Abstract
Fusobacterium nucleatum subsp. nucleatum has been associated with a variety of oral and nonoral infections such as periodontitis, pericarditis, bone infections, and brain abscesses. Several studies have shown the role of plasmin, a plasma serine protease, in increasing the invasive capacity of microorganisms. In this study, we investigated the binding of human plasminogen to F. nucleatum subsp. nucleatum, and its subsequent activation into plasmin. Plasminogen-binding activity of bacterial cells was demonstrated by a solid-phase dot blot assay using an anti-plasminogen antibody. The binding activity was heat resistant and involved cell-surface lysine residues since it was abolished in the presence of the lysine analog ɛ-aminocaproic acid. Activation of plasminogen-coated bacteria occurred following incubation with either streptokinase, urokinase-type plasminogen activator (u-PA), or a Porphyromonas gingivalis culture supernatant. In the case of the P. gingivalis culture supernatant, a cysteine protease was likely involved in the activation. The plasmin activity generated on the cell surface of F. nucleatum subsp. nucleatum could be inhibited by aprotinin. Activation of plasminogen by u-PA was greatly enhanced when plasminogen was bound to bacteria rather than in a free soluble form. u-PA-activated plasminogen-coated F. nucleatum subsp. nucleatum was found to degrade fibronectin, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Tissue inhibitor of metalloproteinase-1 was also degraded by the plasmin activity generated on the bacterial cells. This study suggests a possible role for plasminogen, which is present in affected periodontal sites, in promoting tissue destruction and invasion by nonproteolytic bacteria such as F. nucleatum subsp. nucleatum.
Periodontitis is initiated by an overgrowth of specific bacterial species found at the gingival margin and results in a destruction of the tooth-supporting tissues, including the periodontal ligament and alveolar bone. The presence of gram-negative anaerobic bacteria such as Bacteroides forsythus, Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum, Treponema denticola, and Actinobacillus actinomycetemcomitans in subgingival sites has been associated with the different forms of periodontitis (12). The mechanisms of tissue destruction in periodontal diseases are complex and involve, in part, different bacterial products. Several studies have shown that proteases released by periodontopathogenic bacteria can degrade the principal constituents of periodontal tissues, including collagen, fibronectin, and laminin (11, 16). Bacterial proteases in association with host matrix metalloproteinases (MMPs) released during the inflammatory process may thus play a significant role in the disease progression by increasing the tissue-damaging effect (2, 11, 16, 30).
The fibrinolytic system participates in inflammatory reactions by regulating extracellular proteolysis (2). Plasmin, the proteolytic active form of plasminogen, is the principal mediator of this system. Plasminogen, a single-chain glycoprotein containing lysine-binding domains, is found in high concentrations in human plasma (9, 14). The lysine-binding sites are known to mediate the interaction of plasminogen with fibrin and plasma inhibitor α2-antiplasmin (3, 5). Plasminogen can be converted into plasmin via proteolytic cleavage by two types of plasminogen activators (PAs), tissue-type PA (t-PA) and urokinase-type PA (u-PA) (14). Bacterial products such as streptokinase and staphylokinase can also activate plasminogen into plasmin by a nonproteolytic mechanism (17). Plasmin is a trypsin-like serine protease with a large activity spectrum. It is implicated in different physiological and pathological processes, including ovulation, embryogenesis, and tumor cell invasion (32). The activities of plasmin and its host activators are regulated extracellularly through a number of inhibitors including α2-antiplasmin, PA inhibitor-1 (PAI-1), and PAI-2 (14). In addition to its main function as the lytic agent of fibrin clots, it can also degrade extracellular matrix proteins and activate the kinin cascade and MMPs (7, 13, 25, 27, 28). Both plasminogen and PAs have been found in high concentrations at inflammatory sites during periodontitis and may participate in tissue destruction (15, 19, 33).
Previous studies have shown that a number of bacterial pathogens can both produce PAs and capture the active plasmin on the cell surface (22). Other studies revealed that some bacterial species, including Borrelia burgdorferi (4) and Helicobacter pylori (26), can bind human plasminogen which may be subsequently activated into plasmin by host PAs. These above mechanisms are likely to favor bacterial dissemination and penetration into tissues. Indeed, plasmin-coated bacteria can generate a localized proteolysis that may have an important role in promoting tissue damage and bacterial penetration through the natural host barriers (22).
To our knowledge, plasminogen-binding activity in periodontopathogenic bacteria and the potential role of bacterium-bound plasmin activity in the pathophysiology of periodontitis have not been studied. While oral bacteria were being screened for plasminogen-binding activity, F. nucleatum subsp. nucleatum demonstrated strong activity (unpublished data). The aims of this study were to investigate the plasminogen-binding activity of F. nucleatum subsp. nucleatum and its subsequent activation into plasmin. The potential of plasmin-coated F. nucleatum subsp. nucleatum in promoting tissue destruction was also investigated.
MATERIALS AND METHODS
Bacteria and growth conditions.
The type strains F. nucleatum subsp. nucleatum ATCC 25586, F. nucleatum subsp. vincentii ATCC 49256, and F. nucleatum subsp. polymorphum ATCC 10953 were grown at 37°C for 24 h under anaerobiosis (N2-H2-CO2 [80:10:10]) in Todd-Hewitt broth (BBL Microbiology Systems, Cockeysville, Md.) supplemented with hemin (10 μg/ml) and vitamin K (1 μg/ml). Bacteria were harvested by centrifugation (10,000 × g for 15 min) and resuspended in 50 mM phosphate-buffered saline (PBS), pH 7.2, to an optical density of 2 at 660 nm. This corresponds to a concentration of 2 × 109 cells/ml, as determined by a Petroff-Hausser counting chamber.
Plasminogen-binding assay.
The plasminogen-binding activity of bacteria was evaluated by a solid-phase dot blot immunological procedure. Serial dilutions (1:2) of the bacterial suspensions were applied (5 μl) to a nitrocellulose membrane, and the unreacted sites were blocked by a 1-h incubation in Tris-buffered saline (TBS) (50 mM Tris-HCl–0.5 M NaCl [pH 7.2]) containing 3% gelatin. Thereafter, the membrane was incubated with human plasminogen (Sigma Chemical Co., St. Louis, Mo.), at a concentration of 1.5 × 10−2 U/ml for 90 min, washed three times in TBS containing 0.05% Tween 20 (TTBS), and then incubated with goat anti-human plasminogen antibodies (dilution, 1/1,000; Sigma Chemical Co.) in TBS for 1 h. After being washed twice in TTBS, the membrane was incubated with alkaline phosphatase-conjugated rabbit anti-goat immunoglobulin G antibodies (dilution, 1/5,000; Sigma Chemical Co.) in TBS for 1 h, washed three times in TTBS, and washed once in TBS. The enzymatic reaction was performed in a solution containing nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolylphosphate p-toluidine salt in 100 mM carbonate buffer, pH 9.8. The capacity of F. nucleatum subsp. nucleatum to bind plasminogen present in human serum was also investigated. Bacterial cells immobilized on a nitrocellulose membrane were incubated with human serum (1:3 in PBS), and the binding of plasminogen was evaluated by the assay described above. In one experiment, lipopolysaccharides were isolated from F. nucleatum subsp. nucleatum by the procedure of Darveau and Hancock (6) and tested for plasminogen-binding activity. Lipopolysaccharides were used at a concentration of 500 μg/ml.
Effects of various conditions or treatments on the plasminogen-binding activity of F. nucleatum subsp. nucleatum.
The effects of various conditions or treatments on the plasminogen-binding activity of F. nucleatum subsp. nucleatum were determined by the procedure described above. The effect of cell age was evaluated by using bacteria harvested at various stages during growth (6, 12, 24, 48, and 72 h). During the incubation of plasminogen with cells immobilized on the nitrocellulose membrane, the effect of reactional pH was tested with the following buffers: 50 mM citrate buffer (pHs of 3, 4, 5, and 6), 50 mM phosphate buffer (pH 7), 50 mM Tris-HCl buffer (pHs of 8 and 9), and 50 mM carbonate buffer (pHs of 10 and 11). Cells treated (15 min) at different temperatures (50, 60, 70, or 100°C) or incubated (2 h) with various enzymes (trypsin, chymotrypsin, or proteinase K; 500 μg/ml) were also tested. Finally, plasminogen-binding activity was evaluated in the presence of the chelating agent EDTA (10 mM) or the lysine analog ɛ-aminocaproic acid (10 mM).
Activation of plasminogen bound to F. nucleatum subsp. nucleatum.
Equal volumes of the cell suspension of F. nucleatum subsp. nucleatum and the plasminogen solution (3 × 10−2 U/ml in PBS) were incubated at 37°C for 2 h with gentle shaking. Thereafter, bacteria were washed twice with PBS and further incubated for 1 h at 37°C with either streptokinase (500 U/ml; Sigma Chemical Co.), u-PA (0.15 U/ml in PBS; Sigma Chemical Co.), or a P. gingivalis (ATCC 33277) culture supernatant (24-h culture, final dilution of 1:5 in PBS). Cells were then washed twice in PBS and suspended in half of the initial volume. Aliquots of 100 μl were incubated with the plasmin chromogenic substrate (d-Val-Leu-Lys-p-nitroanilide, 20 μl of a solution at 2 mg/ml in PBS) for 4 h at 37°C. Bacteria were pelleted, and the absorbance of the supernatant was measured at 405 nm. The activation assays were performed twice in duplicate with independent bacterial cultures, and the absorbance (mean ± standard deviation) was calculated. A standard curve was prepared with pure human plasmin (Sigma Chemical Co.) at concentrations ranging from 0.0025 to 0.025 U/ml. Inhibition of plasmin activity generated on the bacterial cell surface was evaluated by incubating cells with either aprotinin (2 μg/ml in PBS) or human serum (1:5 in PBS) for 1 h prior to incubation with the plasmin chromogenic substrate. The activation of plasminogen bound to F. nucleatum subsp. nucleatum by u-PA in the presence of human serum was also tested. Human serum (1:5 in PBS) was added prior to the incubation of plasminogen-coated bacteria with u-PA (0.15 U/ml in PBS). An assay in which cells were not preincubated with plasminogen was also performed. Finally, to test whether the immobilization of plasminogen on the cell surface of F. nucleatum subsp. nucleatum facilitates its activation into plasmin, a similar amount of plasminogen was incubated in the presence or absence of bacteria prior to activation with either u-PA (0.15 U/ml in PBS) or streptokinase (500 U/ml in PBS), as described above. Plasmin activity was monitored by measuring hydrolysis of the chromogenic substrate.
Characterization of the PA produced by P. gingivalis.
To determine the nature of the PA produced by P. gingivalis (ATCC 33277), the culture supernatant was either boiled (15 min) or passed through a microfilter with a nominal molecular mass cutoff of 100 or 300 kDa, prior to incubation with bacterium-bound plasminogen. The effect of adding 1 mM p-chloromercuriphenylsulfonic acid, an inhibitor of cysteine proteases, to the culture supernatant was also tested. The plasmin activity generated on the surface of F. nucleatum subsp. nucleatum was measured as described above.
Degradation of fibronectin by plasmin-coated F. nucleatum subsp. nucleatum.
Equal volumes of the cell suspension of F. nucleatum subsp. nucleatum and plasminogen (3.3 × 10−1 U/ml in PBS) were incubated for 2 h at 37°C. Then, cells were washed once with PBS buffer and suspended in one quarter of the initial volume. After activation or not with u-PA (0.75 U/ml in PBS), cells were washed once and suspended in half of the initial volume. Aliquots (40 μl) of either activated or nonactivated plasminogen-coated bacteria were incubated for 16 h at 37°C with fibronectin (10 μl, 1 mg/ml in PBS). In control tests, proteins were incubated with commercial plasmin at a concentration of 0.05 U/ml in PBS. Solubilizing buffer (30 μl) was added to the cell supernatant (40 μl), and the mixtures were boiled for 10 min. Samples were then analyzed by sodium dodecyl sulfate–8% polyacrylamide gel electrophoresis (SDS–8% PAGE) by using the buffer system of Laemmli (20). Proteins were stained with Coomassie brilliant blue.
Degradation of TIMP-1 by plasmin-coated F. nucleatum subsp. nucleatum.
Cells of F. nucleatum subsp. nucleatum coated with plasminogen and activated by u-PA were prepared as described above and incubated for 4 h at 37°C with an equal volume of tissue inhibitor of metalloproteinase-1 (TIMP-1; 5 μg/ml; Cedarlane Laboratories Ltd., Hornby, Ontario, Canada). In a control test, TIMP-1 was incubated with pure plasmin at a final concentration of 0.05 U/ml in PBS. After incubation, solubilizing buffer was added and the samples were boiled (10 min) and analyzed by SDS–15% PAGE by using the buffer system of Laemmli (20). Proteins were electrophoretically transferred onto a nitrocellulose membrane. The membrane was incubated for 60 min in TBS containing 3% gelatin to block the unreacted sites. Thereafter, the membrane was incubated for 2.5 h with rabbit anti-native human TIMP-1 (Cedarlane Laboratories Ltd.) as the primary antibody at a concentration of 4 μg/ml, and then with goat anti-rabbit immunoglobulin G alkaline phosphatase conjugate (Bio-Rad Laboratories, Mississauga, Ontario, Canada) as the secondary antibody at a dilution of 1/3,000 for 60 min. The enzymatic reaction was developed as described above.
RESULTS
Plasminogen-binding activity.
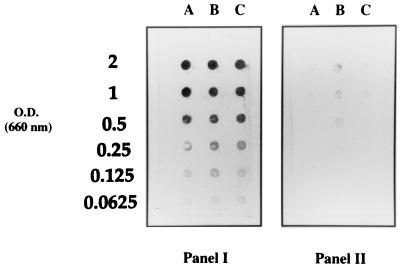
The three subspecies of F. nucleatum (F. nucleatum subsp. nucleatum, F. nucleatum subsp. vincentii, and F. nucleatum subsp. polymorphum) were found to bind human plasminogen, as determined by the solid-phase dot blot assay using an anti-plasminogen antibody (Fig. 1, panel I). Omitting the incubation step with plasminogen revealed a weak, nonspecific attachment of the antibodies to cells of F. nucleatum subsp. vincentii (Fig. 1, panel II). Other oral bacterial species tested (A. actinomycetemcomitans and T. denticola) did not show any plasminogen-binding activity (data not shown). When cells of F. nucleatum subsp. nucleatum immobilized on the nitrocellulose membrane were incubated with human serum instead of pure plasminogen, a binding of the plasminogen present in the serum was noted (data not shown).
FIG. 1.
Plasminogen-binding activity of three subspecies of F. nucleatum determined by a solid-phase dot blot procedure using an anti-plasminogen antibody. Panel I, incubation of cells with human plasminogen. Panel II, control without incubation of cells with plasminogen. Lanes A, F. nucleatum subsp. nucleatum ATCC 25586; lanes B, F. nucleatum subsp. vincentii ATCC 49256; lanes C, F. nucleatum subsp. polymorphum ATCC 10953. Cells suspended at various optical densities in PBS were applied. O.D., optical density.
Further experiments were performed with F. nucleatum subsp. nucleatum ATCC 25586. It appears that younger cells (6-h culture) bound less plasminogen than cells that were older. F. nucleatum subsp. nucleatum was found to bind plasminogen under a pH range of 3 to 9. Although slightly decreased, the binding also occurred at pHs of 10 and 11. Treating cells with proteolytic enzymes did not inhibit the binding. In addition, heat treatment (100°C for 10 min) of cells had no effect on the binding activity. No effect was noticed when bacteria were incubated with plasminogen in the presence of the cation chelator, EDTA. However, in the presence of the lysine analog ɛ-aminocaproic acid, the binding of plasminogen to F. nucleatum subsp. nucleatum was completely inhibited. Finally, lipopolysaccharides isolated from F. nucleatum subsp. nucleatum did not show any plasminogen-binding activity by the dot blot immunological assay.
Activation of plasminogen bound to the bacterial cell surface.
To test whether the plasminogen bound to the cell surface of F. nucleatum subsp. nucleatum can be converted into proteolytically active plasmin, plasminogen-coated bacteria were treated with different sources of activators prior to being incubated with the chromogenic substrate for plasmin. Streptokinase, u-PA, and a P. gingivalis culture supernatant were found to activate plasminogen bound to F. nucleatum subsp. nucleatum to various extents (Table 1). The strongest activations were obtained with streptokinase and u-PA. A standard curve revealed that in our assay conditions, the amounts of plasmin activity generated by streptokinase, u-PA, and P. gingivalis on the bacterial cell surface were 0.015, 0.025, and 0.01 U, respectively. In the control assays, no hydrolysis of the plasmin chromogenic substrate was observed when cells were not preincubated with plasminogen. The PA present in the culture supernatant of P. gingivalis could be completely inhibited by incorporation of p-chloromercuriphenylsulfonic acid in the assay or by heat treatment at 100°C. In order to estimate the molecular mass of the activator, the culture supernatant was passed through microfilters with various pore sizes. The PA produced by P. gingivalis was found to have a molecular mass between 100 and 300 kDa.
TABLE 1.
Activation of plasminogen bound to F. nucleatum subsp. nucleatuma
| Activator | Mean A405 ±
SEMa
|
|
|---|---|---|
| Coated with plasminogen | Uncoated (control) | |
| None | 0.056 ± 0.010 | 0.045 ± 0.012 |
| Streptokinase (500 U/ml) | 0.518 ± 0.031 | 0.051 ± 0.006 |
| Urokinase (0.15 U/ml) | 0.644 ± 0.046 | 0.057 ± 0.007 |
| P. gingivalis culture supernatant (1:5) | 0.171 ± 0.018 | 0.066 ± 0.009 |
Hydrolysis of the plasmin chromogenic substrate by F. nucleatum subsp. nucleatum was measured. Values are the results of two independent experiments performed in duplicate. A405, absorbance at 405 nm.
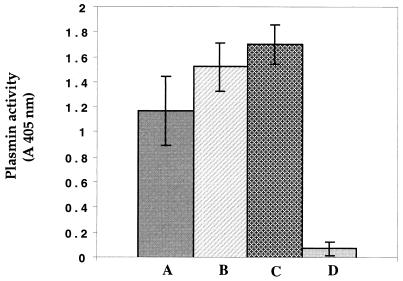
Plasmin activity generated on the bacterial cell surface by streptokinase could be inhibited at a level of approximately 80% by the serine protease inhibitor aprotinin. In the presence of human serum, plasminogen-coated cells incubated with u-PA demonstrated a capacity to hydrolyze the chromogenic substrate (Fig. 2). This level of activity was slightly higher than that observed in the absence of serum. When uncoated cells were incubated with both human serum and u-PA, plasmin activity could be generated, indicating that bacteria can bind plasminogen present in human serum. However, in the absence of u-PA, no enzymatic activity was generated.
FIG. 2.
Effect of human serum on plasmin activity generated on the surface of F. nucleatum subsp. nucleatum. Bars: A, plasminogen-coated cells incubated with u-PA; B, plasminogen-coated cells incubated with u-PA and serum; C, plasminogen-free cells incubated with u-PA and serum; and D, plasminogen-free cells incubated with serum. Plasmin activity was measured by incubation of cells with the chromogenic substrate and determination of the absorbance at 405 nm. Results are the means ± standard deviations (error bars) of three independent experiments.
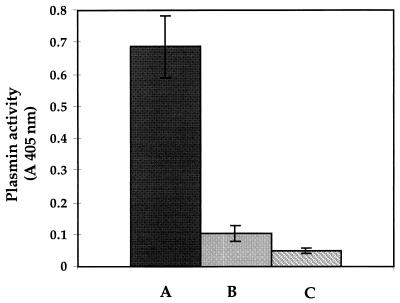
The effect of immobilizing the plasminogen on the cell surface of F. nucleatum subsp. nucleatum on its activation into plasmin was evaluated (Fig. 3). It was found that activation of plasminogen by u-PA was facilitated when plasminogen was attached to F. nucleatum subsp. nucleatum rather than in a soluble form. No such effect was obtained when plasminogen was activated with streptokinase.
FIG. 3.
Effect of immobilization of plasminogen on the surface of F. nucleatum subsp. nucleatum on its activation by u-PA. Bars: A, plasminogen-coated cells incubated with u-PA; B, free soluble plasminogen incubated with u-PA; and C, plasminogen-coated cells without incubation with u-PA. Plasmin activity was measured by incubation of cells with the chromogenic substrate and determination of the absorbance at 405 nm. Results are the means ± standard deviations (error bars) of three independent experiments.
Degradation of host proteins.
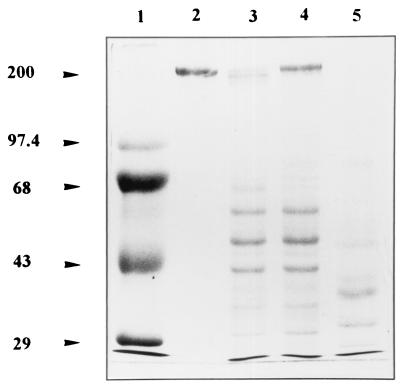
Degradation of fibronectin by F. nucleatum subsp. nucleatum after acquisition of proteolytic plasmin activity was investigated. u-PA-activated plasminogen-coated bacteria were found to degrade fibronectin (Fig. 4). Omitting the step of cell harvesting prior to electrophoresis revealed that the decrease in the intensity of the fibronectin bands was not due to binding of the protein to the cell surface. Cells in which the bound plasminogen was not activated did not show any capacity to degrade the protein. Control tests which used pure plasmin revealed a complete degradation of fibronectin. Incubating u-PA-activated plasminogen-coated bacteria in the presence of TIMP-1 revealed that this protein was highly susceptible (Fig. 5). No degradation occurred when nonactivated plasminogen-coated bacteria were used.
FIG. 4.
Degradation of fibronectin by u-PA-activated plasminogen-coated F. nucleatum subsp. nucleatum. Bacteria were incubated with fibronectin for 16 h at 37°C, and the supernatants were analyzed by SDS-PAGE and Coomassie blue staining. Lanes: 1, molecular mass markers (kDa); 2, fibronectin alone; 3, u-PA-activated plasminogen-coated bacteria with fibronectin; 4, plasminogen-coated bacteria with fibronectin; and 5, pure plasmin with fibronectin. Bands present in the lower part of the gels (lanes 3 and 4) correspond to proteins or lipopolysaccharides released from bacterial cells during the incubation period.
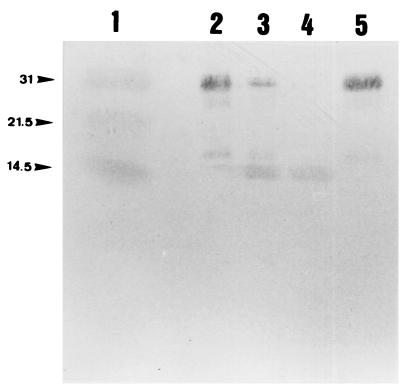
FIG. 5.
Degradation of TIMP-1 by u-PA-activated plasminogen-coated F. nucleatum subsp. nucleatum. Bacteria were incubated with TIMP-1 for 16 h at 37°C, and the supernatants were analyzed by SDS-PAGE and Western immunoblotting using an anti-TIMP-1 antibody. Lanes: 1, molecular mass markers (kDa); 2, TIMP-1 alone; 3, u-PA-activated plasminogen-coated bacteria with TIMP-1; 4, pure plasmin with TIMP-1; and 5, plasminogen-coated bacteria with TIMP-1.
DISCUSSION
The role of plasmin activity in the pathogenesis of several infections has been investigated by different research groups (4, 22, 26). It has been hypothesized that invasive microorganisms can acquire plasmin activity on their cell surface and penetrate through natural host barriers, causing tissue destruction. In this study, we showed that F. nucleatum subsp. nucleatum, a suspected periodontopathogen, can bind human plasminogen on its surface. A consequence of this binding is that cells covered with a host protein such as plasminogen may be protected from the host immune system and thus possess an increased virulence. This activity was inhibited by a lysine analog, ɛ-aminocaproic acid, which suggests that the binding of plasminogen to F. nucleatum subsp. nucleatum involves lysine residues present on the bacterial cell surface. Lysine-binding sites found on the kringles domains of the plasminogen molecule are also responsible for the interaction of plasminogen with other microorganisms, including B. burgdorferi (4) and H. pylori (26). The binding of plasminogen to F. nucleatum subsp. nucleatum did not involve lipopolysaccharides, since this purified cell surface component demonstrated no plasminogen-binding activity by the solid-phase dot blot assay. Furthermore, electrostatic interactions appear not to participate in the binding of plasminogen to F. nucleatum, since sodium chloride was always included at 0.5 M during the incubation of cells with plasminogen. The fact that treatment of bacterial cells with proteases had no effect on the binding of plasminogen suggests that the receptors may be either partially masked by nonproteinaceous surface components or highly resistant to proteolytic enzymes.
Plasminogen bound to F. nucleatum subsp. nucleatum could be converted into proteolytic active plasmin by streptokinase, u-PA, and a P. gingivalis product which is likely a cysteine protease. It is logical to speculate that this mechanism of acquisition of plasmin activity occurs in the subgingival sites, since both plasminogen and plasminogen activators have been detected in high concentrations at inflammatory sites during periodontitis (15, 19, 33). Indeed, the concentrations of PAs have been found to be 100-fold higher in the gingival crevicular fluid than in plasma (19). A recent study by Kinnby et al. (19) revealed that the distribution of PA activity in the gingival crevicular fluid was associated with the clinical status of the periodontal tissue. A significant decrease of t-PA and PAI-2 was observed following periodontal treatment. Distribution of PAs and PAIs in human gingival tissue and gingival fibroblasts was also reported by Xiao et al. (33). They showed by immunohistochemical methods that during inflammation, t-PA was highly expressed in the extracellular matrix of gingival connective tissue. The expression of t-PA by fibroblasts was also stimulated in vitro in the presence of interleukin-1β. Mochan et al. (23) have also reported that interleukin-1β may stimulate the production of PAs by gingival fibroblasts. These studies suggest a possible interaction between inflammatory mediators and the plasmin system that could contribute to increasing the tissue destruction observed in inflammated periodontal sites. Furthermore, lipopolysaccharides from Campylobacter rectus are known to stimulate plasmin activity in the gingival fibroblasts by increasing the amount of u-PA (24). Some studies have measured the level of plasmin activity in gingival crevicular fluid of periodontitis patients (15, 29). Hidaka et al. (15) reported increased activities of plasmin as well as of PAs in samples from diseased periodontal sites. It was also found that periodontal treatments resulted in a marked decrease of the plasmin activity in gingival crevicular fluid (29).
Activation of plasminogen by u-PA was greatly enhanced when plasminogen was bound to F. nucleatum subsp. nucleatum rather than in a free soluble form. This observation was also reported for another pathogenic bacterium, Salmonella enterica (21). This phenomenon suggests that changes in the conformation of the plasminogen molecule occur following its fixation on bacteria and render it more susceptible to cleavage by u-PA. When activation of F. nucleatum subsp. nucleatum-bound plasminogen was performed by treatment with streptokinase instead of u-PA, the amount of plasmin activity generated was similar to that generated with free plasminogen. This is likely related to the fact that the mechanism of activation by streptokinase is not proteolytic, as for the u-PA, but rather mediated by a 1:1 stoichiometric complex that can convert free plasminogen into plasmin (17). F. nucleatum subsp. nucleatum incubated with human serum and u-PA revealed a strong capacity to hydrolyze the plasmin chromogenic substrate. This indicates the ability of bacteria to bind plasminogen present in serum, suggesting that these interactions could function under physiological conditions such as those found in periodontal pockets.
In this study, plasminogen bound to F. nucleatum subsp. nucleatum was activated by a supernatant of P. gingivalis, suggesting a potential mechanism for the generation of plasmin activity in the subgingival sites. Since the activation was inhibited by a cysteine protease inhibitor, it is likely to involve one of the proteases produced by P. gingivalis. In a previous report, an 80-kDa cysteine protease from P. gingivalis was found to activate human plasminogen (10). Furthermore, increased amounts of collagenase and PA, secreted by gingival fibroblasts, were demonstrated in the presence of a 35-kDa protease from P. gingivalis (31). Interestingly, F. nucleatum is known to coaggregate with a wide range of bacteria, including P. gingivalis (18). This could favor the interaction between proteases produced by these microorganisms and the plasminogen that could be attached on the cell surface of F. nucleatum.
Invasive microorganisms could use their capacity to produce proteolytic enzymes to penetrate through host barriers, a critical step for tissue invasion. Other pathogens devoid of such protease activities could capture host protease activities on their surface to acquire invasive properties. Plasmin is a potent protease that can degrade extracellular matrix proteins. This proteolytic activity plays important roles in many physiological and pathological processes, such as cell migration, tissue remodeling, and tumor cell invasion (32). Thus, the ability of F. nucleatum subsp. nucleatum to bind human plasminogen and the possible activation into plasmin by host or bacterial activators could enhance the capacity of this microorganism to degrade the surrounding gingival tissues. In this study, degradation of fibronectin by plasmin-coated F. nucleatum subsp. nucleatum was demonstrated. Earlier studies have shown that invasive infections, such as Lyme disease caused by B. burgdorferi, are associated with the capacity of bacteria to penetrate through host natural barriers (4). B. burgdorferi, a nonproteolytic microorganism, was found to bind human plasminogen which can be converted into active plasmin. Thus, incorporation of a host protease could enhance the capacity of bacteria to spread by contributing to the degradation of extracellular matrix proteins.
Proteolytic activity observed in the subgingival sites results in part from the degranulation of polymorphonuclear (PMN) cells during the inflammatory process (2). Recent studies have demonstrated that secretion and activation of PMN enzymes including MMPs are greatly enhanced by direct interaction of PMN cells with periodontopathogens including F. nucleatum (8). Indeed, F. nucleatum was found to induce in vitro synthesis of high amounts of elastase and collagenase (MMP-8), after interaction with PMN cells (8). This indicates the high potential role of F. nucleatum in promoting tissue destruction during periodontitis by increasing the concentration of MMPs. These proteinases are normally produced under a latent form and are activated by the host when necessary. It is possible that MMPs could be activated by plasmin-coated F. nucleatum subsp. nucleatum, since it was previously demonstrated that pro-MMPs can be activated by plasmin (7, 25). The degradation of TIMP-1 by plasmin-coated F. nucleatum subsp. nucleatum that we observed may also be in part responsible for the high MMP activities observed in periodontal sites during periodontitis.
The phenomenon of acquisition of plasmin activity by F. nucleatum subsp. nucleatum reported in this study may potentially increase the virulence of this bacterial species. Our in vitro results support the hypothesis that once covered with plasmin activity, the bacteria may invade periodontal tissues and participate in its destruction. In addition, the bacteria may use this acquired invasive property to migrate from periodontal pockets via the bloodstream and cause serious infections in various organs of humans, as previously reported (1).
REFERENCES
- 1.Bennett K W, Eley A. Fusobacteria: new taxonomy and related diseases. J Med Microbiol. 1993;39:246–254. doi: 10.1099/00222615-39-4-246. [DOI] [PubMed] [Google Scholar]
- 2.Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J Periodontol. 1993;64:474–484. doi: 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- 3.Christensen U, Clemmensen I. Purification and reaction mechanism of the primary inhibitor of plasmin from human plasma. J Biochem. 1974;175:635–641. doi: 10.1042/bj1750635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman J L, Sellati T J, Testa J E, Kew R R, Furie M B, Benach J L. Borrelia burgdorferibinds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect Immun. 1995;63:2478–2484. doi: 10.1128/iai.63.7.2478-2484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collen D. On the regulation and control of fibrinolysis. Thromb Haemostasis. 1980;43:77–89. [PubMed] [Google Scholar]
- 6.Darveau R P, Hancock R E W. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimuriumstrains. J Bacteriol. 1983;155:831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Declerck Y A, Laug W E. Cooperation between matrix metalloproteinases and the plasminogen activator-plasmin system in tumor progression. Enzyme Protein. 1996;49:72–84. doi: 10.1159/000468617. [DOI] [PubMed] [Google Scholar]
- 8.Ding Y, Haapasalo M, Kerosuo E, Lounatmaa K, Kotiranta A, Sorsa T. Release and activation of human neutrophil matrix metallo- and serine proteinases during phagocytosis of Fusobacterium nucleatum, Porphyromonas gingivalis and Treponema denticola. J Clin Periodontol. 1997;24:237–248. doi: 10.1111/j.1600-051x.1997.tb01837.x. [DOI] [PubMed] [Google Scholar]
- 9.Francis C W, Marder V J. Mechanism of fibrinolysis. In: Williams W, Beutler E, Lichtman A J E, editors. Hematology. New York, N.Y: McGraw-Hill; 1983. pp. 1266–1276. [Google Scholar]
- 10.Grenier D. Degradation of host protease inhibitors and activation of plasminogen by proteolytic enzymes from Porphyromonas gingivalis and Treponema denticola. Microbiology. 1996;142:955–961. doi: 10.1099/00221287-142-4-955. [DOI] [PubMed] [Google Scholar]
- 11.Grenier D, Mayrand D. Proteinases. In: Shah H N, Mayrand D, Genco R J, editors. Biology of the species Porphyromonas gingivalis. Boca Raton, Fla: CRC Press; 1993. pp. 227–243. [Google Scholar]
- 12.Haffajee A D, Socransky S S. Microbial etiological agents of destructive periodontal diseases. Periodontology 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 13.He C S, Wilhelm S M, Pentland A P, Marmer B L, Grant G A, Eisen A Z, Goldberg G I. Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc Natl Acad Sci USA. 1989;86:2632–2636. doi: 10.1073/pnas.86.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hekman C M, Loskutoff D J. Fibrinolytic pathways and endothelium. Semin Thromb Hemost. 1987;13:514–523. doi: 10.1055/s-2007-1003527. [DOI] [PubMed] [Google Scholar]
- 15.Hidaka N, Maeda K, Kawakami C, Aono M, Okada H. Fibrinolytic activity in periodontal disease: the relationship between fibrinolytic activity and severity of periodontal disease. J Periodontol. 1981;52:181–186. doi: 10.1902/jop.1981.52.4.181. [DOI] [PubMed] [Google Scholar]
- 16.Holt S C, Bramanti T E. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med. 1991;2:177–281. doi: 10.1177/10454411910020020301. [DOI] [PubMed] [Google Scholar]
- 17.Johnston K H. Solid and fluid phase assays for bacterial plasminogen activators. J Microbiol Methods. 1993;18:267–274. [Google Scholar]
- 18.Kinder S A, Holt S C. Characterization of coaggregation between Bacteroides gingivalis T22 and Fusobacterium nucleatumT18. Infect Immun. 1989;57:3425–3433. doi: 10.1128/iai.57.11.3425-3433.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinnby B, Matsson L, Lecander I. The plasminogen-activating system in gingival fluid from adults. Scand J Dent Res. 1994;102:334–341. [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Lähteenmäki K, Virkola R, Pouttu R, Kuusela P, Kukkonen M, Korhonen T K. Bacterial plasminogen receptors: in vitro evidence for a role in degradation of the mammalian extracellular matrix. Infect Immun. 1995;63:3659–3664. doi: 10.1128/iai.63.9.3659-3664.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lottenberg R, Minning-Wenz D, Boyle M D P. Capturing host plasmin(ogen): a common mechanism for invasive pathogens? Trends Microbiol. 1994;2:20–24. doi: 10.1016/0966-842x(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 23.Mochan E, Arnor L, Sporer R. Interleukin 1 stimulation of plasminogen activator production in cultured gingival fibroblasts. J Periodontal Res. 1988;23:28–32. doi: 10.1111/j.1600-0765.1988.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 24.Ogura N, Shibata Y, Matsuda U, Oikawa T, Takiguchi H, Izumi H, Abiko Y. Effect of Campylobacter rectusLPS on plasminogen activator-plasmin system in human gingival fibroblast cells. J Periodontal Res. 1995;30:132–140. doi: 10.1111/j.1600-0765.1995.tb01262.x. [DOI] [PubMed] [Google Scholar]
- 25.Okumura Y, Sato H, Seiki M, Kido H. Proteolytic activation of the precursor of membrane type 1 matrix metalloproteinase by human plasmin. A possible cell surface activator. FEBS Lett. 1997;402:181–184. doi: 10.1016/s0014-5793(96)01523-2. [DOI] [PubMed] [Google Scholar]
- 26.Pantzar M, Ljungh A, Wadström T. Plasminogen binding and activation at the surface of Helicobacter pyloriCCUG 17874. Infect Immun. 1998;66:4976–4980. doi: 10.1128/iai.66.10.4976-4980.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salonen E-M, Zitting A, Vaheri A. Laminin interacts with plasminogen and its tissue-type activator. FEBS Lett. 1984;172:29–32. doi: 10.1016/0014-5793(84)80866-2. [DOI] [PubMed] [Google Scholar]
- 28.Salonen E-M, Saksela O, Vartio T, Vaheri A, Nielsen L S, Zeuthen J. Plasminogen and tissue-type plasminogen activator bind to immobilized fibronectin. J Biol Chem. 1985;260:12302–12307. [PubMed] [Google Scholar]
- 29.Talonpoika J, Söderling E, Tiekso J, Paunio K. Gingival crevicular fluid plasmin activity in different clinical conditions and after periodontal treatment. Proc Finn Dent Soc. 1991;87:329–337. [PubMed] [Google Scholar]
- 30.Travis J, Pike R, Imamura T, Potempa J. The role of proteolytic enzymes in the development of pulmonary emphysema and periodontal disease. Am J Respir Crit Care Med. 1994;150:143–146. doi: 10.1164/ajrccm/150.6_Pt_2.S143. [DOI] [PubMed] [Google Scholar]
- 31.Uitto V-J, Larjava H, Heino J, Sorsa T. A protease of Bacteroides gingivalisdegrades cell surface and matrix glycoproteins of cultured gingival fibroblasts and induces secretion of collagenase and plasminogen activator. Infect Immun. 1989;57:213–218. doi: 10.1128/iai.57.1.213-218.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vassalli J-D, Sappino A-P, Belin D. The plasminogen activator/plasmin system. J Clin Investig. 1991;88:1067–1072. doi: 10.1172/JCI115405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao Y, Bunn C L, Bartold P M. Immunohistochemical demonstration of the plasminogen activator system in human gingival tissues and gingival fibroblasts. J Periodontal Res. 1998;33:17–26. doi: 10.1111/j.1600-0765.1998.tb02287.x. [DOI] [PubMed] [Google Scholar]