Abstract
Aluminum (Al) is an important factor in the environment as it is used in agriculture and several industries leading to hazardous effects via oxidative stress. Bromelain is a cheap extract from the byproduct waste of Ananas comosus stem. It has been used in several biological and therapeutic applications. So, this study was undertaken to assess the hepatoprotective potential of bromelain versus oxidative stress induced by aluminum chloride in rats. Results revealed that administration of AlCl3 reduced the body and liver weights and increased Al concentration in the blood and liver tissue. Also, AlCl3 caused valuable changes in hematological parameters and increased TBARS and H2O2 concentrations in rat liver. Enzymatic (SOD, CAT, GPx, GR, and GST) and nonenzymatic (GSH) antioxidants and protein content were significantly decreased. Furthermore, alterations in liver biomarkers such as bilirubin level and enzyme activities in both serum and liver homogenate (LDH, ALP, AST, and ALT) were detected. AlCl3 also caused inflammation as indicated by upregulation of the inflammation-related genes [interleukin 1 beta (IL-1β)], tumor necrosis factor-alpha (TNF-α), as well as matrix metalloproteinase (MMP9), and downregulation of nuclear factor erythroid 2 (Nrf2) expression. In addition, histopathological examination showed significant variations in the liver that confirms the biochemical results. Otherwise, bromelain intake alone slumped lipid peroxidation and gotten better antioxidant status significantly. Moreover, supplementation with bromelain before AlCl3 intoxication restores enzymatic and nonenzymatic antioxidants as well as biochemical indices and tissue architecture with respect to the AlCl3 group. In conclusion, bromelain proved its remarkable protective power to abolish AlCl3 toxicity. So, it might represent a new strategy in the therapy of metal toxicity by its antioxidant capacity.
1. Introduction
Aluminum (Al) is an important metal widely disseminated in various environmental compartments and largely used in daily life leading to many health problems in humans and animals [1]. The major origin of Al is due to its ingestion in different food (corn, yellow and processed cheese, baking powder, and flour) [2, 3]. Aluminum and its different compounds are used in the food industry (processing, packaging, and storage) leading to an increase in their levels in foods [4]. Also, they are used broadly in water purification [5], in medicines such as antacids, and in food additives authorizing their entrance into the body [6] and causing serious health problems in human and animal [7]. As an environmental contaminant, Al exposure generated hurtful effects on different biological systems including blood constituents, nervous, respiratory, skeletal, and immune systems [7]. Additionally, it is of great importance in various free radical-mediated diseases, osteomalacia, nephrotoxicity, and hepatotoxicity [8, 9]. Aluminum toxicity occurred through different mechanisms that encompass increasing blood-brain barrier permeability, interference with phosphorylation-dephosphorylation processes, and interchange of ions metabolism with successive free radicals' production and perturbation of the second messenger system [10]. Al salts may affect enzyme activity like hexokinase, phosphatases, phosphodiesterase, and phosphooxydase [5, 11]. Moreover, Al generates reactive oxygen species (ROS) [12], resulting in oxidative deterioration of lipids, proteins, and DNA.
Great interest is directed to many plants because of their antioxidant potential. Ananas comosus (Pineapple), which belongs to the Bromeliaceae family, is one of them. Waste usage is a promising strategy to get rid of the huge waste from processing. Ananas comosus is largely cultivated in the equatorial regions worldwide and has wide beneficial known effects as antioxidant, anticancer, anti-inflammatory, and antiplatelet impact. A. comosus stem extract is a waste product rich in complex enzymes identified by bromelain which are so important in some clinical applications, especially tumor growth modulation, wound healing, anti-inflammatory effect, antidiarrhea, and digestive help [13–15]. Bromelain has many commercial uses including food industry, pharmaceutical products such as cosmetics, for health benefits, and supplements as well as protein hydrolysates production [16, 17]. Prolonged oral use of bromelain is safe and it can be absorbed easily in the human intestinal tract without any decomposition or activity loss [18, 19]. The utilization of pineapple wastes as a source of bioactive compounds, especially proteolytic enzymes, is an alternative means. Therefore, the present study was designed to assess the potential antioxidant role of bromelain in modulating the harmful impacts induced by aluminum in male rats.
2. Materials and Methods
2.1. Chemicals
Bromelain from A. comosus (Pineapple) stem extract (600 GDU's/g) was purchased from Holland and Barrett, England. Aluminum chloride (AlCl3) was bought from Aldrich Chemical Company (Milwaukee, USA).
2.2. Experimental Design
The experimental design was performed following the US National Institute of Health Guidelines for the Care and Use of Laboratory Animals and Helsinki's declaration of animal ethics as approved by the Research Ethics Committee of Alexandria University (AU14-200204-1-3). Twenty-eight male Wister rats (150–170 g) were bought from the Faculty of Medicine, Alexandria University, Alexandria, Egypt. Rats were distributed randomly in cages seven per each and kept on a commercial diet and provided with tap water ad libitum and acclimated for two weeks (temperature, 21°C; photoperiod, 7 a.m. to 7 p.m.). Animals were classified into four groups: control, bromelain (250 mg/kg), AlCl3 (34 mg/Kg, 1/25 LD50), and bromelain plus AlCl3, respectively. Bromelain was administered one hour before AlCl3 intoxication daily while AlCl3 was given day after day orally for 30 days according to Saxena and Panjwani and El-Demerdash [20, 21], respectively. None of the AlCl3-intoxicated rats showed signs of morbidity or mortality during the study. At the experiment termination, rats were anesthetized using isoflurane and then killed via cervical dislocation, and livers were immediately removed. The liver was divided into two portions: the first portion was fixed in 10% formalin for histopathology examination, and the second portion was stored at -80°C for biochemical analyses.
2.3. Measurement of Aluminum Concentration
The level of AlCl3 was measured in the blood and liver tissue according to the method of Van Ginkel et al. [22] using the atomic absorption spectrometer (Shimadzu, AA6200).
2.4. Blood Samples
Complete blood counts (CBC) were performed in the collected blood samples by automatic methods (Sysmex kx-21n automated hematology analyzer; JAPAN CARE CO., LTD) including hemoglobin (Hb), white blood cells (WBCs), red blood cells (RBCs), platelets and hematocrit, or packed cell volume (PCV). Other blood samples were assembled for serum preparation and were left in a stand position for 30 min for blood clotting at 25°C then centrifuged at 3000 g for 15 min. The serum of each sample was taken and stored at -80°C till utilized in the determination of biochemical parameters.
2.5. Tissue Preparation
Livers were taken away and homogenized in ice-cold 0.01 mol/l sodium-potassium phosphate with 1.15% KCl buffer (pH 7.4). The homogenate was centrifuged at 10,000 g (4°C) for 20 min then the supernatants were taken and utilized for the determination of different assays.
2.6. Determination of TBARS, H2O2, and Glutathione Content
Thiobarbituric acid-reactive substances (TBARS), hydrogen peroxide (H2O2), and reduced glutathione (GSH) content were determined using the methods of Ohkawa et al., Velikova et al., and Ellman, respectively [23–25].
2.7. Determination of Antioxidant Enzyme Activities
The activities of superoxide dismutase (SOD; EC 1.15.1.1), catalase (CAT; EC 1.11.1.6), and glutathione S-transferase (GST; EC 2.5.1.18) were assessed by the methods of Misra and Fridovich, Aebi, and Habig et al. [26–28], respectively. While the activities of glutathione peroxidase (GPx; EC 1.11.1.9) and glutathione reductase (GR; EC 1.6.4.2) were evaluated according to Hafeman et al. [29].
2.8. Determination of Liver Function Biomarkers
Lactate dehydrogenase (LDH; EC 1.1.1.27) and alkaline phosphatase (ALP; EC 3.1.3.1) activities, protein content, and total bilirubin were estimated according to the methods used in the previous research [30–33]. Alanine aminotransferase (ALT; EC 2.6.1.2) and aspartate aminotransferase (AST; EC 2.6.1.1) activities were assayed using kits from Biodiagnostic, Egypt.
2.9. Molecular Analysis by Real-Time PCR
Using the RNeasy mini kit (Qiagen), total RNA was extracted from liver tissue in accordance with the manufacturer's recommendations. 10 μg of RNA were reversely transcribed to produce first-strand cDNA. Real-time PCR was used to evaluate the relative expressions of matrix metallopeptidase 9 (MMP9), nuclear factor erythroid 2 (Nrf2), interleukin 1β (IL-1β), and tumor necrosis factor-α (TNF-α). Gene-specific primers are displayed in Table 1. A Real-Time PCR System (Applied Biosystems, USA) was used for the procedure, which consisted of 40 cycles of denaturation at 95°C for 30 s, annealing for both genes at 59°C for 30 s, and extension at 72°C for 30 s. The results were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression that acts as the internal control and was amplified in the same process [34]. The relative gene expression was calculated using the 2−∆∆Ct method.
Table 1.
Forward and reverse primer sequence used in RT-PCR.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| MMP9 | 5′-GATCCCCAGAGCGTTACTCG-3′ | 5′-GTTGTGGAAACTCACACGCC-3′ |
| Nrf2 | 5′-TTGTAGATGACCATGAGTCGC-3′ | 5′-ACTTCCAGGGGCACTGTCTA-3′ |
| TNF-α | 5′-GCATGATCCGCGACGTGGAA-3′ | 5′-AGATCCATGCCGTTGGCCAG-3′ |
| IL-1β | 5′-CACCTCTCAAGCAGAGCACAG-3′ | 5′-GGGTTCCATGGTGAAGTCAAC-3′ |
| Housekeeping GAPDH | GGTGAAGGTCGGAGTCAACG | TGAAGGGGTCATTGATGGCAAC |
2.10. Histopathological Examinations
Livers were fixed in 10% formalin and serial paraffin sections were obtained to examine the histological changes using hematoxylin and eosin stain [35] then, slides were photographed by light microscope (Olympus BX 41, Japan).
2.11. Statistical Analysis
Data from different groups were presented as means ± standard errors (SEM) and then analyzed utilizing SPSS software (version 22, IBM Co., Armonk, NY). Comparison between groups was performed by ANOVA followed by Tukey's post-hoc test. P value ≤0.05 was approved to be significant.
3. Results
3.1. Body Weight
Final body weight and body weight gain in addition to the absolute liver weight of AlCl3-treated rats were significantly decreased as compared to control. However, bromelain supplementation alleviated this reduction with respect to AlCl3 exposed group. Bromelain alone did not cause any significant change (Figures 1 and 2).
Figure 1.
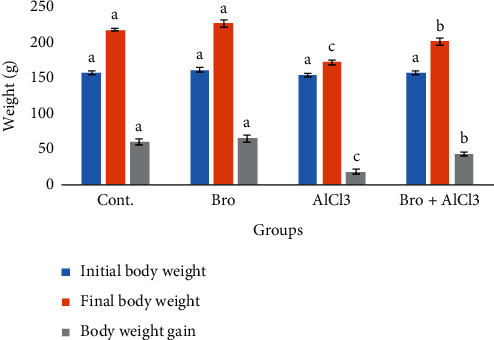
Initial and final body weights and body weight gain in rats of different experimental groups. Values are expressed as means ± SEM; n = 7 for each treatment group. A significant difference between the groups was shown with different superscript letters (a, b, and c), P < 0.05.
Figure 2.
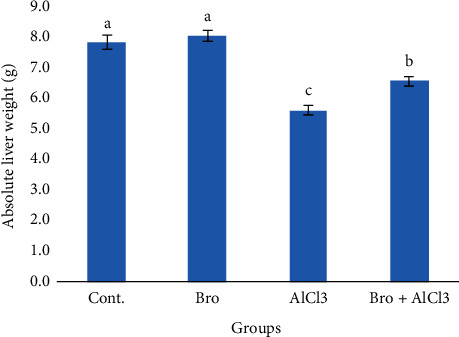
Absolute liver weight in rats of different experimental groups. Values are expressed as means ± standard error (SEM); n = 7 for each treatment group. Values are expressed as means ± SEM; n = 7 for each treatment group. A significant difference between the groups was shown with different superscript letters (a, b, and c), P < 0.05.
3.2. Aluminum Concentration in Rat Liver and Blood
The Al concentration in rat liver and blood was measured after one month of oral AlCl3 administration (Figure 3). The level of Al in the liver and blood of the AlCl3 intoxicated group was increased by +64.21% and +81.45% when compared to the control group, respectively. However, this concentration was significantly decreased in the liver and blood of rats treated with bromelain plus AlCl3 by +38.56% and +47.89% as compared to the AlCl3 intoxicated group, respectively.
Figure 3.
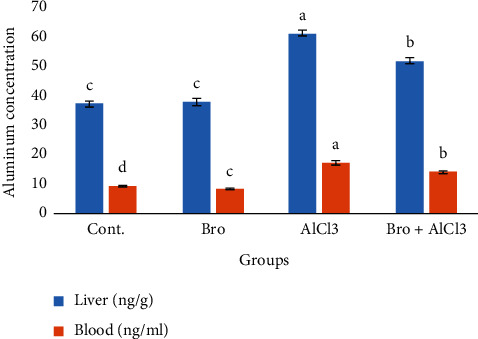
Aluminum concentration in liver and blood of male rats in different experimental groups. Values are expressed as means ± standard error (SEM); n = 7 for each treatment group. Values are expressed as means ± SE; n = 7 for each treatment group. A significant difference between the groups was shown with different superscript letters (a, b, c, and d), P < 0.05.
3.3. Hematological Parameters
Rats administered AlCl3 exhibited a significant decline in RBCs, Hb, PCV, and lymphocytes concentration while WBCs, platelets, and neutrophils increased significantly as compared to the control group. Other blood parameters are not significantly changed. On the other hand, the administration of bromelain alone showed a nonsignificant change in blood parameters as compared to the control group. Rats pretreated with bromelain and then received AlCl3 showed significant restoration near the normal level as compared to AlCl3 treated rats (Table 2).
Table 2.
Effect of bromelain (Bro), aluminum chloride (AlCl3), and their combination (Bro+AlCl3) on the hematological parameters in rats.
| Parameters | Groups | |||
|---|---|---|---|---|
| Cont. | Bro | AlCl3 | Bro+AlCl3 | |
| RBCs (x 106/μL) | 8.03a ± 0.274 | 8.41a ± 0.246 | 5.78b ± 0.264 | 8.33a ± 0.345 |
| WBCs (x 106/μL) | 10.97b ± 0.193 | 11.51b ± 0.418 | 13.23a ± 0.387 | 11.96b ± 0.340 |
| Hemoglobin (g/dl) | 13.20a ± 0.205 | 13.82a ± 0.455 | 9.51c ± 0.197 | 11.79b ± 0.202 |
| Platelets (103/μL) | 427c ± 12.08 | 427c ± 14.86 | 527a ± 13.59 | 470b ± 13.63 |
| PCV (%) | 47.16a ± 1.65 | 47.16a ± 1.42 | 38.10c ± 1.07 | 42.09b ± 1.25 |
| MCV (fl) | 63.58a ± 1.90 | 66.28a ± 1.99 | 65.80a ± 1.99 | 65.47a ± 2.11 |
| MCH (pg) | 18.90a ± 0.620 | 19.18a ± 0.487 | 19.90a ± 0.528 | 19.47a ± 0.603 |
| MCHC (%) | 32.59a ± 0.298 | 32.46a ± 0.301 | 31.93a ± 0.506 | 31.99a ± 0.415 |
| Neutrophils (%) | 19.14b ± 0.459 | 19.7b ± 0.522 | 23.29a ± 0.837 | 20.71b ± 0.680 |
| Lymphocytes (%) | 58.13a ± 0.527 | 56.26ab ± 2.15 | 45.36c ± 1.20 | 52.75b ± 1.28 |
| Eosinophils (%) | 1.57a ± 0.297 | 1.60a ± 0.170 | 1.54a ± 0.177 | 1.58a ± 0.276 |
| Monocytes (%) | 4.19a ± 0.142 | 4.07a ± 0.170 | 4.36a ± 0.180 | 4.17a ± 0.166 |
RBC: red blood cell; WBC: white blood cell; hb: hemoglobin; PCV: packed cell volume; MCV: mean cell volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration. Values are expressed as means ± SEM; n = 7 for each treatment group. abc Mean values within a row not sharing a common superscript letter were significantly different, P < 0.05. Statistically significant variations are compared as follows: Bromelain and AlCl3 groups are compared vs the control group while Bromelain+AlCl3 group is compared vs the AlCl3 group.
3.4. Lipid Peroxidation and Reduced Glutathione Content
Results revealed that the levels of TBARS and H2O2 were significantly (P < 0.05) increased in rats treated with AlCl3 versus control while rats pretreated with bromelain and then intoxicated by AlCl3 presented a significant reduction in TBARS and H2O2 levels as compared to AlCl3 -treated rats. Otherwise, GSH content was significantly decreased in AlCl3 -treated rats. While in the rats' group ingested with both bromelain and AlCl3, induction in GSH content was observed as compared with AlCl3-treated rats. Supplementation with bromelain alone reduced the concentrations of TBARS and H2O2 and induced GSH content in liver homogenate significantly (Table 3).
Table 3.
Effect of bromelain (Bro), aluminum chloride (AlCl3) and their combination (Bro+AlCl3) on the level of thiobarbituric acid reactive substances (TBARS), hydrogen peroxide (H2O2), and reduced glutathione (GSH) content in rat liver.
| Parameters | Groups | |||
|---|---|---|---|---|
| Cont. | Bro | AlCl3 | Bro+AlCl3 | |
| TBARS (nmol/g tissue) % change |
30.20c ± 0.899 | 23.49d ± 0.599 (-22.22%) |
44.49a ± 1.28 (+47.33%) |
36.31b ± 0.928 (+20.25%) |
| H2O2 (μmol/g tissue) % change |
63.65c ± 1.62 | 50.35d ± 1.36 (-20.90) |
90.26a ± 1.45 (+41.81%) |
78.92b ± 2.02 (+23.98%) |
| GSH (mmol/mg protein) % change |
1.72b ± 0.052 | 2.05a ± 0.061 (+19.37%) |
0.99d ± 0.031 (-42.27%) |
1.39c ± 0.041 (-18.95%) |
Values are expressed as means ± SEM; n = 7 for each treatment group. abcd Mean values within a row not sharing a common superscript letter were significantly different, P < 0.05. Statistically significant variations are compared as follows: Bromelain and AlCl3 groups are compared vs control group while Bromelain+AlCl3 group is compared vs AlCl3 group.
3.5. Antioxidant Enzymes
A significant reduction (P < 0.05) in SOD, CAT, GPx, GR, and GST activity was observed in liver homogenate of AlCl3-treated rats. Furthermore, rats taken with bromelain+AlCl3 showed significant alleviation in antioxidant enzyme activities as compared to AlCl3-treated ones (P < 0.05). Moreover, the treatment of rats with bromelain alone improved antioxidant enzyme activities significantly versus the control group (Table 4).
Table 4.
Effect of bromelain (Bro), aluminum chloride (AlCl3), and their combination (Bro+AlCl3) on the activities of antioxidant enzymes in rat liver.
| Parameters | Groups | |||
|---|---|---|---|---|
| Cont. | Bro | AlCl3 | Bro+AlCl3 | |
| SOD (U/mg protein) % change |
75.93b ± 2.25 | 91.38a ± 3.19 (+20.35%) |
38.83d ± 1.16 (-48.86%) |
60.46c ± 1.64 (-20.37%) |
| CAT (μmol/hr/mg protein) % change |
46.07b ± 1.62 | 54.85a ± 1.82 (+19.05%) |
25.39d ± 0.574 (-44.89%) |
35.72c ± 1.08 (-22.47%) |
| GPx (U/mg protein) % change |
1.07b ± 0.038 | 1.26a ± 0.041 (+18.08%) |
0.60d ± 0.021 (-43.66%) |
0.85c ± 0.025 (-20.66%) |
| GR (U/mg protein) % change |
1.23b ± 0.042 | 1.46a ± 0.036 (+18.77%) |
0.70d ± 0.023 (-42.53%) |
0.99c ± 0.033 (-19.46%) |
| GST (μmol/hr/mg protein) % change |
1.26b ± 0.032 | 1.50a ± 0.045 (+19.23%) |
0.67d ± 0.022 (-46.93%) |
0.99c ± 0.027 (-21.22%) |
Values are expressed as means ± SEM; n = 7 for each treatment group. abcd Mean values within a row not sharing a common superscript letter were significantly different, P < 0.05. Statistically significant variations are compared as follows: Bromelain and AlCl3 groups are compared vs control group while Bromelain+AlCl3 group is compared vs AlCl3 group.
3.6. Liver Function Biomarkers
Data showed that AST, ALT, and ALP activities were significantly (P < 0.05) decreased in liver homogenate and increased in rat serum while LDH activity increased in serum and liver homogenates of rats received AlCl3 with respect to control. Protein content was decreased while bilirubin was increased in rat liver homogenate, significantly. Moreover, a significant modulation in enzyme activities, protein and bilirubin contents in rats that received bromelain and then intoxicated with AlCl3 versus AlCl3 group was observed. Bromelain supplementation alone had insignificantly affected the measured parameters (Table 5).
Table 5.
Effect of bromelain (Bro), aluminum chloride (AlCl3), and their combination (Bro+AlCl3) on enzyme activities and protein content in serum and liver of male rats.
| Parameters | Groups | |||
|---|---|---|---|---|
| Cont. | Bro | AlCl3 | Bro+AlCl3 | |
| Serum | ||||
| AST (U/l) % change |
53.12c ± 1.12 | 51.20c ± 1.51 (-3.62%) |
76.88a ± 2.29 (+44.74%) |
65.97b ± 1.75 (+24.19%) |
| ALT (U/l) % change |
58.57c ± 1.28 | 54.70c ± 0.84 (-6.61%) |
82.89a ± 2.42 (+41.54%) |
71.55b ± 2.37 (+22.17%) |
| LDH (U/l) % change |
585c ± 14.32 | 563c ± 14.21 (-3.71%) |
823a ± 18.84 (+40.82%) |
713b ± 20.03 (+21.95) |
| ALP (U/l) % change |
59.01c ± 1.90 | 58.20c ± 2.09 (-1.38%) |
83.80a ± 2.32 (+42.01%) |
69.47b ± 2.05 (+17.72%) |
| Bilirubin (mg/dl) % change |
0.730c ± 0.017 | 0.732c ± 0.016 (+0.25%) |
1.02a ± 0.027 (+39.13%) |
0.871b ± 0.028 (+19.29%) |
| Liver | ||||
| AST (U/mg protein) % change |
125a ± 3.75 | 131.32a ± 3.15 (+4.79%) |
80.43c ± 1.78 (-35.78%) |
102b ± 2.88 (-18.54%) |
| ALT (U/mg protein) % change |
162a ± 5.03 | 154a ± 3.29 (-5%) |
99c ± 3.40 (-38.73%) |
131b ± 4.21 (-19.12%) |
| LDH (U/mg protein) % change |
1001c ± 34.04 | 940c ± 31.17 (-6.07%) |
1366a ± 37.79 (+36.51%) |
1206b ± 27.83 (+20.58%) |
| ALP (U/mg protein) % change |
344a ± 9.98 | 374a ± 11.18 (+8.87%) |
216c ± 6.21 (-37.32%) |
291b ± 7.68 (-15.33%) |
| Protein (mg/g tissue) % change |
194a ± 2.44 | 197a ± 4.29 (+1.48%) |
128c ± 4.45 (-34.15%) |
163b ± 4.02 (+16.10%) |
Values are expressed as means ± SEM; n = 7 for each treatment group. abc Mean values within a row not sharing a common superscript letter were significantly different, P < 0.05. Statistically significant variations are compared as follows: Bromelain and AlCl3 groups are compared vs control group while Bromelain+AlCl3 group is compared vs AlCl3 group.
3.7. Genes Expression
In the hepatic tissues of rats given AlCl3, a considerable upregulation in the mRNA expression of the MMP9, IL-1β, and TNF-α genes and a downregulation of the Nrf2 gene were found in comparison to the control group. On the other hand, the administration of bromelain prior to AlCl3 resulted in a considerable modification in the examined genes as opposed to the AlCl3 group. Furthermore, the intake of bromelain alone increased the Nrf2 mRNA expression in the hepatic tissues in comparison to the control group (Figure 4).
Figure 4.
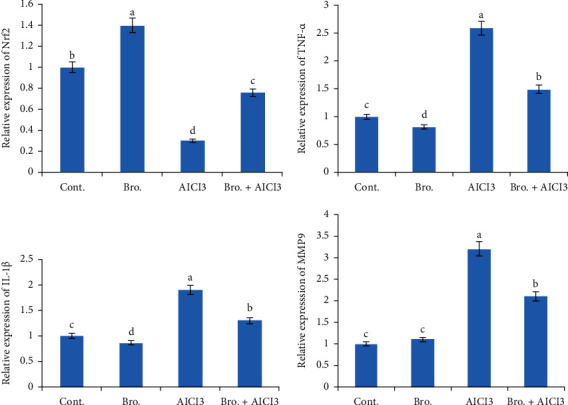
Effect of bromelain and aluminum on the gene expression (IL-1β, TNF-α, Nrf2, and MMP9) in rat liver. Data were normalized to the housekeeping gene (GAPDH). Data are presented as fold change (mean ± SEM; n = 7/group). Groups with different letters are significantly different at P < 0 : 05.
3.8. Liver Histopathology
Light microscopic examination of the liver from control (G1) and bromelain (G2) groups showed normal architecture of hepatic lobules. However, liver sections of the AlCl3 group (G3) revealed enlarged and congested veins, bile ductular proliferation, leukocytic infiltration, activated Kupffer cells, and hepatocytes with pyknotic nuclei. While gradual improvement was observed in liver sections of rats treated with Bromelain+AlCl3 group (G4). Most hepatocytes appeared more or less normal, while some of them still showed cytoplasmic vacuolation (Figure 5).
Figure 5.
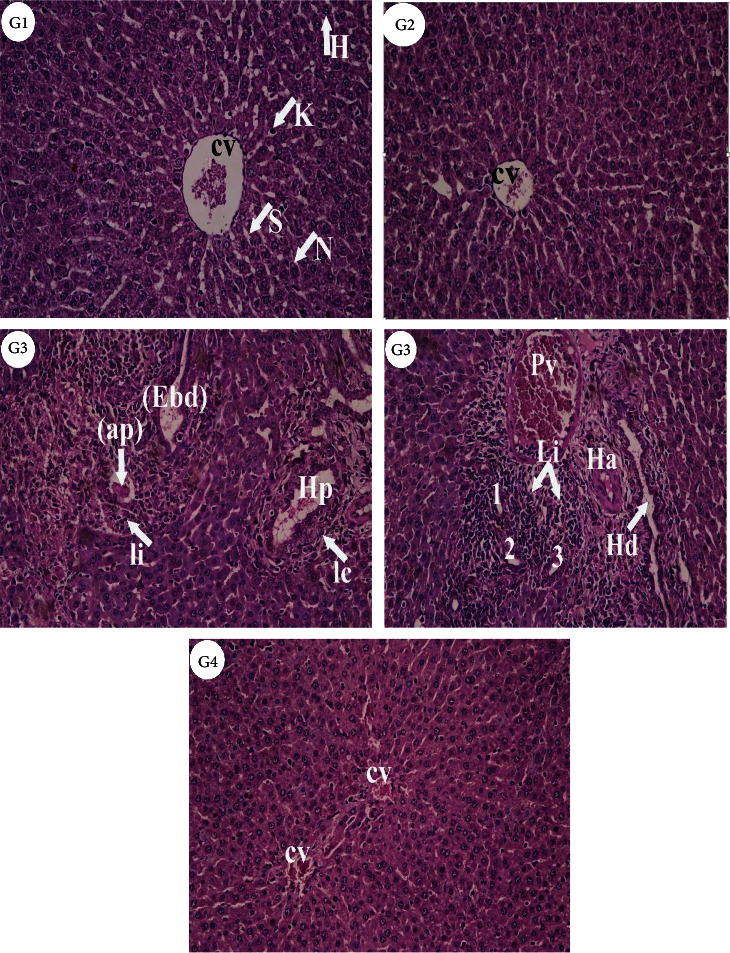
Photomicrographs of liver sections from different experimental groups stained with Hematoxylin & Eosin. Control (G1) and bromelain (G2) rats revealed the normal histological structures of normal hepatocytes (H) with nuclei (N), central vein (CV), sinusoids (S), and Kupffer cells (K) which represented monocytes-macrophage defense system. (G3), AlCl3 treated rats showed inflammation or infiltration (Ii) and enlarged bile ductular (Ebd), apoptic body (ap), branches of hepatic portal vein (Hp) surrounded by inflammatory cells (Ic). Also, leukocytic infiltration (Li) and bile ductular proliferation (1, 2, 3), branch of hepatic artery (Ha), congested portal vein (Pv) and enlarged hepatic duct (Hd) and hyperchromatic nuclei (Hn) were observed. (G4), Bromelain+AlCl3 group revealed more or less normal hepatocytes (H&E X 200).
4. Discussion
In the current investigation, the hepatoprotective role of bromelain from Ananas comosus stem against AlCl3 induced oxidative injury, and also hematological and biochemical perturbations were studied. To our knowledge, few references have indicated the adequacy of bromelain as a natural antioxidant to get rid of xenobiotic toxicity. AlCl3 administration impaired weight gain over the experimental period [36] due to malnutrition induced by the decrease in food consumption or by the toxicity induced by xenobiotics [37]. Also, a significant increase in Al concentration in blood and liver tissue was observed [38–40] in rats that received AlCl3 leading to calcium replacement [41]. Additionally, Al can bind to transferrin and some low molecular weight compound in the blood as citrate [42]. So, it interferes with Fe homeostasis by displacing it from transferrin leading to disturbance in iron metabolism [43]. Interestingly, bromelain supplementation for 30 days improved these parameters due to its antioxidant and chelation properties.
Blood and hematopoietic tissues rank as target organs for toxic effects of environmental chemicals; hence, they offer sensitive and reliable indicators, which could be effectively used to detect the magnitude of biochemical stress [44]. In agreement with the present results, perturbations in blood parameters in rats treated with AlCl3 were observed and this attributed to the inhibition of erythropoiesis and iron metabolism as well as alterations in erythrocyte morphology leading to anemia [45]. Furthermore, leukocytosis observed indicates the improved defense mechanism and immune system against infection induced by xenobiotics [46]. Bromelain is extensively used to improve tissue regeneration [47] and acts as an anti-inflammatory agent [19]. In this work, the administration of bromelain markedly hampered the toxic action produced by AlCl3 on hematological parameters as it is efficient in improving blood circulation and the amendment of arterial diseases [48]. Also, bromelain is used as a natural blood thinner because it prohibits exaggerated stickiness of blood platelets [49] and affects blood coagulation by inducing fibrinolytic capability and by reducing fibrin synthesis [50].
The liver is an important organ rich in mitochondria and plays a significant role in the metabolic process. AlCl3 inhibits the enzymes of oxidative phosphorylation leading to the cessation of energy metabolism [51]. So, oxidative stress and excess ROS production, via the Fenton reaction, have been involved in the mechanism of aluminum toxicity [21, 52] causing hepatocellular damage, apoptosis, and cellular necrosis [53, 54]. ROS Overproduction provokes injury via oxidizing cellular macromolecules such as lipids and proteins and triggering DNA injury [55]. Lipid peroxidation is a highly damaging peroxidative process that occurs in phospholipid compartments of the cellular membrane following metal intoxication [56]. AlCl3-treated rats manifested disruption in the antioxidant status where significant elevation in TBARS and H2O2 accompanied by a reduction in enzymatic (SOD, CAT, GPx, GR, and GST) and nonenzymatic antioxidants (GSH) in the liver homogenate were observed. The level of LPO is specified by the oxidants/antioxidants balance where the oxidants produced can be eliminated by the antioxidants [57, 58]. In agreement, previous authors showed that heavy metals work as oxidants affecting different organs leading to oxidative stress [8, 21, 54, 59–61]. On the other hand, treatment with bromelain given rise to a significant amelioration in oxidative stress markers (TBARS and H2O2) in AlCl3 intoxicated rats and this reflects its immense antioxidant properties and its interaction with heavy metals [8, 62, 63] beside the presence of cysteine, an amino acid with known antioxidant properties. Also, it is an important precursor in the output of glutathione, which protects cells from toxins as free radicals incriminated in AlCl3 toxicity [64]. Thus, bromelain supplementation could overcome AlCl3-induced hepatotoxicity by abolishing oxidative tissue injuries.
Glutathione is a low molecular weight tripeptide with a thiol group. It plays a significant function in cell metabolism and defense versus toxicants [57]. Glutathione can directly remove free radicals or provide detoxification using GSH-dependent enzymes (GST, GR, and GPx) as substrates [65]. Antioxidant enzymes (SOD, CAT, GPx, GR, and GST) have a majestic role in the elimination of ROS and keeping cellular homeostasis for normal cell function as well as act as indicators of oxidative stress [66]. The significant reduction in antioxidant enzymes and GSH might be attributed to Al accumulation observed in liver cells leading to a decline in enzyme protein synthesis [59, 67]. Superoxide dismutase is implicated in the cellular defense versus oxidative injury in aerobic living organisms, where it stimulates the conversion of superoxide anion to O2 and H2O2, which is decomposed by catalase into H2O. GPx protects the membrane lipids from oxidative injury [68] and catalyzes the reaction of hydroperoxide radicals with GSH to form disulfide glutathione (GSSG) [69]. While GST is a detoxifying enzyme that acts to convert xenobiotics into water-soluble nontoxic metabolites easily excreted outside the body [70]. Aluminum may affect the synthesis of GSH through the inhibition of glutathione-synthase and glucose 6-phosphate dehydrogenase activities. Moreover, it retards the conversion of oxidized glutathione (GSSG) into its reduced form (GSH) via GR inhibition [71]. Therefore, the antioxidant defense system is so important in the protection against oxidative stress-induced by Al and disturbing antioxidant enzymes [21, 72] in rat liver via the prohibition of free radicals chain reaction. Otherwise, the observed induction in antioxidant enzyme activities might be related to the decline in radical's generation and accumulation that are prohibited by bromelain [60]. Furthermore, it can protect against the toxic effects of ROS either by preventing their formation or interrupting their attack, via scavenging the reactive metabolites [73]. In accordance, ethanolic extract of A. comosus peel positively improved the antioxidant status by quenching and detoxifying the radicals stimulated by a carcinogenic substance and isoproterenol-caused oxidative injury in rats, respectively [20, 69]. Also, escalation in GSH content helps in the detoxification of ROS, preservation of cell integrity, and cellular components versus oxidation via the glutathione redox cycle due to its reducing features.
Toxic substances are transformed in the liver into less harmful products leading to hepatocytes damage. In the current study, rats treated with AlCl3 showed significant variations in serum and liver ALP, ALT, AST, and LDH activities as well as total bilirubin and protein. These parameters are important biomarkers for hepatocellular damage [74] and its alterations pointed out hepatocytes damage that altered the transport function and membrane permeability as well as leakage of enzymes from the cells to the bloodstream indicating hepatotoxicity [74–76]. Also, lipid peroxidation has a fundamental role in the disruption of hepatocellular membrane integrity, leading to the leakage of cytoplasmic enzymes and this confirmed the possible mechanism of oxidative stress in liver injury induced by Al [77]. Lactate dehydrogenase was significantly increased in AlCl3 intoxicated rats and this agreed with El-Demerdash [21]. This induction may be related to cellular impairment leading to disturbance in the metabolism of carbohydrates and protein as well as energy depletion [78]. Alkaline phosphatase is a critical membrane-bound enzyme in the biological processes used as a biomarker for heavy metals toxicity. It is responsible for the detoxification, metabolism, and biosynthesis of macromolecules which are required for many biological functions. Moreover, the decline in ALP activity in liver homogenate is consistent with the findings of Ochmanski and Barabasz and Szilagyi et al. [5, 11] who referred the change in ALP to the disturbance in bone formation induced by Al in addition to the binding of Al with DNA and RNA, respectively. So, alterations in these enzymes' activity could be expected due to cellular necrosis of the liver, kidney, and lung [79]. Protein is an essential cellular component susceptible to damage by free radicals and its depression might be linked to exaggerated leakage via nephrosis [80] or may be related to a disturbance in protein anabolic and catabolic processes. The elevation in total bilirubin may be due to diminished liver uptake, conjugation, or prolonged bilirubin output from hemolysis [21]. Moreover, results showed that bromelain treatment attenuated hepatotoxicity induced by AlCl3 since it could maintain hepatocytes' integrity and minimize the liver injury caused by AlCl3. Generally, it appears that the effectiveness of bromelain as a hepatoprotector because of its high content of active ingredients (ananasate, beta-sitosterol, campesterol, chlorogenic acid, rutin, naringenin, bromelain, vitamin A, B and C, glycosides, and flavonoids) that have potent antioxidant and anti-inflammatory activities [62, 81].
In agreement with the present results, several authors noted that Al administration significantly increased tissue TNF-α and the rise in cytokine expression suggests that the prooxidant/antioxidant balance has been upset. They also showed that during hepatocyte damage, activated Kupffer cells release growth factors and cytokines that have an encouraging effect on stellate cell activation and proliferation. Additionally, they release inflammatory mediators (TNF-α, IL-1β) that cause inflammatory leukocyte infiltration [59, 82, 83]. MMP-2 and MMP-9, two proteins associated with cell migration, were dramatically upregulated by AlCl3. It has been discovered that human tissue inflammation increases MMP expression. MMPs have a role in the regulation of inflammatory mediators, which attract immune cells to injured tissues [84, 85]. The observed downregulation in the Nrf2 gene's expression level in the hepatic tissues of AlCl3-intoxicated rats is consistent with findings made by Yu et al. [86] and Othman et al. [87], who discovered that Nrf2 deregulation is linked to Al toxicity. One of our most intriguing findings was that giving bromelain before AlCl3 increased the Nrf2 gene's level of expression in rat liver tissue. This would imply that one of bromelain's primary defense mechanisms against AlCl3-induced toxicity is the activation of Nrf2. Furthermore, the bromelain's impact on Nrf2 expression in the liver of the treated rats coincided with the antioxidant enzymes' functions in this investigation.
Histopathological examination of liver sections of AlCl3 intoxicated rats showed several lesions and abnormalities in the hepatocytes as enlarged and congested veins, proliferation and leukocytic infiltration, activated Kupffer cells, and hepatocytes with pyknotic nuclei. This could be attributed to the oxidative toxicity induced by AlCl3, which may have apparently led to severe alterations in liver architecture. Similar observations exhibited severe degenerative alterations in liver hepatocytes with hepatic cord derangement, intrahepatic hemorrhage and inflammatory cells infiltration, karyopyknosis and necrosis [51, 61], brain and testis [12, 88], and heart tissues [89] of rats treated with AlCl3. Here, the observed degenerative alterations in liver tissues may be related to lipid peroxidation and free radicals' accumulation along with perturbation in antioxidant status induced by AlCl3 in rats. So, the histological examination confirmed the biochemical results and proved to be a good marker in liver failure. Based on our results, bromelain can improve most of the studied parameters in AlCl3 intoxicated rats and could restore hepatocyte integrity and decrease liver damage. In general, it appears that the effectiveness of bromelain as a hepatoprotector in AlCl3 toxicity may be referred to its high antioxidant constituents.
5. Conclusion
In conclusion, the current study pointed out that aluminum chloride has the potency to cause liver dysfunction via oxidative injury, alterations in the antioxidant defense system, liver function biomarkers, as well as molecular and histopathological changes. Furthermore, bromelain from A. comosus stem supplementation before aluminum treatment restores its toxic effects by quenching, chelating, and detoxifying the free radicals. So, our findings suggested that bromelain had a powerful antioxidant effect and could be used to develop functional healthy foods with hepatoprotective effects against complications induced by AlCl3.
Data Availability
All data are incorporated in the manuscript.
Conflicts of Interest
The authors have declared no conflict of interest.
Authors' Contributions
El-Demerdash F.M. was responsible for the conceptualization, methodology, manuscript preparation, and publication. Hussien D.M. was responsible for practical work, data generation, and writing the original draft. Ghanem N.F. was responsible for manuscript revision, methodology, and investigation. AL-Farga A.M. was responsible for data analysis and manuscript writing and revision.
References
- 1.Kumar V., Gill K. D. Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology . 2014;41:154–166. doi: 10.1016/j.neuro.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Lione A. The prophylactic reduction of aluminium intake. Food and Chemical Toxicology . 1983;21(1):103–109. doi: 10.1016/0278-6915(83)90277-6. [DOI] [PubMed] [Google Scholar]
- 3.Abbasali K. M., Zhila T., Farshad N. Developmental toxicity of aluminium from high doses of AlCl3 in mice. Journal of Applied Research . 2005;5:575–579. [Google Scholar]
- 4.IPCS (International Programme on Chemical Safety) Safety Evaluation of Certain Food Additives and Contaminants . WHO, Academic Press Inc; 2007. Aluminium from all sources, including food additives; pp. 119–208. [Google Scholar]
- 5.Ochmanski W., Barabasz W. Aluminum occurrence and toxicity for organisms. Przegla̧d Lekarski . 2000;57:665–668. [PubMed] [Google Scholar]
- 6.Yoke R. A. The toxicology of aluminum in the brain: a review. Neurotoxicology . 2000;21:813–828. [PubMed] [Google Scholar]
- 7.Willhite C. C., Karyakina N. A., Yokel R. A., et al. Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts. Critical Reviews in Toxicology . 2014;44(supplement4):1–80. doi: 10.3109/10408444.2014.934439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Demerdash F. M., Baghdadi H. H., Ghanem N. F., Al Mhanna A. B. Nephroprotective role of bromelain against oxidative injury induced by aluminium in rats. Environmental Toxicology and Pharmacology . 2020;80:103–509. doi: 10.1016/j.etap.2020.103509. [DOI] [PubMed] [Google Scholar]
- 9.Shati A. A., Alamri S. A. Role of saffron (Crocus sativus L.) and honey syrup on aluminum- induced hepatotoxicity. Saudi Medical Journal . 2010;31(10):1106–1113. [PubMed] [Google Scholar]
- 10.Agarwal S. K., Ayyash L., Gourley C. S., Levy J., Faber K., Hughes C. L., Jr. Evaluation of the developmental neuroendocrine and reproductive toxicology of aluminium. Food and Chemical Toxicology . 1996;34(1):49–53. doi: 10.1016/0278-6915(95)00088-7. [DOI] [PubMed] [Google Scholar]
- 11.Szilagyi M., Bokori J., Fekete S., Vetesi F., Albert M., Kadar I. Effects of long-term aluminum exposure on certain serum constituents in broiler chickens. European Journal of Clinical Chemistry and Clinical Biochemistry . 1994;32(6):485–486. [PubMed] [Google Scholar]
- 12.Mohamed N. E., Abd El-Moneim A. A. Ginkgo biloba extract alleviates oxidative stress and some neurotransmitters changes induced by aluminum chloride in rats. Nutrition . 2017;35:93–99. doi: 10.1016/j.nut.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Koh J., Kang S. M., Kim S. J., Cha M. K., Kwon Y. J. Effect of pineapple protease on the characteristics of protein fibers. Fibers and Polymers . 2006;7(2):180–185. doi: 10.1007/BF02908264. [DOI] [Google Scholar]
- 14.Chaisakdanugull C., Theerakulkait C., Wrolstad R. E. Pineapple juice and its fractions in enzymatic browning inhibition of banana [Musa (AAA Group) Gros Michel] Journal of Agricultural and Food Chemistry . 2007;55(10):4252–4257. doi: 10.1021/jf0705724. [DOI] [PubMed] [Google Scholar]
- 15.Bitange N. T., Zhang W., Shi Y. X., Wenbin Z. Therapeutic application of pineapple protease (bromelain) Pakistan Journal of Nutrition . 2008;7:513–520. [Google Scholar]
- 16.Uhlig H. Industrial Enzymes and their Applications . New York: John Wiley and Sons, Inc; 1998. [Google Scholar]
- 17.Ketnawa S., Rawdkuen S. Application of bromelain extract for muscle foods tenderization. Food and Nutrition Sciences . 2011;2(5):393–401. doi: 10.4236/fns.2011.25055. [DOI] [Google Scholar]
- 18.Chobotova K., Vernallis A. B., Majid F. A. A. Bromelain's activity and potential as an anti-cancer agent: current evidence and perspectives. Cancer Letters . 2010;290(2):148–156. doi: 10.1016/j.canlet.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Pavan R., Jain S., Shraddha, Kumar A. Properties and therapeutic application of bromelain: a review. Biotechnology Research International . 2012;2012:6. doi: 10.1155/2012/976203.976203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saxena P., Panjwani D. Cardioprotective potential of hydro-alcoholic fruit extract of Ananas comosus against isoproterenol induced myocardial infraction in Wistar albino rats. Journal of Acute Disease . 2014;3(3):228–234. doi: 10.1016/S2221-6189(14)60051-2. [DOI] [Google Scholar]
- 21.El-Demerdash F. M. Antioxidant effect of vitamin E and selenium on lipid peroxidation, enzyme activities and biochemical parameters in rats exposed to aluminium. Journal of Trace Elements in Medicine and Biology . 2004;18(1):113–121. doi: 10.1016/j.jtemb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Van Ginkel M. F., van der Voet G. B., de Wolf F. A. Improved method of analysis for aluminum in brain tissue. Clinical Chemistry . 1990;36(4):658–661. doi: 10.1093/clinchem/36.4.658. [DOI] [PubMed] [Google Scholar]
- 23.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry . 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 24.Velikova V., Yordanov I., Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Science . 2000;151(1):59–66. doi: 10.1016/S0168-9452(99)00197-1. [DOI] [Google Scholar]
- 25.Ellman G. L. Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics . 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 26.Misra H. P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. The Journal of Biological Chemistry . 1972;247(10):3170–3175. doi: 10.1016/S0021-9258(19)45228-9. [DOI] [PubMed] [Google Scholar]
- 27.Aebi H. B. Isolation, purification, characterization, and assay of antioxygenic enzymes [13] Catalase in vitro. Methods in Enzymology . 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 28.Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. The Journal of Biological Chemistry . 1974;249(22):7130–7139. doi: 10.1016/S0021-9258(19)42083-8. [DOI] [PubMed] [Google Scholar]
- 29.Hafeman D. G., Sunde R. A., Hoekstra W. G. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. The Journal of Nutrition . 1974;104(5):580–587. doi: 10.1093/jn/104.5.580. [DOI] [PubMed] [Google Scholar]
- 30.Cabaud P. C., Wroblewski F. Colorimetric measurement of Lactate dehydrogenase activity of body fluids. Journal of Clinical Pathology . 1958;30(3):234–236. doi: 10.1093/ajcp/30.3.234. [DOI] [PubMed] [Google Scholar]
- 31.Principato G. B., Aisa M. C., Talesa V., Rosi G., Giovannini E. Characterization of the soluble alkaline phosphatase from hepatopancreas of Squilla mantis L. Comparative Biochemistry and Physiology. B . 1985;80:801–804. doi: 10.1016/0305-0491(85)90464-X. [DOI] [Google Scholar]
- 32.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry . 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Walters M., Gerade H. An ultramicromethod for the determination of conjugated and total bilirubin in serum or plasma. Microchemical Journal . 1970;15(2):231–243. doi: 10.1016/0026-265X(70)90045-7. [DOI] [Google Scholar]
- 34.Ibrahim M. A., Radwan M. I., Kim H. K., Han J., Warda M. Evaluation of global expression of selected genes as potential candidates for internal normalizing control during transcriptome analysis in dromedary camel (Camelus dromedarius) Small Ruminant Research . 2020;184, article 106050 doi: 10.1016/j.smallrumres.2020.106050. [DOI] [Google Scholar]
- 35.Bancroft J. D., Stevens A. Theory and Practice of Histological Techniques . 3rd. Edinburgh: Churchill Livingstone; 1990. [Google Scholar]
- 36.Balgoon M. J. Assessment of the protective effect of Lepidium sativum against aluminum-induced liver and kidney effects in albino rat. BioMed Research International . 2019;2019:9. doi: 10.1155/2019/4516730.4516730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H., Huang P., Lie T., et al. Reproductive toxicity of acrylamide-treated male rats. Reproductive Toxicology . 2010;29(2):225–230. doi: 10.1016/j.reprotox.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Krewski D., Yokel R. A., Nieboer E., et al. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. Journal of Toxicology and Environmental Health . 2007;10(supplement 1):1–269. doi: 10.1080/10937400701597766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu L., Zhai Q., Yin R., et al. Lactobacillus plantarum CCFM639 alleviate trace element imbalance-related oxidative stress in liver and kidney of chronic aluminum exposure mice. Biological Trace Element Research . 2017;176(2):342–349. doi: 10.1007/s12011-016-0843-8. [DOI] [PubMed] [Google Scholar]
- 40.Drobyshev E. J., Solovyev N. D., Gorokhovskiy B. M., Kashuro V. A. Accumulation patterns of sub-chronic aluminum toxicity model after gastrointestinal administration in rats. Biological Trace Element Research . 2018;185(2):384–394. doi: 10.1007/s12011-018-1247-8. [DOI] [PubMed] [Google Scholar]
- 41.Cochran M., Coates J. H., Elliott D. C. Aluminium interaction with macromolecules and membranes. In: Broe M. E., Coburn J. W., editors. Aluminum and Renal Failure . Netherlands, Dordrecht: Springer; 1990. pp. 139–153. [DOI] [Google Scholar]
- 42.Yokel R. A. Brain uptake, retention, and efflux of aluminum and manganese. Environmental Health Perspectives . 2002;110(supplement 5):699–704. doi: 10.1289/ehp.02110s5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crichton S., Wilmet R., Legssyer R., Ward R. J., Legssyer L. Molecular and cellular mechanisms of iron homeostasis and toxicity in mammalian cells. Journal of Inorganic Biochemistry . 2002;91(1):9–18. doi: 10.1016/S0162-0134(02)00461-0. [DOI] [PubMed] [Google Scholar]
- 44.Lakshmanan S., Rajendran A., Sivasubramaniyan C. Impact of dichlorvos on tissue glycogen and protein content in freshwater fingerlings, Oreochromis mossambicus (Peters) International journal of Research in Environmental science and Technology . 2013;3:19–25. [Google Scholar]
- 45.Ganchev T., Dyankov E., Zacharieva R., Pachalieva I., Velikova M., Kavaldjieva B. Influence of aluminium on erythropoiesis, iron metabolism and some functional characteristics of erythrocytes in rats. Acta Physiologica et Pharmacologica Bulgarica . 1998;23:27–31. [PubMed] [Google Scholar]
- 46.Srivastava P. N., Narain A. Catfish blood chemistry under environmental stress. Experientia . 1985;41(7):955–957. doi: 10.1007/BF01970031. [DOI] [PubMed] [Google Scholar]
- 47.Aichele K., Bubel M., Deubel G., Pohlemann T., Oberringer M. Bromelain down-regulates myofibroblast differentiation in an in vitro wound healing assay. Naunyn-Schmiedeberg's Archives of Pharmacology . 2013;386(10):853–863. doi: 10.1007/s00210-013-0890-z. [DOI] [PubMed] [Google Scholar]
- 48.Al-Sereiti M. R., Abu-Amer K. M., Sen P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Indian Journal of Experimental Biology . 1999;37(2):124–130. [PubMed] [Google Scholar]
- 49.Heinicke R., van der Wal L., Yokoyama M. Effect of bromelain (Ananase) on human platelet aggregation. Experientia . 1972;28(7):844–845. doi: 10.1007/BF01923166. [DOI] [PubMed] [Google Scholar]
- 50.Maurer H. R. Bromelain: biochemistry, pharmacology and medical use. Cellular and Molecular Life Sciences . 2001;58(9):1234–1245. doi: 10.1007/PL00000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu F., Liu Y., Zhao H., et al. Aluminum chloride caused liver dysfunction and mitochondrial energy metabolism disorder in rat. Journal of Inorganic Biochemistry . 2017;174:55–62. doi: 10.1016/j.jinorgbio.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 52.Anane R., Creppy E. E. Lipid peroxidation as pathway of aluminium cytotoxicity in human skin fibroblast cultures: prevention by superoxide dismutase+catalase and vitamins E and C. Human & Experimental Toxicology . 2001;20(9):477–481. doi: 10.1191/096032701682693053. [DOI] [PubMed] [Google Scholar]
- 53.Mailloux R. J., Lemire J., Appanna V. D. Hepatic response to aluminum toxicity: dyslipidemia and liver diseases. Experimental Cell Research . 2011;317(16):2231–2238. doi: 10.1016/j.yexcr.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 54.Ekakitie L. I., Okpoghono J., Orororo O. C., Ekakitie O. A. Ameliorative prowess of bee honey in the tissues of rats administered aluminium nitrate. Scientific African . 2021;12, article e00782 doi: 10.1016/j.sciaf.2021.e00782. [DOI] [Google Scholar]
- 55.Al Olayan E. M., Aloufi A. S., AlAmri O. D., El-Habit O. H., Abdel Moneim A. E. Protocatechuic acid mitigates cadmium-induced neurotoxicity in rats: role of oxidative stress, inflammation and apoptosis. Science of the Total Environment . 2020;723, article 137969 doi: 10.1016/j.scitotenv.2020.137969. [DOI] [PubMed] [Google Scholar]
- 56.AL-Megrin W. A., Alkhuriji A. F., Yousef A. O. S., et al. Antagonistic efficacy of luteolin against lead acetate exposure-associated with hepatotoxicity is mediated via antioxidant, anti-inflammatory, and anti-apoptotic activities. Antioxidants . 2020;9:p. 10. doi: 10.3390/antiox9010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Halliwell B., Gutteridge J. M. C. Free Radicals in Biology and Medicine . 4th. New York: Oxford University Press; 2007. [Google Scholar]
- 58.Liang Y., Dong B., Pang N., Hu J. Abamectin induces cytotoxicity via the ROS, JNK, and ATM/ATR pathways. Environmental Science and Pollution Research . 2020;27(12):13726–13734. doi: 10.1007/s11356-019-06869-2. [DOI] [PubMed] [Google Scholar]
- 59.Ghorbel I., Maktouf S., Kallel C., Chaabouni S. E., Boudawara T., Zeghal N. Disruption of erythrocyte antioxidant defense system, hematological parameters, induction of pro-inflammatory cytokines and DNA damage in liver of co-exposed rats to aluminium and acrylamide. Chemico-Biological Interactions . 2015;236:31–40. doi: 10.1016/j.cbi.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 60.Jebur A. B., El-Demerdash F. M., Kang W. Bromelain from Ananas comosus stem attenuates oxidative toxicity and testicular dysfunction caused by aluminum in rats. Journal of Trace Elements in Medicine and Biology . 2020;62, article 126631 doi: 10.1016/j.jtemb.2020.126631. [DOI] [PubMed] [Google Scholar]
- 61.Hosseini S. M., Hejazian L. B., Amani R., Badeli N. S. Geraniol attenuates oxidative stress, bioaccumulation, serological and histopathological changes during aluminum chloride-hepatopancreatic toxicity in male Wistar rats. Environmental Science and Pollution Research . 2020;27(16):20076–20089. doi: 10.1007/s11356-020-08128-1. [DOI] [PubMed] [Google Scholar]
- 62.Al-Otaibi W. R., ViRk P., ElobEid M. Ameliorative potential of stem bromelain on lead-induced toxicity in Wistar rats. Acta Biologica Hungarica . 2015;66(2):149–160. doi: 10.1556/018.66.2015.2.2. [DOI] [PubMed] [Google Scholar]
- 63.Agarwal S., Chaudhary B., Bist R. Bacoside a and bromelain relieve dichlorvos induced changes in oxidative responses in mice serum. Chemico-Biological Interactions . 2016;254:173–178. doi: 10.1016/j.cbi.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 64.Piste P. Cysteine- master antioxidant. International Journal of Pharmaceutical, Chemical and Biological Sciences . 2013;3:143–149. [Google Scholar]
- 65.Sies H. Glutathione and its role in cellular functions. Free Radical Biology & Medicine . 1999;27(9-10):916–921. doi: 10.1016/S0891-5849(99)00177-X. [DOI] [PubMed] [Google Scholar]
- 66.Gutteridge J. M. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clinical Chemistry . 1995;41(12):1819–1828. doi: 10.1093/clinchem/41.12.1819. [DOI] [PubMed] [Google Scholar]
- 67.Nehru B., Anand P. Oxidative damage following chronic aluminium exposure in adult and pup rat brains. Journal of Trace Elements in Medicine and Biology . 2005;19(2-3):203–208. doi: 10.1016/j.jtemb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 68.Kantola M., Sarranen M., Vanha P. T. Selenium and glutathione peroxidase in seminal plasma of men and bulls. Journal of Reproduction and Fertility . 1988;83(2):785–794. doi: 10.1530/jrf.0.0830785. [DOI] [PubMed] [Google Scholar]
- 69.Kalaiselvi M., Gomathi D., Ravikumar G., Devaki K., Uma C. Ameliorative effect of Ananus comosus peel on 7, 12 dimethylbenz(α)anthracene induced mammary carcinogenesis with reference to oxidative stress. Journal of Acute Disease . 2013;2(1):22–28. doi: 10.1016/S2221-6189(13)60089-X. [DOI] [Google Scholar]
- 70.Ghosh T., Mustafa M. D., Kumar V., et al. A preliminary study on the influence of glutathione S transferase T1 (GSTT1) as a risk factor for late onset Alzheimer's disease in north Indian population. Asian Journal of Psychiatry . 2012;5(2):160–163. doi: 10.1016/j.ajp.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 71.Yeh C. C., Hou M. F., Tsai S. M., et al. Superoxide anion radical, lipid peroxides and antioxidant status in the blood of patients with breast cancer. Clinica Chimica Acta . 2005;361(1-2):104–111. doi: 10.1016/j.cccn.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 72.Muselin F., Trif A., Stana G. L., et al. Protective effects of aqueous extract of Sempervivum tectorum L (Crassulaceae) on aluminium-induced oxidative stress in rat blood. Tropical Journal of Pharmaceutical Research . 2014;13(2):179–184. doi: 10.4314/tjpr.v13i2.2. [DOI] [Google Scholar]
- 73.Jagetia G. C., Rao S. K. Evaluation of the antineoplastic activity of guduchi (Tinospora cordifolia) in Ehrlich ascites carcinoma bearing mice. Biological & Pharmaceutical Bulletin . 2006;29(3):460–466. doi: 10.1248/bpb.29.460. [DOI] [PubMed] [Google Scholar]
- 74.Chen C., Lin B., Qi S., He J., Zheng H. Protective effects of salidroside on lead acetate-induced oxidative stress and hepatotoxicity in Sprague-Dawley rats. Biological Trace Element Research . 2019;191(2):426–434. doi: 10.1007/s12011-019-1635-8. [DOI] [PubMed] [Google Scholar]
- 75.Gokcimen A., Gulle K., Demirin H., Bayram D., Kocak A., Altuntas I. Effects of diazinon at different doses on rat liver and pancreas tissues. Pesticide Biochemistry and Physiology . 2007;87(2):103–108. doi: 10.1016/j.pestbp.2006.06.011. [DOI] [Google Scholar]
- 76.Albasher G., Alsaleh A. S., Alkubaisi N., et al. Red beetroot extract abrogates chlorpyrifos-induced cortical damage in rats. Oxidative Medicine and Cellular Longevity . 2020;2020:13. doi: 10.1155/2020/2963020.2963020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhadauria M. Combined treatment of HEDTA and propolis prevents aluminum induced toxicity in rats. Food and Chemical Toxicology . 2012;50(7):2487–2495. doi: 10.1016/j.fct.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 78.Sivakumari K., Manavalaramanujam R., Ramesh M., Lakshmi R. Cypermethrin toxicity: sublethal effects on enzyme activities in a freshwater fish, Cyprinus carpio var. communis. Journal of Environmental Biology . 1997;18:121–125. [Google Scholar]
- 79.El-Shenawy N. S., Al-Eisa R. A., El-Salmy F., Salah O. Prophylactic effect of vitamin E against hepatotoxicity, nephrotoxicity, haematological indices and histopathology induced by diazinon insecticide in mice. Current Zoology . 2009;55(3):219–226. doi: 10.1093/czoolo/55.3.219. [DOI] [Google Scholar]
- 80.Chatterjea M. N., Shinde R. Text Book of Medical Biochemistry . 5th. New Delhi: Jaypee Brothers. Medical Publishers Ltd; 2002. [Google Scholar]
- 81.Parle M., Goel P. Eat pineapple a day to keep depression at bay. International Journal of Research in Ayurveda and Pharmacy . 2010;1:439–448. [Google Scholar]
- 82.John J., Nampoothiri M., Kumar N., Mudgal J., Nampurath G. K., Chamallamudi M. R. Sesamol, a lipid lowering agent, ameliorates aluminium chloride induced behavioral and biochemical alterations in rats. Pharmacognosy Magazine . 2015;11:p. 327. doi: 10.4103/0973-1296.153086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khalifa M., Safar M. M., Abdelsalam R. M., Zaki H. F. Telmisartan protects against aluminum-induced Alzheimer-like pathological changes in rats. Neurotoxicity Research . 2020;37:275–285. doi: 10.1007/s12640-019-00085-z. [DOI] [PubMed] [Google Scholar]
- 84.Prabhu S. D., Frangogiannis N. G. The biological basis for cardiac repair after myocardial infarction. Circulation Research . 2016;119(1):91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ahmed W., Ibrahim M. A., Helmy N. A., ElKashlan A. M., Elmaidomy A. H., Zaki A. R. Amelioration of aluminum-induced hepatic and nephrotoxicity by Premna odorata extract is mediated by lowering MMP9 and TGF-β gene alterations in Wistar rat. Environmental Science and Pollution Research . 2022;29(48):72827–72838. doi: 10.1007/s11356-022-20735-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu H., Zhang J., Ji Q., et al. Melatonin alleviates aluminium chloride-induced immunotoxicity by inhibiting oxidative stress and apoptosis associated with the activation of Nrf2 signaling pathway. Ecotoxicology and Environmental Safety . 2019;173:131–141. doi: 10.1016/j.ecoenv.2019.01.095. [DOI] [PubMed] [Google Scholar]
- 87.Othman M. S., Fareid M. A., Abdel Hameed R. S., Abdel Moneim A. E. The protective effects of melatonin on aluminum-induced hepatotoxicity and nephrotoxicity in rats. Oxidative Medicine and Cellular Longevity . 2020;2020:12. doi: 10.1155/2020/7375136.7375136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cao Z., Wang P., Gao X., Shao B., Zhao S., Li Y. Lycopene attenuates aluminum-induced hippocampal lesions by inhibiting oxidative stress-mediated inflammation and apoptosis in the rat. Journal of Inorganic Biochemistry . 2019;193:143–151. doi: 10.1016/j.jinorgbio.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 89.Gouda A. S., El-Nabarawy N. A., Ibrahim S. F. Moringa oleifera extract (lam) attenuates aluminium phosphide-induced acute cardiac toxicity in rats. Toxicology Reports . 2018;5:209–212. doi: 10.1016/j.toxrep.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are incorporated in the manuscript.


