Keywords: bedding, hypertension, kidney damage, salt
Abstract
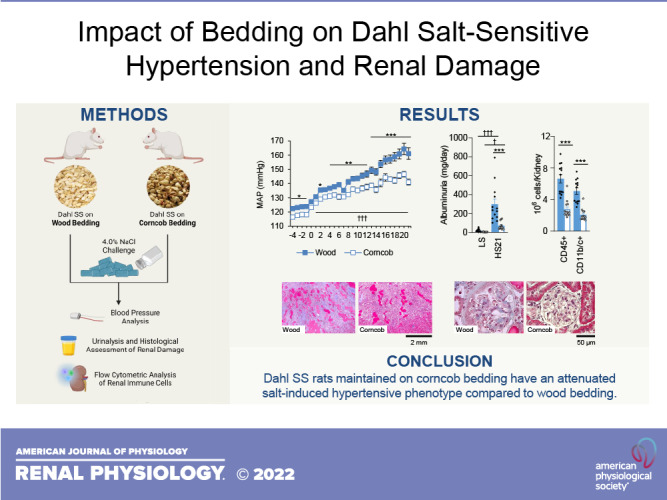
Salt-sensitive hypertension, increases in blood pressure in response to increased salt intake, is associated with an increased risk of morbidity, mortality, and end-organ damage compared with salt-resistant hypertension. The Dahl salt-sensitive (SS) rat mimics the phenotypic characteristics observed in human hypertension when rats are challenged with a high-salt diet. Our previous work demonstrated that environmental factors, such as dietary protein, alter the severity of salt sensitivity in Dahl SS rats and should be an important consideration in experimental design. The present study investigated how the bedding on which animals were maintained (wood vs. corncob) could impact the SS phenotype in the Dahl SS rat. Animals that were maintained on corncob bedding exhibited a significant attenuation in blood pressure and renal end-organ damage in response to a high-salt diet compared with animals maintained on wood bedding. This attenuation was associated with an improvement in renal function and reduction in immune cell infiltration into the kidneys of Dahl SS rats maintained on corncob bedding. These results indicate that the type of bedding impacts the SS phenotype in the Dahl SS rat and that the bedding used in experiments can be a confounding factor to consider during data interpretation and experimental design.
NEW & NOTEWORTHY Results from our present study demonstrate the profound effect of animal bedding on the severity of salt-sensitive hypertension, renal damage, and inflammation in Dahl salt-sensitive rats. This study highlights the important consideration that should be given to environmental factors, namely, the type of bedding in animal facilities, in experimental design and data interpretation.
INTRODUCTION
Hypertension is a major risk factor for cardiovascular diseases, which continues to be the leading cause of mortality in the United States (1). Hypertension affects ∼50% of the United States adult population and is associated with an increased risk of developing kidney disease (2). There is a subset of individuals with hypertension who exhibit salt-sensitive (SS) hypertension, which is associated with increased end-organ damage and higher risk of mortality relative to individuals with salt-resistant hypertension (3). Although the exact mechanisms responsible for SS hypertension remain unclear, it has been shown that environmental factors, such as diet and time of the year, influence the severity of hypertension in both humans (4, 5) and preclinical models (6, 7).
The Dahl SS rat model develops hypertension, albuminuria, and increased renal infiltration of immune cells in response to a high-salt (HS) challenge that mimics observed phenotypes in SS humans. In addition to the Na+ content found within the diet, Na+-independent components of the diet can influence the severity of salt sensitivity in the Dahl SS rat (6–9). By replacing the dietary protein source from an animal-based source to a plant-based source, there is a significant attenuation in salt sensitivity, demonstrating the impact of environmental factors on disease phenotypes in the Dahl SS rat. In addition to dietary manipulations, bedding material has been shown to impact metabolic phenotypes in caloric restriction experiments in rodents (10, 11), demonstrating the importance of all environmental factors.
Our laboratory relocated to Augusta University in July 2019 from the Medical College of Wisconsin, where our rat colony originated. Although relocating our entire colony to a new location would undoubtedly introduce new environmental factors, the first noticeable difference between the institutions was the type of bedding used for animal husbandry. The present study was designed to determine whether bedding modifies the salt-induced hypertensive and renal damage phenotype in the Dahl SS rat. Based on our previous work demonstrating the protective effect of plant-based diets, we used data collected from multiple experiments performed at Augusta University to test the hypothesis that Dahl SS rats housed on corncob-based bedding would exhibit attenuated SS hypertension and renal damage relative to rats maintained on aspen wood-based bedding.
METHODS
Animal Care and Use
All protocols and procedures were in accordance with and approved by the Augusta University Institutional Animal Care and Use Committee. All experiments were performed in male and female inbred Dahl SS rats from a colony maintained at Augusta University (AU); the founders of the AU colony originated from the Medical College of Wisconsin (SS/JrHsdMcwi). Rats were maintained ad libitum on a 0.4% NaCl [low-salt (LS)] AIN-76A purified diet (No. 113755, Dyets) throughout their lives until ∼9 wk of age, followed by a 21-day HS diet challenge (4.0% NaCl, AIN76A, No. 113756, Dyets). The two types of bedding used in this study were aspen wood bedding (aspen, Sani Chips, P. J. Murphy, Wood) and corncob bedding (4B–¼'', Bed-o’Cobs, Corncob). Animals were maintained on their respective bedding for the entirety of their lives. All chow, water, bedding, and cage materials were autoclaved before the animals were placed in the cages. All animals were euthanized between 7:00 and 9:00 AM following the 21-day HS challenge.
Meta-Analysis
To assess the impact of bedding on the SS phenotype in the Dahl SS rat, the data presented are a compilation of Dahl SS rats phenotyped from six different studies (n = 2–11 rats/study) performed in our laboratory from July 2020 to January 2022. The phenotyping of animals placed on corncob or wood bedding was not performed concurrently, and these comparisons were studied at different times. All animals were fed the same highly purified diet during both the LS and HS periods and underwent the same HS protocol detailed previously. All experimental protocols were performed in the same experimental room using the same Data Science Ponemah Telemetry system. Furthermore, all blood pressure data were subjected to the same analyses and thresholds. Blood pressure was measured for 10 s every 2 min throughout the experiment. These intervals were averaged over a 24-h period for each animal, excluding data points when pulse pressures were <10 or >70 mmHg.
Blood Pressure Monitoring and Urinalysis
At 8 wk of age, rats were placed under deep anesthesia by inhalation of isoflurane for instrumentation of radio telemeters (Data Science) into their right carotid arteries for continuous measurement of mean arterial pressure (MAP) as previously described (12, 13). Animals were treated with antibiotics [cefazolin (25 mg/kg)] and an analgesic [Buprenorphine-SR (0.3 mg/kg)] postoperatively. After a recovery period, baseline LS blood pressure measurements were made before a 21-day HS challenge. To assess markers of renal damage and electrolyte excretion, urine was collected via metabolic cages while animals were on LS as well as on HS days 7, 14, and 21. Urine electrolytes were assessed using an autoanalyzer (Alfa Wasserman). Urine albumin was quantified using a fluorescent assay to analyze albumin blue 580 dye (Molecular Probes) on a fluorescent plate reader (FL600, BioTek), and urine protein was quantified using Weichselbaum’s biuret reagent.
Immune Cell Isolation and Flow Cytometric Analysis
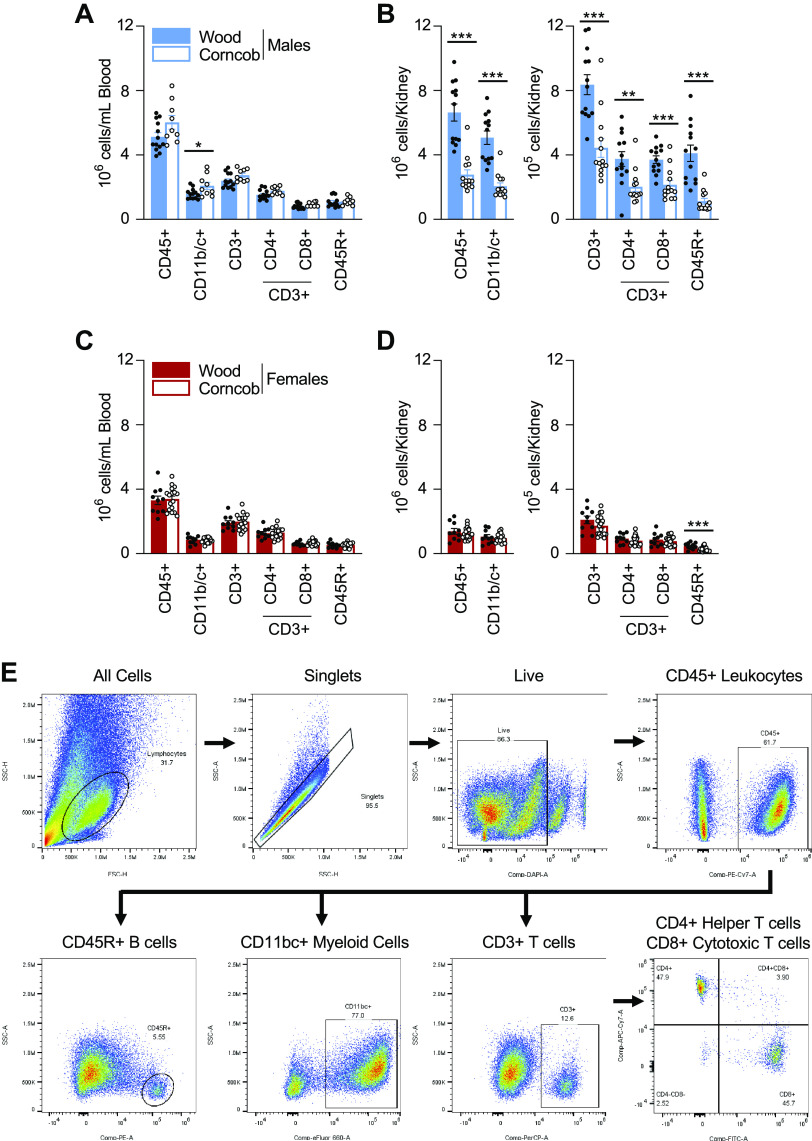
At the end of the study, rats were anesthetized with isoflurane, and abdominal aortas were catheterized for the collection of blood for circulating immune cells. Kidneys were then flushed using heparinized Dulbecco’s PBS to remove blood and circulating immune cells. Immune cells were isolated as previously described (12, 14). In short, circulating immune cells were layered over HisTopaque-1083 (Sigma-Aldrich). Renal immune cells were isolated by mincing the left kidney and incubated in RPMI-1640 media containing l-glutamine, HEPES, collagenase type IV, and DNase. The kidney homogenate was passed through a series of filters to enrich the immune cell populations before being layered over Percoll for density gradient centrifugation. Immune cells were isolated from the mononuclear layer and counted using a hemocytometer, and 1 million cells were taken from each sample and incubated with the extracellular markers anti-CD45 (No. 202214, BioLegend) for leukocytes, anti-CD3 (No. 46-0030-82, eBioscience) for T cells, CD4 (No. 201518, BioLegend) for T helper cells, CD8 (No. 201703, BioLegend) for cytotoxic T cells, anti-CD11b/c (No. 50-0110-82, eBioscience) for monocytes/macrophages, and anti-CD45R (No. 554881, BD Bioscience) for B cells, as well as for live/dead staining via DAPI (No. 422801, BioLegend). All cells were analyzed via flow cytometry (5-Laser AURORA Spectral Cytometer, Cytek) with FlowJo software (Tree Star). An example of our gating strategy is shown in Fig. 4E.
Figure 4.
A and C: isolated circulating immune cells between rats on corncob versus wood bedding in males (A) and females (C). B and D: immune cells isolated from the kidneys of male (B) and female (D) rats on corncob and wood bedding. E: representative gating strategy. CD45+, leukocytes; CD11b/c+, monocytes/macrophages; CD3+, T cells; CD4+, T helper cells; CD8+, cytotoxic T cells; CD45R+, B cells. n = 9–19. Data were analyzed by a t test within sex. Filled symbols indicate salt-sensitive rats on wood; open symbols indicate salt-sensitive rats on corncob. *P < 0.05, **P < 0.01, and ***P < 0.001, corncob vs. wood.
Histological Analysis
Right kidneys were fixed in 10% neutral buffered formalin, paraffin embedded, cut into 5-µm sections, mounted, and stained with Masson’s trichrome. Slides were scanned using a Leica Laser Microdissection Microscope (LMD6). Outer medullary protein cast percentages were determined by color inclusion via ImageJ software (version 1.53k, National Institutes of Health). At least 45 individual glomeruli from eight kidneys within each group were scored based on a semiquantitative index method and assigned an injury score of 0–4 by a blinded scorer.
Statistical Analysis
Data are presented as means ± SE. Data were considered significant if P < 0.05 using a Student’s t test or two-way ANOVA with a Holm–Sidak post hoc test, depending on comparisons made.
RESULTS
Dahl SS Rats Maintained on Corncob Bedding Have an Attenuation in Salt-Induced Hypertension Compared With on Wood Bedding
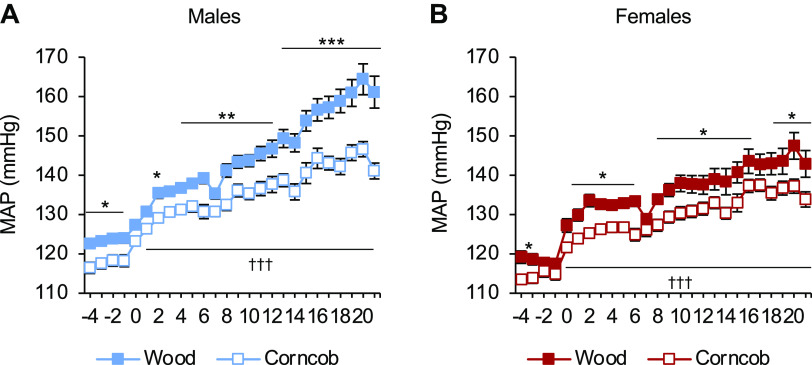
While maintained on LS, there was a significantly lower MAP in the rats maintained on corncob bedding relative to the rats maintained on wood bedding in both sexes. After the HS challenge, both male rats (141 ± 2 vs. 161 ± 4 mmHg, corncob vs. wood; Fig. 1A) and female rats (134 ± 2 vs. 143 ± 3 mmHg, corncob vs. wood) maintained on corncob bedding had a significantly lower MAP relative to rats maintained on wood bedding (Fig. 1B). Furthermore, there was a significant change in blood pressure (ΔHS20-LS-1) between the different bedding types for both male (40 ± 4 vs. 29 ± 2 mmHg, P < 0.05, wood vs. corncob) and female (30 ± 2 vs. 22 ± 2 mmHg, P < 0.05, wood vs. corncob) rats. This demonstrated that the rats maintained on corncob bedding had significantly lower blood pressure than their wood bedding counterparts, regardless of sex. In addition, there was a statistically significant difference in MAP between sexes after allowing for effects of differences in the bedding (P < 0.001) at HS day 21.
Figure 1.
Attenuation in salt-induced hypertension in Dahl salt-sensitive rats on corncob bedding regardless of sex. A and B: daily mean arterial pressure (MAP) averages of male (A) and female (B) Dahl salt-sensitive rats. n = 7–15. Data were analyzed by two-way ANOVA with repeated measures followed by a Holm–Sidak post hoc test for multiple comparisons. *P < 0.05, **P < 0.01, and ***P < 0.001, corncob vs. wood; †††P < 0.001, low salt vs. high salt.
Dahl SS Rats Maintained on Corncob Bedding Have Less Renal Damage Than Rats on Wood Bedding Despite Greater Electrolyte Excretion Following a HS Challenge
Similar to the blood pressure response to HS, male SS rats maintained on corncob bedding developed less renal damage, marked by less proteinuria (Fig. 2A) and albuminuria (Fig. 2B), relative to the rats maintained on wood bedding. Although food and water intake were not measured in these animals, rats maintained on corncob bedding exhibited higher levels of both K+ (Fig. 2C) and Na+ (Fig. 2D) excretion rates after the HS challenge, suggesting a greater consumption of chow than rats maintained on wood bedding. In addition, the urinary flow rate at HS day 21 was comparable between male rats (53.2 ± 2.8 vs. 54.9 ± 3.1 mL/day, wood vs. corncob, P > 0.05), suggesting that water consumption was similar between groups. This same trend was also observed in female Dahl SS rats maintained on corncob bedding relative to wood bedding (Fig. 2, E–H). Urine flow rates were comparable between female rats at HS day 21 as well (40.1.2 ± 6.3 vs. 35.4 ± 3.0 mL/day, wood vs. corncob, P > 0.05). These data suggest that perhaps the corncob-bedded animals might be more proficient at excreting excess electrolytes. Future studies will more precisely examine how the different bedding types impact the renal handling of electrolytes. Similar to the blood pressure response to HS, there was a statistically significant difference in urinary albumin and protein excretion rates between the sexes following a 21-day HS challenge (P < 0.01).
Figure 2.
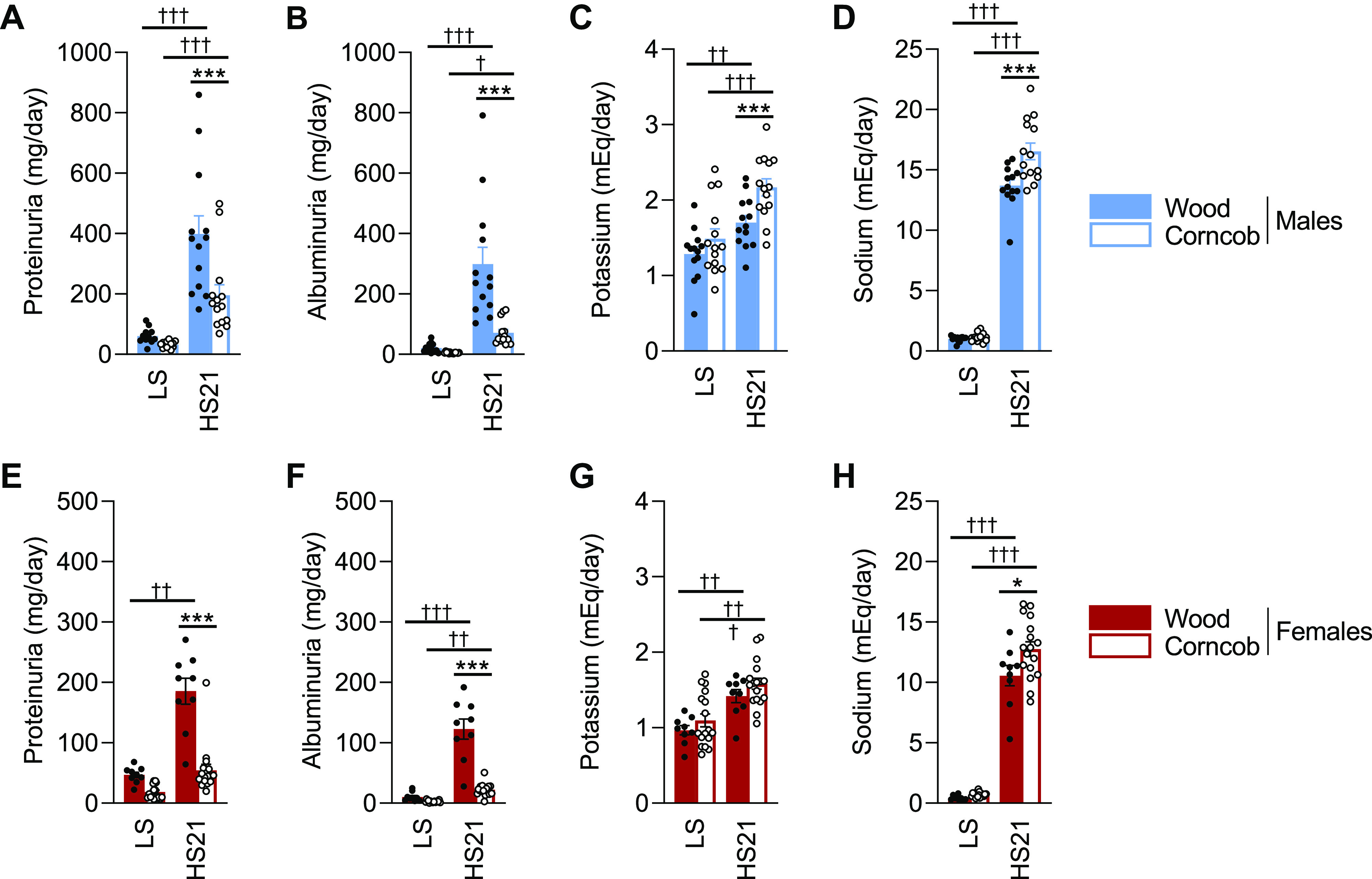
Despite higher excretion rates of K+ and Na+ at the end of the study, Dahl salt-sensitive (SS) rats maintained on corncob bedding exhibited decreased proteinuria and albuminuria compared with those maintained on wood bedding. A−H: baseline and high salt (HS) day 21 (HS21) measurements recorded for urinary protein (A and E), albumin (B and F), K+ excretion (C and G), and Na+ excretion (D and H) in males and females, respectively. n = 9–17. Data were analyzed by two-way ANOVA with repeated measures followed by a Holm–Sidak post hoc test for multiple comparisons. Filled symbols indicate SS rats on wood; open symbols indicate SS rats on corncob. *P < 0.05 and ***P < 0.001, corncob vs. wood; †P < 0.05, ††P < 0.01, and †††P < 0.001, low salt (LS) vs. HS.
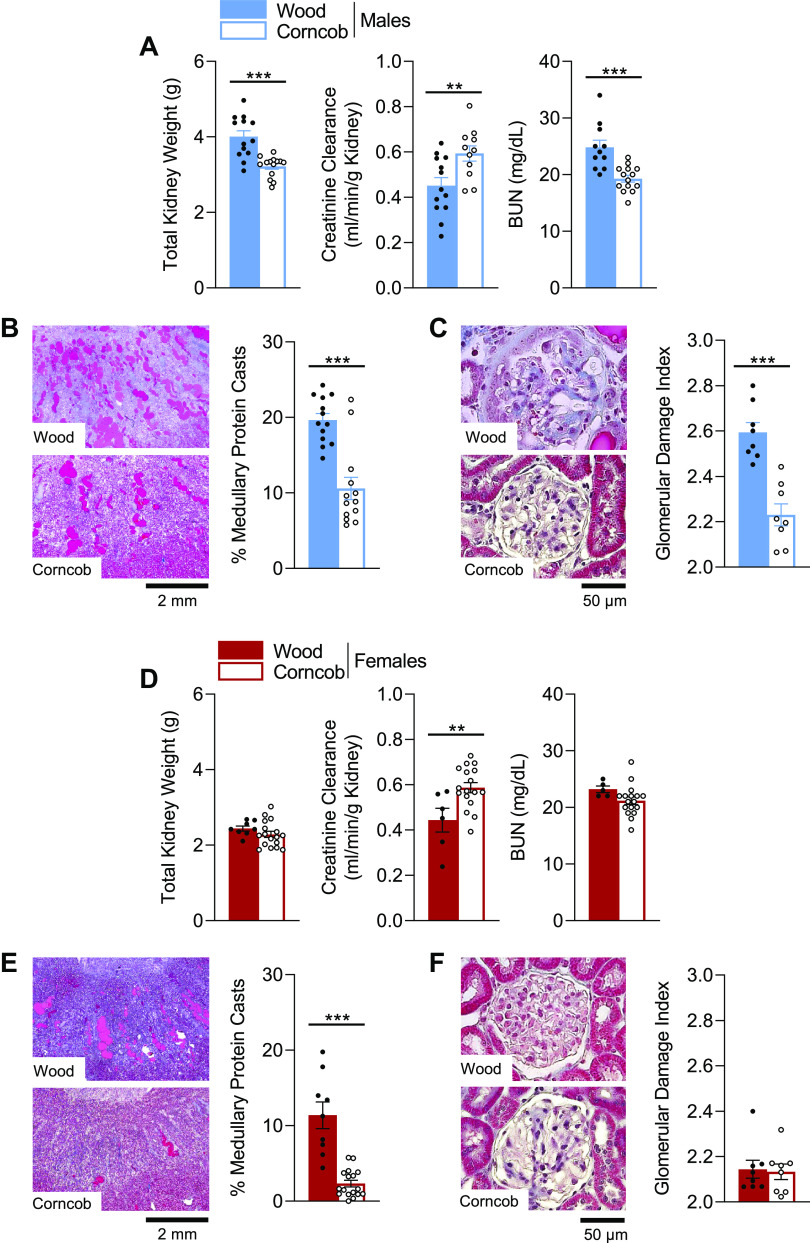
Dahl SS Rats Maintained on Wood Bedding Exhibit Impairments in Renal Function and Histological Damage in Response to a HS Diet
Following HS, male SS rats maintained on wood bedding exhibited significant renal hypertrophy that was accompanied with a decrease in creatinine clearance and an increase in blood urea nitrogen levels relative to rats maintained on corncob bedding (Fig. 3A). These alterations in renal function also corresponded with a significant increase in renal histological damage, indicated by a greater percentage of protein casts in the outer medulla of the kidneys stained with Masson’s trichrome (Fig. 3B) and greater damage to glomeruli (Fig. 3C). In female SS rats, there was no difference observed in total kidney weight or blood urea nitrogen levels between the two bedding groups; however, there was a significant reduction in creatinine clearance in the wood relative to the corncob bedding group (Fig. 3D). There was also significantly less histological damage and medullary protein casts observed in female rats maintained on corncob compared with wood bedding (Fig. 3E), yet there was no difference observed in glomerular injury between female groups (Fig. 3F).
Figure 3.
Indexes of renal damage and function are attenuated in Dahl salt-sensitive (SS) rats maintained on corncob bedding compared with rats maintained on wood bedding. A and D: renal damage assessed by combined kidney weight, creatinine clearance, and blood urea nitrogen (BUN). B, C, E, and F: kidney histological slides stained with Masson’s trichrome and quantification of medullary protein casts (B and E) and glomerular scoring (C and F) demonstrating the protection that corncob bedding provided. n = 5–17. Data were analyzed by a t test within sex. Filled symbols indicate SS rats on wood; open symbols indicate SS rats on corncob. **P < 0.01 and ***P < 0.001, corncob vs. wood.
Greater Infiltration of Immune Cells Into Kidneys of Male Dahl SS Rats Maintained on Wood Bedding Relative to Corncob Bedding, With No Difference Observed in Females
In male rats, there were comparable numbers of circulating immune cells for a majority of the populations between the two bedding groups, except for a slight increase in CD11b/c+ monocytes and macrophages in the rats maintained on corncob bedding (Fig. 4A). Following HS, male rats maintained on corncob bedding had fewer infiltrating immune cells into the kidney across all populations relative to the rats maintained on wood bedding (Fig. 4B). In female rats, there were no differences in the number of immune cells in the circulation between the different bedding types (Fig. 4C). In female kidneys, the only significant difference observed was a reduction in CD45R+ B cells in the rats maintained on corncob bedding compared with wood bedding (Fig. 4D).
DISCUSSION
This study demonstrated the importance of bedding on salt-induced hypertension and renal damage in both male and female Dahl SS rats. Male Dahl SS rats maintained on corncob bedding had a blunted SS phenotype, demonstrated by an attenuation in both blood pressure and renal damage compared with male rats maintained on wood bedding. In addition, there was an accompanying improvement in renal function and less infiltration of immune cells into the kidneys of rats maintained on corncob bedding. Although female Dahl SS rats maintained on corncob bedding also displayed an attenuation in their SS phenotype relative to female Dahl SS rats maintained on wood bedding, there were no differences observed in the number of renal immune cells between these groups, except for CD45R+ B cells.
Environmental factors, such as dietary intake, have been shown to impact the development of hypertension and renal damage in the Dahl SS rat (6–9, 12). For example, a simple dietary protein substitution from an animal-based protein to a gluten- or plant-based protein resulted in a significant attenuation in the SS phenotype in the Dahl SS rat, which was associated with a decrease in the number of infiltrating immune cells in the kidneys, despite being challenged with the same HS diet. These studies demonstrated how important it is to control for environmental factors when designing experiments. Despite trying to control for all factors, there are some factors that are often overlooked when analyzing and interpreting data. Seasonal effects have been shown in humans to contribute to blood pressure variability and overall incidence of cardiovascular disease risk depending on the season (5). There are studies that have demonstrated a role for circadian rhythms in the control of blood pressure (15, 16), yet the time of day when experiments are performed is often not included in the methods of a report. The details for animal husbandry, such as diet composition as well as the type of bedding used in animal facilities, are often underreported in studies (17). It is evident from the present study that environmental factors such as bedding had profound effects on the disease phenotype in Dahl SS rats.
Recently, our group demonstrated a causal role for the gut microbiota to contribute to the severity of salt sensitivity in blood pressure and end-organ damage in the Dahl SS rat (18). There are reports of bedding affecting the composition of the microbiota in rodents due to the animals consuming the bedding during caloric-restricted time periods (10). By consuming the bedding during the restricted feeding, there were alterations in the microbiome composition and metabolic phenotypes in mice maintained on corncob bedding relative to those maintained on wood or cellulose. Similarly, rats placed on corncob bedding had alterations in large bowel fermentation due to ingestion of the bedding (11). We have previously shown that dietary manipulations alter both gene expression and DNA methylation status of renal T cells, shifting the function of these T cells to a more metabolic profile, demonstrating how environmental factors can alter cell function (12, 13). Taking this information into consideration, we can postulate that the attenuation in salt sensitivity observed in Dahl SS rats maintained on corncob bedding could be due to alterations in gut microbiota composition and potentially subsequent shifts in immune cell function. Since these studies were not run concurrently, we cannot claim that these phenotypic differences are solely due to bedding alone. Due to the duration of time, it is possible that other variables, such as seasonal effects or intrastrain variability, could have some impact on the salt sensitivity of Dahl SS rats. Although further investigations are needed to test this hypothesis, it is evident from the present study that all environmental factors should be considered when designing experimental protocols and reported in publications.
GRANTS
This work was supported by National Institutes of Health Grants HL116264, HL137748, and K99HL157549-01A1 and by American Heart Association Grant 19CDA34660184.
DISCLOSURES
J.H.D. is an editor of the American Journal of Physiology-Renal Physiology and was not involved and did not have access to information regarding the peer-review process or final disposition of this article. An alternate editor oversaw the peer review and decision-making process for this article. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
J.H.D., S.D.W., E.C.B., J.M.A., and D.L.M. conceived and designed research; J.H.D., S.D.W., E.C.B., M.C., J.M.A., and D.L.M. performed experiments; J.H.D., S.D.W., E.C.B., M.C., J.M.A., and D.L.M. analyzed data; J.H.D., S.D.W., E.C.B., M.C., J.M.A., and D.L.M. interpreted results of experiments; J.H.D. and J.M.A. prepared figures; J.H.D., S.D.W., E.C.B., and M.C. drafted manuscript; J.H.D., S.D.W., E.C.B., J.M.A., and D.L.M. edited and revised manuscript; J.H.D., S.D.W., E.C.B., M.C., J.M.A., and D.L.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Douglas Taylor, Cari Ross, Levi Trusty, Leah Busbin, and the Division of Laboratory Animals Sciences at Augusta University for the assistance during these experiments.
REFERENCES
- 1. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart Disease and Stroke Statistics-2021 Update: a report from the American Heart Association. Circulation 143: e254–e743, 2021. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2. Thomas G, Felts J, Brecklin CS, Chen J, Drawz PE, Lustigova E, Mehta R, Miller ER 3rd, Sozio SM, Weir MR, Xie D, Wang X, Rahman M. Apparent treatment-resistant hypertension assessed by office and ambulatory blood pressure in chronic kidney disease-a report from the chronic renal insufficiency cohort study. Kidney360 1: 810–818, 2020. doi: 10.34067/KID.0002072020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 37: 429–432, 2001. doi: 10.1161/01.HYP.37.2.429. [DOI] [PubMed] [Google Scholar]
- 4. Richter CK, Skulas-Ray AC, Champagne CM, Kris-Etherton PM. Plant protein and animal proteins: do they differentially affect cardiovascular disease risk? Adv Nutr 6: 712–728, 2015. doi: 10.3945/an.115.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Narita K, Hoshide S, Kario K. Seasonal variation in day-by-day home blood pressure variability and effect on cardiovascular disease incidence. Hypertension 79: 2062–2070, 2022. doi: 10.1161/HYPERTENSIONAHA.122.19494. [DOI] [PubMed] [Google Scholar]
- 6. Mattson DL, Kunert MP, Kaldunski ML, Greene AS, Roman RJ, Jacob HJ, Cowley AW Jr. Influence of diet and genetics on hypertension and renal disease in Dahl salt-sensitive rats. Physiol Genomics 16: 194–203, 2004. doi: 10.1152/physiolgenomics.00151.2003. [DOI] [PubMed] [Google Scholar]
- 7. Mattson DL, Meister CJ, Marcelle ML. Dietary protein source determines the degree of hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 45: 736–741, 2005. doi: 10.1161/01.HYP.0000153318.74544.cc. [DOI] [PubMed] [Google Scholar]
- 8. Abais-Battad JM, Lund H, Fehrenbach DJ, Dasinger JH, Alsheikh AJ, Mattson DL. Parental dietary protein source and the role of CMKLR1 in determining the severity of Dahl salt-sensitive hypertension. Hypertension 73: 440–448, 2019. doi: 10.1161/HYPERTENSIONAHA.118.11994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geurts AM, Mattson DL, Liu P, Cabacungan E, Skelton MM, Kurth TM, Yang C, Endres BT, Klotz J, Liang M, Cowley AW Jr.. Maternal diet during gestation and lactation modifies the severity of salt-induced hypertension and renal injury in Dahl salt-sensitive rats. Hypertension 65: 447–455, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gregor A, Fragner L, Trajanoski S, Li W, Sun X, Weckwerth W, König J, Duszka K. Cage bedding modifies metabolic and gut microbiota profiles in mouse studies applying dietary restriction. Sci Rep 10: 20835, 2020. doi: 10.1038/s41598-020-77831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Le Leu RK, Conlon MA, Bird AR, Clarke JM. Housing experimental rats in solid-based cages with digestible bedding may confound outcomes of nutritional studies. J Sci Food Agric 95: 2155–2158, 2015. doi: 10.1002/jsfa.6919. [DOI] [PubMed] [Google Scholar]
- 12. Abais-Battad JM, Alsheikh AJ, Pan X, Fehrenbach DJ, Dasinger JH, Lund H, Roberts ML, Kriegel AJ, Cowley AW Jr, Kidambi S, Kotchen TA, Liu P, Liang M, Mattson DL. Dietary effects on Dahl salt-sensitive hypertension, renal damage, and the T lymphocyte transcriptome. Hypertension 74: 854–863, 2019. doi: 10.1161/HYPERTENSIONAHA.119.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dasinger JH, Alsheikh AJ, Abais-Battad JM, Pan X, Fehrenbach DJ, Lund H, Roberts ML, Cowley AW Jr, Kidambi S, Kotchen TA, Liu P, Liang M, Mattson DL. Epigenetic modifications in T cells: the role of DNA methylation in salt-sensitive hypertension. Hypertension 75: 372–382, 2020. doi: 10.1161/HYPERTENSIONAHA.119.13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fehrenbach DJ, Dasinger JH, Lund H, Zemaj J, Mattson DL. Splenocyte transfer exacerbates salt-sensitive hypertension in rats. Exp Physiol 105: 864–875, 2020. doi: 10.1113/EP088340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Douma LG, Costello HM, Crislip GR, Cheng KY, Lynch IJ, Juffre A, Barral D, Masten S, Roig E, Beguiristain K, Li W, Bratanatawira P, Wingo CS, Gumz ML. Kidney-specific KO of the circadian clock protein PER1 alters renal Na+ handling, aldosterone levels, and kidney/adrenal gene expression. Am J Physiol Renal Physiol 322: F449–F459, 2022. doi: 10.1152/ajprenal.00385.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hill AM, Crislip GR, Stowie A, Ellis I, Ramsey A, Castanon-Cervantes O, Gumz ML, Davidson AJ. Environmental circadian disruption suppresses rhythms in kidney function and accelerates excretion of renal injury markers in urine of male hypertensive rats. Am J Physiol Renal Physiol 320: F224–F233, 2021. doi: 10.1152/ajprenal.00421.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reinhardt V. Common husbandry-related variables in biomedical research with animals. Lab Anim 38: 213–235, 2004. doi: 10.1258/002367704323133600. [DOI] [PubMed] [Google Scholar]
- 18. Abais-Battad JM, Saravia FL, Lund H, Dasinger JH, Fehrenbach DJ, Alsheikh AJ, Zemaj J, Kirby JR, Mattson DL. Dietary influences on the Dahl SS rat gut microbiota and its effects on salt-sensitive hypertension and renal damage. Acta Physiol (Oxf) 232: e13662, 2021. doi: 10.1111/apha.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]