wolfe M L, Wachsmuth I K, Orskov F, Orskov I, Wilson R A. Clonal relationships among Escherichia colistrains that cause hemorrhagic colitis and infantile diarrhea. Infect Immun. 1993;61:1619–1629. doi: 10.1128/iai.61.5.1619-1629.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wülfing C, Lombardero J, Plückthun A. An Escherichia coliprotein consisting of a domain homologous to FK506-binding proteins (FKBP) and a new metal binding motif. J Biol Chem. 1994;269:2895–2901. [PubMed] [Google Scholar]
- 70.Yu J, Kaper J B. Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coliO157:H7. Mol Microbiol. 1992;6:411–417. doi: 10.1111/j.1365-2958.1992.tb01484.x. [DOI] [PubMed] [Google Scholar]