Abstract
Background
Studies of flares of autoimmune inflammatory rheumatic diseases (AIIRD) after COVID-19 mRNA vaccination are limited by small sample size, short follow up or at risk of selection bias.
Methods
A national retrospective cohort study of consecutive AIIRD patients ≥12 years old, across 8 hospitals who received at least one dose of a COVID-19 mRNA vaccine. Patients were included from the date of 1st vaccine dose and censored at the time of flare or on the date of the clinic visit at least 3 months from cohort entry, whichever came first. Predictors of flare were determined by Cox proportional hazards analysis.
Findings
4627 patients (73% Chinese, 71% female) of median (IQR) age 61 (48, 70) years were included; 42% Rheumatoid arthritis, 14% Systemic lupus erythematosus and 11% Psoriatic arthritis. 47% were in remission, 41% low disease activity, 10% moderate disease activity and 1% in high disease activity. 18% patients flared, of which 11.7% were within the 3-month period of interest. 11.8% patients improved. Median (IQR) time-to-flare was 60 (30, 114) days. 25% flares were self-limiting, 61% mild-moderate and 14% severe. Older patients (53–65 years and >66 years) had a lower risk of flare [HR 0.6 (95% CI 0.5–0.8) and 0.7 (0.6–0.8) respectively]. Patients with inflammatory arthritis and with active disease had a higher risk of flare [HR 1.5 (1.2–2.0) and 1.4 (1.2–1.6), respectively]. Treatment with conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs), immunosuppression and prednisolone was also associated with an increased risk of flare [HR 1.5 (1.1–2), 1.2 (1.1–1.4) and 1.5 (1.2–1.8) for prednisolone ≤7.5 mg respectively].
Interpretation
There was a moderately high rate of AIIRD flares after mRNA vaccination but also improvement in several patients. Severe flares and hospitalisation were rare. Thus, vaccination remains safe and highly recommended.
Keywords: Autoimmune inflammatory rheumatic diseases, COVID vaccines, Registry
1. Introduction
Patients with autoimmune inflammatory rheumatic diseases (AIIRD) have poorer outcomes when infected with SARS-CoV2, including higher rates of hospitalisation, oxygen support, intensive care unit (ICU) admission and mortality [1]. Vaccination is currently the most effective way to reducing mortality, with international and national rheumatology societies recommending COVID-19 vaccination for all patients with AIIRD [[2], [3], [4]]. Although initial clinical trials of COVID-19 vaccines have largely excluded patients with AIIRD [5,6], there is a growing body of evidence that COVID-19 vaccines are safe and effective for this group of patients [[7], [8], [9]]. Universal uptake of vaccination is an essential part of controlling COVID-19 at both the patient and population level. However, vaccine hesitancy has been a major barrier, with fear of side effects being a significant factor [10]. In addition, up to 44% of AIIRD patients have a fear of potentially triggering flares of their rheumatic disease [9].
Rates of flares of AIIRD after COVID-19 vaccination in various studies have been wide-ranging from no flares to up to 18% [[7], [8], [9],[11], [12], [13], [14], [15], [16], [17], [18]]. Contributing factors to this variability include different study designs, varying sample size, varying length of follow-up and definition of “at-risk” period and differences in flare definitions. In addition, most of these studies are at risk of selection bias, as they are based on voluntary physician reports or patient surveys. Higher rates of flares have been described in patients who have history of self-reported flares within the past 6 months, patients with inflammatory arthritides and those who have had past COVID infection [7,8].
Comprehensive inclusion of all vaccinated patients is an important factor when evaluating incidence of flares. In Singapore, COVID-19 vaccination began in January 2021, almost exclusively with the two mRNA vaccines (BNT162b2 Pfizer-BioNTech® COVID-19 vaccine and the mRNA-1273 Moderna® COVID-19 vaccine). Vaccination was offered to all adults, and children 12 years and older by July 2021. By November 2021, 82% of the Singapore population had completed 2 doses of COVID-19 vaccination [19]. As such, we set up the COronavirus National Vaccine registry for ImmuNe diseases SINGapore (CONVIN-SING), to accurately assess flares, and the risk factors for flares, from direct medical record review of all patients with AIIRD attending public sector hospitals in Singapore and who had received an mRNA vaccine against SARS-COV2.
We aimed to determine the proportion of patients with rheumatic disease who flared within 3 months of the 1st dose of an mRNA vaccine, assess time to flare and predictors of flare.
2. Methods
Using a retrospective cohort study design and electronic health record (EHR) review, we included adults and children 12 years of age or older, who had received at least one dose of an mRNA vaccine and who had been diagnosed with an AIIRD prior to vaccination. The AIIRD of interest were pre-defined and are listed in the Supplementary Appendix Table S1. Eight of the nine public hospitals in Singapore participated in this study. A list of patients with AIIRD was extracted using SNOMED diagnosis codes and matched against the National Immunisation Register (NIR) for type and dates of vaccine doses. Matching with the NIR was done in August 2021 and refreshed once in November 2021. We included patients consecutively, in order of the date of the 1st vaccine dose.
Patients entered the cohort from the date of 1st vaccine dose and were censored at the time of flare or on the date of the clinic visit which was at least 3 months from cohort entry, whichever came first. Data were abstracted manually onto a secure web-based portal via manual chart review and included demographics, confirmation of diagnosis, physician-defined baseline disease activity (remission, low, moderate or high), baseline treatment, previous diagnosis of COVID-19, severity and date of flare if any, and hospitalisation for flare. A training session was conducted to standardise data entry across sites. Practice cases were used to harmonise definitions of pre-vaccine disease activity, and adjudication of flare. The primary outcome was a flare of AIIRD between 0 and 3 months after the 1st dose of anti-SARS-CoV mRNA vaccine.
The severity of flare was classified as follows: A mild, self-limiting flare was defined as one that did not require an escalation of treatment or early consult with the specialist, and resolved before the index visit, though the patient may have taken analgesics or non-steroidal anti-inflammatory drugs (NSAID) or self-increased glucocorticoids (GC) for a few days. A mild to moderate, non-self-limiting flare was one that necessitated an earlier specialist consult and/or increase in treatment, but not exceeding prednisolone 20 mg per day and not requiring intramuscular (IM) or intra-articular (IA) GC injection or new biologic or cytotoxic drug (e.g. cyclophosphamide) initiation. A severe flare was one that required hospitalisation or GC more than prednisolone equivalent of 20 mg per day or IM or IA GC, or new initiation of a biologic or cytotoxic agent.
Ethics approval was obtained from the National Healthcare Group (NHG) Domain Specific Review Board (DSRB) Ref: 2021/0043. The requirement for patient consent was waived as the data were collected via EHR review only, without any direct patient contact.
Statistical analyses: Sample size was calculated based on a background flare rate of 8% over 3 months in a rheumatoid arthritis (RA) cohort and power of 90% to detect a 25% relative increase in flares [20]. All analyses were performed using Stata version 16 (StataCorp). Continuous variables are presented as median (interquartile range, IQR) while categorical variables are presented as frequencies and percentages. Predictors of flare and time to flare were assessed using a mixed effect stepwise backward-selection Cox regression model to account for clustering effect of site, adjusting for demographics and relevant covariates. Age (as tertiles), race, AIIRD diagnosis (grouped as inflammatory arthritis (IA), connective tissue disease (CTD), vasculitis and others) treatment [grouped as no disease modifying antirheumatic drugs (DMARDs), hydroxychloroquine only, conventional synthetic (cs)DMARDs (methotrexate, sulfasalazine or leflunomide), immunosuppressants (cyclosporine A, mycophenolate mofetil, mycophenolate sodium, tacrolimus, azathioprine, cyclophosphamide), and biological (b)DMARDs or Janus Kinase inhibitors (JAKi)] and GC dose (grouped as no GC, prednisolone≤7.5 mg, prednisolone >7.5 mg) were entered into the model as categorical variables. Gender, vaccine type and disease activity (grouped as inactive disease for patients in remission or low disease activity and active disease for those with moderate or high disease activity) were used as binary variables. The model was adjusted for number of vaccine doses received. Kaplan Meier curve was generated for time to flare after first dose. The proportion of patients who flared within 3 weeks of the first versus second dose were compared using Chi squared test. For subjects who flared, line graphs by disease group were plotted for time to flare.
3. Results
4627 patients (73.3% Chinese, 71.1% female) of median (IQR) age 61 (48, 70) years were included in the analysis (Table 1 ). 4058 (87.7%) received the BNT162b2 (Pfizer/BioNTech) COVID-19 vaccine and 523 (11.3%) received the mRNA-1273 (Moderna) vaccine, with a median (IQR) interval of 21 (21, 28) days between the two doses. 127 patients (2.7%) had a single vaccine dose. The most common AIIRD diagnoses were RA (1966, 42.4%), systemic lupus erythematosus (SLE) (641, 13.8%) and psoriatic arthritis (486, 10.5%). At the pre-vaccination clinic visit, 2188 (47.3%) patients were in remission, 1897 (41%) had low disease activity, 480 (10.4%) had moderate disease activity and 62 (1.3%) had high baseline disease activity. 1389 (30%) were treated with hydroxychloroquine, 1684 (36%) with methotrexate and 848 (18%) treated with sulfasalazine. 431 (9.3%) were treated with bDMARD/JAKi. The median prednisolone dose was 0 mg (IQR 0,4). Treatment was interrupted for vaccination in only 63 (1.4%) patients. Only 45 (1%) patients had previous COVID-19 infection.
Table 1.
Patient characteristics.
| Baseline characteristics | Whole Cohort (n = 4627) | Stable (n = 3228, 69.8%) |
Flares within 0–3 months of 1st vaccine dose (n = 542, 11.7%) | Flares outside of 0–3 months after 1st vaccine dose (n = 312, 6.8%) | Improved (n = 545, 11.8%) |
|---|---|---|---|---|---|
| Age | 61 | 61 | 58 | 62 | 60 |
| (median years, IQR) | (48, 70) | (47, 70) | (46, 67) | (48, 70) | (51, 67) |
| Ethnicity | |||||
| Chinese | 3394 (73) | 2388 (74) | 395 (73) | 226 (72) | 385 (71) |
| Malay | 486 (11) | 322 (10) | 60 (11) | 31 (10) | 73 (13) |
| Indian | 473 (10) | 321 (10) | 61 (11) | 43 (14) | 48 (9) |
| Others | 274 (6) | 197 (6) | 26 (5) | 12 (4) | 39 (7) |
| Gender | |||||
| Female | 3293 (71) | 2313 (72) | 390 (72) | 205 (66) | 385 (71) |
| Vaccine type | |||||
| Pfizer/BioNTech | 4058 (88) | 2862 (89) | 459 (86) | 271 (87) | 466 (87) |
| Moderna | 523 (11) | 339 (11) | 76 (14) | 39 (13) | 69 (13) |
| Diagnosis | |||||
| Rheumatoid Arthritis | 1966 (42) | 1314 (41) | 264 (49) | 149 (49) | 239 (44) |
| Systemic Lupus Erythematosus | 641 (14) | 496 (15) | 51 (9) | 21 (7) | 73 (13) |
| Psoriatic Arthritis | 486 (11) | 302 (9) | 79 (15) | 41 (13) | 64 (12) |
| Spondyloarthropathies | 484 (10) | 325 (10) | 60 (11) | 46 (15) | 53 (10) |
| Sjogren's Syndrome | 261 (6) | 208 (6) | 22 (4) | 11 (4) | 20 (4) |
| Systemic sclerosis | 159 (3) | 128 (4) | 8 (1) | 7 (2) | 16 (3) |
| Treatment | |||||
| Methotrexate | 1684 (36) | 1088 (34) | 233 (43) | 124 (40) | 239 (44) |
| Sulfasalazine | 848 (18) | 553 (17) | 125 (23) | 73 (23) | 97 (18) |
| Hydroxychloroquine | 1389 (30) | 969 (30) | 154 (28) | 77 (25) | 189 (35) |
| Mycophenolate Mofetil | 296 (6) | 210 (7) | 32 (6) | 13 (4) | 41 (8) |
| Biological DMARDs | 383 (8) | 249 (8) | 57 (11) | 24 (8) | 53 (10) |
| JAK inhibitors | 46 (1) | 24 (1) | 6 (1) | 8 (3) | 8 (1) |
| Prednisolone dose (mg, median, IQR) | 0 (0,4) | 0 (0, 2.5) | 0 (0,5) | 0 (0,5) | 0 (0,5) |
| Baseline Physician Disease Activity | |||||
| Remission | 2188 (47) | 1761 (55) | 179 (33) | 115 (36) | 133 (24) |
| Low Disease Activity | 1897 (41) | 1235 (39) | 257 (47) | 157 (50) | 248 (46) |
| Moderate Disease Activity | 480 (10) | 213 (7) | 92 (17) | 37 (12) | 138 (25) |
| High Disease Activity | 62 (1) | 19 (1) | 14 (3) | 3 (0.96) | 26 (5) |
Data are shown in number (percentages) unless specified.
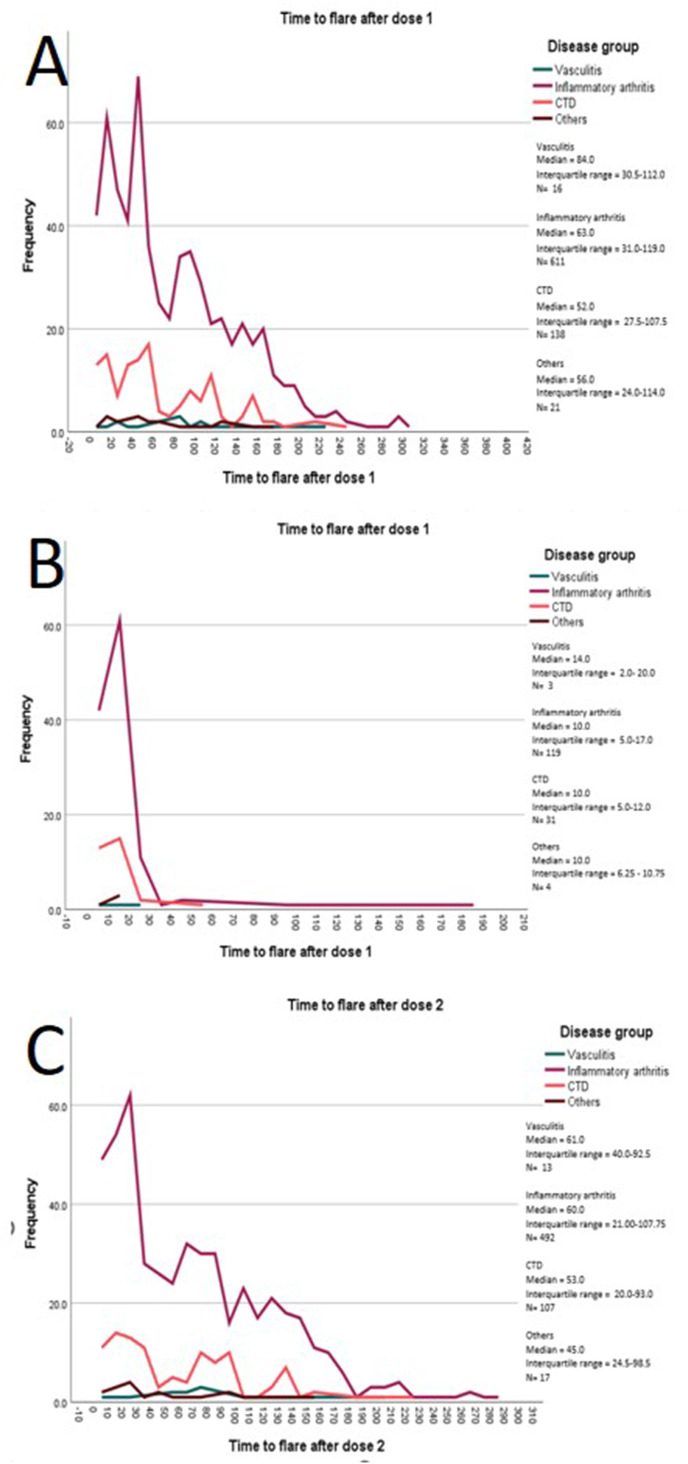
854 (18%) flares were recorded during 20,287 patient-months of follow-up (4.5/100 patient-months, median (IQR) follow up duration 4.3 (3.4, 5.5) months), of which 542 (11.7%) patients flared in the 3-month period of interest (Fig. 1 ). Median (IQR) time to flare was 60 (30–114) days. When examining by disease groups, a biphasic peak was seen for patients with IA (Fig. 2 A). Separating the flares after the 1st dose from those after the 2nd dose, the median time to flare for patients with IA was 10 (5–17) days after the 1st dose (Figs. 2B) and 60 (21–108) days after the 2nd dose (Fig. 2C)].
Fig. 1.
Kaplan-Meier graph - Time to Flare after the First Dose of mRNA Vaccine.
Fig. 2.
Time to flare by disease group. A = All Flares, B = Flares after dose 1 and before dose 2, C = Flares after dose 2 (patients who flared after dose 1 are excluded).
To compare flare rate after the 1st versus the 2nd dose, we limited the period of observation to within 3 weeks of the vaccine, as the 2nd dose was given 3 weeks after the 1st dose in most patients. 144 patients (3.2%) flared after the 1st dose. These patients were censored at the date of flare and hence not included in the susceptible population for flare after the 2nd dose. 159 patients (3.6%) flared after the 2nd dose (chi2 for difference in proportions = 0.23).
134 (24.7%) of all flares were mild and self-limiting, 333 (61.4%) were mild-moderate and 75 (13.8%) were severe. 393 (72.5%) of those who flared required escalation of treatment and 30 (5.5%) required hospital admission. Conversely, 545 patients (11.8%) had improved disease activity after the vaccine.
On Cox regression analysis, patients in the older age tertiles (53–65 and > 66 years) had a lower risk of flare, hazard ratio (HR) 0.6, 95% CI 0.5–0.8, p < 0.001 and 0.7, 95% CI 0.6–0.8 p < 0.001 respectively (Table 2 ). Patients of the minority non-Chinese/non- Malay/non-Indian ethnicity had a lower risk of flare (HR 0.7 95% CI 0.5–0.9). Compared to CTD, patients with IA had a HR of flare of 1.5, 95% CI 1.2–2.0, p = 0.006. Patients with baseline active disease had a 40% higher risk of flare compared to those with inactive disease (HR 1.4, 95% CI 1.2–1.6, p < 0.001). Treatment with csDMARDs, immunosuppressants and prednisolone was also associated with an increased risk of flare [HR 1.5 (1.1–2), 1.2 (1.1–1.4) and 1.5 (1.2–1.8) for prednisolone dose ≤7.5 mg respectively].
Table 2.
Predictors of time to flare.
| Variables | Unadjusted |
Adjusted |
||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Vaccine type | ||||
| BNT162b2 Pfizer-BioNTech | 1.0 | 0.06 | ||
| mRNA-1273 Moderna | 1.2 (1–1.5) | |||
| Age | ||||
| ≤52 | 1.0 | 1.0 | ||
| 53-65 | 0.8 (0.7–0.97) | 0.02 | 0.6 (0.5–0.8) | <0.001 |
| >66 | 0.8 (0.7–0.9) | 0.002 | 0.7 (0.6–0.8) | <0.001 |
| Ethnicity | ||||
| Chinese | 1.0 | 1.0 | ||
| Malay | 1.0 (0.8–1.3) | 0.78 | 0.7 (0.4–1.1) | 0.14 |
| Indian | 1.2 (1–1.5) | 0.08 | 1.0 (0.8–1.3) | 0.75 |
| Others | 0.8 (0.5–1.1) | 0.10 | 0.7 (0.5–0.9) | 0.006 |
| Gender | ||||
| Male | 1.0 | |||
| Female | 0.9 (0.8–1.1) | 0.53 | ||
| Disease group | ||||
| Connective Tissue Disease | 1.0 | 1.0 | ||
| Vasculitis | 1.4 (0.9–2.2) | 0.18 | 1.2 (0.7–1.8) | 0.67 |
| Inflammatory arthritis | 1.9 (1.6–2.2) | <0.001 | 1.5 (1.2–2.0) | 0.001 |
| Others | 1.3 (0.8–2.2) | 0.35 | 1.4 (0.8–2.5) | 0.28 |
| Active disease | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.7 (1.4–2.1) | <0.001 | 1.4 (1.2–1.6) | <0.001 |
| Glucocorticoids | ||||
| None | 1.0 | 1.0 | ||
| Pred≤7.5 mga | 1.4 (1.2–1.6) | <0.001 | 1.5 (1.2–1.8) | <0.001 |
| Pred >7.5 mga | 1.7 (1.2–2.3) | 0.002 | 1.7 (1–3.0) | 0.05 |
| Treatment | ||||
| None | 1.0 | 1.0 | ||
| Hydroxychloroquine only | 0.9 (0.7–1.2) | 0.48 | 1.1 (0.7–1.7) | 0.55 |
| csDMARDsb | 1.9 (1.5–2.3) | <0.001 | 1.5 (1.1–2.0) | 0.005 |
| Immunosuppressantsc | 1.2 (0.9–1.6) | 0.16 | 1.2 (1.1–1.4) | 0.004 |
| bDMARDs or JAKid | 1.9 (1.4–2.5) | <0.001 | 1.3 (0.9–1.7) | 0.11 |
Multivariate analysis has been adjusted for number of vaccine doses. In the adjusted model, 96.8% of subjects (4327 out of 4627 subjects) were used.
Significant p-value in bold.
Or equivalent dose in prednisolone.
csDMARDs = conventional synthetic disease modifying anti-rheumatic drugs which included Methotrexate, Sulfasalazine and Leflunomide.
Immunosuppressants include Cyclosporin A, Cyclophosphamide, Azathioprine, Mycophenolate Mofetil, Mycophenolate Sodium, Tacrolimus.
bDMARDs or JAKi = biological disease modifying anti-rheumatic drugs or Janus Kinase inhibitors.
4. Discussion
We have described post-vaccination flares in an inception cohort of consecutively vaccinated AIIRD patients across Singapore. Flares were seen in 18% of our patients, of which 11.7% were within 3 months of the first vaccine dose, with a median time to flare of 60 days. Conversely, 11.8% had improved disease activity after the vaccine. Only 75 (1.6%) patients had a severe flare, while 30 (0.6% of those vaccinated) required hospitalisation. Given the high morbidity and mortality of COVID-19 in patients with AIIRD, our study strongly supports a favourable risk-benefit ratio for vaccination of these vulnerable patients [7,21].
Our study has several strengths. We included all AIIRD patients from eight of the nine public hospitals in Singapore, sequentially, in order of the date of vaccination. Vaccination data were obtained centrally through the NIR and thus represents a near-complete capture of all eligible AIIRD patients in Singapore. Demographic and clinical data were abstracted manually through detailed EHR review, by trained personnel. Flares were adjudicated by the attending physician, and were graded for severity.
The rate of flares in our cohort is comparable to other studies that evaluated the rate of flare after COVID-19 vaccination, although, as mentioned, these studies were mostly based on patient self-reported symptom questionnaires. These previous studies have reported flare rates between 2.2 and 18.8% [7,13,15,16]. Studies which employed physician assessment were based on voluntary physician reporting of patients at varying intervals post-vaccination. A recent study using linked government vaccine records noted no increased risk of flare with COVID-19 vaccine in patients with RA [11]. However, this study used administrative data on outpatient/hospital visits and prescription patterns as proxies to assess disease flares.
Only 1% of our cohort had previous COVID-19 infection at the time of our study. This was in keeping with the low population prevalence of COVID in Singapore in 2021 (3%, 198,374) [22] due to stringent COVID restrictions mandated by the Singapore government. As in other cohorts, the predominant AIIRDs in our patients were IA (67%) and SLE (13.8%). In studies based on patient-surveys, symptoms such as joint pain, stiffness, myalgia and fatigue were taken to indicate disease flare. While these are important symptoms to elicit from patients, objective assessment by a physician is important to discern whether these symptoms truly represent disease flare. To this end, 75% of our patients who flared were assessed by their rheumatologist to require escalation of treatment, suggesting that they were true flares.
We found that patients with IA were more likely to experience a flare of disease. Our observed 3-month flare rate does appear to be higher than previously reported normalized background yearly flare-rates among patients with stable RA (approximately 7.9% flares per 3-months) [20] and SLE (approximately 3.2% per 3-months) [23]. Common post-vaccination effects such as arthralgias or myalgias leading to tender joints on examination and elevated inflammatory markers may contribute toward a higher perceived disease activity, potentially overestimating the observed flare rates. It is also reassuring to know that over 10% of patients continue to report improvement in their disease activity, as part of their natural disease history, highlighting that patients are still able to have better control of their disease over the 3-months despite receiving vaccination.
It is interesting to observe that treatment with csDMARDs, immunosuppression and a higher dose of prednisolone were associated with flares. Firstly, while only 1.4% patients were documented to have withheld their treatment prior to vaccination, this may be an under-estimation, leading to more flares in patients pausing higher dose treatment. Secondly, patients on such therapy are likely on closer follow up than those who are on minimal treatment, and thus this finding may be due to ascertainment bias.
Vaccine recommendations in people with rheumatic disease, both for COVID-19 vaccination and other non-COVID vaccines, are largely based on vaccine studies conducted in patients with quiescent disease [4,24]. Suboptimal disease control at the time of vaccination has been reported to be associated with reduced vaccine immunogenicity, likely due to the associated therapy [25]. In addition, similar to others using patient-reported flares, we found that suboptimal disease control at the time of vaccination was found to predict post-vaccination flare [7,15].
Older patients had a lower risk of AIIRD flare in our study. This may be partially attributed to reduced vaccine immunogenicity in the elderly population [26]. Other factors, such as disease duration, depth and/or length of remission at the time of vaccination and immunosuppressive medications in this population may have also played a part.
Our study has certain limitations. While the Singapore national guidelines do not recommend interruption of treatment for vaccination [4], suboptimal medication adherence and dose changes by the patients themselves may not have been captured in our retrospective study. Similarly, flares were only captured if reported to, and documented by, the treating physician in the medical record, and the time of flare relative to vaccine administration was usually approximated.
To our knowledge this is the largest and most comprehensive report of post-COVID-19 vaccination flares in AIIRD to date. While the flare rate appears higher than the previously reported background flare rate among stable AIIRD patients, causality cannot be determined from this retrospective observational study. Moreover, we reported a large number of patients with improved disease activity after vaccination, suggesting that both the flares and improvement may just be a part of the natural history of disease, and warrants further study. Patients with IA and suboptimal disease control were at higher risk of post-vaccination flares and may require closer follow up after vaccination. Severe flares and hospitalisation were rare; thus, vaccination remains safe and highly recommended for our vulnerable patients with AIIRD.
Author statement
Margaret MA: Conceptualization, Data curation, Methodology, Supervision, Validation, Writing – original draft; Amelia SANTOSA: Conceptualization, Methodology, Writing – review & editing; Warren FONG: Conceptualization, Data curation, Methodology, Supervision, Validation, Writing – review & editing; Li-Ching CHEW: Writing – review & editing; Andrea HL LOW; Conceptualization, Writing – review & editing; Annie LAW: Writing – review & editing, Yih Jia POH: Writing – review & editing; Siaw Ing YEO: Writing – review & editing; Ying Ying LEUNG: Writing – review & editing; Victoria WW NG: Data curation; Joshua ZE KOH: Data curation; Sen Hee TAY: Conceptualization, Methodology, Writing – review & editing; Anselm MAK: Conceptualization, Methodology, Writing – review & editing; Gim Gee TENG: Writing – review & editing; Chuanhui XU: Data curation; Johnston GX TANG: Data curation: Kok Ooi KONG: Writing – review & editing; Stanley ANGKODJOJO: Data curation, Supervision, Validation, Writing – review & editing; Wei-Rui GOH: Data curation; Tyng Yu CHUAH: Data curation; Nur Emillia ROSLAN: Data curation; Thaschawee ARKACHAISRI: Writing – review & editing; Kai Liang THE: Data curation; Melonie SRIRANGANATHAN: Writing – review & editing; Teck Choon TAN: Data curation; Kee Fong PHANG: Data curation; Qai Ven YAP: Formal analysis; Yiong Huak CHAN: Formal analysis, Supervision; Peter PM CHEUNG: Conceptualization, Methodology, Writing – review & editing; Manjari LAHIRI: Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to acknowledge the Chapter of Rheumatologists, College of Physicians, Academy of Medicine, Singapore.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaut.2022.102959.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Xu C., Yi Z., Cai R., et al. Clinical outcomes of COVID-19 in patients with rheumatic diseases: a systematic review and meta-analysis of global data. Autoimmun. Rev. 2021 doi: 10.1016/j.autrev.2021.102778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curtis J.R., Johnson S.R., Anthony D.D., et al. American college of rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 3. Arthritis Rheumatol. 2021;73(10):e60–e75. doi: 10.1002/art.41928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landewe R.B.M., Kroon F.P.B., Alunno A., et al. EULAR recommendations for the management and vaccination of people with rheumatic and musculoskeletal diseases in the context of SARS-CoV-2: the November 2021 update. Ann. Rheum. Dis. 2022;12:1628–1639. doi: 10.1136/annrheumdis-2021-222006. [DOI] [PubMed] [Google Scholar]
- 4.Santosa A., Xu C., Arkachaisri T., et al. Recommendations for COVID-19 vaccination in people with rheumatic disease: developed by the Singapore Chapter of Rheumatologists. Int J Rheum Dis. 2021;24(6):746–757. doi: 10.1111/1756-185X.14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connolly C.M., Ruddy J.A., Boyarsky B.J., et al. Disease flare and reactogenicity in patients with rheumatic and musculoskeletal diseases following two-dose SARS-CoV-2 messenger RNA vaccination. Arthritis Rheumatol. 2022;74(1):28–32. doi: 10.1002/art.41924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machado P.M., Lawson-Tovey S., Strangfeld A., et al. Safety of vaccination against SARS-CoV-2 in people with rheumatic and musculoskeletal diseases: results from the EULAR Coronavirus Vaccine (COVAX) physician-reported registry. Ann. Rheum. Dis. 2022;81(5):695–709. doi: 10.1136/annrheumdis-2021-221490. [DOI] [PubMed] [Google Scholar]
- 9.Sattui S.E., Liew J.W., Kennedy K., et al. Early experience of COVID-19 vaccination in adults with systemic rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance Vaccine Survey. RMD Open. 2021;7(3) doi: 10.1136/rmdopen-2021-001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko T., Dendle C., Woolley I., et al. SARS-COV-2 vaccine acceptance in patients with rheumatic diseases: a cross-sectional study. Hum. Vaccines Immunother. 2021;17(11):4048–4056. doi: 10.1080/21645515.2021.1958611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X., Tong X., Yeung W.W.Y., et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann. Rheum. Dis. 2022;81(4):564–568. doi: 10.1136/annrheumdis-2021-221571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan Y., Geng Y., Wang Y., et al. Safety and disease flare of autoimmune inflammatory rheumatic diseases: a large real-world survey on inactivated COVID-19 vaccines. Ann. Rheum. Dis. 2022;81(3):443–445. doi: 10.1136/annrheumdis-2021-221736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbhaiya M., Levine J.M., Bykerk V.P., et al. Systemic rheumatic disease flares after SARS-CoV-2 vaccination among rheumatology outpatients in New York City. Ann. Rheum. Dis. 2021;80(10):1352–1354. doi: 10.1136/annrheumdis-2021-220732. [DOI] [PubMed] [Google Scholar]
- 14.Izmirly P.M., Kim M.Y., Samanovic M., et al. Evaluation of immune response and disease status in systemic lupus erythematosus patients following SARS-CoV-2 vaccination. Arthritis Rheumatol. 2022;74(2):284–294. doi: 10.1002/art.41937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rotondo C., Cantatore F.P., Fornaro M., et al. Preliminary data on post market safety profiles of COVID 19 vaccines in rheumatic diseases: assessments on various vaccines in use, different rheumatic disease subtypes, and immunosuppressive therapies: a two-centers study. Vaccines (Basel) 2021;9(7) doi: 10.3390/vaccines9070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peet C.J., Papadopoulou C., Sombrito B.R.M., et al. COVID-19 and autoinflammatory diseases: prevalence and outcomes of infection and early experience of vaccination in patients on biologics. Rheumatol Adv Pract. 2021;5(2):rkab043. doi: 10.1093/rap/rkab043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furer V., Eviatar T., Zisman D., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann. Rheum. Dis. 2021;80(10):1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 18.Braun-Moscovici Y., Kaplan M., Braun M., et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann. Rheum. Dis. 2021;80(10):1317–1321. doi: 10.1136/annrheumdis-2021-220503. [DOI] [PubMed] [Google Scholar]
- 19.MoH Singapore. https://www.moh.gov.sg/covid-19/vaccination/statistics Vaccination Statistics 2022 [updated 26 June 2022. Available from:
- 20.Bechman K., Tweehuysen L., Garrood T., et al. Flares in rheumatoid arthritis patients with low disease activity: predictability and association with worse clinical outcomes. J. Rheumatol. 2018;45(11):1515–1521. doi: 10.3899/jrheum.171375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tzioufas A.G., Bakasis A.D., Goules A.V., et al. A prospective multicenter study assessing humoral immunogenicity and safety of the mRNA SARS-CoV-2 vaccines in Greek patients with systemic autoimmune and autoinflammatory rheumatic diseases. J. Autoimmun. 2021;125 doi: 10.1016/j.jaut.2021.102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medicine J.H.U. Coronavirus resource center 2022. https://coronavirus.jhu.edu/region/singapore [Available from:
- 23.Cho J., Lahiri M., Teoh L.K., et al. Predicting flares in patients with stable systemic lupus erythematosus. Semin. Arthritis Rheum. 2019;49(1):91–97. doi: 10.1016/j.semarthrit.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Furer V., Rondaan C., Heijstek M.W., et al. Update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2019;79(1):39–52. doi: 10.1136/annrheumdis-2019-215882. 2020. [DOI] [PubMed] [Google Scholar]
- 25.Campos L.M., Silva C.A., Aikawa N.E., et al. High disease activity: an independent factor for reduced immunogenicity of the pandemic influenza a vaccine in patients with juvenile systemic lupus erythematosus. Arthritis Care Res. 2013;65(7):1121–1127. doi: 10.1002/acr.21948. [DOI] [PubMed] [Google Scholar]
- 26.Collier D.A., Ferreira I., Kotagiri P., et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596(7872):417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.