Abstract
A large-scale DNA vaccination trial was performed with sheep to investigate whether an antigen targeted by CTLA-4 enhanced and accelerated the humoral immune response. Vaccination with genetically detoxified phospholipase D (ΔPLD) has been shown to be effective, at least partially, against Corynebacterium pseudotuberculosis, the causal agent of caseous lymphadenitis in sheep. CTLA-4 binds to B7 on antigen-presenting cells and thus was used to direct the fusion antigens to sites of immune induction. Here we demonstrated that targeting ΔPLD as a CTLA-4 fusion protein significantly enhanced the speed, magnitude, and longevity of the antibody response compared to that obtained with DNA encoding ΔPLD. While all groups of sheep vaccinated with DNA encoding ΔPLD were afforded better protection against an experimental challenge with C. pseudotuberculosis than those immunized with an irrelevant plasmid or those left unimmunized, the best protection was provided by the targeted DNA vaccine. We propose that targeting antigens to antigen-presenting cells offers a generic strategy for enhancing the efficacy of DNA vaccines.
Since its first demonstration in mice, a number of studies have shown that DNA vaccination can induce specific antibody and cell-mediated immune responses to a variety of bacterial, viral, and parasitic antigens (9). Although nucleic acid vaccination has generated a great deal of interest within veterinary research, there have been few published reports of the use of DNA vaccination for domestic livestock species. One of the first reports demonstrated that cattle immunized with DNA encoding a bovine herpesvirus glycoprotein generated neutralizing-antibody titers sufficient to reduce virus shedding following a herpesvirus infection (6). However, a number of studies have since shown that multiple doses of DNA encoding parasite and viral antigens in sheep (19) and cattle (20) induce responses which are weak and short-lived. Indeed, the level of protection provided by DNA vaccines is often inferior to that provided by conventional and subunit vaccines (19, 20). Recently, we devised a novel approach of directing antigen to sites of immune induction by vaccination with DNA encoding antigen as a CTLA-4 fusion protein (3). This study showed that targeting the antigen as a fusion protein enhanced both the speed and the magnitude of the immune response. On this basis we have used this targeting strategy in the present study, in an attempt to improve the poor immune responses following DNA vaccination in domestic outbred species.
Corynebacterium pseudotuberculosis, a gram-positive intracellular pathogen, is the causative agent of caseous lymphadenitis (CLA) in sheep. Transmission of this disease is believed to occur via skin wounds or by aerosol infection, and it is characterized by the formation of abscesses within the superficial lymph nodes, in addition to those draining the lungs. C. pseudotuberculosis secretes phospholipase D (PLD), which is thought to mediate dissemination of the pathogen within the host by increasing local vascular permeability. This exotoxin has been characterized as a major virulence factor, since PLD-specific antibodies are correlated with protection against CLA (10) and a genetically toxoided PLD (ΔPLD) has been demonstrated to protect sheep from CLA (13). Although a commercial vaccine is currently produced from a formalin-inactivated, PLD-rich C. pseudotuberculosis supernatant, the potential of DNA to elicit a long-lived humoral and cytotoxic T-lymphocyte response following a single dose in mice (7) offers a great advantage over such conventional attenuated vaccines.
In this study we investigated whether the genetically attenuated ΔPLD delivered as a DNA vaccine could provide protection against CLA and whether the protective humoral response could be accelerated and enhanced by targeting ΔPLD as a CTLA-4 fusion protein.
MATERIALS AND METHODS
Generation of DNA constructs.
A pCI plasmid (Promega) encoding a CD5 leader (CD5L)–human immunoglobulin (hIg) fusion protein (3) was used to construct the plasmids encoding the proteins used in the vaccine trial: bovine (bo) CTLA-4–hIg, boCTLA-4–hIg–ΔPLD, and CD5L–hIg–ΔPLD. CD5L refers to the leader sequence of the human CD5 molecule, which is required to ensure the secretion of the hIg–ΔPLD fusion protein. By using site-specific mutagenesis, the PLD gene was inactivated by replacing a histidine with a serine residue within the enzyme active site (22); this ΔPLD gene was then subcloned into pCI.
boCTLA-4 was generated by reverse transcription-PCR from total RNA isolated from bovine peripheral blood mononuclear cells stimulated for 24 h with concanavalin A, as previously described (5). The forward (positions 1 to 21) and reverse (667 to 643) primers were designed from the published sequence (accession no. X15070) for boCTLA-4 (18). The PCR product was cloned into Zeroblunt according to the manufacturer's instructions (Invitrogen), and the sequence was verified by using an Applied Biosystems automated sequencer. The extracellular domain of boCTLA-4 was amplified from this clone by using Pfu polymerase (Stratagene) for 30 cycles of 30 s at 94°C, 30 s at 55°C, and 2 min at 72°C, followed by 1 cycle of 30 s at 94°C, 30 s at 55°C, and 10 min at 72°C. The forward primer, GGGCTCGAGATGGCTTGCTCTGGATTCCAGAGTC (positions 1 to 25), incorporated an XhoI restriction site (in italics) immediately upstream of the transcription initiation codon (boldfaced), while the reverse primer, GGGGGATCCGAATCCGGGCATGGTTCTGG (positions 477 to 454), incorporated a BamHI site (in italics) partially incorporating the last codon of the extracellular domain of CTLA-4 (underlined). This digested PCR product was ligated to an XhoI/ClaI pCI backbone, together with a BamHI/ClaI-digested DNA encoding hIg, to create a single open reading frame encoding the fusion protein boCTLA-4–hIg.
To generate the ΔPLD constructs, the signal sequence of ΔPLD was removed by PCR using the forward (ATAATAACGCGTGCGCCTGTTGTGCATAACCCA) and reverse (ATAATATCTAGATCACCACGGGTTATCCTCTTCG) primers, which incorporated restriction sites (in italics) for cloning. This product was cloned into the MluI/XbaI sites of boCTLA-4–hIg and CD5L–hIg to create the constructs boCTLA-4–hIg–ΔPLD and CD5L–hIg–ΔPLD.
All DNA constructs were verified by using an Applied Biosystems automated sequencer, and the large-scale production of endotoxin-free DNA was carried out with a commercial kit (Qiagen).
Analysis of protein expression.
Plasmid constructs (1 μg) were transfected into COS-M6 cells (5 × 105) by using the Lipofectamine-Plus reagent according to the protocol supplied (Gibco-BRL). Three days posttransfection, the serum-free supernatants were harvested and used to establish whether the plasmid constructs had expressed functional proteins. The supernatants from COS-M6 cells transfected with the plasmid encoding ΔPLD were analyzed by Western blotting using a sheep polyclonal antibody raised against PLD (13) and were compared to native PLD expressed in COS-M6 cells and C. pseudotuberculosis. To establish the level of glycosylation, the ΔPLD protein was incubated at 37°C for 24 h with three N-deglycosidases (H, F, and A) and one O-deglycosidase (Boehringer) prior to Western blot analysis.
The supernatants from COS-M6 cells transfected with the plasmids encoding CTLA-4 fusion proteins (boCTLA-4–hIg and boCTLA-4–hIg–ΔPLD) were analyzed for their abilities to bind to ovine lymphocytes isolated from afferent lymph by using flow cytometry as previously described (15). Afferent lymph was obtained by cannulation of the pseudoafferent duct following the removal of the prescapular lymph node (11).
Serological assays.
Sera were collected from the sheep at weekly intervals and assayed for the presence of antibodies to ΔPLD and hIg by a standard enzyme-linked immunosorbent assay (ELISA). Recombinant ΔPLD was expressed in a PLD-negative strain of C. pseudotuberculosis grown in CLA medium (kindly provided by CSL Limited, Parkville, Australia) and dialyzed against phosphate-buffered saline (PBS). The ΔPLD represented >90% of the protein in these culture supernatants, as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and was used to coat the ELISA plates at a 1/10 dilution. For the detection of anti-hIg antibodies, the plates were coated with 5 μg of hIg protein (CSL Limited)/ml. The sera were diluted in twofold steps, usually starting at 1/100, although 1/10 dilutions were also used to increase the sensitivity of the ELISA for the detection of low levels of antibody to ΔPLD and hIg. Antibody titers were calculated by linear regression on a double logarithmic scale by using the linear part of the graph, and the titer was defined as the dilution that resulted in an optical density of 0.3. An arbitrary value of 1 was given when no antibody titer could be determined.
Sheep vaccination and experimental challenge.
Three-month-old crossbred merino ewes were selected from a flock with no history of vaccination or CLA. Animals were prescreened for the presence of antibodies to ΔPLD and to a whole-cell C. pseudotuberculosis lysate by ELISA, and negative animals were divided into seven groups of 10 sheep. At day 0, animals received an intramuscular injection in the left quadricep of 500 μg of plasmid DNA (encoding either ΔPLD, boCTLA-4–hIg, boCTLA-4–hIg–ΔPLD, or CD5L–hIg–ΔPLD) in 5 ml of PBS. Control animals received either the commercial vaccine Glanvac 3 (CSL Limited) or the plasmid pCI or were left unimmunized. Glanvac 3 contains formalin-inactivated toxins from two clostridial species and the C. pseudotuberculosis PLD component. All animals received a second injection of the same vaccine, at the same dose, 4 weeks later. All sheep were challenged 6 weeks after the primary immunization with a pathogenic wild-type strain of C. pseudotuberculosis, C231. Bacterial cultures were grown to late-log phase in brain heart infusion (BHI) broth (Difco Laboratories), and 106 CFU of C. pseudotuberculosis C231 was injected just above the coronet of the right hind lateral claw. A full necropsy of all animals was performed 6 weeks postchallenge to assess the efficacy of the treatments. The left and right hind popliteal, inguinal, and prefemoral lymph nodes were dissected to be visually assessed for the characteristic abscesses associated with CLA. In addition, the lungs were palpated and samples were taken from the abscesses identified in all sites for bacterial culture on BHI plates containing sheep erythrocytes and Rhodococcus equi supernatant (12) to confirm the presence of C. pseudotuberculosis. Protection was defined by the absence of the characteristic CLA abscesses after animal numbers were matched with treatments at the end of the necropsy.
Statistical analysis.
Statistical analysis was performed with the Systat program. The nonparametric Mann-Whitney U test was performed, and P values below 0.05 were considered significant.
RESULTS
Expression of ΔPLD in eukaryotic cells.
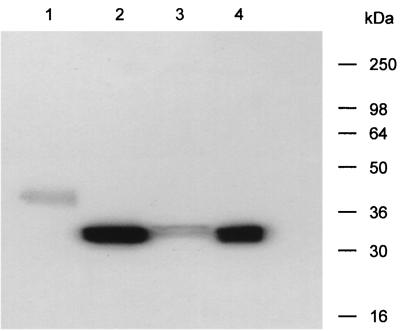
ΔPLD is a bacterial gene product and was cloned into pCI, retaining the prokaryote signal sequence. To ensure that this protein was expressed and secreted in eukaryotic cells, ΔPLD was transiently expressed in COS-M6 cells. Supernatants from these COS-M6 cells reacted with anti-PLD sera in a Western blot to reveal a band of 37 kDa (Fig. 1). This was larger than the size expected for native PLD and for ΔPLD expressed in C. pseudotuberculosis, 31 kDa (Fig. 1). Furthermore, when ΔPLD expressed in COS-M6 cells was treated with deglycosidases, the apparent molecular mass of ΔPLD decreased from 37 to 31 kDa (Fig. 1). However, the glycosylation of PLD in eukaryotic cells did not affect functional activity, as evidenced by the fact that the native PLD expressed in COS-M6 cells displayed the characteristic hemolytic activity associated with the native C. pseudotuberculosis PLD (data not shown).
FIG. 1.
Western blot analysis of ΔPLD expressed by COS-M6 cells and C. pseudotuberculosis. Supernatants from either COS-M6 cells transfected with plasmid DNA encoding ΔPLD (lane 1) or C. pseudotuberculosis cultures expressing ΔPLD (lane 2) were incubated with N- and O-deglycosidases prior to Western blot analysis with hyperimmune anti-PLD sheep sera (lanes 3 and 4, respectively).
Evaluation of CTLA-4 fusion proteins.
To establish whether the construct encoding boCTLA-4–hIg–ΔPLD encoded a functional CTLA-4–hIg fusion protein, this vector was transiently expressed in COS-M6 cells and the supernatants were evaluated by staining of ovine afferent lymphocytes using flow cytometry. COS-M6 supernatants containing the boCTLA-4–hIg–ΔPLD fusion protein stained 21% of the afferent lymphocytes, as determined by using an anti-hIg–fluorescein isothiocyanate conjugate (data not shown). However, supernatants from COS-M6 cells transfected with the construct encoding CD5L–hIg–ΔPLD failed to stain ovine lymphocytes. Furthermore, the ΔPLD component of the boCTLA-4–hIg–ΔPLD fusion protein did not affect the binding of boCTLA-4, as shown by the fact that a similar percentage of cells stained with a boCTLA-4–hIg fusion protein. Double staining revealed that the majority of cells expressing CD80/CD86 as determined by staining with the boCTLA-4–hIg fusion proteins had a dendritic-cell phenotype, as indicated by their large size and expression of high levels of major histocompatibility complex (MHC) class I, MHC class II, and CD1b (data not shown).
Improvement in the antibody response to hIg by targeting.
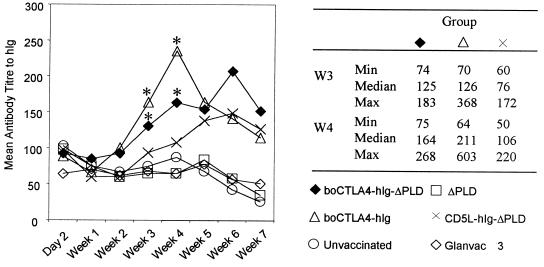
To determine the antibody responses to both hIg and ΔPLD, the animals were bled weekly and specific titers were determined by ELISA. Titers of antibody to hIg following DNA vaccination reflect the antibody response generated to the hIg part of the fusion proteins boCTLA-4–hIg, boCTLA-4–hIg–ΔPLD, and CD5L–hIg–ΔPLD. By directly comparing the antibody responses to hIg of the groups of animals immunized with boCTLA-4–hIg–ΔPLD and boCTLA-4–hIg, on the one hand, with that of the group receiving the DNA encoding CD5L–hIg–ΔPLD, on the other hand, it is possible to evaluate the effect of targeting hIg as a boCTLA-4 fusion protein. In the animals that received the DNA encoding CD5L–hIg–ΔPLD there was a weak antibody response to hIg (titers of <100), although the levels increased following a DNA boost at week 4, reaching a peak at week 6 (Fig. 2). In contrast, the antibody response in the animals immunized with boCTLA-4–hIg was detected earlier (week 3) and reached a peak response at week 4, which was two times greater (P = 0.007) than the antibody titers generated in the animals immunized with CD5L–hIg–ΔPLD. A similar significantly (P < 0.05) enhanced early antibody response was observed at weeks 2, 3, 4, and 5 for the animals that received the boCTLA-4–hIg–ΔPLD construct (Fig. 2). The antibody response to hIg was undetectable in all groups by week 10 (data not shown).
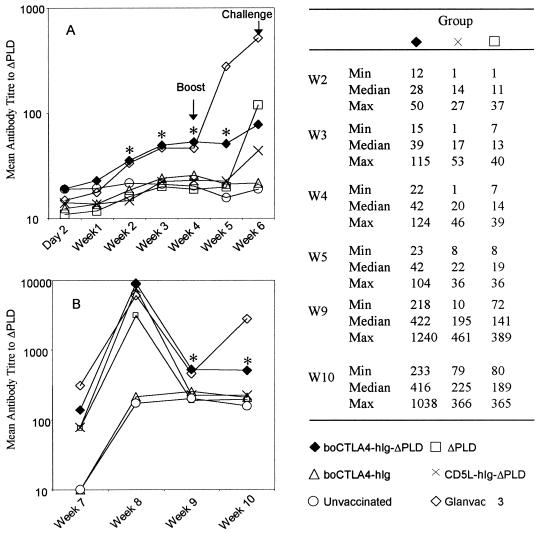
FIG. 2.
Kinetics of production of antibody to hIg following immunization. Animals were bled weekly, and the sera were tested for the presence of antibodies to hIg by a specific ELISA. The groups of animals that had significantly (P < 0.05) higher titers of antibody to hIg compared to control groups are indicated by asterisks, and the relevant minimum (Min), maximum (Max), and median values are shown. W, week.
Improvement of the antibody response to ΔPLD by targeting.
The titers of antibody to ΔPLD were low in all the treatment groups (<1,000) for the first 6 weeks prior to the experimental challenge with C. pseudotuberculosis (Fig. 3A). Indeed, the only two groups that had significant titers of antibody to ΔPLD in the first 4 weeks were those immunized with either the DNA encoding boCTLA-4–hIg–ΔPLD or Glanvac 3. These antibody titers were detected as early as 2 weeks postimmunization and were significantly enhanced (P < 0.05) compared to those of all the other treatment groups up to week 5. Although the antibody titers for the Glanvac 3 control group increased following the boost at week 4, there appeared to be no boost effect in the animals immunized with the DNA encoding boCTLA-4–hIg–ΔPLD. In contrast, the mean antibody titers in the animals immunized with DNA encoding ΔPLD had increased to levels similar to those of the animals receiving boCTLA-4–hIg–ΔPLD by week 6.
FIG. 3.
Kinetics of production of antibody to ΔPLD following immunization. Animals were bled weekly, and the sera were tested for the presence of antibodies to ΔPLD by a specific ELISA. To increase the sensitivity of the ELISA, the starting dilution of the sera was 1/10 for the first 6 weeks (A) and 1/100 for weeks 7 to 10 (B). Groups of animals that received DNA vaccines and had significantly (P < 0.05) higher titers of antibody to ΔPLD compared to control groups are indicated by asterisks, and the relevant minimum (Min), maximum (Max), and median values are shown. W, week.
Challenge with C. pseudotuberculosis was performed 6 weeks post-primary immunization, and the mean antibody titers for all the groups of animals are shown in Fig. 3B. All groups immunized with DNA encoding ΔPLD had been successfully primed against native PLD, as shown by the fact that by 2 weeks postchallenge the mean antibody titers had increased to >5,000. Furthermore, this postchallenge antibody response generated by all the DNA constructs encoding ΔPLD was equivalent to the peak antibody titers generated by the formalin-inactivated protein vaccine Glanvac 3. However, the antibody response to ΔPLD was short-lived in the groups of animals immunized with ΔPLD and CD5L–hIg–ΔPLD; these antibody titers dropped back to the level of the control group receiving DNA encoding boCTLA-4–hIg by week 9 (3 weeks postchallenge). In contrast, when ΔPLD was targeted as a boCTLA-4 fusion protein, the antibody titers remained at levels significantly higher than those of the groups immunized with DNA encoding CD5L–hIg–ΔPLD (P = 0.007) and ΔPLD (P = 0.003) at week 10 (Fig. 3).
Necropsy examination of sheep.
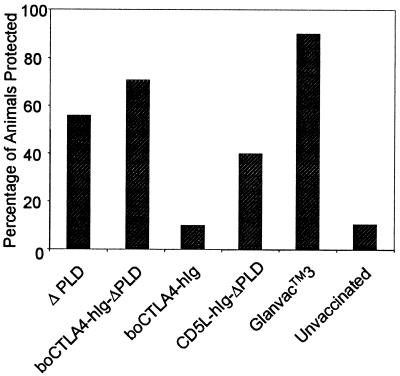
The disease CLA is characterized by the formation of abscesses in the lymphatic system draining the site of a C. pseudotuberculosis infection. Therefore, to evaluate the protective efficacy of the individual treatments, a necropsy of all animals was performed at 6 weeks postchallenge in order to visually assess them for abscess formation in all the major draining lymph nodes. Hemolytic C. pseudotuberculosis organisms were isolated from all of the abscesses tested from the draining popliteal lymph node in 9 of 10 animals in the unvaccinated group, indicating that the experimental challenge with C. pseudotuberculosis had been successful. Furthermore, 90% of the animals receiving Glanvac 3 lacked any signs of CLA, confirming previous reports describing the level of protection provided by this commercial vaccine. Although there were quantitative differences in the level of protection provided by the different DNA vaccination treatments (Fig. 4), due to the group sizes these differences were not statistically significant. DNA encoding ΔPLD or CD5L–hIg–ΔPLD provided reasonable levels of protection (56 and 40%, respectively), while targeting ΔPLD as a boCTLA-4 fusion protein provided the highest level of protection (70%).
FIG. 4.
Percentage of animals protected from an experimental challenge with C. pseudotuberculosis. Six weeks after primary immunization with the various treatments, animals were challenged with 106 CFU of a pathogenic wild-type strain of C. pseudotuberculosis. A necropsy was performed at week 12 to assess the level of abscess formation in the popliteal, inguinal, and prefemoral lymph nodes. Protection was defined by the absence of the characteristic CLA abscesses after animal numbers were matched with treatments at the end of the necropsy.
DISCUSSION
Recently it was demonstrated that targeting an antigen (hIg) as a CTLA-4 fusion protein significantly enhanced the speed and magnitude of antibody response in mice (3). These results have been confirmed in the present study in an outbred population of large animals and extended by the use of a challenge system to a clear demonstration of the efficacy of this DNA vaccination strategy. Moreover, we have shown that targeting ΔPLD as a CTLA-4 fusion protein provided sheep with a level of protection against an experimental challenge with C. pseudotuberculosis similar to that provided by a formalin-inactivated subunit vaccine. Indeed, this is the first animal trial that has clearly demonstrated the efficacy of DNA vaccination in an outbred domestic species such as sheep. This study clearly illustrates the generic nature of this targeting strategy for enhancing the immune response and the subsequent protection provided by DNA vaccines.
DNA vaccination has been shown to induce a long-lived antibody and cell-mediated response in mice, and there have been numerous reports demonstrating the efficacy of this approach against viral and parasitic infections (9). In contrast, there have been few reports of the use of DNA vaccination against bacterial infections, although the potential of this approach has been demonstrated for Mycobacterium tuberculosis (16), Mycoplasma pulmonis (2), and Clostridium tetani (1). It has been suggested that the use of DNA vaccination against bacterial infections may be complicated by fundamental differences between prokaryotic and eukaryotic genes (e.g., codon usage) and gene products (e.g., due to different cellular machinery), leading to the poor expression of a conformationally correct protein (21). However, in the present study the prokaryotic signal sequence of ΔPLD was shown to be functional in eukaryotic cells, resulting in the successful secretion of this protein. Furthermore, the glycosylated ΔPLD was capable of priming the immune response to native PLD, as the antibody responses peaked 2 weeks after challenge with a wild-type strain of C. pseudotuberculosis.
CTLA-4 has a high affinity for its ligands, CD80 and CD86 (formerly B7-1 and B7-2). CD80 and CD86 are expressed at high levels by antigen-presenting cells (APC), which are potent initiators of immune responses. The weak immune responses that are often associated with DNA vaccines are likely due to the low level of expression of the antigen in transfected cells. Therefore, it is likely that the enhanced immune response observed when ΔPLD was delivered as a CTLA-4 fusion protein was due to targeting of the low levels of antigen to APC. Indeed, it has been proposed that a possible mechanism of DNA vaccination is the uptake of the DNA and/or the protein secreted by transfected myocytes by specialized APC (dendritic cells), which subsequently present the antigen to lymphocytes in the draining lymph nodes (4). The findings of this study would support this as a mechanism, as dendritic cells expressing high levels of CD80/CD86 were identified in the afferent lymph.
Although the titers of antibody to ΔPLD were enhanced when ΔPLD was targeted as a CTLA-4 fusion protein, the antibody responses to ΔPLD were low in all groups prior to challenge. This appears to be a characteristic feature of the antibody response to PLD, as similar kinetics have been shown for Glanvac and ΔPLD delivered by a live recombinant vector (13). Despite the apparent role of antibodies to proteins secreted by C. pseudotuberculosis in protective immunity, no correlation between the magnitude of the antibody response and protection has been shown (10). Indeed, the differences in the level of protection between the different groups receiving ΔPLD constructs or Glanvac 3 do not seem to correlate with the antibody titers, which were in the same order of magnitude for all groups 2 weeks postchallenge. It is possible, however, that higher titers of antibody to ΔPLD at the time of challenge, or the increased longevity of the antibody response in the groups immunized with either CTLA-4–hIg–ΔPLD or Glanvac 3, accounts for the increased levels of protection.
The ΔPLD used in the current study was inactivated by replacing a histidine with a serine residue within the enzyme active site (22). Recently it has been shown that this recombinant ΔPLD protein induces an antibody response in sheep similar to that induced by Glanvac following a challenge with C. pseudotuberculosis (14). However, the level of protection provided by this recombinant ΔPLD was poor (43%) (14), albeit similar to the level of protection provided when ΔPLD was delivered as a DNA construct in the present study. Cell-mediated immunity may also play a role in the protective immunity to C. pseudotuberculosis (23), which would be in agreement with the findings of studies of mice that have established that the induction of a T-helper 1 response is important in resistance to a number of facultatively intracellular bacteria (17). Indeed, cell-mediated immune responses characterized by the production of gamma interferon have been established following immunization with the wild-type strain of C. pseudotuberculosis (12). Given that targeting an antigen as a CTLA-4 fusion protein has previously been shown to enhance T-helper responses (3), it is intriguing to speculate that the increased protection of the animals immunized with CTLA-4–hIg–ΔPLD was due, in part, to an enhanced cell-mediated immune response.
An important feature of the use of DNA is clearly the longevity of the immune response, which has been demonstrated to last as long as 18 months in mice vaccinated against hepatitis B (7). However, a study with sheep could find no evidence for the maintenance of antibody levels following vaccination with DNA, or following a subsequent boost with recombinant protein (20). A similar short-lived antibody response was also demonstrated in some primates following nucleic acid vaccination (8). Although it is unclear whether this is a feature of particular antigens, the DNA vaccination protocols for domestic animals have not been fully optimized, and this may, in part, account for the weak, short-lived responses observed in sheep. However, although optimizing factors such as the volume, dose, and route of administration may improve the longevity of the immune response, it is clear that targeting ΔPLD as a CTLA-4 fusion protein had a significant effect on increasing the length of the humoral immune response generated to ΔPLD.
This study has clearly demonstrated the efficacy of vaccines using DNA encoding antigens as CTLA-4 fusion proteins for targeting to APC. This approach enhances the speed, magnitude, and longevity of the immune response and offers a novel strategy for overcoming the weak immune responses that have been associated with DNA vaccines.
ACKNOWLEDGMENTS
This work was supported by the Cooperative Research Centre for Vaccine Technology and by the NHMRC, Canberra, Australia.
REFERENCES
- 1.Anderson R, Goa X M, Papakonstantinopoulou A, Roberts M, Dougan G. Immune response in mice following immunization with DNA encoding fragment C of tetanus toxin. Infect Immun. 1996;64:3168–3173. doi: 10.1128/iai.64.8.3168-3173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry M A, Lai W C, Johnston S A. Protection against mycoplasma infection using expression-library immunization. Nature. 1995;377:632–635. doi: 10.1038/377632a0. [DOI] [PubMed] [Google Scholar]
- 3.Boyle J S, Brady J L, Lew A M. Enhanced responses to a DNA vaccine encoding a fusion antigen that is directed to sites of immune induction. Nature. 1998;392:408–411. doi: 10.1038/32932. [DOI] [PubMed] [Google Scholar]
- 4.Casares S, Inaba K, Brumeanu T-D, Steinman R M, Bona C A. Antigen presentation by dendritic cells after immunisation with DNA encoding a major histocompatibility complex class II-restricted viral epitope. J Exp Med. 1997;186:1481–1486. doi: 10.1084/jem.186.9.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaplin P J, Entrican G, Gelder K I, Collins R A. Cloning and biologic activities of a bovine interferon-α isolated from a rotavirus infected calf. J Interferon Cytokine Res. 1996;16:25–30. doi: 10.1089/jir.1996.16.25. [DOI] [PubMed] [Google Scholar]
- 6.Cox G M, Zamb T J, Babiuk L A. Bovine herpesvirus 1: immune responses in mice and cattle injected with plasmid DNA. J Virol. 1993;67:5664–5667. doi: 10.1128/jvi.67.9.5664-5667.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis H L, Mancini M, Michel M L, Whalen R G. DNA-mediated immunization to hepatitis B surface antigen: longevity of primary response and effect of boost. Vaccine. 1996;14:910–915. doi: 10.1016/0264-410x(95)00255-y. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly J J, Friedman A, Martinez D, Montgomery D L, Shiver J W, Motzel S L, Ulmer J B, Liu M A. Preclinical efficacy of a prototype DNA vaccine: enhanced protection against antigenic drift in influenza virus. Nat Med. 1995;1:583–587. doi: 10.1038/nm0695-583. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 10.Eggleton D G, Haynes J A, Middleton H D, Cox J C. Immunisation against caseous lymphadenitis: correlation between Corynebacterium pseudotuberculosistoxoid content and protective efficacy in combined clostridial-corynebacterial vaccines. Aust Vet J. 1991;68:322–325. doi: 10.1111/j.1751-0813.1991.tb03088.x. [DOI] [PubMed] [Google Scholar]
- 11.Hall J G, Morris B. The output of cells in lymph from the popliteal node in sheep. J Exp Physiol. 1962;47:360–369. doi: 10.1113/expphysiol.1962.sp001620. [DOI] [PubMed] [Google Scholar]
- 12.Hodgson A L, Krywult J, Corner L A, Rothel J S, Radford A J. Rational attenuation of Corynebacterium pseudotuberculosis: potential cheesy gland vaccine and live delivery vehicle. Infect Immun. 1992;60:2900–2905. doi: 10.1128/iai.60.7.2900-2905.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodgson A L, Tachedjian M, Corner L A, Radford A J. Protection of sheep against caseous lymphadenitis by a single oral dose of live recombinant Corynebacterium pseudotuberculosis. Infect Immun. 1994;62:5275–5280. doi: 10.1128/iai.62.12.5275-5280.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodgson L M, Carter K, Tachedjian M, Krywult J, Corner L A, McColl M, Cameron A. Efficacy of an ovine caseous lymphadenitis vaccine formulated using a genetically inactive form of the Corynebacterium pseudotuberculosisphospholipase D. Vaccine. 1999;17:802–808. doi: 10.1016/s0264-410x(98)00264-3. [DOI] [PubMed] [Google Scholar]
- 15.Howard C J, Sopp P, Brownlie J, Kwong L S, Parsons K R, Taylor G. Identification of two distinct populations of dendritic cells in afferent lymph that vary in their ability to stimulate T cells. J Immunol. 1998;159:5372–5382. [PubMed] [Google Scholar]
- 16.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D'Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann S H. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 18.Parsons K P, Young J R, Collins R A, Howard C J. Cattle CTLA-4, CD28 and chicken CD28 bind CD86: MYPPPY is not conserved in cattle CD28. Immunogenetics. 1996;43:388–391. doi: 10.1007/BF02199808. [DOI] [PubMed] [Google Scholar]
- 19.Rothel J S, Boyle D B, Both G W, Pye A D, Waterkeyn J G, Wood P R, Lightowlers M W. Sequential nucleic acid and recombinant adenovirus vaccination induces host-protective immune responses against Taenia ovisinfection in sheep. Parasite Immunol. 1997;19:221–227. doi: 10.1046/j.1365-3024.1997.d01-200.x. [DOI] [PubMed] [Google Scholar]
- 20.Schrijver R S, Langedijk J P, Keil G M, Middel W G, Maris-Veldhuis M, Oirschot J T, Rijsewijk F A. Immunisation of cattle with BHV1 vector vaccine or a DNA vaccine both encoding for the G protein of BRSV. Vaccine. 1997;15:1908–1916. doi: 10.1016/s0264-410x(97)00129-1. [DOI] [PubMed] [Google Scholar]
- 21.Strugnell R A, Drew D, Mercieca J, Dinatale S, Firez N, Dunstan S J, Simmons C P, Vadolas J. DNA vaccines for bacterial infections. Immunol Cell Biol. 1997;75:364–369. doi: 10.1038/icb.1997.57. [DOI] [PubMed] [Google Scholar]
- 22.Tachedjian M, Krywult J, Moore R J, Hodgson A L. Caseous lymphadenitis vaccine development: site-specific inactivation of the Corynebacterium pseudotuberculosisphospholipase D gene. Vaccine. 1995;18:1785–1792. doi: 10.1016/0264-410x(95)00144-p. [DOI] [PubMed] [Google Scholar]
- 23.Walker J, Jackson H J, Eggleton D G, Meeusen E N, Wilson M J, Brandon M R. Identification of a novel antigen from Corynebacterium pseudotuberculosisthat protects sheep against caseous lymphadenitis. Infect Immun. 1994;62:2562–2567. doi: 10.1128/iai.62.6.2562-2567.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]