Background:
Proton-pump inhibitors (PPIs) and vonoprazan are recommended as first-line therapies for erosive esophagitis (EE). However, it is uncertain how the magnitude of efficacy and safety of first-line therapy, the choice of individual PPIs or vonoprazan in the treatment of EE remains controversial. This study aimed to evaluate the efficacy and safety of vonoprazan and PPIs in healing esophageal mucosal injury in patients with EE.
Methods:
Relevant databases were searched to collect randomized controlled trials of proton pump inhibitors and vonoprazan in the treatment of reflux esophagitis up to December 2021. Studies on standard-dose PPIs or vonoprazan that were published in Chinese or English and assessed healing effects in EE were included in the analysis. Stata16.0 was used to conduct a network Meta-analysis to evaluate the efficacy and safety of the treatment.
Results:
A total of 41 literatures were included with 11,592 enrolled patients. For the endoscopic cure rate, all the PPIs and vonoprazan significantly improve compared to Placebo; Based on the surface under the cumulative ranking curve, Ilaprazole ranked first, followed by esomeprazole, vonoprazan, pantoprazole, lansoprazole, omeprazole, rabeprazole and placebo therapy ranked the last. For the rate of adverse events, there was no significant difference among all the PPIs, vonoprazan, and placebo.
Conclusions:
Ilaprazole, esomeprazole and vonoprazan have more advantages in mucosal erosion healing, there was no significant difference in the comparative safety among all interventions.
Keywords: erosive esophagitis, proton-pump inhibitors, vonoprazan
1. Introduction
Erosive esophagitis (EE) is classified as a type of gastroesophageal reflux disease (GERD), which generally involves changes in the barrier between the stomach and the esophagus, esophageal clearance dysfunction and weakened epithelial defense function, increased esophageal sensitivity.[1,2] The corresponding symptoms or mucosal damage are caused by the reflux of gastric contents from the stomach into the esophagus, including heartburn, regurgitation, odynophagia, nausea, chest pain and coughing. Complications may deteriorate and even further develop into esophageal strictures, Barrett’s esophagus, and even esophageal adenocarcinoma without adequate treatment.[3,4]
Currently, the main treatments for EE include drug therapy, surgery, and lifestyle and dietary modifications, and drug therapy is one of the essential therapeutic methods. Notably, standard-dose vonoprazan and proton-pump inhibitors (PPIs) were recommended as first-line therapy for EE by guidelines.[5–8] However, the choice of individual PPIs drug or vonoprazan for the treatment of EE is still controversial. Previous studies have compared PPIs and vonoprazan for Heartburn Symptoms in EE,[9] but few studies have investigated the healing of esophageal mucosal injuries. Therefore, we conducted this study to evaluate the comparative efficacy and safety of vonoprazan and PPIs for healing esophageal mucosal injury in EE.
2. Materials and Methods
The study referred to the recommended method by the Cochrane Handbook for Systematic Reviews of Interventions,[10] and reported according to the Preferred reporting items for systematic reviews and meta-analyses statement.[11]
2.1. Data sources and searches
PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure, China Biology Medicine disc, VIP database, and the Wanfang database were searched to identify randomized controlled trials of PPIs and vonoprazan in the treatment of EE up to December 2021. The search strategy searched for PubMed can be found in Table S1 (Supplemental Digital Content, http://links.lww.com/MD/H938, which shows the search strategies).
2.2. Eligibility criteria and study selection
Two authors independently screened the title and abstract of citations for relevant literature. Any disagreement would consult another author and solve by discussion. The inclusion criteria were the followings:
Patients: patients with EE. The definition of EE: patients have the condition symptoms with the presence of esophageal abnormalities by endoscopy. Exclude refractory EE or resistance to previous PPIs treatment.
Interventions and comparisons: We included studies which had at least 2 of the following interventions: omeprazole 20 mg/d, lansoprazole 30 mg/d, pantoprazole 40 mg/d, rabeprazole 20 mg/d, esomeprazole 40 mg/d, ilaprazole 10 mg/d, vonoprazan 20 mg/d, and placebo.
Outcomes: Four to eight weeks endoscopic cure rate and rate of adverse events.
Study design: We included randomized clinical trials in Chinese or English.
2.3. Data extraction and quality assessment
We independently extracted relevant information, including study characteristics (year of publication, duration), population (sample size, age, male-to-female ratio, patient demographics), description of interventions (drug class, name, dose), and outcomes. The number of healed patients or the observed healing rate by endoscopy at 4 to 8 weeks and the number or rate of adverse events were extracted for each treatment group from each study. Intention-to-treat data were collected for all outcomes when possible; otherwise, Full Analysis Set data were collected. The risk of bias was assessed using the Cochrane Risk of Bias Tool for randomized clinical trials.[10]
2.4. Data synthesis and analysis
We evaluated assumptions of transitivity and consistency before conducting data synthesizing.[12] For transitivity assumption, the study method and patient characters at baseline for each study were compared.[13,14] For the consistency assumption, The overall inconsistency was tested by using global approach and local approach.[15] When the global P value in the global approach was more than 0.05 and the inconsistency factor approaches the lower limit of 0 or 95% confidence interval (CI) includes 0 or near 0, we considered that the consistency is accepted.[15–17] If there was good transitivity across the study and no significant inconsistency, we would conduct network meta-analyses with random model.[18] The efficacy and safety of them were ranked by using the surface under the cumulative ranking curve (SUCRA).[19,20] For the same outcome, the larger SUCRA and the higher the rank probability, the better efficacy and safety of the treatment regimen. Funnel plot was used to assess publication bias.[19] Moreover, sensitivity analyses were conducted to examine the robustness of results by excluding studies with a high risk of bias.
All data analyses were conducted through STATA software (version 16; StataCorp LP, College Station, TX).
3. Results
3.1. Study characteristics and risk of bias
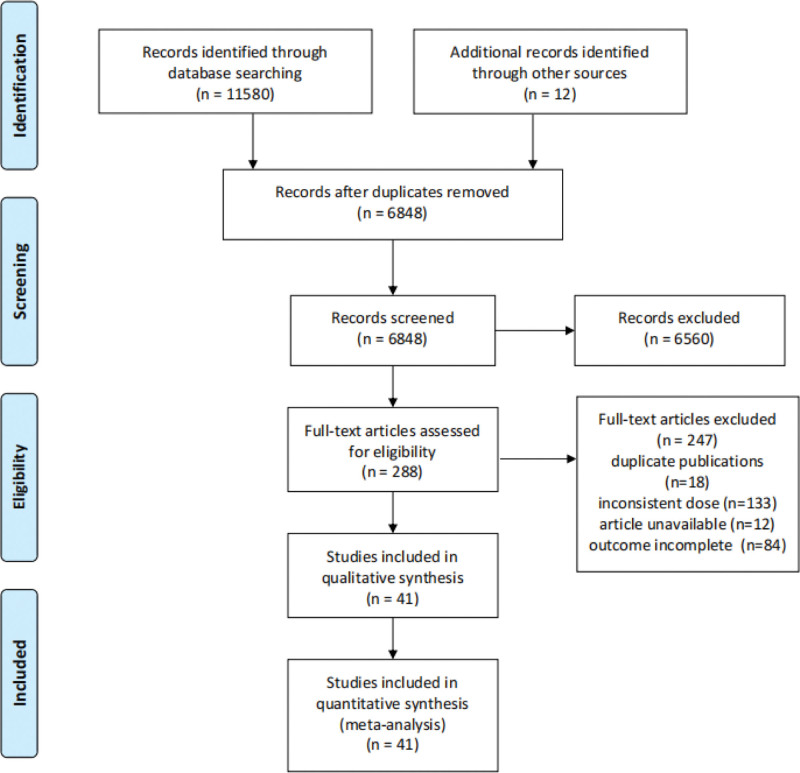
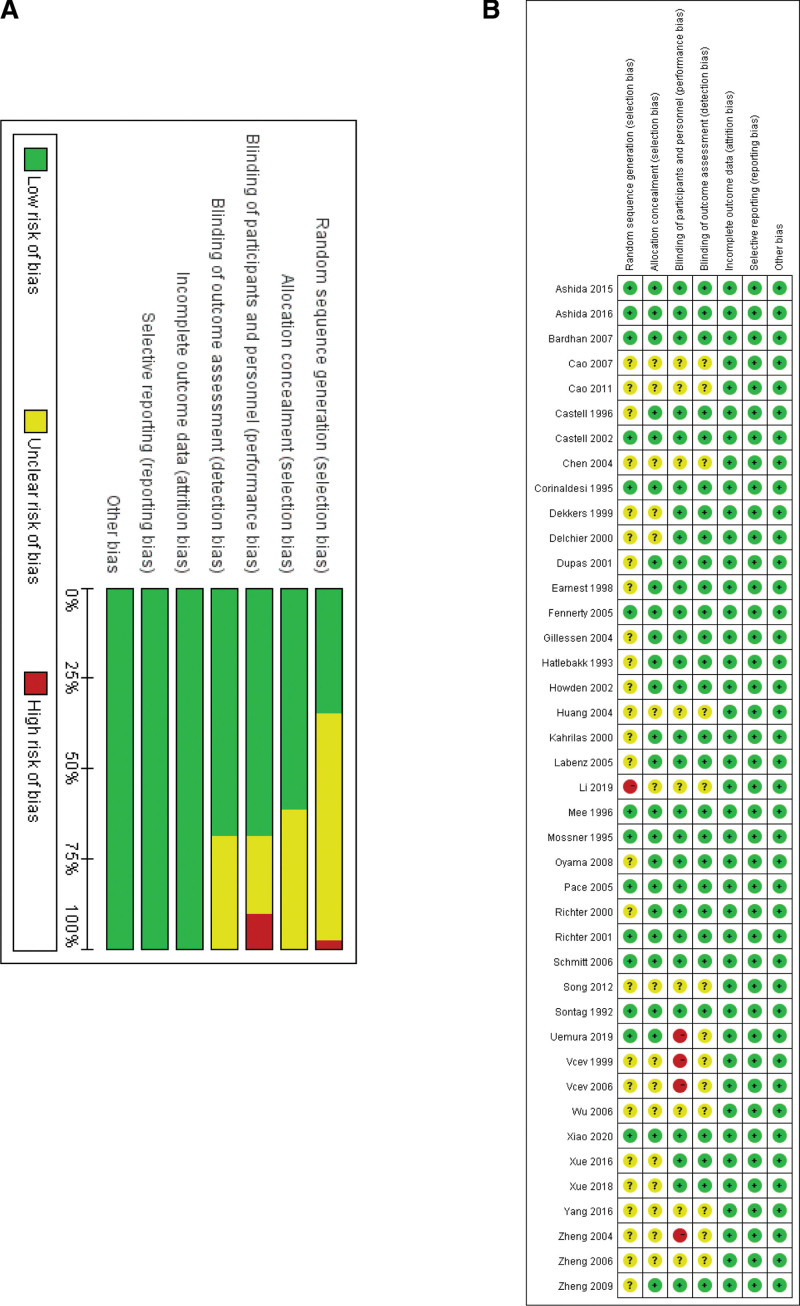
Figure 1 presented the election process, the systematic literature search identified 11,592 potential studies from the databases, and we imported citations into EndNoteX9. After excluding duplicates, we screened 6848 records by reading all titles and abstracts based on the selection criteria. And then, we reviewed full texts of 288 potentially relevant articles. Forty-one randomized controlled trials ultimately were included with 11,592 enrolled patients.[21–61] The characteristics of the included studies were shown in Table S2 (Supplemental Digital Content, http://links.lww.com/MD/H939, which presents the characteristics of included studies). As shown in Figure 2, only 14 studies have a low risk of bias in random sequence generation.[21,23,24,26,34,35,41,43,44,46,54,55,59,61] Twenty-five studies have used appropriate allocation concealment.[21–27,31–37,41–44,46,50,51,54,55,59,61] Twenty-eight studies have a low risk of blinding of participants, personnel and outcome assessment.[21–37,41–44,46,50,51,54–56,58,61] All 41 studies have complete data and none selectively reported the findings, and it was unclear whether other sources of bias existed.[21–61]
Figure 1.
Flow chart of study selection.
Figure 2.
Risk of bias graph and summary. Green for low risk of bias (+), yellow for unclear risk of bias (?) and red for high risk of bias (-).
3.2. Rate of healing at 4 weeks
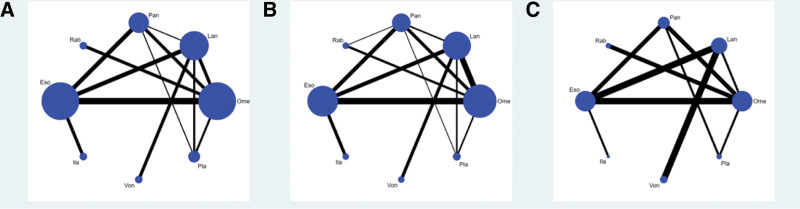
Thirty-three studies have reported the endoscopic cure rate at 4 weeks, and 8 interventions were involved, including omeprazole, lansoprazole, pantoprazole, rabeprazole, esomeprazole, ilaprazole, and vonoprazan. The network plot was shown in Figure 3.
Figure 3.
Network plots. Network plots for healing rates at 4 and 8 wk (A and B), network plot for adverse event rate (C). Ome, 20 mg/d; Pan, 40 mg/d; Lan, 30 mg/d; Rab, 20 mg/d; Ila, 10 mg/d; Eso, 40 mg/d; Von, 20 mg/d. Eso = esomeprazole, Ila = ilaprazole, Lan = lansoprazole, Ome = omeprazole, Pan = pantoprazole, PLA = placebo, Rab = rabeprazole, Von = vonoprazan.
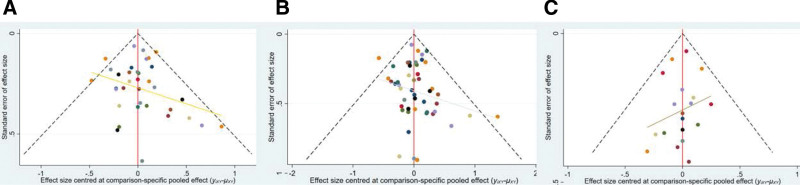
The area of nodes indicates the number of studies included in the corresponding nodes, and the width of the lines connecting nodes suggested the number of relevant data. No inconsistency was detected through global (P = .7526) and loop-specific approach in the endoscopic cure rate at 4 weeks (Table 1). The results of the network meta-analysis (NMA) indicated that in terms of the 4-week endoscopic cure rate (Table 2): PPIs and vonoprazan significantly improved the 4-week healing rate compared to placebo. The cure rate of omeprazole was lower than esesomeprazole (odds ratio [OR] = 0.68, 95% CI = 0.59–0.79) and ilaprazole (OR = 0.72, 95% CI = 0.61–0.86), lansoprazole was lower than esomeprazole (OR = 0.72, 95% CI = 0.61–0.86), Pantoprazole was lower than esomeprazole (OR = 0.78, 95% CI = 0.65–0.94), Rabeprazole was lower than esomeprazole (OR = 0.61, 95% CI = 0.41–0.91). Table 3 presented the ranking of all interventions for the endoscopic cure rate at 4 weeks based on the SUCRA. Ilaprazole ranked first followed by esomeprazole, vonoprazan, pantoprazole, lansoprazole, omeprazole, rabeprazole and placebo therapy ranked the last. The funnel plot was indicated in Figure 4.
Table 1.
Inconsistency factors of all comparisons.
| Outcome | Cycle | IF and 95% CI | P value |
|---|---|---|---|
| 4 wk healing rates | Ome-Pan-Pla | IF = 0.637, 95% CI (0.00, 1.51) | .153 |
| Ome-Lan-Pan | IF = 0.404, 95% CI (0.00, 1.01) | .190 | |
| Lan-Pan-Pla | IF = 0.383, 95% CI (0.00, 1.29) | .410 | |
| Lan-Pan-Eso | IF = 0.302, 95% CI (0.00, 0.82) | .250 | |
| Ome-Pan-Eso | IF = 0.213, 95% CI (0.00, 0.77) | .455 | |
| Ome-Lan-Pla | IF = 0.194, 95% CI (0.00, 0.78) | .518 | |
| Ome-Lan-Eso | IF = 0.086, 95% CI (0.00, 0.58) | .736 | |
| 8 wk healing rates | Ome-Pan-Rab | IF = 1.017, 95% CI (0.00, 2.85) | .277 |
| Ome-Pan-Eso | IF = 0.541, 95% CI (0.00, 1.23) | .124 | |
| Ome-Lan-Eso | IF = 0.417, 95% CI (0.00, 0.88) | .077 | |
| Ome-Lan-Pan | IF = 0.404, 95% CI (0.00, 1.07) | .237 | |
| Lan-Pan-Eso | IF = 0.303, 95% CI (0.00, 0.95) | .356 | |
| Ome-Pan-Pla | IF = 0.117, 95% CI (0.00, 0.96) | .785 | |
| Lan-Pan-Pla | IF = 0.104, 95% CI (0.00, 1.00) | .820 | |
| Ome-Lan-Pla | IF = 0.224, 95% CI (0.00, 0.80) | .448 | |
| Adverse event rates | Ome-Pan-Von | IF = 0.414, 95% CI (0.00, 1.46) | .437 |
| Ome-Pan-Eso | IF = 0.387, 95% CI (0.00, 1.12) | .302 | |
| Ome-Lan-Eso | IF = 0.173, 95% CI (0.00, 0.83) | .608 |
CI = confidence interval; Eso = esomeprazole, 40 mg/d; IF = inconsistency factor; Ila = ilaprazole, 10 mg/d; Lan = lansoprazole, 30 mg/d; Ome = omeprazole, 20 mg/d; Pan = pantoprazole, 40 mg/d; PLA = placebo; Rab = rabeprazole, 20 mg/d; Von = vonoprazan 20 mg/d.
Table 2.
Results of network meta-analyses for the rate of healing at 4 and 8 wk.
| OR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
| Ome | 1.17 (0.94, 1.45) | 1.31 (1.01, 1.69)* | 0.84 (0.54, 1.32) | 1.56 (1.29, 1.89)* | 1.73 (1.11, 2.71)* | 1.52 (0.91, 2.55) | 0.09 (0.06, 0.13)* |
| 0.94 (0.78, 1.14) | Lan | 1.12 (0.84, 1.48) | 0.72 (0.44, 1.19) | 1.34 (1.08, 1.66)* | 1.48 (0.94, 2.34) | 1.30 (0.81, 2.09) | 0.08 (0.05, 0.11)* |
| 0.87 (0.71, 1.08) | 0.93 (0.74, 1.17) | Pan | 0.64 (0.39, 1.07) | 1.20 (0.94, 1.51) | 1.33 (0.83, 2.12) | 1.16 (0.67, 2.01) | 0.07 (0.05, 0.10)* |
| 1.13 (0.78, 1.64) | 1.19 (0.79, 1.81) | 1.29 (0.84, 1.97) | Rab | 1.86 (1.14, 3.02)* | 2.06 (1.10, 3.87)* | 1.81 (0.91, 3.58) | 0.11 (0.06, 0.19)* |
| 0.68 (0.59, 0.79)* | 0.72 (0.61, 0.86)* | 0.78 (0.65, 0.94)* | 0.61 (0.41, 0.91)* | Eso | 1.11 (0.74, 1.66) | 0.97 (0.58, 1.63) | 0.06 (0.04, 0.08)* |
| 0.63 (0.42, 0.94)* | 0.67 (0.44, 1.01) | 0.72 (0.48, 1.09) | 0.56 (0.32, 0.97)* | 0.92 (0.64, 1.34) | Ila | 0.88 (0.46, 1.69) | 0.05 (0.03, 0.09)* |
| 0.74 (0.48, 1.14) | 0.78 (0.53, 1.16) | 0.84 (0.53, 1.33) | 0.65 (0.37, 1.16) | 1.08 (0.70, 1.66) | 1.17 (0.66, 2.06) | Von | 0.06 (0.03, 0.11)* |
| 9.33 (6.65, 13.11)* | 9.90 (7.07, 13.86)* | 10.67 (7.42, 15.33)* | 8.29 (5.01, 13.72)* | 13.66 (9.68, 19.29)* | 14.78 (8.89, 24.56)* | 12.67 (7.55, 21.29)* | Pla |
Results of 4 wk healing rates were listed in right upper triangles and results of 8 wk healing rates were listed in left lower triangles.
CI = confidence interval; Eso = esomeprazole, 40 mg/d; Ila = ilaprazole, 10 mg/d; Lan = lansoprazole, 30 mg/d; Ome = omeprazole, 20 mg/d; OR = odds ratio; Pan = pantoprazole, 40 mg/d; PLA = placebo; Rab = rabeprazole, 20 mg/d; Von = vonoprazan 20 mg/d.
Significant difference between two treatments.
Table 3.
The results of SUCRA.
| Treatment | 4 wk healing rates | 8 wk healing rates | Adverse event rate | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SUCRA | Pr. Best | MeanRank | Pr.Best | MeanRank | SUCRA | Pr. Best | MeanRank | ||
| Ome | 31.6 | 0.0 | 5.8 | 27.7 | 0.0 | 6.1 | 65.9 | 12.1 | 3.4 |
| Lan | 41.7 | 0.0 | 5.1 | 45.7 | 0.0 | 4.8 | 57.4 | 7.3 | 4.0 |
| Pan | 54.3 | 0.1 | 4.2 | 59.9 | 1.6 | 3.8 | 47.5 | 10.2 | 4.7 |
| Rab | 24.4 | 0.3 | 6.3 | 20.5 | 0.2 | 6.6 | 54.3 | 26.2 | 4.2 |
| Eso | 85.3 | 21.4 | 2.0 | 82.2 | 14.3 | 2.2 | 57.9 | 3.7 | 3.9 |
| Ila | 88.8 | 55.1 | 1.8 | 88.1 | 53.9 | 1.8 | 45.2 | 24.4 | 4.8 |
| Von | 73.9 | 23.2 | 2.8 | 75.9 | 30.0 | 2.7 | 41.0 | 7.4 | 5.1 |
| Pla | 0.0 | 0.0 | 8.0 | 0.0 | 0.0 | 8.0 | 30.7 | 8.8 | 5.8 |
Eso = esomeprazole, 40 mg/d; Ila = ilaprazole, 10 mg/d; Lan = lansoprazole, 30 mg/d; Ome = omeprazole, 20 mg/d; Pan = pantoprazole, 40 mg/d; PLA = placebo; Pr. Best = probability of being the best; Rab = rabeprazole, 20 mg/d; SUCRA = the surface under the cumulative ranking curve; Von = vonoprazan 20 mg/d.
Figure 4.
Funnel plots. Funnel plots for healing rates at 4 and 8 wk (A and B), funnel plot for adverse event rate (C).
3.3. Rate of healing at 8 weeks
Forty-one studies have reported the endoscopic cure rate at 8 weeks. No inconsistency was detected through global (P = .4973) and loop-specific approach (Table 1).
The results of the NMA indicated that in terms of 8-week endoscopic cur-e rate (Table 2): pantoprazole is higher than omeprazole (OR = 1.31, 95% CI = 1.01–1.69), esomeprazole was higher than omeprazole (OR = 1.56, 95% CI = 1.29–1.89), lansoprazole (OR = 1.34, 95% CI = 1.08–1.66), and rebeprazole (OR = 1.86, 95% CI = 1.14–3.02). Ilaprazole was higher than omeprazole (OR = 1.73, 95% CI = 1.11–2.71) and rabeprazole (OR = 2.06, 95% CI = 1.10–3.87). The 8-week endoscopic cure rat-e of PPIs and vonoprazan were significantly higher than the placebo.
Table 3 presented the ranking of all interventions for the endoscopic cure rate at 8 weeks based on the SUCRA. Ilaprazole ranked first followed by esomeprazole, vonoprazan, pantoprazole, lansoprazole, omeprazole, rabeprazole and placebo therapy ranked the last. The funnel plot was indicated in Figure 4.
3.4. Rate of adverse events
Adverse event rates were reported in 20 studies involving all 8 interventions, forming 5 loops. No inconsistency was detected through global (P = .1909) approach. However, the result of loop-specific approach indicated that cyclo lansoprazole- pantoprazole-esomeprazole presented inconsistency (Table S3, Supplemental Digital Content, http://links.lww.com/MD/H940, which presents the results of the loop-specific approach to adverse event rate). Therefore, we tested for inconsistency based on the node-splitting method (Table S4, Supplemental Digital Content, http://links.lww.com/MD/H941, which presents the results of node-splitting method on adverse event rate). The results showed that the inconsistency might come from the study by Dupas et al,[33] which was the only head-to-head study of lansoprazole and pantoprazole reporting adverse event rates. There may be a small sample effect, so this study was excluded. The remaining 19 literature were analyzed, and the evidence relationship diagram was shown in Figure 3. No inconsistency was detected through global (P = .6737) and loop-specific approach (Table 1). As shown in Table 4, there was no significant difference in the incidence of adverse events among all the PPIs, vonoprazan, and placebo. The results of SUCRA (Table 3) indicated that the relative ranking safety was: omeprazole ranked first followed by esomeprazole, lansoprazole, rabeprazole, pantoprazole, ilaprazole, vonoprazan and placebo. The funnel plot was indicated in Figure 4.
Table 4.
Results of network meta-analyses for adverse event rate.
| OR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
| Ome | |||||||
| 0.97 (0.78, 1.21) | Lan | ||||||
| 0.93 (0.68, 1.28) | 0.96 (0.69, 1.34) | Pan | |||||
| 0.97 (0.61, 1.52) | 0.99 (0.60, 1.64) | 1.04 (0.60, 1.80) | Rab | ||||
| 0.97 (0.83, 1.14) | 1.00 (0.87, 1.16) | 1.05 (0.77, 1.41) | 1.01 (0.62, 1.63) | Eso | |||
| 0.90 (0.46, 1.76) | 0.93 (0.48, 1.80) | 0.97 (0.47, 1.98) | 0.93 (0.41, 2.09) | 0.92 (0.48, 1.77) | Ila | ||
| 0.91 (0.65, 1.27) | 0.93 (0.72, 1.21) | 0.98 (0.64, 1.49) | 0.94 (0.53, 1.66) | 0.93 (0.69, 1.26) | 1.01 (0.49, 2.06) | Von | |
| 0.82 (0.51, 1.32) | 0.84 (0.51, 1.40) | 0.88 (0.56, 1.38) | 0.85 (0.44, 1.64) | 0.84 (0.52, 1.36) | 0.91 (0.41, 2.05) | 0.90 (0.51, 1.59) | Pla |
CI = confidence interval; Eso = esomeprazole, 40 mg/d; Ila = ilaprazole, 10 mg/d; Lan = lansoprazole, 30 mg/d; Ome = omeprazole, 20 mg/d; OR = odds ratio; Pan = pantoprazole, 40 mg/d; PLA = placebo; Rab = rabeprazole, 20 mg/d; Von = vonoprazan 20 mg/d.
3.5. Sensitivity analysis
The results of sensitivity analyses based on data from studies with a low risk of bias, indicating the robustness of the results (Tables S5 and S6, Supplemental Digital Content, http://links.lww.com/MD/H942, which present the sensitivity analyses results of subgroup analysis of primary outcomes).
4. Discussion
In this study, we performed a NMA to evaluate the comparative efficacy and safety of PPIs and vonoprazan for treating EE. Moreover, we have ranked order based on efficacy and safety.
The results indicated that all the PPIs and vonoprazan significantly improved the endoscopic cure rate compared to placebo, endoscopic cure rates were higher at 8 weeks than 4 weeks for all interventions. Notably, in terms of endoscopic cure rates at 4 and 8 weeks, the NMA results showed some statistical differences between partial interventions, but frequently the 95% CI are relatively close to the 1. For example, statistically significant results were observed for pantoprazole compared with omeprazole in 8-week healing rate, but the effect was almost borderline. We should be cautious in interpreting the results and avoid exaggerating intervention differences.
According to SUCRA rank results, ilaprazole, esomeprazole, and vonoprazan might have more advantages in mucosal erosion healing. The advantages could be interpreted by its property of acid control.[62] Ilaprazole, esomeprazole and vonoprazan produced relatively good effects on acid suppression and maintained PH > 4 for a relatively long time, which could promote mucosal healing.[63–66]
Ilaprazole is a new drug approved in China which was not included in previous studies. The results of our study are consistent with a NMA of 25 articles by Li et al,[62] showed that standard-dose esomeprazole was superior in mucosal erosion healing to other PPIs. Miyazaki’s analysis indicated that the GERD-healing effect of vonoprazan is higher than rabeprazole but not higher than other PPIs,[67] which was inconsistent with our study. On the 1 hand, this could be attributed to the dose of esomeprazole in their studies was 20 mg/d. On the other hand, the included participants in their studies were GERD, which involved non-EE.
For the rate of adverse events, our results were totally the same as the previous studies,[62,68] there was no significant difference among all interventions.
There are several limitations to this study. Firstly, literature-based meta-analyses include heterogeneity and bias based on each study. Therefore, we performed sensitivity analyses to examine the robustness of the results by excluding studies with a high risk of bias. Secondly, the outcomes of the majority include studies have not been reported according to severity grading under endoscopy, and the grading systems differed among studies so that we can not assess the efficacy for patients with different severity of EE. Finally, most of the included studies have not reported the results of patients with CYP2C19 genotype and Helicobacter pylori infection, which meant we could not evaluate the impact of genetic polymorphism and Helicobacter pylori infection on drug therapy for EE.
Therefore, further high-quality, large-sample, and comprehensive clinical trials of vonoprazan and PPIs are required to confirm the efficacy and safety of the treatment of EE.
5. Conclusion
We have conducted NMA to analysis the efficacy and safety of vonoprazan and PPIs in EE. The results suggested that ilaprazole, esomeprazole, and vonoprazan have more advantages compared with other PPIs in mucosal erosion healing, while the safety of vonoprazan was similar to PPIs. To further confirm our conclusions, high-quality, large-sample and multi-center studies are required to provide more evidence.
Author contributions
All authors contributed to the study conception and design. Project development: Jisheng Chen and Sensen Yang. Data collection or management: Sensen Yang and Weishang Deng. Data analysis and interpretation: Sensen Yang and Zeyu Xie. Manuscript writing: Sensen Yang and Weishang Deng. Manuscript editing: Jisheng Chen.
Data curation: Sensen Yang.
Investigation: Weishang Deng, Jisheng Chen.
Methodology: Sensen Yang.
Resources: Weishang Deng.
Software: Sensen Yang.
Validation: Zeyu Xie.
Visualization: Zeyu Xie.
Writing – original draft: Sensen Yang, Jisheng Chen.
Writing – review & editing: Jisheng Chen.
Supplementary Material
Abbreviations:
- CI =
- confidence interval
- EE =
- erosive esophagitis
- GERD =
- gastroesophageal reflux disease
- NMA =
- network meta-analysis
- OR =
- odds ratio
- PPIs =
- proton-pump inhibitors
- SUCRA =
- surface under the cumulative ranking curve
This study was supported by the National Key Specialty Construction Project (Clinical Pharmacy) and the High-level Clinical Key Specialty of Guangdong Province, and the funders were the central finance subsidy fund for the improvement of medical services and guarantee capacity, code Z155080000004; the Guangzhou Minsheng Science and Technology Research Program Project, code 201803010096.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental Digital Content is available for this article.
How to cite this article: Yang S, Deng W, Xie Z, Chen J. Efficacy and safety of proton pump inhibitors versus vonoprazan in treatment of erosive esophagitis: A PRISMA-compliant systematic review and network meta-analysis. Medicine 2022;101:47(e31807).
Contributor Information
Sensen Yang, Email: 875003716@qq.com.
Weishang Deng, Email: davidson95@qq.com.
Zeyu Xie, Email: 578365488@qq.com.
References
- [1].Chinese Society of Gastroenterology, Chinese Medical Association. Chinese expert consensus of gastroesophageal reflux disease in China in 2020. Chin J Dig. 2020;40:649–63. [Google Scholar]
- [2].Dunbar KB, Agoston AT, Odze RD, et al. Association of acute gastroesophageal reflux disease with esophageal histologic changes. JAMA. 2016;315:2104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut. 2018;67:1351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fass R, Boeckxstaens GE, El-Serag H, et al. Gastro-oesophageal reflux disease. Nat Rev Dis Primers. 2021;7:55. [DOI] [PubMed] [Google Scholar]
- [5].Iwakiri K, Fujiwara Y, Manabe N, et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2021. J Gastroenterol. 2022;57:267–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jung HK, Tae CH, Song KH, et al. 2020 Seoul consensus on the diagnosis and management of gastroesophageal reflux disease. J Neurogastroenterol Motil. 2021;27:453–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hunt R, Armstrong D, Katelaris P, et al. World Gastroenterology Organisation Global Guidelines: GERD Global Perspective on Gastroesophageal reflux disease. J Clin Gastroenterol. 2017;51:467–78. [DOI] [PubMed] [Google Scholar]
- [8].Katz PO, Dunbar KB, Schnoll-Sussman FH, et al. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2022;117:27–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Oshima T, Igarashi A, Nakano H, et al. Network meta-analysis comparing vonoprazan and proton pump inhibitors for heartburn symptoms in erosive esophagitis. J Clin Gastroenterol. 2022;56:493–504. [DOI] [PubMed] [Google Scholar]
- [10].Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3. [updated February 2022]. Cochrane, 2022. Available at: www.training.cochrane.org/handbook. [access date May 31, 2022]. [Google Scholar]
- [11].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shim S, Yoon BH, Shin IS, et al. Network meta-analysis: application and practice using Stata. Epidemiol Health. 2017;39:e2017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Baker SG, Kramer BS. The transitive fallacy for randomized trials: if A bests B and B bests C in separate trials, is A better than C? [published correction appears in BMC Med Res Methodol. 2003 Oct 29;3(1):23]. BMC Med Res Methodol. 2002;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen L, Chen Y, Li B. The efficacy and safety of proton-pump inhibitors in treating patients with non-erosive reflux disease: a network meta-analysis. Sci Rep. 2016;6:32126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Higgins JP, Jackson D, Barrett JK, et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3:98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].White IR, Barrett JK, Jackson D, et al. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3:111–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Higgins JPT, Jackson D, Barrett JL, et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3:98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].White IR. Multivariate random-effects meta-analysis. Stata J. 2009;9:40–56. [Google Scholar]
- [19].Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One. 6654;8:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang J, Ge L, Hill M, et al. Standard-dose proton pump inhibitors in the initial non-eradication treatment of duodenal ulcer: systematic review, network meta-analysis, and cost-effectiveness analysis. Front Pharmacol. 2019;9:1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sontag SJ, Hirschowitz BI, Holt S, et al. Two doses of omeprazole versus placebo in symptomatic erosive esophagitis: the U.S. Multicenter Study. Gastroenterology. 1992;102:109–18. [DOI] [PubMed] [Google Scholar]
- [22].Hatlebakk JG, Berstad A, Carling L, et al. Lansoprazole versus omeprazole in short-term treatment of reflux oesophagitis. Results of a Scandinavian multicentre trial. Scand J Gastroenterol. 1993;28:224–8. [DOI] [PubMed] [Google Scholar]
- [23].Corinaldesi R, Valentini M, Belaïche J, et al. Pantoprazole and omeprazole in the treatment of reflux oesophagitis: a European multicentre study. Aliment Pharmacol Ther. 1995;9:667–71. [DOI] [PubMed] [Google Scholar]
- [24].Mössner J, Hölscher AH, Herz R, et al. A double-blind study of pantoprazole and omeprazole in the treatment of reflux oesophagitis: a multicentre trial. Aliment Pharmacol Ther. 1995;9:321–6. [DOI] [PubMed] [Google Scholar]
- [25].Castell DO, Richter JE, Robinson M, et al. Efficacy and safety of lansoprazole in the treatment of erosive reflux esophagitis. The Lansoprazole Group. Am J Gastroenterol. 1996;91:1749–57. [PubMed] [Google Scholar]
- [26].Mee AS, Rowley JL. Rapid symptom relief in reflux oesophagitis: a comparison of lansoprazole and omeprazole. Aliment Pharmacol Ther. 1996;10:757–63. [DOI] [PubMed] [Google Scholar]
- [27].Earnest DL, Dorsch E, Jones J, et al. A placebo-controlled dose-ranging study of lansoprazole in the management of reflux esophagitis. Am J Gastroenterol. 1998;93:238–43. [DOI] [PubMed] [Google Scholar]
- [28].Dekkers CP, Beker JA, Thjodleifsson B, et al. Double-blind comparison [correction of Double-blind, placebo-controlled comparison] of rabeprazole 20 mg vs. omeprazole 20 mg in the treatment of erosive or ulcerative gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 1999;13:49–57. [DOI] [PubMed] [Google Scholar]
- [29].Vcev A, Stimac D, Vceva A, et al. Pantoprazole versus omeprazole in the treatment of reflux esophagitis. Acta Med Croatica. 1999;53:79–82. [PubMed] [Google Scholar]
- [30].Delchier JC, Cohen G, Humphries TJ. Rabeprazole, 20 mg once daily or 10 mg twice daily, is equivalent to omeprazole, 20 mg once daily, in the healing of erosive gastrooesophageal reflux disease. Scand J Gastroenterol. 2000;35:1245–50. [DOI] [PubMed] [Google Scholar]
- [31].Kahrilas PJ, Falk GW, Johnson DA, et al. Esomeprazole improves healing and symptom resolution as compared with omeprazole in reflux oesophagitis patients: a randomized controlled trial. The Esomeprazole Study Investigators. Aliment Pharmacol Ther. 2000;14:1249–58. [DOI] [PubMed] [Google Scholar]
- [32].Richter JE, Bochenek W. Oral pantoprazole for erosive esophagitis: a placebo-controlled, randomized clinical trial. Pantoprazole US GERD Study Group. Am J Gastroenterol. 2000;95:3071–80. [DOI] [PubMed] [Google Scholar]
- [33].Dupas JL, Houcke P, Samoyeau R, et al. Pantoprazole versus lansoprazole in French patients with reflux esophagitis. Gastroenterol Clin Biol. 2001;25:245–50. [PubMed] [Google Scholar]
- [34].Richter JE, Kahrilas PJ, Johanson J, et al. Efficacy and safety of esomeprazole compared with omeprazole in GERD patients with erosive esophagitis: a randomized controlled trial. Am J Gastroenterol. 2001;96:656–65. [DOI] [PubMed] [Google Scholar]
- [35].Castell DO, Kahrilas PJ, Richter JE, et al. Esomeprazole (40 mg) compared with lansoprazole (30 mg) in the treatment of erosive esophagitis. Am J Gastroenterol. 2002;97:575–83. [DOI] [PubMed] [Google Scholar]
- [36].Howden CW, Ballard ED, 2nd, Robieson W. Evidence for therapeutic equivalence of lansoprazole 30mg and esomeprazole 40mg in the treatment of erosive oesophagitis. Clin Drug Investig. 2002;22:99–109. [DOI] [PubMed] [Google Scholar]
- [37].Gillessen A, Beil W, Modlin IM, et al. 40 mg pantoprazole and 40 mg esomeprazole are equivalent in the healing of esophageal lesions and relief from gastroesophageal reflux disease-related symptoms. J Clin Gastroenterol. 2004;38:332–40. [DOI] [PubMed] [Google Scholar]
- [38].Chen Y, Xu W, Huang W. Comparison of the efficacy of esomeprazole and omeprazole in the treatment of reflux esophagitis. Zhejiang Clin Med. 2004;12:1064–5. [Google Scholar]
- [39].Huang P, Lu H. Comparison of the efficacy of lansoprazole and omeprazole on reflux esophagitis. J Math Med. 2004;2:131–2. [Google Scholar]
- [40].Zheng Y, Zhang D, Hou X, et al. Comparison of curative effect between rabeprazole and gentorazole in the treatment of reflux esophagitis. Mod Prev Med. 2004;5:695–6. [Google Scholar]
- [41].Fennerty MB, Johanson JF, Hwang C, et al. Efficacy of esomeprazole 40 mg vs. lansoprazole 30 mg for healing moderate to severe erosive oesophagitis. Aliment Pharmacol Ther. 2005;21:455–63. [DOI] [PubMed] [Google Scholar]
- [42].Labenz J, Armstrong D, Lauritsen K, et al. A randomized comparative study of esomeprazole 40 mg versus pantoprazole 40 mg for healing erosive oesophagitis: the EXPO study. Aliment Pharmacol Ther. 2005;21:739–46. [DOI] [PubMed] [Google Scholar]
- [43].Pace F, Annese V, Prada A, et al. Rabeprazole is equivalent to omeprazole in the treatment of erosive gastro-oesophageal reflux disease. A randomised, double-blind, comparative study of rabeprazole and omeprazole 20 mg in acute treatment of reflux oesophagitis, followed by a maintenance open-label, low-dose therapy with rabeprazole. Dig Liver Dis. 2005;37:741–50. [DOI] [PubMed] [Google Scholar]
- [44].Schmitt C, Lightdale CJ, Hwang C, et al. A multicenter, randomized, double-blind, 8-week comparative trial of standard doses of esomeprazole (40 mg) and omeprazole (20 mg) for the treatment of erosive esophagitis. Dig Dis Sci. 2006;51:844–50. [DOI] [PubMed] [Google Scholar]
- [45].Vcev A, Begić I, Ostojić R, et al. Esomeprazole versus pantoprazole for healing erosive oesophagitis. Coll Antropol. 2006;30:519–22. [PubMed] [Google Scholar]
- [46].Bardhan KD, Achim A, Riddermann T, et al. A clinical trial comparing pantoprazole and esomeprazole to explore the concept of achieving “complete remission” in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2007;25:1461–9. [DOI] [PubMed] [Google Scholar]
- [47].Wu X, Zhai S, Zhang G. Clinical observation of lansoprazole in the treatment of reflux esophagitis. Guangxi Med. 2006;5:689–90. [Google Scholar]
- [48].Zheng X, Chen R. Clinical comparative observation of esomeprazole in the treatment of reflux esophagitis. Huaxia Med. 2006;3:447–8. [Google Scholar]
- [49].Cao Y, Chen Y. Observation on the curative effect of esomeprazole in the treatment of reflux esophagitis. J Gastroenterol Hepatol. 2007;3:208–12. [Google Scholar]
- [50].Reflux Esophagitis Phase III Study (Initial Treatment)[EB/OL]. 2013. Available at: https://clinicaltrials.gov/show/NCT00633932 [access date January 31, 2022].
- [51].Zheng RN. Comparative study of omeprazole, lansoprazole, pantoprazole and esomeprazole for symptom relief in patients with reflux esophagitis. World J Gastroenterol. 2009;15:990–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cao G. Comparison of the efficacy of esomeprazole and omeprazole in the treatment of reflux esophagitis. Chin Commun Phys (Medicine). 2011;13:46. [Google Scholar]
- [53].Song F, Hui H, Shen L, et al. Efficacy and safety of ilaprazole in the treatment of 180 cases of reflux esophagitis. Chin J New Drugs. 2012;21:905–7. [Google Scholar]
- [54].Ashida K, Sakurai Y, Nishimura A, et al. Randomised clinical trial: a dose-ranging study of vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the treatment of erosive oesophagitis. Aliment Pharmacol Ther. 2015;42:685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ashida K, Sakurai Y, Hori T, et al. Randomised clinical trial: vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment Pharmacol Ther. 2016;43:240–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Xue Y, Qin X, Zhou L, et al. A Randomized, double-blind, active-controlled, multi-center study of ilaprazole in the treatment of reflux esophagitis. Clin Drug Investig. 2016;36:985–92. [DOI] [PubMed] [Google Scholar]
- [57].Yang H. Analysis of the clinical efficacy of lansoprazole in the treatment of patients with reflux esophagitis. World Latest Med Inf Digest. 2016;16:5–6. [Google Scholar]
- [58].Xue Y, Qin X, Zhou L, et al. A randomized, double blind, controlled, multi center study of Ilaparazole in the treatment of reflux esophagitis-Phase III clinical trial. Contemp Clin Trials. 2018;68:67–71. [DOI] [PubMed] [Google Scholar]
- [59].Uemura N, Kinoshita Y, Haruma K, et al. 1-year interim analysis results of vision trial: a randomized, open-label study to evaluate a long-term safety of vonoprazan as maintenance treatment in patients with erosive esophagitis. Gastroenterology. 2019;156:S-302–3. [Google Scholar]
- [60].Biqian L, Chen Y. Analysis of the clinical value of ilaprazole in the treatment of reflux esophagitis. Psychol Monthly. 2019;14:183. [Google Scholar]
- [61].Xiao Y, Zhang S, Dai N, et al. Phase III, randomised, double-blind, multicentre study to evaluate the efficacy and safety of vonoprazan compared with lansoprazole in Asian patients with erosive oesophagitis. Gut. 2020;69:224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Li MJ, Li Q, Sun M, et al. Comparative effectiveness and acceptability of the FDA-licensed proton pump inhibitors for erosive esophagitis: a PRISMA-compliant network meta-analysis. Medicine (Baltimore). 2017;96:e8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shin JS, Lee JY, Cho KH, et al. The pharmacokinetics, pharmacodynamics and safety of oral doses of ilaprazole 10, 20 and 40 mg and esomeprazole 40 mg in healthy subjects: a randomised, open-label crossover study. Aliment Pharmacol Ther. 2014;40:548–61. [DOI] [PubMed] [Google Scholar]
- [64].Wang H, Shao F, Liu X, et al. Efficacy, safety and pharmacokinetics of ilaprazole infusion in healthy subjects and patients with esomeprazole as positive control. Br J Clin Pharmacol. 2019;85:2547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Rohss K, Lind T, Wilder-Smith C. Esomeprazole 40 mg provides more effective intragastric acid control than lansoprazole 30 mg, omeprazole 20 mg, pantoprazole 40 mg and rabeprazole 20 mg in patients with gastro-oesophageal reflflux symptoms. Eur J Clin Pharmacol. 2004;60:531–9. [DOI] [PubMed] [Google Scholar]
- [66].Sakurai Y, Mori Y, Okamoto H, et al. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects--a randomised open-label cross-over study. Aliment Pharmacol Ther. 2015;42:719–30. [DOI] [PubMed] [Google Scholar]
- [67].Miyazaki H, Igarashi A, Takeuchi T, et al. Vonoprazan versus proton-pump inhibitors for healing gastroesophageal reflux disease: a systematic review. J Gastroenterol Hepatol. 2019;34:1316–28. [DOI] [PubMed] [Google Scholar]
- [68].Cheng Y, Liu J, Tan X, et al. Direct comparison of the efficacy and safety of vonoprazan versus proton-pump inhibitors for gastroesophageal reflux disease: a systematic review and meta-analysis. Dig Dis Sci. 2021;66:19–28. [DOI] [PubMed] [Google Scholar]