Abstract
Type 2 immunity is activated in response to both allergens and helminth infection. It can be detrimental or beneficial, and there is a pressing need to better understand its regulation. The immunosuppressive cytokine IL-10 is known as a T helper 2 (Th2) effector molecule, but it is currently unclear whether IL-10 dampens or promotes Th2 differentiation during infection. Here we show that helminth infection in mice elicits IL-10 expression in both the intestinal lamina propria and the draining mesenteric lymph node, with higher expression in the infected tissue. In vitro, exogenous IL-10 enhanced Th2 differentiation in isolated CD4+ T cells, increasing expression of GATA3 and production of IL-5 and IL-13. The ability of IL-10 to amplify the Th2 response coincided with its suppression of IFNγ expression and in vivo we found that, in intestinal helminth infection, IL-10 receptor expression was higher on Th1 cells in the small intestine than on Th2 cells in the same tissue, or on any Th cell in the draining lymph node. In vivo blockade of IL-10 signalling during helminth infection resulted in an expansion of IFNγ+ and Tbet+ Th1 cells in the small intestine and a coincident decrease in IL-13, IL-5 and GATA3 expression by intestinal T cells. These changes in Th2 cytokines correlated with reduced expression of type 2 effector molecules, such as RELMα, and increased parasite egg production. Together our data indicate that IL-10 signalling promotes Th2 differentiation during helminth infection at least in part by regulating competing Th1 cells in the infected tissue.
Introduction
Gastrointestinal helminths infect more than 1.5 billion people per year1 and type 2 immune responses are critical for parasite expulsion2,3 and subsequent wound healing4. The same type 2 responses can be harmful in contexts such as allergy and atopic asthma5. Better understanding of type 2 immunity is important both to optimise anti-helminthic strategies, such as vaccination, and to accelerate new therapeutic approaches to atopic diseases. Type 2 immunity is initiated when antigen or allergen exposure coincides with the release of alarmins such as interleukin (IL) -25, IL-33 or thymic stromal lymphopoietin6. Alarmins promote the activation of type 2 innate lymphoid cells (ILC2) and the recruitment and activation of dendritic cells (DCs) that direct T helper 2 (Th2) cell differentiation7–9. Th2 cells secrete cytokines such as IL-4, IL-5 and IL-13, which drive further Th2 polarisation, direct B cell class-switching, recruit effector cells such as eosinophils, basophils and mast cells, and stimulate goblet cell hyperplasia, mucus secretion, epithelial turnover and increased smooth muscle reactivity10,11. Th2 cytokines show spatial patterning, with IL-4 concentrated in the lymph node and IL-5 and IL-13 in the effector tissues12–15, reflecting the timing of their production and their distinct target cells16. If cytokine production becomes chronic or excessive, type 2 immunity can drive fibrosis, scar formation and loss of tissue function. Regulatory mechanisms are therefore an inherent part of type 2 immunity, balancing protective immunity and immunopathology.
IL-10 is a key regulatory cytokine. It was first described as a Th2 effector cytokine, secreted by isolated Th2 clones17,18. Neonates have a Th2 bias that correlates with high expression of IL-1019,20. DC-derived IL-10 has been reported to promote antigen-specific Th2 responses in a model of allergic dermatitis21, and IL-10-dependent induction of STAT3 and Blimp-1 has recently been shown to be essential for the development of inflammatory Th2 cells in the lung during asthma22,23. IL-10 has also been reported to support antibody isotype switching24 and to amplify mast cell activity25–27. However, IL-10 is known foremost as a suppressive cytokine, particularly in Th1 responses. IL-10-deficient mice develop spontaneous colitis28, driven by exaggerated IFNγ and IL-17 responses to commensal bacteria29–31. Protozoan, viral and bacterial infections in IL-10-deficient mice also show potent increases in Th1 and Th17 cytokines, associated with rapid pathogen clearance but also with acute and often fatal immunopathology32–34. The original description of IL-10 as a Th2 effector molecule in vitro may reflect its ability to limit Th1 differentiation, especially in vitro where Th1 and Th2 responses are mutually antagonistic18. The impact of IL-10 on Th2 responses in vivo, in the context of mixed T cell responses, is less clear.
IL-10 expression increases in the draining lymph node during infection with the murine helminth Heligmosomoides polygyrus35–37, and it is essential for host survival during infection with the whipworm Trichuris muris38. The Th2 response to Nippostrongylus brasiliensis infection also requires IL-4 dependent IL-10 signalling39. IL-10 has different effects at different stages of Trichinella spiralis infection, promoting intestinal mast cell accumulation and clearance of adult worms, but suppressing the immune response against larvae encysted in peripheral muscle25. The tissue location of IL-10 activity may be influential. The impact of IL-10 is dependent on timing, location and cell type34,40 and yet the cells that IL-10 targets at the site of helminth infection, and the intestinal networks by which it acts, are still uncertain. Previous studies of T cell regulation during helminth infection have focused on lymph node responses, limited by the technical difficulties created by the extensive mucus production, oedema, and tissue fragility in the helminth-infected gut. We and others recently published new protocols for successful isolation of intestinal immune cells during active type 2 immune responses40–42. Here, we have used these technical advances to interrogate the impact of IL-10 on the regulation of the Th2 immune response in the infected tissue during an enteric helminth infection.
Our data show that, during infection with the intestinal helminth parasite H. polygyrus, IL-10 is a striking feature of the immune response in the infected intestinal tissue. We demonstrate in vitro that IL-10 promotes Th2 cytokine expression in unpolarised cells in part by suppressing IFNγ expression. We show in vivo that H. polygyrus infection includes an intestinal Th1 response that is limited by direct IL-10 signalling. Surface expression of the IL-10 receptor was higher on Th1 cells in the infected small intestine than on Th2 cells locally or in the draining lymph node, and in vivo blockade of IL-10 signalling during H. polygyrus infection resulted in enhanced Th1 and reduced Th2 activity in the small intestine. Together our data suggest a regulatory loop in helminth-infected, intestinal tissue in which IL-10 suppresses competing Th1 cells to promote Th2 immunity.
Results
IL-10 expression increases in H. polygyrus infection and is higher in the small intestine than the draining lymph node
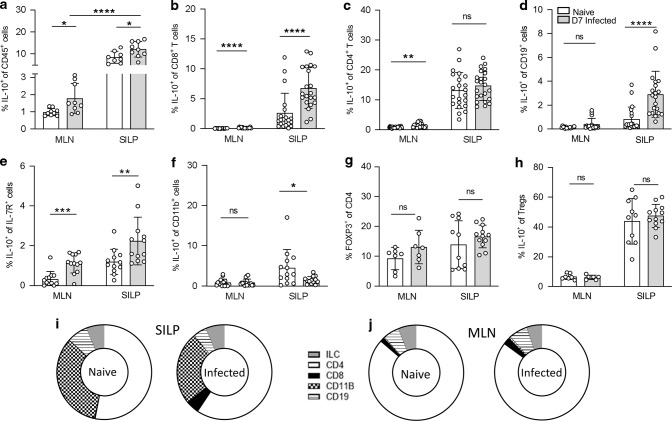
To investigate the impact of IL-10 on the immune response to intestinal helminth infection, we first assessed the location of IL-10 expression during infection with the enteric roundworm, Heligmosomoides polygyrus. IL-10 production has previously been shown to increase during H. polygyrus infection in cells of the draining, mesenteric lymph node (MLN)35–37,43. Using IL-10 reporter mice44,45, we saw a significant increase in the percentage of CD45+ IL-10+ cells upon infection in the MLN (Fig. 1a) but also in the small intestine lamina propria (SILP). Expression was significantly higher in the small intestine compared with MLN (Fig. 1a). We then analysed the cells producing IL-10 in both the SILP and MLN. Our gating strategies are shown in Figs. S1 and S2. In the SILP, the percentage of CD8+ T cells, B cells and ILCs expressing IL-10 increased at day 7 (D7) post-infection with H. polygyrus compared to naïve controls, whereas the proportion of IL-10+ CD4+ T cells remained unchanged and there was a small decrease in IL-10+ myeloid cells (Fig. 1a–f). In the MLN, the percentage of IL-10+ CD8+ T cells, CD4+ T cells and ILCs increased upon infection, while both IL-10+ B cells and myeloid cells remained unchanged between naïve and infected samples (Fig. 1d, f). IL-10 expression in both naïve and infected mice was higher in the SILP compared to the MLN in all cell types (Fig. 1a–f). We also examined the presence of Foxp3+ regulatory T cells (Tregs) in both tissues (Fig. 1g, h). Neither the frequency of Tregs nor their IL-10 expression was significantly affected by infection in either the SILP or MLN at this early timepoint, although IL-10 was again more strongly expressed in the SILP than the MLN (Fig. 1h). We then compared the contribution of each cell type to the total IL-10+ population (Fig. 1i, j). In the SILP, CD4+ T cells and myeloid cells made up the majority of CD45+ IL-10+ cells in both naïve and infected mice. The proportion of IL-10+ cells that were CD8+ T cells also expanded in the SILP during infection (Fig. 1i). In the MLN, CD4+ T cells were the dominant population of CD45+ IL-10+ cells in both naïve and infected animals, although the proportion of B cells and ILCs within the IL-10+ pool increased slightly upon infection (Fig. 1j). Together, these data show multiple sources of IL-10 active during H. polygyrus infection and demonstrate high IL-10 expression at the site of infection, in the SILP.
Fig. 1. IL-10 expression increases in the MLN and small intestine during H. polygyrus infection.
Il10gfp-foxp3rfp B6 mice were infected with 200 L3 H. polygyrus and 7 days later the small intestine and MLN were collected for analysis. Percentage of IL-10+ cells within a CD45+, b CD8+, c CD4+, d CD19+, e IL-7R+ Lineage- (ILCs), f CD11b+ and h FOXP3+ cells of the MLN and SILP of naïve and D7 infected mice. g Percentage of FOXP3+ cells within the CD4+ population in the MLN and SILP of naïve and D7 infected mice. Changes in proportion of IL-10 producing cells from total CD45+ IL-10+ cells in the (i) SILP and (j) MLN from naïve and D7 infected mice. Graphed data are shown with mean ± 1 SD and are pooled from 2–4 independent experiments with n = 2–5 per experiment. Statistical significance was calculated by Student’s t test where data were normally distributed (a, c (SILP)) and Mann–Whitney U test where data were not normally distributed (b, c (MLN), d–f). (Significance *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
IL-10 enhances Th2 differentiation in vitro
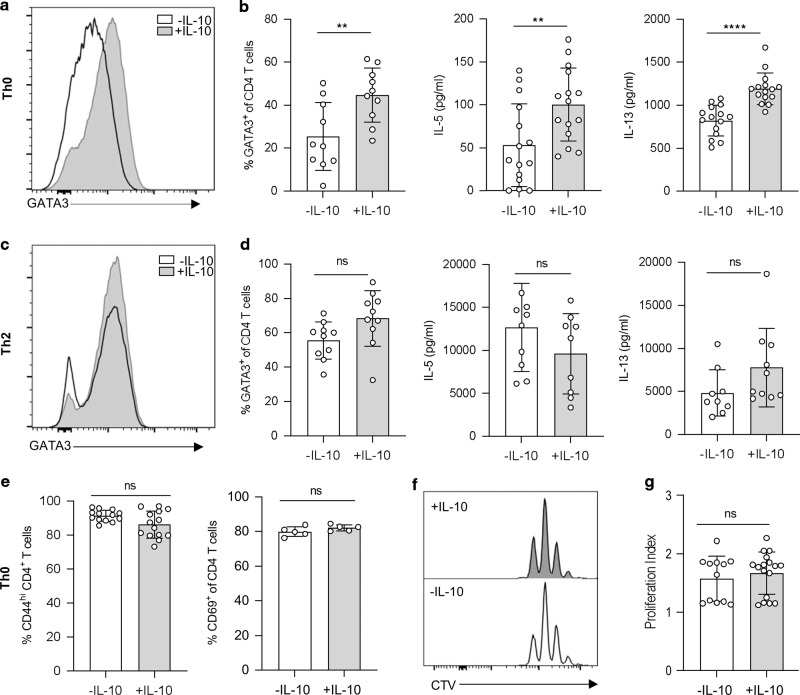
To determine the functional importance of IL-10 expression during H. polygyrus infection, we first considered whether direct IL-10 signalling to CD4+ T cells could contribute to Th2 polarisation. We stimulated purified CD4+ T cells with αCD3, αCD28 and IL-2 in vitro (Th0 cultures) (shown in Fig. S3) and added recombinant IL-10. The presence of IL-10 caused a significant increase in the expression of GATA3, IL-5 and IL-13 (Fig. 2a, b), showing that, in an unpolarised CD4+ T cell, IL-10 can enhance Th2 differentiation. When we added IL-10 to polarised Th2 cell cultures (CD4+ T cells stimulated with αCD3, αCD28, IL-2, IL-4 and anti-IFNγ), the presence of IL-10 did not cause any further increase in expression of GATA3 or of the effector cytokines IL-5 and IL-13 (Fig. 2c, d), perhaps reflecting the high levels of these cytokines already produced under polarising conditions. The impact of IL-10 in enhancing Th2 differentiation in Th0 cultures appeared to be on polarisation rather than on activation or proliferation, since neither CD44, CD69 nor cell division were significantly different in Th0 cells cultured in the presence or absence of IL-10 (Fig. 2e–g). Together, these data show that IL-10 can act directly on CD4+ T cells to promote Th2 polarisation, particularly in sub-maximal polarisation conditions, and that this occurs independently of activation and proliferation.
Fig. 2. IL-10 enhances Th2 differentiation independently of activation and proliferation.
In vitro polarised Th0 and Th2 cells were cultured with αCD3, αCD28 and IL-2 for 4 days with or without IL-10. a Representative histogram of GATA3 staining from IL-10 stimulated and unstimulated Th0 cells. b Percentage of CD4+ GATA3+ CD4 T cells (left) and concentration (pg/ml) of IL-5 (middle) and IL-13 (right) in Th0 culture supernatants. c Representative histogram of GATA3 staining from IL-10 stimulated and unstimulated Th2 cells. d Percentage of CD4+ GATA3+ T cells (left) and concentration (pg/ml) of IL-5 (middle) and IL-13 (right) in Th2 culture supernatants. e Percentage of CD44hi (left) and CD69+ (right) CD4 T cells from IL-10 stimulated and unstimulated Th0 cultures. f Representative histograms of Cell Trace Violet staining from IL-10 stimulated (top) and unstimulated (bottom) Th0 cultures. g Proliferation index of IL-10 stimulated and unstimulated Th0 cultures. Graphed data are shown with mean ± 1 SD and are pooled from three independent experiments with n = 4–5 per experiment. Statistical significance was calculated by Student’s t test where data were normally distributed (b, d, e) and Mann–Whitney U test where data were not normally distributed (g). (Significance **p < 0.01, ****p < 0.0001).
Th2 induction by IL-10 in vitro coincides with suppression of IFNγ
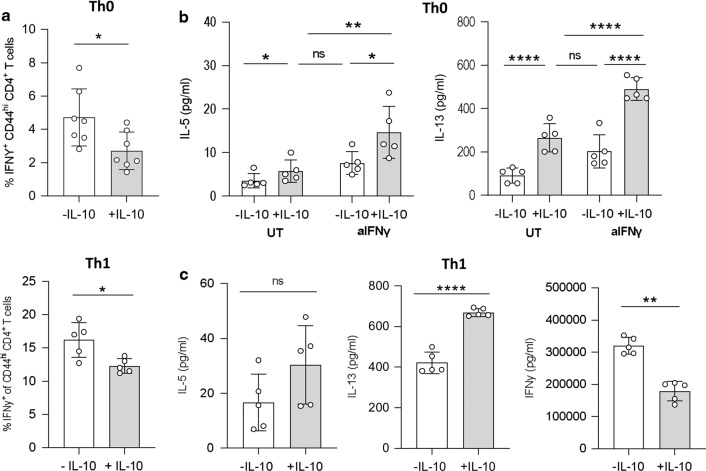
An inverse relationship between IL-10 and IFNγ has been well described18,33,46–48, and we next aimed to determine if the Th2 skewing effects of IL-10 could be due to IFNγ-mediated suppression. The addition of exogenous IL-10 decreased IFNγ expression in the unpolarised Th0 cells, as well as in polarised Th1 cultures (purified CD4+ T cells stimulated with αCD3, αCD28 IL-2 and IL-12) (Fig. 3a, c). We then treated Th0 cells with or without IL-10 while blocking IFNγ signalling, using an anti-IFNγ antibody, to test whether the absence of IFNγ signalling could replicate the Th2 polarisation induced by IL-10 alone. Indeed, Th0 cells cultured without IL-10 but in the absence of IFNγ signalling showed equivalent Th2 polarisation to Th0 cells stimulated with IL-10 alone (Fig. 3b). However, dual treatment of Th0 cells with IL-10 and anti-IFNγ was synergistic and induced higher secretion of IL-5 and IL-13 than each intervention alone (Fig. 3b). Interestingly, the partial reduction in IFNγ production seen even in strongly polarised Th1 cultures (Fig. 3a, c) also corresponded with a rebound in IL-13 expression (Fig. 3c), although any increase in IL-5 expression did not reach statistical significance (Fig. 3c). Together, these data suggest that the ability of IL-10 to enhance Th2 polarisation correlates with its ability to limit IFNγ expression.
Fig. 3. IL-10 suppresses IFNγ expression when promoting Th2 differentiation.
In vitro polarised Th0 cells were cultured with αCD3, αCD28 and IL-2 only, and Th1 cells with the addition of IL-12, for 4 days with or without exogenous IL-10. a The percentage of IFNγ+ CD44hi CD4+ T cells measured in Th0 (top) and Th1 (bottom) cultures. b The concentration (pg/ml) of IL-5 and IL-13 measured in culture supernatants of in vitro polarised Th0 cells cultured as above and with or without anti-IFNγ. c The concentration (pg/ml) of IL-5, IL-13 and IFNγ measured in culture supernatants of in vitro polarised Th1 cells cultured with or without exogenous IL-10. Graphed data are shown with mean ± 1 SD and are representative of two independent experiments with n = 5 per experiment. Statistical significance was calculated by Student’s t test and one-way ANOVA with Tukey’s post-test for multiple comparisons between groups (Significance *p < 0.05, **p < 0.01, ****p < 0.0001).
In the infected intestine, IL-10 receptor expression is higher on Th1 cells than Th2
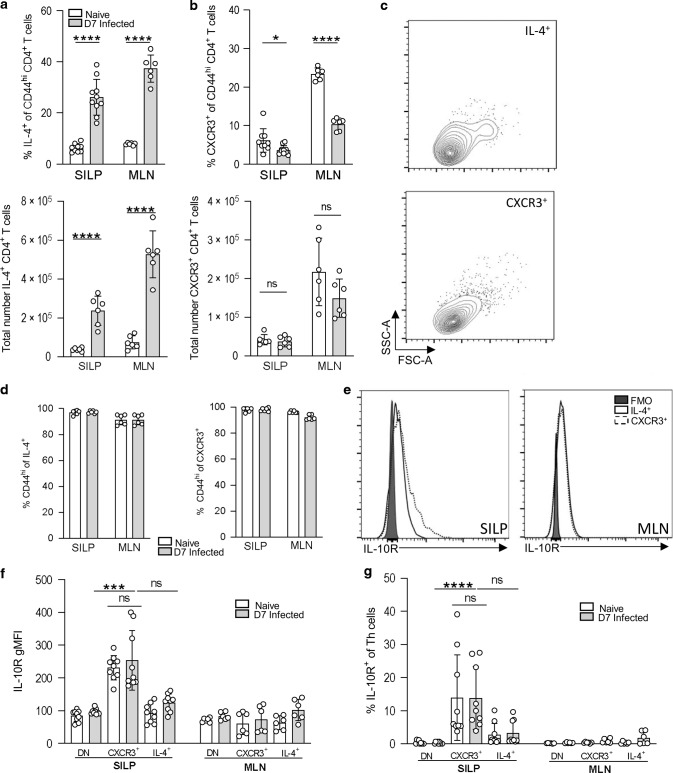
Our in vitro data suggested that IL-10 may promote the Th2 response in part by suppressing IFNγ. To compare the potential IL-10 responsiveness of Th1 and Th2 cells in vivo, we first measured the expression of IL-10R1, which has been shown to correlate closely with changes in cellular responsiveness to IL-1048,49. We infected B6.4get IL-4 reporter mice50 with H. polygyrus and identified Th cell subsets using CXCR3 as a marker of Th1 cells and IL-4-GFP as an indicator of Th2 cells. Our gating and isotype controls are shown in Supplementary Figs. S4–S7. As expected in a helminth infection, the frequency and number of IL-4+ CD4+ Th2 cells increased in both the MLN and small intestine upon H. polygyrus infection (Fig. 4a, b). We were able to define a small population of CXCR3+ CD4+ Th1 cells in the MLN and SILP (Fig. 4a, b), the frequency of which decreased in both the MLN and small intestine as the Th2 cells expanded (Fig. 4a, b). Both CXCR3+ Th1 cells and IL-4/GFP+ Th2 cells appeared activated, as indicated by scatter profile (Fig. 4c) and expression of CD44 (Fig. 4d). The numbers of IL-10+ Th1 and Th2 cells in the small intestine were similar for both populations (Supplementary Fig. S7), but CXCR3+ CD4+ Th1 cells in the small intestine showed significantly higher expression of IL-10R1 than did IL-4/GFP+ Th2 cells, both in frequency and intensity, in infected and uninfected animals (Fig. 4e–g). IL-10R1 expression was significantly higher on Th1 cells in the intestine than in the draining MLN, while the lower level of IL-10R1 expression on Th2 cells was similar in both tissues (Fig. 4e–g). These data indicate that high IL-10R expression is a feature of Th1 cells in the small intestinal mucosa, but not in the draining LN, even during intestinal helminth infection.
Fig. 4. Th1 cells in the small intestine during H. polygyrus infection show high IL-10R expression.
B6.4get mice were infected with 200 L3 H. polygyrus and 7 days post-infection the small intestine and MLN collected for analysis. a Percentage (top) and total number (bottom) of IL-4(GFP)+ Th cells (TCRβ + CD4+ CD44+) in the SILP. b Percentage (top) and total number (bottom) of CXCR3+ Th cells in the SILP. c Representative flow cytometry scatter plot of IL-4+ (top) and CXCR3+ (bottom) Th cells from the SILP of D7 infected mice. d Percentage of CD44hi of IL-4(GFP)+ (left) and CXCR3+ (right) from the SILP and MLN. e Representative overlaid histograms of IL-10R expression of IL-4+ and CXCR3+ Th cells compared to the IL-10R FMO control, in the SILP and MLN of day 7 infected mice. f Geometric mean of IL-10R expression and (g) percentage of IL-10+ cells of IL-4(GFP)+, CXCR3+ and DN (double negative) Th cells in the MLN and SILP. Graphed data are shown with means ± 1 SD and are pooled from three independent experiments with n = 3 per experiment. Statistical significance was calculated by Student’s t test and Kruskal–Wallis test with Dunn’s post-test for multiple comparisons between groups. (Significance *p < 0.05, ***p < 0.001, ****p < 0.0001).
IL-10 signalling blockade in helminth infection leads to Th1 expansion in the infected tissue
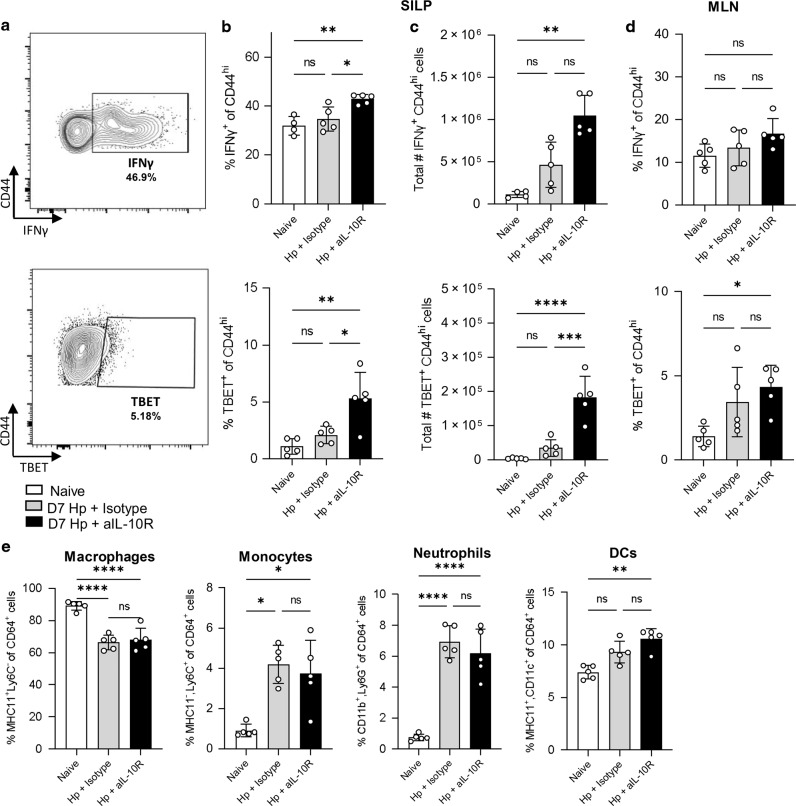
Our data so far had shown that IL-10 expression and IL-10 receptor expression were both concentrated at the site of infection and suggested that the primary T cell target of IL-10 signalling during H. polygyrus infection may be Th1 cells in the small intestine. To test the impact of IL-10 during infection, we disrupted signalling using a blocking antibody against the IL-10R1. IL-10 blockade during H. polygyrus infection caused an increase in the frequency and number of IFNγ+ CD4+ CD44hi Th1 cells in the intestinal tissue (Fig. 5a–c), but no change in the draining MLN (Fig. 5d). Staining for Tbet+ CD4+ CD44hi Th1 cells gave the same result (Fig. 5a–d). We did not observe any significant differences in myeloid cell populations in the intestinal tissue during IL-10R blockade (Fig. 5e) (gating shown in Supplementary Fig. S6). These data indicate that, during H. polygyrus infection, IL-10 acts to limit Th1 expansion and IFNγ expression in the small intestine.
Fig. 5. IL-10 signalling blockade in H. polygyrus infection leads to expansion of Th1 cells in the small intestine.
C57BL/6 mice were infected with 200 L3 H. polygyrus and, at D-1, D2 and D5, treated with anti-IL-10R mAb or a matched isotype control. Seven days post-infection the small intestine and MLN were collected for analysis. a Representative flow cytometry plot of IFNγ (top) and TBET (bottom) staining. b Percentage of IFNγ+ (top) and TBET + (bottom) of CD44hi CD4+ T cells in the SILP. c Total number of IFNγ+ (top) and TBET+ (bottom) of CD44hi CD4+ T cells in the SILP. d Percentage of IFNγ+ (top) and TBET + (bottom) of CD44hi CD4+ T cells in the MLN. e Percentage of Macrophages (MHCII+ Ly6C−), Monocytes (MHCII− Ly6C+), Neutrophils (CD11b+ Ly6G+) and DCs (MHCII+ CD11c+) within the CD64+ population in the SILP. Graphed data are shown with mean ± 1 SD and are representative of 1–3 independent experiments with n = 4–5 per experiment. Statistical significance was calculated by ANOVA followed by a Tukey’s post-test for multiple comparisons between groups where data were normally distributed (b, c (TBET), d, e (macrophages and neutrophils)) and Kruskal–Wallis test with Dunn’s post-test for multiple comparisons between groups where data were not normally distributed (c (IFNγ), e (monocytes and DCs). (Significance *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Immune competition in the small intestine during H. polygyrus infection is regulated by IL-10
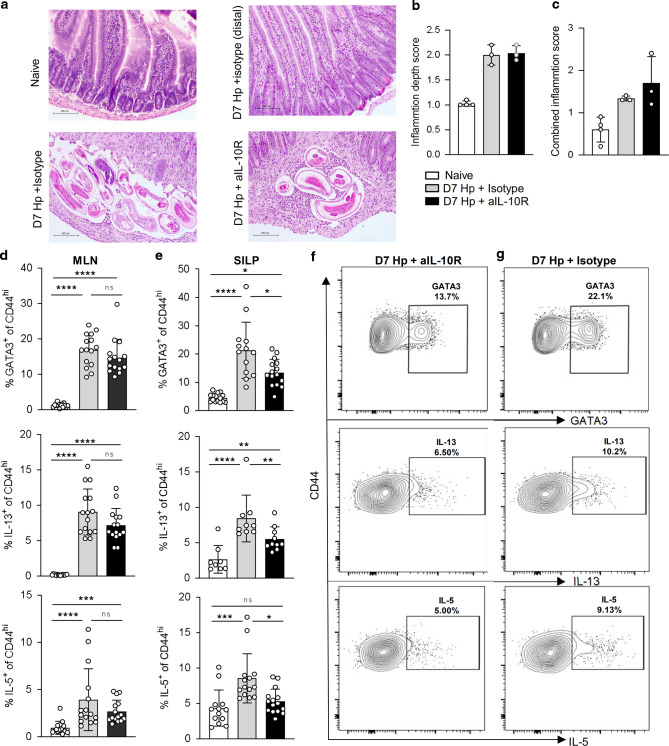
Our observation that IL-10 controls IFNγ expression in the small intestine during H. polygyrus infection prompted two hypotheses. The first was that IL-10 signalling might regulate tissue pathology. H. polygyrus causes only limited pathology in laboratory mice51–53, but parasite larvae cross the intestinal wall twice during infection: around day 2 as they penetrate into the sub-mucosa, where they encyst, mature and moult; and again at D7-8 as the adult worms move back into the intestinal lumen, where they persist by twisting themselves around the surface of the host villi. We predicted that, at D7, the effect of the enhanced IFNγ expression in the absence of IL-10 signalling would be to exaggerate pathology around the encysted larvae or at sites of epithelial disruption. In contrast, our data showed that IL-10 blockade had little effect on intestinal pathology at this timepoint. In infected animals, the severity of inflammation was very variable among areas of the tissue sampled, varying from very mild and mostly mucosal at sites distant from the parasite (Fig. 6a, top row), to severe and submucosal around the encysted parasites (Fig. 6a, bottom left). Comparing pathology in infected animals treated with IL-10R1 blocking mAb versus those infected but given the isotype control did not reveal any significant differences (Fig. 6a, bottom row). When we quantified the histology sections, infection was associated with an increase in both combined inflammation and inflammation depth score, but there was no difference in pathology between mice that were infected with or without disrupted IL-10 signalling (Fig. 6b, c).
Fig. 6. IL-10 signalling promotes the intestinal Th2 response in H. polygyrus infection.
C57BL/6 mice were infected with 200 L3 H. polygyrus and at D-1, D2 and D5 treated with anti-IL-10R mAb or isotype control, and 7 days post-infection the small intestine and MLN collected for analysis. a Representative H&E staining of the duodenum from naïve (top left), D7 infected + isotype control parasite area (bottom left), D7 infected + isotype control distal area (top right) and D7 infected + anti-IL-10R mAb parasite area (bottom right). Histology scoring of (b) inflammation depth and (c) combined inflammation score from the three treatment groups. d Percentage of GATA3+ (top) IL-13+ (middle) IL-5+ (bottom) of CD4+ CD44hi T cells in the MLN. e Percentage of GATA3+ (top) IL-13+ (middle) IL-5+ (bottom) of CD4+ CD44hi T cells in the SILP. Representative staining in the SILP of GATA3 (top), IL-13 (middle) and IL-5 (bottom) from H. polygyrus infected (f anti-IL-10R treated, g isotype control treated). Graphed data are shown with mean ± 1 SD and are pooled from 2–3 independent experiments with n = 4–5 per experiment. Statistical significance was calculated by ANOVA followed by a Tukey’s post-test for multiple comparisons between groups where data were normally distributed (d (IL-5, IL-13) and e (GATA3)) and Kruskal–Wallis test with Dunn’s post-test for multiple comparisons between groups where data were not normally distributed (d (GATA3), e (IL-5, IL-13). (Significance *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Our second hypothesis was that the impact of IL-10 during H. polygyrus infection would be to reduce immune competition between Th1 and Th2 cell subsets and thus allow the Th2 response to expand. To assess this, we repeated the infection in the presence or absence of the anti-IL-10R1 blocking antibody and measured the Th2 response. In the MLN, infection induced a clear Th2 response, shown by increased expression of the Th2 master transcription factor GATA3 and the Th2 cytokines IL-5 and IL-13 in CD4+ CD44hi cells, and this response was not affected by the blockade of IL-10 signalling (Fig. 6d). Th2 immunity in the SILP, however, was significantly decreased during IL-10R1 blockade compared with isotype treated controls (Fig. 6e–g). Together these data demonstrate that IL-10 signalling limits IFNγ expression by Th1 cells in the small intestine during H. polygyrus infection, and suggest that this restriction on IFNγ corresponds with a local expansion of Th2 immune activity in the infected tissue site.
Early IL-10 signalling blockade in H. polygyrus infection alters anti-parasite immunity
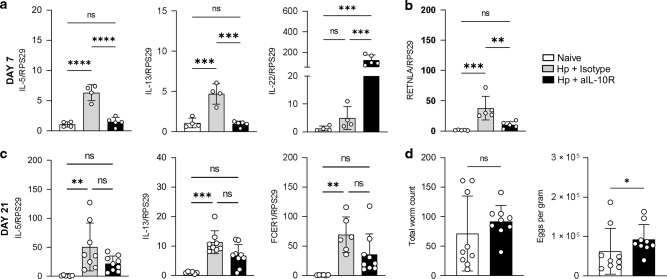
To test whether the changes in T cell immunity imposed by the anti-IL-10R1 blockade correlated with changes in epithelial or anti-parasite immunity, we first examined gene expression in the duodenum at day 7 of infection following the anti-IL-10R1 treatment (or isotype control) on days −1, 2 and 5. Consistent with changes in the T cell response (Fig. 6), Il5 and Il13 gene expression in unfractionated duodenal tissue increased upon infection but was curtailed by IL-10R1 blockade, becoming similar to levels seen in naïve mice (Fig. 7a). In contrast, gene expression of Il22, a cytokine known to be negatively regulated by IL-10, increased 100-fold during IL-10R1 blockade (Fig. 7a). We then examined early markers of type 2 effector activation and saw a similar pattern at day 7: Retlna gene expression, associated with alternative activation of myeloid cells and the type 2 wound healing response, increased on infection but was ablated by the IL-10R1 signalling blockade (Fig. 7b). To assess the impact of these early changes in immunity, we also examined the animals at a time when adult parasites are detectable in the lumen of the gut. IL-10 signalling was again disrupted by aIL-10R1 injections on days −1, 2 and 5, and tissues collected on day 21 (Fig. 7c, d). Il5 and Il13 expression remained elevated in whole duodenal tissue at day 21 of infection, but this increase above naïve control was not statistically significant when IL-10 signalling was disrupted. The same pattern was observed with gene expression for Fcer1, a marker of type 2 effector cell infiltration (Fig. 7c). Finally, we measured the parasite burden at this timepoint. There was no statistical difference in the number of worms present in infected mice in which IL-10 signalling had been disrupted during early infection, compared to infected mice given an isotype control, but parasite egg production was significantly increased in the absence of early IL-10 signalling (Fig. 7d). These data suggest that the regulation of T cell differentiation achieved by IL-10 signalling early in H. polygyrus infection has a significant impact on intestinal immunity and subsequent parasite fitness.
Fig. 7. IL-10 signalling promotes anti-parasite immunity in H. polygyrus infection.
C57BL/6 mice were infected with 200 L3 H. polygyrus and at D-1, D2 and D5 treated with anti-IL-10R mAb or isotype control. Seven or 21 days post-infection the small intestine was collected for analysis. a Gene expression of IL-5 (left), IL-13 (middle) and IL-22 (right) in the duodenum on day 7. b Retnla gene expression in the duodenum on day 7. c Gene expression of IL-5 (left), IL-13 (middle), FCER1 (right) in the duodenum on day 21. All gene expression is standardised against the housekeeping gene (RSP29) and is presented as a fold change relative to mean expression in naïve controls. d Total worm count (left) and eggs per gram of stool (right) from the small intestine at day 21. Graphed data are shown with mean ± 1 SD and are pooled from 2–3 independent experiments with n = 4–5 per experiment. For a–c, statistical significance was calculated by ANOVA followed by a Tukey’s post-test for multiple comparisons between groups where data were normally distributed (a–c (IL-5 and FcER1) and Kruskal–Wallis test with Dunn’s post-test for multiple comparisons between groups where data were not normally distributed (c (IL-13)). For d, statistical significance was calculated by Kolmogorov–Smirnov test to compare the relative distributions (Significance *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Discussion
Understanding the regulation of Th2 immunity is important for a variety of diseases, most prominently helminth infection and allergy. IL-10 is a key regulatory cytokine, but while it is well established that IL-10 is suppressive in type 1 immune settings18,33,54–56, its role during a type 2 immune response is less well understood. IL-10 expression is known to increase in the lymph nodes and blood during type 2 immune responses22,35,36,38,57,58, but it has been suggested both to promote and to restrict Th2 immunity59,60. Here we show that, during infection with the helminth H. polygyrus, the intestinal immune response involves both Th1 and Th2 activity and intestinal IL-10 balances these responses, promoting Th2 cytokine expression by limiting local Th1 cells.
The original identification of IL-10 was as an effector cytokine of Th2 immunity18, but there have been mixed reports of its regulatory impact on Th2 cells. He et al. recently argued that IL-10 signalling is critical for the development of the Th2 response in a murine model of asthma22, and Coomes et al. have previously reported that IL-10 promotes full Th2 differentiation in allergic airway inflammation23. In contrast, others have reported that IL-10 inhibits Th2 activity in the lung23,61. Even within a single parasite infection with Trichinella spiralis, IL-10 can both suppress Th2 immunity in infected muscle and promote Th2 activity in infected instestine25. Site-specific variation in IL-10 signalling complements our growing understanding of the importance of tissue-specific regulation of immunity62,63. One of the location-dependent factors that could influence IL-10’s impact might be the presence or absence of an underlying Th1 response. Despite containing fewer bacteria than the colon, the small intestine still has an abundant microbiome64 and any breach of the intestinal epithelium provides an opportunity for bacterial translocation and the stimulation of anti-bacterial immunity. In H. polygyrus infection, larvae and adult worms burrow into and out of the intestinal wall of the small intestine at days 1–2 and 7–8 of infection. Barrier disruption in the presence of intestinal bacteria has been hypothesised to lead to IFNγ expression65, and our data showing Th1 expansion in the H. polygyrus-infected intestine when IL-10 is blocked provide new experimental evidence. Bacterial translocation has been reported in other infections that damage the integrity of the intestinal wall, such as in Toxoplasma gondii, where the microbiota-specific T cell response has been shown to amplify the parasite-specific Th1 response to infection66. In helminth infection, where protective immunity is Th2 biased, a Th1 component to the anti-parasite immune response may instead act as a competitive inhibitor39.
H. polygyrus infection is also associated with a shift in the balance of bacterial species in the intestine, favouring an expansion of Lactobacillae67, and this altered microbiome might alone be immunogenic65,67,68. In Trichuris muris infection, Duque-Correa et al. have reported that IL-10 can influence both the composition of the intestinal microbiome and the translocation of those bacteria across the intestinal wall69. Here we report that IL-10 in H. polygyrus infection regulates an intestinal Th1 response, and it will be interesting in future studies to assess whether the underlying Th1 response is a reaction to helminth- and/or bacterial-derived stimuli.
In many protozoan and bacterial infections, the role of IL-10 in limiting IFNγ expression is critical for host survival, suppressing damaging immunopathology33,34,70,71. IL-10 has also been proposed to limit tissue pathology in the intestine by promoting epithelial cell proliferation and subsequent colonic wound repair, via WISP-1 signalling72. Despite these data, we did not see significant changes in intestinal pathology in H. polygyrus infection when IL-10 signalling was blocked. Pathology in H. polygyrus infection is both mild and patchy, concentrated around the granulomas that encase developing larvae10,73. H. polygyrus has recently also been shown to promote epithelial repair in intestinal organoids, reducing goblet and tuft cell differentiation and favouring the development of enterocytes74, which may have limited our ability to observe an impact of IL-10 on epithelial structure. However, our data did reveal striking differences in intestinal cytokine profiles early in infection and altered intestinal immunity and parasite fecundity at later timepoints, likely reflecting reduced fitness of adult worms. Together our data suggest that, in H. polygyrus infection, IL-10 suppresses IFNγ to enhance Th2 function and increase anti-parasite immunity. Interestingly, the ability of IL-10 to promote the Th2 response was observed only in the SILP and not in the draining lymph node, complementing a growing understanding of the importance of tissue-specific regulation of immunity62,63.
When we examined the sources of intestinal IL-10 during helminth infection, our data suggested that multiple cell types are involved. Th1 cells are known to express IL-10 in a number of parasite infections54,55,75 and IL-10 signalling is often autocrine55,76. Th2 cells, CD8+ T cells, B cells and myeloid cell populations have all also been shown to be capable of releasing IL-10 during infection (reviewed in34). In this study we focused on the early, larval stages of H. polygyrus infection, when the helminth larvae traffic through and within the intestinal wall. The regulatory networks activated at this time might persist or be modified once the worm exits the intestinal wall, but our data on intestinal gene expression and parasite numbers at day 21 of infection suggest that the cytokine balance established during the first seven days of infection has a lasting impact.
Immune regulation through the balance of opposing T cell cytokines is a common feature of infection. In mice, H. polygyrus infection of MyD88−/− animals leads to reduced IFNγ expression, heightened IL-4, and accelerated parasite expulsion77. Mice without a functional IL-4 receptor show exaggerated IFNγ recall responses during H. polygyrus infection, compared with wildtype controls39. Cytokine exclusion is often less absolute in humans, but a recent report of a child with an inherited Tbet deficiency described elevated Th2 cytokine production78. The mechanisms of regulation by opposing Th1 and Th2 cytokines can include direct molecular inhibition of signalling within the CD4+ T cell, such as STAT1-driven induction of Tbet, and Tbet mediated suppression of GATA379. Cytokine competition can also be achieved through different conditioning of antigen presenting cells, recruitment of different effector cells, or alteration of metabolic profiles80. Our data emphasise that such cytokine competition is a key feature of the intestinal immune response during enteric helminth infection, and that IL-10 is a key regulator of this process.
Our observation of high expression of the IL-10 receptor on Th1 cells in the intestine, greater than on intestinal Th2 cells or on all T cells in the draining lymph node, suggested strong, local IL-10 signalling to Th1 cells. Surface expression of cytokine receptors reflects both gene expression and surface binding, internalisation and recycling81 and, in vitro, when exogenous cytokines are added at supraphysiological concentrations, active signalling can result in loss of surface expression of the receptors82,83. In vivo, active cytokine concentrations are lower and cell surface receptor stripping is less commonly observed84; instead, increased receptor expression is associated with increased signalling48,85. Macrophage expression of IL-10R1 determines their responsiveness to exogenous IL-1049. Decreased IL-10R1 expression on peripheral T cells in lupus patients correlates with heightened T cell activity, suggesting that reduced receptor expression is associated with reduced IL-10 function86. Our data show high expression of IL-10R1 on Th1 cells in the intestine, and increased frequency of intestinal Tbet+ or IFNγ+ T cells when IL-10 signalling is blocked. Together these data suggest that understanding receptor expression will be an important step in targeting attempts to use IL-10 therapeutically, which has been challenging and highly context-dependent34,87.
In summary, we have shown that IL-10 promotes the intestinal Th2 response to a helminth infection. We show that IL-10 can signal directly to CD4+ T cells to increase Th2 differentiation, but that IL-10 production and IL-10 receptor expression are both concentrated in the infected tissue rather than the MLN. High expression of the IL-10R by Th1 cells in the small intestine suggests that these cells are sensitive to IL-10 signalling and subsequent IL-10 mediated suppression, providing an indirect mechanism in which IL-10 promotes the intestinal Th2 response and reduces parasite fitness. Our data provide new insight into the complexity of tissue-based regulation during a Th2 immune response and suggest that IL-10 may be an interesting candidate for therapeutic targeting in Th2 dominated diseases such as allergy, asthma and helminth infection.
Methods
Mice and infection
C57BL/6 mice were purchased from Envigo (Huntingdon, UK). B6.4get mice were kindly provided by Professor Judi Allen (University of Manchester) and bred in-house at the University of Glasgow. These mice were first developed by Mohrs et al.50. Il10gfp-foxp3rfp B6 mice were bred in-house (University of Glasgow). These mice express two separate transgenes: IRES-eGFP inserted at the last exon and before the polyadenylation site of the Il10 gene44 and IRES-RFP inserted at this site of the Foxp3 gene45. For each experiment mice were sex-matched and used at age 6–12 weeks. Animals were maintained in individually ventilated cages under standard animal house conditions at the University of Glasgow and procedures were performed under a UK Home Office licence (Project number 70/8483) in accordance with UK Home Office regulations and following review by the University of Glasgow Ethics Committee. Mice were acclimatised for 1 week after arrival in the animal unit before use. For infections, H. polygyrus (also known as H. polygyrus bakeri or H. bakeri) was maintained in the laboratory as described88, and experimental animals were infected with 200 L3 larvae by oral gavage.
Isolation of cells
Lamina propria leucocytes were isolated as described previously40. The MLN was harvested and crushed through a 70 μm filter to obtain a single cell suspension. For experiments where myeloid cells were analysed, MLNs were digested for 40 min in a shaking incubator using 1 mg/ml collagenase D (Merck) in RPMI. Cells were counted and dead cell exclusion carried out using trypan blue.
In vitro CD4+ T cell culture and proliferation
Negative selection of CD4+ T cells from naïve splenocytes was carried out using the MojoSort™ magnetic cell separation system (Biolegend). CD4+ T cells were re-suspended in in RPMI 1640 supplemented with 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mM L-glutamine and stimulated in a 96-well plate with plate bound αCD3 (1 μg/ml), soluble αCD28 (1 μg/ml) and appropriate stimulation and polarisation cocktails. Polarisation cocktails: Th0: 20 ng/ml IL-2, Th2: IL-2 (20 ng/m), IL-4 (40 ng/ml) (Thermo Fisher), αIFNγ (1 μg/ml) (Biolegend). Th1: IL-2 (20 ng/m), IL-12 (10 ng/ml) (Thermo Fisher). Cells were then cultured for 4 days 37 °C, 5% CO2. For IL-10 stimulation, IL-10 (Thermo Fisher) was added at 10 ng/ml. For assessing CD4 T cell proliferation the CellTrace™ Violet Cell Proliferation Kit (Thermo Fisher) was used according to manufacturer’s guidelines.
Ex vivo re-stimulation
To measure cytokine production following ex vivo re-stimulation, unfractionated MLN cells were resuspended at 5 × 106 cells/ml in RPMI 1640 supplemented with 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mM L-glutamine. In total, 500,000 cells were then added to each well, coated with αCD3 (1 μg/ml). Cultures were incubated (37 °C, 5% CO2) for 3 days and then supernatants collected for further analysis.
Cytokine measurement in supernatants
Supernatants were collected from in vitro T cell cultures or ex vivo stimulated cultures and stored at −20 °C until further analysis. For cytokine measurements, supernatants were diluted 1/200 in sterile filtered FACS buffer (PBS, 2 mM EDTA and 10% FCS). Cytokines (IL-5 and IL-13) were measured using BD™ CBA Flex Sets (BD Biosciences) according to manufacturer’s guidelines. The cytometric bead array was analysed using the MACSQuant® Analyser (Miltenyi Biotec). Analysis was performed using FlowJo (Treestar).
Flow cytometry and intracellular cytokine staining
To measure cytokine production immediately ex vivo, cells were stimulated and then stained for flow cytometry. In total, 3 × 106 cells were resuspended in in 500 μl of RPMI 1640 supplemented with 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine and 2 μl/ml solution of cell stimulation cocktail and protein transport inhibitors (Invitrogen eBioscience™ Cell Stimulation Cocktail plus protein transport inhibitors (500X)). After 4 h of stimulation, cells were washed and stained with Fixable viability dye eFluor 780 or 506 (eBioscience), to enable dead cell exclusion, and then with anti-mouse CD16/32 Antibody (BioLegend) as an FC block, to reduce non-specific binding. Samples were next stained for surface markers for 20 min at 4 °C with the following antibodies (all from BioLegend, unless otherwise noted): PerCP-Cy5.5-conjugated anti-TCRβ (clone H57–597), APC-CY7- or AlexaFluor 647-conjugated anti-B220 (RA3-6B2), APC-CY7- or AlexaFluor 700-conjugated anti-MHCII (M5/114.15.2, eBioscience), APC-conjugated anti-IL-7Rα (A7R34), BV421 or FITC-conjugated anti-CD44 (IM7), PE-Cy7-conjugated anti-CXCR3 (CXCR3-173), PE-conjugated IL-10R (1B1.3a), APC-Cy7-conjugated anti-CD19 (6D5), BUV395- or BV421-conjugated CD45 (30-F11, BD Bioscience), BV711-conjugated anti-CD4 (RM4-5), PE-Cy7-conjugated anti-CD8 (53.6.7), BV505-conjugated anti-CD4 (RM4-5), FITC-conjugated anti-CD69 (H1.2F3), AlexaFluor 647-conjugated anti-CD3 (17A2), PerCP-Cy5.5-conjugated anti-CD11c (N418), BV421- or BV605-conjugated anti-CD11b (M1/70), BV510-conjugated anti-Ly6C (HK1.4), PE-conjugated anti-Ly6G (1A8), and PE-Cy7-conjugated anti-CD64 (X54-5/7.1). Samples were then permeabilised and fixed for intracellular cytokine staining using 150 μl of BD Cytofix/Cytoperm™ for 20 min at 4 °C. Samples were stained using 50 μl of intracellular anti-cytokine antibody stain: PE-Cy7-conjugated anti-IL-13 (eBio13A, Invitrogen), PE-conjugated anti-IL-5 (TRFK5, BioLegend), e450-conjugated anti-IFNγ (XMG1.2, Invitrogen)) or appropriate isotype controls for 1 h at room temperature in the dark). When including staining for intracellular transcription factors, samples were then permeabilised and fixed intracellularly using the eBioscience™ Foxp3/Transcription Factor Staining Kit (Thermo Fisher) for 1 h at room temperature in the dark. Samples were stained with 100 μl of intracellular anti-transcription factor stain: eFluor 450-conjugated anti-Foxp3 (FJK-16s, Thermo Fisher), PE-Cy7-conjugated anti-T-bet (eBio4B10, Thermo Fisher) and PE-conjugated anti-GATA3 (TWAJ, Thermo Fisher) for 1.5 h at room temperature in the dark. For anti-GFP retrieval, cells were permeabilised and fixed for anti-GFP staining using 150 μl of BD Cytofix/Cytoperm™ for 20 min at 4 °C. Samples were then stained with anti-GFP (FM264G, BioLegend) for 45 min at 4 °C. Cells were then further permeabilised using the eBioscience™ Foxp3/Transcription kit, as described above, for transcription factor staining. All samples were acquired immediately after staining on either a BD LSRII flow cytometer or a BD LSR FORTESSA running FACS-Diva software (BD Biosciences). Analysis was performed using FlowJo (Treestar).
RNA extraction and real-time PCR
In total, 1 cm section of the top of the duodenum was collected, placed in RNA later (Qiagen) and kept at 4 °C. RNA was purified using RNEASY Mini Kit (Qiagen) and its concentration determined using a Nanodrop 1000. cDNA was generated using the High Capacity cDNA Reverse Transcription Kit (Invetrogen). For real-time PCR, PowerUp™ SYBR™ Green Master Mix (Applied Biosystems) and QuantStudio 6 Flex Real-time PCR system (Applied Biosystems) were used. Values were normalised to ribosomal protein S29 (RPS29) and expression of gene of interest determined using the 2−ΔΔC(t) method. The following primers were used (all primers are shown 5′-3′); Rps29 Fwd, ACGGTCTGATCCGCAAATAC, Rev, CATGATCGGTTCCACTTGGT; Il13 Fwd, CCTGGCTCTTGCTTGCCTT, Rev, GGTCTTGTGTGATGTTGCTCA; Il5 Fwd, CTCTGTTGACAAGCAATGAGACG, Rev, TCTTCAGTATGTCTAGCCCCTG; Il22 Fwd, TTTCCTGACCAAACTCAGCA, Rev, CTGGATGTTCTCGTCGTCAC; Retnla Fwd TATGAACAGATGGGCCTCCT, Rev, GGCAGTTGCAAGTATCTCCAC; Fcer1 Fwd, CCTTTCCTGCTATGGGAACA, Rev GTCTGAAGGAGCAGCCAAT.
IL-10R1 monoclonal antibody blockade
IL-10R signalling was blocked using an IL-10R1 monoclonal antibody (Clone 1b1.3a BioXcell). An anti-HRP rat IgG1 antibody (BioXcell) was used as the isotype-matched control. In total, 200 μl of a 2.5 mg/ml stock solution of each antibody was injected intraperitoneally at days −1, 2, and 5 of H. polygyrus infection. Infected mice treated with anti-IL-10R or isotype were kept in mixed cages.
Histology and scoring
The first 6 cm of the duodenum were collected, sliced into 1 cm pieces and placed in 10% neutral buffered formalin. Samples were fixed overnight, trimmed and embedded in paraffin wax. Tissue sections were collected on frosted glass slides and stained with haematoxylin and eosin. The depth of inflammation, and a combined score of depth and severity of inflammation, were evaluated blindly by a certified pathologist (V.G.) in several high-power fields of two intestinal sections per animal using a protocol established to previously89. The scoring system was from 0-4: 0—Minimal and mucosal, 1—Mild and mucosal/submucosal or minimal transmural, 2—Moderate and mucosal/submucosal, mild transmural or marked and mucosal, 3—Marked mucosal/submucosal or moderate and transmural, 4—Marked and transmural.
Parasite counts
Assessment of H. polygyrus parasite load was conducted on day 21 of infection. To count adult worms, small intestines from stomach to caecum were removed and opened longitudinally for examination on a dissecting microscope. To count parasite eggs, two to three faecal pellets were collected from the colon, weighed, and dissolved overnight in 1 ml dH20. Samples were mixed with 1 ml saturated salt solution (>400 g NaCl in 1 l dH20) and then counted using a McMaster egg counting chamber. Data are presented as eggs per gram of faecal material.
Statistical analysis
All statistical analysis was carried out using GraphPad Prism (version 8 or 9) and data are presented with mean + standard deviation. All data sets were tested for normality using the Shapiro–Wilk normality test and where data were normally distributed, a Student’s t test was used for comparison between two groups and a one-way ANOVA with Tukey’s multiple comparison correction for comparisons between three or more groups. Where data was not normally distributed, a Mann–Whitney U test was used to compare medians between two groups, a Kolmogorov–Smirnov test was used to compare distributions of the two groups, and a Kruskal–Wallis test with Dunn’s multiple comparison correction was used for comparisons between three or more groups. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns = not significant.
Supplementary information
Acknowledgements
We thank David Dow, Joanne Battersby and staff in the Wolfson Research Unit for animal husbandry; Nicola Britton and Claire Ciancia for H. polygyrus larvae and life cycle maintenance, and for advice and discussion; Allan Mowat for critical review of the manuscript; the Flow Core at the University of Glasgow and especially Diane Vaughan for flow cytometry support; Lucy McShane and Lotus Westerhof for sample processing; and Anna Heawood and Jen McCavitt for assistance with qPCR. This work was supported by the MRC (grant MR/S009779/1) and funds from the University of Glasgow, including a University of Glasgow MVLS PhD studentship awarded to H.C.W. R.M.M. also received Wellcome Trust support through an Investigator Grant (Ref 219530), and the Wellcome Trust core-funded Wellcome Centre for Integrative Parasitology (Ref: 104111).
Author contributions
H.C.W. conceived and refined the experimental and conceptual design of the study, conducted experiments, analysed data and prepared the manuscript. V.G. is a veterinary pathologist and conducted all analysis of the histopathology and developed a scoring system for histological samples. A.T.A., O.J.R., J.M.C., A.L.S. and G.A.H. performed experiments and acquired data. S.W.F.M. provided critical expertise and edited the manuscript. R.M.M. contributed to the conceptual design of the study, provided critical expertise and edited the manuscript. G.P.W. conceived and refined the experimental and conceptual design of the study, performed parasite counts, analysed data, and prepared and edited the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41385-022-00513-y.
References
- 1.Hotez PJ, et al. Helminth infections: the great neglected tropical diseases. J. Clin. Investig. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gause WC, Urban JF, Stadecker MJ. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 2003;24:269–277. doi: 10.1016/S1471-4906(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 3.Urban JF, Jr., Katona IM, Finkelman FD. Heligmosomoides polygyrus: CD4+ but not CD8+ T cells regulate the IgE response and protective immunity in mice. Exp. Parasitol. 1991;73:500–511. doi: 10.1016/0014-4894(91)90074-7. [DOI] [PubMed] [Google Scholar]
- 4.Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat. Rev. Immunol. 2013;13:607–614. doi: 10.1038/nri3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 6.Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Gold MJ, et al. Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, Th2-inducing allergen exposures. J. Allergy Clin. Immunol. 2014;133:1142–1148. doi: 10.1016/j.jaci.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 8.Mayer JU, et al. Different populations of CD11b(+) dendritic cells drive Th2 responses in the small intestine and colon. Nat. Commun. 2017;8:15820–15820. doi: 10.1038/ncomms15820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redpath SA, et al. Functional specialization of intestinal dendritic cell subsets during Th2 helminth infection in mice. Eur. J. Immunol. 2018;48:87–98. doi: 10.1002/eji.201747073. [DOI] [PubMed] [Google Scholar]
- 10.Anthony RM, et al. Protective immune mechanisms in helminth infection. Nat. Rev. Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maizels RM, Pearce EJ, Artis D, Yazdanbakhsh M, Wynn TA. Regulation of pathogenesis and immunity in helminth infections. J. Exp. Med. 2009;206:2059–2066. doi: 10.1084/jem.20091903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang H-E, et al. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat. Immunol. 2011;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J. Exp. Med. 2009;206:1001–1007. doi: 10.1084/jem.20090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Dyken SJ, et al. A tissue checkpoint regulates type 2 immunity. Nat. Immunol. 2016;17:1381–1387. doi: 10.1038/ni.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochiai S, et al. Thymic stromal lymphopoietin drives the development of IL-13(+) Th2 cells. Proc. Natl Acad. Sci. USA. 2018;115:1033–1038. doi: 10.1073/pnas.1714348115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redpath SA, Heieis G, Perona-Wright G. Spatial regulation of IL-4 signalling in vivo. Cytokine. 2015;5:51–56. doi: 10.1016/j.cyto.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the Interleukin-10 Receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 18.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halonen M, et al. Th1/Th2 patterns and balance in cytokine production in the parents and infants of a large birth cohort. J. Immunol. 2009;182:3285–3293. doi: 10.4049/jimmunol.0711996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forsthuber T, Yip HC, Lehmann PV. Induction of Th1 and Th2 immunity in neonatal mice. Science. 1996;271:1728. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 21.Laouini D, et al. IL-10 is critical for Th2 responses in a murine model of allergic dermatitis. J. Clin. Investig. 2003;112:1058–1066. doi: 10.1172/JCI18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He K, et al. Blimp-1 is essential for allergen-induced asthma and Th2 cell development in the lung. J. Exp. Med. 2020;217:e20190742. doi: 10.1084/jem.20190742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coomes SM, et al. CD4 + Th2 cells are directly regulated by IL-10 during allergic airway inflammation. Mucosal Immunol. 2017;10:150–161. doi: 10.1038/mi.2016.47. [DOI] [PubMed] [Google Scholar]
- 24.Malisan F, et al. Interleukin-10 induces immunoglobulin G isotype switch recombination in human CD40-activated naive B lymphocytes. J. Exp. Med. 1996;183:937–947. doi: 10.1084/jem.183.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helmby H, Grencis RK. Contrasting roles for IL-10 in protective immunity to different life cycle stages of intestinal nematode parasites. Eur. J. Immunol. 2003;33:2382–2390. doi: 10.1002/eji.200324082. [DOI] [PubMed] [Google Scholar]
- 26.Thompson-Snipes L, et al. Interleukin 10: a novel stimulatory factor for mast cells and their progenitors. J. Exp. Med. 1991;173:507–510. doi: 10.1084/jem.173.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghildyal N, et al. IL-10 induces transcription of the gene for mouse mast cell protease-1, a serine protease preferentially expressed in mucosal mast cells of Trichinella spiralis-infected mice. J. Immunol. 1992;149:2123. [PubMed] [Google Scholar]
- 28.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-P. [DOI] [PubMed] [Google Scholar]
- 29.Berg DJ, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J. Clin. Investig. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yen D, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Investig. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kullberg MC, et al. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect. Immun. 1998;66:5157–5166. doi: 10.1128/IAI.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sellon RK, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 1998;66:5224–5231. doi: 10.1128/IAI.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J. Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 34.Redpath SA, Fonseca NM, Perona-Wright G. Protection and pathology during parasite infection: IL-10 strikes the balance. Parasite Immunol. 2014;36:233–252. doi: 10.1111/pim.12113. [DOI] [PubMed] [Google Scholar]
- 35.Finney CAM, Taylor MD, Wilson MS, Maizels RM. Expansion and activation of CD4+CD25+ regulatory T cells in Heligmosomoides polygyrus infection. Eur. J. Immunol. 2007;37:1874–1886. doi: 10.1002/eji.200636751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Setiawan T, et al. Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine. Infect. Immun. 2007;75:4655–4663. doi: 10.1128/IAI.00358-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redpath SA, et al. ICOS controls Foxp3(+) regulatory T-cell expansion, maintenance and IL-10 production during helminth infection. Eur. J. Immunol. 2013;43:705–715. doi: 10.1002/eji.201242794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schopf LR, Hoffmann KF, Cheever AW, Urban JF, Wynn TA. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J. Immunol. 2002;168:2383–2392. doi: 10.4049/jimmunol.168.5.2383. [DOI] [PubMed] [Google Scholar]
- 39.Balic A, Harcus YM, Taylor MD, Brombacher F, Maizels RM. IL-4R signaling is required to induce IL-10 for the establishment of Th2 dominance. Int. Immunol. 2006;18:1421–1431. doi: 10.1093/intimm/dxl075. [DOI] [PubMed] [Google Scholar]
- 40.Webster HC, Andrusaite AT, Shergold AL, Milling SWF, Perona-Wright G. Isolation and functional characterisation of lamina propria leukocytes from helminth-infected, murine small intestine. J. Immunological Methods. 2020;477:112702. doi: 10.1016/j.jim.2019.112702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferrer-Font L, et al. High-dimensional analysis of intestinal immune cells during helminth infection. eLife. 2020;9:e51678. doi: 10.7554/eLife.51678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jarjour NN, et al. BHLHE40 promotes Th2 cell–mediated antihelminth immunity and reveals cooperative CSF2RB family cytokines. J. Immunol. 2020;204:923. doi: 10.4049/jimmunol.1900978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hang L, et al. Heligmosomoides polygyrus bakeri infection activates colonic Foxp3 + T cells enhancing their capacity to prevent colitis. J. Immunol. 2013;191:1927. doi: 10.4049/jimmunol.1201457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamanaka M, et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 45.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc. Natl Acad. Sci. USA. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu X, et al. IFN-γ suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity. 2006;24:563–574. doi: 10.1016/j.immuni.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 47.Gazzinelli RT, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J. Immunol. 1996;157:798. [PubMed] [Google Scholar]
- 48.Shouval DS, et al. Interleukin 10 receptor signaling: master regulator of intestinal mucosal homeostasis in mice and humans. Adv. Immunol. 2014;122:177–210. doi: 10.1016/B978-0-12-800267-4.00005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Lanzenauer SH, et al. Interleukin-10 receptor-1 expression in monocyte-derived antigen-presenting cell populations: dendritic cells partially escape from IL-10’s inhibitory mechanisms. Genes Immun. 2015;16:8–14. doi: 10.1038/gene.2014.69. [DOI] [PubMed] [Google Scholar]
- 50.Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23:419–429. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu S-K. Pathology of nematospiroides dubius. I. Primary infections in C3H and webster mice. Exp. Parasitol. 1965;17:123–135. doi: 10.1016/0014-4894(65)90016-0. [DOI] [PubMed] [Google Scholar]
- 52.Reynolds LA, Filbey KJ, Maizels RM. Immunity to the model intestinal helminth parasite Heligmosomoides polygyrus. Semin Immunopathol. 2012;34:829–846. doi: 10.1007/s00281-012-0347-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monroy FG, Enriquez FJ. Heligmosomoides polygyrus: a model for chronic gastrointestinal helminthiasis. Parasitol. Today. 1992;8:49–54. doi: 10.1016/0169-4758(92)90084-F. [DOI] [PubMed] [Google Scholar]
- 54.Villegas-Mendez A, et al. Parasite-specific CD4+ IFN-γ+ IL-10+ T cells distribute within both lymphoid and nonlymphoid compartments and are controlled systemically by interleukin-27 and ICOS during blood-stage malaria infection. Infect. Immun. 2015;84:34–46. doi: 10.1128/IAI.01100-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jankovic D, et al. Conventional T-bet+ Foxp3- Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J. Exp. Med. 2007;204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perona-Wright G, et al. Persistent loss of IL-27 responsiveness in CD8+ memory T cells abrogates IL-10 expression in a recall response. Proc. Natl Acad. Sci. USA. 2012;109:18535–18540. doi: 10.1073/pnas.1119133109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marinho FV, et al. Schistosoma mansoni Tegument (Smteg) induces IL-10 and modulates experimental airway inflammation. PLoS ONE. 2016;11:e0160118–e0160118. doi: 10.1371/journal.pone.0160118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dias FF, et al. Identification of piecemeal degranulation and vesicular transport of MBP-1 in liver-infiltrating mouse eosinophils during acute experimental. Front Immunol. 2018;9:3019. doi: 10.3389/fimmu.2018.03019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 2000;164:6406. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 60.Hawrylowicz CM, O’Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat. Rev. Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 61.Golebski K, et al. Induction of IL-10-producing type 2 innate lymphoid cells by allergen immunotherapy is associated with clinical response. Immunity. 2021;54:291–307. doi: 10.1016/j.immuni.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 62.Hu W, Pasare C. Location, location, location: tissue-specific regulation of immune responses. J. Leukoc. Biol. 2013;94:409–421. doi: 10.1189/jlb.0413207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molofsky AB, Locksley RM. Tissue immunity broadcasts near and far. Nat. Rev. Immunol. 2020;20:93–94. doi: 10.1038/s41577-019-0250-4. [DOI] [PubMed] [Google Scholar]
- 64.Bowcutt R, et al. Heterogeneity across the murine small and large intestine. World J. Gastroenterol. 2014;20:15216–15232. doi: 10.3748/wjg.v20.i41.15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rapin A, Harris NL. Helminth-bacterial interactions: cause and consequence. Trends Immunol. 2018;39:724–733. doi: 10.1016/j.it.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 66.Hand TW, et al. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science. 2012;337:1553–1556. doi: 10.1126/science.1220961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reynolds LA, et al. Commensal-pathogen interactions in the intestinal tract: lactobacilli promote infection with, and are promoted by, helminth parasites. Gut Microbes. 2014;5:522–532. doi: 10.4161/gmic.32155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walk ST, Blum AM, Ewing SA-S, Weinstock JV, Young VB. Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus. Inflamm. Bowel Dis. 2010;16:1841–1849. doi: 10.1002/ibd.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duque-Correa MA, et al. Exclusive dependence of IL-10Rα signalling on intestinal microbiota homeostasis and control of whipworm infection. PLOS Pathog. 2019;15:e1007265. doi: 10.1371/journal.ppat.1007265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suzuki Y, et al. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J. Immunol. 2000;164:5375. doi: 10.4049/jimmunol.164.10.5375. [DOI] [PubMed] [Google Scholar]
- 71.Arai T, et al. Effects of in vivo administration of anti-IL-10 monoclonal antibody on the host defence mechanism against murine Salmonella infection. Immunology. 1995;85:381–388. [PMC free article] [PubMed] [Google Scholar]
- 72.Morhardt TL, et al. IL-10 produced by macrophages regulates epithelial integrity in the small intestine. Sci. Rep. 2019;9:1223. doi: 10.1038/s41598-018-38125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Menge DM, et al. Mapping of chromosomal regions influencing immunological responses to gastrointestinal nematode infections in mice. Parasite Immunol. 2003;25:341–349. doi: 10.1046/j.1365-3024.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 74.Drurey C, et al. Intestinal epithelial tuft cell induction is negated by a murine helminth and its secreted products. J. Exp. Med. 2022;219:e20211140. doi: 10.1084/jem.20211140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Belkaid Y, et al. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 2001;194:1497–1506. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jankovic D, Kugler DG, Sher A. IL-10 production by CD4+ effector T cells: a mechanism for self-regulation. Mucosal Immunol. 2010;3:239–246. doi: 10.1038/mi.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reynolds LA, et al. MyD88 signaling inhibits protective immunity to the gastrointestinal helminth parasite Heligmosomoides polygyrus. J. Immunol. 2014;193:2984–2993. doi: 10.4049/jimmunol.1401056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang R, et al. High Th2 cytokine levels and upper airway inflammation in human inherited T-bet deficiency. J. Exp. Med. 2021;218:e20202726. doi: 10.1084/jem.20202726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Usui T, et al. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J. Exp. Med. 2006;203:755–766. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Butcher MJ, Zhu J. Recent advances in understanding the Th1/Th2 effector choice. Fac. Rev. 2021;10:30–30. doi: 10.12703/r/10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cendrowski J, Mamińska A, Miaczynska M. Endocytic regulation of cytokine receptor signaling. Cytokine Growth Factor Rev. 2016;32:63–73. doi: 10.1016/j.cytogfr.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 82.Perona-Wright G, Mohrs K, Mohrs M. Sustained signaling by canonical helper T cell cytokines throughout the reactive lymph node. Nat. Immunol. 2010;11:520–526. doi: 10.1038/ni.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perona-Wright G, Mohrs K, Mayer KD, Mohrs M. Differential regulation of IL-4Ralpha expression by antigen versus cytokine stimulation characterizes Th2 progression in vivo. J. Immunol. 2010;184:615–623. doi: 10.4049/jimmunol.0902408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ingram JT, Yi JS, Zajac AJ. Exhausted CD8 T cells downregulate the IL-18 receptor and become unresponsive to inflammatory cytokines and bacterial co-infections. PLoS Pathog. 2011;7:e1002273. doi: 10.1371/journal.ppat.1002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Y, Wei SH, Ho AS, de Waal Malefyt R, Moore KW. Expression cloning and characterization of a human IL-10 receptor. J. Immunol. 1994;152:1821. [PubMed] [Google Scholar]
- 86.Cui HD, et al. Interleukin-10 receptor expression and signalling were down-regulated in CD4+ T cells of lupus nephritis patients. Clin. Exp. Immunol. 2011;165:163–171. doi: 10.1111/j.1365-2249.2011.04424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rallis KS, et al. Cytokine-based cancer immunotherapy: challenges and opportunities for IL-10. Anticancer Res. 2021;41:3247. doi: 10.21873/anticanres.15110. [DOI] [PubMed] [Google Scholar]
- 88.Johnston CJC, et al. Cultivation of Heligmosomoides polygyrus: an immunomodulatory nematode parasite and its secreted products. J. Vis. Exp. 2015;98:e52412. doi: 10.3791/52412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Erben U, et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014;7:4557–4576. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.