Abstract
Compounds with antiendotoxin properties have been extensively studied for their potential as therapeutic agents for sepsis attributable to gram-negative bacteria. However, with the increasing incidence of gram-positive sepsis, there is interest in identifying compounds with a broad spectrum of action against both gram-positive and gram-negative bacteria. A series of synthetic α-helical cationic peptides related to bee melittin and silk moth cecropin have previously been shown to bind lipopolysaccharide (LPS) with high affinity, inhibit LPS-induced tumor necrosis factor alpha (TNF-α) production in vitro and in vivo, and kill gram-negative bacteria. In this study, we analyzed whether these peptides were active against gram-positive bacteria; whether they could bind to lipoteichoic acid (LTA), the major proinflammatory structure on gram-positive bacteria; and whether they could block the ability of LTA to promote the release of cytokines by the RAW 264.7 murine macrophage cell line. We found that the cationic peptides demonstrated moderate growth-inhibitory activity toward gram-positive bacteria. In addition, the peptides bound LTA with high affinity. This correlated with the ability of the peptides to block LTA-induced production of TNF and interleukin-6 by RAW 264.7 cells but did not correlate with their ability to kill the bacteria. The peptides also effectively inhibited LTA-induced TNF production in a whole human blood assay. The peptides were also able to partly block the ability of heat-killed Staphylococcus aureus, as well as soluble products of live S. aureus, to stimulate cytokine production by macrophages. Our results indicate that these cationic peptides may be useful to prevent sepsis and inflammation caused by both gram-negative and gram-positive bacteria.
Sepsis is associated with the presence of pathogenic microorganisms or their toxins in the blood. It can result from infections with either gram-negative or gram-positive bacteria. Sepsis due to a gram-negative bacterium (gram-negative sepsis) is usually caused by the release of a bacterial outer membrane component, endotoxin (lipopolysaccharide [LPS]). The systemic release of cytokines, in particular tumor necrosis factor alpha (TNF-α), can result in septic shock and death. Gram-positive sepsis is also presumed to be due to the release of bacterial cell wall components. A number of gram-positive cell wall constituents, including lipoteichoic acid (LTA) (10), peptidoglycan (PG) (16), Streptococcus rhamose-glucose polymers (24), and Staphylococcus capsular polysaccharide (23), have been shown to stimulate the production of inflammatory mediators in vitro. When injected into animals, these gram-positive cell wall components elicit many of the characteristic features of septic shock, including cytokine production, leukocytopenia, circulatory failure, multiple-organ dysfunction syndrome, and mortality (3, 14, 15, 18, 31). PG has also been shown to enhance the toxicity of endotoxin in animals (26). The increasing incidence of gram-positive-microorganism-induced septic shock (2) indicates that there is a need to develop therapeutic strategies to prevent the activation of inflammatory cells by components of gram-positive cell walls.
Two of the major gram-positive cell wall components that are known to stimulate the production of inflammatory mediators are PG and LTA. PG is an essential constituent of the gram-positive cell wall, while LTAs are associated with the cell walls of most, but not all, gram-positive bacteria (6, 7). PG is a polymer of alternating GlcNAc and MurNAc residues with tetrapeptide side chains, cross-linked in gram-positive bacteria by short peptides. LTAs are amphipathic compounds which typically consist of a repeating glycerol phosphate backbone that is substituted with d-alanine, sugars such as glucose, and a single lipid side chain that intercalates into the cytoplasmic membrane (7). Both LTA and PG are released spontaneously into the culture medium during growth of gram-positive bacteria (25). Moreover, β-lactam antibiotics such as penicillin enhance the release of LTA and PG (12, 29). Thus, the release of LTA and PG from gram-positive bacteria may promote septic shock during bacterial infections and during subsequent antibiotic treatment.
Despite their structural differences, LTA and PG both activate macrophages and polymorphonuclear leukocytes by binding to CD14 (4, 11, 32), a surface receptor that mediates responses to LPS (27, 28). Thus, substances that bind to bacterial components and ablate their ability to bind to CD14 would be good candidates for use as anti-inflammatory agents. Compounds with a broad spectrum of binding to both gram-positive and gram-negative bacterial products would be extremely useful in this regard.
We and others have previously shown that cationic peptides can bind to LPS and neutralize its ability to stimulate the production of inflammatory cytokines (8, 22). In particular, we have focused on derivatives of an α-helical peptide that is a hybrid of silk moth cecropin and bee melittin (1). The parent peptide, CEME, contains the N-terminal 8 amino acids of cecropin followed by the first 18 amino acids of melittin. CEME and its derivatives have strong antimicrobial activity against gram-negative bacteria, bind LPS with a high affinity, block LPS-induced macrophage activation in vitro, and block LPS-induced toxicity in mice (8, 19, 22).
In this study, we have investigated whether these synthetic cationic peptides have antimicrobial activity toward gram-positive bacteria, whether they can bind LTA, and whether they can block the ability of LTA, PG, or heat-killed Staphylococcus aureus to induce the production of inflammatory mediators by the RAW 264.7 murine macrophage cell line. We have also tested the ability of the peptides to work in vivo, in a whole-blood assay. Our results indicate that several of these cationic peptides can kill gram-positive bacteria and prevent the production of TNF-α and interleukin-6 (IL-6) in response to heat-killed gram-positive bacteria or purified gram-positive bacterial cell wall components. Thus, these cationic peptides may have therapeutic potential for the treatment of gram-positive sepsis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains were grown on Meuller-Hinton medium supplemented with 1.5% (wt/vol) agar, with the exception of Streptococcus pyogenes, which was grown on Todd-Hewitt medium. The strains used in this study were S. aureus RN4220, ATCC 25293, and SAP0017 (methicillin-resistant S. aureus), as well as clinical S. aureus isolates received from A. Chow (Department of Medicine, University of British Columbia), Staphylococcus epidermidis (a clinical isolate from A. Chow), Streptococcus pyogenes ATCC 19615, Enterococcus faecalis ATCC 29212, Bacillus subtilis (lab strain), Listeria monocytogenes NCTC 7973, Cornyebacterium xerosis (lab strain), and Escherichia coli UB1005 (20).
Bacterial products.
LTA from S. aureus, Streptococcus pyogenes, and B. subtilis, as well as PG peptide from the cell wall of S. aureus (d-ala–isoglutaminyl–l-lys–d-ala–d-ala), were purchased from Sigma Chemical Co (St. Louis, Mo.). PG from Micrococcus luteus was purchased from Wako (Osaka, Japan). LTA and PG were resuspended in endotoxin-free water (Sigma). The Limulus amoebocyte lysate assay (Sigma) was performed on the LTA and PG preparations to confirm that lots were not significantly contaminated by endotoxin. The level of endotoxin contamination was less than 1 ng/ml, a concentration that did not cause significant cytokine production (<0.2 ng/ml) in the RAW cell assay. Heat-killed S. aureus was prepared by boiling the bacterial cells for 10 min and then washing them three times with phosphate-buffered saline. The efficacy of the heat treatment was confirmed by culturing the bacteria overnight to ensure that there was no growth.
Cationic peptides.
The cationic peptides were synthesized at the University of British Columbia service facility by Fmoc [N-(9-fluorenyl)methoxycarbonyl] chemistry (22). The amino acid sequences of the peptides are found in Table 1 in the single-letter amino acid code.
TABLE 1.
Peptide amino acid sequences
| Peptide | Amino acid sequencea | Charge | % Hydrophobic amino acids |
|---|---|---|---|
| CP26 | KWKSFIKKLTSAAKKVVTTAKPLISS | +7 | 46 |
| CEME | KWKLFKKIGIGAVLKVLTTGLPALIS | +5 | 69 |
| CEMA | KWKLFKKIGIGAVLKVLTTGLPALKLTK | +7 | 64 |
| CP29 | KWKSFIKKLTTAVKKVLTTGLPALIS | +6 | 50 |
| CM5 | KLFKKIGIGAVLKVLTTGLPALKLTK | +6 | 65 |
| CM7 | KLWKLFKKIGIGAVLKVLTTGLPALKLTK | +7 | 66 |
| CP203 | KWKSFIKKLTSAAKKVLTTGLPALIS | +6 | 54 |
| CP207 | KWKSFIKKLTSVLKKVVTTAKPLISS | +7 | 46 |
| CP208 | KKKSFIKLLTSAKVSVLTTAKPLISS | +6 | 46 |
| CPα2 | KWKKFIKKIGIGAVLKVLTTGLPALKLTKK | +9 | 60 |
Single-letter amino acid code.
Determination of MICs.
The MICs of each peptide for a range of microorganisms were determined by the modified broth dilution method (33). Experiments were performed with Mueller-Hinton medium (with the exception of those involving Streptococcus pyogenes, which required Todd-Hewitt medium) in 96-well polypropylene microtiter plates (Costar, Cambridge, Mass.). Wells were inoculated with 10-μl volumes containing approximately 2 × 106 to 2 × 107 CFU of the test organism/ml. Samples of the bacterial inoculum were plated to ensure that they were within the proper range of concentrations. The MICs were determined after 18 h of incubation of the plates at 37°C. The MIC was considered to be the lowest peptide concentration at which growth was inhibited.
Determination of LTA binding affinity.
The relative binding affinity of each peptide for LTA was determined by modifying the LPS binding assay described previously by Moore et al. (17). Dansyl polymyxin B (DPX) was used at a concentration of 2.5 μM to obtain 90 to 100% of the maximum fluorescence when bound to LTA. The DPX and 5 μg of S. aureus LTA were mixed in 1 ml of 5 mM HEPES (pH 7.2). Fluorescence was measured by the use of a fluorescence spectrophotometer. Sequential additions of synthetic peptide, in 5-μl volumes, were made to the reactions mixtures, and the resulting decreases in DPX fluorescence were determined.
Cell culture.
The murine macrophage cell line RAW 264.7 was obtained from the American Type Culture Collection (Manassas, Va.). The cells were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum and passaged as described previously (13). For stimulation with bacterial products, the cells were plated at a density of 106/well in 24-well plates, incubated overnight to permit adherence, and then washed with fresh medium before stimulation. The cells were stimulated with LTA, PG peptide, or heat-killed S. aureus. To stimulate the RAW 264.7 cells with soluble products of live S. aureus, the bacteria were grown overnight in Mueller-Hinton medium, diluted in phosphate-buffered saline to a concentration of 107 to 108 per ml, and added to a Transwell diffusion chamber (Costar) in which a 0.2-μm-pore-size membrane separated the bacteria from the RAW 264.7 cells. In all cases, the supernatants of the RAW 264.7 culture were collected and used for cytokine enzyme-linked immunosorbent assays (ELISAs).
Whole-blood assay.
Blood from three donors was collected by venipuncture into tubes (Becton Dickinson, Franklin Lakes, N.J.) containing 14.3 USP units of heparin/ml of blood. Whole blood was stimulated with a 1-μg/ml solution of S. aureus LTA in the presence or absence of peptide (50 μg/ml) in polypropylene tubes at 37°C for 6 h. The samples were centrifuged for 10 min at 2,000 × g, and the plasma was stored at −20°C until analyzed by ELISA.
Cytokine assays.
The concentrations of TNF-α and IL-6 in the RAW 264.7 supernatants were determined by ELISA (Endogen, Hornby, Ontario, Canada) in accordance with the manufacturer's suggestions. The concentration of TNF in serum was determined by ELISA (R&D Systems, Minneapolis, Minn.).
RESULTS
Antimicrobial activity of the peptides.
CEME is an synthetic α-helical cationic peptide which contains the N-terminal 8 amino acids of silk moth cecropin followed by the first 18 amino acids of bee melittin (Table 1). CEME and some of its derivatives have previously been shown to have strong antimicrobial activity against a broad range of gram-negative bacteria (22). In this study, we asked whether CEME and/or its derivatives were capable of killing a variety of gram-positive bacteria, including clinically relevant pathogens. The peptides tested included CEME, a variant of CEME that is modified at the C terminus (CEMA), and two amphipathic derivatives of CEME (CP26 and CP29), as well as six other peptide variants with sequences related to CEME (Table 1). We have previously shown that all of these peptides, with the exception of CP208, are potent inhibitors of gram-negative bacterial growth (22). The MICs of these peptides for E. coli ranged from 0.5 to 2 μg/ml, with the exception of CP208, which had an MIC of 32 μg/ml. In contrast to their strong activity against E. coli, we found that the antimicrobial activity of the peptides toward gram-positive bacteria differed widely depending on the bacterium (Table 2). In general, most of the CEME-related cationic peptides, with the exception of CP208, had good activity against C. xerosis and L. monocytogenes, while many of the peptides had good activity against Streptococcus pyogenes, S. epidermidis, and a variety of S. aureus lab strains and clinical isolates. Note that the MICs of peptides that did have significant antimicrobial activity were often in the range of 4 to 16 μg/ml, which is considerably higher than their MICs for E. coli, which ranged from 0.5 to 2 μg/ml. Nevertheless, several of the CEME-related peptides had significant antimicrobial activity against multiple gram-positive bacteria.
TABLE 2.
Activity of cationic antimicrobial peptides against gram-positive bacteria and E. colia
| Strain | MIC (μg/ml)b
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CP26 | CEME | CEMA | CP29 | CP203 | CP207 | CP208 | CM5 | CM7 | CPα2 | PBc | |
| S. aureus | |||||||||||
| RN4220 | 64 | 4 | 4 | 8 | 8 | 16 | >64 | 64 | 4 | 16 | 32 |
| 25923 | >64 | 8 | 8 | 16 | 16 | 32 | >64 | >64 | 16 | 16 | 32 |
| SAP0017 (MRSAe) | 64 | 4 | 4 | 16 | 8 | 16 | >64 | >64 | 8 | 16 | 32 |
| Clinical isolate | >64 | 8 | 16 | 16 | 8 | 16 | >64 | >64 | 16 | 16 | 16 |
| Clinical isolate | >64 | 4 | 16 | 16 | 8 | 16 | >64 | >64 | 16 | 32 | 16 |
| S. epidermidis | 16 | 4 | 4 | 8 | 4 | 8 | >64 | 32 | 8 | 16 | 16 |
| S. pyogenes 19615 | 16 | 8 | 8 | 8 | 4 | 8 | >64 | 16 | 8 | 16 | 8 |
| E. faecalis 29212 | >64 | 32 | 32 | 64 | 32 | 64 | >64 | >64 | 16 | 64 | >64 |
| B. subtilis | 32 | 8 | 8 | 8 | 16 | 16 | >64 | 64 | 8 | 16 | 64 |
| L. monocytogenes | 32 | 4 | 4 | 4 | 4 | 8 | 64 | 64 | 4 | 8 | 4 |
| C. xerosis | 2 | 4 | 4 | 4 | 2 | 2 | 32 | 4 | 4 | 4 | 2 |
| E. coli UB1005d | 1 | 2 | 2 | 2 | 2 | 2 | 32 | 5 | 2 | 2 | 0.5 |
Bacterial strains were cultured with serial twofold dilutions of the various peptides (0.5 to 64 μg/ml) for 18 h as described in Materials and Methods.
The MIC represents the minimal concentration of peptide that completely inhibited growth. The values represent the averages of data from three experiments.
PB, polymyxin B.
MICs for this organism were taken from reference 22.
MRSA, methicillin-resistant S. aureus.
Of the cationic peptides tested, CEME had the lowest MICs for the gram-positive bacteria that were tested, while CEMA and CP203 were only slightly less effective. CM7 also had significant antimicrobial activity against gram-positive bacteria, although it was less effective than CEME, CEMA, or CP203. CM7 was also the only peptide that had significant activity against E. faecalis. Despite being highly effective against gram-negative bacteria, CP29, CP207, and CPα2 had only modest antimicrobial activity against most of the gram-positive bacteria, while CP26 and CP208 had little activity. Note that all of the cationic peptides with the exception of CP208 were highly active against C. xerosis. Thus, some of these CEME-related cationic peptides have significant activity against a broad range of gram-positive and gram-negative bacteria while others are active only against gram-negative bacteria and C. xerosis.
In addition to testing CEME-like peptides, we also asked whether the cationic lipopeptide polymyxin B displayed antimicrobial activity toward gram-positive bacteria (Table 2). Polymyxin B is generally considered to be a gram-negative-selective drug which has very low MICs (0.1 to 2 μg/ml) for gram-negative bacteria such as E. coli. We found that while polymyxin B had good antimicrobial activity toward C. xerosis and L. monocytogenes as well as modest activity toward Streptococcus pyogenes, it had minimal antimicrobial activity against other gram-positive bacteria. Thus, while polymyxin B has antimicrobial activity toward some species of gram-positive bacteria, it does not have as broad a range as CEME and some of its derivatives, such as CEMA and CP203.
Binding of CEME-related peptides to S. aureus LTA.
CEME-related cationic peptides have been shown to bind to purified E. coli LPS in vitro (22). The ability of the peptides to bind LPS is likely to play a significant role in their ability to neutralize LPS that is shed from bacteria and thereby prevent inflammatory responses. Since LTA has some structural analogy (being anionic and acylated) to LPS, we asked whether the CEME-related peptides could bind to purified LTA in vitro. To do this, we modified the DPX fluorescence assay that we had previously used to monitor the binding of these peptides to LPS. When excited at 340 nm, DPX fluoresces at 485 nm. This fluorescence is increased when DPX binds to LPS and is reduced when CEME-related peptides bind to LPS and displace the DPX. Since we observed a similar increase in DPX fluorescence when LTA was added, we were able to perform an analogous displacement assay to determine whether the CEME-related peptides could bind to purified LTA.
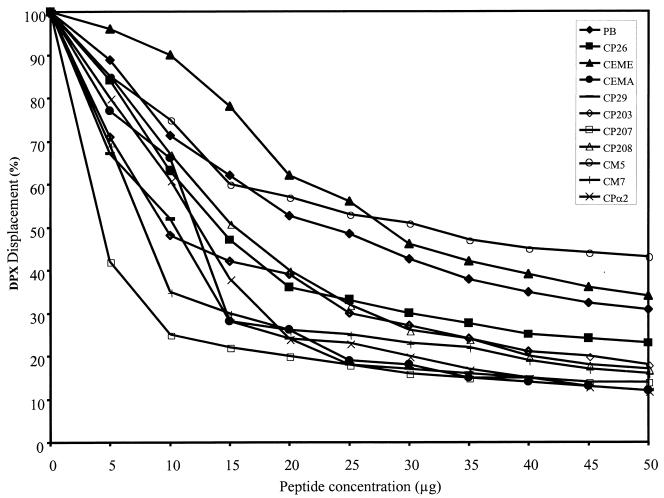
Figure 1 shows that the CEME-related peptides were able to displace up to 90% of the bound DPX from purified LTA. In previous studies, we found that these peptides displaced only about 50% of the DPX from E. coli O111:B4 LPS (17, 22). Most of the peptides had a higher affinity for LTA than polymyxin B, with CM5 and CEME being exceptions. The abilities of the peptides to bind LTA did not correspond with their MICs for gram-positive bacteria (Table 2), since CEME was the most effective peptide of this series against gram-positive bacteria but had a relatively low affinity for S. aureus LTA compared to some of the other peptides. Conversely, CP207 had the highest affinity for purified LTA, but its MICs for gram-positive bacteria were in general fourfold higher than those of CEME. Furthermore, CP208 also exhibited good affinity for purified LTA, even though it was unable to kill gram-positive bacteria. These results indicate that the ability to bind LTA is probably not the major mechanism by which CEME-related peptides kill gram-positive bacteria. The exact mechanism by which cationic peptides kill bacteria is not known. Nevertheless, the ability of these peptides to bind LTA could prevent LTA that is shed by bacteria from inducing inflammatory responses. To test this hypothesis, we asked whether the CEME-related peptides could block the ability of soluble LTA to induce the production of inflammatory cytokines by macrophages.
FIG. 1.
Binding affinities of the peptides for S. aureus LTA as measured by the DPX displacement assay. Purified soluble S. aureus LTA (5 μg/ml) was incubated with 2.5 μM DPX, and the fluorescence at 485 nm was measured. Peptides were added in increments of 5 μg/ml, and the DPX fluorescence was measured after each addition. Each data point represents the mean of values from three independent experiments. The standard errors of the means were all less than 10%. PB, polymyxin B.
CEME-related peptides inhibit LTA-induced cytokine secretion.
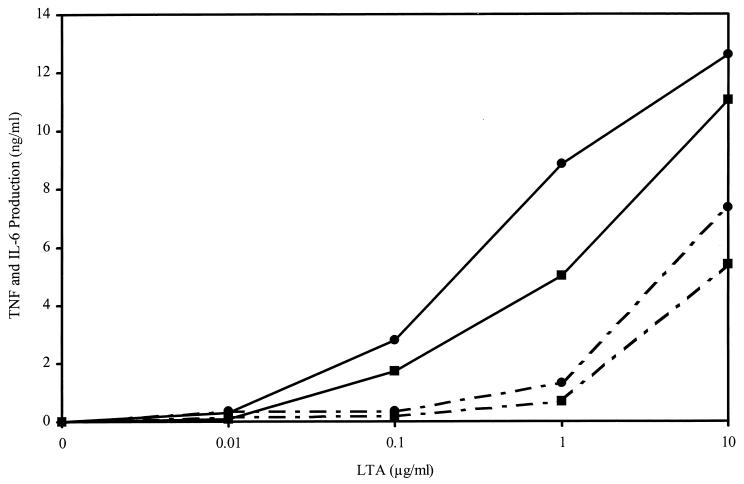
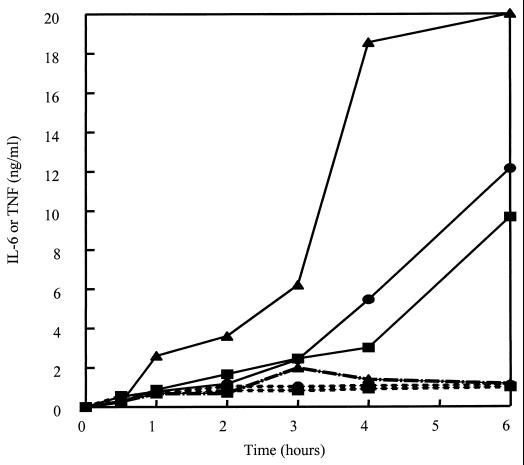
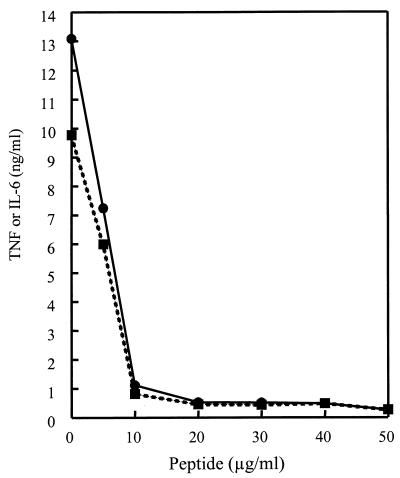
Previous studies have demonstrated that LTA results in many of the characteristics of septic shock when injected into animals (3, 14, 15, 18, 31). Consistent with this observation, LTA also induces the production of inflammatory cytokines by macrophages in vitro (10). Therefore, we asked whether the cationic peptides could block LTA-induced cytokine production by the murine macrophage cell line RAW 264.7. Figure 2 shows that LTA stimulated the release of TNF-α and IL-6 by RAW 264.7 cells. Maximal TNF-α and IL-6 production was observed after 6 h, and 0.1 μg/ml was the minimal concentration of S. aureus LTA that induced significant cytokine production. CEMA (20 μg/ml) significantly blocked cytokine production elicited by LTA at 0.1 μg/ml (Table 3) or 1 μg/ml (Fig. 3). When the cells were stimulated with LTA at 10 μg/ml, CEMA was not as effective, resulting in only approximately 50% inhibition. The production of TNF-α and IL-6 in response to stimulation at 1 μg/ml (Fig. 3) was completely suppressed over the entire 24-h observation period by 20-μg/ml CEMA (data not shown). Figure 4 shows that a 10-μg/ml concentration of CEMA was sufficient to cause nearly complete inhibition of production of TNF-α and IL-6 by RAW 264.7 cells stimulated with LTA at 1 μg/ml. The PG peptide and M. luteus PG were also tested for their ability to induce the production of TNF and IL-6 by RAW 264.7 cells. Addition of these substances at concentrations of 100 ng to 10 μg/ml did not result in significant levels of TNF and IL-6 (data not shown), and so peptide inhibition experiments were not performed. Nevertheless, our results show that CEMA is a potent inhibitor of LTA-induced production of inflammatory cytokines.
FIG. 2.
Production of TNF-α (●) and IL-6 (■) by RAW 264.7 cells incubated for 6 h with the indicated doses of S. aureus LTA in the absence (solid line) or presence (broken line) of 20 μg of CEMA. TNF-α and IL-6 concentrations in the cell supernatant were determined by ELISA. The levels of TNF-α and IL-6 produced by macrophages incubated in medium alone for 6 h were less than 0.3 ng/ml. The experiment was repeated three times with similar results, and the data from one representative experiment are shown.
TABLE 3.
CEME-related peptides inhibit LTA-stimulated production of TNF and IL-6 by RAW 264.7 cellsa
| Peptide | % Inhibition of cytokine
induction (± SE) by:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
E. coli LPS (100
ng/ml)b
|
S. aureus
LTA
|
B. subtilis LTA (1
μg/ml)
|
S. pyogenes LTA (1 μg/ml)
|
|||||||
| 100 ng/ml
|
1 μg/ml
|
|||||||||
| TNF | IL-6 | TNF | IL-6 | TNF | IL-6 | TNF | IL-6 | TNF | IL-6 | |
| PB | 98 | 98 | 99 ± 1 | 98 ± 1 | 96 ± 1 | 93 ± 4 | 93 ± 2 | 91 ± 1 | 97 ± 1 | 99 ± 1 |
| CP26 | 90 | 90 | 48 ± 19 | 76 ± 1 | 52 ± 12 | 50 ± 15 | 23 ± 17 | 42 ± 11 | 68 ± 3 | 78 ± 6 |
| CEME | 94 | 76 | 99 ± 1 | 92 ± 2 | 86 ± 10 | 92 ± 6 | 90 ± 2 | 94 ± 2 | 96 ± 1 | 98 ± 1 |
| CEMA | 90 | 82 | 94 ± 4 | 97 ± 2 | 91 ± 4 | 87 ± 2 | 86 ± 2 | 88 ± 1 | 96 ± 1 | 97 ± 2 |
| CP29 | 98 | 96 | 99 ± 1 | 95 ± 3 | 90 ± 2 | 95 ± 3 | 76 ± 14 | 89 ± 4 | 96 ± 1 | 99 ± 1 |
| CM5 | 42 | 44 | 65 ± 2 | 88 ± 1 | 64 ± 12 | 73 ± 8 | 77 ± 1 | 67 ± 3 | 80 ± 4 | 75 ± 5 |
| CM7 | 99 | 95 | 99 ± 1 | 95 ± 1 | 96 ± 2 | 95 ± 4 | 83 ± 4 | 91 ± 6 | 90 ± 4 | 94 ± 5 |
| CP203 | 98 | 90 | 98 ± 1 | 99 ± 1 | 90 ± 2 | 93 ± 3 | 70 ± 14 | 77 ± 12 | 87 ± 6 | 93 ± 3 |
| CP207 | 97 | 93 | 92 ± 6 | 96 ± 1 | 83 ± 3 | 84 ± 10 | 72 ± 15 | 73 ± 12 | 69 ± 11 | 89 ± 3 |
| CP208 | 0 | 7 | 46 ± 5 | 61 ± 1 | 14 ± 8 | 19 ± 9 | 26 ± 8 | 29 ± 5 | 23 ± 9 | 37 ± 13 |
| CPα2 | 93 | 94 | 97 ± 1 | 89 ± 6 | 89 ± 5 | 85 ± 3 | 83 ± 5 | 88 ± 6 | 93 ± 1 | 95 ± 2 |
RAW 264.7 cells were cultured with the indicated concentrations of LTA from different bacteria in the presence or absence of the various CEME-related peptides (20 μg/ml) or polymyxin B (PB; 20 μg/ml). After 6 h, cell supernatants were collected and analyzed for TNF-α and IL-6 content by ELISA. With the exception of those for E. coli, the data are presented as mean percent inhibition of cytokine production ± the standard error of the mean for triplicate samples. The 100% value ranged from 16 to 20 ng/ml for LPS and from 9 to 12 ng/ml for LTA. Values for medium-only controls as well as medium plus peptide were always less than 0.3 ng/ml.
Data taken from reference (22).
FIG. 3.
Production of TNF-α () and IL-6 (■) by RAW 264.7 cells stimulated with S. aureus LTA at 1 μg/ml or E. coli O111:B4 LPS at 100 ng/ml (▴ [TNF-α production]) for 1 to 6 h in the absence (solid line) or presence (broken line) of 20 μg of CEMA. Supernatant was removed at each time point and assayed for cytokine levels. The experiment was repeated three times with similar results, and the data from one representative experiment are shown.
FIG. 4.
Production of TNF-α (solid lines) and IL-6 (dotted lines) by RAW 264.7 cells stimulated with S. aureus LTA at 1 μg/ml and with increasing doses of CEMA. The experiment was repeated three times with similar results, and the data from one representative experiment are shown.
Having shown that the CEMA peptide can block the ability of S. aureus LTA to stimulate TNF-α and IL-6 production by RAW 264.7 cells, we wished to extend these results and determine whether the other CEME-related peptides could block LTA-stimulated cytokine production. In addition to testing their ability to block cytokine production stimulated by S. aureus LTA at either 100 ng/ml or 1 μg/ml (Table 3), we also tested the peptides for their ability to block cytokine production induced by LTAs from B. subtilis and Streptococcus pyogenes (Table 3). In this way, we could determine whether some or all of the peptides had the ability to neutralize LTAs from a broad spectrum of gram-positive bacteria. As a control, we used 10 to 100 mM phorbol 12-myristate 13-acetate (PMA; Sigma) to stimulate the cells and found that the peptides did not block cytokine production (∼0.6 ng/ml) caused by PMA (data not shown). We found that almost all of the CEME-related peptides were very potent inhibitors of LTA-stimulated TNF-α and IL-6 production. The exceptions were CP26 and CP208, which caused only partial inhibition of cytokine production. These two peptides also have little or no antimicrobial activity toward gram-positive bacteria. Nevertheless, many of the CEME-related peptides were potent antagonists of LTAs from a broad spectrum of gram-positive bacteria. Thus, these peptides not only have antimicrobial activity against both gram-negative and gram-positive bacteria but also block the ability of the major cell wall components released from these bacteria (LPS and LTA) to stimulate inflammatory responses.
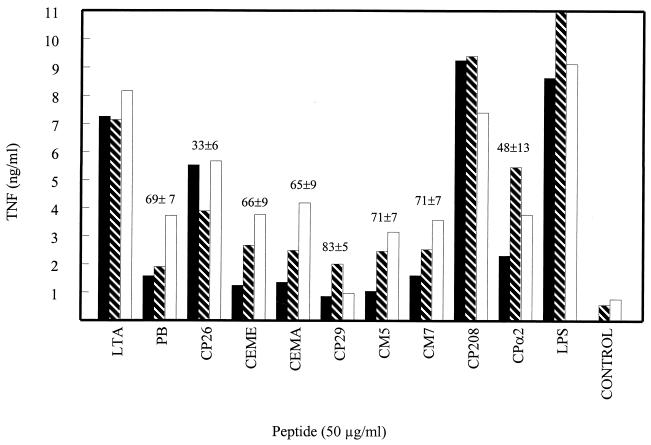
Effect of cationic peptides on RAW 264.7 cell production of TNF and IL-6 in response to S. aureus.
We next examined whether the CEME-related peptides could block the induction of TNF-α and IL-6 production by intact heat-killed S. aureus or by soluble products of S. aureus. These stimuli may better reflect a physiological encounter between macrophages and bacteria than the addition of purified LTA to cultures. Incubating RAW 264.7 cells with intact heat-killed S. aureus for 6 h resulted in secretion of very high levels of TNF-α (approximately 20 ng/ml). When RAW 264.7 cells were exposed to soluble products of live S. aureus by culturing the macrophages and the bacteria in separate compartments of Transwell dishes, the macrophages also produced significant amounts of TNF-α (approximately 2.2 ng/ml). Neither the intact heat-killed S. aureus nor the soluble products of S. aureus caused significant (above that evident with medium alone) production of IL-6. At a concentration of 50 μg/ml (found optimal from a dose-response curve [data not shown]), a number of the CEME-related peptides significantly decreased the ability of the intact heat-killed S. aureus and the S. aureus soluble products to stimulate TNF-α production (Fig. 5). The CEMA, CM7, CPα2, CP29, and CP203 peptides were the most effective at inhibiting S. aureus-stimulated TNF-α production, decreasing the level of TNF-α secretion by more than 50%. Interestingly, the CEME peptide itself was somewhat less effective at blocking S. aureus-stimulated TNF-α production, even though it had the lowest MICs for S. aureus of all the peptides. Nevertheless, many of the CEME-related peptides were able to reduce the ability of S. aureus or its products to cause TNF-α release.
FIG. 5.
RAW 264.7 cells were incubated with boiled S. aureus (solid bars) or live S. aureus separated from the RAW 264.7 cells by a filter (open bar) for 6 h. The supernatant was collected and measured for TNF-α by ELISA. The data are presented as percent inhibition of cytokine production ± the standard error of the mean for triplicate samples. PB, polymyxin B.
Effect of cationic peptides on whole-blood stimulation by LTA.
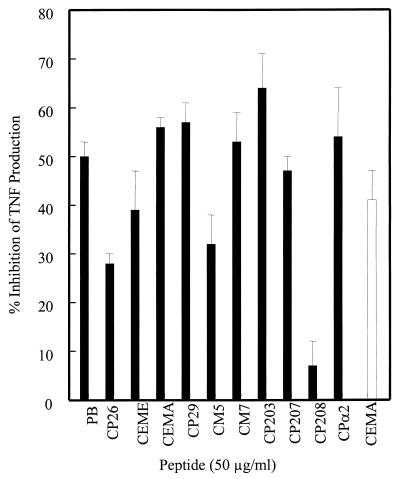
Although CEMA and CEME were shown to be effective in a mouse model of endotoxic shock (22), it was of interest to determine the effectiveness of the peptides against LTA in a more realistic model. They were tested in an ex vivo assay in which LTA and peptide were added to blood samples from human volunteers and, after incubation of the blood specimens for 6 h, the sera were separated and tested for TNF levels by ELISA. It was found that the peptides effectively inhibited LTA-induced TNF production (Fig. 6), although not quite as well as in the RAW cell assay. CP29 was the most effective peptide, inhibiting TNF production by 83%, whereas CP26, CP208, and CPα2 were relatively ineffective. Therefore, the peptides were effective at reducing LTA-induced stimulation of TNF both in vitro and in human blood.
FIG. 6.
Production of TNF-α by whole blood stimulated with E. coli O111:B4 LPS at 100 ng/ml or S. aureus LTA at 1 μg/ml and/or peptide at 50 μg/ml was measured by ELISA after a 6-h incubation. The three different types of bar represent the three donors. The numbers above the bars represent the average amounts of TNF inhibition by the peptides ± standard errors. PB, polymyxin B.
DISCUSSION
In this study, CEME-related cationic peptides were tested for their ability to kill gram-positive bacteria, their ability to bind LTA, and their ability to neutralize cytokine production by macrophages stimulated with products of gram-positive bacteria. Many of the peptides had activity against gram-positive bacteria, although a few peptides had no antimicrobial activity. All of the peptides were able to bind LTA, and many were able block the production of cytokines by macrophages stimulated with different species of LTA. The CEME-related peptides were also effective in blocking cytokine production by RAW 264.7 cells stimulated with heat-killed S. aureus.
It was of interest to determine if the peptides have potential therapeutic value for the treatment of gram-positive sepsis as they have shown for treatment of gram-negative sepsis. Although the peptides had better antimicrobial activity against gram-negative bacteria, some of the peptides were still active against gram-positive bacteria. It is likely that structural differences among these peptides determine whether they can interact with surface structures of various gram-positive and gram-negative bacteria. The different abilities of the various CEME-related peptides to kill gram-positive bacteria may point to structural features that are important for this process. CP26 and CP29 were designed to be more amphipathic than CEME, a property which might help them intercalate into membranes. Although CP29 still had reasonable antimicrobial activity toward gram-positive bacteria, it was less effective than CEME. CP26, while being very effective against E. coli, had no antimicrobial activity toward gram-positive bacteria. Thus, an amphipathic nature, by itself, is not sufficient for these cationic peptides to kill gram-positive bacteria. Except for three conservative amino acid changes, the major difference between CP26 and CP29 is that the C terminus of CP29 is identical to that of CEME (PALIS [single-letter code]) while the C terminus of CP26 consists of PLISS. Consistent with the idea that the C-terminal PALIS sequence confers greater activity toward gram-positive bacteria, CP203, which also has a C-terminal PALIS sequence, was somewhat more potent than CP207, which has a C-terminal PLISS sequence. Despite having the same C-terminal PLISS sequence as CP26, CP207 had modest activity against gram-positive bacteria while CP26 did not. These two peptides differ only at 2 amino acids, at positions 12 and 13, where CP207 has a valine and a leucine whereas CP26 has two alanine residues. The presence of the larger hydrophobic side chains at residues 12 and 13 in CP207 may therefore be important for activity against gram-positive bacteria. Both CEME and CEMA have an alanine and a valine at positions 12 and 13, suggesting that one larger side chain may be sufficient. CP203, while having a PALIS sequence at the C terminus, has alanine residues at positions 12 and 13. Thus, the CEME-like peptides that have the highest antimicrobial activity against gram-positive bacteria have a C-terminal PALIS sequence and valines or leucines instead of alanines at positions 12 and 13.
A hydrophobic residue at position 2 appears to be important for a CEME-like peptide to have antimicrobial activity against gram-positive and gram-negative bacteria. CP208, which has a lysine at position 2, had no antimicrobial activity against either gram-positive or gram-negative bacteria. A tryptophan at position 2 appears to be optimal for antimicrobial activity. CM5, which has a leucine at this position, has little activity toward gram-positive bacteria and reduced activity toward E. coli. CM7 also has a leucine at position 2, but changes elsewhere, including a tryptophan at position 3, in the peptide may compensate for this to some extent since CM7 has modest activity against gram-positive bacteria and good activity toward gram-negative bacteria.
Although the mechanism of action of these CEME-related peptides against gram-positive bacteria is not clearly understood, it does not appear to be related to their ability to bind LTA, since the relative abilities of these peptides to bind LTA did not correspond to their MICs. Even though many of the peptides had a higher affinity for S. aureus LTA than did CEME, it was the most effective of the peptides at killing S. aureus and other gram-positive bacteria. While the ability of the peptides to bind LTA may not be important for their antimicrobial activity, it is likely to be important for reducing the ability of shed LTA to stimulate inflammatory reactions.
LTA, at high doses, has been shown to stimulate the production of cytokines by the murine macrophage cell line RAW 264.7 (9). In this report, we have shown that the CEME-related peptides are able to significantly inhibit the production of TNF-α and IL-6 by RAW 264.7 cells. Moreover, the peptides were able to block cytokine production induced by LTAs from different species of gram-positive bacteria. The abilities of the peptides to reduce the production of TNF-α and IL-6 by LTA-stimulated RAW 264.7 cells corresponded somewhat to their MICs. For example, CP26, which has virtually no activity against gram-positive bacteria, was relatively ineffective at reducing macrophage activation by LTA. On the other hand, CP26 has much lower MICs against gram-negative bacteria and is also much more effective at inhibiting cytokine production in response to LPS. Similarly, CP208 had no antimicrobial activity against gram-positive bacteria and also did not block LTA-induced cytokine production. Moreover, the peptides that were more active against S. aureus than they were against B. subtilis were slightly less effective at inhibiting cytokine production by macrophages stimulated with B. subtilis LTA as opposed to S. aureus LTA. The peptides were also effective at reducing the production of TNF in human blood in response to LTA. It was not surprising that the levels of inhibition were lower than those evident in the RAW cell system, since blood has many factors which might inhibit the peptides. Polymyxin B was found to inhibit LTA-induced cytokine production, partly in contrast to the findings of other researchers (5, 31). This could be due to the concentrations of polymyxin B used in this study (20 to 50 μg/ml), which were much higher than those used previously (5 to 10 μg/ml). It is reasonable to assume that LTA and polymyxin B could interact, as suggested by the DPX assay, based on the negatively charged lipid structure of LTA and the cationic structure of polymyxin B. Since LTA, like LPS, has both a polyanionic and a lipidic nature, it seems reasonable that it should be able to interact with polymyxin B and DPX, although the kinetics of binding of DPX to LTA suggested a lower affinity than that observed for DPX-LPS binding. We have previously found that some antibiotics and other peptides with no charge do not work in the DPX assay. The PG preparations tested were not effective in stimulating macrophages to produce TNF and IL-6; therefore, no conclusions can be drawn with regard to the effectiveness of the peptides in PG-induced cell stimulation. In other studies, PG has been used in conjunction with LTA to cause sepsis-like conditions in animals (3). This most likely mimics a physiological situation in which PG attached to LTA is released from gram-positive bacteria.
The results of this study demonstrate that the CEME-related α-helical peptides tested are not only effective against gram-negative sepsis but also may have therapeutic value in the treatment of gram-positive sepsis. These cecropin-melittin hybrids have demonstrated a broad range of activities, including antibacterial activity, antiendotoxin activity, the ability to synergize with other antibiotics, and efficacy in animal models of infection (22). Although there have been many new therapies for gram-negative sepsis examined in recent years (21, 30), very little progress has been achieved in finding new therapies for gram-positive sepsis. In this report, we have shown that CEME-related peptides can kill gram-positive bacteria, bind LTA released from gram-positive bacteria, and block cytokine release by macrophages stimulated with gram-positive bacteria or their products. The effects of the peptides on soluble LTA are significant, since LTA is released by bacteria during normal growth and LTA release is enhanced by β-lactam antibiotics. Although the CEME-related peptides lack the potency of some antibiotics, they are able to bind bacterial products released by gram-positive bacteria and significantly reduce macrophage activation. Thus, CEME-related peptides may be an important tool for the prevention of gram-positive sepsis, by themselves or in concert with other antibiotics.
ACKNOWLEDGMENTS
This research was funded by grants from the Canadian Bacterial Diseases Network (to R.E.W.H.) and from the Medical Research Council (MRC) of Canada to M.R.G. M.G.S. was supported by a studentship from the MRC. M.R.G. is the recipient of an MRC scholarship, and R.E.W.H. is the recipient of an MRC Distinguished Scientist Award.
REFERENCES
- 1.Boman H G, Wade D, Boman I A, Wahlin B, Merrifield R B. Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett. 1989;259:103–106. doi: 10.1016/0014-5793(89)81505-4. [DOI] [PubMed] [Google Scholar]
- 2.Bone R C. Gram-positive organisms and sepsis. Arch Intern Med. 1994;154:26–34. [PubMed] [Google Scholar]
- 3.De Kimpe S J, Kengatharan M, Thiemermann C, Vane J R. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureusact in synergy to cause shock and multiple organ failure. Proc Natl Acad Sci USA. 1995;92:10359–10363. doi: 10.1073/pnas.92.22.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dziarski R, Tapping R I, Tobias P S. Binding of bacterial peptidoglycan to CD14. J Biol Chem. 1998;273:8680–8690. doi: 10.1074/jbc.273.15.8680. [DOI] [PubMed] [Google Scholar]
- 5.English B K, Patrick C C, Orlicek S L, McCordic R, Shenep J L. Lipoteichoic acid from viridans streptococci induces the production of tumor necrosis factor and nitric oxide by murine macrophages. J Infect Dis. 1996;174:1348–1351. doi: 10.1093/infdis/174.6.1348. [DOI] [PubMed] [Google Scholar]
- 6.Fischer W. Physiology of lipoteichoic acids in bacteria. Adv Microb Physiol. 1988;29:233–302. doi: 10.1016/s0065-2911(08)60349-5. [DOI] [PubMed] [Google Scholar]
- 7.Fischer W, Mannsfeld T, Hagen G. On the basic structure of poly(glycerophosphate) lipoteichoic acids. Biochem Cell Biol. 1990;68:33–43. doi: 10.1139/o90-005. [DOI] [PubMed] [Google Scholar]
- 8.Gough M, Hancock R E W, Kelly N M. Antiendotoxin activity of cationic peptide antimicrobial agents. Infect Immun. 1996;64:4922–4927. doi: 10.1128/iai.64.12.4922-4927.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grunfeld C, Marshall M, Shigenaga J K, Moser A H, Tobias P, Feingold K R. Lipoproteins inhibit macrophage activation by lipoteichoic acid. J Lipid Res. 1999;40:245–252. [PubMed] [Google Scholar]
- 10.Heumann D, Barras C, Severin A, Glauser M P, Tomasz A. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect Immun. 1994;62:2715–2721. doi: 10.1128/iai.62.7.2715-2721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heumann D, Glauser M P, Calandra T. Molecular basis of host-pathogen interaction in septic shock. Curr Opin Microbiol. 1998;1:49–55. doi: 10.1016/s1369-5274(98)80142-2. [DOI] [PubMed] [Google Scholar]
- 12.Horne D, Tomasz A. Release of lipoteichoic acid from Streptococcus sanguis: stimulation of release during penicillin treatment. J Bacteriol. 1979;137:1180–1184. doi: 10.1128/jb.137.3.1180-1184.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly N M, Young L, Cross A S. Differential induction of tumor necrosis factor by bacteria expressing rough and smooth lipopolysaccharide phenotypes. Infect Immun. 1991;59:4491–4496. doi: 10.1128/iai.59.12.4491-4496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kengatharan K M, De Kimpe S, Robson C, Foster S J, Thiermermann C. Mechanism of gram-positive shock: identification of peptidoglycan and lipoteichoic acid moieties essential in the induction of nitric oxide synthase, shock, and multiple organ failure. J Exp Med. 1998;188:305–315. doi: 10.1084/jem.188.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Roy D, Morand P, Lengacher S, Celio M, Grau G E, Glauser M P, Heumann D. Streptococcus mitis cell walls and lipopolysaccharide induce lethality in d-galactosamine-sensitized mice by a tumor necrosis factor-dependent pathway. Infect Immun. 1996;64:1846–1849. doi: 10.1128/iai.64.5.1846-1849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattsson E, Rollof J, Verhoef J, Van Dijk H, Fleer A. Serum-induced potentiation of tumor necrosis factor alpha production by human monocytes in response to staphylococcal peptidoglycan: involvement of different serum factors. Infect Immun. 1994;62:3837–3843. doi: 10.1128/iai.62.9.3837-3843.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore R A, Bates N C, Hancock R E W. Interaction of polycationic antibiotics with Pseudomonas aeruginosalipopolysaccharide and lipid A studied by using dansyl-polymyxin. Antimicrob Agents Chemother. 1986;29:496–500. doi: 10.1128/aac.29.3.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Natanson C, Danner R L, Elin R J, Hosseini J M, Peart K W, Banks S M, MacVittie T J, Walker R I, Parrillo J E. Role of endotoxemia in cardiovascular dysfunction and mortality. Escherichia coli and Staphylococcus aureuschallenges in a canine model of human septic shock. J Clin Investig. 1989;83:243–251. doi: 10.1172/JCI113866. . (Erratum, 83:1087.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piers K L, Brown M H, Hancock R E W. Improvement of outer membrane-permeabilizing and lipopolysaccharide-binding activities of an antimicrobial cationic peptide by C-terminal modification. Antimicrob Agents Chemother. 1994;38:2311–2316. doi: 10.1128/aac.38.10.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocque W J, Fesik S W, Haug A, McGroarty E J. Polycation binding to isolated lipopolysaccharide from antibiotic-hypersusceptible mutant strains of Escherichia coli. Antimicrob Agents Chemother. 1988;32:308–313. doi: 10.1128/aac.32.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogy M A, Moldawer L L, Oldenburg H S, Thompson W A, Montegut W J, Stackpole S A, Kumar A, Palladino M A, Marra M N, Lowry S F. Anti-endotoxin therapy in primate bacteremia with HA-1A and BPI. Ann Surg. 1994;220:77–85. doi: 10.1097/00000658-199407000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott M G, Yan H, Hancock R E W. Biological properties of structurally related α-helical cationic antimicrobial peptides. Infect Immun. 1999;67:2005–2009. doi: 10.1128/iai.67.4.2005-2009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soell M, Diab M, Haan-Archipoff G, Beretz A, Herbelin C, Poutrel B, Klein J-P. Capsular polysaccharide types 5 and 8 of Staphylococcus aureusbind specifically to human epithelial (KB) cells, endothelial cells, and monocytes and induce release of cytokines. Infect Immun. 1995;63:1380–1386. doi: 10.1128/iai.63.4.1380-1386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soell M, Lett E, Holveck F, Scholler M, Wachsmann D, Klein J-P. Activation of human monocytes by streptococcal rhamnose glucose polymers is mediated by CD14 antigen, and mannan binding protein inhibits TNF-α release. J Immunol. 1995;154:851–860. [PubMed] [Google Scholar]
- 25.Soto A, Evans T J, Cohen J. Proinflammatory cytokine production by human peripheral blood mononuclear cells stimulated with cell-free supernatants of viridans streptococci. Cytokine. 1996;8:300–304. doi: 10.1006/cyto.1996.0040. [DOI] [PubMed] [Google Scholar]
- 26.Takada H, Kawabata Y, Kawata S, Kusomoto S. Structural characteristics of peptidoglycan fragments required to prime mice for induction of anaphylactoid reactions by lipopolysaccharides. Infect Immun. 1996;64:657–659. doi: 10.1128/iai.64.2.657-659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 28.Ulevitch R J, Tobias P S. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr Opin Immunol. 1999;11:19–22. doi: 10.1016/s0952-7915(99)80004-1. [DOI] [PubMed] [Google Scholar]
- 29.van Langevelde P, van Dissel J T, Ravensbergen E, Appelmelk B J, Schrijver I A, Groeneveld P H P. Antibiotic-induced release of lipoteichoic acid and peptidoglycan from Staphylococcus aureus: quantitative measurements and biological reactivities. Antimicrob Agents Chemother. 1998;42:3073–3078. doi: 10.1128/aac.42.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Zee K J, Moldawer L L, Oldenburg H S, Thompson W A, Stackpole S A, Montegut W J, Rogy M A, Meschter C, Gallati H, Schiller C D, Richter W F, Loetscher H, Ashkenazi A, Chamow S M, Wurm F, Calvano S E, Lowry S F, Lesslauer W. Protection against lethal Escherichia coli bacteremia in baboons (Papio anubis) by pretreatment with a 55-kDa TNF receptor (CD120a)-Ig fusion protein, Ro 45-2081. J Immunol. 1996;156:2221–2230. [PubMed] [Google Scholar]
- 31.Wakabayashi G, Gelfand J A, Jung W K, Connolly R J, Burke J F, Dinarello C A. Staphylococcus epidermidis induces complement activation, tumor necrosis factor and interleukin-1, a shock-like state and tissue injury in rabbits without endotoxemia. Comparison to Escherichia coli. J Clin Investig. 1991;87:1925–1935. doi: 10.1172/JCI115218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weidemann B, Schletter J, Dziarski R, Kusumoto S, Stelter F, Rietschel E T, Flad H-D, Ulmer A J. Specific binding of soluble peptidoglycan and muramyldipeptide to CD14 on human monocytes. Infect Immun. 1997;65:858–864. doi: 10.1128/iai.65.3.858-864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu M, Hancock R E W. Interaction of the cyclic antimicrobial cationic peptide bactenecin with the outer and cytoplasmic membrane. J Biol Chem. 1999;274:29–35. doi: 10.1074/jbc.274.1.29. [DOI] [PubMed] [Google Scholar]