Dear Editor,
Since the emergence of the Omicron BA.1.1.529 variant of SARS-CoV-2 in November 20211, a number of Omicron sublineages with increased antibody evasion capacity and transmissibility have been identified and caused regional and global outbreaks, including BA.1.1, BA.2, BA.2.12.1 and BA.4/52–4. While BA.4 and BA.5 share identical spike (S) sequence, for unknown reasons BA.5 outcompeted BA.4 in many regions, with a global prevalence of 77.1% as of epidemiological week 40 (3rd–9th October 2022) (https://reliefweb.int/report/world/coronavirus-disease-covid-19-weekly-epidemiological-update-26-october-2022).
Recently, a new variant related to BA.4/5, designated BA.4.6, has emerged and expanded in the United States where BA.5 dominates [80.3% prevalence as of 31st October 2022 (https://cov-spectrum.org/explore/United%20States/AllSamples/from=2022-07-01&to=2022-11-01/variants?nextcladePangoLineage=ba.5*&)], rising from < 2% of sequences in early July to 11.7% as of 31st October 2022 (https://cov-spectrum.org/explore/United%20States/AllSamples/from=2022-07-01&to=2022-11-01/variants?nextcladePangoLineage=BA.4.6&). Compared to BA.4/5, BA.4.6 contains two extra mutations in the Spike protein (S), R346T in the Receptor Binding Domain (RBD) and N658S in the C-terminal domain. The R346T mutation has raised concern for enhanced antibody evasion over BA.4/5, as the R346K mutation in BA.1.1 reduced serum neutralization compared to BA.1 and impaired the activity of a number of monoclonal antibodies (mAbs)2. Here, we study the neutralization profile of BA.4.6 using Pfizer-BioNtech vaccine serum, BA.1, BA.2, and BA.4/5 vaccine breakthrough immune serum (characteristics of sample donors are shown in Supplementary Table S1), as well as panels of mAbs. Remarkably, we show further antibody evasion of BA.4.6, providing guidance for vaccine design and the use of therapeutic monoclonals.
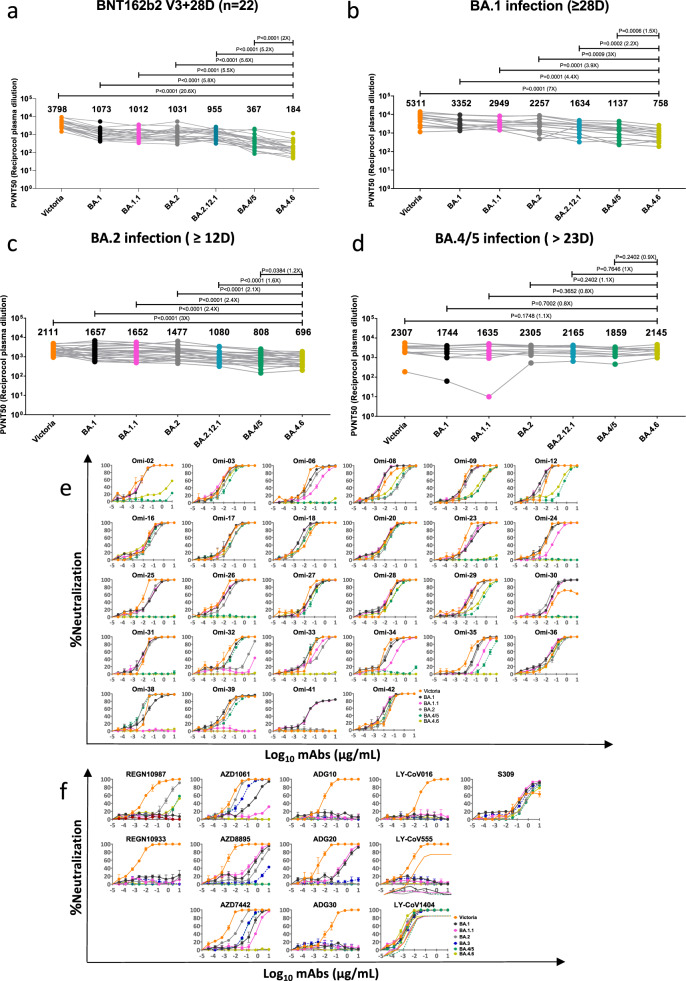
To evaluate the antibody evasion capacity of BA.4.6, we constructed a panel of pseudotyped lentiviruses5 expressing the S gene from BA.4.6 and other SARS-CoV-2 variants together with early pandemic Wuhan-related strain, Victoria, used as a control. Firstly, we examined the neutralization profile with sera collected 4 weeks following a third dose of the Pfizer-BioNtech vaccine BNT162b2 (n = 22). Compared to BA.4/5, neutralization titers against BA.4.6 were reduced twofold (P < 0.0001) for BNT162b2 sera (Fig. 1a).
Fig. 1. Characterisation of BA.4.6 by pseudoviral neutralization assay.
a–d Pseudoviral neutralization assays of BA.4.6 by vaccine, BA.1, BA.2, and BA.4.5 immune serum. IC50 values for the indicated viruses using serum obtained from vaccinees 28 days following their third dose of Pfizer BNT162b2 vaccine (n = 22, a). IC50 values for the indicated viruses against serum from volunteers suffering vaccine breakthrough BA.1 (n = 14, b), BA.2 (n = 23, c) and BA.4/5 (n = 11, d) infections. Geometric mean titers are shown above each column. The Wilcoxon matched-pairs signed rank test was used for the analysis and two-tailed P values were calculated. e Neutralization curves for a panel of 28 monoclonal antibodies made from samples taken from vaccinees infected with BA.1 against BA.4.6 were compared with Victoria, BA.1, BA.1.1, BA.2, BA.4/5, and BA.2.75 variants. Error bars represent means ± SEM of repeat experiments. f Neutralization curves for a panel of 12 commercial monoclonal antibodies against same variants. IC50 ± SEM values are shown in Supplementary Table S2.
Next, we assayed the neutralization profile for serum samples collected from vaccinees infected with BA.1 [samples (n = 16), taken ≥ 28 days following symptom onset], BA.2 [samples (n = 23), taken ≥ 12 days following symptom onset] or BA.4/5 [samples (n = 11, all but one vaccinated), taken > 23 days following symptom onset] (Fig. 1b–d). Neutralization titers against BA.4.6 were significantly reduced compared to BA.4/5 for both breakthrough BA.1 (1.5-fold; P = 0.0006) and BA.2 (1.2-fold; P = 0.0384) serum samples. Notably, BA.4.6 was able to effectively escape neutralization by serum samples from BA.1 breakthrough infections, showing a substantial reduction in titers compared to BA.1 (4.4-fold; P = 0.0001), BA.2 (threefold; P = 0.0009) and BA.4/5 (1.5-fold; P = 0.0006). A small non-significant increase in neutralization titers against BA.4.6 was observed in the BA.4/5 breakthrough cohort compared to BA.4/5. Of note, the single serum sample from the unvaccinated BA.4/5 convalescent showed lower levels of neutralization to most variants, especially BA.1 and BA1.1. It is not clear why the BA.2 and BA.4/5 neutralization titers using BA.2 and BA.4/5 serum respectively were not higher than titers for other SARS-CoV-2 variants as one might expect.
To further characterize the antigenic escape properties of BA.4.6, we performed pseudoviral assays on a panel of potent human mAbs generated from BA.1 breakthrough convalescents2 (Fig. 1e). In general, the neutralization profiles of BA.4.6 were similar to those of BA.4/5. However, the residual activity of Omi-35 (IC50 = 1.687 µg/mL) was further knocked out for BA.4.6, and the potency of Omi-32 and Omi-33 against BA.4/5 (IC50 = 0.035 and 0.013 µg/mL, respectively) was completely impaired for BA.4.6. The loss in activity of Omi-32 could be explained by the disruption of the interaction between CDR-H1 and R346 by the R346T mutation, as illustrated by previous structural analysis2.
Finally, we evaluated the neutralization activities of a number of mAbs in clinical use (Fig. 1f). The potency of AZD1061/cilgavimab against BA.4/5 was completely knocked out against BA.4.6, leading to a total loss in activity of AZD7742/Evusheld (a combination of AZD1061/cilgavimab and AZD8895/tixagevimab which is already inactive against BA.4/5). The activity of S309/sotrovimab [no longer authorized by the U.S. food and drug administration (FDA) for COVID-19 treatment since April 2022 due to its inefficacy against BA.2] was further reduced compared to BA.2 and BA.4/5. This, therefore, leaves LY-CoV1404/bebtelovimab the only option for the treatment of BA.4.6.
In summary, BA.4.6 showed further reduction in neutralization by serum from triple dose Pfizer vaccinees, as well as from BA.1 and BA.2 vaccine breakthrough convalescents compared to BA.4/5, which is in line with recent reports6. Notably, BA.4.6 does not seem to be more resistant to neutralization by serum from BA.4/5 breakthrough infection compared to other variants. This altogether suggests that there is a strong likelihood of infection or breakthrough infection by BA.4.6 unless one has been triply vaccinated and recovered from BA.4/5 infections, which seems to provide some protection against BA.4.6. Mutation R346T has been acquired by a number of emerging SARS-CoV-2 strains, notably BA.7 a derivative of BA.5 which is increasing in a number of locations (https://cov-spectrum.org/explore/United%20Kingdom/AllSamples/Past6M/variants?nextcladePangoLineage=bf.7*&).
Bivalent booster vaccination, combining the ancestral strain with Omicron BA.1 is being rolled out in the UK (https://www.gov.uk/government/news/pfizerbiontech-bivalent-covid-19-booster-approved-by-uk-medicines-regulator), and has been recently authorized by FDA (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use). It remains to be seen how effective these bivalent boosters are at preventing BA.4.6 infection. Finally, BA.4.6 has further impaired the activity of Evusheld which remained active against BA.4/5; as a result, now only LY-CoV1404/bebtelovimab retains potency against all circulating SARS-CoV-2 variants.
Supplementary information
Acknowledgements
This work was supported by the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Science (CIFMS), China (grant number: 2018-I2M-2-002) to D.I.S. and G.R.S. We are also grateful for support from Schmidt Futures, the Red Avenue Foundation, and the Oak Foundation. G.R.S. was supported by Wellcome. J.R. is supported by Wellcome (101122/Z/13/Z), D.I.S. and E.E.F. by the UKRI MRC (MR/N00065X/1). D.I.S. and G.R.S. are Jenner Investigators. This is a contribution from the UK Instruct-ERIC Centre. The convalescent sampling was supported by the Medical Research Council (grant MC_PC_19059, awarded to the ISARIC-4C consortium, with a full contributor list available at https://isaric4c.net/about/authors/), the National Institutes for Health, Oxford Biomedical Research Centre and an Oxfordshire Health Services Research Committee grant. OPTIC Consortium: Christopher Conlon, Alexandra Deeks, John Frater, Lisa Frending, Siobhan Gardiner, Anni Jämsén, Katie Jeffery, Tom Malone, Eloise Phillips, Lucy Rothwell, and Lizzie Stafford. The Wellcome Centre for Human Genetics is supported by the Wellcome Trust (grant 090532/Z/09/Z). The computational aspects of this research were supported by the Wellcome Trust Core Award Grant Number 203141/Z/16/Z and the NIHR Oxford BRC. The Oxford Vaccine work was supported by UK Research and Innovation, Coalition for Epidemic Preparedness Innovations, National Institute for Health Research (NIHR), NIHR Oxford Biomedical Research Centre, and Thames Valley and South Midland’s NIHR Clinical Research Network. We thank the Oxford Protective T cell Immunology for COVID-19 (OPTIC) Clinical team for participant sample collection and the Oxford Immunology Network Covid-19 Response T cell Consortium for laboratory support. We acknowledge the rapid sharing of Victoria, B.1.1.7, and B.1.351, which was isolated by scientists within the National Infection Service at PHE Porton Down, and the B.1.617.2 virus was kindly provided Wendy Barclay and Thushan De Silva. We thank The Secretariat of National Surveillance, Ministry of Health Brazil for assistance in obtaining P.1 samples. This work was supported by the UK Department of Health and Social Care as part of the PITCH (Protective Immunity from T cells to Covid-19 in Health workers) Consortium, the UK Coronavirus Immunology Consortium (UK-CIC), and the Huo Family Foundation. The views expressed in this article are those of the authors and not necessarily those of the National Health Service (NHS), the Department of Health and Social Care (DHSC), the NIHR, the Medical Research Council (MRC) or Public Health, England. Diamond Light Source provided time on Beamline I03 under Proposal lb27009 for COVID-19 Rapid Access and we thank beamline staff for support.
Author contributions
A.D.G., R.D., R.N., and C.L. performed neutralization assays. A.J.M., S.J.D., and T.L. assisted with the patient samples and vaccine trials. G.R.S. and D.I.S. conceived the study. G.R.S., D.I.S., and J.H. wrote the initial manuscript draft with other authors providing editorial comments. All authors read and approved the manuscript.
Conflict of interest
G.R.S. sits on the GSK Vaccines Scientific Advisory Board, is a consultant to Astra Zeneca, and is a founder member of RQ Biotechnology. Oxford University holds intellectual property related to the Oxford-Astra Zeneca vaccine and SARS-CoV-2 mAb discovered in G.R.S’s laboratory. T.L. is named as an inventor on a patent application covering this SARS-CoV-2 vaccine and was a consultant to Vaccitech for an unrelated project whilst the study was conducted. S.J.D. is a Scientific Advisor to the Scottish Parliament on COVID-19.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Aiste Dijokaite-Guraliuc, Raksha Das
Contributor Information
Jiandong Huo, Email: huojiandong@gird.cn.
David I. Stuart, Email: dave@strubi.ox.ac.uk
Gavin R. Screaton, Email: gavin.screaton@medsci.ox.ac.uk
Supplementary information
The online version contains supplementary material available at 10.1038/s41421-022-00493-0.
References
- 1.Dejnirattisai, W. et al. Cell185, 467–484.e15 (2022). [DOI] [PMC free article] [PubMed]
- 2.Nutalai, R. et al. Cell185, 2116–2131.e18 (2022). [DOI] [PMC free article] [PubMed]
- 3.Tuekprakhon, A. et al. Cell185, 2422–2433.e13 (2022). [DOI] [PMC free article] [PubMed]
- 4.Cao, Y. et al. Nature608, 593–602 (2022). [DOI] [PMC free article] [PubMed]
- 5.Di Genova C, et al. Bio Protoc. 2020;11:e4236. doi: 10.21769/BioProtoc.4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao, Y. et al. bioRxiv, 10.1101/2022.09.15.507787.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.