Abstract
The coronavirus disease 2019 (COVID-19) pandemic has seen an increase in global cases of severe acute respiratory distress syndrome (ARDS), with a concomitant increased demand for extracorporeal membrane oxygenation (ECMO). Outcomes of patients with severe ARDS due to COVID-19 infection receiving ECMO support are evolving. The need for surge capacity, practical and ethical limitations on implementing ECMO, and the prolonged duration of ECMO support in patients with COVID-19-related ARDS has revealed limitations in organization and resource utilization. Coordination of efforts at multiple levels, from research to implementation, resulted in numerous innovations in the delivery of ECMO.
Keywords: ECMO, Acute respiratory distress syndrome, ARDS, COVID-19, Respiratory failure
Key points
-
•
The coronavirus disease 2019 (COVID-19) pandemic has posed challenges on multiple levels in the implementation of extracorporeal membrane oxygenation (ECMO) support for patients with severe acute respiratory distress syndrome around the world.
-
•
Real-time data gathering and new approaches to research have helped evaluate the utility of ECMO during an ongoing pandemic.
-
•
Regional, national, and international coordination has been crucial in knowledge sharing, research collaboration, and development of guidelines.
Introduction
The use of extracorporeal membrane oxygenation (ECMO) in the management of severe acute respiratory distress syndrome (ARDS) has been well established in recent years1, 2, 3, 4 but the ongoing coronavirus disease 2019 (COVID-19) pandemic has presented new limitations in knowledge and has complicated the implementation of ECMO. Severe COVID-19 frequently presents with acute respiratory failure in the form of ARDS, and the pandemic has been characterized by surges in the volume of critically ill patients with ARDS worldwide.
Episodic surges in patients with COVID-19-related ARDS have been accompanied by strain both on health-care resources (eg, beds, staffing, medical supplies) and in the ability to provide equitable access to care, all of which is further exaggerated when considering the application of ECMO, a highly resource-intensive and specialized technology.
As the pandemic has evolved, the medical community has learned not only about the role of ECMO for COVID-19 from a clinical standpoint but also about how to gather knowledge in real-time about the use of ECMO for a novel disease, the limitations in our ability to equitably deliver health-care resources across the globe, and how to devise best strategies for care in light of substantial resource constraints. The use of ECMO for cardiac or circulatory failure, including for extracorporeal cardiopulmonary resuscitation, has been relatively limited in the setting of COVID-19; we will focus on the use of ECMO for respiratory failure.
Early Experience
Registry data and large cohort studies from early in the pandemic suggested patients managed with ECMO for COVID-19-related ARDS had a mortality rate comparable to similar patients with ARDS before COVID-19 (Table 1 ); mortality rates of 31% at 60 days in a large single-center experience from Paris, France,5 33.2% at 60 days in a cohort study across 60 hospitals in the United States,6 and an estimated 37.4% in-hospital mortality at 90 days from the Extracorporeal Life Support Organization (ELSO) Registry, including 213 hospitals across 36 countries.7 A meta-analysis of studies spanning December 1, 2019, through January 10, 2021, tallying 1896 patients, reported an in-hospital mortality rate of 37.1%8; these analyses were heavily weighted by the aforementioned large cohort studies.
Table 1.
Early experience of extracorporeal membrane oxygenation for coronavirus disease 2019–related acute respiratory distress syndrome
| Data Source | Timeframe | Number of Patients | Mortality |
|---|---|---|---|
| Single-center observational experience from Paris, France5 | Patients admitted between March 8 and May 2, 2020 | 83 | 31% at 60 d |
| Cohort study across 60 hospitals in the United States6 | Patients admitted between March 1 and July 1, 2020 | 190 | 33.2% at 60-d |
| ELSO registry, including 213 hospitals across 36 countries7 | Patients in whom ECMO was initiated between Jan 16 and May 1, 2020 | 1035 | 37.4% in-hospital mortality at 90-d |
| Meta-analysis of 22 studies8 | December 1, 2019, through January 10, 2021 | 1896 | 37.1% in-hospital mortality |
Evolving Mortality Over Time
Despite encouraging data early on, additional data gathered as the pandemic continued suggested increasing mortality and duration of ECMO over time. An analysis of a survey from the European chapter of ELSO found a mortality of 56% for patients with COVID-19 managed with ECMO between September 15, 2020, and March 8, 2021, as compared with 47% before that time period.9 Similarly, data from 24 centers in Spain and Portugal suggested a higher in-hospital mortality after June 30, 2020 (60.1%) than before that date (41.1%).10 The Paris-Sorbonne University Hospital Network found a 90-day mortality of 48% in patients after July 1, 2020, as compared with 36% before July (HR 2.27, 95% CI 1.02–5.07).11 Further analysis of the ELSO registry database, encompassing 4812 patients, also noted a higher 90-day in-hospital mortality for patients after May 1, 2020, as compared with earlier (51.9% vs 36.9%, RR 0.82, 95% CI 0.7–0.96), with a longer duration of ECMO support in the latter cohort (20 vs 14 days).12 Data published from Germany demonstrated a high in-hospital mortality (68%) for all patients supported with ECMO (n = 3,397) for COVID-19-related ARDS from the start of the pandemic through May 31, 2021, despite a lack of resource constraints.13 An updated meta-analysis of 52 studies (18,211 patients) reporting data between December 1, 2019, to January 26, 2022, revealed a pooled mortality rate of 48.8% (95% CI 44.8–52.9%) among patients with COVID-19 receiving ECMO, with increasing mortality in the second half of 2020 (46.4%) compared with the first half (41.2%), and an even higher mortality (62%) in the first half of 2021. Predictors of increased mortality included age, later time of enrollment, higher proportion of patients receiving corticosteroids, and reduced duration of ECMO run.14
Several potential reasons for increasing mortality over time have been speculated, including greater selection over time for treatment-refractory disease (those who did not respond to COVID-19-directed therapies that were increasingly used during the course of the pandemic, eg, corticosteroid therapy),9, 10, 11, 12 increased use of noninvasive ventilatory support before intubation, and ECMO (which may contribute to preendotracheal intubation self-inflicted lung injury),10, 11, 12 an increase in superimposed bacterial pneumonia in the setting of immunosuppressive treatments for COVID-19,10 , 15 , 16 emergence of SARS-CoV-2 variants with differential effects on prognosis, increased use of ECMO by less experienced centers,10 , 12 and variations in patient selection criteria for ECMO.10
Although the mortality of patients managed with ECMO may have increased during the course of the pandemic, ECMO may still benefit selected patients with severe COVID-19-related ARDS.17, 18, 19 A multicenter international emulation trial, which applies principles of randomized controlled trials (RCTs) to the analysis of observational data, including 7345 patients between January 3, 2020, and August 29, 2021, found a reduction in 60-day in-hospital mortality with a risk ratio of 0.78 (95% CI 0.75–0.82). Adherence adjusted mortality, which accounts for adherence to the treatment assignment, was 26% for patients managed with ECMO as compared with conventional treatment (33.2%). Secondary analyses suggested ECMO was most effective in patients aged younger than 65 years, those with a Pao 2 to Fio 2 ratio of less than 80 mm Hg, a driving pressure greater than 15 cmH2O, or during the first 10 days of mechanical ventilation. Although this was not a traditional RCT, the emulation design allowed for a more rigorous analysis of real-world effectiveness of ECMO than a traditional observational study.17 Additionally, a study conducted in the United Kingdom demonstrated an absolute mortality reduction of 18.2% (44% vs 25.8%; OR 0.44; 95% CI 0.29–0.68, P < .001) for those receiving ECMO compared with matched controls.18 These data should be interpreted cautiously, as residual confounding may have accounted for some of the benefit, especially as those receiving ECMO were managed at highly specialized centers, whereas those not offered ECMO remained in their original facilities for ongoing care.
The true efficacy of ECMO for severe COVID-19-related ARDS remains uncertain in the absence of high-quality RCTs. The fact that RCTs could not be implemented despite thousands of ECMO cases highlights the challenges faced in conducting research, especially RCTs, during a constantly changing pandemic with substantial limitations in infrastructure and resources, including staffing and time.20 Ongoing evaluation of emerging data will be necessary to help determine optimal patient selection and management strategies; until then, use of conventional inclusion and exclusion criteria based on pre-COVID-19 ECMO data,1 , 21 modified by factors identified in large registry analyses to be predictive of outcomes, seems to be a reasonable approach.1 , 22
Clinical care
Patient Selection
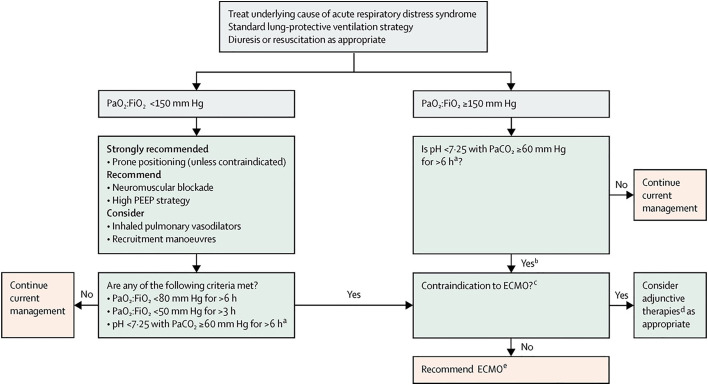
Criteria for ECMO initiation in patients with COVID-19-related ARDS remain the same as those recommended before the pandemic,21, 22, 23, 24, 25 and fall within the standard approach to ARDS algorithm (Fig. 1 ), because there is no good evidence to support deviation from these preestablished guidelines when resources are available. Outcomes with delayed initiation may in fact be worse and can lead to longer duration of ECMO support, which may in turn offset the benefit of an attempt of conservation of resources. However, resource constraints during the pandemic have overwhelmed the ability to provide ECMO at varying times in different regions of the world. As such, selection criteria may need to be more flexible and potentially stringent at any given time, depending on available resources and coordination locally. Criteria may need to evolve as well, as increasing knowledge arises regarding prognostic factors.26
Fig. 1.
Algorithm flowsheet for the management of ARDS including indications for ECMO. PEEP, positive end-expiratory pressure. Pao2:Fio2, ratio of partial pressure of oxygen in arterial blood to the fractional concentration of oxygen in inspired air. ECMO = extracorporeal membrane oxygenation. Paco2, partial pressure of carbon dioxide in arterial blood. aWith respiratory rate increased to 35 breaths per minute and mechanical ventilation settings adjusted to keep a plateau airway pressure of 32 cm or less of water. bConsider neuromuscular blockade. cThere are no absolute contraindications that are agreed on except end-stage respiratory failure when lung transplantation will not be considered; exclusion criteria used in the extracorporeal membrane oxygenation for severe acute respiratory distress syndrome (EOLIA)EOLIA trial1 can be taken as a conservative approach to contraindications to ECMO. dFor example, neuromuscular blockade, high PEEP strategy, inhaled pulmonary vasodilators, recruitment maneuvers, high-frequency oscillatory ventilation. eRecommend early ECMO as per EOLIA trial criteria; salvage ECMO, which involves deferral of ECMO initiation until further decompensation (as in the crossovers to ECMO in the EOLIA control group), is not supported by the evidence but might be preferable to not initiating ECMO at all in such patients.
(Reprinted with permission from Elsevier. The Lancet, February 2019, 7 (2), 108-110.)
Cannulation and Transport
Conventional cannulation strategies are generally recommended for patients undergoing ECMO initiation for severe COVID-19-related ARDS because there is a paucity of data to support alternative strategies.22 These include two-site or single-site, dual-lumen venovenous (V-V) cannulation, with additional arterial support depending on whether there is concomitant cardiogenic shock.23 Some centers have used a veno-pulmonary artery configuration through a single dual-lumen catheter inserted through the internal jugular or subclavian vein in an attempt to provide right ventricular protection27 because some reports suggest a higher incidence of right ventricular dysfunction in patients with COVID-19-related ARDS28 but more data is needed to support this strategy either with a dual-lumen cannula or with dual-site cannulation with 2 separate cannulae. Mobile ECMO or ECMO transport with cannulation at the originating hospital and transfer to another institution seems to be safe from a health-care exposure standpoint when accompanied by protocols incorporating adequate protective measures.29 , 30 However, it is important to note that the coordination and feasibility of ECMO transport varies across regions and, consequently, rates of transport do as well. This may be due in part to differences in practice, training, certification, and regulation.
Ongoing care while receiving extracorporeal membrane oxygenation
Management strategies of patients requiring ECMO support for COVID-19-related ARDS remain similar to those recommended before the pandemic (see Fig. 1) but specific factors should be considered (Fig. 2 ).23 , 31 From a safety and feasibility standpoint, several procedures and techniques have been successfully performed during the course of the pandemic. Endotracheal extubation while awake during ECMO has been performed, although data are very limited27; in contrast, awake ECMO without intubation in highly selected patients has been associated with potentially worse outcomes in one small cohort.32 Prone positioning5 , 33 , 34 and early mobilization27 seem feasible in patients with COVID-19 requiring ECMO support; however, data remain too limited to support specific recommendations. Percutaneous tracheostomy seems to be safe in patients with COVID-19.35 From an infection control standpoint, there is no evidence to suggest virions can be expelled through an ECMO circuit.36 Cytokine removal devices, which use an absorber to remove excess cytokines from whole blood, have been proposed as adjunctive therapies in patients receiving ECMO for COVID-19-related ARDS. However, clinicians should proceed cautiously and consider only using cytokine-removal devices in such patients in the setting of research, given that recent evidence suggests possible harm.37
Fig. 2.
Specific considerations for ECMO for COVID-19-related ARDS that may differ from ECMO for non-COVID-19 ARDS. RV, right ventricular.
(From Brodie D, Abrams D, MacLaren G, et al. Extracorporeal Membrane Oxygenation during Respiratory Pandemics: Past, Present, and Future. Am J Respir Crit Care Med. 2022;205(12):1382-1390.)
COVID-19 has been associated with coagulopathy, including an increase in risk of both significant thrombosis and bleeding.38 , 39 Hematological complications—including circuit clotting,40 , 41 pulmonary embolism,5 and intracranial hemorrhage42, 43, 44, 45—have been reported as occurring more frequently in patients with COVID-19 supported with ECMO than in non-COVID ECMO cases but when normalized to ECMO run duration, such complication rates seem similar to historical data.12 In the setting of observational data, the similar normalized rates must be interpreted with caution. Multiple centers have adjusted their anticoagulation thresholds but there are insufficient data to support anticoagulation strategies and monitoring other than usual practices.46 Additionally, there is no evidence to suggest different blood transfusion thresholds for patients with COVID-19 during ECMO support.47
Extracorporeal Membrane Oxygenation as a Bridge to Lung Transplantation
COVID-19-related ARDS has often demonstrated a more prolonged recovery process than what is typically seen for ARDS of other causes, and the duration of ECMO support for patients with severe disease has increased as the pandemic has evolved.12 Lung transplantation may be considered in select patients who have persistent severe respiratory failure—assuming otherwise appropriate candidacy with preserved extrapulmonary organ function—and may be particularly relevant for those who are unable to wean from ECMO, with initial reports demonstrating posttransplant outcomes comparable to those with non-COVID end-stage lung disease.48 , 49 However, determination of the potential for recovery of native lung function and optimal timing of transplantation remain areas of uncertainty that warrant further investigation.50 , 51
Surge capacity and extracorporeal membrane oxygenation
Crisis Standards of Care
During the pandemic, surges in case volume often led to the implementation of contingency or crisis standards of care, requiring triage of critical care resources, including intensive care unit beds, medical supplies, and staffing. At the same time, there was an increased demand for ECMO—a highly resource-intensive intervention with potential for prolonged use of critical care services52—due to the high incidence of severe, refractory ARDS among patients with COVID-19. Many institutions, in an effort to provide medical care to the greatest number of patients, became more stringent with patient selection for ECMO—or abandoned the use of ECMO altogether.53, 54, 55, 56 For those centers that continued to perform ECMO despite resource constraints, nontraditional staffing models were helpful in maintaining operations.57 In settings of reduced ECMO capacity, it may be necessary to apply more stringent exclusion criteria based on patient characteristics associated with increased mortality and longer ECMO run duration (Fig. 3 ).22 Predesigned triage systems may be useful in standardizing which patients should receive ECMO at varying levels of capacity and may also help achieve equitable access to ECMO by establishing allocation policies that avoid discrimination based on age, race, ethnicity, disability, or socioeconomic status.55
Fig. 3.
Patient selection and contingency flowsheet for ECMO during a pandemic. Contraindications algorithm for V-A and V-V ECMO use (COVID-19 and non-COVID-19) during a pandemic based on system capacity. aThe impact of duration on high-flow nasal cannula and/or noninvasive mechanical ventilation in addition to invasive mechanical ventilation is unknown. COVID-19, coronavirus disease 2019; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; Paco2, partial pressure of carbon dioxide in arterial blood; Pao2:Fio2, ratio of partial pressure of oxygen in arterial blood to the fractional concentration of oxygen in inspired air; PEEP, positive end-expiratory pressure; V-A, venoarterial; V-V, venovenous.
(From Badulak J, Antonini MV, Stead CM, et al. Extracorporeal Membrane Oxygenation for COVID-19: Updated 2021 Guidelines from the Extracorporeal Life Support Organization. ASAIO J. 2021;67(5):485-495.)
Regional and National Coordination
During the course of the pandemic, the coordination of ECMO programs at regional and national levels was leveraged to more effectively standardize ECMO candidacy and allocate resources. In Paris, a regional network coordinated care of 17 hospitals to pool resources, systematize ECMO candidacy evaluations, and expanded mobile ECMO capacity in an effort to improve resource utilization, streamline workflow for clinicians, optimize management of patients before ECMO initiation, and facilitate data collection.58 , 59 Chile used a National Advisory Commission to help coordinate ECMO referrals, provide consistent patient selection, optimize capacity, and distribute educational materials.60 The United Kingdom modified a preexisting national system to balance ECMO cases among centers in order to help manage capacity.61 The creation or utilization of existing regional or national ECMO networks has been encouraged by the ELSO, which has an “ECMO Availability Map” to help guide such coordination efforts.22 , 62 The purported effectiveness of these examples also provides a rationale for establishing new networks where none currently exists. However, the ability to coordinate ECMO across a region will depend, in large part, on the established health-care systems in that area.
Development of new extracorporeal membrane oxygenation centers
Initial guidance early in the pandemic recommended against the development of new ECMO centers,24 , 63 given the concerns during the implementation of a resource-heavy intervention in inexperienced centers that may be dealing with simultaneous surge capacity issues. Retrospective data from new ECMO centers developed in the Middle East and India—under the guidance of established centers—did report acceptable survival in these new programs overall compared with established ECMO centers (55% vs 45%, OR 1.65; 95% CI 0.75–3.67), although, as the authors note, patient selection likely differed between the centers with selection bias favoring the new centers. In light of this relative success, and recognizing the potential need for ECMO in regions that otherwise would not have access to ECMO, ELSO has updated its guidance to recommend that establishment of new ECMO centers may be considered in select cases where regional resources exist to support these programs, there is sufficiently high demand, and there is close collaboration with experienced centers to optimize outcomes.22
Evaluating effectiveness of extracorporeal membrane oxygenation in an ongoing pandemic
Surveillance and Study Design Approaches
Early data during a pandemic of a novel disease must be interpreted with caution given the potential for misleading data due to study designs and population characteristics. Determining the effectiveness of ECMO during an evolving pandemic requires ongoing surveillance as well as design and implementation of studies that have the ability to assess both short-term and long-term outcomes, and especially patient-centered outcomes.64 National and international registries are useful in centralizing data and may serve as platforms for analysis and dissemination of information.31 As the course of a pandemic takes shape and data begins to accumulate, especially results from clinical trials, the community must learn to pivot toward practices that are more evidence-based.
Although traditional RCTs are considered the “gold standard” for providing credible, unbiased evidence for the efficacy of an intervention, they can be difficult to organize and perform in real-time during a rapidly evolving pandemic, especially with a resource-intensive therapy such as ECMO, substantial limitations in staffing and funding to conduct such trials, and a perceived lack of clinical equipoise for randomization. Several different study designs have been used during the COVID-19 pandemic in an effort to approximate an RCT using observational data (Table 2 ), including emulation trials,6 , 17 registry RCTs, and matched-pair analyses.18 Adaptive platform trials and weighted lottery systems can also provide a more rapid assessment of ECMO efficacy, the latter of which can provide potentially large sample sizes and a more equitable approach to resource allocation.65 Given that temporal changes during the pandemic—including emergence of viral variants and evolution of management strategies—are likely to impact ECMO efficacy and effectiveness, more adaptive and flexible study designs are likely to have the greatest success in providing high-quality evidence in a timely fashion.20
Table 2.
Study designs in a pandemic, pros and cons
| Study Design | Pros | Cons |
|---|---|---|
| RCT |
|
|
| Emulation of target trial or RCT |
|
|
| Registry RCT |
|
|
| Matched-pair analyses |
|
|
| Adaptive platform trial |
|
|
Communication and Collaboration
Through the course of the pandemic, regional, national, and international coordination has been crucial in knowledge sharing, research collaboration, and development of guidelines. Remote learning and communication became a key component of health-care provider education throughout the pandemic. Educational webinars and conferences by ELSO and other ECMO networks have been used to disseminate new data to both experienced and new ECMO centers and practitioners.22
Pediatric Access to Extracorporeal Membrane Oxygenation During the Pandemic
Children were generally less susceptible to severe illness associated with COVID-19 infection, although a new postviral pathologic process, multisystem inflammatory syndrome in children, was identified during the pandemic.66, 67, 68 Access to ECMO for children with non-COVID-19 critical illness, congenital anomalies and emergent perioperative indications, in addition to the infrequent pediatric patients who were critically ill with COVID-19, was preserved in many regions and recommended in guidelines.22 , 69, 70, 71, 72, 73 In addition, many established pediatric ECMO centers expanded their admission criteria to facilitate the care of adult patients with COVID-19 who required ECMO and offload regional centers at capacity.69 , 70 , 74 Although there are many examples of successful shared resource allocation protocols, particular care was required to address the unique challenges of assessing mortality risk in the neonatal and pediatric populations. The COVID-19 pandemic highlighted that at times of critical care shortages, protocols that ensure equity across the life span should be used.72 , 75
Health-Care Providers and Extracorporeal Membrane Oxygenation
The COVID-19 pandemic has affected health-care workers in a multitude of ways, notably through occupational stress and provider burnout.76 , 77 Surge situations and crisis standards of care have only amplified the pressure placed on health-care providers through unfavorable changes in staffing models and increases in provider responsibilities, ethical dilemmas, and patient mortality, among others. Contingency and crisis standards for ECMO implementation during the COVID-19 pandemic have added to provider stress through a potentially heavy ethical burden of rationing care, potentially resulting in an inability, during surge conditions, to provide ECMO to those who meet standard criteria for initiation and would otherwise have received ECMO under less strained conditions. Prolonged ECMO run times and longer than usual time-to-recovery for these patients with ARDS12 can also contribute to health-care provider burnout. Triage committees have been proposed to relieve bedside providers of these burdens by objectively and independently setting standards and developing guidelines for rationing decisions. However, operationalizing a triage committee amid a crisis is a complex and fraught undertaking that requires direct input from community leaders in order to address ethical and legal issues, along with the need for equity. The major function of an oversight committee under crisis conditions may simply be to offload the overburdened clinicians on the front lines.54
Summary
The COVID-19 pandemic has led to a marked increase in cases of ARDS globally, leading to a concomitant increase in demand for ECMO support. The utilization of ECMO during the pandemic has been complicated by numerous factors, including limited knowledge of ECMO-related outcomes, challenges in performing real-time, high-quality research in an ongoing evolving pandemic, difficulties in patient selection during episodic severe capacity restraints, the ethical dilemmas of rationing care, and excess strain not only on health-care systems but on providers as well. As the pandemic eventually recedes and resources become more readily and consistently available to provide ECMO, both coordinated research and tailoring of guidelines will be necessary to understand the role of ECMO, provide the best care possible to patients with severe COVID-19-related ARDS, and to anticipate needs for future potential pandemics.
Clinics care points
-
•
The COVID-19 pandemic has led to an increase in severe ARDS cases globally, increasing the demand for ECMO.
-
•
Outcomes of patients with COVID-19 managed with ECMO have evolved during the pandemic, with early data suggesting mortality rates similar to those with non-COVID-19 causes of ARDS but later data suggesting increasing mortality and longer ECMO duration of support over time.
-
•
Eligibility criteria, cannulation, and management strategies for patients with severe ARDS requiring ECMO thus far remain largely the same for patients with COVID-19.
-
•
Episodic surge capacity requirements have often led to crisis standards of care, which may include the rationing or tailoring of eligibility criteria of ECMO, particularly with respect to more stringent exclusion criteria.
-
•
Regional, national, and international collaboration will continue to help inform ECMO providers, disseminate new information and aid in resource allocation.
-
•
Ongoing surveillance data and new research techniques will continue to inform the role of ECMO in the management of COVID-19-related ARDS.
Disclosure
D. Brodie receives research support from and consults for LivaNova. He has been on the medical advisory boards for Abiomed, Xenios, Medtronic, Inspira, and Cellenkos. He is the President-elect of the ELSO and the Chair of the Executive Committee of the International ECMO Network. P.M.A. Alexander reported receiving grant funding from the National Institute of Child Health and Human Development, National Institutes of Health, Pediatric ECMO Anticoagulation Collaborative, outside the submitted work and serving on the Board of the Extracorporeal Life Support Organization as Treasurer.
References
- 1.Combes A., Hajage D., Capellier G., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 2.Goligher E.C., Tomlinson G., Hajage D., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc bayesian analysis of a randomized clinical trial. JAMA. 2018;320(21):2251–2259. doi: 10.1001/jama.2018.14276. [DOI] [PubMed] [Google Scholar]
- 3.Munshi L., Walkey A., Goligher E., et al. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med. 2019;7(2):163–172. doi: 10.1016/S2213-2600(18)30452-1. [DOI] [PubMed] [Google Scholar]
- 4.Combes A., Peek G.J., Hajage D., et al. ECMO for severe ARDS: systematic review and individual patient data meta-analysis. Intensive Care Med. 2020;46(11):2048–2057. doi: 10.1007/s00134-020-06248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt M., Hajage D., Lebreton G., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med. 2020;8(11):1121–1131. doi: 10.1016/S2213-2600(20)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaefi S., Brenner S.K., Gupta S., et al. Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med. 2021;47(2):208–221. doi: 10.1007/s00134-020-06331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbaro R.P., MacLaren G., Boonstra P.S., et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396(10257):1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramanathan K., Shekar K., Ling R.R., et al. Extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Crit Care. 2021;25(1):211. doi: 10.1186/s13054-021-03634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broman L.M., Eksborg S., Lo Coco V., et al. Extracorporeal membrane oxygenation for COVID-19 during first and second waves. Lancet Respir Med. 2021;9(8):e80–e81. doi: 10.1016/S2213-2600(21)00262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riera J., Roncon-Albuquerque R., Jr., Fuset M.P., et al. Increased mortality in patients with COVID-19 receiving extracorporeal respiratory support during the second wave of the pandemic. Intensive Care Med. 2021;47(12):1490–1493. doi: 10.1007/s00134-021-06517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt M., Langouet E., Hajage D., et al. Evolving outcomes of extracorporeal membrane oxygenation support for severe COVID-19 ARDS in Sorbonne hospitals, Paris. Crit Care. 2021;25(1):355. doi: 10.1186/s13054-021-03780-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbaro R.P., MacLaren G., Boonstra P.S., et al. Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international extracorporeal life support organization registry. Lancet. 2021;398(10307):1230–1238. doi: 10.1016/S0140-6736(21)01960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karagiannidis C., Slutsky A.S., Bein T., et al. Complete countrywide mortality in COVID patients receiving ECMO in Germany throughout the first three waves of the pandemic. Crit Care. 2021;25(1):413. doi: 10.1186/s13054-021-03831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling R., Ramanathan K., et al. Evolving outcomes of extracorporeal membrane oxygenation during the first 2 years of the COVID-19 pandemic: a systematic review and meta-analysis. Crit Care. 2022;26:147. doi: 10.1186/s13054-022-04011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abrams D., Grasselli G., Schmidt M., et al. ECLS-associated infections in adults: what we know and what we don't yet know. Intensive Care Med. 2020;46(2):182–191. doi: 10.1007/s00134-019-05847-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carbonell R., Urgelés S., Rodríguez A., et al. Mortality comparison between the first and second/third waves among 3,795 critical COVID-19 patients with pneumonia admitted to the ICU: a multicentre retrospective cohort study. Lancet Reg Health Eur. 2021;11:100243. doi: 10.1016/j.lanepe.2021.100243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urner M., Barnett A.G., Bassi G.L., et al. Venovenous extracorporeal membrane oxygenation in patients with acute covid-19 associated respiratory failure: comparative effectiveness study. Bmj. 2022;377:e068723. doi: 10.1136/bmj-2021-068723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whebell S., Zhang J., Lewis R., et al. Survival benefit of extracorporeal membrane oxygenation in severe COVID-19: a multi-centre-matched cohort study. Intensive Care Med. 2022;48(4):467–478. doi: 10.1007/s00134-022-06645-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gannon W.D., Stokes J.W., Francois S.A., et al. Association between availability of ECMO and mortality in COVID-19 patients eligible for ECMO: a natural experiment. Am J Respir Crit Care Med. 2022;205(11):1354–1357. doi: 10.1164/rccm.202110-2399LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granholm A., Alhazzani W., Derde L.P.G., et al. Randomised clinical trials in critical care: past, present and future. Intensive Care Med. 2022;48(2):164–178. doi: 10.1007/s00134-021-06587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abrams D., Ferguson N.D., Brochard L., et al. ECMO for ARDS: from salvage to standard of care? Lancet Respir Med. 2019;7(2):108–110. doi: 10.1016/S2213-2600(18)30506-X. [DOI] [PubMed] [Google Scholar]
- 22.Badulak J., Antonini M.V., Stead C.M., et al. Extracorporeal membrane oxygenation for COVID-19: updated 2021 guidelines from the extracorporeal life support organization. Asaio j. 2021;67(5):485–495. doi: 10.1097/MAT.0000000000001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brodie D., Slutsky A.S., Combes A. Extracorporeal life support for adults with respiratory failure and related indications: a review. Jama. 2019;322(6):557–568. doi: 10.1001/jama.2019.9302. [DOI] [PubMed] [Google Scholar]
- 24.Shekar K., Badulak J., Peek G., et al. Extracorporeal life support organization coronavirus disease 2019 interim guidelines: a consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. Asaio j. 2020;66(7):707–721. doi: 10.1097/MAT.0000000000001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tonna J.E., Abrams D., Brodie D., et al. Management of adult patients supported with venovenous extracorporeal membrane oxygenation (VV ECMO): guideline from the extracorporeal life support organization (ELSO) Asaio j. 2021;67(6):601–610. doi: 10.1097/MAT.0000000000001432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brodie D., Abrams D., MacLaren G., et al. ECMO during respiratory pandemics: past, present, and future. Am J Respir Crit Care Med. 2022;205(12):1382–1390. doi: 10.1164/rccm.202111-2661CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mustafa A.K., Alexander P.J., Joshi D.J., et al. Extracorporeal membrane oxygenation for patients with COVID-19 in severe respiratory failure. JAMA Surg. 2020;155(10):990–992. doi: 10.1001/jamasurg.2020.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Creel-Bulos C., Hockstein M., Amin N., et al. Acute cor pulmonale in critically ill patients with covid-19. N Engl J Med. 2020;382(21):e70. doi: 10.1056/NEJMc2010459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salas de Armas I.A., Akkanti B.H., Janowiak L., et al. Inter-hospital COVID ECMO air transportation. Perfusion. 2021;36(4):358–364. doi: 10.1177/0267659120973843. [DOI] [PubMed] [Google Scholar]
- 30.Rafiq M.U., Valchanov K., Vuylsteke A., et al. Regional extracorporeal membrane oxygenation retrieval service during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic: an interdisciplinary team approach to maintain service provision despite increased demand. Eur J Cardiothorac Surg. 2020;58(5):875–880. doi: 10.1093/ejcts/ezaa327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Extracorporeal life support organization (ELSO). Resources > guidelines. https://www.elso.org/Resources/Guidelines.aspx Available at: Accessed April 1, 2022.
- 32.Mang S., Reyher C., Mutlak H., et al. Awake extracorporeal membrane oxygenation for COVID-19-induced acute respiratory distress syndrome. Am J Respir Crit Care Med. 2022;205(7):847–851. doi: 10.1164/rccm.202105-1189LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia B., Cousin N., Bourel C., et al. Prone positioning under VV-ECMO in SARS-CoV-2-induced acute respiratory distress syndrome. Crit Care. 2020;24(1):428. doi: 10.1186/s13054-020-03162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giani M., Martucci G., Madotto F., et al. Prone positioning during venovenous extracorporeal membrane oxygenation in acute respiratory distress syndrome. A multicenter cohort study and propensity-matched analysis. Ann Am Thorac Soc. 2021;18(3):495–501. doi: 10.1513/AnnalsATS.202006-625OC. [DOI] [PubMed] [Google Scholar]
- 35.Rosano A., Martinelli E., Fusina F., et al. Early percutaneous tracheostomy in coronavirus disease 2019: association with hospital mortality and factors associated with removal of tracheostomy tube at ICU discharge. A cohort study on 121 patients. Crit Care Med. 2021;49(2):261–270. doi: 10.1097/CCM.0000000000004752. [DOI] [PubMed] [Google Scholar]
- 36.Dres M., Burrel S., Boutolleau D., et al. SARS-CoV-2 does not spread through extracorporeal membrane oxygenation or dialysis membranes. Am J Respir Crit Care Med. 2020;202(3):458–460. doi: 10.1164/rccm.202004-1339LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Supady A., Weber E., Rieder M., et al. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): a single centre, open-label, randomised, controlled trial. Lancet Respir Med. 2021;9(7):755–762. doi: 10.1016/S2213-2600(21)00177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnes G.D., Burnett A., Allen A., et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50(1):72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yusuff H., Zochios V., Brodie D. Thrombosis and coagulopathy in COVID-19 patients requiring extracorporeal membrane oxygenation. Asaio j. 2020;66(8):844–846. doi: 10.1097/MAT.0000000000001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bemtgen X., Zotzmann V., Benk C., et al. Thrombotic circuit complications during venovenous extracorporeal membrane oxygenation in COVID-19. J Thromb Thrombolysis. 2021;51(2):301–307. doi: 10.1007/s11239-020-02217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo Z., Sun L., Li B., et al. Anticoagulation management in severe coronavirus disease 2019 patients on extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth. 2021;35(2):389–397. doi: 10.1053/j.jvca.2020.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masur J., Freeman C.W., Mohan S. A double-edged sword: neurologic complications and mortality in extracorporeal membrane oxygenation therapy for COVID-19-related severe acute respiratory distress syndrome at a tertiary care center. AJNR Am J Neuroradiol. 2020;41(11):2009–2011. doi: 10.3174/ajnr.A6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Usman A.A., Han J., Acker A., et al. A case series of devastating intracranial hemorrhage during venovenous extracorporeal membrane oxygenation for COVID-19. J Cardiothorac Vasc Anesth. 2020;34(11):3006–3012. doi: 10.1053/j.jvca.2020.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zahid M.J., Baig A., Galvez-Jimenez N., et al. Hemorrhagic stroke in setting of severe COVID-19 infection requiring Extracorporeal Membrane Oxygenation (ECMO) J Stroke Cerebrovasc Dis. 2020;29(9):105016. doi: 10.1016/j.jstrokecerebrovasdis.2020.105016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heman-Ackah S.M., Su Y.S., Spadola M., et al. Neurologically devastating intraparenchymal hemorrhage in COVID-19 patients on extracorporeal membrane oxygenation: a case series. Neurosurgery. 2020;87(2) doi: 10.1093/neuros/nyaa198. E147-e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McMichael A.B.V., Ryerson L.M., Ratano D., et al. 2021 ELSO adult and pediatric anticoagulation guidelines. Asaio j. 2022;68(3):303–310. doi: 10.1097/MAT.0000000000001652. [DOI] [PubMed] [Google Scholar]
- 47.Ramanathan K., MacLaren G., Combes A., et al. Blood transfusion strategies and ECMO during the COVID-19 pandemic - authors' reply. Lancet Respir Med. 2020;8(5):e41. doi: 10.1016/S2213-2600(20)30174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bharat A., Machuca T.N., Querrey M., et al. Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respir Med. 2021;9(5):487–497. doi: 10.1016/S2213-2600(21)00077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roach A., Chikwe J., Catarino P., et al. Lung transplantation for covid-19-related respiratory failure in the United States. N Engl J Med. 2022;386(12):1187–1188. doi: 10.1056/NEJMc2117024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bermudez C.A., Crespo M.M. The case for prolonged ECMO for COVID-19 ARDS as a bridge to recovery or lung transplantation. Transplantation. 2022;106(4):e198–e199. doi: 10.1097/TP.0000000000004063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cypel M., Keshavjee S. When to consider lung transplantation for COVID-19. Lancet Respir Med. 2020;8(10):944–946. doi: 10.1016/S2213-2600(20)30393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Combes A., Brodie D., Bartlett R., et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med. 2014;190(5):488–496. doi: 10.1164/rccm.201404-0630CP. [DOI] [PubMed] [Google Scholar]
- 53.Supady A., Badulak J., Evans L., et al. Should we ration extracorporeal membrane oxygenation during the COVID-19 pandemic? Lancet Respir Med. 2021;9(4):326–328. doi: 10.1016/S2213-2600(21)00131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Supady A., Brodie D., Curtis J.R. Ten things to consider when implementing rationing guidelines during a pandemic. Intensive Care Med. 2021;47(5):605–608. doi: 10.1007/s00134-021-06374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Supady A., Curtis J.R., Abrams D., et al. Allocating scarce intensive care resources during the COVID-19 pandemic: practical challenges to theoretical frameworks. Lancet Respir Med. 2021;9(4):430–434. doi: 10.1016/S2213-2600(20)30580-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abrams D., Lorusso R., Vincent J.L., et al. ECMO during the COVID-19 pandemic: when is it unjustified? Crit Care. 2020;24(1):507. doi: 10.1186/s13054-020-03230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agerstrand C., Dubois R., Takeda K., et al. Extracorporeal membrane oxygenation for coronavirus disease 2019: crisis standards of care. Asaio j. 2021;67(3):245–249. doi: 10.1097/MAT.0000000000001376. [DOI] [PubMed] [Google Scholar]
- 58.Lebreton G., Schmidt M., Ponnaiah M., et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med. 2021;9(8):851–862. doi: 10.1016/S2213-2600(21)00096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levy D., Lebreton G., Pineton de Chambrun M., et al. Outcomes of patients denied extracorporeal membrane oxygenation during the COVID-19 pandemic in greater Paris, France. Am J Respir Crit Care Med. 2021;204(8):994–997. doi: 10.1164/rccm.202105-1312LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diaz R.A., Graf J., Zambrano J.M., et al. Extracorporeal membrane oxygenation for COVID-19-associated severe acute respiratory distress syndrome in Chile: a nationwide incidence and cohort study. Am J Respir Crit Care Med. 2021;204(1):34–43. doi: 10.1164/rccm.202011-4166OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Camporota L., Meadows C., Ledot S., et al. Consensus on the referral and admission of patients with severe respiratory failure to the NHS ECMO service. Lancet Respir Med. 2021;9(2):e16–e17. doi: 10.1016/S2213-2600(20)30581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Extracorporeal Life Support Organization (ELSO) Membership > ECMO availability Map. https://www.elso.org/Membership/ECMOAvailabilityMap.aspx Available at: Accessed April 1, 2022.
- 63.Bartlett R.H., Ogino M.T., Brodie D., et al. Initial ELSO guidance document: ECMO for COVID-19 patients with severe cardiopulmonary failure. Asaio j. 2020;66(5):472–474. doi: 10.1097/MAT.0000000000001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.MacLaren G., Fisher D., Brodie D. Treating the most critically ill patients with COVID-19: the evolving role of extracorporeal membrane oxygenation. Jama. 2022;327(1):31–32. doi: 10.1001/jama.2021.22580. [DOI] [PubMed] [Google Scholar]
- 65.White D.B., Angus D.C. A proposed lottery system to allocate scarce COVID-19 medications: promoting fairness and generating knowledge. Jama. 2020;324(4):329–330. doi: 10.1001/jama.2020.11464. [DOI] [PubMed] [Google Scholar]
- 66.Lu X., Zhang L., Du H., et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feldstein L.R., Rose E.B., Horwitz S.M., et al. Multisystem inflammatory syndrome in U.S. Children and adolescents. N Engl J Med. 2020;383(4):334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feldstein L.R., Tenforde M.W., Friedman K.G., et al. Characteristics and outcomes of US children and adolescents with Multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325(11):1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gerall C., Cheung E.W., Klein-Cloud R., et al. Allocation of resources and development of guidelines for extracorporeal membrane oxygenation (ECMO): experience from a pediatric center in the epicenter of the COVID-19 pandemic. J Pediatr Surg. 2020;55(12):2548–2554. doi: 10.1016/j.jpedsurg.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DeFazio J.R., Kahan A., Fallon E.M., et al. Development of pediatric surgical decision-making guidelines for COVID-19 in a New York City children's hospital. J Pediatr Surg. 2020;55(8):1427–1430. doi: 10.1016/j.jpedsurg.2020.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cho H.J., Ogino M.T., Jeong I.S., et al. Pediatric intensive care preparedness and ECMO availability in children with COVID-19: an international survey. Perfusion. 2021;36(6):637–639. doi: 10.1177/0267659120981810. [DOI] [PubMed] [Google Scholar]
- 72.Lemmon M.E., Truog R.D., Ubel P.A. Allocating resources across the life span during COVID-19-integrating neonates and children into crisis standards of care protocols. JAMA Pediatr. 2021;175(4):347–348. doi: 10.1001/jamapediatrics.2020.5215. [DOI] [PubMed] [Google Scholar]
- 73.Cleary A., Chivers S., Daubeney P.E., et al. Impact of COVID-19 on patients with congenital heart disease. Cardiol Young. 2021;31(1):163–165. doi: 10.1017/S1047951120004345. [DOI] [PubMed] [Google Scholar]
- 74.Yager P.H., Whalen K.A., Cummings B.M. Repurposing a pediatric ICU for adults. N Engl J Med. 2020;382(22):e80. doi: 10.1056/NEJMc2014819. [DOI] [PubMed] [Google Scholar]
- 75.Emanuel E.J., Persad G., Upshur R., et al. Fair allocation of scarce medical resources in the time of covid-19. N Engl J Med. 2020;382(21):2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 76.Myran D.T., Cantor N., Rhodes E., et al. Physician health care visits for mental health and substance use during the COVID-19 Pandemic in Ontario, Canada. JAMA Netw Open. 2022;5(1):e2143160. doi: 10.1001/jamanetworkopen.2021.43160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pfefferbaum B., North C.S. Mental health and the Covid-19 pandemic. N Engl J Med. 2020;383(6):510–512. doi: 10.1056/NEJMp2008017. [DOI] [PubMed] [Google Scholar]