Abstract
Insulin resistance in the vasculature is a hallmark of type 2 diabetes (T2D), and blunting of insulin-induced vasodilation is its primary consequence. Individuals with T2D exhibit a marked impairment in insulin-induced dilation in resistance arteries across vascular beds. Importantly, reduced insulin-stimulated vasodilation and blood flow to skeletal muscle limits glucose uptake and contributes to impaired glucose control in T2D. The study of mechanisms responsible for the suppressed vasodilatory effects of insulin has been a growing topic of interest for not only its association with glucose control and extension to T2D but also its relationship with cardiovascular disease development and progression. In this mini-review, we integrate findings from recent studies by our group with the existing literature focused on the mechanisms underlying endothelial insulin resistance in T2D.
Keywords: diabetes, glucose control, insulin resistance, obesity, vascular dysfunction
INTRODUCTION
Eight out of 10 patients with type 2 diabetes (T2D) die from cardiovascular disease (1). A primary connection between T2D and cardiovascular disease is insulin resistance (2), a classic feature of T2D that is categorized by a diminished ability of peripheral tissues to regulate glucose homeostasis in response to insulin. Beyond its role in glucose uptake, insulin also produces vasodilatory effects that augment its delivery and that of glucose to metabolically active tissues such as skeletal muscle (3–13). Specifically, insulin acts on endothelial cells to stimulate downstream insulin receptor (IR) substrate 1/2 (IRS-1/2)-phosphatidylinositol 3-kinase (PI3K) signaling, resulting in the activation of endothelial nitric oxide (NO) synthase (eNOS), NO production, and vasodilation (10–12, 14, 15). Concurrently, insulin stimulates the Ras/mitogen-activated protein kinase (MAPK) signaling pathway, causing the production of the vasoconstrictor peptide, endothelin-1 (ET-1) (10–12, 14, 15). Increased vascular tone stemming from ET-1, in conjunction with sympathetic vasoconstriction, counteracts NO-mediated vasodilation during hyperinsulinemia (15–26). Blunting of insulin-induced dilation and blood flow is a distinguished feature of obesity and T2D (4, 6, 9, 18, 23, 25–34).
In healthy individuals, insulin-mediated increases in skeletal muscle blood flow account for as much as 35% of total insulin-stimulated skeletal muscle glucose uptake (3). However, in T2D, abnormalities in vasomotor reactivity to insulin lead to a reduction in glucose uptake by skeletal muscle, contributing to impaired glucose homeostasis (4, 9). Notably, evidence from preclinical models indicates that vascular insulin resistance is an early event in the disease process that occurs before systemic insulin resistance (18, 29, 30, 35). Indeed, accumulating evidence suggests that disruption of endothelial insulin signaling can directly contribute to whole body insulin resistance (36, 37). For example, mice with genetic deletion of the IRS-2 in endothelial cells exhibit impairments in insulin action in peripheral target tissues (e.g., reduced insulin-stimulated skeletal muscle glucose uptake) (37). Loss of endothelial insulin signaling is also shown to impair insulin actions in different brain regions and predisposes mice to overeating and obesity (36). We recently showed that vascular insulin resistance can be quickly triggered when young healthy men transition to an obesogenic lifestyle characterized by reduced ambulatory activity and increased consumption of sugar-sweetened beverages (38). This suggests that endothelial insulin resistance can occur acutely and may participate in the development of T2D if triggering events persist.
Impaired insulin signaling in endothelial cells is also linked to cardiovascular derangements (14, 39–41). For example, recent data in humans indicate that endothelial insulin resistance is associated with left ventricular diastolic dysfunction and arterial stiffening (40). Moreover, genetic deletion of the IR in endothelial cells causes atherosclerotic lesions in the aorta of ApoE−/− mice (41). Conversely, data are also available demonstrating that restoration of endothelial IR expression alone is sufficient to prevent vascular dysfunction in a genetic mouse model of insulin resistance (42), whereas selective activation of the IR-PI3K branch results in protection from atherosclerosis (43). Taken together, current literature suggests that defects in endothelial insulin signaling may represent a causal factor in the pathogenesis of T2D and atherosclerosis, thus underscoring the urgency to identify molecular targets and strategies that could restore the vascular actions of insulin (14, 44–47). Indeed, reinstating optimal endothelial insulin signaling may be paramount in the protection against metabolic and cardiovascular disease. In the present mini-review, we summarize new insights pertaining to mechanisms of endothelial insulin resistance in T2D recently put forth by our group and integrate these findings with the existing literature. We begin with alterations observed at the level of the IR, followed by changes in the intracellular signaling pathway and culminate with mechanisms outside canonical insulin signaling that we show also affect insulin-induced vasodilation. The goal is to spark innovative ideas and follow-up studies that will ultimately lead to effective therapeutic strategies to insulin resensitize the vasculature in T2D.
ROLE OF A DISINTEGRIN AND METALLOPROTEINASE-17
Individuals with T2D exhibit a chronic proinflammatory state, characterized by increased levels of inflammatory cytokines and proteolytic enzymes such as a disintegrin and metalloproteinase-17 (ADAM17) (48). Along these lines, current reports indicate that the expression of ADAM17 is elevated in the endothelium of resistance arteries from obese mice and humans (49). ADAM17 is a membrane-bound enzyme that cleaves the ectodomain of multiple transmembrane proteins. For example, ADAM17 cleaves pro-TNFα from the cell surface and converts it to its mature soluble form (50). Thus, elevated levels of TNFα in T2D plasma are suggestive of increased ADAM17 activity (48, 51). ADAM17 is a nonspecific sheddase, and thus, beyond its role in cleaving pro-TNFα, it has multiple membrane-anchored molecules as substrates including growth factors, cytokines, and cell adhesion molecules (52). One such well-known substrate of ADAM17 is angiotensin-converting enzyme 2, and this molecular interaction has been an area of recent attention given its implication in COVID-19 pathogenesis (53–56).
We reasoned that the IR may also be a substrate for ADAM17. Specifically, we formulated the hypothesis that, in T2D, increased ADAM17 activity sheds the IR ectodomain from endothelial cells and renders the endothelium unresponsive to insulin. Consistent with this hypothesis, we recently reported that vessels from bariatric patients with T2D exhibit impaired insulin-induced dilation and reduced extracellular IRα content relative to vessels from body mass index-matched non-T2D patients (27). Notably, these differences occurred in conjunction with increased ADAM17 expression in T2D vessels. Following up on the potential link between ADAM17 and IR, we found that activation of ADAM17 in human endothelial cells, caused by the protein kinase-C activator, PMA, diminishes the presence of IRα within cell membrane patches where ADAM17 is clustered and increases the shedding of IR ectodomain into the cell culture supernatant (27). Furthermore, ADAM17 activation blunts endothelial insulin signaling, as evidenced by reduced phosphorylation of Akt and eNOS (27). Importantly, ADAM17 inhibition using TAPI-0 prevents the PMA-provoked impairments in endothelial insulin signaling and insulin-induced vasodilation in visceral resistance arteries (27). Taken together, these findings suggest that ADAM17-mediated shedding of the IR ectodomain from the endothelial surface may be an important mechanism mediating vascular insulin resistance in T2D.
ROLE OF THE MAPK AND ET-1 PATHWAY
As mentioned earlier, obesity and T2D are associated with impairments in insulin-induced vasodilation and blood flow (4, 6, 9, 18, 23, 25–34). Although vasoconstriction in response to insulin does not always occur, it has been reported in rodent models of insulin resistance and in humans (18, 21, 24). A principal mechanism implicated in this aberrant vascular response is the imbalance in endothelial insulin signaling, typified by a reduction in PI3K-Akt with either no alteration or heightened MAPK-ET-1 signaling. This discriminatory blunting of PI3K-Akt signaling is known as selective insulin resistance (14, 57), a culprit of metabolic dysfunction and cardiovascular disease.
Increased secretion of insulin occurs as a compensatory mechanism to counterbalance insulin resistance. However, because activation of MAPK remains, persistent insulin signaling may produce detrimental effects in the vasculature via heightened ET-1 production and vasoconstriction. It is plausible that the coexistence of chronic hyperinsulinemia and selective insulin resistance (i.e., impaired PI3K activation) is a trigger of insulin-stimulated vasoconstriction and vascular remodeling in the setting of obesity and T2D. This is consistent with reports indicating that prolonged ET-1 stimulation induces inward remodeling and stiffening of the resistance vasculature (58, 59). To investigate the joint roles of sustained insulin stimulation and dampened PI3K activation in regulating vasomotor responses to insulin, we conducted a number of experiments in isolated skeletal muscle resistance arteries from pigs (24). We tested the hypothesis that high insulin levels combined with blunted PI3K activity would elicit a vasoconstriction response to insulin, resulting from a heightened activation of the countercurrent MAPK-ET-1 pathway. As expected, we found that inhibition of PI3K signaling by itself caused a reduction in insulin-induced vasodilation (24). Importantly, although sustained insulin stimulation without inhibition of PI3K signaling (i.e., unrestricted insulin signaling) did not affect vasomotor responses to insulin, sustained insulin stimulation complemented with inhibition of PI3K exerted a vasoconstriction response (24). Together, these observations suggest that the constraint of the PI3K pathway may be a critical step underpinning the increased insulin-induced vasoconstriction that can manifest in the context of insulin resistance and compensatory hyperinsulinemia. Remarkably, we observed that insulin-induced vasoconstriction in our model of selective insulin resistance is rescued by MAPK inhibition and ET-1 receptor antagonism (24). These findings support the idea that strategies to reestablish the balance between insulin-stimulated PI3K and MAPK activation should be considered for the amelioration of vascular insulin resistance, vascular remodeling, and consequent cardiovascular disease.
In alignment with these findings, we have unpublished data demonstrating that inhibition of the ETA receptor with BQ-123 restores insulin-induced dilation in isolated skeletal muscle resistance arteries from T2D pigs, with no further enhancement detected in arteries from healthy control pigs. The finding that ETA signaling contributes to impaired insulin-induced dilation in T2D is translatable to humans. In studies conducted at Southern Denmark University, we demonstrated that intra-arterial (femoral artery) administration of BQ-123 increases leg blood flow during hyperinsulinemia in individuals with T2D (26). Of note, the magnitude of this increase in blood flow was greater in T2D subjects compared with controls. We also showed that ETA receptor antagonism increased skeletal muscle glucose uptake in T2D, an increase also present in control participants (26). We did not observe an additional benefit by antagonizing both the ETA and ETB receptors relative to selective ETA inhibition alone (26). Together, these observations indicate that enhanced activation of the ETA receptor limits skeletal muscle blood flow and glucose uptake during hyperinsulinemia in T2D individuals, with the minimal additional role of the ETB receptor. The notion that ET-1 signaling is implicated in glucose metabolism is consistent with our recent work using preclinical mouse models in which we found that mice overexpressing ET-1 in endothelial cells exhibit impaired glucose tolerance, whereas chronic oral administration of bosentan, an ETA/ETB receptor antagonist, improves glucose tolerance in Western diet-fed mice (60).
An apparent paradox that has not yet been fully resolved is how insulin signaling leads to the overproduction of ET-1 if the IR is depleted by ADAM17 in T2D. A conceivable scenario for reconciliation is that mechanisms that govern the overproduction of ET-1 in T2D remain active despite reduced insulin signaling and/or by mechanisms independent of insulin. Along these lines, we showed that ET-1 protein content is greater in skeletal muscle biopsy samples of T2D individuals, relative to healthy control subjects, even before systemic insulin infusion (25). Blunting of endothelial insulin signaling as a result of IR shedding is compounded by cytosolic mechanisms that further dampen insulin signaling specifically through the PI3K/Akt/eNOS pathway. Reduced NO bioavailability may contribute to increased ET-1 production. In this regard, we previously demonstrated that chronic systemic NOS inhibition in rats increases aortic expression of ET-1 (61); conversely, studies from others show that shear stress-induced downregulation of ET-1 in endothelial cells is NO-dependent (62). Thus, it is possible that the link between NO and ET-1 further contributes to increased ET-1 production in T2D, despite ADAM17 shedding of IR. Additional mechanisms by which ET-1 is upregulated in T2D are discussed below.
ROLE OF TRAF3 INTERACTING PROTEIN 2 (TRAF3IP2)
Although increased ET-1 signaling contributes to impaired insulin-induced vasodilation and glucose disposal, the molecular mechanisms central to endothelial synthesis of ET-1 in the setting of T2D are not well understood. In this regard, we provided the first evidence that TRAF3IP2 mediates the production of ET-1 in endothelial cells when exposed to high glucose (63). TRAF3IP2 is a proinflammatory cytoplasmic adaptor protein that has been identified as a candidate molecule implicated in endothelial dysfunction (64), atherosclerosis (65), and pathological stress pathways in vascular cells (63, 66, 67). We also showed that ET-1 signaling can upregulate TRAF3IP2 in endothelial cells and that ET-1-induced endothelial inflammation and monocyte adhesion are mediated by TRAF3IP2 (63). Such findings suggest that a detrimental feedback loop exists in which TRAF3IP2 stimulates ET-1 production and ET-1 signaling enhances TRAF3IP2-dependent proinflammatory pathways. These findings also support the idea that TRAF3IP2 should be considered as a putative therapeutic target to subdue ET-1 formation and resulting diabetic vascular complications.
Consistent with this notion, we recently documented that TRAF3IP2 plays a causal role in obesity-associated vascular insulin resistance and dysfunction (68). Specifically, we showed that ectopic expression of TRAF3IP2 blunts insulin signaling in endothelial cells and diminishes endothelium-dependent vasorelaxation in isolated aortic rings (68). Furthermore, we showed that male mice lacking TRAF3IP2 are protected against Western diet-induced impairments in insulin-induced relaxation, as well as from increased blood pressure and arterial stiffening (68). All this supports that TRAF3IP2 may be a promising therapeutic target in vasculometabolic derangements associated with obesity and T2D, particularly in males.
ROLE OF SHEAR STRESS
Because exercise-associated improvements in vascular insulin actions are typically conferred within the active skeletal muscle, where a notable increase in vascular shear stress occurs, it is reasonable to postulate that shear stress is an important determinant of vascular insulin sensitivity (69, 70). In this regard, we recently conducted a number of experiments to determine whether shear stress exerts insulin-sensitizing effects in the vasculature.
First, we showed in cultured endothelial cells that increased shear stress for 1 h produced a shift in insulin signaling characterized by an augmented activation of eNOS relative to MAPK (71). Furthermore, we found in isolated skeletal muscle resistance arteries that exposure to shear stress for 1 h produced a succeeding increase in insulin-induced vasodilation without alterations in endothelium-independent dilation (71). Next, the significance of these ex vivo observations was substantiated by demonstrating that single leg heating for 1 h (and resulting increase in blood flow and likely shear stress) caused a subsequent augmentation in leg blood flow and calf microvascular perfusion in response to hyperinsulinemia in healthy individuals (71). These findings can also have positive implications to individuals with vascular insulin resistance. We recently observed that T2D subjects undergoing lower-body heating via water immersion for 7 consecutive days (i.e., 1-h session/day) exhibited a partial restoration of leg blood flow responses to an oral glucose load (32). Of note, these improvements were not associated with an induction of endothelial expression of heat shock protein 72 (HSP72), nor modulation of HSP72 in cultured endothelial cells resulted in changes in insulin signaling (32). As such, it is conceivable that the observed vascular benefits following 7 days of lower-body heating were mostly attributed to repeated increases in blood flow and shear stress.
In alignment with the idea that increased shear stress promotes vascular insulin sensitivity, we recently reported that the reverse may also be true; that is, physical inactivity-induced “knockdown” of shear stress in the leg vasculature triggers vascular insulin resistance (38). In this regard, we showed that when healthy young men adopt an obesogenic lifestyle for 10 days that includes a daily reduction of step counts from >10,000 to <5,000, insulin-stimulated leg blood flow responses become impaired (38). In aggregate, findings from this series of studies are consistent with the hypothesis that blood flow-induced shear stress may be a determinant of vascular insulin sensitivity. Interestingly, young healthy women showed protection from the development of vascular insulin resistance associated with the short-term adoption of an obesogenic lifestyle (38). Further research is needed to elucidate the mechanisms by which the female sex confers such vascular protection.
Shear stress is detected by mechanosensitive endothelial luminal structures, such as the glycocalyx, a mesh of interwoven and negatively charged glycoproteins and proteoglycans that participate in converting mechanical forces into biochemical signals via mechanotransduction (72–74). In accordance, an intact glycocalyx is required for appropriate shear stress mechanotransduction in endothelial cells. Using sidestream darkfield imaging, Vink et al. (75) have elegantly demonstrated greater thickness of the erythrocyte-perfused boundary region in patients with T2D, relative to healthy subjects, indicative of glycocalyx degradation. Actually, endothelial glycocalyx degradation is a classic feature of T2D likely attributable to multiple factors in the diabetic milieu, including increased inflammation and enzymatic activity, among other mechanisms (72). In fact, it has been shown that increased ADAM17 activity cleaves glypican-1 (76), a mechanosensitive component of the glycocalyx (77). Thus, it is possible that degradation of the endothelial glycocalyx in T2D precludes shear stress mechanotransduction and, consequently, the insulin-sensitizing effects of shear stress become lost. However, this hypothesis remains to be tested. Also unknown is whether T2D-associated shedding of both the IR and mechanosensitive structures from the endothelial surface synergistically lead to a more profound vascular insulin-resistant phenotype.
The credence that increased blood flow and shear stress on the vascular endothelium can exert vascular insulin-sensitizing effects led us to examine the potential benefits of increased walking on skeletal muscle vascular insulin sensitivity in patients with T2D (31). We posited that suppressed leg blood flow responses to insulin in T2D would be ameliorated by a supervised walking intervention consisting of 45-min walking sessions, 5 days per week for 8 wk. However, in contrast to our hypothesis, we found that such walking intervention was not a sufficient stimulus to overhaul skeletal muscle vascular insulin resistance in our cohort of obese subjects with T2D, despite a demonstrated cardiorespiratory adaptation and reduction in HbA1c (31). From this work, we concluded that more refined physical activity interventions that involve the recruitment of the maximum number of muscle fibers in large muscle groups may be necessary for repairing skeletal muscle insulin resistance in T2D. We also posit that restoration of the glycocalyx in T2D may be required for exercise and blood flow-induced shear stress to enhance vascular insulin sensitivity.
CONCLUSIONS
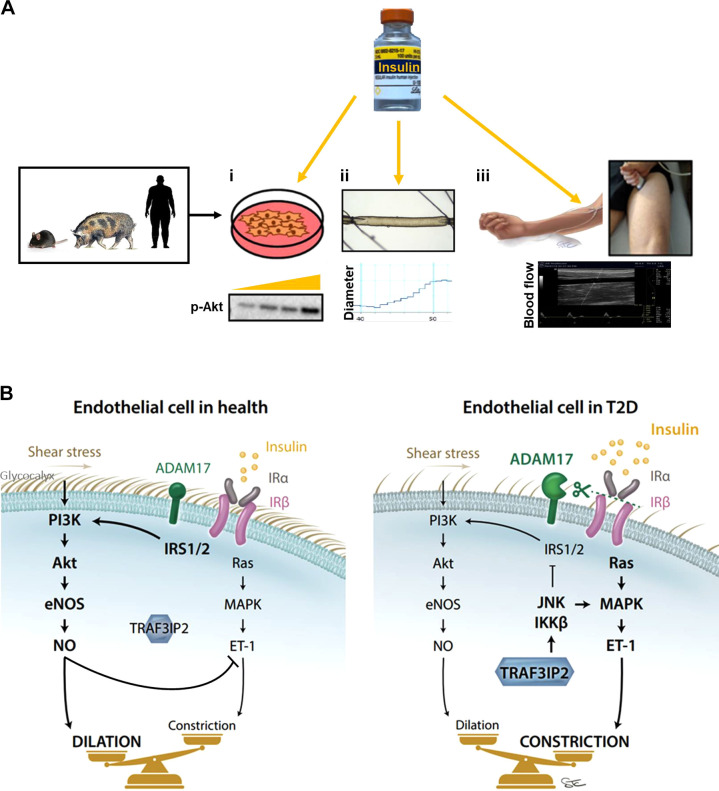
Over the past few years, our group has conducted a number of studies to advance the understanding of mechanisms underlying vascular insulin resistance in T2D. These studies have used a variety of complimentary experimental approaches including in vitro and ex vivo preparations involving insulin stimulation of cultured endothelial cells and isolated arteries from animals and humans, and in vivo systemic insulin infusion studies coupled with ultrasound-based measurements of leg blood flow in human participants (Fig. 1A). Collectively, findings from these studies highlight that endothelial insulin resistance may be caused by a number of molecular players (both intracellular and extracellular) acting in an additive or synergistic fashion. Indeed, as summarized in Fig. 1B, compared with healthy insulin-sensitive endothelial cells, the “diabetic endothelium” could be portrayed as having the following characteristics: 1) increased activation of ADAM17 and shedding of the IR from the endothelial surface leading to impaired overall insulin signaling; 2) further impairment of IRS-PI3K-Akt insulin pathway by TRAF3IP2-mediated activation of JNK and IKKβ, also causing an overactivation of the MAPK-ET-1 pathway; and 3) degraded glycocalyx limiting the mechanotransduction of shear stress forces and provoking a further dampened signal through the PI3K-Akt-eNOS pathway. At the functional level, the accretion of all these molecular events culminates with increased vascular tone during hyperinsulinemia.
Figure 1.
Translational approaches (A) used to study endothelial insulin signaling (i), insulin-induced dilation (ii), and insulin induced-blood flow (iii), as well as summary of putative mechanisms (B) contributing to endothelial insulin resistance in type 2 diabetes (T2D).
GRANTS
This work was supported, in part, by National Institutes of Health Grants R01HL137769 (to J.P.), R01HL151384 and R01HL153264 (to L.A.M.-L. and J.P.), and R21DK116081 and R01HL142770 (to C.M.-A.) and salary support from Veterans Affairs Merit Grant 1I01CX002399 (for J.P. and C.M.-A.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.P. drafted manuscript; C.M.-A. and L.A.M.-L. edited and revised manuscript; J.P., C.M.-A., and L.A.M.-L. approved final version of manuscript.
REFERENCES
- 1. Laakso M. Cardiovascular disease in type 2 diabetes: challenge for treatment and prevention. J Intern Med 249: 225–235, 2001. doi: 10.1046/j.1365-2796.2001.00789.x. [DOI] [PubMed] [Google Scholar]
- 2. Abdul-Ghani MA, Jayyousi A, DeFronzo RA, Asaad N, Al-Suwaidi J. Insulin resistance the link between T2DM and CVD: basic mechanisms and clinical implications. Curr Vasc Pharmacol 17: 153–163, 2019. doi: 10.2174/1570161115666171010115119. [DOI] [PubMed] [Google Scholar]
- 3. Baron AD, Brechtel-Hook G, Johnson A, Cronin J, Leaming R, Steinberg HO. Effect of perfusion rate on the time course of insulin-mediated skeletal muscle glucose uptake. Am J Physiol Endocrinol Physiol 271: E1067–E1072, 1996. doi: 10.1152/ajpendo.1996.271.6.E1067. [DOI] [PubMed] [Google Scholar]
- 4. Baron AD, Laakso M, Brechtel G, Edelman SV. Mechanism of insulin resistance in insulin-dependent diabetes mellitus: a major role for reduced skeletal muscle blood flow. J Clin Endocrinol Metab 73: 637–643, 1991. doi: 10.1210/jcem-73-3-637. [DOI] [PubMed] [Google Scholar]
- 5. Clark MG, Wallis MG, Barrett EJ, Vincent MA, Richards SM, Clerk LH, Rattigan S. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Physiol 284: E241–E258, 2003. doi: 10.1152/ajpendo.00408.2002. [DOI] [PubMed] [Google Scholar]
- 6. Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 55: 1436–1442, 2006. doi: 10.2337/db05-1373. [DOI] [PubMed] [Google Scholar]
- 7. Clerk LH, Vincent MA, Lindner JR, Clark MG, Rattigan S, Barrett EJ. The vasodilatory actions of insulin on resistance and terminal arterioles and their impact on muscle glucose uptake. Diabetes Metab Res Rev 20: 3–12, 2004. doi: 10.1002/dmrr.414. [DOI] [PubMed] [Google Scholar]
- 8. Hernandez Schulman I, Zhou M. Vascular insulin resistance: a potential link between cardiovascular disease and metabolic disease. Curr Hypertens Rep 11: 48–55, 2009. doi: 10.1007/s11906-009-0010-0. [DOI] [PubMed] [Google Scholar]
- 9. Laakso M, Edelman S, Brechtel G, Baron A. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes 41: 1076–1083, 1992. doi: 10.2337/diab.41.9.1076. [DOI] [PubMed] [Google Scholar]
- 10. Mather K. The vascular endothelium in diabetes - a therapeutic target? Rev Endocr Metab Disord 14: 87–99, 2013. doi: 10.1007/s11154-013-9237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mather KJ, Steinberg HO, Baron AD. Insulin resistance in the vasculature. J Clin Invest 123: 1003–1004, 2013. doi: 10.1172/JCI67166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord 14: 5–12, 2013. doi: 10.1007/s11154-012-9229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 53: 1418–1423, 2004. doi: 10.2337/diabetes.53.6.1418. [DOI] [PubMed] [Google Scholar]
- 14. King GL, Park K, Li Q. Selective insulin resistance and the development of cardiovascular diseases in diabetes: the 2015 Edwin Bierman Award Lecture. Diabetes 65: 1462–1471, 2016. doi: 10.2337/db16-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 28: 463–491, 2007. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 16. Bourque SL, Davidge ST, Adams MA. The interaction between endothelin-1 and nitric oxide in the vasculature: new perspectives. Am J Physiol Regul Integr Comp Physiol 300: R1288–R1295, 2011. doi: 10.1152/ajpregu.00397.2010. [DOI] [PubMed] [Google Scholar]
- 17. Eringa EC, Stehouwer CD, Merlijn T, Westerhof N, Sipkema P. Physiological concentrations of insulin induce endothelin-mediated vasoconstriction during inhibition of NOS or PI3-kinase in skeletal muscle arterioles. Cardiovasc Res 56: 464–471, 2002. doi: 10.1016/s0008-6363(02)00593-x. [DOI] [PubMed] [Google Scholar]
- 18. Eringa EC, Stehouwer CD, Roos MH, Westerhof N, Sipkema P. Selective resistance to vasoactive effects of insulin in muscle resistance arteries of obese Zucker (fa/fa) rats. Am J Physiol Endocrinol Physiol 293: E1134–E1139, 2007. doi: 10.1152/ajpendo.00516.2006. [DOI] [PubMed] [Google Scholar]
- 19. Eringa EC, Stehouwer CD, Van Nieuw Amerongen G, Ouwehand L, Westerhof N, Sipkema P. Vasoconstrictor effects of insulin in skeletal muscle arterioles are mediated by ERK1/2 activation in endothelium. Am J Physiol Heart Circ Physiol 287: H2043–H2048, 2004. doi: 10.1152/ajpheart.00067.2004. [DOI] [PubMed] [Google Scholar]
- 20. Eringa EC, Stehouwer CD, Walburg K, Clark AD, van Nieuw Amerongen GP, Westerhof N, Sipkema P. Physiological concentrations of insulin induce endothelin-dependent vasoconstriction of skeletal muscle resistance arteries in the presence of tumor necrosis factor-α dependence on c-Jun N-terminal kinase. Arterioscler Thromb Vasc Biol 26: 274–280, 2006. doi: 10.1161/01.ATV.0000198248.19391.3e. [DOI] [PubMed] [Google Scholar]
- 21. Gogg S, Smith U, Jansson PA. Increased MAPK activation and impaired insulin signaling in subcutaneous microvascular endothelial cells in type 2 diabetes: the role of endothelin-1. Diabetes 58: 2238–2245, 2009. doi: 10.2337/db08-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Limberg JK, Soares RN, Padilla J. Role of the autonomic nervous system in the hemodynamic response to hyperinsulinemia-implications for obesity and insulin resistance. Curr Diab Rep 22: 169–175, 2022. doi: 10.1007/s11892-022-01456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lteif A, Vaishnava P, Baron AD, Mather KJ. Endothelin limits insulin action in obese/insulin-resistant humans. Diabetes 56: 728–734, 2007. doi: 10.2337/db06-1406. [DOI] [PubMed] [Google Scholar]
- 24. Olver TD, Grunewald ZI, Ghiarone T, Restaino RM, Sales ARK, Park LK, Thorne PK, Ganga RR, Emter CA, Lemon PWR, Shoemaker JK, Manrique-Acevedo C, Martinez-Lemus LA, Padilla J. Persistent insulin signaling coupled with restricted PI3K activation causes insulin-induced vasoconstriction. Am J Physiol Heart Circ Physiol 317: H1166–H1172, 2019. doi: 10.1152/ajpheart.00464.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reynolds L, Credeur DP, Manrique C, Padilla J, Fadel PJ, Thyfault JP. Obesity, type 2 diabetes, and impaired insulin stimulated blood flow: role of skeletal muscle NO synthase and endothelin-1. J Appl Physiol (1985) 122: 38–47, 2017. doi: 10.1152/japplphysiol.00286.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Young BE, Padilla J, Finsen SH, Fadel PJ, Mortensen SP. Role of endothelin-1 receptors in limiting leg blood flow and glucose uptake during hyperinsulinemia in type 2 diabetes. Endocrinology 163: bqac008, 2022. doi: 10.1210/endocr/bqac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghiarone T, Castorena-Gonzalez JA, Foote CA, Ramirez-Perez FI, Ferreira-Santos L, Cabral-Amador FJ, de la Torre R, Ganga RR, Wheeler AA, Manrique-Acevedo C, Padilla J, Martinez-Lemus LA. ADAM17 cleaves the insulin receptor ectodomain on endothelial cells and causes vascular insulin resistance. Am J Physiol Heart Circ Physiol 323: H688–H701, 2022. doi: 10.1152/ajpheart.00039.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heise T, Magnusson K, Heinemann L, Sawicki PT. Insulin resistance and the effect of insulin on blood pressure in essential hypertension. Hypertension 32: 243–248, 1998. doi: 10.1161/01.hyp.32.2.243. [DOI] [PubMed] [Google Scholar]
- 29. Katakam P, Tulbert C, Snipes J, Erdös B, Miller A, Busija D. Impaired insulin-induced vasodilation in small coronary arteries of Zucker obese rats is mediated by reactive oxygen species. Am J Physiol Heart Circ Physiol 288: H854–H860, 2005. doi: 10.1152/ajpheart.00715.2004. [DOI] [PubMed] [Google Scholar]
- 30. Olver TD, Grunewald ZI, Jurrissen TJ, MacPherson REK, LeBlanc PJ, Schnurbusch TR, Czajkowski AM, Laughlin MH, Rector RS, Bender SB, Walters EM, Emter CA, Padilla J. Microvascular insulin resistance in skeletal muscle and brain occurs early in the development of juvenile obesity in pigs. Am J Physiol Regul Integr Comp Physiol 314: R252–R264, 2018. doi: 10.1152/ajpregu.00213.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park LK, Parks EJ, Pettit-Mee RJ, Woodford ML, Ghiarone T, Smith JA, Sales ARK, Martinez-Lemus LA, Manrique-Acevedo C, Padilla J. Skeletal muscle microvascular insulin resistance in type 2 diabetes is not improved by eight weeks of regular walking. J Appl Physiol (1985) 129: 283–296, 2020. doi: 10.1152/japplphysiol.00174.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pettit-Mee RJ, Power G, Cabral-Amador FJ, Ramirez-Perez FI, Soares RN, Sharma N, Liu Y, Christou DD, Kanaley JA, Martinez-Lemus LA, Manrique-Acevedo C, Padilla J. Endothelial HSP72 is not reduced in type 2 diabetes nor is it a key determinant of endothelial insulin sensitivity. Am J Physiol Regul Integr Comp Physiol 323: R43–R58, 2022. doi: 10.1152/ajpregu.00006.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rajapakse NW, Chong AL, Zhang WZ, Kaye DM. Insulin-mediated activation of the l-arginine nitric oxide pathway in man, and its impairment in diabetes. PLoS One 8: e61840, 2013. doi: 10.1371/journal.pone.0061840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tack CJ, Ong MK, Lutterman JA, Smits P. Insulin-induced vasodilatation and endothelial function in obesity/insulin resistance. Effects of troglitazone. Diabetologia 41: 569–576, 1998. doi: 10.1007/s001250050948. [DOI] [PubMed] [Google Scholar]
- 35. Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol 28: 1982–1988, 2008. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Konishi M, Sakaguchi M, Lockhart SM, Cai W, Li ME, Homan EP, Rask-Madsen C, Kahn CR. Endothelial insulin receptors differentially control insulin signaling kinetics in peripheral tissues and brain of mice. Proc Natl Acad Sci USA 114: E8478–E8487, 2017. doi: 10.1073/pnas.1710625114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, Inoue M, Itoh S, Takamoto I, Sasako T, Kumagai K, Kawai T, Hashimoto S, Kobayashi T, Sato M, Tokuyama K, Nishimura S, Tsunoda M, Ide T, Murakami K, Yamazaki T, Ezaki O, Kawamura K, Masuda H, Moroi M, Sugi K, Oike Y, Shimokawa H, Yanagihara N, Tsutsui M, Terauchi Y, Tobe K, Nagai R, Kamata K, Inoue K, Kodama T, Ueki K, Kadowaki T. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab 13: 294–307, 2011. doi: 10.1016/j.cmet.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 38. Smith JA, Soares RN, McMillan NJ, Jurrissen TJ, Martinez-Lemus LA, Padilla J, Manrique-Acevedo C. Young women are protected against vascular insulin resistance induced by adoption of an obesogenic lifestyle. Endocrinology 163: bqac137, 2022. doi: 10.1210/endocr/bqac137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gage MC, Yuldasheva NY, Viswambharan H, Sukumar P, Cubbon RM, Galloway S, Imrie H, Skromna A, Smith J, Jackson CL, Kearney MT, Wheatcroft SB. Endothelium-specific insulin resistance leads to accelerated atherosclerosis in areas with disturbed flow patterns: a role for reactive oxygen species. Atherosclerosis 230: 131–139, 2013. doi: 10.1016/j.atherosclerosis.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 40. Masaki N, Ido Y, Yamada T, Yamashita Y, Toya T, Takase B, Hamburg NM, Adachi T. Endothelial insulin resistance of freshly isolated arterial endothelial cells from radial sheaths in patients with suspected coronary artery disease. J Am Heart Assoc 8: e010816, 2019. doi: 10.1161/JAHA.118.010816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu I-H, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, Clermont A, Geraldes P, Dall'Osso C, Wagers AJ, Huang PL, Rekhter M, Scalia R, Kahn CR, King GL. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein e null mice. Cell Metab 11: 379–389, 2010. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sengupta A, Patel PA, Yuldasheva NY, Mughal RS, Galloway S, Viswambharan H, Walker AMN, Aziz A, Smith J, Ali N, Mercer BN, Imrie H, Sukumar P, Wheatcroft SB, Kearney MT, Cubbon RM. Endothelial insulin receptor restoration rescues vascular function in male insulin receptor haploinsufficient mice. Endocrinology 159: 2917–2925, 2018. doi: 10.1210/en.2018-00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kanter JE, Kramer F, Barnhart S, Duggan JM, Shimizu-Albergine M, Kothari V, Chait A, Bouman SD, Hamerman JA, Hansen BF, Olsen GS, Bornfeldt KE. A novel strategy to prevent advanced atherosclerosis and lower blood glucose in a mouse model of metabolic syndrome. Diabetes 67: 946–959, 2018. doi: 10.2337/db17-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Physiol 301: E252–E263, 2011. doi: 10.1152/ajpendo.00186.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kanter JE, Bornfeldt KE. Evidence stacks up that endothelial insulin resistance is a culprit in atherosclerosis. Circ Res 113: 352–354, 2013. doi: 10.1161/CIRCRESAHA.113.301998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pierce GL. Targeting vascular endothelial cell insulin resistance in type 2 diabetes mellitus: is protein kinase cβ the bullseye for reducing vascular risk in diabetes? Circulation 127: 16–18, 2013. doi: 10.1161/CIRCULATIONAHA.112.150813. [DOI] [PubMed] [Google Scholar]
- 47. Williams KJ, Wu X. Imbalanced insulin action in chronic over nutrition: clinical harm, molecular mechanisms, and a way forward. Atherosclerosis 247: 225–282, 2016. [Erratum in Atherosclerosis 328: 60–61, 2021]. doi: 10.1016/j.atherosclerosis.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 48. Liu C, Feng X, Li Q, Wang Y, Li Q, Hua M. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: a systematic review and meta-analysis. Cytokine 86: 100–109, 2016. doi: 10.1016/j.cyto.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 49. Dou H, Feher A, Davila AC, Romero MJ, Patel VS, Kamath VM, Gooz MB, Rudic RD, Lucas R, Fulton DJ, Weintraub NL, Bagi Z. Role of adipose tissue endothelial ADAM17 in age-related coronary microvascular dysfunction. Arterioscler Thromb Vasc Biol 37: 1180–1193, 2017. doi: 10.1161/ATVBAHA.117.309430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su JL, Becherer JD. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-α. Nature 385: 733–736, 1997. [Erratum in Nature 386: 738, 1997]. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 51. Horiuchi K. A brief history of tumor necrosis factor α–converting enzyme: an overview of ectodomain shedding. Keio J Med 62: 29–36, 2013. doi: 10.2302/kjm.2012-0003-re. [DOI] [PubMed] [Google Scholar]
- 52. Arribas J, Esselens C. ADAM17 as a therapeutic target in multiple diseases. Curr Pharm Des 15: 2319–2335, 2009. doi: 10.2174/138161209788682398. [DOI] [PubMed] [Google Scholar]
- 53. Canale MP, Menghini R, Martelli E, Federici M. COVID-19-associated endothelial dysfunction and microvascular injury: from pathophysiology to clinical manifestations. Card Electrophysiol Clin 14: 21–28, 2022. doi: 10.1016/j.ccep.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hedges JF, Snyder DT, Robison A, Grifka-Walk HM, Blackwell K, Shepardson K, Kominsky D, Rynda-Apple A, Walcheck B, Jutila MA. An ADAM17-neutralizing antibody reduces inflammation and mortality while increasing viral burden in a COVID-19 mouse model. Front Immunol 13: 918881, 2022. doi: 10.3389/fimmu.2022.918881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lartey NL, Valle-Reyes S, Vargas-Robles H, Jiménez-Camacho KE, Guerrero-Fonseca IM, Castellanos-Martínez R, Montoya-García A, García-Cordero J, Cedillo-Barrón L, Nava P, Filisola-Villaseñor JG, Roa-Velázquez D, Zavala-Vargas DI, Morales-Ríos E, Salinas-Lara C, Vadillo E, Schnoor M. ADAM17/MMP inhibition prevents neutrophilia and lung injury in a mouse model of COVID-19. J Leukoc Biol 111: 1147–1158, 2022. doi: 10.1002/JLB.3COVA0421-195RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang K, Deng H, Song B, He J, Liu S, Fu J, Zhang L, Li D, Balaji KS, Mei Z, Cheng J, Fu J. The correlation between immune invasion and SARS-COV-2 entry protein ADAM17 in cancer patients by bioinformatic analysis. Front Immunol 13: 923516, 2022. doi: 10.3389/fimmu.2022.923516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest 104: 447–457, 1999. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bakker EN, van der Meulen ET, van den Berg BM, Everts V, Spaan JA, VanBavel E. Inward remodeling follows chronic vasoconstriction in isolated resistance arteries. J Vasc Res 39: 12–20, 2002. doi: 10.1159/000048989. [DOI] [PubMed] [Google Scholar]
- 59. Wang X, Ohnishi M, Wada A, Tsutamoto T, Sawaki M, Fujii M, Matsumoto T, Yamamoto T, Kurokawa K, Yamada H, Kinoshita M. Endothelin-1 promotes vascular structural remodeling during the progression of heart failure prevention of vascular remodeling using a specific endothelin-converting enzyme inhibitor. Life Sci 69: 2477–2488, 2001. doi: 10.1016/s0024-3205(01)01333-9. [DOI] [PubMed] [Google Scholar]
- 60. Jurrissen TJ, Grunewald ZI, Woodford ML, Winn NC, Ball JR, Smith TN, Wheeler AA, Rawlings AL, Staveley-O'Carroll KF, Ji Y, Fay WP, Paradis P, Schiffrin EL, Vieira-Potter VJ, Fadel PJ, Martinez-Lemus LA, Padilla J. Overproduction of endothelin-1 impairs glucose tolerance but does not promote visceral adipose tissue inflammation or limit metabolic adaptations to exercise. Am J Physiol Endocrinol Physiol 317: E548–E558, 2019. doi: 10.1152/ajpendo.00178.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Padilla J, Jenkins NT, Thorne PK, Lansford KA, Fleming NJ, Bayless DS, Sheldon RD, Rector RS, Laughlin MH. Differential regulation of adipose tissue and vascular inflammatory gene expression by chronic systemic inhibition of NOS in lean and obese rats. Physiol Rep 2: e00225, 2014. doi: 10.1002/phy2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Morawietz H, Talanow R, Szibor M, Rueckschloss U, Schubert A, Bartling B, Darmer D, Holtz J. Regulation of the endothelin system by shear stress in human endothelial cells. J Physiol 525: 761–770, 2000. doi: 10.1111/j.1469-7793.2000.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Padilla J, Carpenter A, Das ND, Kandikattu HK, Lopez-Ongil S, Martinez-Lemus LA, Siebenlist U, DeMarco VG, Chandrasekar B. TRAF3IP2 mediates high glucose-induced endothelin-1 production as well as endothelin-1-induced inflammation in endothelial cells. Am J Physiol Heart Circ Physiol H52–H64, 2017. doi: 10.1152/ajpheart.00478.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Valente AJ, Irimpen AM, Siebenlist U, Chandrasekar B. OxLDL induces endothelial dysfunction and death via TRAF3IP2: inhibition by HDL3 and AMPK activators. Free Radic Biol Med 70: 117–128, 2014. doi: 10.1016/j.freeradbiomed.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sakamuri S, Higashi Y, Sukhanov S, Siddesha JM, Delafontaine P, Siebenlist U, Chandrasekar B. TRAF3IP2 mediates atherosclerotic plaque development and vulnerability in ApoE(-/-) mice. Atherosclerosis 252: 153–160, 2016. doi: 10.1016/j.atherosclerosis.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mummidi S, Das NA, Carpenter AJ, Yoshida T, Yariswamy M, Mostany R, Izadpanah R, Higashi Y, Sukhanov S, Noda M, Siebenlist U, Rector RS, Chandrasekar B. RECK suppresses interleukin-17/TRAF3IP2-mediated MMP-13 activation and human aortic smooth muscle cell migration and proliferation. J Cell Physiol 234: 22242–22259, 2019. doi: 10.1002/jcp.28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Venkatesan B, Valente AJ, Das NA, Carpenter AJ, Yoshida T, Delafontaine JL, Siebenlist U, Chandrasekar B. CIKS (Act1 or TRAF3IP2) mediates high glucose-induced endothelial dysfunction. Cell Signal 25: 359–371, 2013. doi: 10.1016/j.cellsig.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Grunewald ZI, Ramirez-Perez FI, Woodford ML, Morales-Quinones M, Mejia S, Manrique-Acevedo C, Siebenlist U, Martinez-Lemus LA, Chandrasekar B, Padilla J. TRAF3IP2 (TRAF3 interacting protein 2) mediates obesity-associated vascular insulin resistance and dysfunction in male mice. Hypertension 76: 1319–1329, 2020. doi: 10.1161/HYPERTENSIONAHA.120.15262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Olver TD, Laughlin MH, Padilla J. Exercise and vascular insulin sensitivity in the skeletal muscle and brain. Exerc Sport Sci Rev 47: 66–74, 2019. doi: 10.1249/JES.0000000000000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Padilla J, Olver TD, Thyfault JP, Fadel PJ. Role of habitual physical activity in modulating vascular actions of insulin. Exp Physiol 100: 759–771, 2015. doi: 10.1113/EP085107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Walsh LK, Ghiarone T, Olver TD, Medina-Hernandez A, Edwards JC, Thorne PK, Emter CA, Lindner JR, Manrique-Acevedo C, Martinez-Lemus LA, Padilla J. Increased endothelial shear stress improves insulin-stimulated vasodilatation in skeletal muscle. J Physiol 597: 57–69, 2019. doi: 10.1113/JP277050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Foote CA, Soares RN, Ramirez-Perez FI, Ghiarone T, Aroor A, Manrique-Acevedo C, Padilla J, Martinez-Lemus L. Endothelial glycocalyx. Comprehensive Physiology 12: 1–31, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pahakis MY, Kosky JR, Dull RO, Tarbell JM. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun 355: 228–233, 2007. doi: 10.1016/j.bbrc.2007.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tarbell JM, Pahakis MY. Mechanotransduction and the glycocalyx. J Intern Med 259: 339–350, 2006. doi: 10.1111/j.1365-2796.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 75. Groen BB, Hamer HM, Snijders T, van Kranenburg J, Frijns D, Vink H, van Loon LJ. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J Appl Physiol (1985) 116: 998–1005, 2014. doi: 10.1152/japplphysiol.00919.2013. [DOI] [PubMed] [Google Scholar]
- 76. Kawahara R, Granato DC, Yokoo S, Domingues RR, Trindade DM, Paes Leme AF. Mass spectrometry-based proteomics revealed Glypican-1 as a novel ADAM17 substrate. J Proteomics 151: 53–65, 2017. doi: 10.1016/j.jprot.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 77. Bartosch AMW, Mathews R, Mahmoud MM, Cancel LM, Haq ZS, Tarbell JM. Heparan sulfate proteoglycan glypican-1 and PECAM-1 cooperate in shear-induced endothelial nitric oxide production. Sci Rep 11: 11386, 2021. doi: 10.1038/s41598-021-90941-w. [DOI] [PMC free article] [PubMed] [Google Scholar]