Abstract
Salt-tolerant halophytes have shown potential for biorefinery and agricultural use in salt-affected soils, increasing the value of marginal lands. They could provide a bio-based source for compounds obtained from the petrochemical industry or an alternative for biomass currently imported overseas. Salicornia europaea, Tripolium pannonicum and Crithmum maritimum were cultivated in hydroponic systems under various salinity conditions, harvested green but not food-grade, and fractionated to green juice and fibre residue. Obtained fractions were characterised for contents of carbohydrates, Klason lignin, crude protein, organic acids, lipids, and minerals to evaluate the biomass’ suitability for biorefinery. Significant differences were observed in the biomass yield and the composition of the biomass fractions from different cultivation salinities. High concentrations of crude protein were found. Thus, these species could have the potential for green protein production. Fractions rich in carbohydrates could be used for lignocellulose processing and processes utilising micro-organisms.
Subject terms: Chemical engineering, Crop waste, Biofuels, Salt
Introduction
Soil salinisation has been reported as one of the major factors to the degradation of agricultural land1. The Food and Agriculture Organization of the United Nations (FAO) estimates that 79 million hectares are considered either salt-affected or sodic in Europe, covering 3.9% of the total land area2,3. Worldwide, salt-affected soils consider more than 100 countries, and total area of saline and sodic soils is estimated to be more than 1 billion hectares2. According to The United States Department of Agriculture, 10 million hectares of farmland is lost every year due to over-irrigation and poor water management4. Most conventional crops are glycophytes, meaning that their growth is inhibited in the presence of salt, and a 50% decrease in biomass yields have been reported for rice, durum wheat and barley at salinities of 80 mM NaCl, 100 mM NaCl and 120 mM NaCl, respectively4. FAO defines saline soils as areas, where the electric conductivity of soil extract is 4 dS/m or higher2, which corresponds to approximately 40 mM NaCl. Besides economic losses, soil salinisation is a threat to food security, as it creates challenges to meet the demand for food for the world’s increasing population.
The natural habitat of halophytes are seashores, marshes and salt deserts, and utilisation of these naturally salt-tolerant plants in agricultural applications is a key to re-valorise these salt-affected marginal lands, which are not suitable for conventional farming3,4. Flowers and Colmer5 define halophytes as plants that can complete their full life cycle and reproduce under the salinity of 200 mM or more, and these type of plants cover approximately 1% of known plant species. Some halophytes can yield as much biomass as traditional crops under full seawater irrigation6. Several cultivation practises for saline agriculture has been set for halophytes, including field or greenhouse cultivation with brackish or seawater irrigation, constructed wetlands, or saline hydroponic systems6,7. Hence, the irrigation water from valuable freshwater resources may not be needed for cultivation. Due to their capability to grow in hydroponics and constructed wetlands, plants can also be used to bio-filter excessive nutrients8 or residues of antimicrobial compounds9 from aquaculture effluents. Combining aquaculture with Salicornia cultivation has also been evaluated for its potential to reduce halophyte production costs10. The most suitable cultivation system depends on the species, salinity of the irrigation water, and available soil type7.
Halophytes have previously been used as medicinal plants, and nowadays, fresh tips of many edible species are sold for culinary use4,11. After harvesting for food, the remaining fraction of halophyte is often seen as waste as the plant lignifies, trapping high concentrations of salt within the plant structure. Due to this high salt concentration, halophytes are suitable for animal feed only when incorporated with other feed sources. The halophyte-supplemented feed has been tested for aquacultures12, chicken13,14 and ruminants15,16.
According to the IPCC 2021 report17, humans as a society have to aim for net-zero carbon dioxide emission by 2050 in order to limit global warming to 1.5 °C. As a part of this green transition, it is necessary to find bio-based alternatives to the variety of products currently obtained from the petrochemical industry. Considering bioenergy, Salicornia spp. have been tested for their potential for bioethanol18,19 and biodiesel20,21 production, and Tripolium pannonicum has been tested for biogas production9,22. As the production of only biofuels is rarely feasible, value-added products can be introduced to the biorefinery process. These multi-product systems are seen as the most robust option for the future23,24. The utilisation of residual fractions for bioenergy production would also lead to a zero-waste biorefinery.
Green biorefinery, where fresh but non-food grade biomass is fractionated to green juice and fibre residue, could provide an opportunity to produce protein-rich feed supplements25 and nutraceutical compounds, such as polyunsaturated fatty acids and carotenoids26,27. This approach has been tested previously for Salicornia sinus-persica and Salicornia bigelovii19,28. One of the key compounds to be valorised in green biorefinery is protein and halophytes could provide a source for locally produced feed. The demand for high-quality plant-derived protein is increasing and farmers are currently strongly dependent on imported sources, such as soybean, for their livestock25. High-value bioactive compounds suitable for cosmetics and pharmaceutical ingredients can also be found from Salicornia spp.27,29–32, Tripolium pannonicum33,34 and Crithmum maritimum35,36 extracts, and these compounds have exhibited antioxidant activity, anti-inflammatory and antimicrobial effects, anti-obesity properties, and even cancer-preventing capabilities.
Halophytes could provide a valuable feedstock for multi-product green biorefinery. Regardless of their potential, halophytes are currently underutilised in agriculture and industrial applications. In this study, three halophyte plants native to European seashores are cultivated and characterised: S. europaea (glasswort, marsh samphire, sea asparagus, pickleweed or sea beans), T. pannonicum (sea aster, previously defined in taxonomy as Aster tripolium) and C. maritimum (sea fennel or rock samphire). These species represent different plant families (Amarathaceae, Asteraceae, and Apiaceae, respectively) and they have all suggested as potential crops for halophyte-based agriculture by Ventura et al.4. The focus is to study the effect of cultivation salinity on biomass yields and the chemical composition of biomass fractions. The plant material was characterised for the contents of carbohydrates, Klason lignin, organic acids, crude protein, lipids, and minerals, for the further evaluation of the species’ suitability for green biorefinery and different types of processes.
Methods
Biomass cultivation
The plants were cultivated in a greenhouse at the Institute of Botany, Leibniz University Hannover, Germany (52°23′42″N; 9°42′13″E), with temperatures varying between 14 °C (minimum temperature during the night) and 35 °C (maximum temperature during the day). The seeds of T. pannonicum (Jacq.) Dobrocz. were collected with official permission at the North Sea, Germany (53°29′13″N; 8°03′16″E). The formal identification of the species was carried out at the Institute of Botany, Leibniz University Hannover, and the voucher specimen was deposited in the herbarium with specimen number TP20191001. Seeds of S. europaea L. var. Aprica were obtained from Serra Maris bvba, Belgium, and the seeds of C. maritimum L. were obtained from mother plants grown at the Institute of Botany, Leibniz University Hannover. The agronomic handling from sowing through transplanting was carried out as described by Buhmann et al.37. S. europaea and T. pannonicum were cultivated with different NaCl concentrations in hydroponic systems: 0, 10, 20, 30, and 40 g/l NaCl (corresponding to 0, 171, 342, and 685 mM NaCl, respectively). It was noted that C. maritimum did not survive under the highest salinities. Hence, it was grown with lower salinities: 0, 5, 10, 15, and 20 g/l NaCl (corresponding to 0, 86, 171, 257, and 342 mM NaCl, respectively). All plants were cultivated in polypropylene containers (400 mm × 300 mm × 175 mm) with a capacity of 16 l, and each container had 13 l of Hoagland solution containing: 606 mg/l KNO3, 944 mg/l Ca(NO3)2·4H2O, 230 mg/l NH4H2PO4, 246 mg/l MgSO4·7H2O, 3.73 mg/l KCl, 1.55 mg/l H3BO3, 0.34 mg/l MnSO4·H2O, 0.58 mg/l ZnSO4·7H2O, 0.12 mg/l CuSO4·5H2O, 0.12 mg/l MoNa2O4·2H2O, and 9.16 mg/l Fe-EDDHA (0.56 mg/l Fe). Small compressors constantly aerated the water, and one air stone was placed in the middle of each tank. The hypocotyl was fixed with soft foam in 35 mm holes. The water level was adjusted constantly in each tank with tap water to compensate for evapotranspiration. Each experimental unit consisted of eight plants per container and three replicates (separate containers) per treatment. Plants were exposed to 14 h of artificial light from sodium vapour lamps (SON-T Agro 400, Philips), and the light intensity ranged from 65 to 850 µmol m−2 s−1 depending on the time of the year, the time of the day, and the weather conditions. The cultivation time in hydroponic systems was 5 weeks for T. pannonicum and S. europaea and 11 weeks for C. maritimum. Plants were harvested partly lignified, weighed, immediately frozen in liquid nitrogen to inhibit metabolism, and then kept at − 80 °C.
Biomass fractionation and processing
Biomass processing and characterisation were performed in AAU Energy, Aalborg University, Denmark. Harvested aerial parts of biomass were thawed and fractionated to green juice and fibre residue by using a horizontal single-auger juicer. Both fractions were recovered to pre-weighed containers. The contents of dry matter (DM) and ash in green juice and fibre residue fractions were determined using the analytical protocols by National Renewable Energy Laboratory (NREL)38,39. The juice was analysed unfiltered and contained small suspended solid particles, which passed through the particle retention of the juicer. For storage, the fibre residue was dried overnight at 60 °C in a fan oven, knife-milled to particle size < 2 mm and kept at room temperature in dry conditions protected from light. Green juice was frozen after fractionation and kept at − 40 °C before composition analysis.
Characterisation methods
Crude protein determination
The crude protein content of the biomass was determined from homogenised DM by measuring the total nitrogen content using an elemental analyser and applying the Jones conversion factor of 6.0040.
Extraction of lipids
The lipid content was defined as a lipid-enriched non-polar fraction in the biomass. For solid biomasses, a dried sample (3–5 g) was weighed onto a cellulose thimble. Lipids were extracted with 250 ml n-hexane using the Soxhlet apparatus with a 100 ml extraction chamber. After extraction, the solvent was recovered using a rotary evaporator, and extracted non-polar compounds were weighed.
In order to extract non-polar compounds from green juice, liquid–liquid extraction was performed by mixing a juice sample (10 ml) with n-hexane (20 ml) in Falcon tubes. Tubes were kept in a nutation mixer for 1 h at room temperature, and juice solids and liquid phases were separated afterwards by centrifuging for 15 min with 4000 rpm (SL16, Thermo Scientific). The non-polar fraction was recovered, the solvent evaporated in the fume hood, and the lipid-enriched fraction was weighed.
Total carbohydrates and organic acids
To analyse the lignocellulosic fraction of solid samples, subsequential 10 h water and 8 h ethanol extractions were performed to remove non-structural compounds. Extraction was performed in a similar setup as used for lipid extraction, and one sample (5 g) was used from each biomass batch. The extracted material was recovered and weighed. Strong acid hydrolysis and determination of structural carbohydrates and Klason lignin in the extractive-free biomass were carried out in duplicate using a protocol by NREL41. Carbohydrates from juice fractions were measured after weak acid hydrolysis, where the juice sample (10 g) was mixed with H2SO4 (10 ml, 8%) in Pyrex tubes and autoclaved at 121 °C for 10 min. Hydrolysis and analytics were run as duplicates for samples and recovery standards. Free sugar monomers and the concentration of organic acids were also determined directly from fresh, untreated juice samples using a protocol by NREL42.
All hydrolysates and fresh juice samples were filtered through 0.22 µm syringe filters. Sugars and organic acids were analysed with high-performance liquid chromatography (1260 Infinity II, Agilent Technologies), using H2SO4 mobile phase (0.005 M), organic acid column (Aminex HPX-87H, Rio-Rad Laboratories Inc.), and refractive index detector. Separated sugars were glucose, xylose and arabinose, and the calculations for the concentrations of sugars were performed as described by Alassali et al.19. Analysed organic acids were lactic acid, acetic acid, malic acid, succinic acid and glycolic acid.
Mineral analysis
The minerals present in the ash fraction were analysed at the scientific service centre CELABOR, Belgium. Ash samples were digested in acidic conditions under pressure (40 bar) at 240 °C using a microwave system in compliance with EN 13805:2014 standard. Concentrations of the following minerals were measured using inductively coupled plasma atomic emission spectrometry (8300 DV ICP-AES, Perkin Elmer) with a method adapted from EN 11885 standard: aluminium, antimony, arsenic, barium, cadmium, cobalt, chromium, copper, iron, potassium, magnesium, manganese, molybdenum, sodium, nickel, lead, silver, selenium, titanium, zinc, phosphorus, strontium, vanadium, thallium and calcium. Inductively coupled plasma with mass spectrometry (820 ICP-MS CRI, Varian) with a method adapted from EN 15763 standard was used to determine the concentrations of rubidium, scandium and yttrium. Detected minerals with a concentration higher than 100 ppm in DM were reported. Analysis was run only once to each ash sample; thus, results are presented in Supplementary Information.
Statistical methods
All analyses were carried out as triplicates unless stated otherwise. All results, excluding fractionation yields and results from the mineral analysis, are given as mean values with standard deviation. One-way analysis of variance (ANOVA) coupled with Tukey honest significance test was used to test the statistical significance of differences between results from biomass batches cultivated in different salinity conditions.
Ethics
The study complies with local and national guidelines.
Results
Biomass yields
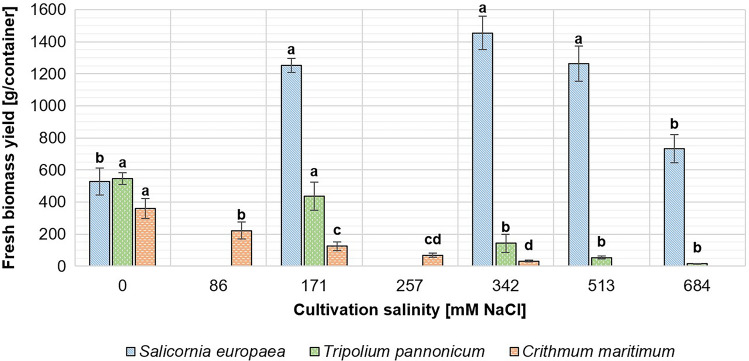
In all plant species, significant differences were observed between biomass yields from different cultivation salinities (p < 0.001). The lowest total yield of S. europaea biomass (1586 g) was gained from the non-saline cultivation conditions, whereas the highest total biomass yield (4365 g) was achieved with a cultivation salinity of 342 mM NaCl.
On the other hand, there was an inverse relationship between cultivation salinity and T. pannonicum yield. The total amounts decreased from 1641 g of fresh biomass (0 mM NaCl) to 44 g (684 mM NaCl). Similarly, the total C. maritimum yield decreased from 1440 g (0 mM NaCl) to 126 g (324 mM NaCl). C. maritimum also exhibited lower biomass production compared to two other species. Obtained biomass yields are presented in Fig. 1.
Figure 1.
Yield of fresh halophyte biomass cultivated under different salinities. Different letters above the bars stand for significantly different (p < 0.05) results calculated individually for each plant species.
Fractionation and dry matter determination
After fractionation, green juice covered 84.6–90.2 w/w% out of the total biomass. Biomass yields of T. pannonicum and C. maritimum cultivated in high salinities (> 171 mM NaCl) were too low to perform the fractionation process. Hence, these plants were considered only as whole shrubs. In S. europaea and T. pannonicum, the juice fraction increased when cultivation salinity increased, whereas, in C. maritimum, the juice fraction decreased.
There was a positive correlation between S. europaea juice DM content and cultivation salinity, as water-soluble salt is mainly present in the juice fraction. The total DM content of T. pannonicum and C. maritimum biomasses increased as cultivation salinity increased, and significant changes were observed between samples (p < 0.001). In contrast, the changes in the total DM content of S. europaea were non-significant (p < 0.058). Halophyte fractions and their respective DM contents are summarised in Table 1. The characterisation results for compounds in juice and fibre residue fractions are given on the basis of their respective DM and the total DM calculated from these two fractions (whole biomass).
Table 1.
Green juice and fibre residue fractions obtained from halophyte biomass and their respective dry matter (DM) contents, and the total DM content of the biomass.
| Species | Salinity [mM NaCl] | Juice [w/w%] | Fibres [w/w%] | DMjuice [w/w%] | DMfibres [w/w%] | DMwhole [w/w%] |
|---|---|---|---|---|---|---|
| S. europaea | 0 | 84.61 | 15.39 | 4.71 ± 0.03c | 27.24 ± 1.18a | 9.15 ± 0.65a |
| 171 | 85.71 | 14.28 | 4.71 ± 0.04c | 23.00 ± 0.28b | n/a | |
| 342 | 85.29 | 14.71 | 5.10 ± 0.00bc | 21.80 ± 1.16b | 8.02 ± 0.38a | |
| 513 | 86.42 | 13.58 | 5.26 ± 0.39ab | 21.11 ± 0.54b | 7.85 ± 0.74a | |
| 684 | 90.23 | 9.77 | 5.82 ± 0.12a | 21.54 ± 1.32b | 8.05 ± 0.04a | |
| T. pannonicum | 0 | 74.97 | 25.03 | 3.87 ± 0.03b | 23.78 ± 0.20b | 8.07 ± 0.19c |
| 171 | 82.51 | 17.49 | 4.17 ± 0.04a | 26.30 ± 1.23a | 7.96 ± 0.67c | |
| 342 | n/a | n/a | n/a | n/a | 11.45 ± 0.39b | |
| 513 | n/a | n/a | n/a | n/a | 11.47 ± 0.47b | |
| 684 | n/a | n/a | n/a | n/a | 14.65 ± 0.22a | |
| C. maritimum | 0 | 70.35 | 29.65 | 5.58 ± 0.02b | 27.22 ± 0.68a | 11.06 ± 0.22b |
| 86 | 70.46 | 29.54 | 5.39 ± 0.00c | 27.15 ± 0.35a | 11.77 ± 0.53b | |
| 171 | 66.97 | 33.03 | 7.44 ± 0.00a | 26.06 ± 0.61a | 13.56 ± 0.54a | |
| 257 | n/a | n/a | n/a | n/a | 13.40 ± 0.14a | |
| 342 | n/a | n/a | n/a | n/a | 14.79 ± 0.98a |
Due to small biomass yields, batches of T. pannonicum and C. maritimum cultivated in high salinity conditions were not fractionated. Results are expressed as [w/w%] on a fresh weight basis. Different letters denote significantly different (p < 0.05) results calculated individually for each plant species and its biomass fractions.
Composition of halophyte biomasses
Salicornia europaea
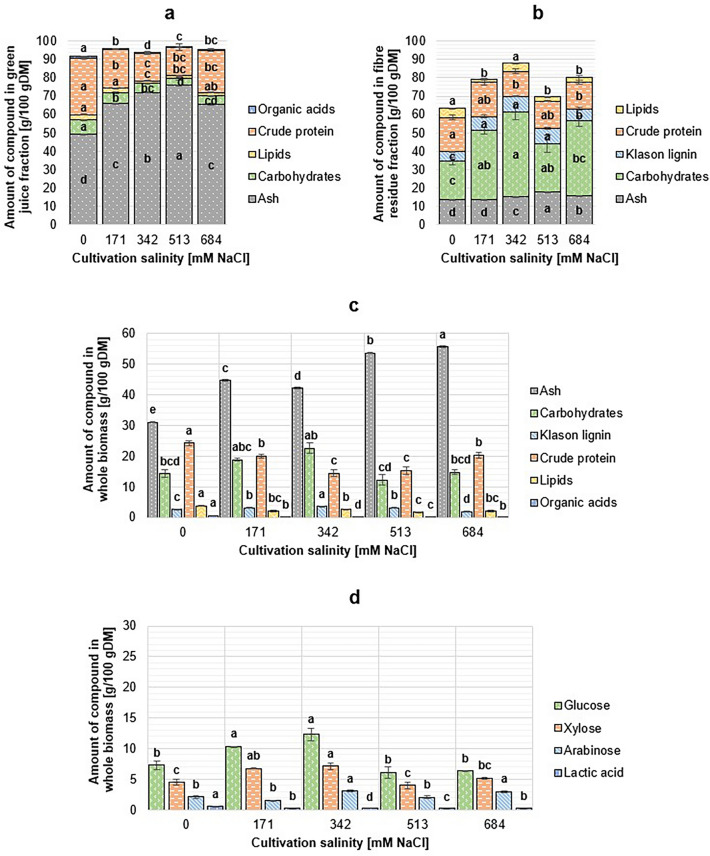
The composition of S. europaea biomass fractions are shown in Fig. 2. The crude protein content of S. europaea was relatively high, and considering the biomass fractions separately, the changes in the crude protein content were more significant in the juice fraction (p < 0.001) than in the pulp fraction (p < 0.011). Out of the total crude protein, > 60% was present in the juice fractions, except for the S. europaea cultivated at 513 mM NaCl, where most of the protein was found in the fibre residue fraction. The lipid content of green S. europaea was low, 1.7–3.7 g/100 g DM. Based on the results, no clear relationship between cultivation salinity and the lipid content of the halophyte biomass was observed.
Figure 2.
Chemical composition of Salicornia europaea green juice (a), fibre residue (b) and whole biomass (c), and total sugar profile (d). DM: dry matter. The content of a compound in whole biomass was calculated from juice and fibre residue fractions. Different letters above the bars denote significantly different (p < 0.05) results calculated individually for each biochemical group.
The analysis showed the content of carbohydrates in the biomass being highest when the biomass was cultivated in more optimal conditions in terms of biomass yield (342 mM NaCl). Significant differences were observed for all types of sugars measured in the hydrolysates from juice and fibre residue (p < 0.001). Contrary to the total carbohydrate content, in the green juice fractions, the concentration of carbohydrates determined from hydrolysates had an inverse relationship to cultivation salinity, the concentration of sugars being highest in the juice obtained from 0 mM NaCl cultivated plants. Therefore, the carbohydrates from the fibre fraction are more pronounced in the total carbohydrate content of the biomass. Overall, the Klason lignin content was very low in all S. europaea fibre residues. Similarly to the total carbohydrates, the lignin content correlated with the obtained biomass yields (more lignocellulose from more optimal cultivation salinity). In S. europaea plants, even the highest share of lignocellulose was only 26.1% of the total biomass composition (342 mM NaCl). The average composition of lignocellulose was 43.5% cellulose (glucose), 41.3% hemicellulose (xylose and arabinose) and 15.2% Klason lignin.
In the fresh juice (non-hydrolysed sample), low concentrations of free sugar monomers were detected. The glucose concentration was the highest in the juice from biomass grown in 0 mM NaCl (2.15 ± 0.01 g/100 g DM) and the lowest in the juice from 513 mM NaCl cultivated biomass (0.91 ± 0.79 g/100 g DM). Arabinose was not present in any fresh juice samples, and xylose was detected only from juice samples from 171 mM NaCl (2.06 ± 0.02 g/100 g DM) and 684 mM NaCl (1.29 ± 0.00 g/100 g DM) cultivated biomass. The results of the organic acid analysis showed only low amounts of lactic acid in the fresh juice samples (< 1.00 g/100 g DM in total DM), whereas other acids were not detected in the analysis.
The ash content of S. europaea increased as the cultivation salinity increased due to the high amount of water-soluble salts in the juice fraction. This can be observed when fractions are considered separately: ash content of fibre residue fraction varied between 13.7 and 18.2 g/100 g DM, but the ash content of the juice increased from 49.31 ± 0.19 g/100 g DM (0 mM NaCl) up to 76.02 ± 0.11 g/100 g DM (513 mM NaCl).
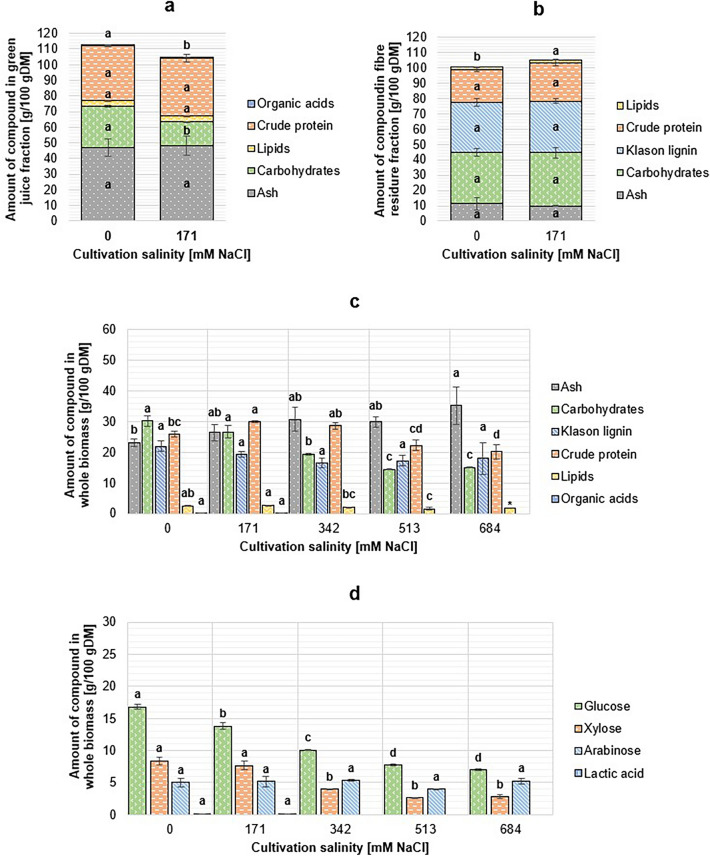
Tripolium pannonicum
Overall, increased cultivation salinity affected the biomass yield and size of fractions rather than the chemical composition of T. pannonicum plants. An inverse relationship was observed between cultivation salinity and the total crude protein content, and the differences between samples were significant (p < 0.001). However, considering the green juice and fibre residue fractions from lower salinities (0 mM and 171 mM NaCl) individually, the crude protein content was stable, and changes were non-significant (p = 0.259 and p = 0.063, respectively). The total lipid content of T. pannonicum varied between 1.59 ± 0.46 g/100 g DM (513 mM NaCl) and 2.81 ± 0.04 g/100 g DM (171 mM NaCl). Despite the significant differences in the total lipid content of biomass samples (p = 0.002), it was not possible to observe a clear relationship between cultivation salinity and lipid content based on the obtained results. Due to the small amount of biomass available, it was not possible to run the lipid extraction in triplicate for 684 mM NaCl salinity grown biomass. The changes in the lipid content were more pronounced in the fibre residue fraction, as in the juice fractions, the lipid content stayed nearly constant and changes were non-significant (p = 0.488).
Significant differences were observed in the total carbohydrate content of samples from different cultivation conditions (p < 0.001). As sugars were determined from the extractive-free fraction of the solid samples, only the amount of structural carbohydrates was determined for the samples considered whole (not fractionated). An inverse relationship between cultivation salinity and the content of structural carbohydrates was observed, but only samples cultivated in the two highest salinities were significantly different to the others. Considering the types of sugars separately, the increased cultivation salinity caused significant changes in total glucose and xylose contents of total DM (p < 0.001), but there were no significant differences in the arabinose content of biomass (p = 0.171). The Klason lignin content of T. pannonicum was up to 21.97 ± 1.70 g/100 g DM (171 mM NaCl), and the changes between biomass samples were non-significant (p = 0.072).
High concentrations of free sugar monomers were detected from fresh T. pannonicum juice, xylose being the most abundant sugar monomer with concentrations of 16.43 ± 0.32 g/100 g DM and 7.99 ± 0.08 g/100 g DM in green juice from 0 mM NaCl and 171 mM NaCl cultivated biomasses, respectively. Fresh green juice was also rich in glucose, but arabinose was not detected in the samples. Only a low amount of lactic acid was measured from fresh juice; other acids were not detected.
The significant difference in the total ash content was only observed in the samples cultivated in the lowest and the highest salinity (p = 0.020), whereas changes between other samples were non-significant. When considered separately, the green juice fractions showed no significant change in the ash content (p = 0.829). During the composition analysis, the cumulative mass balance of T. pannonicum samples exceeded 100%, which is suggested to be caused by the overestimated amount of crude protein and large standard deviations in the ash content results. The composition of T. pannonicum biomass fractions are shown in Fig. 3.
Figure 3.
Chemical composition of Tripolium pannonicum green juice (a), fibre residue (b) and whole biomass (c), and total sugar profile (d). DM: dry matter. For 0 and 171 mM NaCl salinity cultivated samples, the content of a compound in whole biomass was calculated from juice and fibre residue fractions. Only structural carbohydrates were analysed for samples cultivated in 342, 513, and 684 mM NaCl. Biomass samples which were not fractionated were not analysed for their contents of organic acids. Different letters above the bars denote significantly different (p < 0.05) results calculated individually for each biochemical group. *Not possible to give the standard deviation for the sample.
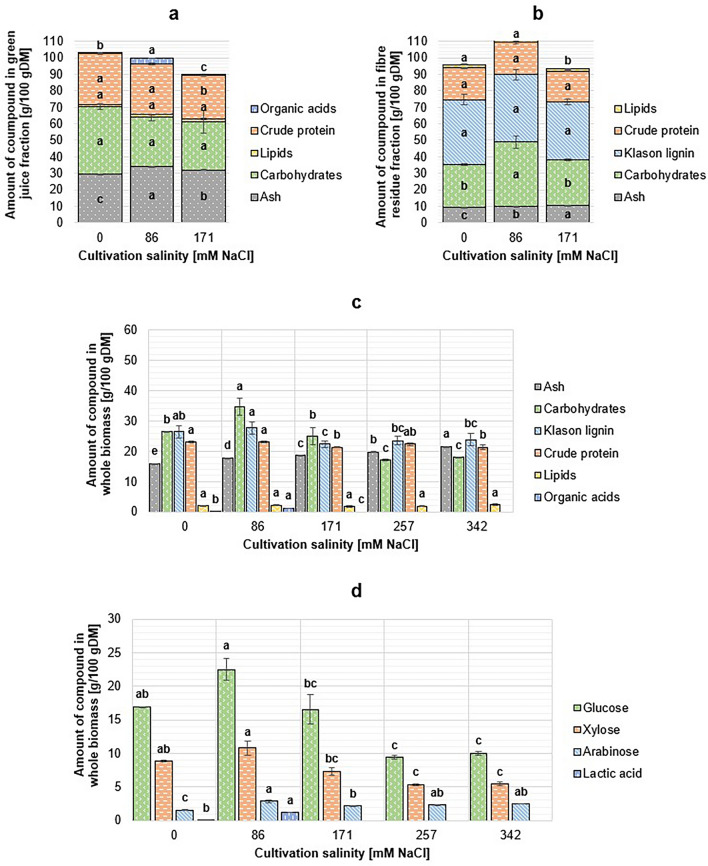
Crithmum maritimum
The composition of C. maritimum biomass fractions is shown in Fig. 4. Cultivation salinity affected mainly the biomass yield and less the chemical composition of C. maritimum. The cumulative mass balance exceeded 100% during the composition analysis of all fibre residue fractions and the juice fraction from biomass grown with 0 mM NaCl. Significant changes (p = 0.002) were observed in plants’ total crude protein content, which varied between 21.3 and 23.1 g/100 g DM. When fractions were considered separately, differences were statistically significant in green juice samples (p < 0.001) and an inverse relationship between crude protein content and cultivation salinity was observed, whereas no significant differences were observed in the crude protein content of fibre residue fractions (p = 0.070). The total lipid content of C. maritimum was low (< 2.5 g/100 g DM) in all samples, with changes between biomass batches being non-significant (p = 0.045). Most of the total lipids (> 60%) were present in the fibre residue fraction after the screw press. In the green juice fractions, the lipid content was < 2.2 g/100 g DM, and the differences between juice samples were also non-significant (p = 0.475).
Figure 4.
Chemical composition of Crithmum maritimum green juice (a), fibre residue (b) and whole biomass (c), and total sugar profile (d). DM: dry matter. For 0, 86, and 171 mM NaCl salinity cultivated samples, the content of a compound in whole biomass was calculated from juice and fibre residue fractions. Only structural carbohydrates were analysed for samples cultivated in 257 and 342 mM NaCl. Due to the small concentration of organic acids (< 0.1 g/100 g DM), all bars are not visible in the graphs. Biomass samples which were not fractionated were not analysed for their contents of organic acids. Different letters above the bars denote significantly different (p < 0.05) results calculated individually for each biochemical group.
Significant changes were observed in the total carbohydrate content of the biomass samples (p < 0.001). The content of carbohydrates was highest in the plants cultivated in 86 mM NaCl, the total amount of sugars being 34.71 ± 2.86 g/100 g DM. Significant changes were observed in the total contents of glucose (p < 0.001), xylose (p < 0.001), and arabinose (p = 0.002) in total DM of C. maritimum. However, when hydrolysed juice samples were considered separately, only non-significant changes were observed in the concentrations of glucose (p = 0.510) and xylose (p = 0.051), and arabinose was detected only in the sample from 171 mM NaCl cultivated plants in very low concentration (< 0.1 g/100 g DM). Considering the amounts of structural carbohydrates in solid samples, only the pulp fraction from 86 mM NaCl cultivation salinity was significantly different, whereas the carbohydrate content in other solid samples remained nearly constant. Klason lignin content of total DM varied from 22.37 ± 1.09 g/100 g DM (171 mM NaCl) to 27.68 ± 1.99 g/100 g DM (86 mM NaCl) with the p-value of 0.004.
Fresh C. maritimum juice was rich in free sugar monomers, and concentration varied between samples (p < 0.001). In these samples, glucose was the most abundant sugar, and the concentration reached up to 22.14 ± 0.20 g/100 g DM (0 mM NaCl). The amount of xylose was also found to be high, being 18.63 ± 0.47 g/100 g DM in the green juice from plants cultivated with 0 mM NaCl and > 10.00 g/100 g DM in other fresh juice samples. Arabinose was not detected in any of the fresh juice samples. The largest amount of lactic acid was detected from the 86 mM NaCl cultivated sample (3.77 ± 0.00 g/100 g DM), but in other juice samples, lactic acid concentrations were low (< 0. 1 g/100gDM).
Cultivation salinity is directly related to the ash content of the C. maritimum biomass (p < 0.001).
Discussion
Halophytes can be divided into two groups: obligate halophytes, which need salt to produce the highest biomass yields, and facultative halophytes, which tolerate salt but have optimal growth in low salinities. In general, dicotyledonous halophyte plants have shown higher salt tolerance compared to monocotyledonous grasses and glass-like species43. As an obligate halophyte, S. europaea requires salt for optimal growth, and the highest obtained biomass yield from cultivation at 342 mM NaCl salinity (approximately 34 dS/m) aligns with the previous studies reporting the optimal salinity range for Salicornia spp. to be 200–400 mM NaCl (corresponding to approximately 20–40 dS/m)44–47. In the cultivation study by Araus et al.48, brackish water (25 dS/m) irrigation lead to taller plants and higher S. europaea biomass production compared to seawater (40 dS/m) irrigation. Adaptability to changing environments was shown, as S. europaea exhibited sufficient growth in various salinities. In the study by Cárdenas-Pérez et al.47 no significant anatomical changes were observed in S. europaea cultivated at 200–800 mM salinity, whereas changes in plant cells were observed with extreme salinity treatments (0 mM and 1000 mM NaCl). S. europaea exposed to high salinity stress (700 mM) has also exhibited recovery after the stress and as high production of fresh biomass as control plants49. Salicornia spp. are fully adapted to flooding conditions, which has also been shown to enhance growth44.
Unlike Salicornia spp., T. pannonicum and C. maritimum are considered facultative halophytes. Uno et al.50 and Ueda et al.51 have reported T. pannonicum growth inhibition in salinities above 300 mM NaCl. Obtained results are aligned with these observations, as biomass yield was significantly lower when salinity increased to 342 mM NaCl. Wiszniewska et al.52 also obtained the optimal T. pannonicum production at non-saline conditions but reported a non-significant difference in yields in 150 mM and 300 mM NaCl cultivated plants. Turcios et al.22 reported T. pannonicum being able to withstand salinities up to 770 mM (45 g/l NaCl). Even if T. pannonicum survived under 684 mM NaCl salinity, the biomass yield was very low, and only small changes in the chemical composition of the biomass were observed. Al-Hawija et al.53 also reported T. pannonicum seeds being able to germinate under salinity up to 600 mM, but the germination percentage decreased from 78 to 24% compared to non-saline conditions. Considering obtained results and previous literature, C. maritimum tolerates lower NaCl concentrations than the other two species. This can be explained by the type and the natural habitat of the species. According to eHALOPH database54, S. europaea and T. pannonicum are classified as hydrohalophytes found in salt marshes, which can be affected by tidal changes in coastal areas, whereas C. maritimum is a chasmophyte found in rocky seashores. In addition, plants have several mechanisms to continue their growth and development under salt stress conditions, including the restriction of Na+ uptake and exclusion, cellular compartmentalisation of Na+ in the vacuole, antioxidant regulation, compatible solutes (osmolytes), morphological adaptations, among others. Germination strategies of halophyte seeds also show high variability depending on plant population and their natural habitat55. The mechanism used depends on the group of plants, glycophytes or halophytes, and on each species, resulting in a wide range of tolerance to salinity. For example, Ben Amor et al.56 and Ben Hamed et al.57 reported a significant reduction in C. maritimum yields in salinities higher than 200 mM NaCl and at 300 mM NaCl, respectively, but showed enhanced or unchanged growth under moderate 50 mM NaCl and 100 mM NaCl salinities, respectively. Similarly, Martins-Noguerol et al.58 defined the optimal cultivation salinity for C. maritimum in greenhouse conditions to be 50 mM NaCl (approximately 5 dS/m). Regardless of the lower total biomass production and longer cultivation time, the utilisation of C. maritimum could be feasible, as the plant has been reported to be rich in valuable bioactive compounds35,36.
It must be taken into consideration that the cultivation of plants in different growth media (hydroponic system or cultivation in soil) can also lead to different plant growth responses and biochemical compositions. However, the use of hydroponic cultivation has increased in importance worldwide due to the known advantages, mainly in the efficient use of the resources, being necessary to carry out research in this field. In addition, under hydroponic conditions and for research purposes, the concentrations of salt and nutrients can be easily and precisely controlled, allowing an accurate comparison between the different treatments. The cultivation system to select also depends on other factors. Different cultivation systems provide varying capabilities to control the salinity7, which among other things, such as targeted products, has to be taken into account when choosing the cultivation practices and following processing methods. As S. europaea is an annual plant, it could provide an interesting species for crop rotation, where it would be used in the remediation process and to uptake the excessive salt from the substrate, which may inhibit the growth of other crops.
This study presents a broad overview of the composition of green fractionated halophyte biomasses grown in the hydroponic system, providing information for planning potential biorefinery processes. The crude protein content of studied halophytes was relatively high, and these species could have the potential for protein production. Results for total crude protein content for S. europaea were aligned with amounts previously reported for Salicornia spp.19,26. The content of soluble protein in S. europaea has previously shown to be relatively stable and content to decrease only when exposed to very high salinities47. The crude protein content was lower in S. europaea samples cultivated in more optimal conditions regarding biomass yield; thus, the actual crude protein content was 12.7% higher in plants cultivated in 171 mM NaCl than plants cultivated in 342 mM NaCl. Therefore, the cultivation in 171 mM NaCl could be more desirable for a biorefinery targeting maximum protein production when assuming that all crude protein could be extracted as true protein and changes in the biomass yields would be significant. Even if the total crude protein content is nearly the same in 171 mM NaCl and 684 mM NaCl cultivated samples, due to significantly lower biomass yield obtained with 684 mM, the amount of total protein would decrease. Therefore this cultivation condition cannot be suggested. For T. pannonicum, the crude protein content was higher than previously reported for species22 and six other species in the Asteraceae family59. The crude protein content of T. pannonicum DM was comparable to widely used legumes, such as chickpea (24.0 g/100 g), lentil (26.1 g/100 g) or green pea (24.9 g/100 g)60, which makes it the most interesting species for protein production. However, the presence of non-protein free amino acids (e.g. asparagine) may cause an overestimation in protein content61, as well as high content of nitrate, which depending on cultivation practices, may also become an anti-nutritional factor in T. pannonicum4. Nitrogen is also present in chlorophylls, and high concentration may affect the estimation of protein content. Therefore, an amino acid analysis would be needed to carry out in further investigations. Also, C. maritimum showed relatively high crude protein content, and previous studies have shown optimal cultivation conditions to increase the content of essential amino acids in the plant58.
Low lipid content was measured from all studied plant species, which is typical for succulent halophytes62. For T. pannonicum, the total lipid content was lower than values previously reported in the literature22. Abiotic stresses, such as high or low salinity, could increase the antioxidant capacity and production of certain protective non-polar compounds, such as carotenoids, in plants26,47,63. Therefore, analysis of fatty acid profile and characterisation of other non-polar compounds (e.g. pigments and tocopherols) from lipid-enriched fractions could be desired to evaluate the feasibility of lipid separations as part of the biorefinery process.
With respect to total biomass yield, optimal cultivation conditions seem to increase the lignocellulosic fraction in the S. europaea biomass. This can be linked to the larger plant size obtained from the cultivation under optimal salinity47,48. Therefore, biomass cultivated in these conditions could be more suitable for biorefinery targeting cellulose and hemicellulose derivatives, cellulose being present mainly in the lignified stems10. Regardless of the significantly higher carbohydrate content in C. maritimum from 86 mM NaCl salinity cultivation, the actual amount of carbohydrates in obtained fresh biomass was still higher in plants cultivated in 0 mM NaCl due to higher biomass yield. Klason lignin content of S. europaea was found to be low, and it is aligned with acid-insoluble lignin contents previously reported for Salicornia species18,19,64. Low lignin content may indicate biomass to be non-recalcitrant, allowing less severe processing conditions, especially after the removal of extractive material. In C. maritimum and T. pannonicum biomass, Klason lignin content was higher than S. europaea, and the insoluble lignin content was aligned with 18.2 g/100 g DM previously reported for T. pannonicum22. Studied facultative halophytes had a high concentration of available sugars in the juice, making them interesting for processes utilising micro-organisms. Compared to forage alfalfa juice, which has been suggested as media for lactic acid fermentation65, the amount of available glucose is similar in T. pannonicum and nearly triple in C. maritimum cultivated in moderate salinities. The high amount of available xylose could also make T. pannonicum a potential feedstock for the production of pentose-derived platform chemicals.
Halophytes are known to accumulate salt and other minerals in their tissues, and high ash contents were also measured from studied biomass. Ushakova et al.66 showed increased sodium intake and decreased potassium, calcium, and magnesium uptake of S. europaea, which aligns with obtained results. Studied halophytes, especially C. maritimum and S. europaea, were rich in calcium (see Supplementary Table S1 and Supplementary Table S3), which has been shown to have an essential protective role in the salt tolerance of plants growing in saline conditions67. Compared to the other studied species cultivated in the same salinities, C. maritimum exhibited lower salt accumulation.
Conclusion
S. europaea, T. pannonicum, and C. maritimum were cultivated in different salinity conditions, and S. europaea yielded the most biomass in 342 mM NaCl (approximately 34 dS/m) salinity, whereas facultative halophyte species exhibited the highest biomass production in non-saline conditions. T. pannonicum, and especially S. europaea, could be potential crops due to their higher biomass yields and shorter cultivation time. Obtained biomass was fractionated to green juice and fibre residue, and the chemical composition of the fractions were analysed. Significant differences were observed between biomass batches cultivated under different salinities, and this study is the first one to report the composition of the species after green fractionation. All species exhibited high crude protein content. Therefore, they can be seen as potential feedstocks for biorefinery targeting green protein production. Obtained results can be used to plan possible processing routes for halophyte-based biorefinery. Still, halophytes and their suitability for different applications should be further explored as a part of the green transition and development of marginal lands.
Supplementary Information
Author contributions
L.S.S.H. prepared the original manuscript draft. J.P. obtained the permission to collect seeds and carried out the formal identification of the species. A.T. cultivated and harvested the biomass. L.S.S.H. ran the composition analysis, except the mineral analysis, which was performed by S.K. All authors reviewed the manuscript.
Funding
This study is part of AQUACOMBINE research project. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No. 862834. Any results of this project reflect only this consortium’s view and the European Commission is not responsible for any use that may be made of the information it contains.
Data availability
The data generated and analysed during the study is available from the corresponding author on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-24865-4.
References
- 1.Daliakopoulos IN, Tsanis IK, Koutroulis A, Kourgialas NN, Varouchakis AE, Karatzas GP, Ritsema CJ. The threat of soil salinity: A European scale review. Sci. Total Environ. 2016;573:727–739. doi: 10.1016/j.scitotenv.2016.08.177. [DOI] [PubMed] [Google Scholar]
- 2.FAO Intergovernmental Technical Panel on Soils. Status of the World’s Soil Resources. ISBN 9789251090046 (2015).
- 3.EIP-AGRI Focus Group Soil Salinisation: Final report. https://ec.europa.eu/eip/agriculture/en/publications/eip-agri-focus-group-soil-salinisation-final.
- 4.Ventura Y, Eshel A, Pasternak D, Sagi M. The development of halophyte-based agriculture: Past and present. Ann. Bot. 2014;115:529–540. doi: 10.1093/aob/mcu173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flowers TJ, Colmer TD. Salinity tolerance in halophytes. New Phytol. 2008;179:945–963. doi: 10.1111/j.1469-8137.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- 6.Glenn EP, Brown JJ, Blumwald E. Salt tolerance and crop potential of halophytes. CRC. Crit. Rev. Plant Sci. 1999;18:227–255. doi: 10.1080/07352689991309207. [DOI] [Google Scholar]
- 7.Ventura Y, Sagi M. Halophyte crop cultivation: The case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013;92:144–153. doi: 10.1016/j.envexpbot.2012.07.010. [DOI] [Google Scholar]
- 8.Shpigel M, Ben-Ezra D, Shauli L, Sagi M, Ventura Y, Samocha T, Lee J. Constructed wetland with Salicornia as a biofilter for mariculture effluents. Aquaculture. 2013;412–413:52–63. doi: 10.1016/j.aquaculture.2013.06.038. [DOI] [Google Scholar]
- 9.Turcios AE, Weichgrebe D, Papenbrock J. Uptake and biodegradation of the antimicrobial sulfadimidine by the species Tripolium pannonicum acting as biofilter and its further biodegradation by anaerobic digestion and concomitant biogas production. Bioresour. Technol. 2016;219:687–693. doi: 10.1016/j.biortech.2016.08.047. [DOI] [PubMed] [Google Scholar]
- 10.Cárdenas-Pérez S, Piernik A, Chanona-Pérez JJ, Grigore MN, Perea-Flores MJ. An overview of the emerging trends of the Salicornia L. genus as a sustainable crop. Environ. Exp. Bot. 2021;191:104606. doi: 10.1016/j.envexpbot.2021.104606. [DOI] [Google Scholar]
- 11.Petropoulos SA, Karkanis A, Martins N, Ferreira ICFR. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 2018;74:69–84. doi: 10.1016/j.tifs.2018.02.006. [DOI] [Google Scholar]
- 12.Belal IEH, Al-Dosari M. Replacement of fish meal with Salicornia meal in feeds for Nile Tilapia Oreochromis niloticus. J. World Aquac. Soc. 1999;30:285–289. doi: 10.1111/j.1749-7345.1999.tb00877.x. [DOI] [Google Scholar]
- 13.Attia FM, Alsobayel AA, Kriadees MS, Al-Saiady MY, Bayoumi MS. Nutrient composition and feeding value of Salicornia bigelovii torr meal in broiler diets. Anim. Feed Sci. Technol. 1997;65:257–263. doi: 10.1016/S0377-8401(96)01074-7. [DOI] [Google Scholar]
- 14.Jiao Y, Hosseindoust A, Zhang W-L, Kim I-H. Effects of Salicornia herbacea on growth performance, meat quality, excreta microbial populations, and noxious gas emissions in broiler chicks. J. Poult. Sci. 2019;56:44–51. doi: 10.2141/jpsa.0170210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdal MS. Salicornia production in Kuwait. World Appl. Sci. J. 2009;6:1033–1038. [Google Scholar]
- 16.Ahmed MH, Salem AZM, Zeweil HS, Sun XZ, Kholif AE, Elghandour MMY, Bahar MSI. Growth performance and carcass characteristics of lambs fed halophytes as a partial or whole replacement of berseem hay. Small Rumin. Res. 2015;128:1–9. doi: 10.1016/j.smallrumres.2015.05.004. [DOI] [Google Scholar]
- 17.Masson-Delmotte, V. P. Z. et al. IPCC, 2021: summary for policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (2021).
- 18.Cybulska I, Chaturvedi T, Brudecki GP, Kádár Z, Meyer AS, Baldwin RM, Thomsen MH. Chemical characterization and hydrothermal pretreatment of Salicornia bigelovii straw for enhanced enzymatic hydrolysis and bioethanol potential. Bioresour. Technol. 2014;153:165–172. doi: 10.1016/j.biortech.2013.11.071. [DOI] [PubMed] [Google Scholar]
- 19.Alassali A, Cybulska I, Galvan AR, Thomsen MH. Wet fractionation of the succulent halophyte Salicornia sinus-persica, with the aim of low input (water saving) biorefining into bioethanol. Appl. Microbiol. Biotechnol. 2017;101:1769–1779. doi: 10.1007/s00253-016-8049-8. [DOI] [PubMed] [Google Scholar]
- 20.Sharma R, Wungrampha S, Singh V, Pareek A, Sharma MK. Halophytes as bioenergy crops. Front. Plant Sci. 2016;7:1372. doi: 10.3389/fpls.2016.01372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folayan AJ, Anawe PAL, Ayeni AO, Arellano-Garcia H. Synthesis and characterization of Salicornia bigelovii and Salicornia brachiata halophytic plants oil extracted by supercritical CO2 modified with ethanol for biodiesel production via enzymatic transesterification reaction using immobilized Candida antarctica lipase catalyst in tert-butyl alcohol (TBA) solvent. Cogent Eng. 2019;6:1625847. doi: 10.1080/23311916.2019.1625847. [DOI] [Google Scholar]
- 22.Turcios AE, Weichgrebe D, Papenbrock J. Effect of salt and sodium concentration on the anaerobic methanisation of the halophyte Tripolium pannonicum. Biomass Bioenergy. 2016;87:69–77. doi: 10.1016/j.biombioe.2016.01.013. [DOI] [Google Scholar]
- 23.Severo IA, Siqueira SF, Deprá MC, Maroneze MM, Zepka LQ, Jacob-Lopes E. Biodiesel facilities: What can we address to make biorefineries commercially competitive? Renew. Sustain. Energy Rev. 2019;112:686–705. doi: 10.1016/j.rser.2019.06.020. [DOI] [Google Scholar]
- 24.Torres, A. I., Ashraf, M. T., Chaturvedi, T., Schmidt, J. E. & Stephanopoulos, G. Hydrothermal pretreatment: Process modeling and economic assessment within the framework of biorefinery processes. In Hydrothermal Processing in Biorefineries: Production of Bioethanol and High Added-Value Compounds of Second and Third Generation Biomass, 207–235 ISBN 9783319564579 (Springer International Publishing, 2017).
- 25.Santamaría-Fernández M, Lübeck M. Production of leaf protein concentrates in green biorefineries as alternative feed for monogastric animals. Anim. Feed Sci. Technol. 2020;268:114605. doi: 10.1016/j.anifeedsci.2020.114605. [DOI] [Google Scholar]
- 26.Lima AR, Castañeda-Loaiza V, Salazar M, Nunes C, Quintas C, Gama F, Pestana M, Correia PJ, Santos T, Varela J, et al. Influence of cultivation salinity in the nutritional composition, antioxidant capacity and microbial quality of Salicornia ramosissima commercially produced in soilless systems. Food Chem. 2020;333:127525. doi: 10.1016/j.foodchem.2020.127525. [DOI] [PubMed] [Google Scholar]
- 27.Isca VMS, Seca AML, Pinto DCGA, Silva H, Silva AMS. Lipophilic profile of the edible halophyte Salicornia ramosissima. Food Chem. 2014;165:330. doi: 10.1016/j.foodchem.2014.05.117. [DOI] [PubMed] [Google Scholar]
- 28.Christiansen AHC, Lyra DA, Jørgensen H. Increasing the value of Salicornia bigelovii green biomass grown in a desert environment through biorefining. Ind. Crops Prod. 2021;160:113105. doi: 10.1016/j.indcrop.2020.113105. [DOI] [Google Scholar]
- 29.Giordano R, Saii Z, Fredsgaard M, Hulkko LSS, Poulsen TBG, Thomsen ME, Henneberg N, Zucolotto SM, Arendt-Nielsen L, Papenbrock J, et al. Pharmacological insights into halophyte bioactive extract action on anti-inflammatory, pain relief and antibiotics-type mechanisms. Molecules. 2021;26:3140. doi: 10.3390/molecules26113140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cybulska I, Brudecki GP, Alassali A, Thomsen MH, Brown JJ. Phytochemical composition of some common coastal halophytes of the United Arab Emirates. Emirates J. Food Agric. 2014;26:1046–1056. doi: 10.9755/ejfa.v26i12.19104. [DOI] [Google Scholar]
- 31.Chaturvedi, T., Christiansen, A. H. C., Gołębiewska, I. & Thomsen, M. H. Salicornia species: current status and future potential. In Future of Sustainable Agriculture in Saline Environments, 461–482 (CRC Press, 2021).
- 32.Hulkko LSS, Chaturvedi T, Thomsen MH. Extraction and quantification of chlorophylls, carotenoids, phenolic compounds, and vitamins from halophyte biomasses. Appl. Sci. 2022;12:840. doi: 10.3390/app12020840. [DOI] [Google Scholar]
- 33.Chai W, Chen L, Lian XY, Zhang Z. Anti-glioma efficacy and mechanism of action of tripolinolate A from Tripolium pannonicum. Planta Med. 2018;84:786–794. doi: 10.1055/s-0044-101038. [DOI] [PubMed] [Google Scholar]
- 34.Wubshet SG, Schmidt JS, Wiese S, Staerk D. High-resolution screening combined with HPLC-HRMS-SPE-NMR for identification of potential health-promoting constituents in sea aster and searocket—New nordic food ingredients. J. Agric. Food Chem. 2013;61:8616–8623. doi: 10.1021/jf402949y. [DOI] [PubMed] [Google Scholar]
- 35.Meot-Duros L, Magné C. Antioxidant activity and phenol content of Crithmum maritimum L. leaves. Plant Physiol. Biochem. 2009;47:37–41. doi: 10.1016/j.plaphy.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 36.GeneralićMekinić I, Blažević I, Mudnić I, Burčul F, Grga M, Skroza D, Jerčić I, Ljubenkov I, Boban M, Miloš M, et al. Sea fennel (Crithmum maritimum L.): Phytochemical profile, antioxidative, cholinesterase inhibitory and vasodilatory activity. J. Food Sci. Technol. 2016;53:3104–3112. doi: 10.1007/s13197-016-2283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buhmann A, Hellmueller L, Louis B. Popular culture and communication practice. Commun. Res. Trends. 2015;34:4. [Google Scholar]
- 38.Sluiter, A. et al.NREL/TP-510-42621 Analytical procedure—Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples 3–5 (2008).
- 39.Sluiter, A., et al.NREL/TP-510-42622 Analytical Procedure—Determination of Ash in Biomass; (2005).
- 40.Jones, D. Factors for Converting Percentages of Nitrogen in Foods and Feeds into Percentages of Proteins, 16–21 (United States Dep. Agric., 1931).
- 41.Sluiter, A. et al.NREL/TP-510-42618 Analytical Procedure—Determination of Structural Carbohydrates and Lignin in Biomass (2012).
- 42.Sluiter, A. et al.Laboratory Analytical Procedure (LAP) Issue Date : 12/08/2006 Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples (2008).
- 43.Glenn EP, O’Leary W. Relationship between salt accumulation and water content of dicotyledonous halophytes. Plant Cell Environ. 1984;7:253–261. doi: 10.1111/1365-3040.ep11589448. [DOI] [Google Scholar]
- 44.van Andel J, Huiskes AHL, Blom CWPM, Rozema J. Vegetation between land and sea: Structure and processes. Q. Rev. Biol. 1989;64:73–74. doi: 10.1086/416161. [DOI] [Google Scholar]
- 45.Lv S, Jiang P, Chen X, Fan P, Wang X, Li Y. Multiple compartmentalization of sodium conferred salt tolerance in Salicornia europaea. Plant Physiol. Biochem. 2012;51:47–52. doi: 10.1016/j.plaphy.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 46.Katschnig D, Broekman R, Rozema J. Salt tolerance in the halophyte Salicornia dolichostachya Moss: Growth, morphology and physiology. Environ. Exp. Bot. 2013;92:32–42. doi: 10.1016/j.envexpbot.2012.04.002. [DOI] [Google Scholar]
- 47.Cárdenas-Pérez S, RajabiDehnavi A, Leszczyński K, Lubińska-Mielińska S, Ludwiczak A, Piernik A. Salicornia europaea L. functional traits indicate its optimum growth. Plants. 2022;11:1051. doi: 10.3390/plants11081051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Araus JL, Rezzouk FZ, Thushar S, Shahid M, Elouafi IA, Bort J, Serret MD. Effect of irrigation salinity and ecotype on the growth, physiological indicators and seed yield and quality of Salicornia europaea. Plant Sci. 2021;304:110819. doi: 10.1016/j.plantsci.2021.110819. [DOI] [PubMed] [Google Scholar]
- 49.Calone R, Mircea D-M, González-Orenga S, Boscaiu M, Lambertini C, Barbanti L, Vicente O. Recovery from salinity and drought stress in the perennial Sarcocornia fruticosa vs. the annual Salicornia europaea and S. veneta. Plants. 2022;11:1058. doi: 10.3390/plants11081058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uno Y, Kanechi M, Inagaki N, Sugimoto M, Maekawa S. The evaluation of salt tolerance during germination and vegetative growth of asparagus, table beet and sea aster. Engei Gakkai zasshi. 1996;65:579–585. doi: 10.2503/jjshs.65.579. [DOI] [Google Scholar]
- 51.Ueda A, Kanechi M, Uno Y, Inagaki N. Photosynthetic limitations of a halophyte sea aster (Aster tripolium L.) under water stress and NaCl stress. J. Plant Res. 2003;116:65–70. doi: 10.1007/s10265-002-0070-6. [DOI] [PubMed] [Google Scholar]
- 52.Wiszniewska A, Koźmińska A, Hanus-Fajerska E, Dziurka M, Dziurka K. Insight into mechanisms of multiple stresses tolerance in a halophyte Aster tripolium subjected to salinity and heavy metal stress. Ecotoxicol. Environ. Saf. 2019;180:12–22. doi: 10.1016/j.ecoenv.2019.04.059. [DOI] [PubMed] [Google Scholar]
- 53.Al-Hawija BN, Partzsch M, Hensen I. Effects of temperature, salinity and cold stratification on seed germination in halophytes. Nord. J. Bot. 2012;30:627–634. doi: 10.1111/j.1756-1051.2012.01314.x. [DOI] [Google Scholar]
- 54.Santos J, Al-Azzawi M, Aronson J, Flowers TJ. eHALOPH a database of salt-tolerant plants: Helping put halophytes to work. Plant Cell Physiol. 2016;57:e10–e10. doi: 10.1093/pcp/pcv155. [DOI] [PubMed] [Google Scholar]
- 55.Gul B, Ansari R, Flowers TJ, Khan MA. Germination strategies of halophyte seeds under salinity. Environ. Exp. Bot. 2013;92:4–18. doi: 10.1016/j.envexpbot.2012.11.006. [DOI] [Google Scholar]
- 56.Ben Amor N, Ben Hamed K, Debez A, Grignon C, Abdelly C. Physiological and antioxidant responses of the perennial halophyte Crithmum maritimum to salinity. Plant Sci. 2005;168:889–899. doi: 10.1016/j.plantsci.2004.11.002. [DOI] [Google Scholar]
- 57.Ben Hamed K, Castagna A, Salem E, Ranieri A, Abdelly C. Sea fennel (Crithmum maritimum L.) under salinity conditions: A comparison of leaf and root antioxidant responses. Plant Growth Regul. 2007;53:185–194. doi: 10.1007/s10725-007-9217-8. [DOI] [Google Scholar]
- 58.Martins-Noguerol R, Matías L, Pérez-Ramos IM, Moreira X, Muñoz-Vallés S, Mancilla-Leytón JM, Francisco M, García-González A, DeAndrés-Gil C, Martínez-Force E, et al. Differences in nutrient composition of sea fennel (Crithmum maritimum) grown in different habitats and optimally controlled growing conditions. J. Food Compos. Anal. 2022;106:104266. doi: 10.1016/j.jfca.2021.104266. [DOI] [Google Scholar]
- 59.García-Herrera P, Sánchez-Mata MC, Cámara M, Fernández-Ruiz V, Díez-Marqués C, Molina M, Tardío J. Nutrient composition of six wild edible Mediterranean Asteraceae plants of dietary interest. J. Food Compos. Anal. 2014;34:163–170. doi: 10.1016/j.jfca.2014.02.009. [DOI] [Google Scholar]
- 60.Iqbal A, Khalil IA, Ateeq N, Sayyar Khan M. Nutritional quality of important food legumes. Food Chem. 2006;97:331–335. doi: 10.1016/j.foodchem.2005.05.011. [DOI] [Google Scholar]
- 61.Moore JC, DeVries JW, Lipp M, Griffiths JC, Abernethy DR. Total protein methods and their potential utility to reduce the risk of food protein adulteration. Compr. Rev. Food Sci. Food Saf. 2010;9:330–357. doi: 10.1111/j.1541-4337.2010.00114.x. [DOI] [PubMed] [Google Scholar]
- 62.Patel MK, Pandey S, Brahmbhatt HR, Mishra A, Jha B. Lipid content and fatty acid profile of selected halophytic plants reveal a promising source of renewable energy. Biomass Bioenergy. 2019;124:25–32. doi: 10.1016/j.biombioe.2019.03.007. [DOI] [Google Scholar]
- 63.Ghanem A-MFM, Mohamed E, Kasem AMMA, El-Ghamery AA. Differential salt tolerance strategies in three halophytes from the same ecological habitat: Augmentation of antioxidant enzymes and compounds. Plants. 2021;10:1100. doi: 10.3390/plants10061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cybulska I, Brudecki GP, Brown JJ, Hulkko LSS, Al Hosani S, Hedegaard Thomsen M. Comparative study of chemical composition of the halophyte species native to the Persian (Arabian) gulf. BioResources. 2021;16:5524–5537. doi: 10.15376/biores.16.3.5524-5537. [DOI] [Google Scholar]
- 65.Santamaría-Fernández M, Schneider R, Lübeck M, Venus J. Combining the production of l-lactic acid with the production of feed protein concentrates from alfalfa. J. Biotechnol. 2020;323:180–188. doi: 10.1016/j.jbiotec.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 66.Ushakova SA, Kovaleva NP, Gribovskaya IV, Dolgushev VA, Tikhomirova NA. Effect of NaCl concentration on productivity and mineral composition of Salicornia europaea as a potential crop for utilization NaCl in LSS. Adv. Space Res. 2005;36:1349–1353. doi: 10.1016/j.asr.2004.09.017. [DOI] [Google Scholar]
- 67.Hadi MR, Karimi N. The role of calcium in plants’ salt tolerance. J. Plant Nutr. 2012;35:2037–2054. doi: 10.1080/01904167.2012.717158. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated and analysed during the study is available from the corresponding author on request.