Abstract
To evaluate the efficacy and safety of topical oxygen therapy (TOT) in diabetic foot ulcers (DFUs), researchers systematically retrieved relevant studies from PubMed, EMBASE, Web of Science, CENTRAL and ClinicalTrials.gov. Relevant studies were searched from database inception to January 2022. Two researchers independently screened the literature, extracted data and assessed the quality of the included studies. Statistical analysis was performed in Stata 16.0. A total of seven RCTs involving 614 participants were included. Compared with the control group, the TOT group had a higher healing rate (RR = 1.63, 95% CI [1.33, 2.00]). According to descriptive analysis, TOT reduced the ulcer area and improved healing durability and quality of life. Furthermore, it had no effect on the occurrence of adverse events. However, it was unclear whether it would be able to reduce the healing time. The existing evidence suggests that TOT is effective and safe for chronic DFUs. Further studies are warranted to validate our findings.
Keywords: diabetic foot ulcer, meta‐analysis, systematic review, topical oxygen therapy
1. INTRODUCTION
Diabetic foot ulcers (DFUs) are the most frequently recognised and notorious diabetic complication. 1 Between 19% and 34% of diabetic patients are likely to be affected by a DFU in their lifetime. 1 DFUs are a major cause of amputation in diabetic patients, and 85% of amputations are related to DFUs. 2 They have the potential to not only diminish patients' quality of life 3 but also their life expectancy. DFU patients have a 2.5‐fold higher risk of death at 5 years than diabetes patients without foot ulcers. 4 Frequent medical visits and hospital admissions result from a high recurrence rate.
Oxygen is a crucial element in the wound healing process. 5 It has been shown that wounds and tissue injuries cause the injured area to become hypoxic, presumably due to the disruption of the vasculature and increased oxygen consumption. 6 Acute hypoxia initiates wound healing, whereas chronic hypoxia impairs neovascularisation. 7 Increasing amounts of evidence have suggested that increased hypoxic conditions and impaired cellular responses to hypoxia are essential pathogenic factors of delayed wound healing in DFU. 8 To increase oxygenation, the rationale for applying exogenous oxygen to a wound can trigger healing responses that have been hindered by hypoxia. 9 At present, different technologies for supplying oxygen to wounds have been developed. Topical oxygen therapy (TOT) can ameliorate oxygen deficiency by directly delivering oxygen to the wound bed without relying on an (impaired) vascular system or respiratory system. 5 , 10 , 11 Studies have shown that TOT is beneficial for numerous cellular mechanisms required for wound healing (eg, antibacterial mechanisms, collagen production and epithelial migration). 12
To date, a number of systematic reviews also found that TOT could promote DFU healing. 13 , 14 , 15 Among them, two reviews included non‐randomised trials, and another review only searched two databases. Furthermore, recently three further RCTs were published that were not included in the previous systematic reviews. 16 , 17 , 18 Therefore, this study aimed to perform an updated meta‐analysis and impact clinical decision‐making.
2. MATERIALS AND METHODS
The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) statement. 19 The corresponding protocol was registered in PROSPERO (CRD42020152750).
2.1. Data sources and searches
To identify relevant RCTs, two researchers systematically retrieved studies from PubMed, CENTRAL, EMBASE (via OVID), Web of Science (core) and ClinicalTrials.gov. After reading some relevant literatures, medical subject heading (MeSH) terms, entry terms and keywords were used to build filters. Taking PubMed as an example, the retrieval strategy is as follows: ((((("Diabetic Foot"[Mesh]) OR "Foot Ulcer"[Mesh])) OR ((diabet*[Title/Abstract]) AND (((((((ulcer*[Title/Abstract]) OR foot[Title/Abstract]) OR feet[Title/Abstract]) OR wound*[Title/Abstract]) OR amputat*[Title/Abstract]) OR defect*[Title/Abstract]) OR gangrene[Title/Abstract])))) AND ((((((((((((((topical[Title/Abstract]) OR local*[Title/Abstract]) OR partial[Title/Abstract]) OR regional[Title/Abstract]) OR percutaneous[Title/Abstract]) OR transcutaneous[Title/Abstract]) OR transdermal[Title/Abstract]) OR skin[Title/Abstract]) OR ulcer*[Title/Abstract]) OR wound*[Title/Abstract]) OR continuous diffusion[Title/Abstract]))) AND (((((oxygen therap*[Title/Abstract]) OR oxygen treatment[Title/Abstract]) OR oxygen supply[Title/Abstract]) OR oxygen delivery[Title/Abstract]) OR oxygen[Title])). Retrieval strategies were adjusted according to different databases. The references of the included studies were manually searched. The retrieval period was from database inception to January 2022.
2.2. Study selection
Inclusion criteria: (a) Participant: patients with DFUs and corresponding podiatric grading; (b) intervention: TOT alone or combined with standard of care (SOC); (c) control: SOC; (d) outcome: number of complete ulcer healing, ulcer area, healing time, adverse event, follow‐up and quality of life (QOL); (e) study design: clinical RCT. Exclusion criteria: (a) lack of key data; (b) abstract; (c) as for interim reports or bilingual publications, the most comprehensive was retained; (d) clinical trial protocol.
2.3. Data extraction
Two researchers independently accomplished the same tasks. First, duplicated literature was removed. After reading the titles and abstracts, studies that obviously did not meet the aforementioned criteria were removed. Then, the researchers identified relevant studies by perusing the full text. Finally, two researchers checked each other's work. Discrepancies were resolved through discussion.
Two researchers independently extracted data from the included studies, and the principle of intention‐to‐treat (ITT) analysis was followed. The following data were collected: characteristics of the included study (first author, publication year, sample size, details of TOT, therapeutic regimens of the control group, curative time), baseline characteristics (country, sex, age, HbA1c, podiatric grade, duration of DFU, ulcer area, ankle‐brachial index) and outcomes (as shown in inclusion criteria).
2.4. Assessment of risk of bias
Applying the Cochrane risk of bias assessment tool, the quality of each included study was evaluated based on the following items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other biases. 20 Two researchers independently assessed each item and checked each other's work. If there were any disagreements, the researchers discussed and resolved them.
2.5. Statistical analysis
Extracted data were imported into Stata 16.0 (Stata Corp, College Station, TX, USA) for meta‐analysis, and heterogeneity tests were performed by Q‐tests and the I 2 index. If the heterogeneity test results were P ≥ .1 and I 2 ≤ 50%, a fixed‐effects model was used; otherwise, a random‐effects model was used. In the absence of key data, descriptive analysis was performed. Relative risk (RR) and 95% confidence interval (CI) were used for dichotomous data. Mean difference and 95% CI were presented for continuous data, but the measurement units were inconsistent, so standardised mean difference and 95% CI were used. Sensitivity analysis was performed by excluding individual studies one at a time and recalculating pooled estimates. Harbord's modified linear regression test and funnel plot were used to analyse potential publication bias. 21 All analyses were conducted with Stata 16.0, and a P‐value less than .05 was considered significant.
3. RESULTS
3.1. Study selection
A total of 2681 potential studies were obtained. First, 983 duplicate studies were removed. After reading the titles and abstracts, 1654 studies that did not meet the inclusion criteria were also removed. Eligible studies were retained after perusing the full‐text of the remaining 44 studies. Finally, seven studies 16 , 17 , 18 , 22 , 23 , 24 , 25 were qualitatively analysed and quantitatively analysed, as shown in Figure 1.
FIGURE 1.
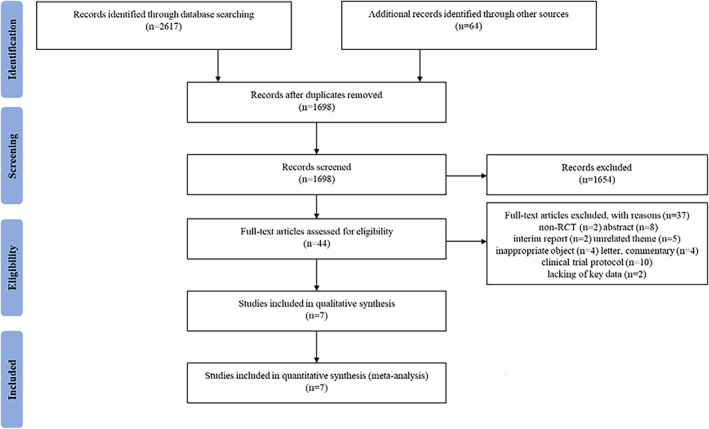
Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) flow chart
3.2. Study characteristics
A total of seven studies 16 , 17 , 18 , 22 , 23 , 24 , 25 were included, including 614 DFU patients. Among them, four studies 17 , 22 , 23 , 24 were conducted in America, and the other three studies 16 , 18 , 25 came from China, India and Canada. The sample sizes ranged from 20 to 146. The publication years ranged from 2016 to 2021. There were 451 males and 153 females in the included studies, with a sex ratio of 2.95:1. Five studies 16 , 17 , 22 , 24 , 25 reported continuous diffusion of oxygen therapy and the remaining studies used intermittent TOT. Four studies 22 , 23 , 24 , 25 adopted the Texas grade, from I A to III D, and the others 16 , 17 , 18 adopted the Wagner grade, from 1 to 3. The curative time ranged from 6 to 12 weeks, with an average of 10 weeks. Six studies 17 , 18 , 22 , 23 , 24 , 25 were registered on clinical trial platforms, and four studies 17 , 22 , 23 , 24 were multicentre RCTs. Other characteristics of the included studies are shown in Tables 1 and 2.
TABLE 1.
Baseline characteristics of the included studies
| Author | Country | Sex | Age | HbA1c | DFU | DFU | DFU | ABI | Sample size T/C |
|---|---|---|---|---|---|---|---|---|---|
| Year | M/F | Mean ± SD (year) | Mean ± SD (%) | Grading | Duration | Ulcer area (cm2) | |||
| Yu et al 2016 25 | Canada | 17/3 |
[T] 57 ± (9.5) [C] 58 ± (9.5) |
[T] 8.6 ± (2.3) [C] 7.3 ± (0.5) |
Texas (I A~III D) |
[T] 47.4 ± (23.4) wk [C] 46.2 ± (17.9) wk |
[T] 1.37 ± (0.95) [C] 1.68 ± (1.31) |
[T] 1.10 ± (0.19) [C] 0.96 ± (0.20) |
10/10 |
| Driver et al 2017 22 | America |
[T] 43/18 a [C] 47/14 |
[T] 58.6 ± (12.31) [C] 58.8 ± (9.4) |
[T] 8.0 ± (1.7) [C] 7.9 ± (1.7) |
Texas (I A) |
[T] 17.7 ± (12.8) wk [C] 14.9 ± (12.5) wk |
[T] 2.0 ± (1.7) [C] 2.3 ± (1.7) |
[T] 1.0 ± (0.2) [C] 1.0 ± (0.2) |
65/63 |
| Niederauer et al 2018 24 | America |
[T] 59/15 [C] 54/18 |
[T] 56.1 ± (10.1) [C] 56.6 ± (14.4) |
[T] 8.4 ± (1.6) [C] 8.3 ± (2.0) |
Texas (I A) |
[T] 131.6 ± (89.2) d [C] 143.8 ± (97.7) d |
[T] 3.54 ± (1.68) [C] 3.89 ± (2.02) |
[T] 1.05 ± (0.14) [C] 1.02 ± (0.15) |
74/72 |
| Frykberg et al 2020 23 | America |
[T] 32/4 [C] 31/6 |
[T] 64.6 ± (10.3) [C] 61.9 ± (9.5) |
[T] 8.43 ± (1.75) [C] 8.14 ± (1.49) |
Texas (I A~II C) |
[T] 160.3 ± (96) d [C] 174.6 ± (94) d |
[T] 3.02 ± (2.66) [C] 3.22 ± (2.54) |
[T] 1.07 ± (0.23) [C] 1.00 ± (0.23) |
36/37 |
| He et al 2021 16 | China |
[T] 24/16 a [C] 23/17 |
[T] 63.5 ± (10.1) [C] 63.1 ± (9.3) |
[T] 7.91 ± (1.17) [C] 7.85 ± (1.23) |
Wagner (2, 3) |
[T] 3.03~4.68 mo [C] 2.00~5.80 mo |
[T] 35.35 ± (20.83) [C] 42.07 ± (38.55) |
NR | 40/42 |
| Serena et al 2021 17 | America |
[T] 54/26 a [C] 53/11 |
[T] 64.20 ± (14.15) [C] 62.29 ± (12.56) |
NR | Wagner (1‐3) |
[T] 24.46 ± (22.62) wk [C] 23.77 ± (17.85) wk |
[T] 2.86 ± (2.93) [C] 3.47 ± (4.12) |
NR | 81/64 |
| Anirudh et al 2021 18 | India |
[T] 7/3a [C] 7/2 |
[T] 58.7 ± (5.7) [C] 57.1 ± (9.9) |
[T] 8.7 ± (0.8) [C] 8.0 ± (1.6) |
Wagner (2, 3) | NR |
[T] 11.7~31.50 b [C] 10.4~27.50 |
[T] 0.77 ± (0.09) [C] 0.79 ± (0.09) |
10/10 |
Abbreviations: ABI, ankle brachial index; C, control group; d, day; DFU, diabetic foot ulcer; mo, month; NR, not reported; SD, standard deviation; T, TOT group; TOT, topical oxygen therapy; wk, week; y, year.
The gender of lost subjects were not described.
Inter quartile range.
TABLE 2.
Intervention protocols of the included studies
| Author | Trial group | Control group | Detail of TOT | Therapeutic regimens of the control group | Curative time | Outcomes |
|---|---|---|---|---|---|---|
| Year | (week) | |||||
| Yu et al 2016 25 | TOT | SOC | The Oxygen Delivery System was placed directly on the wound surface and attached to the active Natrox Oxygen Generator using the tubing provided. | Sharp debridement and antimicrobial dressings, offloading standard practice. | 8 | ①② |
| Driver et al 2017 22 | TOT + SOC | SOC + Placebo | Continuous administration of oxygen (>98%) to the wound site using a 15‐d device changed every 15 d. | Removal of necrotic or infected tissue, wound cleaning, establishment of adequate blood circulation, maintenance of a moist wound environment, offloading, management of wound infection, nutritional support and glycaemic control. | 12 | ①③④ |
| Niederauer et al 2018 24 | TOT + SOC | SOC + Placebo | TransCu O2 System continuously generate pure (>99.9%), humidified oxygen at flow rates of 3~15 mL/h and delivers it directly to the wound bed environment within the MWT dressing system by tubing. | Wound cleaning, MWT, offloading, aggressive debridement. | 12 | ①③④⑤ |
| Frykberg et al 2020 23 | TOT + SOC | SOC + Placebo | The affected limb was put into the air chamber, humidified oxygen was cycled between 10 and 50 mb within the chamber. A 10 L/min oxygen concentrator was used. Patients treated themselves at home for 90 min daily five times per week. | Debridement, offloading, MWT. | 12 | ①②③④⑤⑥ |
| He et al 2021 16 | TOT + SOC | SOC | A micro‐oxygen supply device (Greens O‐4‐3, China) was used to generate high purity oxygen, which was continuously delivered to the center surface of the wound at a constant flow rate through oxygen connection tubing. | Control of blood pressure, blood glucose and lipid levels, treatment of peripheral neuropathy, nutritional support and correction of hypoproteinaemia and electrolyte imbalance, offloading, antibiotic treatments, debridement, moist wound dressing. | 8 | ①②③⑤ |
| Serena et al 2021 17 | TOT + SOC | SOC | The Natrox Oxygen Generator delivers a pure oxygen flow rate of 15 mL/h. The oxygen delivery system allows wound exudate to pass through to the secondary dressing while allowing the diffusion of oxygen across the wound bed. | Wound cleaning, sharp debridement, offloading, moisture balance. | 12 | ①②④ |
| Anirudh et al 2021 18 | TOT + SOC | SOC | The device is designed to deliver topical oxygen at a controlled temperature of 42°C, a concentration of 93% (±3%), and a flow rate of 1 L/min. This protocol was repeated alternate day of every week for 6 wk (18 sessions). | Wound cleaning, debridement, glycaemic control, antimicrobials. | 6 | ②④⑤⑥ |
Note: ①, number of complete ulcer healing; ②, ulcer area; ③, healing time; ④, adverse event; ⑤, follow‐up; ⑥, quality of life.
Abbreviations: MWT, moist wound therapy; SOC, standard of care; TOT, topical oxygen therapy.
3.3. Assessment of risk of bias
The risk of bias is shown in Figure 2. The main bias comes from selective reporting and other biases. Two studies 16 , 25 had a high risk of selective reporting. This was because adverse events were not routinely reported. Other bias was at high risk. The main reason was that two studies 24 , 25 accepted the funding of companies. One study 25 had a high risk of attrition bias. Moreover, all studies provided details about random sequence generation. Six studies 17 , 18 , 22 , 23 , 24 , 25 reported allocation concealment. Three studies 22 , 23 , 24 described their blinded methods and placebo‐controlled.
FIGURE 2.
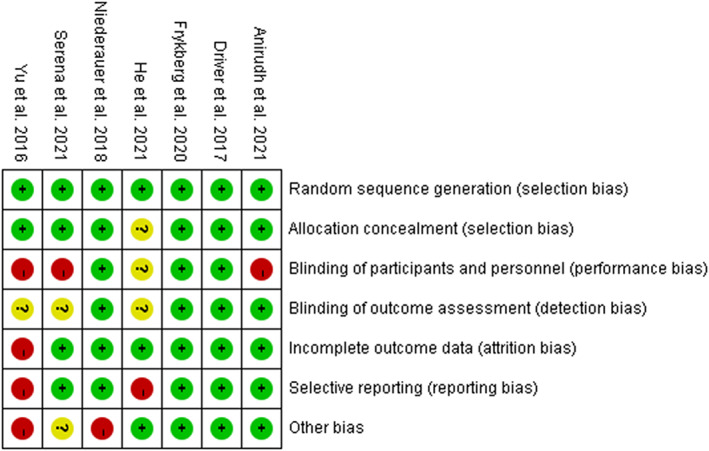
Risk of bias summary
3.4. Data analysis
3.4.1. Number of complete ulcer healing
Six studies 16 , 17 , 22 , 23 , 24 , 25 reported the effect of TOT on the number of complete ulcer healing of DFUs after 8‐week or 12‐week treatment. Therefore, we conducted a subgroup analysis at 8 weeks and 12 weeks. And, He et al provided that clinical outcomes of DFU wound between the two groups after 8 and 12 weeks of treatment. 16 There was heterogeneity (P = .096, I 2 = 46.5%) among these studies. A fixed‐effects model was used. Meta‐analysis showed that TOT could improve the number of ulcer that completely healed compared with the control group (RR = 1.63, 95% CI [1.33, 2.00], P < .00001) (Figure 3).
FIGURE 3.
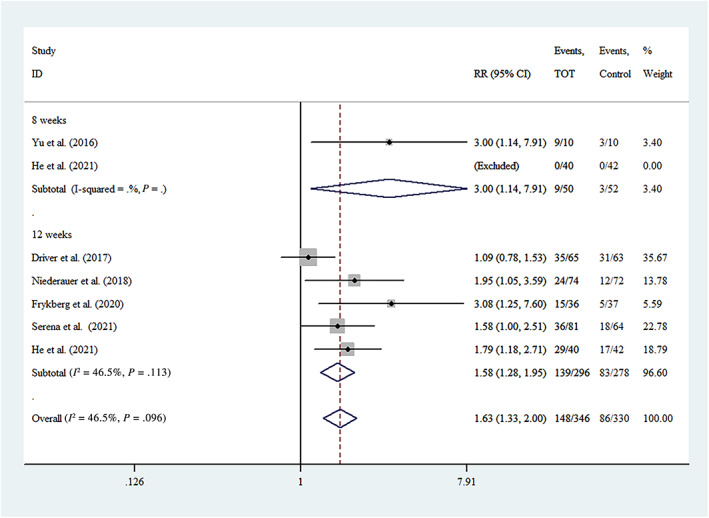
Forest plot of number of complete ulcer healing
3.4.2. Ulcer area
Five studies 16 , 17 , 18 , 23 , 25 reported on ulcer area. Two studies 18 , 25 found that TOT significantly decreased the mean ulcer area from baseline values. Another study 23 indicated that the mean absolute reduction in ulcer area from baseline was 1.97 cm2 for the TOT arm compared with 0.40 cm2 for the sham arm (t(df) = 2.12, P = .041). Although the other two studies 16 , 17 showed that the TOT group had a higher ulcer reduction rate than the control group, their intervention durations were inconsistent, so meta‐analysis was not conducted. Hence, TOT helped reduce the ulcer area.
3.4.3. Healing time
Due to the inconsistent units and reporting, we conducted descriptive analysis on healing time. Four studies 16 , 22 , 23 , 24 reported ulcer healing time. One trial 23 found that TOT did not shorten the healing time compared with the control group (TOT 8.2 ± 4.2 vs control 6.3 ± 1.9 weeks, P = .35). Similarly, one study 22 also reported that the median time to complete closure in the per‐protocol population group was 63 days for the TOT and 77 days for the control group (P > .05); however, for the ≥65‐year‐old subgroup, the median time to closure was 35 days for the TOT and 70 days for the control group (P < .05). In contrast, the time to 50% DFU closure was significantly shorter in patients who received TOT (mean 18.4 vs 28.9 days, P = .001) in one trial. 24 Another study 16 also found that the average wound healing times were 48.39 ± 13.32 days in the TOT group and 76.42 ± 32.78 days in the control group (P < .05). Whether TOT could shorten ulcer healing time was unclear.
3.4.4. Adverse events
Five studies 17 , 18 , 22 , 23 , 24 reported on adverse events. One study 18 reported no adverse events in the intervention group compared to three adverse events in the control group. The other four studies 17 , 22 , 23 , 24 showed that the adverse events were similar between the two groups. Only one included study 22 described three cases of adverse events that might have been caused by TOT device. The remaining studies did not report any adverse events related to TOT. Thus, there are reasons to believe TOT has no clinical risk.
3.4.5. Follow‐up
Four studies 16 , 18 , 23 , 24 were followed up. During the 1‐year follow‐up, one study 16 reported that six patients and no patients in the control and TOT groups, respectively, underwent amputation. During the 12‐week follow‐up, one trial 18 reported no amputation in the TOT group compared to two amputations in the control participants. Frykberg's study 23 showed that only 1 of 15 healed ulcers (6.7%) in the TOT group recurred, compared with 2 of 5 healed ulcers (40%) in the control group(P = .07) after 1 year. Another study 24 indicated that there were no significant differences between the treatment arms (P = .83) at the 12‐week follow‐up. Although there were different follow‐up periods and indicators in the above studies, we concluded that TOT might contribute to ulcer durability.
3.4.6. Quality of life
Two studies 18 , 23 reported on quality of life. One study 18 showed that patients in the TOT group had a better quality of life using the IVDP‐QOL questionnaire after 6‐week treatment. The IVDP‐QOL score decreased from 7.5 to 4.44. Another study 23 also showed that the greatest improvement was seen for the well‐being component using the CWIS QOL index, with mean score difference between baseline and the end of 12 weeks of treatment in the TOT arm of 9.1 compared with 20.1 in the sham arm (t(df) = 2.18, P = .033). Although they used different questionnaires to investigate the participants' quality of life, we still found that TOT helped to improve their quality of life.
3.5. Sensitivity and heterogeneity analysis
In the sensitivity analysis of the number of complete ulcer healings, individual studies were excluded one by one, and the heterogeneity was mainly related to Driver's study. 22 After excluding that study, the heterogeneity was I 2 = 0% (RR = 1.93, 95% CI [1.49, 2.49]) (Figure 4). This may be related to the fact that the patients in that study had a relatively mild condition. Two recent studies 23 , 24 showed that chronic or larger DFUs were more suitable for TOT. In summary, the above result was robust.
FIGURE 4.
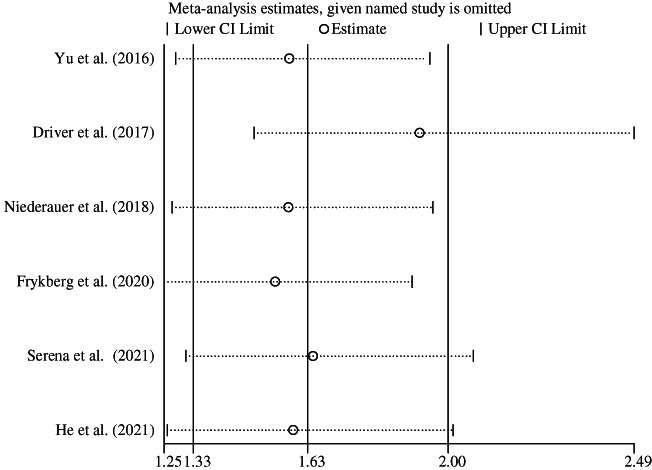
Sensitivity analysis of number of complete ulcer healing
3.6. Publication bias analysis
Publication bias is that the publication or non‐publication of research findings, depending on the nature and direction of the results. 26 Generally, statistically significant results were more likely to be published. The funnel plot of ulcer‐complete healing number displayed an apparent asymmetry that suggested the presence of a potential publication bias (Figure 5). Moreover, Harbord's modified linear regression test also indicated that there was a risk of publication bias (P = .02). Hence, there is reason to believe that some studies with negative results may not have been published. To reduce the risk of publication bias, more databases and grey literatures needed to be searched.
FIGURE 5.
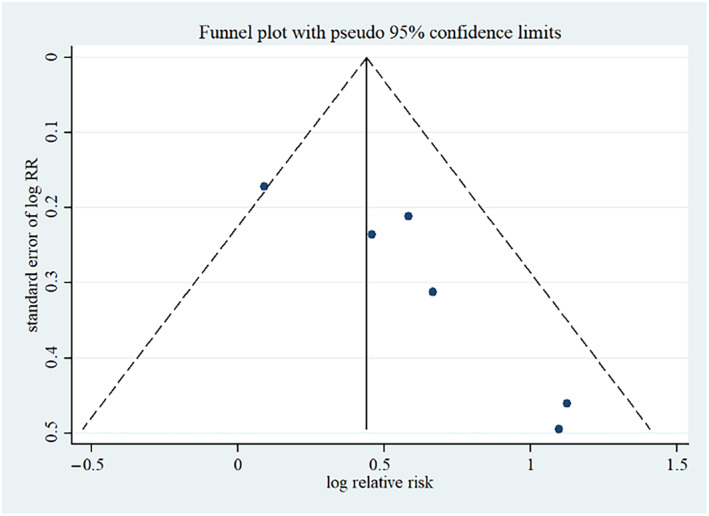
Funnel plot of number of complete ulcer healing
4. DISCUSSION
Currently, SOC alone may not be sufficient to prevent and treat DFUs, and novel adjunctive therapies are urgently needed. 27 For all this, it is also critical to emphasise that TOT (as for adjunctive therapy) must be administered in conjunction with optimal wound care. 28 In other words, DFU patients still need to receive SOC combined with other adjunctive therapies. This combination therapy can not only achieve better therapeutic effects but also complies with ethical principles. In this review, seven RCTs with 614 patients were analysed in our meta‐analysis. All studies have been published since 2016. Six included studies used combination therapy in the trial arm. There were nearly three times as many men as women in the included studies. More studies believe that a 12‐week course is more reasonable. The findings of this review provide a detailed summary of the present evidence of TOT for the treatment of DFUs. This result indicated that compared with SOC, TOT was beneficial for promoting ulcer healing without increasing the incidence of adverse events.
Unfortunately, there were four predefined outcomes that were qualitatively analysed. Although these studies indicated TOT helped to reduce the ulcer area, Serena et al reported that for the ITT analysis, there was no statistical difference in percentage reduction in ulcer area (TOT 46.38 ± 100.24 vs control 41.50 ± 69.82, P = .72). 17 With respect to healing time, two included studies 22 , 23 displayed that TOT did not shorten the healing time, but two other studies 16 , 24 showed opposite results. It is worth noting that SOC, run‐in period, definition of ulcer healing time were different in these studies. Therefore, we were not certain whether TOT could reduce the time to ulcer healing. This indicator will need to be given more consideration in future studies. We also summarised durability of the included studies. Four included studies 16 , 18 , 23 , 24 found that TOT helped to increase healing durability during the 12‐week or 12‐month follow‐up period. In fact, healing durability may lower the rate of readmission. Only two studies 18 , 23 offered significant data on quality of life. They employed various questionnaires and presented the results in various ways. As a result, a meta‐analysis was ruled out. Furthermore, future studies should take into account the subjective results of patients, such as psychological states and patient satisfaction. This was due to the fact that patient compliance required a better treatment experience.
Previous reviews were consistent with our results, but there were some distinctions. Nataraj et al 13 included two RCTs, two non‐RCTs and one case report, without meta‐analysis. Likewise, Connaghan et al 14 performed a meta‐analysis, but four included studies were non‐RCTs. Meanwhile, Thanigaimani et al 15 also conducted a similar meta‐analysis, but only two databases were retrieved. Moreover, two included studies 24 , 29 were from the same study protocol and research team. Hence, there is a risk of duplication data. Unlike previous studies, our review only included RCTs. Apart from healing rate and adverse events, our review showed more outcomes. Because podiatric grading can reflect illness severity, all included studies reported on paediatric grading. These factors can improve the credibility of our study.
Although all included studies were RCTs, there were also some weaknesses. First, inclusion and exclusion criteria were not identical for all included studies, yet baselines between the two groups were similar in each study, or the wounds in the TOT group were more serious, implying that TOT had an effect. However, three included studies 16 , 18 , 25 did not have a run‐in period for chronic wound screening. This might reduce the homogeneity of participants. Second, inconsistent intervention lead to clinical heterogeneity. Offloading, for example, is an important element DFU treatment. However, two included studies 16 , 18 did not use an offloading device. Third, different intervention durations (eg, 6 weeks, 8 weeks, 12 weeks) also resulted in clinical heterogeneity. Future studies should be registered on a clinical trial platform and give intermediate indicators in order to investigate the appropriate period of intervention. Fourth, some studies relied on in‐home care, while others relied on outpatient treatment. Patients' compliance with home care should be evaluated and reported in order to follow treatment regimens. Fifth, due to small number of studies included, no subgroup analysis of continuous diffusion oxygen therapy and intermittent TOT was undertaken. Furthermore, additional randomised double‐blind placebo‐controlled trials should be considered to strengthen the credibility of research results by reducing the influence of patients' and researchers' subjective elements on study results. Therefore, the findings should be interpreted cautiously.
5. CONCLUSION
The existing evidence suggests that TOT is effective and safe for DFU patients. TOT may be an alternative for some chronic DFUs. In the future, more high‐quality studies are needed to support or refute the findings. Additionally, the mechanism, optimal treatment and economic evaluation still need to be further explored.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENT
This work was supported by West China Nursing Discipline Development Special Fund project of Sichuan University, Grant Number: HXHL19005; Popularization and application project of Sichuan Provincial Health Commission, Grant Number: 20PJ023.
Sun X‐K, Li R, Yang X‐L, Yuan L. Efficacy and safety of topical oxygen therapy for diabetic foot ulcers: An updated systematic review and meta‐analysis. Int Wound J. 2022;19(8):2200‐2209. doi: 10.1111/iwj.13830
Funding information Popularization and application project of Sichuan Provincial Health Commission, Grant/Award Number: 20PJ023; West China Nursing Discipline Development Special Fund project of Sichuan University, Grant/Award Number: HXHL19005
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367‐2375. [DOI] [PubMed] [Google Scholar]
- 2. Lepäntalo M, Apelqvist J, Setacci C, et al. Chapter V: diabetic foot. Eur J Vasc Endovasc Surg. 2011;42(Suppl 2):S60‐S74. [DOI] [PubMed] [Google Scholar]
- 3. Snyder RJ, Hanft JR. Diabetic foot ulcers‐‐effects on QOL, costs, and mortality and the role of standard wound care and advanced‐care therapies. Ostomy Wound Manage. 2009;55(11):28‐38. [PubMed] [Google Scholar]
- 4. Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population‐based cohort from the United Kingdom. Diabet Med. 2016;33(11):1493‐1498. [DOI] [PubMed] [Google Scholar]
- 5. de Smet GHJ, Kroese LF, Menon AG, et al. Oxygen therapies and their effects on wound healing. Wound Repair Regen. 2017;25(4):591‐608. [DOI] [PubMed] [Google Scholar]
- 6. Ruthenborg RJ, Ban JJ, Wazir A, Takeda N, Kim JW. Regulation of wound healing and fibrosis by hypoxia and hypoxia‐inducible factor‐1. Mol Cells. 2014;37(9):637‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010;163(2):257‐268. [DOI] [PubMed] [Google Scholar]
- 8. Catrina SB, Zheng X. Disturbed hypoxic responses as a pathogenic mechanism of diabetic foot ulcers. Diabetes Metab Res Rev. 2016;32(Suppl 1):179‐185. [DOI] [PubMed] [Google Scholar]
- 9. Lantis J. Oxygen therapy: evidence base. J Wound Care. 2020;29(Sup5b):S11‐S22. [DOI] [PubMed] [Google Scholar]
- 10. Dissemond J, Kröger K, Storck M, Risse A, Engels P. Topical oxygen wound therapies for chronic wounds: a review. J Wound Care. 2015;24(2):53‐63. [DOI] [PubMed] [Google Scholar]
- 11. Winfeld B. Topical oxygen and hyperbaric oxygen therapy use and healing rates in diabetic foot ulcers. Wounds. 2014;26(5):E39‐E47. [Google Scholar]
- 12. Kaufman H, Gurevich M, Tamir E, Keren E, Alexander L, Hayes P. Topical oxygen therapy stimulates healing in difficult, chronic wounds: a tertiary centre experience. J Wound Care. 2018;27(7):426‐433. [DOI] [PubMed] [Google Scholar]
- 13. Nataraj M, Maiya AG, Karkada G, et al. Application of topical oxygen therapy in healing dynamics of diabetic foot ulcers ‐ a systematic review. Rev Diabet Stud. 2019;15:74‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Connaghan F, Avsar P, Patton D, O'Connor T, Moore Z. Impact of topical oxygen therapy on diabetic foot ulcer healing rates: a systematic review. J Wound Care. 2021;30(10):823‐829. [DOI] [PubMed] [Google Scholar]
- 15. Thanigaimani S, Singh T, Golledge J. Topical oxygen therapy for diabetes‐related foot ulcers: a systematic review and meta‐analysis. Diabet Med. 2021;38(8):e14585. [DOI] [PubMed] [Google Scholar]
- 16. He S, Liang C, Yi C, Wu M. Therapeutic effect of continuous diffusion of oxygen therapy combined with traditional moist wound dressing therapy in the treatment of diabetic foot ulcers. Diabetes Res Clin Pract. 2021;174:108743. [DOI] [PubMed] [Google Scholar]
- 17. Serena TE, Bullock NM, Cole W, et al. Topical oxygen therapy in the treatment of diabetic foot ulcers: a multicentre, open, randomised controlled clinical trial. J Wound Care. 2021;30(Sup5):S7‐S14. [DOI] [PubMed] [Google Scholar]
- 18. Anirudh V, Kamath DY, Ghosh S, et al. Topical controlled warm oxygen therapy delivered through a novel device (KADAM™) to treat diabetic foot ulcers: a randomized controlled, open, pilot trial. Indian J Surg. 2021;83:907‐914. doi: 10.1007/s12262-021-03057-w [DOI] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 21. Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443‐3457. [DOI] [PubMed] [Google Scholar]
- 22. Driver VR, Reyzelman A, Kawalec J, French M. A prospective, randomized, blinded, controlled trial comparing transdermal continuous oxygen delivery to moist wound therapy for the treatment o diabetic foot ulcers. Ostomy Wound Manage. 2017;63(4):12‐28. [PubMed] [Google Scholar]
- 23. Frykberg RG, Franks PJ, Edmonds M, et al. A multinational, multicenter, randomized, double‐blinded, placebo‐controlled trial to evaluate the efficacy of cyclical topical wound oxygen therapy (TWO2) in the treatment of chronic diabetic foot ulcers: the TWO2 study. Diabetes Care. 2020;43(3):616‐624. [DOI] [PubMed] [Google Scholar]
- 24. Niederauer MQ, Michalek JE, Liu Q, Papas KK, Lavery LA, Armstrong DG. Continuous diffusion of oxygen improves diabetic foot ulcer healing when compared with a placebo control: a randomised, double‐blind, multicentre study. J Wound Care. 2018;27(Suppl 9):S30‐S45. [DOI] [PubMed] [Google Scholar]
- 25. Yu J, Lu S, McLaren AM, Perry JA, Cross KM. Topical oxygen therapy results in complete wound healing in diabetic foot ulcers. Wound Repair Regen. 2016;24(6):1066‐1072. [DOI] [PubMed] [Google Scholar]
- 26. Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester, UK: John Wiley & Sons; 2019. [Google Scholar]
- 27. Aldana PC, Khachemoune A. Diabetic foot ulcers: appraising standard of care and reviewing new trends in management. Am J Clin Dermatol. 2020;21(2):255‐264. [DOI] [PubMed] [Google Scholar]
- 28. Frykberg RG. Topical wound oxygen therapy in the treatment of chronic diabetic foot ulcers. Medicina (Kaunas). 2021;57(9):917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Niederauer MQ, Michalek JE, Armstrong DG. A prospective, randomized, double‐blind multicenter study comparing continuous diffusion of oxygen therapy to sham therapy in the treatment of diabetic foot ulcers. J Diabetes Sci Technol. 2017;11(5):883‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


