Abstract
Multisystem inflammatory syndrome in children (MIS-C) is a new illness that evolved during the COVID-19 pandemic with initial reports of severe disease including use of extracorporeal membrane oxygenation and death. Institutions rapidly assembled task forces to develop treatment algorithms. At the national/international levels, collaboratives and associations assembled consensus writing groups to draft guidelines. These guidelines and algorithms were initially on the basis of expert opinion and small case series. Some groups used the Delphi approach, and the resultant guidelines often mimicked those for other conditions that resembled MIS-C, like Kawasaki disease (KD). For instance, intravenous immunoglobulin (IVIG), a known effective treatment for KD, was recommended for MIS-C. Early in the pandemic many favoured IVIG over steroids as first-line therapy. As evidence evolved so did some guidelines, which now endorse the dual use of IVIG with steroids as first-line therapy. In contrast, withholding immunotherapy became an option for some MIS-C patients with mild symptoms. Herein, we review guidelines and discuss the evidence informing early recommendations, how this has evolved, the role and limitations of expert opinion and observational data, and the importance of leveraging existing research infrastructures, such as the intensive care unit collaborative (Overcoming COVID-19 surveillance registry), and the International Kawasaki Disease Registry. Finally, we discuss strategies to rapidly develop, deploy, and adapt clinical trials evaluating the treatment of such rare conditions in children, which might include alternatives to conventional clinical trial design. The emergence of MIS-C during the COVID-19 pandemic has highlighted unmet needs regarding research of a new condition.
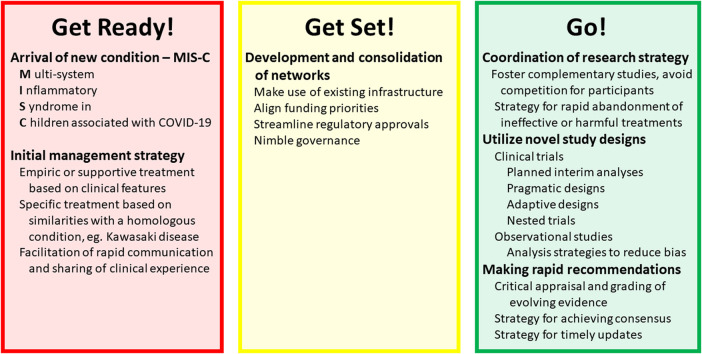
Graphical abstract
Résumé
Le syndrome inflammatoire multisystémique chez les enfants (SIME) est une nouvelle maladie qui s’est manifestée pendant la pandémie de COVID-19. Les premiers rapports faisaient état d’une maladie grave nécessitant parfois l’utilisation de l’oxygénation par membrane extracorporelle, et provoquant parfois la mort. Les établissements ont rapidement mis sur pied des groupes de travail ayant pour but de créer des algorithmes de traitement. À l’échelle nationale et internationale, des collectifs et des associations ont formé des groupes pour rédiger des lignes directrices préliminaires. Ces lignes directrices et ces algorithmes reposaient au départ sur l’avis d’experts et sur de petites séries de cas. Certains groupes ont adopté l’approche Delphi, et les lignes directrices qui en ont résulté étaient souvent très semblables à celles d’autres maladies ressemblant au SIME, comme la maladie de Kawasaki (MK). Par exemple, l’administration d’immunoglobulines par voie intraveineuse (IgIV), un traitement efficace pour la MK, a été recommandée dans les cas de SIME. Au début de la pandémie, nombre de personnes ont favorisé les IgIV plutôt que les stéroïdes comme traitement de première intention. De nouvelles données ont amené les instances à réviser certaines lignes directrices et à préconiser l’utilisation en première intention d’IgIV en association avec des stéroïdes. En contrepartie, l’absence d’immunothérapie est devenue une option pour certains patients atteints du SIME qui présentaient des symptômes légers. Dans la présente publication, nous passons en revue les lignes directrices et discutons des données qui ont servi de base aux premières recommandations; nous examinons leur évolution, les rôles et les limites des avis d’experts et des données observationnelles, et l’importance d’utiliser les infrastructures de recherche existantes, comme la collaboration entre les services de soins intensifs (registre de surveillance Overcoming COVID-19), et l’International Kawasaki Disease Registry (registre international de la maladie de Kawasaki). Enfin, nous discutons des stratégies à utiliser pour élaborer, déployer et adapter rapidement des essais cliniques sur le traitement de ce type de pathologie rare chez les enfants, y compris les solutions de rechange aux protocoles d’essais cliniques conventionnels. L’émergence du SIME pendant la pandémie de COVID-19 a mis en lumière des besoins non satisfaits quant à la recherche sur une nouvelle maladie.
Early experience during the COVID-19 pandemic prompted the prediction that children would be largely spared from the severe disease noted in adults.1 However, several weeks after peak incidences of COVID-19 in Europe, global reports appeared describing a postinfectious inflammatory syndrome in children after exposure to SARS-CoV-2. Children with the syndrome exhibited clinical features overlapping with Kawasaki disease (KD) in addition to a shock-like presentation, often requiring intensive care management. The syndrome has had various names but for the purpose of this review, we will refer to the US Centers for Disease Control and Prevention term, multisystem inflammatory syndrome in children (MIS-C).2, 3, 4, 5, 6 MIS-C also has multiple case definitions with recent call for development of international consensus on diagnostic criteria.7 Initial reports characterized MIS-C as a systemic vasculitis with a propensity for myocardial involvement.8 Emergence of new SARS-CoV-2 variants and the effect of diagnostic and treatment algorithms have modified the clinical presentation. Thus, treatment algorithms developed from various professional societies will require adaptation. We present the readers with a review of the basis for these algorithms and their evolution in response to a changing disease landscape. Finally, we discuss how we can improve decision-making during the initial experience with a new condition, including a review of how clinical trials were expedited, and a discussion of the role of observational data and expert opinion.
Basis for Management Decisions Early in the Pandemic
Faced with the rapid progression of an evolving post-COVID-19 severe illness along with reported cases of mortality and severe morbidity including the need for extracorporeal membrane oxygenation,5 centres across the globe quickly formed task forces to develop institutional clinical practice guidelines and algorithms to help front-line providers diagnose and promptly treat children who present with signs and symptoms suggestive of MIS-C.9 , 10 Within months, treatments were leading to excellent outcomes.11
Initially, in the absence of evidence supporting best treatment practices, treatment decisions were largely influenced by the management of KD, because of the significant overlap noted in presenting signs and symptoms.12 Both conditions are characterized by severe inflammatory disease, triggered by an environmental agent, which in the case of MIS-C is SARS-CoV-2.13 They might both have a genetic predisposition, albeit with different ethnic/race groups disproportionately affected in the 2 processes, and both affect the heart.13 , 14 They might present with similar clinical and laboratory features like fever, conjunctivitis, skin rash, and elevated inflammatory markers.13 Because diagnostic criteria in the United States allow for treatment in the presence of only 24 hours of fever, some treatment algorithms supported the early initiation of intravenous immunoglobulin (IVIG) in the presence of other supporting symptoms.10 KD signs and symptoms can arise at different time points throughout the illness and, thus, the diagnosis might be delayed.15 Thus, considering the approximate 35-year experience with IVIG for the treatment of KD, including the known effectiveness in reducing the incidence of coronary artery aneurysms from 25% to 4%,15 institutional task forces and society guidelines largely supported prompt IVIG therapy as the first-line treatment for MIS-C (Tables 1 and 2 ).16, 17, 18, 19
Table 1.
Evolution of the American College of Rheumatology MIS-C clinical management guidelines with regard to immune modulation therapy
| American College of Rheumatology clinical guidance-version 112 | American College of Rheumatology clinical guidance-version 216 | American College of Rheumatology clinical guidance-version 317 | |
|---|---|---|---|
| Date of online publication | October 3, 2020 | February 15, 2021 | February 3, 2022 |
| Primary immunomodulation therapy | IVIG and/or glucocorticoids If contraindicated then anakinra (I.V. or SC) |
IVIG | IVIG and low- to moderate-dose glucocorticoids |
| Adjunct immunomodulation therapy | Glucocorticoids If contraindicated then anakinra (I.V. or SC) |
Low- to moderate-dose glucocorticoids | See above |
| Indication for adjunct Immunomodulation therapy | N/A | Shock and/or organ-threatening disease | |
| Rescue immunomodulation therapy | Anakinra (I.V. or SC) | Low- to moderate-dose steroids (if not already given) High-dose glucocorticoids Anakinra (I.V. or SC), in patients with MIS-C and features of MAS, or in patients with contraindications to long-term use of glucocorticoids Second dose of IVIG is not recommended |
High-dose glucocorticoids Anakinra (preferred anticytokine therapy) Infliximab (except in patients with features of MAS) Second dose of IVIG is not recommended |
| Indication for rescue immunomodulation therapy | Refractory to IVIG and/or glucocorticoids | Refractory to IVIG and/or low- to moderate-dose glucocorticoids “Persistent fevers and/or ongoing and significant end organ involvement” |
Persistent fevers and/or ongoing and significant end organ involvement |
| Special comment | Immunomodulatory treatment may be withheld in some patients with mild symptoms Patients might require a 2- to 3-week, or even longer, taper of immunomodulatory medications, guided by serial laboratory and cardiac evaluations |
Immunomodulatory treatment may be withheld in some patients with mild symptoms Patients might require a 2- to 3-week, or even longer, taper of immunomodulatory medications, guided by serial laboratory and cardiac evaluations |
Immunomodulatory treatment may be withheld in some patients with mild symptoms Patients might require a 2- to 3-week, or even longer, taper of immunomodulatory medications, guided by serial laboratory and cardiac evaluations |
MIS-C was first described on April 26, 2020.
I.V., intravenous; IVIG, intravenous immunoglobulin; MAS, macrophage activation syndrome; MIS-C, multisystem inflammatory syndrome in children; N/A, not available; SC, subcutaneous.
Table 2.
Comparison of current published society MIS-C clinical management guidelines with regard to immune modulation therapy
| PIMS-TS National Consensus Management Study Group18 | Guidance from the Rheumatology Study Group of the Italian Society of Pediatrics19 | American College of Rheumatology clinical guidance-version 317 | Practice recommendations in Switzerland29 | |
|---|---|---|---|---|
| Date of online publication | September 18, 2020 | February 8, 2021 | May 26, 2021 | |
| Primary immunomodulation therapy | IVIG | IVIG | IVIG and low-moderate dose glucocorticoids | IVIG |
| Adjunct immunomodulation therapy | KD phenotype: High dose glucocorticoids | Glucocorticoids Anakinra |
See above | Glucocorticoids |
| Indication for adjunct immunomodulation therapy | KD phenotype: High-risk children including those younger than 12 months and those with coronary artery changes | Glucocorticoids: in case of heart involvement, severe disease, impending sHLH or toxic shock syndrome Anakinra: in case of severe sHLH or shock with cardiac failure |
||
| Rescue immunomodulation therapy | Second dose of IVIG High-dose glucocorticoids Biological therapy should be considered as a third-line option in children who do not respond to IVIG and glucocorticoids (for those recruited in the RECOVERY trial tocilizumab or standard of care, if not recruited in RECOVERY trial the agent of choice for KD phenotype is infliximab and for nonspecific presentation phenotype, the choice is left to the clinician to choose from the following agents: tocilizumab, anakinra, and infliximab) |
Second dose of IVIG Anakinra |
High-dose glucocorticoids Anakinra (preferred anticytokine therapy) Infliximab (except in patients with features of MAS) Second dose of IVIG is not recommended |
Anakinra Other biologics (tocilizumab, infliximab) Second dose of IVIG |
| Indication for rescue immunomodulation therapy | Indication for second dose of IVIG: not responded or partially responded to the first dose Indication for high-dose glucocorticoids: unwell 24 hours after infusion of IVIG, particularly with ongoing pyrexia |
Persistent disease activity 48 hours after first-line treatment | Persistent fever and/or ongoing and significant end organ involvement | No clinical improvement 24-36 hours after IVIG treatment with persistent fever and/or inflammation |
| Special comment | All children who meet the criteria for the RECOVERY trial should be invited to participate in the first stage of randomization for the trial Management is divided according to phenotype: KD phenotype vs nonspecific presentation phenotype For the nonspecific presentation phenotype, indications for therapy include: evidence of CAA, meeting the criteria for toxic shock syndrome, evidence of progressive disease, and extended duration of fever (> 5 days). In other words those not meeting above criteria can be observed. No guidance was provided on tapering immunomodulatory medications |
Although different doses of steroids were suggested depending on severity and cardiac involvement, no guidance was provided on tapering immunomodulatory medications | Immunomodulatory treatment may be withheld in some patients with mild symptoms Patients might require a 2- to 3-week, or even longer, taper of immunomodulatory medications, guided by serial laboratory and cardiac evaluations |
The guidelines suggest considering immunomodulation therapy (IVIG, prednisolone) in patients who present with undefined inflammatory phenotype (not shock or KD phenotype) In other words, immunomodulatory treatment may be withheld in some patients Slow wean of steroids, taper over 2-6 weeks depending on the clinical course and considering the clinical and biochemical (such as CRP, D-dimer, and ferritin levels) response |
MIS-C was first described on April 26, 2020.
CAA, coronary artery abnormality; CRP, C-reactive protein; IVIG, intravenous immunoglobulin; KD, Kawasaki disease; MAS, macrophage activation syndrome; MIS-C, multisystem inflammatory syndrome in children; PIMS-TS, pediatric inflammatory multisystem syndrome temporally associated with COVID-19; RECOVERY, Randomised Evaluation of COVID-19 Therapy; sHLH, secondary hemophagocytic lymphohistiocytosis.
To further highlight how early management decisions were more on the basis of expert opinion and clinician experience with other diseases, one should consider how recommendations for steroid use in the management of children with MIS-C evolved. MIS-C shows similarities to other inflammatory diseases, including rheumatological conditions such as macrophage activation syndrome.20 Cytopenias, coagulopathy, and elevated ferritin and interleukin levels are shared features for MIS-C and macrophage activation syndrome, albeit to a different degree.20 Accordingly, some centres prioritized the use of steroids alone or in combination with IVIG.21 , 22 In contrast, early in the pandemic some argued against the use of steroids in the management of MIS-C, evoking caution for steroid use in adults with active COVID-19 infection and lack of support for routine use in myocarditis, a common complication of MIS-C.8 , 23, 24, 25, 26 For some centres, anakinra, an interleukin-1 receptor antagonist, was the preferred agent, and was typically used as an adjunct or second-line therapy rather than corticosteroids.8
Current Management Algorithms and How They Evolved During the Pandemic
Now within the third year of the pandemic, clinicians have more familiarity with the presentation and clinical course of MIS-C. The importance of expeditious initiation of immunomodulatory treatment remains a cornerstone in all of the currently published guidelines for treatment. As previously noted in Table 2, most published recommendations include combination therapy of IVIG and steroids as the first-line therapy for MIS-C. Nonetheless, there has been much more practice variation regarding the use of steroids, with 1 early survey showing that only 14% of respondents used steroids for all patients.27 The benefits of steroids were related to their potential effectiveness in treating cytokine storm syndrome, and their added utility for patients with a shock-like presentation. However, there were initial concerns regarding immunosuppression in a potentially septic and bacteremic/viremic patient, as well as the overall concerns regarding adverse effects, including hyperglycemia, hypertension, agitation, hospital-acquired infection, and hip osteonecrosis.28 , 29 The presence and severity of adverse effects also increases with increased duration and dose, necessitating careful consideration of the risk vs benefits of steroid therapy.30 , 31 Other contributing factors were the lack of consensus on the specific indication, dosage, or type of steroids to use, institutional preference and comfort level with using other biologics, and practitioner reluctance to use steroids in the setting of KD translating to reluctance for MIS-C. One of the major turning points for steroid use came about with the publication of a nonrandomized cohort study by Son et al., which showed superior cardiovascular outcomes at hospital discharge in a group treated with IVIG with steroids compared with IVIG alone.32 They also reported that adjunctive therapy was used less frequently in the combination group compared with the single-regimen group, suggestive of a decreased need for escalation of therapy. These findings have been noted in other studies,22 including some studies that have suggested that the use of steroids as monotherapy might be adequate treatment.33 Although current data are compelling, the observational nature of these studies continues to result in practice variation and, at times, conflicting results and overall low level of evidence.34 , 35 Another consideration is the variation in the use of a tapering course of steroids (Table 2). The recommendations for a taper remained relatively broad and nonspecific (ranging from 2 to 6 weeks, depending on clinical response), consistent with the low level of supporting evidence.17, 18, 19 , 29
To address the diagnostic and treatment issues some professional societies convened multidisciplinary task forces to provide guidance on the management of MIS-C primarily on the basis of expert consensus. For instance, the American College of Rheumatology (ACR) task force was composed of 9 pediatric rheumatologists, 2 adult rheumatologists, 2 pediatric cardiologists, 2 pediatric infectious disease specialists, and 1 pediatric critical care physician.12 , 16 , 17 The ACR task force developed consensus using a modified Delphi process on the basis of the principle that forecasts (or decisions) from a structured group of individuals are more accurate than those from unstructured groups. The ACR used 2 rounds of anonymous voting and 2 webinars. The committee applied a 9-point Likert scale to determine each statement’s appropriateness (median scores of 1-3 as inappropriate, 4-6 as uncertain, and 7-9 as appropriate). Consensus was then rated as low, moderate, or high on the basis of vote dispersion along the numeric scale. Approved ACR guidance statements required moderate or high levels of consensus. The ACR statement was intended as a “living document,” which would be modified in response to emerging data.12 , 16 , 17
Although recommendations for first-line immunomodulatory therapy are approaching a relative consensus, choices for adjunct and/or escalation therapy remain highly variable. Some centres used a tiered treatment algorithm on the basis of disease severity at presentation, although there is no consensus for classification of severity, and each centre would develop their own criteria for severity and what warranted adjunctive therapy.36 Published recommendations continue to note use of a wide variety of medications and leave these decisions to the discretion of the treating provider (Table 2). Like KD, data to support a preferred treatment for refractory disease are lacking. In contrast to KD, a second dose of IVIG for MIS-C is not recommended by some (Table 2).37
Evidence-Based Medicine to Support the Currently Used Therapies and Effect of the Management on Clinical Outcomes Including Cardiac Outcomes (Short-term) With No Clinical Trials
As discussed earlier, upon the initial reports of MIS-C in early 2020, there was a lack of evidence and experience in treating this novel condition. Management was on the basis of overlapping clinical features of MIS-C and other inflammatory syndromes, such as KD, and fortunately, patients often responded well to these established treatments.38 , 39 The following summarizes the current evidence, or lack of, since these initial reports with a focus on the 3 tenets of MIS-C management: treatment of shock, immunomodulatory therapies, and thromboprophylaxis.
Shock
Shock is a common presentation among MIS-C patients, with signs of cardiogenic, distributive, or hypovolemic shock. Regardless of the specific cause, patients should be treated with fluid resuscitation. Because of the significant risk of left ventricular dysfunction in patients with MIS-C, clinicians should be aware of potential dysfunction upon administering fluid and reassess frequently. There are no studies that have compared vasoactive agents for patients with fluid-refractory shock, and guidance is on the basis of existing protocols for pediatric shock. For pediatric septic shock, the Surviving Sepsis guidelines40 suggest using either epinephrine or norepinephrine rather than dopamine. Two studies have shown a lower risk of mortality41 and less organ dysfunction42 among those who received epinephrine instead of dopamine. However, with the concern of myocardial dysfunction in patients with MIS-C, the β-agonist actions of epinephrine might be more favourable than the increase in systemic vascular resistance from norepinephrine.
Immunomodulatory therapy
Primary therapy
There have been no randomized clinical trials that have compared immunomodulatory therapies. In an early nonrandomized series from France 18 patients who received IVIG alone were compared with 22 patients who received IVIG and intravenous methylprednisolone (0.8 mg/kg/d for 5 days). Dual therapy was associated with a faster recovery time for left ventricular systolic function (2.9 vs 5.4 days), along with decreased length of stay in the intensive care unit (ICU); (3.4 vs 5.3 days).43 In a subsequent retrospective study with propensity-matched analysis using the national surveillance system in France, 32 children received IVIG and methylprednisolone and 64 received IVIG alone.22 The methylprednisolone dose was 0.8-1 mg/kg every 12 hours (maximum 30 mg for 12 hours) for 5 days, although 4 patients received a bolus of 15-30 mg/kg/d for 3 days. Patients who received dual therapy had a more severe presentation, yet they had a more favourable outcome. Treatment with IVIG and methylprednisolone was associated with lower risk of treatment failure compared with IVIG alone (9% vs 38%) and decreased likelihood of requiring intensification of therapy (9% vs 31%), vasoactive agents (6% vs 23%), or acute left ventricular dysfunction (17% vs 35%), along with shorter duration of stay in the ICU (4 vs 6 days).
The larger US-based Overcoming COVID-19 surveillance registry performed a propensity score-matched comparison of 103 patients who received IVIG and glucocorticoids vs 103 who received IVIG alone.32 Methylprednisolone was the most common glucocorticoid received, primarily 2 mg/kg/d with fewer receiving pulse doses of 10-30 mg/kg/d. Dual therapy was associated with a lower risk of cardiovascular dysfunction on or after day 2, defined as a composite of left ventricular dysfunction or shock resulting in use of vasopressors (17% vs 31%). Left ventricular dysfunction rates were lower among those who received dual therapy (8% vs 17%), as was the likelihood of receiving vasoactive agents (13% vs 24%) or second-line therapy (34% vs 70%).
An analysis by the Best Available Treatment Study (BATS) Consortium, published simultaneously with the Overcoming COVID-19 study, provided what seemed to be conflicting results.35 In an international observational cohort study of patients with suspected or confirmed MIS-C, 246 patients received primary treatment with IVIG alone, 208 IVIG and glucocorticoids, and 99 glucocorticoids alone. The primary outcomes were: (1) a composite of inotropic support or mechanical ventilation by day 2 or later or death; and (2) reduction in disease severity by day 2. There were no significant differences in outcomes among the treatment groups. A subgroup analysis among patients who met World Health Organization criteria for MIS-C and received glucocorticoids alone had a lower risk of requiring respiratory support by day 2 or later or death compared with those who received IVIG alone (odds ratio, 0.3 [0.1-0.85]). However, there were several potential limitations. The study included many patients with suspected MIS-C who did not meet diagnostic criteria, and only 12% of patients had left ventricular systolic dysfunction, less than reported in other series, suggestive of milder disease in this cohort.
In a more recent single-centre retrospective study the potential for steroids as monotherapy, including standard therapy (2 mg/kg/d methylprednisolone) or pulse dosing was reviewed.33 Propensity score analysis was used to compare differing treatments for 179 patients with MIS-C (68 with steroids alone, 111 with IVIG and steroids). Steroid monotherapy was associated with similar rates of treatment failure but shorter steroid course duration (5 vs 10 days) and shorter hospital length of stay (5 vs 6 days).
Escalation of therapy
Patients with MIS-C might decompensate quickly, and there should be a low threshold to escalate therapy. There are no clinical trials that have compared treatment for refractory disease, although the most common reported therapies include high-dose glucocorticoids, anakinra, and infliximab, with a few earlier studies reporting the use of tocilizumab.32 At least for 1 guideline, 2 doses of IVIG are not recommended because of the risk of volume overload and hemolytic anemia.17
In a small single-centre study including 33 patients, 22 received ICU care. Although all 22 received IVIG, 12 received second-line therapy with infliximab, all showing improvement with no adverse reactions.44 A subsequent larger single-centre retrospective study including 72 children with MIS-C, 20 received IVIG alone and 52 received IVIG and infliximab (10 mg/kg).45 Although infliximab was used as primary therapy in this instance rather than intensification, dual therapy was associated with less additional therapy (31% vs 65%), shorter duration of ICU stays (1.8 vs 3.3 days), decreased development of left ventricular systolic dysfunction (4% vs 20%), and faster decrease in C-reactive protein levels.
Thromboprophylaxis
Pediatric patients with acute COVID-19 and MIS-C are presumed to have increased risk for thrombosis. In a multicentre, retrospective study of 138 MIS-C patients, 9 (6.5%) developed a thrombotic event: stroke (n = 1), intracardiac thrombosis (n = 1), and deep venous thrombosis (n = 7), all in the setting of central venous catheters.46 One patient with a thrombotic event died whereas 2 others who received anticoagulation had major bleeding events. Across the entire study of MIS-C and acute COVID-19 patients, age ≥ 12 years old and a D-dimer 5 times greater than the upper limit of normal were associated with thrombotic events.
Antiplatelet treatment with low-dose aspirin is generally recommended for all patients, on the basis of a similar recommendation for KD, as well as the likelihood of platelet activation and evidence of microvascular thrombotic events in adults with acute COVID-19.47, 48, 49 It is less clear when and how aggressive thromboprophylaxis should be applied. Although pediatric patients with acute COVID-19 appear to have a much lower risk of thrombotic complications compared with adults, patients with MIS-C, as well as older patients (age > 12 years), a history of cancer, or the presence of a central venous catheter, were at the highest risk in 1 multicentre study.46 Left ventricular dysfunction is also believed to be a risk factor for cardiac thrombosis, with guidelines suggesting a need for anticoagulation if there is at least moderate left ventricular dysfunction.17 , 50
However, current recommendations for additional thromboprophylaxis in addition to low-dose aspirin are on the basis of expert consensus, and include consideration of pre-COVID-19 risk factors, presence of cardiovascular abnormalities (severe ventricular dysfunction, large coronary aneurysms, etc), markedly elevated D-dimer (> 5 times the upper limit of normal), and risk factors for hospital-associated venous thrombosis and thromboembolism.51
Facilitating Clinical Decision-Making and Building Evidence With a New Condition
As discussed earlier, the COVID-19 pandemic and the subsequent first descriptions of MIS-C arrived suddenly and dramatically.52 The first reported cases of MIS-C seemed particularly severe. This was presumably due to delays in presentation, diagnostic uncertainties, and a lack of realization early on of the need for prompt immunomodulatory therapy, together with the fact that the health care system was reeling from the effects of severe acute COVID-19 cases in adults.5 The assumption that children were largely spared the consequences of severe COVID-19 complications was quickly dashed, when the association with a previous COVID-19 infection was determined.13 , 53
How Can We Inform Decision-Making During the Initial Experience With a New Condition?
For the first cases of MIS-C, care was appropriately supportive and empiric specific to the complications that were observed in individual patients. Because this appeared to be a new condition, 3 important strategies were deployed that were aimed at informing clinical care and discovery. First, sharing of information was widespread, initially by peer-reviewed journals pivoting to prioritize rapid review and publication, often through open access to case reports and series.54 In addition, increased use of preprint services expedited dissemination, albeit at the expense of peer-review and the occasional retraction of incorrect or misleading information.55 Second, multicentre data collections were quickly organized. These took the form of public health surveillance and reporting as well as the formation or leveraging of existing networks of clinicians, investigators, and institutions.56 These efforts, together with the huge shift to virtual meetings, also facilitated greater and more rapid communication and sharing, as well as the critical examination of pooled data. For example, the International Kawasaki Disease Registry collaborators held frequent open virtual webinars to review and discuss recent literature, and to provide a forum for open sharing and discussion regarding participants’ experience with clinical challenges related to MIS-C and KD, in addition to pivoting data collection to include MIS-C and acute KD patients.57 Of particular importance for surveillance and scientific inquiry, expert opinion and the evaluation of pooled data were used to define and refine initial case definitions for this new condition.5 , 8 , 58
The third strategy was to look for homologous conditions. It was immediately apparent that a large proportion of presumed MIS-C patients also met diagnostic criteria for KD.59 This homology led to the use of IVIG as initial therapy, the evidence-based standard of care for initial therapy of KD, followed by steroids and other inflammatory therapies in the event of insufficient response.10 The strategy appeared to be effective. However, without knowing the exact underlying pathophysiology of MIS-C, the development and use of alternative or adjunctive specific therapies, and the creation of treatment algorithms, is challenging. In addition, when 1 therapy quickly becomes the standard of care it becomes difficult to study what might be more specific and effective alternatives. This appears to be the case regarding whether IVIG or steroids or both together would be the more effective initial therapy for MIS-C.60
The Strengths and Challenges of Clinical Trials
Clinical decision-making should be on the basis of critical appraisal and synthesis of the best available research evidence. However, clinician and institutional expertise and experience together with patient preferences for treatments and outcomes also should be incorporated to personalize the decision.61 This concept was first developed in the early 1990s by David Sackett and colleagues, and has stood the test of time to become the ideal approach to clinical practice,61 albeit with calls for refinements and expansion of scope.62 There is a hierarchy of evidence, with systematic reviews and meta-analyses being at the top, which ideally should be on the basis of the next level of evidence being randomized controlled clinical trials. Clinical trials produce high-quality evidence for 2 main reasons. First, random allocation to treatments, if successful and the sample size is large enough, reduces bias by balancing known and unknown confounders equally among the groups to be compared. Hence, they provide the best evidence as to whether the intervention causally affected the outcome. Second, the study design is inherently prospective in nature, allowing for standardization of study procedures and measurements and strategies to mitigate loss to follow-up. However, clinical trials are resource-intensive, have restrictive inclusion and exclusion criteria that often limit generalizability, only allow for the study of the randomized interventions, and are usually powered to detect the effect on a specific outcome. They are also prone to give equivocal answers, usually focused on average effect and not balancing different benefits and risks or specifying patient and clinical characteristics that mediate the effect of the interventions. In addition, real-world generalizability of the results depend on the degree of deviation of the clinical circumstance/patient being treated and the study design/participant population. Clinical trials are also particularly challenging for the study of rare conditions and rare or time-related outcomes (especially long-term outcomes), which has been true for KD and MIS-C and for the outcome of coronary artery involvement and cardiovascular events.63, 64, 65, 66 The need for head-to-head or adjunctive trials necessitates a large participant population to generate necessary power for the study design, providing an added challenge for pediatric clinical trials of uncommon disease processes.
For ethical reasons, the comparator to a new intervention must include the minimal current standard of care. Lacking equipoise, placebo-controlled trials, particularly those in children, can be ethically challenged. In some cases, the new intervention must be provided as an adjunctive therapy to the standard of care to meet clinical equipoise requirements. This strategy makes it difficult to determine if a new intervention is superior to the standard of care alone. With the first cases of MIS-C, the rapid adoption of the empiric use of IVIG as the standard of care made it difficult to study the use of steroids alone as first-line therapy.60 This rapid acceptance of a standard of care without strong evidence has further impeded the study of newer biologic agents, such as infliximab, etanercept, and anakinra, which are currently reserved for patients not responsive to the current standard of care therapy or used as an adjunct therapy.
Expediting Clinical Trials During a Pandemic
Because randomized clinical trials provide the highest quality of research evidence, how might they be rapidly conceived, implemented, and adapted to provide timely answers in new and perhaps rapidly evolving clinical scenarios? First, they must take advantage of existing infrastructure and expertise. Relevant consortia and networks of investigators might be engaged and empowered. They could be augmented by external experts, particularly if a multispecialty and interdisciplinary approach is needed. Some of these might be methodologic experts, particularly those with expertise in novel trial approaches/designs and statistical methods. Merging of networks might be desired. Relevant existing patient registries might pivot to prioritize the new patient population for data collection. There are precedents for nesting clinical trials within existing registries to provide efficiency in recruitment, data collection, and study management at lower cost.67
Regarding study costs, funding agencies need to rapidly adapt and prioritize research into the new condition, which is what happened with the onset of the COVID-19 pandemic. However, investigators need to be prepared to respond rapidly to these prioritized requests for proposals, and funding agencies need to be prepared to provide expedited review and funding decisions. Both would have to ensure that the best science be put forward, evaluated, and funded, and that this is not compromised by the compressed time lines. Clinical trials must negotiate numerous regulatory hurdles that might need to be carefully expedited, including review and approvals from governmental regulatory bodies such as Health Canada and the US Food and Drug Administration, negotiation of multi-institutional contracts and data-sharing agreements, and obtaining institutional ethics review board approvals. The entire research governance structure might need to go into overdrive, together with strategies and oversight to minimize and mitigate risk.
Because of the rarity of MIS-C, conventional and timely randomized clinical trial designs would not likely be feasible because of the small participant numbers limiting statistical power to reliably detect relative treatment effects. This persistent problem has plagued pediatric clinical trials, has hampered acquisition of the necessary evidence required to support regulatory approvals for pediatric drug use, and has made it difficult to support strong recommendation statements in clinical practice guidelines.68 , 69
Because of the importance of quickly developing high-quality and reliable evidence on which to base clinical care for a new condition, a clear strategy is needed. Rather than relying on funding agencies to solely determine which investigator-initiated trials are performed, a clearinghouse is needed for coordination across agencies. Such a strategy would provide for rapid deployment of overlapping and complementary trials (perhaps shorter and smaller in scope, perhaps powered for reasonably justified surrogate end points), avoidance of competition for enrollment, and facilitation of sharing of expertise and resources. Therapies identified to be ineffective or potentially harmful should be abandoned as quickly as possible. Nimble governance would be essential to quickly and efficiently respond to findings and changes in the condition, such as with the emergence of new variants of the SARS-CoV-2 virus or the additional effect of vaccination.
Alternatives to conventional clinical trials might have a particular utility for evaluating new therapies for rare diseases. These might include pragmatic trials, use of cluster randomization, stepped wedge designs, factorial designs, and Bayesian methods.70 , 71 Another strategy would be to use adaptative trial designs,72 those that adapt or are modified on the basis of new information that emerges as the trial progresses. That information most commonly derives from different types of prespecified interim analysis strategies. Noncomparative analyses might lead to adjustments in sample size informed by improved estimates of variance or of baseline risk of the outcome. The interim analyses could be performed comparatively to assess for rigourous prespecified criteria for stopping a trial early for either efficacy, futility, or safety (group sequential design). If comparative interim analyses show futility in the overall group but efficacy in a prespecified subpopulation then further enrollment might be restricted for that subpopulation, known as adaptive enrichment. Comparative interim analyses might also be used to plan modifications to treatment arms, such as dose escalation, or adding or deleting certain treatment arms, often compared with a common control or comparator arm. Adaptations can be applied to treatment arm allocation on the basis of comparative interim analyses, such as consecutive allocation aimed to balance baseline covariates between treatment arms or basing consecutive allocation on accumulating outcome data that might favour allocation to the more beneficial treatment (play the winner designs). All of these adaptation strategies must be preplanned and strictly monitored to maintain the overall integrity and rigour of the trial. In addition, because the adaptations described all entail interim analyses, a detailed statistical strategy must be in place to compensate or adjust for an increasing probability of a type 1 error. These adaptation techniques could be applied in the setting of rapidly evolving clinical scenarios and emergence of new treatment strategies, such as was seen with the COVID-19 pandemic and MIS-C.
What Is the Role of Observational Data?
Observational data can be very useful to determine the clinical disease spectrum within a new condition. Such data can identify variations in clinical presentation and serve to refine case definitions, which were somewhat diverse for MIS-C after the onset of the pandemic. Observational data sets can also define the natural and modified history of a condition, which is critical information for determining patient prognosis and prediction of health care system resource requirements. Prospective cohort studies with defined inclusion and exclusion criteria and standardized and adjudicated data collection provide the optimum observational data. Patient-reported outcomes should be formally elicited, and patient advocates actively engaged. For optimal estimates of incidence and risk, population-based studies with a defined denominator and complete reporting are needed. This limits the utility of passive surveillance systems (incomplete reporting) and administrative data (lack of granularity and standardization).
The primary limitation of using observational data to determine or compare outcomes of interventions is that the allocation to intervention type is nonrandom and, hence, subject to bias, because patients might get one intervention over another because of factors that might also influence the outcome. A number of statistical methods can be used to make the comparison fairer, but they only partially adjust for differences, and can only adjust for factors that were measured, in contrast to randomization, which ideally creates equal groups balanced according to unmeasured factors as well. Introduction of these biases represents the primary reason that studies using observational data to make nonrandomized comparisons often yield differing results. A recent example for MIS-C were 3 multi-institutional observational studies regarding initial immunomodulatory treatment with IVIG alone vs IVIG with steroids. The study by Son et al. included 518 patients and noted, as mentioned earlier, a relative benefit of IVIG with steroids on left ventricle dysfunction at and after 2 days.32 The comparison incorporated propensity score matching and covariate adjustment, and a further analysis using inverse probability weighting, both yielding similar results. The study by McArdle et al. included 614 patients and did not note a relative benefit of either steroids alone or in combination with IVIG over IVIG alone on a composite outcome of ventilatory or inotropic support or death.35 Likewise, these investigators used a similar statistical adjustment approach. The reasons for the disparity in the results for these 2 observational studies were explored by the combined teams of investigators. They noted differences in disease severity and cardiovascular involvement among these 2 studies, which also used differing case definitions.73 A further multi-institutional study by Ouldali et al. included 181 patients and noted a relative benefit of IVIG with steroids over IVIG alone on treatment failure, defined as persistent or recurrent fever, as well as other clinically relevant outcomes.22 These investigators used propensity score matching for statistical adjustment, with further analyses incorporating covariates and using inverse probability weighting. Because of the potential for residual bias for each of these 3 studies, it is difficult to know where the true answer lies.
How Do We Make Recommendations?
Recommendations for management strategies must be on the basis of accurate and reliable evidence of effectiveness and safety. However, early in the experience with a new condition this evidence might be lacking, indirect, incomplete, or at risk of being inaccurate. Expert opinion can be used as a starting point, but it must be used cautiously. The search for relevant experts should be broad and diverse, and all perspectives should be represented. The questions to be addressed should be carefully specified, and the starting point should be from a critical appraisal of what is already known as published in the literature where possible. Methods for reaching consensus should be fair, inclusive, and transparent, such as with the Delphi approach and others.74
Eventually, research evidence will emerge and either complement, refine, or replace expert opinion alone. Many studies, including observational studies, will make concluding statements that highlight the need for further research, but some will include statements regarding the clinical relevance of the findings and then proceed to make a recommendation. These recommendations should be viewed with caution and only in light of critical appraisal of the specific study.
Cumulative evidence and experience can be synthesized into higher-level collections of recommendations that might have broader applicability. This is the process for development of guidelines, for which there are guidelines for creating guidelines (www.agreetrust.org). Pivotal to the process is an underlying prespecified and detailed review and critical appraisal of the published research evidence, aimed at addressing key questions that are defined according to the patient, population, or problem at hand, the intervention and its comparators, and the outcomes. The review might be performed by experts independent of those drafting the recommendation statements. An evidence review should culminate in the production of evidence tables, which ultimately guide decisions as to the wording and grading of recommendation statements which is in alignment with the overall quantity and quality of the appraised evidence. These tables should be published with the guideline statement and kept alive and updated with new evidence periodically. Recommendation statements relevant to MIS-C are characterized by lower classes, reflecting the magnitude and uncertainties in the size of the treatment effect or association, which are accompanied by lower levels of evidence, reflecting uncertainties about the precision of that treatment effect or association.12 , 16, 17, 18, 19 , 29
As might be seen in rare diseases, such as MIS-C, the evidence is often lacking or of suboptimal quality, yet guidance is needed.75 To that end, some advocated for the development of rapid guidelines but caution was raised in the adaptation of rapid guidelines because they often lack clarity around reason for development, how the quality of evidence was assessed, and how management of conflicts was handled.76 Gaps in recommendations can lead to inconsistencies and uncertainties in clinical practice, which can affect patient outcomes. It is necessary to provisionally fill these gaps, and we are often left with expert opinion. However, great caution is needed. Expert opinion is often on the basis of anecdotal practice that has not been systematically reviewed and appraised. It might be skewed by outlier experiences, firmly held opinions and outside influences, and it might be biased by local circumstances and practices. If expert opinion is to be used to fill recommendation gaps, it must be used with the provisions previously described.
The conclusion of any guideline development project should not only include an accompanying plan for knowledge translation, but also a detailed accounting of the evidence gaps and the research strategies to address them. This can be done by identifying gaps and challenges during the evidence review and incorporating findings into evidence tables that are updated. Methodologists should be included in this activity to inform research approaches. The results should be specified a priori as an output in addition to the guideline itself. Guidelines are often developed by organizations that fund research, and the results should be used to inform funding priorities. Finally, the research strategy needs to have its own knowledge translation and implementation plan.
Penultimately, recommendations and guidelines are really on the basis of the average treatment effects as derived from research evidence. Optimally, decisions regarding management need to move from this approach toward management that is precision-based.77
Conclusion
Although results have been encouraging, the optimal treatment of children with MIS-C remains to be determined. Current therapies are on the basis of expert opinion, similarities to other pediatric conditions like KD, and multiple observational studies. Herein, we have provided guidance on developing future clinical trials for such a rare condition to help inform optimal treatment strategies.
Acknowledgements
The authors are grateful for Angela J. Doty, MD, for her editorial assistance and for everyone treating and researching MIS-C along with our patients and their families.
Funding Sources
Dr Harahsheh is supported by a subagreement from the Johns Hopkins University with funds provided by grant number R61HD105591 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development and the Office of the Director, National Institutes of Health. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development, the Office of the Director, National Institutes of Health, the National Institutes of Health, the National Institute of Biomedical Imaging and Bioengineering, the National Heart, Lung, and Blood Institute, or the Johns Hopkins University.
Disclosures
Dr Harahsheh serves as a scientific advisory board member of OP2 Drugs.
Footnotes
See page 812 for disclosure information.
References
- 1.Adhikari S.P., Meng S., Wu Y.J., et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9:29. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeBiasi R.L., Song X., Delaney M., et al. Severe coronavirus disease-2019 in children and young adults in the Washington, DC, metropolitan region. J Pediatr. 2020;223:199–203.e1. doi: 10.1016/j.jpeds.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauer R.M., Lee J., Clarke W.R. Predicting adult cholesterol levels from measurements in childhood and adolescence: the Muscatine Study. Bull N Y Acad Med. 1989;65:1127–1142. [discussion: 1154-60] [PMC free article] [PubMed] [Google Scholar]
- 4.de Ferranti S.D., Steinberger J., Ameduri R., et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association. Circulation. 2019;139:e603–e634. doi: 10.1161/CIR.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 5.Whittaker E., Bamford A., Kenny J., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harahsheh A.S., Dahdah N., Newburger J.W., et al. Missed or delayed diagnosis of Kawasaki disease during the 2019 novel coronavirus disease (COVID-19) pandemic. J Pediatr. 2020;222:261–262. doi: 10.1016/j.jpeds.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molloy E.J., Nakra N., Gale C., Dimitriades V.R., Lakshminrusimha S. Multisystem inflammatory syndrome in children (MIS-C) and neonates (MIS-N) associated with COVID-19: optimizing definition and management. Pediatr Res. 2022;(Sep 1):1–10. doi: 10.1038/s41390-022-02263-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harahsheh A.S., Krishnan A., DeBiasi R.L., et al. Cardiac echocardiogram findings of severe acute respiratory syndrome coronavirus-2-associated multi-system inflammatory syndrome in children. Cardiol Young. 2022;32:718–726. doi: 10.1017/S1047951121003024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tritt A., Abda I., Dahdah N. Review of MIS-C clinical protocols and diagnostic pathways: towards a consensus algorithm. CJC Pediatric and Congenital Heart Disease. 2022;1:86–93. doi: 10.1016/j.cjcpc.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeBiasi R.L., Harahsheh A.S., Srinivasalu H., et al. Multisystem inflammatory syndrome of children: sub-phenotypes, risk factors, biomarkers, cytokine profiles and viral sequencing. J Pediatr. 2021;237:125–135.e18. doi: 10.1016/j.jpeds.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Feldstein L.R., Tenforde M.W., Friedman K.G., et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325:1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henderson L.A., Canna S.W., Friedman K.G., et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 1. Arthritis Rheumatol. 2020;72:1791–1805. doi: 10.1002/art.41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loke Y.H., Berul C.I., Harahsheh A.S. Multisystem inflammatory syndrome in children: is there a linkage to Kawasaki disease? Trends Cardiovasc Med. 2020;30:389–396. doi: 10.1016/j.tcm.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hejazi O.I., Loke Y.H., Harahsheh A.S. Short-term cardiovascular complications of multi-system inflammatory syndrome in children (MIS-C) in adolescents and children. Curr Pediatr Rep. 2021;9:93–103. doi: 10.1007/s40124-021-00258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCrindle B.W., Rowley A.H., Newburger J.W., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 16.Henderson L.A., Canna S.W., Friedman K.G., et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 2. Arthritis Rheumatol. 2021;73:e13–e29. doi: 10.1002/art.41616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson L.A., Canna S.W., Friedman K.G., et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 3. Arthritis Rheumatol. 2022;74:e1–e20. doi: 10.1002/art.42062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harwood R., Allin B., Jones C.E., et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc Health. 2021;5:133–141. doi: 10.1016/S2352-4642(20)30304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cattalini M., Taddio A., Bracaglia C., et al. Childhood multisystem inflammatory syndrome associated with COVID-19 (MIS-C): a diagnostic and treatment guidance from the Rheumatology Study Group of the Italian Society of Pediatrics. Ital J Pediatr. 2021;47:24. doi: 10.1186/s13052-021-00980-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee P.Y., Day-Lewis M., Henderson L.A., et al. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J Clin Invest. 2020;130:5942–5950. doi: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harthan A.A., Nadiger M., McGarvey J.S., et al. Early combination therapy with immunoglobulin and steroids is associated with shorter ICU length of stay in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19: a retrospective cohort analysis from 28 U.S. hospitals. Pharmacotherapy. 2022;42:529–539. doi: 10.1002/phar.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouldali N., Toubiana J., Antona D., et al. Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA. 2021;325:855–864. doi: 10.1001/jama.2021.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y., Yu Y., Chen S., Liao Y., Du J. Corticosteroids and intravenous immunoglobulin in pediatric myocarditis: a meta-analysis. Front Pediatr. 2019;7:342. doi: 10.3389/fped.2019.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caldeira D., Lopes L.R., Vaz-Carneiro A., Costa J. Cochrane corner: corticosteroids for viral myocarditis. Rev Port Cardiol. 2015;34:65–67. doi: 10.1016/j.repc.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Julia P., Young H.H., Buckberg G.D., Kofsky E.R., Bugyi H.I. Studies of myocardial protection in the immature heart. II: Evidence for importance of amino acid metabolism in tolerance to ischemia. J Thorac Cardiovasc Surg. 1990;100:888–895. [PubMed] [Google Scholar]
- 26.Belhadjer Z., Meot M., Bajolle F., et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 27.Elias M.D., McCrindle B.W., Larios G., et al. Management of multisystem inflammatory syndrome in children associated with COVID-19: a survey from the International Kawasaki Disease Registry. CJC Open. 2020;2:632–640. doi: 10.1016/j.cjco.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weusten B.L., Jacobs J.W., Bijlsma J.W. Corticosteroid pulse therapy in active rheumatoid arthritis. Semin Arthritis Rheum. 1993;23:183–192. doi: 10.1016/s0049-0172(05)80039-3. [DOI] [PubMed] [Google Scholar]
- 29.Schlapbach L.J., Andre M.C., Grazioli S., et al. Best practice recommendations for the diagnosis and management of children with pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS; Multisystem Inflammatory Syndrome in Children, MIS-C) in Switzerland. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.667507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Min K.H., Rhee C.K., Jung J.Y., Suh M.W. Characteristics of adverse effects when using high dose short term steroid regimen. Korean J Audiol. 2012;16:65–70. doi: 10.7874/kja.2012.16.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schacke H., Docke W.D., Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 32.Son M.B.F., Murray N., Friedman K., et al. Multisystem inflammatory syndrome in children - initial therapy and outcomes. N Engl J Med. 2021;385:23–34. doi: 10.1056/NEJMoa2102605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villacis-Nunez D.S., Jones K., Jabbar A., et al. Short-term outcomes of corticosteroid monotherapy in multisystem inflammatory syndrome in children. JAMA Pediatr. 2022;176:576–584. doi: 10.1001/jamapediatrics.2022.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris S.I., Balaban R.S., Barrett L., Mandel L.J. Mitochondrial respiratory capacity and Na+ and K+-dependent adenosine triphosphatase-mediated ion transport in the intact renal cell. J Biol Chem. 1981;256:10319–10328. [PubMed] [Google Scholar]
- 35.McArdle A.J., Vito O., Patel H., et al. Treatment of multisystem inflammatory syndrome in children. N Engl J Med. 2021;385:11–22. doi: 10.1056/NEJMoa2102968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jonat B., Gorelik M., Boneparth A., et al. Multisystem inflammatory syndrome in children associated with coronavirus disease 2019 in a children’s hospital in New York City: patient characteristics and an institutional protocol for evaluation, management, and follow-up. Pediatr Crit Care Med. 2021;22:e178–e191. doi: 10.1097/PCC.0000000000002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hering J.P., Schroder T., Singer D., Hellige G. Influence of pH management on hemodynamics and metabolism in moderate hypothermia. J Thorac Cardiovasc Surg. 1992;104:1388–1395. [PubMed] [Google Scholar]
- 38.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verdoni L., Mazza A., Gervasoni A., et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss S.L., Peters M.J., Alhazzani W., et al. Surviving Sepsis Campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. 2020;21:e52–e106. doi: 10.1097/PCC.0000000000002198. [DOI] [PubMed] [Google Scholar]
- 41.Ventura A.M., Shieh H.H., Bousso A., et al. Double-blind prospective randomized controlled trial of dopamine versus epinephrine as first-line vasoactive drugs in pediatric septic shock. Crit Care Med. 2015;43:2292–2302. doi: 10.1097/CCM.0000000000001260. [DOI] [PubMed] [Google Scholar]
- 42.Ramaswamy K.N., Singhi S., Jayashree M., Bansal A., Nallasamy K. Double-blind randomized clinical trial comparing dopamine and epinephrine in pediatric fluid-refractory hypotensive septic shock. Pediatr Crit Care Med. 2016;17:e502–e512. doi: 10.1097/PCC.0000000000000954. [DOI] [PubMed] [Google Scholar]
- 43.Belhadjer Z., Auriau J., Meot M., et al. Addition of corticosteroids to immunoglobulins is associated with recovery of cardiac function in multi-inflammatory syndrome in children. Circulation. 2020;142:2282–2284. doi: 10.1161/CIRCULATIONAHA.120.050147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdel-Haq N., Asmar B.I., Deza Leon M.P., et al. SARS-CoV-2-associated multisystem inflammatory syndrome in children: clinical manifestations and the role of infliximab treatment. Eur J Pediatr. 2021;180:1581–1591. doi: 10.1007/s00431-021-03935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cole L.D., Osborne C.M., Silveira L.J., et al. IVIG compared to IVIG plus infliximab in multisystem inflammatory syndrome in children. Pediatrics. 2021;148 doi: 10.1542/peds.2021-052702. e2021052702. [DOI] [PubMed] [Google Scholar]
- 46.Whitworth H., Sartain S.E., Kumar R., et al. Rate of thrombosis in children and adolescents hospitalized with COVID-19 or MIS-C. Blood. 2021;138:190–198. doi: 10.1182/blood.2020010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ankola A.A., Bradford V.R., Newburger J.W., et al. Coagulation profiles and viscoelastic testing in multisystem inflammatory syndrome in children. Pediatr Blood Cancer. 2021;68 doi: 10.1002/pbc.29355. [DOI] [PubMed] [Google Scholar]
- 48.Sharathkumar A.A., Faustino E.V.S., Takemoto C.M. How we approach thrombosis risk in children with COVID-19 infection and MIS-C. Pediatr Blood Cancer. 2021;68 doi: 10.1002/pbc.29049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chow J.H., Khanna A.K., Kethireddy S., et al. Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with coronavirus disease 2019. Anesth Analg. 2021;132:930–941. doi: 10.1213/ANE.0000000000005292. [DOI] [PubMed] [Google Scholar]
- 50.Giglia T.M., Massicotte M.P., Tweddell J.S., et al. Prevention and treatment of thrombosis in pediatric and congenital heart disease: a scientific statement from the American Heart Association. Circulation. 2013;128:2622–2703. doi: 10.1161/01.cir.0000436140.77832.7a. [DOI] [PubMed] [Google Scholar]
- 51.Goldenberg N.A., Sochet A., Albisetti M., et al. Consensus-based clinical recommendations and research priorities for anticoagulant thromboprophylaxis in children hospitalized for COVID-19-related illness. J Thromb Haemost. 2020;18:3099–3105. doi: 10.1111/jth.15073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boekholdt S.M., Hovingh G.K., Mora S., et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol. 2014;64:485–494. doi: 10.1016/j.jacc.2014.02.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harahsheh A.S., Dahdah N., Newburger J.W., et al. Reply. J Pediatr. 2020;224:184–185.e1. doi: 10.1016/j.jpeds.2020.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Downing S.E., Lee J.C., Taylor J.F.N. Cardiac function and metabolism during cholinergic stimulation in the newborn lamb. Am J Physiol. 1977;233:H451–H457. doi: 10.1152/ajpheart.1977.233.4.H451. [DOI] [PubMed] [Google Scholar]
- 55.Ravinetto R., Caillet C., Zaman M.H., et al. Preprints in times of COVID19: the time is ripe for agreeing on terminology and good practices. BMC Med Ethics. 2021;22:106. doi: 10.1186/s12910-021-00667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feldstein L.R., Rose E.B., Horwitz S.M., et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parrish M.D., Ayres N.A., Kendrick B.T., Fixler D.E. Maturational differences in the silated isovolumic rabbit heart. Am J Physiol. 1986;251:H1143–H1148. doi: 10.1152/ajpheart.1986.251.6.H1143. [DOI] [PubMed] [Google Scholar]
- 58.Haney E.M., Huffman L.H., Bougatsos C., Freeman M., Steiner R.D., Nelson H.D. Screening and treatment for lipid disorders in children and adolescents: systematic evidence review for the US Preventive Services Task Force. Pediatrics. 2007;120:e189–e214. doi: 10.1542/peds.2006-1801. [DOI] [PubMed] [Google Scholar]
- 59.McCrindle B.W., Manlhiot C. SARS-CoV-2-related inflammatory multisystem syndrome in children: different or shared etiology and pathophysiology as Kawasaki disease? JAMA. 2020;324:246–248. doi: 10.1001/jama.2020.10370. [DOI] [PubMed] [Google Scholar]
- 60.DeBiasi R.L. Immunotherapy for MIS-C - IVIG, glucocorticoids, and biologics. N Engl J Med. 2021;385:74–75. doi: 10.1056/NEJMe2108276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sackett D.L., Rosenberg W.M., Gray J.A., Haynes R.B., Richardson W.S. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71–72. doi: 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sheridan D.J., Julian D.G. Achievements and limitations of evidence-based medicine. J Am Coll Cardiol. 2016;68:204–213. doi: 10.1016/j.jacc.2016.03.600. [DOI] [PubMed] [Google Scholar]
- 63.McCrindle B.W., Rowley A.H. Improving coronary artery outcomes for children with Kawasaki disease. Lancet. 2019;393:1077–1078. doi: 10.1016/S0140-6736(18)33133-7. [DOI] [PubMed] [Google Scholar]
- 64.McCrindle B.W., Selamet Tierney E.S. Acute treatment for Kawasaki disease: challenges for current and future therapies. J Pediatr. 2017;184:7–10. doi: 10.1016/j.jpeds.2017.01.072. [DOI] [PubMed] [Google Scholar]
- 65.Tremoulet A.H., Jain S., Jaggi P., et al. Infliximab for intensification of primary therapy for Kawasaki disease: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet. 2014;383:1731–1738. doi: 10.1016/S0140-6736(13)62298-9. [DOI] [PubMed] [Google Scholar]
- 66.Portman M.A., Dahdah N.S., Slee A., et al. Etanercept with IVIg for acute Kawasaki disease: a randomized controlled trial. Pediatrics. 2019;143 doi: 10.1542/peds.2018-3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J., Nong L., Wei Y., Qin S., Zhou Y., Tang Y. Association of interleukin-12 polymorphisms and serum IL-12p40 levels with osteosarcoma risk. DNA Cell Biol. 2013;32:605–610. doi: 10.1089/dna.2013.2098. [DOI] [PubMed] [Google Scholar]
- 68.Torok R.D., Li J.S., Kannankeril P.J., et al. Recommendations to enhance pediatric cardiovascular drug development: report of a multi-stakeholder think tank. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harris K.C., Mackie A.S., Dallaire F., et al. Unique challenges of randomised controlled trials in pediatric cardiology. Can J Cardiol. 2021;37:1394–1403. doi: 10.1016/j.cjca.2021.06.013. [DOI] [PubMed] [Google Scholar]
- 70.Psioda M.A., Xue X. A Bayesian adaptive two-stage design for pediatric clinical trials. J Biopharm Stat. 2020;30:1091–1108. doi: 10.1080/10543406.2020.1821704. [DOI] [PubMed] [Google Scholar]
- 71.Wang Y., Travis J., Gajewski B. Bayesian adaptive design for pediatric clinical trials incorporating a community of prior beliefs. BMC Med Res Methodol. 2022;22:118. doi: 10.1186/s12874-022-01569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ellis D., Thomas R.C. Direct measurement of the intracellular pH of mammalian cardiac muscle. J Physiol. 1976;262:755–771. doi: 10.1113/jphysiol.1976.sp011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Melgar M., Seaby E.G., McArdle A.J., et al. Treatment of multisystem inflammatory syndrome in children: understanding differences in results of comparative effectiveness studies. ACR Open Rheumatol. 2022;4:804–810. doi: 10.1002/acr2.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones J., Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311:376–380. doi: 10.1136/bmj.311.7001.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neumann I., Schunemann H.J. Guideline groups should make recommendations even if the evidence is considered insufficient. CMAJ. 2020;192:E23–E24. doi: 10.1503/cmaj.190144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kowalski S.C., Morgan R.L., Falavigna M., et al. Development of rapid guidelines: 1. Systematic survey of current practices and methods. Health Res Policy Syst. 2018;16:61. doi: 10.1186/s12961-018-0327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tonelli M.R., Shirts B.H. Knowledge for precision medicine: mechanistic reasoning and methodological pluralism. JAMA. 2017;318:1649–1650. doi: 10.1001/jama.2017.11914. [DOI] [PubMed] [Google Scholar]