Abstract
Hyoscyamine 6β-hydroxylase (H6H) is a bifunctional non-heme 2-oxoglutarate/Fe2+-dependent dioxygenase that catalyzes the two final steps in the biosynthesis of scopolamine. Based on high resolution crystal structures of H6H from Datura metel, detailed information on substrate binding was obtained that provided insights into the onset of the enzymatic process. In particular, the role of two prominent residues was revealed – Glu-116 that interacts with the tertiary amine located on the hyoscyamine tropane moiety and Tyr-326 that forms CH–π hydrogen bonds with the hyoscyamine phenyl ring. The structures were used as the basis for QM/MM calculations that provided an explanation for the regioselectivity of the hydroxylation reaction on the hyoscyamine tropane moiety (C6 vs. C7) and quantified contributions of active site residues to respective barrier heights.
Introduction
Tropane alkaloids have been used as both medicine and poison since antiquity.1 Even today, they are widely used in medicine, either as natural products or as (semi)synthetic derivatives.2 Within this large group of alkaloids comprising approximately 200 compounds, hyoscyamine and scopolamine (Fig. 1) are probably the most known substances because of their common use, inter alia, as eye pupil widening agents, anesthetic and anti-Parkinson drugs. Of the two, scopolamine displays a weaker effect on the central nervous system and is better tolerated than hyoscyamine, but it is also less abundant in nature. Hence, ongoing research activity is directed towards the enhancement of scopolamine production, which can be achieved by various means, e.g. metabolic engineering of plants,3 the use of recombinant Escherichia coli for biotransformations,4,5 the search for more active forms of the enzyme hyoscyamine 6β-hydroxylase (H6H)6 and the redesign of H6H by random and site-directed mutagenesis.5
Fig. 1.
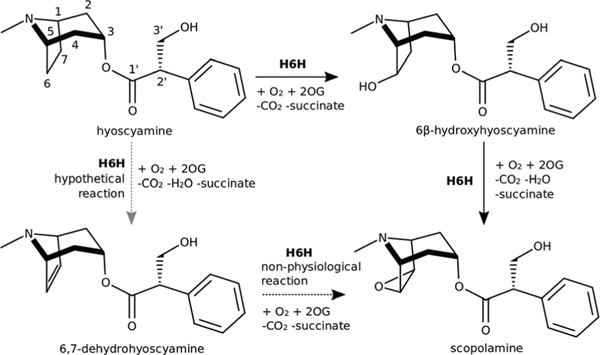
Reactions catalysed by H6H. MarvinSketch was used to draw structures and reactions.11
H6H belongs to the 2-oxoglutarate/Fe2+-dependent dioxygenases (ODDs) family and converts hyoscyamine into scopolamine by catalysing two consecutive reactions: the hydroxylation of hyoscyamine to 6β-hydroxyhyoscyamine and its subsequent dehydrogenation to the final product scopolamine (Fig. 1).7 Notably, 6β-hydroxyhyoscyamine – the intermediate product of these transformations – has unique pharmacologi-cal properties that make it an attractive drug, especially for the treatment of organophosphate poisoning.8,9 The formation of this product follows an OH rebound mechanism,10 which is the most established among ODDs. The epoxidation reaction mechanism leading to the final product is more elusive and has already been widely investigated.
A study of H6H from Atropa belladonna indicated that 6β-hydroxyhyoscyamine is not the sole substrate for the reaction as the enzyme was also shown to catalyze the direct epoxidation of 6,7-dehydrohyoscyamine to scopolamine.12 Recently, hyoscyamine diol derivatives with hydroxyl groups at C6 and C7, as well as a substrate analogue with the bicyclic moiety enlarged by one CH2 group, were tested as potential substrates of H6H from Hyoscyamus niger.13 None of the tested diols was converted into epoxide, which argues against the dehydration reaction as the last step of scopolamine biosynthesis and further corroborates the mechanism whereby 6β-hydroxyhyoscyamine is directly converted into scopolamine with preservation of the hydroxyl oxygen.14 Instead, the trans diol was oxidised to 7-keto-6β-hydroxyhyoscyamine, whereas the substrate analogue with an enlarged ring was oxidised to alcohols and diols but not epoxides. These latter findings indicate that H6H abstracts exclusively exo hydrogens from C6 or C7 and the epoxidation reaction requires syn-periplanar arrangement between the hydroxyl group and the adjacent H-atom to be abstracted by ferryl species.
Other enzymes from the ODD family are well known to catalyze multiple reactions in sequence, e.g. trifunctional clavaminic acid synthase (CAS) from Streptomyces clavuligerus converts deoxyguanidinoproclavaminate into clavaminate in the biosynthesis of clavulanic acid, an inhibitor of serine β-lactamases.15–17 Bifunctional LolO oxygenase from endophytic fungi Neotyphodium uncinatum catalyses the conversion of 1-exo-1-acetamidopyrrolizidine to N-acetylnorloline in the biosynthesis of loline alkaloids, which show wide antiparasitic effects against invertebrate herbivores in plants.18–20 Finally, AsqJ from Aspergillus nidulans performs two oxidative reactions on 4′-methoxycyclopeptin (desaturation and epoxidation), thus leading to the formation of a quinolone antibiotic.21–23 This peculiar feature of various reactions catalyzed by a single enzyme remains of high interest for chemists, as it raises a fundamental question about the origin of enzymatic reaction specificity, as well as for biochemists focused on performing multiple step reactions in one-pot biosynthesis.
Although extensive studies have been performed on H6H originating from numerous species of the Solanaceae family: Anisodus spp.,24–26 Atropa spp.,12,27–30 Brugmansia spp.,31,32 Datura spp.,33,34 Duboisia myoporoides,35 Hyoscyamus spp.,7,36–40 Mandragora spp.41 and Scopolia spp.;6,42,43 so far no structural information has been reported for this enzyme. Here, we fill this gap and present crystal structures of full-length H6H from Datura metel complexed with N-oxalylglycine and hyoscyamine (H6H:NOG:Hyo, 1.91 Å) as well as its N-terminally truncated form in complex with 2-oxoglutarate (tH6H:2OG, 1.31 Å) and N-oxalylglycine and hyoscyamine (tH6H:NOG:Hyo, 1.12 Å). The factors determining the H6H reaction preference towards hydroxylation vs. desaturation were investigated by QM/MM methods. The regioselectivity of hydroxylation catalysed by H6H was further studied and residues crucial for hydroxylation at the C6 vs. C7 position of the hyoscyamine tropane moiety were identified. Since 7β-hydroxyhyoscyamine is also observed as a natural product of the H6H reaction,10,40 albeit only as a tiny fraction when compared to 6β-hydroxyhyoscyamine, it indicates that it may be possible to tune the product specificity of the enzymatic reaction by redesigning the H6H substrate binding pocket. Hopefully, detailed structural information about H6H revealed herein will allow for rational redesign of the enzyme into a more robust biocatalyst and spawn further fundamental studies on this bifunctional enzyme.
Results
Crystal structures of the full length H6H and N-terminally truncated H6H
Three crystal structures of H6H from D. metel were determined by molecular replacement and refined: full-length H6H complexed with NOG and hyoscyamine at 1.91 Å resolution, tH6H in complex with 2-OG at 1.31 Å resolution, and tH6H with NOG and hyoscyamine at 1.12 Å resolution. The H6H and tH6H complexes crystallized in space group P212121 with one monomer in the asymmetric unit and estimated solvent contents of 48.8%, 46.5% and 46.7% for H6H:NOG:Hyo, tH6H:2OG and tH6H:NOG:Hyo, respectively.44,45
The determined structure of full-length H6H revealed the flexible nature of 32 N-terminal residues (35 including Ser-Asn-Ala remaining after cleavage of a purification tag by TEV protease). No interpretable electron density is observed for this part of the protein. Later, this part was removed from the H6H construct used for crystallization trials. There is also no clear electron density for two loops (121–126, 208–214 in H6H:NOG:Hyo; 122–126, 208–215 in tH6H:NOG:Hyo) and a few residues at the N- and C-termini. Interestingly, these two loops are discernible in the tH6H:2OG density, although they are modelled with high B-factors. Thus, it may be suspected that their increased flexibility is related to substrate recognition and binding. The N-terminal truncation of the polypeptide chain did not influence the overall protein fold. The structures of H6H:NOG:Hyo and tH6H:NOG:Hyo can be superposed with a r.m.s.d. of 0.43 Å based on 297 aligned Cα-atom pairs.46,47 A comparison of full-length H6H and tH6H with respect to binding modes of NOG and hyoscyamine shows that the active site arrangement is well preserved, therefore further discussion will be focused on the structural features of tH6H, because of the higher resolution of the structure.
The overall structure of tH6H
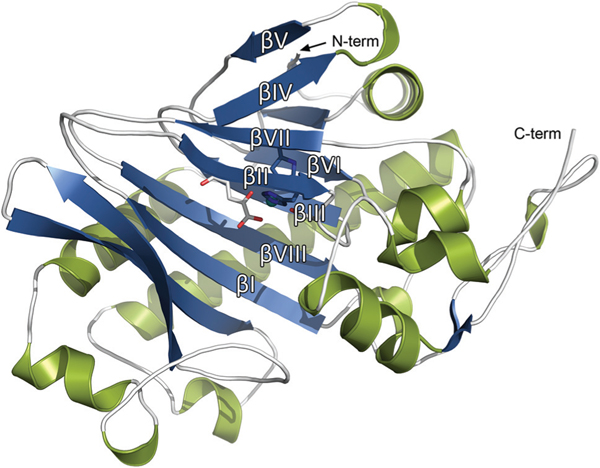
The protein folds into a double-stranded β-helix (DSBH) motif, the so called “jelly-roll” fold, which is typical and highly conserved in the ODD family.48 The DSBH core is composed of eight antiparallel β-strands divided into a major (βI, βIII, βVI, βVIII) and minor β-sheet (βII, βIV, βV, βVII) (Fig. 2). The secondary structure of tH6H is composed of 14 β-strands that are connected by a series of loops, α- and 310 helices. The major β-sheet of the DSBH motif is extended by four β-strands at the N-terminal region.
Fig. 2.
Overall view at tH6H:2OG secondary structure in STRIDE interpretation with DSBH fold labelled.52,53
The structure of tH6H is additionally stabilized in two ways – by one disulphide bond and by Sr2+ ions from the crystallization cocktail. The latter, which was identified based on an anomalous signal in the diffraction data collected on tH6H: NOG:Hyo crystals (Table S4†), stabilizes the crystal contacts by bridging the side chains of Glu-92*, Gln-95*, the main chain of Gly-98*, Gly-67 and three water molecules. An additional electron density peak that may also be interpreted as Sr2+ is found in the tH6H:2OG and tH6H:NOG:Hyo structures. It was finally assigned as Sr2+ and refined with lower occupancy only in the case of the tH6H:2OG complex. This additional Sr2+ ion is located in close proximity to Lys-303 and Asp-240. In the case of the tH6H:NOG:Hyo complex, only weak anomalous signal is present at this position and the coordination sphere does not clearly indicate that the ion is Sr2+, so it was finally assigned as an unknown atom (UNX). The stabilizing disulphide bond is formed between Cys-121 and Cys-205 and is located in close proximity to the flexible loops.
There is a relatively low sequence identity between H6H and the closest homologs with structures deposited in the PDB, though structural similarities are apparent, especially to: thebaine 6-O-demethylase (T6ODM, PDB ID: 5O7Y; 32.3%),49 anthocyanidin synthase (ANS, PDB ID: 2BRT; 29.8%)50 and feruloyl-CoA 6-hydroxylase (F6H, PDB ID: 4XAE; 28.9% amino acid sequence identity).51 The similarity manifests itself mainly in the DSBH core and in the helical region in the middle of the scaffolding.
Active site and co-substrate binding pocket of tH6H complexes
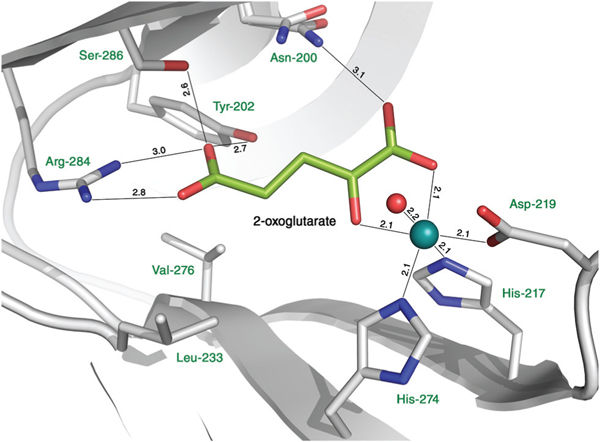
A Ni2+ ion, which is the surrogate for the natively present Fe2+ ion, is coordinated by the side chains of His-217 (strand βII), Asp-219 (loop βII/βIII) and His-274 (strand βVII) that form a metal binding His-X-Asp⋯His motif, which is highly conserved in the ODD family (Fig. 3). Both 2-OG and NOG coordinate the metal ion in a bidentate manner. The metal coordination sphere is completed by a water molecule. The hydrophobic residues Leu-226, Leu-233 and Val-276 line the co-substrate binding pocket along with Asn-200, Tyr-202, Arg-284 and Ser-286 that form hydrogen bonds with 2-OG and NOG. Comparing the NOG and 2-OG binding modes of tH6H and the closest homologs with known 3D structures (T6ODM, ANS, F6H), only Leu-226 and Val-276 present in tH6H are exchanged to other residues, yet all exchanges are conservative. In the ANS structure, Leu-226 of tH6H is replaced by isoleucine, while Val-276 present in tH6H is substituted by the smaller alanine in the case of T6ODM and by glycine in the case of ANS.
Fig. 3.
Active site of tH6H:2OG complex. The nickel ion is shown in cyan. For clarity of visualization only the water molecule that is directly involved in metal coordination is shown.
Metal in the active site of tH6H
ICP-OES analysis revealed a high nickel content and the residual presence of iron in tH6H protein samples. To fully confirm that nickel is present in the active center of tH6H: NOG:Hyo, energy scans were performed in the vicinity of nickel and iron absorption K-edges (Fig. S2†) followed by data collection at 7.05 keV, 7.25 keV, 8.24 keV and 8.44 keV.54 The ratios of metal/sulfur peak values in anomalous difference maps were compared with theoretical values calculated with CROSSEC implemented in CCP455 and they were consistent with the presence of nickel (Table S4†). Consequently, a nickel ion was modelled in the active sites of tH6H:2OG and tH6H: NOG:Hyo. Previous study on ODDs showed that iron to nickel substitution does not significantly change the active site arrangement.56
Hyoscyamine binding cavity in tH6H:NOG:Hyo
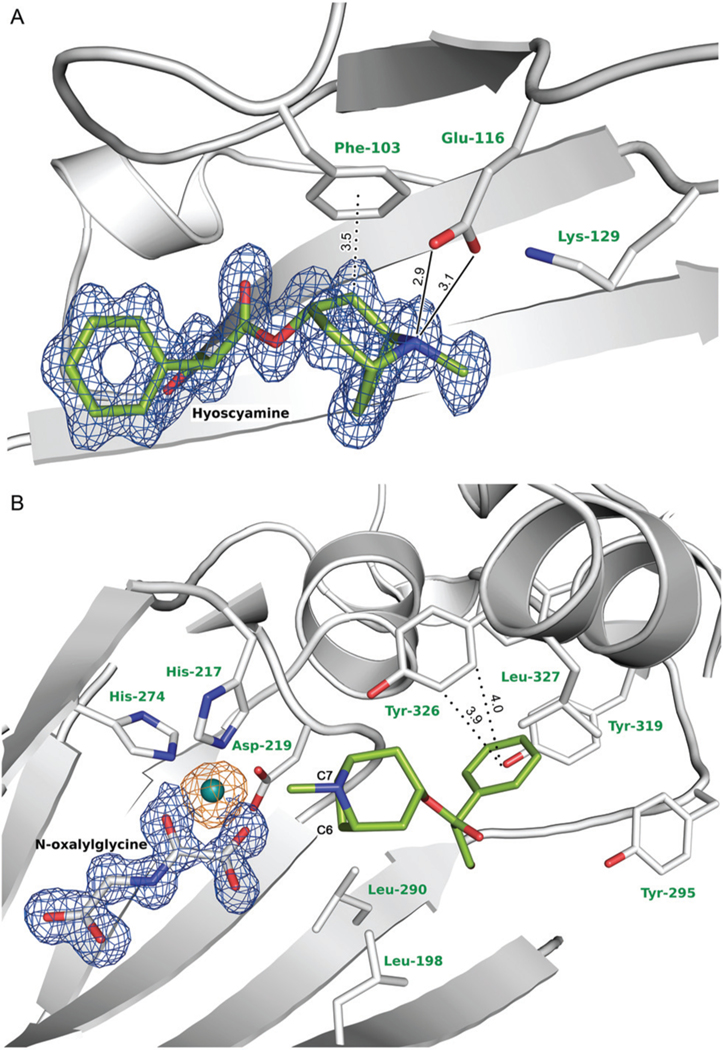
Hyoscyamine is clearly visible in the electron density map with average B-factor of 12.5 Å2. The carbon C7 of the hyoscyamine tropane moiety is located at a distance of 4.5 Å to the metal ion, while C6 is slightly further away with a distance of 4.8 Å. The binding cavity of hyoscyamine is formed mostly by hydrophobic amino acids with some key exceptions (Fig. 4). The phenyl ring of hyoscyamine is bound in an aromatic cage formed by Phe-103, Tyr-295, Tyr-319, Phe-322 and Tyr-326, of which the most prominent is Tyr-326 which forms CH–π hydrogen bonds with the phenyl ring of hyoscyamine in an edge-to-face bidentate manner (Fig. 4(B)). The tropane moiety is stabilized by hydrophobic interactions with Leu-198 and Leu-290, and a CH–π hydrogen bond with Phe-103 (Fig. 4(A)), while Glu-116 takes part in a direct electrostatic interaction with the tertiary amine group located on the tropane moiety. Glu-116 is located between two lysine residues (Lys-129 and Lys-330) that stabilize the arrangement of Glu-116 and hyoscyamine through water bridges. Notably, Glu-116 and Lys-129 are located in close proximity to the loop 122–126 that was not modelled due to lack of interpretable electron density. Comparison of the tH6H:2OG and tH6H:NOG:Hyo structures clearly indicates that Glu-116 shifts towards hyoscyamine upon substrate binding. This shift enables hydrogen bonding with distances of 2.9 Å and 3.1 Å between the Glu-116 carboxyl group and hyoscyamine tertiary amine group. Three additional water molecules present in close proximity to the OH-group of hyoscyamine further reduce the hydrophobic character of the binding pocket.
Fig. 4.
The active center and the hyoscyamine binding cavity. Hyoscyamine and NOG are shown with the 2mFO-DFC electron density map contoured at 1 r.m.s.d. (in blue), while the anomalous difference electron density map based on the data collected at 8.44 keV and indicating the presence of nickel ion is contoured at 5 r.m.s.d. (in orange). Water molecules have been omitted for clarity. Hydrogen and CH–π hydrogen bonds between hyoscyamine and Phe-103, Glu-116 and Tyr-326, main residues involved in the ligand binding, are shown as solid and dotted lines, respectively. The hyoscyamine binding pocket can be inspected interactively at molstack.bioreproducibility.org/project/view/jrLcoq1txzy6kbIzCkt8/(accession code: CW7U),57 while detailed hyoscyamine binding pocket analysis is shown in Fig. S3.†
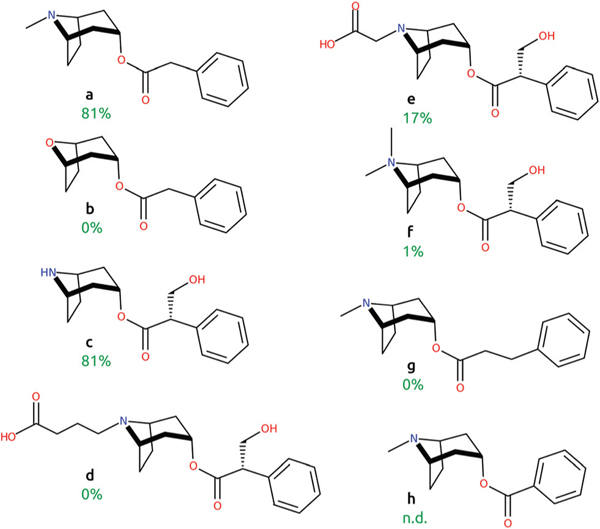
Notably, previous studies showed 81% of H6H activity towards phenylacetyltropine (Fig. 5, compound a), although when the amino group of the phenylacetyltropine tropane moiety was replaced with oxygen (b), neither enzymatic activity nor inhibition of H6H was detected.12,58 The latter substrate modification involves the removal of the methyl group bound to the heteroatom and a change of the ionizable amino group into the non-ionizable ether group. Interestingly, H6H shows substantial (81%) activity against norhyoscyamine (c), which substitutes the methyl group of the tertiary amine in hyoscyamine with hydrogen, and thus contains a secondary rather than tertiary amine. Also, hyoscyamine analogs with bulky modifications to the tropane moiety were previously investigated as H6H substrates,58 yet they were processed by the enzyme very slowly or not at all. Relative activities of 0%, 17% and 1% as compared to hyoscyamine were reported towards noratropine-N-butyric acid (d), noratropine-N-acetic acid (e) and atropine methyl bromide (f). These previous results and this current study indicate a high importance of the interaction between Glu-116 and the ionizable and presumably positively charged amino group of hyoscyamine, since removal of the amine completely abolished H6H activity and ligand binding. H6H activity towards compounds of different length (with an added or removed methylene group between the phenyl ring and ester group) was also previously studied.12 Extending the molecule by one additional CH2 group (g) resulted in a loss of the enzymatic activity of H6H, while shortening the linker by one CH2 group (h) gave very low conversion yields, disabling its quantification by HPLC. Taken together, these modifications of hyoscyamine most likely adversely affected the synergy between the two key hyoscyamine – protein interactions: the phenyl ring – hydrophobic pocket and the amino group – Glu-116.
Fig. 5.
Comparison of previously studied hyoscyamine analogues (a – phenylacetyltropine, b – 8-Oxabicyclo[3.2.1]octan-3-yl 2-phenylacetate, c – norhyoscyamine, d – noratropine-N-butyric acid, e – noratropine-N-acetic acid, f – atropine methyl bromide, g – phenylpropionyltropine, h – benzoyltropine).12,58 Relative activities towards selected ligands are indicated in green. N.d. stands for not determined.
Recently, a detailed analysis of the sequence and activity of H6H from Mandragora species suggested that the Gly-220-Cys substitution may result in a deactivation of H6H.41 Mandragora officinarum, which harbors this substitution, accumulates high amounts of hyoscyamine and, at the same time, produces neither anisodamine nor scopolamine. As the mutation site is directly next to the metal-ligating Asp-219, the substitution may affect the active site arrangement. In the structure of tH6H from D. metel, Gly-220 is located in close proximity to the phenyl ring of hyoscyamine. The substitution of the small glycine residue by the more bulky cysteine may disturb hyoscyamine binding through re-arrangement of the hydrophobic cavity. In particular, the closely placed Tyr-326 may be amenable to a conformational change and, as a result, stabilization of the hyoscyamine phenyl ring may be diminished.
Hydrogen atom transfer – reaction mechanism as revealed by QM/MM
The QM/MM study of H6H catalysed hydroxylation of hyoscyamine was performed on an Fe containing model of the protein. As has been mentioned above, Fe2+ is the natural cofactor of the enzyme. After a 20 ns MD simulation of the tH6H:2OG:Hyo complex, the bond lengths between Fe2+ and its first shell ligands remained within 0.1 Å of the lengths observed in the crystal structure of the nickel-containing tH6H:2OG complex. The QM/MM optimisation of the complex resulted in a slight increase of the distance between Fe2+ and the oxo O atom of 2OG, from 2.1 Å to 2.3 Å (for details see Fig. S4†). However, this value remains in reasonable agreement with the one determined experimentally. Moreover, comparison of the geometry of the QM/MM optimised active site of tH6H with the geometries of X-ray structures of other known ODDs with Fe2+ modelled in the active site: ANS (PDB ID: 1GP5),50 CAS (PDB ID: 1DRY)16 and carbapenem synthase (CarC, PDB ID: 1NX4),59 shown in Fig. S4C–S4E,† confirmed that the Fe to Ni substitution has a limited impact on the geometry of the active site, which lends credence to the employed computational approach.
In the QM/MM optimised structure of the activated enzyme–substrate complex (S) the ferryl species is six coordinated with its oxo group located trans to His-274 and a water ligand bound trans to His-217. Indirect evidence for water coordination comes from a recent kinetics study, which revealed substantial incorporation of solvent-derived oxygen into the hydroxylation product.40 The C6-bound exo H atom of hyoscyamine is positioned 2.23 Å from the oxo ligand, whereas the C7-bound exo H atom is located slightly further at a distance of 2.84 Å (3.18 Å and 3.50 Å for C6–O and C7–O distances, respectively). The position of the C6-bound H atom is more appropriate for the initial hydrogen atom transfer (HAT) as the Fe–O⋯H angle equals 141°, which facilitates entering the usually energetically preferred σ-channel.60,61 The Fe–O...H angle for the C7-bound H totals to only 94°, which seems more likely to invoke the π-channel usually associated with a higher energy barrier.62
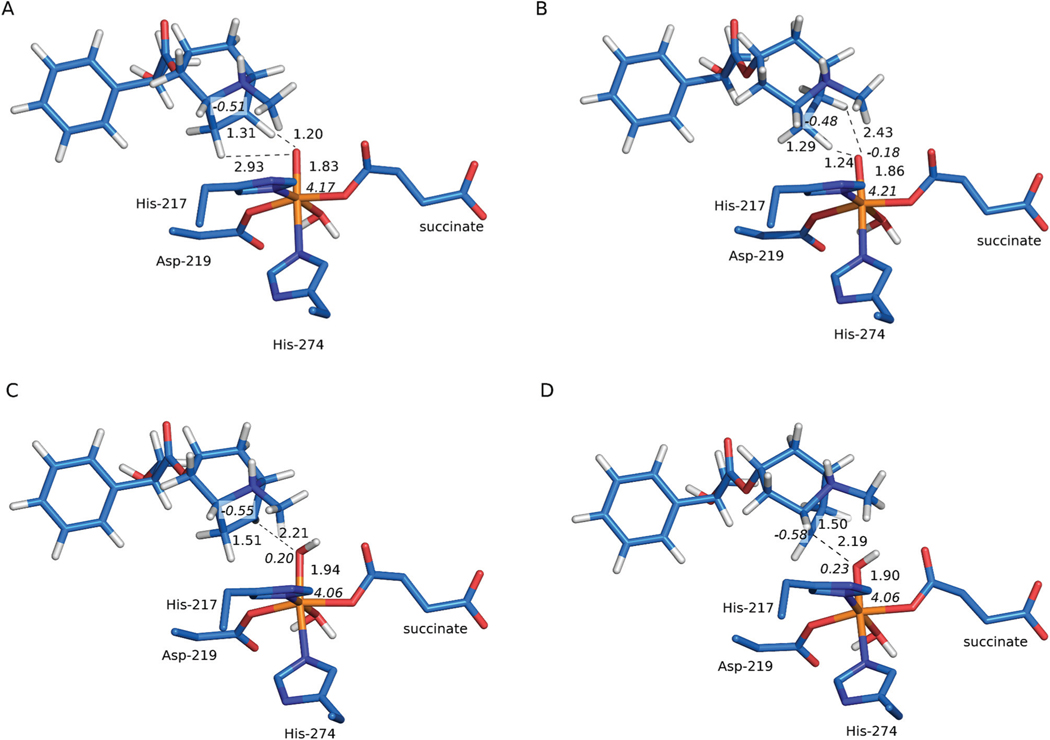
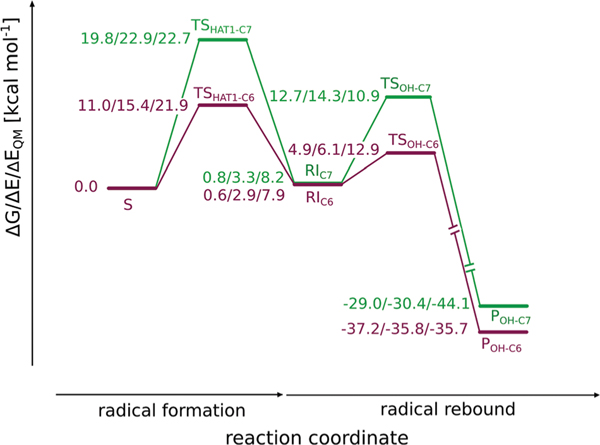
The transition state structure associated with HAT at the C6 position (TSHAT1-C6) requires only a modest shift of the substrate, the H⋯O distance shortens to 1.20 Å and the C6–H bond length increases to 1.31 Å (see Fig. 6(A)). The Gibbs free energy barrier for the process totals to 11.0 kcal mol−1 (as shown in Fig. 7). The spin populations on Fe (4.17) and the C6 atom of the substrate (−0.51) are consistent with the σ-channel pathway, which is also reflected by the geometry of the transition state structure, i.e. Fe–O⋯H angle, which is 129° and the elongated Fe–O bond (from 1.63 Å in S to 1.83 Å in TSHAT1-C6). Formation of the radical intermediate (RIC6) is slightly endoergic by 0.6 kcal mol−1. In the intermediate the spin density is located on C6 (−0.96) and the high-spin Fe3+ (4.25), in accordance with the electronic structure of TSHAT1-C6.
Fig. 6.
QM/MM optimised structures for TSHAT1-C6 (A), TSHAT1-C7 (B), TSOH-C6 (C) and TSOH-C7 (D). Distances are given in Å and spin populations of absolute value larger than 0.1 are given in italics. The figure was rendered with PyMOL,63 for clarity only the QM region and the MM carboxylate of succinate is shown.
Fig. 7.
Reaction energy profiles for H6H-catalysed hydroxylation at the C6 and C7 position. The energy values, i.e. Gibbs free energy (ΔG), relative potential energy (ΔE) calculated at the ONIOM(B3LYP-D3/def2-TZVP, Amber) level with electronic embedding and the relative potential energy of the QM part (ΔEQM) obtained with the B3LYP-D3/def2-TZVP method (mechanical embedding) are given in kcal mol−1.
Alternatively, HAT at the C7 position (TSHAT1-C7) requires crossing a significantly higher ΔG barrier of 19.8 kcal mol−1 (consult Fig. 7). The geometry of the transition state structure features an Fe–O⋯H angle of 110°, which is characteristic for the π-channel pathway.64 The spin populations on Fe (4.21) and the C7 atom of the substrate (−0.48) indicate that the process proceeds via the S = 5/2 Fe3+ mechanism and an investigation of the natural orbitals for spin density (shown in Fig. S-C6(C) and (D)†) reveals that the α electron of the σC–H orbital is accepted by the π*(Fe-dxz/yz) orbital. This is consistent with the π-channel with S(Fe) = 5/2 and S(substrate) = −1/2, which is enabled by mixing of the ground state with an excited state, the latter characterised by an α-dxz/yz electron excited into the α-dz2 orbital.65 In the case of the H6H catalysed reaction, the excitation is simultaneous with hydrogen atom transfer. A slightly obtuse angle that facilitates overlap between the σC–H and the π*(Fe-dxz/yz) orbitals is obtained thanks to a moderate shift of the tropane ring (see Fig. S-C8†). The geometry of the transition state structure features a relatively short O⋯H distance of 1.24 Å and C7–H bond elongated to 1.29 Å (as presented in Fig. 6(B)). Free energy of the resultant β radical – Fe3+ (S = 5/2) complex RIC7 (0.8 kcal mol−1) is comparable with that for RIC6 (0.6 kcal mol−1). The spin density in the intermediate totals to 4.26 on Fe3+, and −0.98 on the C7 radical.
The transition state leading to the S = 3/2 Fe3+ and α carbon-centered radical (TS′HAT1-C7) lies 6.9 kcal mol−1 higher in ΔG than TSHAT1-C7. The resultant intermediate spin Fe3+ species RIC7(π) is relatively unstable with ΔG of 17.8 kcal mol−1 and undergoes a relaxation to RIC7(σ).
The barrier associated with HAT, at the C6 position is rate-determining, which is a common feature of Fe4+-oxo catalysed reactions.66–70 Also, the height of the barrier (11.0 kcal mol−1) aligns with those previously reported for aliphatic hydrogen atom abstraction by nonheme iron dioxygenases, which span from ca. 10 kcal mol−1, cf. flavanone-3β-hydroxylase (8.4 kcal mol−1),71 AlkB (9.0 kcal mol−1)72 or CarC (9.9 kcal mol−1)70 to ca. 20 kcal mol−1.73,74 Similar values were also obtained for cytochrome P450 catalysed reactions75–77 and synthetic nonheme iron-oxo complexes.78,79
This reaction step also determines the regioselectivity of the overall process as TSHAT1-C6 lies ca. 8.8 kcal mol−1 lower in energy than TSHAT1-C7, thus favouring reaction at the C6 position. Experimental results show that a minor (2% of total yield) C7 hydroxylated side product is also formed in vitro,10 which indicates that HAT at C7 is also feasible, however to a much lower extent. Through transition state theory, this product ratio corresponds to ΔΔG‡ = 2.3 kcal mol−1. The calculated ΔΔG between the two barriers is too high to explain this observation quantitatively, therefore it is tempting to speculate that the substrate can adopt another (less stable) position within the binding cavity, which is more favourable for HAT at the C7 position.
Factors favouring HAT at C6
The difference in barriers obtained for the C6 and C7 reaction channels can be attributed to the electronic properties of the reactants, i.e. the substrate and iron cofactor, as well as electrostatic and van der Waals interactions between the substrate and the binding cavity of the protein. Comparison of the QM-only energies of TSHAT1-C6 and TSHAT1-C7 obtained at the B3LYP-D3/def2-TZVP level with mechanical embedding (see Table S-C4†) indicates that the inherent properties of the “reactants” favour HAT at C6 by ca. 0.8 kcal mol−1. Bond dissociation energies (BDEs) of C6–H and C7–H bonds of hyoscyamine are very similar (94.3 kcal mol−1 and 94.0 kcal mol−1, respectively), which is to be expected as the tropane ring is a meso entity. Even though the position of the substrate with respect to the active site is such that the α electron of the cleaved C–H bond is accepted by different orbitals of oxoferryl, i.e. α-σ*(Fe-dz2) (for HAT at C6) and α-π*(Fe-dxz/yz) (for HAT at C7), the inherent preference towards HAT at C6, expressed by this 0.8 kcal mol−1 difference in QM-only contributions to barrier heights, is only slight. A DFT study for the model complex (1,1,1-tris{2-[N2-(1,1,3,3-tetramethylguanidino)]ethyl} amine, TMG3tren) Fe4+=O has also shown that the σ- and π-channel pathways may be associated with similar barriers.65,72
The specific substrate-cofactor disposition observed for TSHAT1-C7 with an Fe–O⋯H angle of 110° and a slightly shifted tropane ring, which moderately increases the inherent barrier for C7–H bond activation, is obtained only when the binding pocket of the protein is taken into account. Optimisation of the two transition states using a minimal cluster model, consisting only of the substrate and the first coordination shell of iron, revealed that in TSHAT1-C7 the substrate can adopt a position that facilitates HAT following the σ-pathway channel and such a TS is associated with a barrier of 9.9 kcal mol−1, which is 3.2 kcal mol−1 lower than the respective barrier for TSHAT1-C6. The difference can be attributed to the different positions of the substrate, which result in varied interactions between the substrate and the first shell ligands of the iron cofactor.
The role of the binding cavity is not only to impose the geometry of the enzyme–substrate complex, but also to stabilise the transition state associated with HAT at the C6 position and possibly also to differentiate further between the barriers for C6 and C7 HAT. Incorporating partial charges into the QM description (electronic embedding) lowers the QM-only barrier of TSHAT1-C6 by 8.6 kcal mol−1, which corresponds to the stabilizing effect of QM-MM electrostatic interactions. For TSHAT1-C7 this stabilization is much smaller, i.e. 3.6 kcal mol−1. Therefore, in the H6H catalysed hydroxylation reaction the C7 pathway is impeded by the protein environment (second coordination shell residues), which fits into the negative catalysis paradigm.80 Similar effects have been described for flavonol synthase (FLS), also a member of the ODD family,81 and enoyl-thioester reductase Etr1p.82 For the latter protein, a single residue was pinpointed as responsible for the effect of negative catalysis.
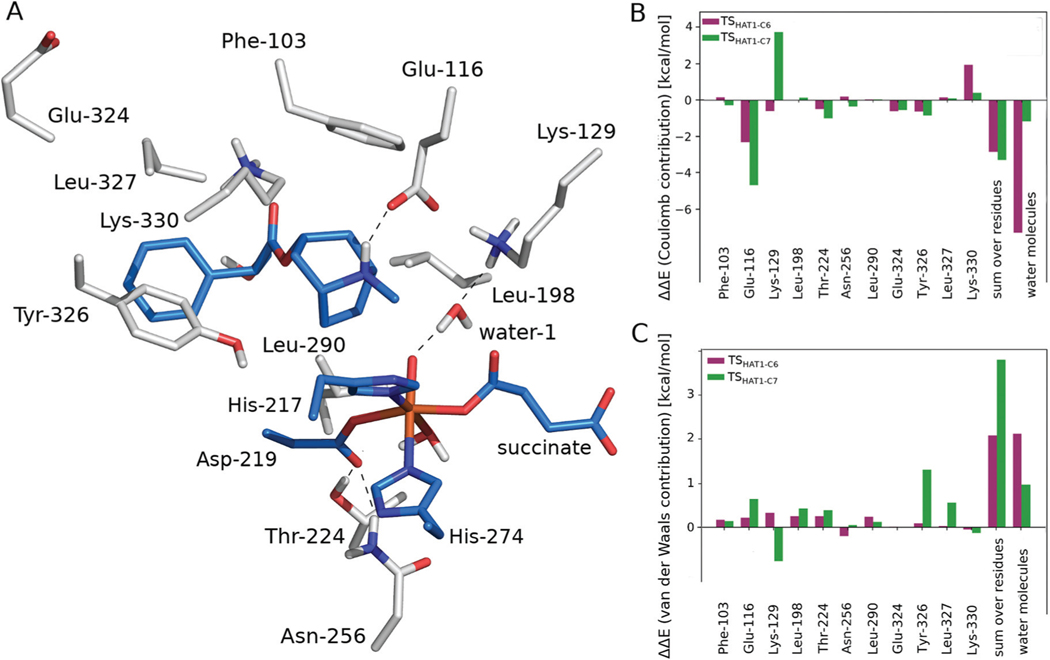
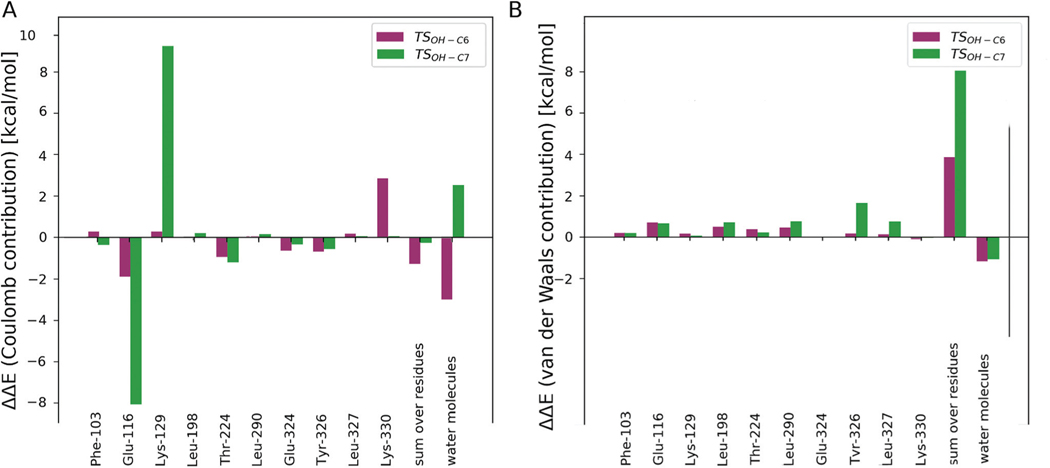
To assess the contributions of individual amino acid residues towards tuning barriers heights, amino acid side chains in the vicinity of the active site and water molecules surrounding the substrate located within 6 Å of hyoscyamine or the Fe ion, were sequentially removed from the MM part and such “computational variants” were subjected to ONIOM calculations. The results (presented in Fig. 8) indicate that the stabilisation of TSHAT1-C6 comes mostly from electrostatic interactions with adjacent waters (it totals to 7.3 kcal mol−1 and does not differ much from the stabilisation effect calculated for all waters in the MM part, i.e. 7.7 kcal mol−1). The most pronounced contribution comes from a water molecule that is hydrogen bonded to Lys and the oxo ligand (6.2 kcal mol−1). The presence of this water molecule could be partly responsible for the regioselectivity of HAT, as its electrostatic contribution to the ONIOM energy of TSHAT1-C7 is markedly less stabilising (by 1.0 kcal mol−1). Notably, a water molecule in this position was observed in the crystal structure and it remained there during 20 ns of MD simulation. To further examine the effect of this water molecule, additional calculations at the ONIOM(B3LYP-D3/def2-TZVP, Amber) level were performed with electronic embedding incorporating the water and the adjacent Lys-129 into the QM part. As a result, the barrier for HAT at the C6 position was lowered by 0.1 kcal mol−1 and the barrier for HAT at C7 increased by 1.4 kcal mol−1 (see Table S-C5†), thus confirming that the model used here is capable of reproducing most of the stabilisation/destabilisation effects of these key second shell residues.
Fig. 8.
The amino acid side chains (shown as grey sticks) interacting directly with the substrate or the iron ligands (blue sticks) (A). The Coulomb (B) and van der Waals (C) contributions of the presented residues’ side chains to the relative potential energy barrier (ΔE, calculated at the ONIOM (B3LYP-D3/def2-TZVP, Amber) level with mechanical embedding) for TSHAT1-C6 and TSHAT1-C7.
The amino acid residues that are involved in electrostatic interactions with the QM region generally lower both barriers by a comparable amount (ca. 3 kcal mol−1). However, some of the amino acid residues should be highlighted as possibly responsible for the preference of C6 as the reaction site.
The amino acid residue whose effect on the ONIOM energy differs noticeably for TSHAT1-C6 versus TSHAT1-C7 is Tyr-326, making it a promising target for mutagenesis aimed at switching the regioselectivity of HAT. This residue stabilises the first TS by 0.5 kcal mol−1 and destabilises the latter by 0.4 kcal mol−1. The difference originates from van der Waals interactions between the residue and the tropane ring of the substrate. In the structure for TSHAT1-C7 the ring moves away from Tyr-236 to facilitate O–H bond formation, which weakens vdW interactions (as shown in Fig. S-C8†). Also, Lys-129 has an impact on the energetics of HAT via stabilisation of the TS associated with the reaction at C6 (−0.6 kcal mol−1) and destabilisation of the one involving C7 (3.8 kcal mol−1). The majority of the effect comes from electrostatic interactions and can be again attributed to the shift of the positively charged tropane ring toward the Lys-129 side chain in TSHAT1-C7. This observation suggests that these two residues, i.e. Tyr-326 and Lys-129, might be partially responsible for the regioselectivity of HAT.
On the other hand, Lys-330 acts in an opposing way, increasing the ONIOM energy of TSHAT1-C6 (by 1.9 kcal mol−1, mostly via electrostatic interactions) and only slightly destabilising TSHAT1-C7 (by 0.3 kcal mol−1). Such behaviour makes it an interesting target, alongside Tyr-326 and Lys-129, for point mutation aimed at switching the regioselectivity of the H6H catalysed reaction.
Regioselectivity of oxygen rebound
In the optimised structure of the radical intermediate RIC6, the C6 atom hosting the unpaired electron is positioned 3.04 Å away from the O atom of the Fe3+–OH moiety, slightly closer than in the enzyme–substrate complex (cf. 3.18 Å C6⋯O distance in structure of S). For the C7-centered radical RIC7, the atom hosting the unpaired electron (C7) moves away from the OH moiety as compared to S (the C7⋯O distance increases from 3.50 to 3.68). Both geometries can facilitate the oxygen rebound, however the former seems better suited for the process.
The transition states associated with radical rebound at C6 (TSOH-C6) and C7 (TSOH-C7) feature a shortened C⋯O distance (2.21 and 2.19 Å, for TSOH-C6 and TSOH-C7, respectively, see Fig. 6(C) and (D)). In both cases the spin population on the radical carbon decreases to ca. −0.5. The QM-only energy barriers for the processes are also comparable, 10.9 and 12.9 kcal mol−1 for TSOH-C6 and TSOH-C7, respectively. This corresponds with similar C6/C7–O bond dissociation energies, i.e. 87.7 and 89.9 kcal mol−1, which is again to be expected for a meso entity.
Taking interactions within the binding pocket into account reveals that the OH-rebound reaction proceeding at the C6 position is facilitated (by 6.7 kcal mol−1) by the surroundings, whereas the oxygen rebound involving the C7 atom is destabilised (by 3.4 kcal mol−1). It is consistent with the C6/C7 regioselectivity during HAT described above and the rationale for this effect is also similar. In the optimised structure of TSOH-C6 the substrate hardly shifts its position to promote contact between the C6 atom and OH moiety (see Fig. 6(C)). Such a geometry is stabilised mostly by electrostatic interactions with Glu-116 and surrounding water molecules (as shown in Fig. 8 (A) and 9). On the other hand, making close contact between the C7 atom and the OH moiety at TSOH-C7 requires a shift of the tropane ring, which is analogous to the one observed for TSHAT1-C7 and leads to unfavourable van der Waals interactions with the adjacent Tyr-326 and destabilising electrostatic interactions with water molecules and Lys-129, although this effect is largely compensated by Glu-116 stabilising the position of the substrate (see Fig. 9).
Fig. 9.
The Coulomb (A) and van der Waals (B) contributions of the significant residues’ side chains (as shown in Fig. 8(A)) to the relative potential energy barrier (ΔE, calculated at the ONIOM(B3LYP-D3/def2-TZVP, Amber) level with mechanical embedding) for TSOH-C6 and TSOH-C7.
It can be summarized that for the most prevalent geometry of the enzyme–substrate complex, interactions between the “reactants”, i.e. the substrate and the iron cofactor, and the surrounding protein residues and water molecules determine the preference for C6 as the reaction site, whereas the electronic properties of hyoscyamine and the reactive ferryl species (alongside their relative positioning) do not dictate the regioselectivity of the reactions. The effects that determine regioselectivity originate from only a few residues (i.e. Lys-129, Tyr-326, Lys-330), which suggests that they could be promising targets for H6H redesign aiming at switching the oxygen rebound regioselectivity.
Discussion
The results provided here describe the crystal structures of H6H, a bifunctional ODD enzyme involved in the final steps of scopolamine biosynthesis in the Solanaceae family, at very high resolution. H6H catalyzes two reactions: hydroxylation of hyoscyamine and the subsequent dehydrogenation of 6β-hydroxyhyoscyamine to scopolamine. However, a small fraction of 7β-hydroxyhyoscyamine was previously detected in the experiments, which raises questions about H6H regioselectivity.10 Recently, Pan et al. showed that hydroxylation at the C7 position of hyoscyamine takes place in only 1.5% of cases, although the frequency increased to 31.4% when C6-deuterated hyoscyamine was used in the experiments.40 This effect diminished when hyoscyamine had deuterium at both C6 and C7 positions of the tropane moiety (hydroxylation at C7 again dropped to 1.8%). The fact that the presence of deuterium is able to alter the hydroxylation preference indicates that regioselectivity of the H6H reaction depends on a subtle competition between two pathways, a phenomenon which has been previously reported for other ODDs.73,83
The detailed structural information provided here allowed us to shed light on the molecular basis of H6H specificity towards hyoscyamine. We have established that Glu-116 is responsible for recognition of the tertiary amine located on the hyoscyamine tropane moiety. This interaction is further stabilized by two Lys residues (Lys-129 and Lys-330) and water bridges, whereas the phenyl ring of the substrate is trapped in aromatic surroundings and forms CH–π hydrogen bonds with Tyr-326. The shape and size of the cavity limit the length of the substrate. Shift of Glu-116 is observed after hyoscyamine binding in tH6H:NOG:Hyo as compared to the structure of the tH6H:2OG complex. As Glu-116 is responsible for recognition of the amine group, it is possible that a simple modification of this residue would allow the enzyme to accommodate a wider range of compounds.
The regioselectivity of H6H and its reaction mechanism were studied further with QM/MM methods. Partitioning the barrier heights into contributions from: (a) electronic energy of the substrate – iron cofactor (QM) subsystem, (b) electrostatic and van der Waals interactions between the QM subsystem and the residues from its nearest surroundings and (c) other interactions, revealed that most of the differences decisive for the observed regioselectivity of the H6H catalytic reaction come from electrostatic interactions between the QM subsystem and several key residues. This observation may serve as useful guidance for attempts to redesign the enzyme. Particularly interesting targets for mutagenesis are Lys-129, Lys-330 and Tyr-326, exchange of which may allow modification of hyoscyamine positioning in the binding pocket and switch the H6H regioselectivity towards hydroxylation at the C7 position of the tropane moiety. Consistent with the results of recent kinetic measurements,40 the QM/MM results indicate that both hydrogen atom transfer and the subsequent OH-rebound steps are substantially slower for C7 compared to C6 of hyoscyamine.
Materials and methods
H6H expression and purification
The codon-optimized DNA sequence of H6H from Datura metel (Uniprot ID: Q6EZB3) was synthesized by GenScript (Piscataway, NJ). The gene was later cloned into pMCSG19 by a ligation independent cloning protocol (LIC)84 and H6H expressed as MBP-(His)6-H6H fusion protein with a Tobacco Etch Virus (TEV) protease cleavage site between (His)6 and H6H. BL21(DE3) Magic Escherichia coli cells were transformed with pMCSG19_H6H plasmid and single colonies were picked up and grown overnight in 50 mL LB medium. After inoculation of the TB medium, cells were grown at 37 °C until OD600 reached approximately 1.5. After the culture was cooled to 16 °C, overproduction of H6H was induced with 0.2 mM IPTG and performed overnight.
The cells were harvested by centrifugation at 4000 rcf for 1 hour. Pellets were resuspended in 50 mM Tris-HCl, 500 mM NaCl, pH 7.8, 10% (v/v) glycerol and, after incubation on ice with lysozyme (Carl Roth; cat. no. 8259) and DNAse (Roche; cat. no. 10104159001), homogenized in the presence of protease inhibitor cocktail (Roche; cat. no. 5056489001). The cell debris was then removed by centrifugation at 40 000 rcf for 1 hour and the supernatant was applied to Ni-NTA Superflow resin (Qiagen; cat. no. 30430) in a HiScale 16/20 column (GE Healthcare; cat. no. 28964441) attached to a ÄKTA Start Protein Purification System. The column was washed with 10 column volumes of 50 mM Tris-HCl, 500 mM NaCl, 10 mM imidazole, pH 7.8, 5% (v/v) glycerol and finally MBP-(His)6-H6H fusion protein was eluted with 50 mM Tris-HCl, 500 mM NaCl, and 250 mM imidazole, at pH 7.8, 5% (v/v) glycerol.
The MBP-(His)6-H6H fusion protein was dialyzed overnight against 50 mM Tris-HCl, 500 mM NaCl, pH 7.8, 5% (v/v) glycerol with addition of (His)6-TEV protease. MBP-(His)6 and (His)6-TEV were removed by an additional purification step on a nickel affinity column, whereas flow through that contained H6H was concentrated and loaded on a Superdex 75 16/600 column attached to a Bio-Rad NGC Chromatography System. Fractions containing H6H were pooled together and concentrated using centrifugal filter units with 10 kDa MWCO (Millipore; cat. no. UFC9010). The concentration of H6H was estimated by absorbance at 280 nm with an extinction coefficient as calculated by protparam.85 The purity of samples was estimated by SDS-PAGE.86
tH6H expression and purification
Recently, Fischer et al. indicated that truncation of H6H from A. belladonna at the N-terminus would facilitate obtaining a more compact protein without changing the activity of H6H.31 Our XtalPred analysis on the crystallizability of H6H from D. metel confirmed that this disordered and flexible N-terminal region may hinder H6H stabilization and crystallization.87–89 With this in mind, we truncated the H6H from D. metel by 33 amino acids at the N-terminus. A H6H gene lacking 99 nucleotides was cloned into a pMCSG19 vector using LIC protocol (Fig. S1 and 2†). As described previously, BL21(DE3)Magic E. coli cells were transformed with pMCSG19_tH6H plasmid and single colonies were picked up. Aiming to produce protein with a nickel ion in the active center, tH6H production was performed in minimal medium supplemented with vitamins and NiCl2.90,91 100 mL of the minimal medium supplemented with the vitamin cocktail was inoculated with BL21(DE3)Magic harboring pMCSG19_tH6H from an overnight culture in LB medium. This additional intermediate step was performed to minimize the iron concentration in the medium at the protein production step. Finally, cells were grown at 37 °C in 1L of the minimal medium supplemented with the vitamin cocktail until OD600 reached approximately 1. After the culture was cooled to 16 °C, overproduction of H6H was induced with 0.2 mM IPTG. After 10 minutes, NiCl2 was added to a final concentration of 2.5 μM and protein production was continued overnight. tH6H samples were purified as described previously for H6H. Again, the concentration of tH6H was estimated by absorbance at 280 nm and the purity of samples was estimated by SDS-PAGE.
Crystallization
Preliminary crystallization conditions were examined by a sitting-drop vapour diffusion method set up by hand or with use of Mosquito (TTP Labtech) and Crystal Phoenix (Art Robbins Instruments) protein crystallization robots at 4 °C or 20 °C with the following commercially available crystallization screens: Index, Crystal, Salt Rx, PEG/Ion (Hampton Research), Morpheus, PACT premier, LMB, Midas, PGA, SG1 (Molecular Dimensions), Nucleix (Qiagen), MCSG, PurePEGs (Anatrace). Diffracting crystals of H6H were grown from only one crystallization condition (Index screen 90: 200 mM sodium formate, 20% (w/v) PEG 3350) after 9 months at 4 °C. The crystallization condition was further optimized. Addition of JBScreen Detergents and JBScreen Buffers was tested (Jena Bioscience), but crystal quality was not improved. Setting up an Additive Screen (Hampton Research) pointed to SrCl2 as a stabilizing reagent that allowed us to obtain crystals diffracting up to 2 Å resolution in less than a month. During subsequent experiments, the concentration of SrCl2 was optimized.
The tH6H crystallization was carried out using conditions that yielded diffracting H6H crystals. Crystallization drops were set up by hand on polystyrene MRC Maxi 48-well Plates (Swissci) in protein-reservoir ratio 1 μL : 1 μL and placed at 20 °C. tH6H:NOG:Hyo seeds were prepared with Crystal Crusher and Seed Bead (Hampton Research). Series of dilutions were prepared and used in the crystallization process of tH6H:2OG complex (1.3 μL of protein solution, 1 μL of mother liquor, 0.3 μL of 100× diluted seeds stock). Plateshaped crystals appeared within 1–7 days depending on the chosen ligands and most of them diffracted up to a resolution of 1.3–1.6 Å. Final crystallization conditions are specified in Table S2.† Before flash-cooling, all crystals were incubated in cryo-solutions (crystallization solutions supplemented with 20% (v/v) ethylene glycol).
Full-length H6H data collection and structure determination
Diffraction data of H6H:NOG:Hyo crystals were collected at beamline P13 operated by EMBL Hamburg at the PETRA III storage ring (DESY, Hamburg, Germany)92 at an energy of 12.7 keV. Data were collected using the MXCuBE293 user interface and processed with HKL-2000.94 The structure of the H6H complex with NOG and hyoscyamine was solved by molecular replacement implemented in MoRDa,95 which output a homology model based on PDB entry 1GP6 (ANS from A. thaliana).50 Missing amino acids were rebuilt with ARP/wARP implemented in HKL-300096,97 and manually in later stages.
tH6H data collection and structure determination
Diffraction data were collected at beamline BL14.1 at the BESSY II electron storage ring operated by the Helmholtz-Zentrum Berlin, which is equipped with a Pilatus 6M detector.98 The diffraction data were collected using MXCuBE293 and processed with XDS implemented in XDSAPP.95,99–104 The diffraction images have been deposited to IRRMC and can be accessed at proteindiffraction.org.105 The tH6H:NOG:Hyo structure was solved by molecular replacement with PHASER106 implemented in CCP4i2 based on the H6H:NOG: Hyo model, while tH6H:2OG was solved based on the tH6H: NOG:Hyo model. Data collection statistics are summarized in Table 1.
Table 1.
Data collection and processing statistics
| PDB ID | 6TTM | 6TTN | 6TTO |
|---|---|---|---|
| H6H:ligand | H6H:NOG:Hyo | tH6H:NOG:Hyo | tH6H:2OG |
| Data collection | |||
| Beamline | P13 | BL14.1 | BL14.1 |
| PETRA III | BESSY II | BESSY II | |
| Detector | Pilatus 6M | Pilatus 6M | Pilatus 6M |
| No. of frames | 1050 | 3600 | 1800 |
| Exposure time/frame [s] | 0.04 | 0.35 | 0.30 |
| Wavelength [Å] | 0.9763 | 0.9184 | 0.9184 |
| Temperature [K] | 100 | 100 | 100 |
| Space group | P212121 | P212121 | P212121 |
| α = β = γ [°] | 90 | 90 | 90 |
| a, b, c [Å] | 68.5, 70.8, 78.6 | 39.9, 79.9, 104.7 | 39.9, 79.8, 104.4 |
| Resolution [Å] | 50.00–1.91 (1.94–1.91) |
43.78–1.12 (1.18–1.12) |
43.70–1.31 (1.39–1.31) |
| Rmeas[%] | 4.3 (39.2) | 9.0 (173.4) | 7.9 (136.6) |
| I/σ (I) | 25.1 (2.0) | 15.2 (1.2) | 14.6 (1.3) |
| CC(1/2) | 99.8 (89.7) | 99.9 (46.0) | 99.9 (57.8) |
| Completeness [%] | 96.9 (90.4) | 99.2 (95.0) | 99.8 (99.4) |
| Multiplicity | 3.9 (4.0) | 12.4 (9.1) | 6.5 (6.6) |
| B (Wilson) [A2] | 31.2 | 15.9 | 20.3 |
| Mosaicity [°] | 0.50 | 0.11 | 0.11 |
| ISa | — | 20.0 | 31.5 |
Model refinement
Model refinement was performed with REFMAC5,107,108 phenix.refine100,109 and Coot,110 while the quality of the models was assessed against wwPDB111 and Molprobity.112,113 Partial occupancies were refined with occupancy refinement implemented in Phenix. Resolution cut-offs were checked against paired refinement implemented in PDB-redo.114 Validation of anisotropic refinement was performed with the PARVATI server.115 Refinement statistics are summarized in Table 2. Interpretation of five ions (two in the case of H6H: NOG:Hyo and three in the case of tH6H:NOG:Hyo) remained ambiguous and they were finally assigned as unknown atoms for deposition. Some of these atoms were previously refined as Na+ ions or water molecules, but CheckMyMetal116,117 validation revealed these metal-binding sites have geometries inconsistent with Na+ ions or weak anomalous signal was present at the location of the presumable water molecules. Additionally, in the case of the tH6H:NOG:Hyo model, one ion present at crystal contact was assigned as UNX since only weak anomalous signal is present at this position and the coordination sphere is not fully occupied, whereas in the tH6H:2OG complex this ion was interpreted as Sr2+.
Table 2.
Refinement and validation statistics
| PDB ID | 6TTM | 6TTN | 6TTO |
|---|---|---|---|
| Refinement | |||
| Resolution range [Å] | 49.24–1.91 | 43.78–1.12 | 43.70–1.31 |
| Completeness for range [%] | 96.9 | 99.1 | 99.8 |
| Number of reflections | 29 374 | 129 191 | 81 462 |
| Rwork/Rfree | 0.159/0.200 | 0.127/0.146 | 0.137/0.164 |
| Number of non-hydrogen atoms | 2679 | 3200 | 3215 |
| Average B factor [Å2] | 46.2 | 18.4 | 20.5 |
| Structure quality | |||
| R.m.s deviations | |||
| Bond lengths [Å] | 0.017 | 0.011 | 0.010 |
| Bond angles [°] | 1.4 | 1.1 | 1.1 |
| Ramachandran statistics | |||
| Favoured [%] | 98.0 | 98.3 | 98.4 |
| Allowed [%] | 99.7 | 100.0 | 100.0 |
| Outliers [%] | 0.3 | 0.0 | 0.0 |
| Molprobity analysis | |||
| Clashscore | 2.65 (99th) | 3.27 (88th) | 5.65 (83th) |
| Poor rotamers [%] | 0.0 | 0.0 | 0.3 |
| Favoured rotamers [%] | 96.9 | 99.0 | 97.7 |
| Molprobity score | 1.05 (100th) | 1.15 (94th) | 1.29 (92nd) |
Identification of metal ions
tH6H was produced in minimal medium that should not contain iron, yet ICP-OES analysis performed on a tH6H sample revealed some residual iron content (0.4 μM as compared to 17.8 μM tH6H and 20.7 μM nickel concentration). Consequently, tH6H:Hyo complex was not crystallized with the co-substrate (2-OG) but rather with an inactive co-substrate analog (NOG) as H6H:NOG:Hyo complex previously. Preliminary results of metal identification in the structure of full-length H6H:NOG:Hyo indicated the presence of nickel and strontium ions. The detailed metal content was further analyzed at BL14.1 at the BESSY II electron storage ring. X-ray fluorescence spectra were measured and analyzed with XFEplot.118 For tH6H:NOG:Hyo crystals, the X-ray energy scans were performed in the vicinity of iron (7.062 keV–7.152 keV) and nickel (8.283 keV–8.373 keV) absorption K-edges (Fig. S2†). Additional diffraction data were collected at 7.05 keV, 7.25 keV, 8.24 keV, 8.44 keV. All datasets were processed up to completeness of approximately 95% with XDS implemented in XDSAPP and anomalous difference maps were analyzed with CCP4i2 (Table S4†). Data collection statistics are summarized in Table S3.†
Model preparation and MD simulations
The model of the protein was based on the crystal structure of tH6H with Ni2+, NOG and hyoscyamine bound in the cavity. The loop regions (residues: 119–128 and 206–215) were built and refined with MODELLER,119 the best result was chosen based on the DOPE score. The 33 amino acids on the N-terminus were not rebuilt. The credibility of the prediction was assessed on the basis of the DOPE energy – RMSD relationship (see Fig. S-C1†). The model of the active protein was constructed by replacing Ni2+, NOG by Fe2+ and 2OG, respectively. The protonation state of titratable residues was assigned with PROPKA 3.0 (as part of the PDB2PQR web server).120 Later, the protein was placed in a box of TIP3P water121 with a minimum 10 Å between any atom of the tH6H model and the edge of the box. Crystallographic waters were retained. The charge of the system was neutralised by adding 12 Na+ ions. The minimisation and MD simulation were performed with the Amber ff14SB force field,122 and the active site of the protein was described with the 12–6-4 LJ-type non-bonded model.123
MD simulations were preceded by minimisation of the system. First, the position of water molecules and Na+ ions were optimised, while the solute was restrained with a 500 kcal mol−1 harmonic potential. The potential was reduced to 10 kcal mol−1 in the next step and completely removed in the final minimisation. MD simulation was initiated by heating the system from 0 to 300 K in 100 ps (NVT), followed by a 1 ns density equilibration (NTP). During these steps the backbone of the solute was restrained with a 1 kcal mol−1 harmonic potential. The 20 ns production run was executed at constant temperature (300 K) and pressure (1 atm) with a time step of 2 fs. The bonds involving hydrogen atoms were constrained using the SHAKE algorithm.124 The system reached equilibrium after 1 ns (see Fig. S-C2†) and the stable part of the trajectory was clustered with use of an agglomerative method.125 A representative structure of the dominant cluster was optimised within the ONIOM scheme as implemented in Gaussian 16126 following the method described in the next subsection.
To obtain the product of oxidative cleavage of 2OG, 2OG was replaced by succinate with the oxygens of the carboxyl groups located approximately in the positions of the oxo O atom and the C atom of the carboxyl groups of 2OG, the oxo iron ligand was added trans to His-274 and an equatorial water molecule trans to His-217 was retained to reproduce the most likely octahedral geometry of Fe4+. The new model was optimised with the ONIOM method. The resultant structure was the starting point for a new MD simulation performed in the same manner as previously enumerated. The active site was described with a bonded model due to a lack of nonboned parameters for oxoferryl species. The bonded parameters and the atomic charges for the residues ligating Fe4+ ion were calculated as described previously elsewhere.23 The 21 ns production run was performed in three repetitions, the stable parts of the trajectories (4–21 ns, consult Fig. S-C3–S-C5†) were clustered with an agglomerative method. In the representative structures of the dominant clusters, the active sites and their immediate surroundings adopted very similar conformations and the (three) RMSD values calculated for the backbone of the structures total to ca. 0.6, therefore only one structure was chosen for further QM/MM calculations.
QM/MM calculations
The QM/MM calculations were performed using the ONIOM method from the Gaussian 16 program. The model for the calculations consisted of the protein with its ligands and water molecules located not further than 20 Å from the Fe ion. The QM part of the system involved the Fe4+ ion and is first coordination shell: the oxo ligand, the side chains of residues His217, Asp-219, His-274 and the equatorial water molecule, and the hyoscyamine cation. The MM part was described with the Amber force field and the spin unrestricted B3LYP-D3 method127,128 was used for the QM part. First, the geometries were optimised with the def2-SVP basis set129 and mechanical embedding scheme. In the next step the charge-update procedure was employed to account for polarisation of the QM part by the MM layer and also changes of atomic charges along the reaction coordinate. The ESP was calculated for the QM part of each stationary point with the MM part modelled as point charges. In accordance with electronic embedding scheme, the charges within 2 bonds of the QM region were removed. The RESP procedure130 was used to derive new atomic charges for the system. The structures with a new set of charges were optimised and frequency calculations were performed at the same level of theory. For the stationary points with updated charges, single-point calculations were performed at the B3LYP-D3/def2-TZVP level of theory with mechanical and electronic embedding. The reported energy values (if not stated otherwise) are ONIOM (B3LYP-D3/def2-TZVP, Amber) energies obtained within an electronic embedding scheme with Gibbs free energy corrections computed with the ONIOM(B3LYP-D3/def2-SVP, Amber) method and mechanical embedding. Optimised structures will be deposited in the ioChem database and are available from the authors upon request.
Supplementary Material
Acknowledgements
This research project was supported by SONATA-BIS grant no. UMO-2014/14/E/NZ1/00053 from the National Science Centre, Poland and PL-Grid Infrastructure. Computations were performed in the AGH Cyfronet Supercomputer Centre. We acknowledge the MCB Structural Biology Core Facility (supported by the TEAM TECH CORE FACILITY/2017-4/6 grant from the Foundation for Polish Science) for valuable support, particularly Dr. Przemysław Grudnik, Dr. Piotr Wilk and MSc Elżbieta Wątor. Diffraction data were collected at beamline BL14.1 at the BESSY II electron storage ring operated by the Helmholtz-Zentrum Berlin.98 We would particularly like to acknowledge the support of Dr. Martin Gerlach and Dr. Piotr Wilk during these experiments. Diffraction data were also collected at beamline P13 operated by EMBL Hamburg at the PETRA III storage ring (DESY, Hamburg, Germany). We would like to thank Dr. David von Stetten for assistance in using this beamline. AK would like to acknowledge the financial support of the Erasmus+ Program and the Fulbright Program. We would like to thank Dr. Maksymilian Chruszcz for helpful discussions.
Abbreviations
- H6H
Hyoscyamine 6β-hydroxylase
- ODD 2
oxoglutarate/Fe2+-dependent dioxygenase
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/d0dt00302f
Conflicts of interest
There are no conflicts to declare.
References
- 1.Holzman RS, Anesthesiology, 1998, 89, 241–249. [DOI] [PubMed] [Google Scholar]
- 2.Grynkiewicz G. and Gadzikowska M, Pharmacol. Rep, 2008, 60, 439–463. [PubMed] [Google Scholar]
- 3.Palazón J, Navarro-Ocaña A, Hernandez-Vazquez L. and Mirjalili M, Molecules, 2008, 13, 1722–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardillo AB, Perassolo M, Sartuqui M, Rodríguez Talou J. and Giulietti AM, Biochem. Eng. J, 2017, 125, 180–189. [Google Scholar]
- 5.Cao Y-D, He Y-C, Li H, Kai G-Y, Xu J-H and Yu H-L, J. Biotechnol, 2015, 211, 123–129. [DOI] [PubMed] [Google Scholar]
- 6.Lan X, Zeng J, Liu K, Zhang F, Bai G, Chen M, Liao Z. and Huang L, Biochem. Biophys. Res. Commun, 2018, 497, 25–31. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto T, Matsuda J. and Yamada Y, FEBS Lett., 1993, 329, 35–39. [DOI] [PubMed] [Google Scholar]
- 8.Poupko JM, Baskin SI and Moore E, J. Appl. Toxicol, 2007, 27, 116–121. [DOI] [PubMed] [Google Scholar]
- 9.Eisenkraft A. and Falk A, Br. J. Pharmacol, 2016, 173, 1719–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ushimaru R, Ruszczycky MW and Liu H, J. Am. Chem. Soc, 2019, 141, 1062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MarvinSketch was used for drawing structures and reactions. MarvinSketch, Version 18.20, ChemAxon, 2018. [Google Scholar]
- 12.Li J, Van Belkum MJ and Vederas JC, Bioorg. Med. Chem, 2012, 20, 4356–4363. [DOI] [PubMed] [Google Scholar]
- 13.Ushimaru R, Ruszczycky MW, Chang W-C, Yan F, Liu Y. and Liu H-W, J. Am. Chem. Soc, 2018, 140, 7433–7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto T, Kohno J. and Yamada Y, Phytochemistry, 1989, 28, 1077–1082. [Google Scholar]
- 15.Borowski T, de Marothy S, Broclawik E, Schofield CJ and Siegbahn PEM, Biochemistry, 2007, 46, 3682–3691. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Ren J, Stammers DK, Baldwin JE, Harlos K. and Schofield CJ, Nat. Struct. Biol, 2000, 7, 127–133. [DOI] [PubMed] [Google Scholar]
- 17.Krol WJ, Basak A, Salowe SP and Townsend CA, J. Am. Chem. Soc, 1989, 111, 7625–7627. [Google Scholar]
- 18.Pan J, Bhardwaj M, Zhang B, Chang W, Schardl CL, Krebs C, Grossman RB and Bollinger JM, Biochemistry, 2018, 57, 2074–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan J, Bhardwaj M, Faulkner JR, Nagabhyru P, Charlton ND, Higashi RM, Miller AF, Young CA, Grossman RB and Schardl CL, Phytochemistry, 2014, 98, 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blankenship JD, Spiering MJ, Wilkinson HH, Fannin FF, Bush LP and Schardl CL, Phytochemistry, 2001, 58, 395–401. [DOI] [PubMed] [Google Scholar]
- 21.Chang WC, Li J, Lee JL, Cronican AA and Guo Y, J. Am. Chem. Soc, 2016, 138, 10390–10393. [DOI] [PubMed] [Google Scholar]
- 22.Bräuer A, Beck P, Hintermann L. and Groll M, Angew. Chem., Int. Ed, 2016, 55, 422–426. [DOI] [PubMed] [Google Scholar]
- 23.Wojdyla Z. and Borowski T, J. Biol. Inorg. Chem, 2018, 23, 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kai G, Zhang A, Guo Y, Li L, Cui L, Luo X, Liu C. and Xiao J, Mol. Biosyst, 2012, 8, 2883–2890. [DOI] [PubMed] [Google Scholar]
- 25.Kai G, Liu Y, Wang X, Yang S, Fu X, Luo X. and Liao P, Biotechnol. Lett, 2011, 33, 1361–1365. [DOI] [PubMed] [Google Scholar]
- 26.Liu T, Zhu P, Cheng K, Meng C. and He H, Planta Med., 2005, 71, 249–253. [DOI] [PubMed] [Google Scholar]
- 27.el Jaber-Vazdekis N, González C, Ravelo ÁG and Zárate R, Plant Physiol. Biochem, 2009, 47, 20–25. [DOI] [PubMed] [Google Scholar]
- 28.el Jaber-Vazdekis N, Barres ML, Ravelo AG and Zárate R, J. Nat. Prod, 2008, 71, 2026–2031. [DOI] [PubMed] [Google Scholar]
- 29.Zárate R, el Jaber-Vazdekis N, Medina B. and Ravelo ÁG, Biotechnol. Lett, 2006, 28, 1271–1277. [DOI] [PubMed] [Google Scholar]
- 30.Xia K, Liu X, Zhang Q, Qiang W, Guo J, Lan X, Chen M. and Liao Z, Plant Physiol. Biochem, 2016, 106, 46–53. [DOI] [PubMed] [Google Scholar]
- 31.Fischer C, Kwon M, Ro D-K, van Belkum MJ and Vederas JC, MedChemComm, 2018, 9, 888–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cardillo AB, Talou J. and Giulietti AM, Microb. Cell Fact, 2008, 7, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiang W, Hou Y, Li X, Xia K. and Liao Z, Yaoxue Xuebao, 2015, 50, 1346–1355. [PubMed] [Google Scholar]
- 34.Pramod KK, Singh S. and Jayabaskaran C, Plant Physiol. Biochem, 2010, 48, 966–970. [DOI] [PubMed] [Google Scholar]
- 35.Kohnen KL, Sezgin S, Spiteller M, Hagels H. and Kayser O, Plant Cell Physiol., 2018, 59, 107–118. [DOI] [PubMed] [Google Scholar]
- 36.Dehghan E, Reed DW, Covello PS, Hasanpour Z, Palazon J, Oksman-Caldentey K-M and Ahmadi FS, Plant Cell Rep., 2017, 36, 1615–1626. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto T, Hayashi A, Amano Y, Kohno J, Iwanari H, Usuda S. and Yamada Y, J. Biol. Chem, 1991, 266, 4648–4653. [PubMed] [Google Scholar]
- 38.Zhang L, Ding R, Chai Y, Bonfill M, Moyano E, Oksman-Caldentey K-M, Xu T, Pi Y, Wang Z, Zhang H, Kai G, Liao Z, Sun X. and Tang K, Proc. Natl. Acad. Sci. U. S. A, 2004, 101, 6786–6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hashimoto T, Kohno J. and Yamada Y, Plant Physiol., 1987, 84, 144–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan J, Wenger ES, Matthews ML, Pollock CJ, Bhardwaj M, Kim AJ, Allen BD, Grossman RB, Krebs C. and Bollinger JM, J. Am. Chem. Soc, 2019, 141, 15153–15165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlesinger D, Davidovich Rikanati R, Volis S, Faigenboim A, Vendramin V, Cattonaro F, Hooper M, Oren E, Taylor M, Sitrit Y, Inbar M. and Lewinsohn E, Plant Sci., 2019, 283, 301–310. [DOI] [PubMed] [Google Scholar]
- 42.Kim YD, Kang SM, Min JY, Choi WK, Jeong MJ, Karigar CS and Choi MS, J. Nat. Prod, 2010, 73, 147–150. [DOI] [PubMed] [Google Scholar]
- 43.Zhao K, Zeng J, Zhao T, Zhang H, Qiu F, Yang C, Zeng L, Liu X, Chen M, Lan X. and Liao Z, Front. Plant Sci, 2017, 8, 1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matthews BW, J. Mol. Biol, 1968, 33, 491–497. [DOI] [PubMed] [Google Scholar]
- 45.Kantardjieff KA and Rupp B, Protein Sci., 2003, 12, 1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krissinel E, Henrick K. and IUCr, Acta Crystallogr., Sect. D: Biol. Crystallogr, 2004, 60, 2256–2268. [DOI] [PubMed] [Google Scholar]
- 47.Krissinel E. and Henrick K, Multiple Alignment of Protein Structures in Three Dimensions, in Computational Life Sciences. CompLife 2005. Lecture Notes in Computer Science, ed. Berthold MR, Glen RC, Diederichs K, Kohlbacher O. and Fischer I, Springer, Berlin, Heidelberg, 2005, vol. 3695, pp. 67–78, ISBN: 978–3-540–29104-6. [Google Scholar]
- 48.Aik W, McDonough MA, Thalhammer A, Chowdhury R. and Schofield CJ, Curr. Opin. Struct. Biol, 2012, 22, 691–700. [DOI] [PubMed] [Google Scholar]
- 49.Kluza A, Niedzialkowska E, Kurpiewska K, Wojdyla Z,Quesne M, Kot E, Porebski PJ and Borowski T, J. Struct. Biol, 2018, 202, 229–235. [DOI] [PubMed] [Google Scholar]
- 50.Wilmouth RC, Turnbull JJ, Welford RWD, Clifton IJ, Prescott AG and Schofield CJ, Structure, 2002, 10, 93–103. [DOI] [PubMed] [Google Scholar]
- 51.Sun X, Zhou D, Kandavelu P, Zhang H, Yuan Q, Wang B-C, Rose J. and Yan Y, Sci. Rep, 2015, 5, 10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heinig M. and Frishman D, Nucleic Acids Res., 2004, 32, W500–W502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roth Emma, “Convert Stride Secondary Structure to PDB format” can be found under http://www.canoz.com/sdh/STRIDEtoPDBsecondarystruct.pl, 2010. [Google Scholar]
- 54.Handing KB, Niedzialkowska E, Shabalin IG, Kuhn ML, Zheng H. and Minor W, Nat. Protoc, 2018, 13, 1062–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cromer DT, J. Appl. Crystallogr, 1983, 16, 437–437. [Google Scholar]
- 56.Horton JR, Upadhyay AK, Hashimoto H, Zhang X. and Cheng X, J. Mol. Biol, 2011, 406, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Porebski PJ, Sroka P, Zheng H, Cooper DR and Minor W, Protein Sci., 2018, 27, 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hashimoto T. and Yamada Y, Eur. J. Biochem, 1986, 164, 277–285. [DOI] [PubMed] [Google Scholar]
- 59.Clifton IJ, Doan LX, Sleeman MC, Topf M, Suzuki H, Wilmouth RC and Schofield CJ, J. Biol. Chem, 2003, 278, 20843–20850. [DOI] [PubMed] [Google Scholar]
- 60.Ye S, Geng C-Y, Shaik S. and Neese F, Phys. Chem. Chem. Phys, 2013, 15, 8017. [DOI] [PubMed] [Google Scholar]
- 61.Bernasconi L. and Baerends EJ, Eur. J. Inorg. Chem, 2008, 2008, 1672–1681. [Google Scholar]
- 62.Geng C, Ye S. and Neese F, Angew. Chem., Int. Ed, 2010, 49, 5717–5720. [DOI] [PubMed] [Google Scholar]
- 63.The PyMOL Molecular Graphics System, Version 1.8, Schrödinger LLC, 2015. [Google Scholar]
- 64.de Visser SP, J. Am. Chem. Soc, 2006, 128, 15809–15818. [DOI] [PubMed] [Google Scholar]
- 65.Srnec M, Wong SD, England J, Que L. and Solomon EI, Proc. Natl. Acad. Sci. U. S. A, 2012, 109, 14326–14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Timmins A, Saint-André M. and De Visser SP, J. Am. Chem. Soc, 2017, 139, 9855–9866. [DOI] [PubMed] [Google Scholar]
- 67.Ma G, Zhu W, Su H, Cheng N. and Liu Y, ACS Catal., 2015, 5, 5556–5566. [Google Scholar]
- 68.Shah DD, Conrad JA and Moran GR, Biochemistry, 2013, 52, 6097–6107. [DOI] [PubMed] [Google Scholar]
- 69.Bollinger JM, Price JC, Hoffart LM, Barr EW and Krebs C, Eur. J. Inorg. Chem, 2005, 2005, 4245–4254. [Google Scholar]
- 70.Borowski T, Broclawik E, Schofield CJ and Siegbahn PEM, J. Comput. Chem, 2006, 27, 740–748. [DOI] [PubMed] [Google Scholar]
- 71.Zeb N, Rashid MH, Mubarak MQE, Ghafoor S. and de Visser SP, J. Inorg. Biochem, 2019, 198, 110728. [DOI] [PubMed] [Google Scholar]
- 72.Quesne MG, Latifi R, Gonzalez-Ovalle LE, Kumar D. and de Visser SP, Chem. – Eur. J, 2014, 20, 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Srnec M. and Solomon EI, J. Am. Chem. Soc, 2017, 139, 2396–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Timmins A. and de Visser SP, Catalysts, 2018, 8, 314. [Google Scholar]
- 75.Ji L, Faponle AS, Quesne MG, Sainna MA, Zhang J, Franke A, Kumar D, van Eldik R, Liu W. and de Visser SP, Chem. – Eur. J, 2015, 21, 9083–9092. [DOI] [PubMed] [Google Scholar]
- 76.Shaik S, Kumar D. and De Visser SP, J. Am. Chem. Soc, 2008, 130, 10128–10140. [DOI] [PubMed] [Google Scholar]
- 77.Faponle AS, Quesne MG and de Visser SP, Chem. – Eur. J, 2016, 22, 5478–5483. [DOI] [PubMed] [Google Scholar]
- 78.Kwon E, Cho K-B, Hong S. and Nam W, Chem. Commun, 2014, 50, 5572–5575. [DOI] [PubMed] [Google Scholar]
- 79.Rana S, Biswas JP, Sen A, Clémancey M, Blondin G, Latour JM, Rajaraman G. and Maiti D, Chem. Sci, 2018, 9, 7843–7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vögeli B. and Erb TJ, Curr. Opin. Chem. Biol, 2018, 47, 94–100. [DOI] [PubMed] [Google Scholar]
- 81.Ghafoor S, Mansha A. and de Visser SP, J. Am. Chem. Soc, 2019, 141, 20278–20292. [DOI] [PubMed] [Google Scholar]
- 82.Rosenthal RG, Vögeli B, Quade N, Capitani G, Kiefer P, Vorholt JA, Ebert MO and Erb TJ, Nat. Chem. Biol, 2015, 11, 398–400. [DOI] [PubMed] [Google Scholar]
- 83.Matthews ML, Neumann CS, Miles LA, Grove TL, Booker SJ, Krebs C, Walsh CT and Bollinger JM, Proc. Natl. Acad. Sci. U. S. A, 2009, 106, 17723–17728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eschenfeldt WH, Lucy S, Millard CS, Joachimiak A. and Mark ID, in Methods in molecular biology, NIH Public Access, Clifton, N.J, 2009, vol. 498, pp. 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD and Bairoch A, The Proteomics Protocols Handbook, Humana Press, Totowa, NJ, 2005. [Google Scholar]
- 86.Laemmli UK, Nature, 1970, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- 87.Slabinski L, Jaroszewski L, Rychlewski L, Wilson IA, Lesley SA and Godzik A, Bioinformatics, 2007, 23, 3403–3405. [DOI] [PubMed] [Google Scholar]
- 88.Slabinski L, Jaroszewski L, Rodrigues APC, Rychlewski L, Wilson IA, Lesley SA and Godzik A, Protein Sci., 2007, 16, 2472–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jahandideh S, Jaroszewski L. and Godzik A, ActaCrystallogr., Sect. D: Biol. Crystallogr, 2014, 70, 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miller JH, Experiments in molecular genetics. Assay of ß Galactosidase, Cold Spring Harbor Laboratory Press, 1972. [Google Scholar]
- 91.Miłaczewska A, Kot E, Amaya JA, Makris TM, Zając M, Korecki J, Chumakov A, Trzewik B, Kędracka-Krok S, Minor W, Chruszcz M. and Borowski T, Chem. – Eur. J, 2018, 24, 5225–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cianci M, Bourenkov G, Pompidor G, Karpics I, Kallio J, Bento I, Roessle M, Cipriani F, Fiedler S. and Schneider TR, J. Synchrotron Radiat, 2017, 24, 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oscarsson M, Beteva A, Flot D, Gordon E, Guijarro M, Leonard G, McSweeney S, Monaco S, Mueller-Dieckmann C, Nanao M, Nurizzo D, Popov AN, von Stetten D, Svensson O, Rey-Bakaikoa V, Chado I, Chavas LMG, Gadea L, Gourhant P, Isabet T, Legrand P, Savko M, Sirigu S, Shepard W, Thompson A, Mueller U, Nan J, Eguiraun M, Bolmsten F, Nardella A, Milàn-Otero A, Thunnissen M, Hellmig M, Kastner A,Schmuckermaier L Gerlach M, Feiler C, Weiss MS, Bowler LW, Gobbo A, Papp G, Sinoir J, McCarthy AA, Karpics I, Nikolova M, Bourenkov G, Schneider T, Andreu J, Cuní G, Juanhuix J, Boer R, Fogh R, Keller P, Flensburg C, Paciorek W, Vonrhein C, Bricogne G. and de Sanctis D, J. Synchrotron Radiat, 2019, 26, 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Otwinowski Z. and Minor W, Methods Enzymol., 1997, 276, 307–326. [DOI] [PubMed] [Google Scholar]
- 95.Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AGW, McCoy A, McNicholas SJ, Murshudov GN, Pannu NS, Potterton EA, Powell HR, Read RJ, Vagin A. and Wilson KS, Acta Crystallogr., Sect. D: Biol. Crystallogr, 2011, 67, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perrakis A, Morris R. and Lamzin VS, Nat. Struct. Biol, 1999, 6, 458–463. [DOI] [PubMed] [Google Scholar]
- 97.Minor W, Cymborowski M, Otwinowski Z. and M Chruszcz, Acta Crystallogr., Sect. D: Biol. Crystallogr, 2006, 62, 859–866. [DOI] [PubMed] [Google Scholar]
- 98.Mueller U, Förster R, Hellmig M, Huschmann FU, Kastner A, Malecki P, Pühringer S, Röwer M, Sparta K, Steffien M, Ühlein M, Wilk P. and Weiss MS, Eur. Phys. J. Plus, 2015, 130, 141. [Google Scholar]
- 99.Kabsch W, Acta Crystallogr., Sect. D: Biol. Crystallogr, 2010, 66, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L-W, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH and IUCr, Acta Crystallogr., Sect. D: Biol. Crystallogr, 2010, 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Diederichs K, Acta Crystallogr., Sect. D: Biol. Crystallogr, 2006, 62, 96–101. [DOI] [PubMed] [Google Scholar]
- 102.Krug M, Weiss MS, Heinemann U. and Mueller U, J. Appl. Crystallogr, 2012, 45, 568–572. [Google Scholar]
- 103.Vaguine AA, Richelle J. and Wodak SJ, Acta Crystallogr., Sect. D: Biol. Crystallogr, 1999, 55, 191–205. [DOI] [PubMed] [Google Scholar]
- 104.Sparta KM, Krug M, Heinemann U, Mueller U. and Weiss MS, J. Appl. Crystallogr, 2016, 49, 1085–1092. [Google Scholar]
- 105.Grabowski M, Langner KM, Cymborowski M, Porebski PJ, Sroka P, Zheng H, Cooper DR, M D. Zimmerman, M.-A. Elsliger, S. K. Burley and W. Minor, Acta Crystallogr., Sect. D: Struct. Biol, 2016, 72, 1181–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC and Read RJ, J. Appl. Crystallogr, 2007, 40, 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murshudov GN, Skubák P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, Winn MD, Long F. and Vagin AA, Acta Crystallogr., Sect. D: Biol. Crystallogr, 2011, 67, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AGW, McCoy A, McNicholas SJ, Murshudov GN, Pannu NS, Potterton EA, Powell HR, Read RJ, Vagin A. and Wilson KS, Acta Crystallogr., Sect. D: Biol. Crystallogr, 2011, 67, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, Mustyakimov M, Terwilliger TC, Urzhumtsev A, Zwart PH, Adams PD and IUCr, Acta Crystallogr., Sect. D: Biol. Crystallogr, 2012, 68, 352–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Emsley P, Lohkamp B, Scott WG and Cowtan K, Acta Crystallogr., Sect. D: Biol. Crystallogr, 2010, 66, 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Young JY, Westbrook JD, Feng Z, Sala R, Peisach E, Oldfield TJ, Sen S, Gutmanas A, Armstrong DR, Berrisford JM, Chen L, Chen M, Di Costanzo L, Dimitropoulos D, Gao G, Ghosh S, Gore S, Guranovic V, Hendrickx PMS, Hudson BP, Igarashi R, Ikegawa Y, Kobayashi N, Lawson CL, Liang Y, Mading S, Mak L, Mir MS, Mukhopadhyay A, Patwardhan A, Persikova I, Rinaldi L, Sanz-Garcia E, Sekharan MR, Shao C, Swaminathan GJ, Tan L, Ulrich EL, van Ginkel G, Yamashita R, Yang H, Zhuravleva MA, Quesada M, Kleywegt GJ, Berman HM, Markley JL, Nakamura H, Velankar S. and Burley SK, Structure, 2017, 25, 536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen VB, Arendall WB, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS and Richardson DC, Acta Crystallogr., Sect. D: Biol. Crystallogr, 2010, 66, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Williams CJ, Headd JJ, Moriarty NW, Prisant MG, Videau LL, Deis LN, Verma V, Keedy DA, Hintze BJ, Chen VB, Jain S, Lewis SM, Arendall WB, Snoeyink J, Adams PD, Lovell SC, Richardson JS and Richardson DC, Protein Sci., 2018, 27, 293–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Joosten RP, Long F, Murshudov GN and Perrakis A, IUCrJ, 2014, 1, 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zucker F, Champ PC and Merritt EA, Acta Crystallogr., Sect. D: Biol. Crystallogr, 2010, 66, 889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zheng H, Cooper DR, Porebski PJ, Shabalin IG, Handing KB and Minor W, Acta Crystallogr., Sect. D: Biol. Crystallogr, 2017, 73, 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zheng H, Chordia MD, Cooper DR, Chruszcz M, Müller P, Sheldrick GM and Minor W, Nat. Protoc, 2013, 9, 156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.XFEplot – A GUI application for X-Ray fluorescence spectrum analysis, Version 2.1, Helmholtz-Zentrum Berlin. [Google Scholar]
- 119.Fiser A, Do RKG and Šali A, Protein Sci., 2000, 9, 1753–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dolinsky TJ, Nielsen JE, McCammon JA and Baker NA, Nucleic Acids Res., 2004, 32, 665–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW and Klein ML, J. Chem. Phys, 1983, 79, 926–935. [Google Scholar]
- 122.Maier JA, Martinez C, Kasavajhala K, Wickstrom L, Hauser KE and Simmerling C, J. Chem. Theory Comput, 2015, 11, 3696–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li P, Song LF and Merz KM, J. Phys. Chem. B, 2015, 119, 883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ryckaert JP, Ciccotti G. and Berendsen HJ, J. Comput. Phys, 1977, 23, 327–341. [Google Scholar]
- 125.Roe DR and Cheatham TE, J. Chem. Theory Comput, 2013, 9, 3084–3095. [DOI] [PubMed] [Google Scholar]
- 126.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci A, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega M, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr., Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB and Fox DJ, Gaussian, Inc., Wallingford CT, 2016. [Google Scholar]
- 127.Becke AD, J. Chem. Phys, 1993, 98, 5648–5652. [Google Scholar]
- 128.Grimme S, Ehrlich S. and Goerigk L, J. Comput. Chem, 2011, 32, 1456–1465. [DOI] [PubMed] [Google Scholar]
- 129.Weigend F. and Ahlrichs R, Phys. Chem. Chem. Phys, 2005, 7, 3297–3305. [DOI] [PubMed] [Google Scholar]
- 130.Wang J, Cieplak P. and Kollman PA, J. Comput. Chem, 2000, 21, 1049–1074. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.