Abstract
BALB/c mice are susceptible to progressive infection with Leishmania major due to the preferential development of CD4+ T cells that secrete Th2 cytokines. Although Th2 cell development and susceptibility are disrupted by blockade of CD86 function early in infection, CD28-deficient BALB/c mice remain susceptible to leishmaniasis. We therefore examined whether the alternative CD86 ligand, CTLA4, contributes to the expression of susceptibility. BALB/c mice treated for 2 weeks of infection with anti-CTLA4 monoclonal antibody developed more rapidly progressive disease than sham-treated mice, whereas normally resistant C57BL/6 mice were unaffected. The draining lymph node cells of anti-CTLA4-treated BALB/c mice produced up to sixfold more interleukin-4 (IL-4) and IL-13 than control mice in the first 2 weeks of infection, but IFN-γ synthesis was reciprocally decreased. Anti-CTLA4 treatment of BALB/c mice pretreated with neutralizing anti-IL-4 antibody or genetically deficient in IL-4 also caused significant worsening of leishmaniasis. Exacerbation in IL-4 KO mice was associated with increased IL-13 and decreased gamma interferon (IFN-γ) and inducible nitric oxide synthase (iNOS) mRNA expression in vivo. These data indicate that anti-CTLA4 antibody induced earlier and more-polarized Th2 responses in susceptible BALB/c mice infected with L. major. The mechanism of disease worsening was partially IL-4 independent, indicating that increased IL-13 and/or decreased IFN-γ production may have disrupted nitric oxide-based microbicidal responses. We conclude that CTLA4 significantly modulates Th2 development in murine leishmaniasis and that the Th2-polarizing effects of anti-CTLA4 treatment result in IL-4-independent exacerbation of disease.
Susceptible BALB/c mice fail to contain cutaneous infection with Leishmania major due to the inappropriate and early expansion of Th2-like CD4+ T cells that produce interleukin-4 (IL-4) and IL-13 (35). Although gamma interferon (IFN-γ)-producing Th1 CD4+ T cells are also generated, the presence of IL-4 disables unipolar Th1 development by disrupting IL-12 receptor function (18, 39) and directly antagonizes IFN-γ-dependent cure by interfering with the microbicidal activation of parasitized macrophages (27). The engagement of T-cell CD28 and/or CTLA4 by CD80/CD86 accessory cell molecules contributes to dysfunctional T-cell development in progressive leishmaniasis. Specifically, antibody-mediated neutralization of CD86 or CTLA4-Ig-mediated inhibition of both CD80 and CD86 prevents the development of IL-4-producing T-cell responses in L. major-infected BALB/c mice and restores the ability to heal cutaneous disease (6, 9, 11). Similar forms of CD86 blockade also disrupt the predisposition towards Th2 cytokine responses in murine models of helminthic infection and allergy (13, 20, 38). These effects presumably reflect interruption of critical regulatory signals generated by T-cell molecules CD28 and CTLA-4 (CD152) after interactions with CD80 and CD86. Mechanisms proposed for costimulation-dependent Th2 development in leishmaniasis or helminthic infection include biasing effects dependent on the intensity of T-cell activation, the rate of cell proliferation, or other effects unique to interactions between CD86 and its ligands (3, 14, 25). However, CD28 knockout (KO) mice on BALB/c and C57BL/6 backgrounds demonstrate no change in their respective susceptibility and resistance to L. major (5). Although unintended effects of CD28 deficiency might include recruitment of alternative costimulatory pathways capable of substituting for CD28 in T-cell regulation (45), the preserved susceptibility of CD28 KO BALB/c mice raises questions about how the alternative CD86/CD80 ligand, CTLA4, might contribute to distinct leishmania-induced T-cell responses.
CTLA4 is transiently displayed on activated T cells, binds with high affinity to CD80 and CD86, and functions to inhibit both T-cell proliferation and IL-2 synthesis during the primary T-cell response (23, 42, 43). These effects appear to be mediated both indirectly by suppression of CD28 costimulatory signals and directly by inhibition of T-cell receptor-dependent tyrosine kinases necessary for cellular activation (4, 7, 12, 26, 28). Consistent with these observations made in vitro, neutralization of CTLA4 by either intact or Fab fragments of monoclonal antibody (MAb) enhances superantigen- and antigen-specific T-cell proliferation in vivo (19, 21, 24). Similarly, CTLA4 KO mice develop autoimmune pathology due to unrestricted CD4+ T-cell expansions in vivo (40). The effects of CTLA4 on T-cell differentiation toward different cytokine-producing phenotypes remain incompletely defined and may be distinct for the experimental model used. For instance, anti-CTLA4 antibody treatments worsen autoimmune diseases by enhancing proinflammatory Th1-type responses in predisposed hosts (19) but also enhance Th2-type T-cell responses and subsequent worm expulsion in mouse models of intestinal helminth infection (31). These findings therefore suggest that CTLA4 nonselectively modulates T-cell phenotypes independently determined by the stimulus or by host-dependent biases. In the current studies, we test the hypothesis that CTLA4 differentially affects Th1 and Th2 cytokine responses in susceptible BALB/c mice infected with L. major. We demonstrate that intact anti-CTLA4 antibody markedly accelerates Th2 development and the progression of murine leishmaniasis, confirming a recent report showing similar results (37). We further extend these findings to describe IL-4-independent increases in IL-13 synthesis that are induced by anti-CTLA4 treatment and that correlate with worsening of disease in IL-4-deficient mice.
MATERIALS AND METHODS
Mice.
Four- to six-week-old female C57BL/6J and BALB/cByJ mice were purchased from Jackson Laboratories (Bar Harbor, Maine) and housed in the Cleveland VA Medical Center or Case Western Reserve University animal facilities under specific-pathogen-free conditions. IL-4 KO BALB/cJ-IL4tm2Nnt mice (33) were obtained from Jackson Laboratories.
Parasite cultivation and mouse infection.
L. major (WHO strain WHOM/IR/-/173) were grown in M199 medium (BioWhittaker, Walkersville, Md.) containing antibiotics, supplemental glutamine, and 30% fetal calf serum (HyClone Laboratories, Logan, Utah) as described previously (36). Stationary-phase promastigotes were injected into the hind feet of recipient mice at a dose of 2 × 106 organisms/footpad to initiate infection. The course of infection was monitored by measuring the thickness of footpad swelling weekly by using a dial gauge caliper.
Reagents.
Hybridoma cells producing neutralizing anti-CTLA4 MAb (UC10-4F10-11, hamster immunoglobulin G [IgG] group 1κ) were provided by J. Bluestone (University of Chicago). Anti-CD86 (GL1, rat IgG2b), anti-IL-4 (11B11, rat IgG1), and neutralizing anti-MHC II (M5/114 rat IgG specific for I-Ab,d and I-Ed) were obtained from the American Type Culture Collection ATCC. Monoclonal hamster IgG and rat IgG were purified from conditioned media or ascites by using HiTrap protein A and protein G columns, respectively (Pharmacia, Piscataway, N.J.). Immunopurified normal rat IgG was obtained from Sigma Chemical Co. (St. Louis, Mo.), and anti-TNP hamster IgG, group 1κ, was obtained from Pharmingen (San Diego, Calif.).
Culture of lymph node cells.
Lymph node cells harvested from uninfected or infected mice were washed three times, counted, and suspended in Dulbecco modified Eagle medium (DMEM; BioWhittaker) containing antibiotics, 2 mM glutamine, 0.1 mM nonessential amino acids, and 10% fetal bovine serum (FBS) and was buffered at pH 7.4 with 10 mM HEPES. Cells were aliquoted into flat-bottom 96-well culture plates at 106 cells per well and cultured for 48 h in DMEM–10% FBS. Stimuli included 10 μg of soluble Leishmania major promastigote antigen (SLA) per ml. Where indicated, 10 μg of anti-IL-4 receptor MAb (M-1; Genzyme Corp.) per ml was added to the culture to prevent loss of assayable IL-4 due to receptor binding (16). Conditioned media were removed at 48 h for enzyme-linked immunosorbent assay (ELISA) measurement of cytokines.
Cytokine ELISAs.
Culture supernatants were assayed for murine cytokines by using double-sandwich MAb ELISA techniques as previously described (29). IL-13 was assayed by using a commercial kit (Quantikine M; R&D; Minneapolis, Minn.).
Quantitative parasite cultures.
Approximately 0.2 g of footpad tissue were minced in 2 ml of M199 medium, crushed through a number 200 stainless steel screen, and disrupted by using a Ten-Broeck homogenizer. Footpad or lymph node suspensions were serially diluted fivefold in promastigote growth medium (M199–20% FBS) and incubated in flat-bottom 96-well plates at 26°C in humidified room air. Individual wells were examined by using an inverted microscope at ×200 power at 2-day intervals for the presence of motile promastigotes. Data represent the geometric mean and standard error of the last positive reciprocal dilution for each experimental group.
Statistics.
Significance was assessed by using the Mann-Whitney rank sum or the Student's t test.
RESULTS
Effects of anti-CTLA4 MAb treatment on the course of L. major infection in BALB/c and C57BL/6 mice.
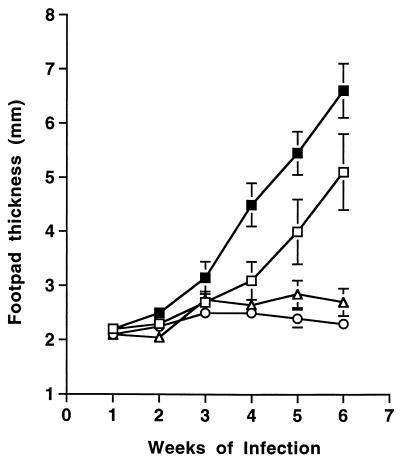
To determine whether CTLA4 activity contributed significantly to the distinct outcomes of leishmaniasis in different strains of inbred mice, susceptible BALB/c and resistant C57BL/6 mice were injected with either 0.3 mg of anti-CTLA4 MAb or nonspecific rat IgG on days 0 and 7 after infection in the hind feet with 2 × 106 L. major promastigotes. Cutaneous lesions, as measured by footpad thickening, developed at a significantly accelerated pace in BALB/c mice treated with anti-CTLA4 antibody compared to control BALB/c mice (Fig. 1). Anti-CTLA4 also caused accelerated disease in comparison to control mice treated with 0.3 mg of isotype-matched hamster anti-TNP antibody (data not shown). Footpad thickening appeared at least a week earlier than control mice, and the size of the lesions continued to exceed that of controls by at least a millimeter throughout the rest of the infection course. In contrast, anti-CTLA4-treated C57BL/6 mice remained fully resistant to infection relative to rat IgG-treated controls, although statistically significant and transient increases in footpad thickening were observed in one of four additional experiments (data not shown).
FIG. 1.
Treatment with anti-CTLA4 MAb accelerates the progression of cutaneous leishmaniasis in normally susceptible BALB/c mice but does not affect the outcome of disease in normally resistant C57BL/6 mice. Groups of five infected BALB/c mice were injected intraperitoneally on days 0 and 7 of infection with 0.3 mg of rat IgG (□) or anti-CTLA4 MAb (■). Groups of five C57BL/6 mice were similarly treated with rat IgG (○) and anti-CTLA4 MAb (▵). Shown are the mean and standard error of the mean for footpad thicknesses measured at weekly intervals after injection of 2 × 106 L. major promastigotes into both hind feet. Differences in footpad size for BALB/c mice were statistically significant (P < 0.05) from week 3 onward, whereas differences in C57BL/6 footpad sizes were not significantly different.
Accelerated cellular expansions and polarization of the developing Th2 response after anti-CTLA4 blockade.
We next compared the antigen-specific cytokine response of draining lymph node cells obtained from control and anti-CTLA4 treated BALB/c mice in the first 2 weeks of infection. Results were also compared to those of BALB/c mice treated with 0.5 mg of anti-CD86 MAb on days 0 and 7 of infection, an intervention that partially protects against progressive leishmaniasis (6, 15a). Consistent with the well-characterized and functionally opposed effects of these antibodies on lymphocyte expansion in vivo (21), the total numbers of cells obtained from popliteal lymph nodes draining the cutaneous site of infection at 14 days increased 5.7-fold in anti-CTLA4-treated mice relative to uninfected controls (Table 1). This increase was significantly greater (P < 0.05) than those observed in control mice and anti-CD86-treated mice, which only increased 2.3- and 1.6-fold at 14 days of infection, respectively.
TABLE 1.
Effects of anti-CTLA4 MAb on infection-related increases in lymph node cell numbers
| Treatment | Lymph node cell count
(10−7) at:
|
||
|---|---|---|---|
| 0 days | 7 days | 14 days | |
| Control | 0.23 ± 0.01 | 0.55 ± 0.04 | 0.53 ± 0.09 |
| Anti-CTLA4 | NDc | 0.76 ± 0.21 | 1.32 ± 0.11a |
| Anti-CD86 | ND | 0.32 ± 0.03 | 0.37 ± 0.10b |
Significant differences between control and anti-CTLA4 groups (P < 0.05; Mann-Whitney U test).
Significant differences between control and anti-CD86 groups (P < 0.05; Mann-Whitney U test).
ND, not determined.
Differences in the capacity of lymph node cells to produce cytokine in response to leishmania antigen were also observed. At 7 days of infection, cultured lymph node cells from anti-CTLA4-treated mice produced from 1.5 to 5 times more IL-4 in response to soluble leishmania antigen than did the cells from control mice (Fig. 2). There was no detectable production of IFN-γ at 7 days of infection in any of these groups. By 14 days of infection, anti-CTLA4-treated BALB/c mice continued to produce higher levels of IL-4 than did control BALB/c mice (ranging from 1.6- to 2-fold in three experiments) but remained markedly deficient in IFN-γ (6- to 10-fold decreases). Anti-CD86 treatment had the opposite effect, reducing early production of IL-4 while supporting the development of normal IFN-γ responses. Similar effects were observed on the antigen-specific response of IL-13, another Th2 cytokine with IL-4-like activity in vivo (1, 41). In each of two experiments, antigen-stimulated IL-13 levels were increased up to sixfold in lymph node cultures derived from anti-CTLA4-treated BALB/c mice.
FIG. 2.
Treatment with anti-CTLA4 MAb enhances IL-4 and inhibits IFN-γ cytokine responses in the draining lymph nodes of BALB/c mice at 7 and 14 days of infection. Groups of five BALB/c mice were infected with 2 × 106 L. major promastigotes in both hind feet and then treated on days 0 and 7 with 0.3 mg of anti-CTLA4 or anti-CD86 as described above. Draining lymph node cells were harvested on days 7 and 14 for culture and ELISA analysis of antigen-induced cytokine production. Data are shown as the mean ± the standard error of the mean (SEM) cytokine concentrations and are representative of two experiments. Naive BALB/c lymph node cells produced between 0 and 1 ng of IFN-γ per ml and ≤0.05 ng of IL-4 and IL-13 per ml under these conditions (data not shown).
Delayed effects of anti-CTLA4 MAb on Th1 and Th2 cytokines: IL-13 recall responses are IL-4 independent.
When lymph node responses were tested at 4 weeks of infection, prior treatment with anti-CTLA4 was less clearly associated with distinct patterns of cytokine production (Table 2). Although IL-4 and IL-13 production were no longer significantly increased, anti-CTLA4-treated BALB/c mice still maintained a twofold lower capacity for antigen-induced IFN-γ production (P < 0.05). Control infected C57BL/6 mice produced similar amounts of IFN-γ compared to BALB/c mice but generated ninefold less IL-4 and sixfold less IL-13. Delayed effects of anti-CTLA4 MAb in C57BL/6 mice were distinct from those seen in BALB/c; treatment caused significant increases in both IL-4 and IL-13 production, but no change in IFN-γ synthesis. Recall IL-13 production was IL-4 independent in all strains, as indicated by preserved IL-13 levels when neutralizing anti-IL-4R antibody was added to culture. The addition of anti-MHC II antibody to culture inhibited both IL-4 and IL-13 generation by more than 95%, confirming that these cytokines were produced by CD4+ T cells (data not shown).
TABLE 2.
Delayed effects of anti-CTLA4 treatment on production of Th1 and Th2 cytokinesa
| Group | Condition | Mean cytokine production
(ng/ml) ± SEM
|
||
|---|---|---|---|---|
| IL-4b | IFN-γ | IL-13 (+ anti-IL-4R MAbb) | ||
| BALB/c | Control | 5.6 ± 0.26 | 18.0 ± 6.2 | 1.32 ± 0.13 (1.33 ± 0.11) |
| BALB/c | Anti-CTLA4 | 5.8 ± 0.16 | 8.4 ± 2.5c | 1.26 ± 0.20 (1.31 ± 0.19) |
| C57BL/6 | Control | 0.67 ± 0.13 | 20.2 ± 3.1 | 0.23 ± 0.09 (0.33 ± 0.01) |
| C57BL/6 | Anti-CTLA4 | 1.5 ± 0.32c | 24.8 ± 4.6 | 0.48 ± 0.08† (0.57 ± 0.05)c |
Popliteal lymph node cells (106/well) were harvested at 4 weeks after cutaneous infection with 2 × 106 L. major promastigotes and cultured with 20 μg of SLA per ml for 48 h. Data represent mean ± the SEM for ELISA data obtained from five mice per group. Where indicated, mice were treated with 0.3 mg of anti-CTLA4 MAb on days 0 and 7 of infection. Findings are representative of two experiments.
Culture media included 10 μg of M-1 neutralizing anti-IL-4R MAb per ml.
Significant differences between control and anti-CTLA4 MAb treated groups (P < 0.05; Mann-Whitney U test).
Normally protective anti-IL-4 antibody treatments fail to reverse the accelerated course of leishmaniasis in anti-CTLA4-infected mice.
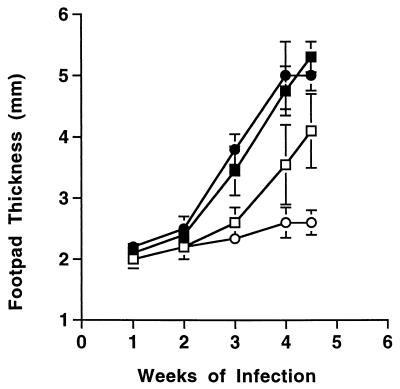
Because the earlier appearance of Th2 polarized cytokine responses correlated with accelerated progression of leishmaniasis in anti-CTLA4-treated BALB/c mice, we tested whether in vivo neutralization of IL-4 during early infection would restore curative immunity in the presence of anti-CTLA4 antibody. Cotreatment of mice with 1.0 mg of neutralizing anti-IL-4 MAb 11B11 on days 0 and 7 of infection protected control BALB/c mice against progressive disease, as previously described (17), but did not benefit anti-CTLA4-treated mice (Fig. 3). Consistent with the observed differences in lesion size, cutaneous parasite burdens were increased by 62-fold in anti-CTLA4-treated mice relative to control BALB/c mice at 4 weeks of infection (differences significant; P = 0.02). Anti-IL-4 MAb treatment reduced parasite numbers over 200-fold in infected BALB/c mice relative to control mice (P < 0.01) but did not significantly reduce the infectious load in mice that had been coinjected with anti-CTLA4 (P = 0.05) (Table 3).
FIG. 3.
Treatment with anti-IL-4 antibody does not reverse the exacerbative effects of CTLA4 blockade in L. major-infected BALB/c mice. Groups of five mice were infected with 2 × 106 promastigotes of L. major and injected on days 0 and 7 with saline (□) and 0.3 mg of anti-CTLA4 MAb (■). Other groups of mice were treated with 1.0 mg of neutralizing anti-IL-4 MAb 11B11 alone (○) or anti-CTLA4 MAb in combination with anti-IL-4 MAb (●). Shown are the mean ± the SEM footpad thicknesses at weekly intervals.
TABLE 3.
IL-4-independent effects of anti-CTLA4 antibody on cutaneous parasite loada
| Expt and mouse strain | Treatment | Parasite load (log10 ± SEM) | P valueb |
|---|---|---|---|
| Expt 1 | |||
| BALB/c | Saline | 5.54 ± 0.46 | |
| BALB/c | Anti-CTLA4 | 7.33 ± 0.29 | 0.02 |
| BALB/c | Anti-IL-4 | 3.15 ± 0.19 | <0.001 |
| BALB/c | Anti-CTLA4 plus anti-IL-4 | 6.99 ± 0.40 | 0.05 |
| Expt 2 | |||
| BALB/c | Saline | 9.20 ± 0.17 | |
| BALB/c IL-4 KO | Saline | 6.75 ± 0.18 | 0.005 |
| BALB/c IL-4 KO | Anti-CTLA4 | 8.53 ± 0.46 | 0.25 (0.02) |
Shown are the logarithmic mean numbers ± the SEM of parasites per gram of tissue as determined by limiting dilution culture for leishmania. Mice were infected for 4 weeks with 2 × 106 promastigotes of L. major. Where indicated, mice were treated with 0.3 mg of anti-CTLA4 MAb on days 0 and 7 of infection or with 1 mg of neutralizing anti-IL-4 MAb 11B11 on day 0.
P values were determined by use of the Student's t test (n = five mice each) and compare the indicated experimental groups with their respective BALB/c controls. Parentheses in experiment 2 indicate significantly increased parasite loads in anti-CTLA4-treated IL-4 KO mice compared to IL-4 KO controls.
Anti-CTLA4 MAb causes early disease exacerbation in IL-4 KO BALB/c mice.
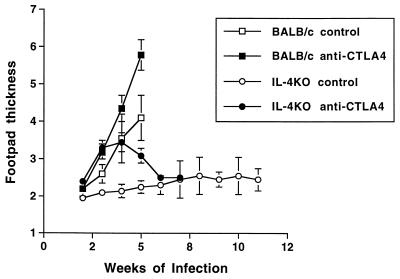
IL-4 production in anti-CTLA4-treated BALB/c mice might have either recovered late in infection or exceeded the neutralizing capacity of the anti-IL-4 antibody used. We therefore tested whether anti-CTLA4 MAb would also exacerbate infection in BALB/c mice genetically deficient in IL-4 (Fig. 4). This mouse strain was originally generated from a BALB/c embryonic cell line and is susceptible to progressive infection with the Friedlin (WHOM/IL/80/Friedlin) strain of L. major but not the 173 strain (WHOM/IR/-/173) employed in our studies (2, 22, 33)]. As expected, IL-4 KO BALB/c mice were resistant to a standard inoculum of L. major 173 promastigotes (2 × 106 per hind foot). However, treatment with 0.5 mg of anti-CTLA4 on days 0 and 7 of infection resulted in rapid exacerbation of disease that was sustained for 5 weeks and that was followed by late recovery. Irreversible disease exacerbation was again observed in wild-type BALB/c mice receiving the same dose of anti-CTLA4. A separate experiment confirmed exacerbation of disease in anti-CTLA4-treated IL-4 KO mice that correlated with 60-fold increases (P = 0.02) in cutaneous parasite burden compared to infected control IL-4 KO mice (Table 3).
FIG. 4.
Transient exacerbation of leishmaniasis is induced by anti-CTLA4 MAb in normally resistant BALB/c IL-4 KO mice. Groups of five mice each were infected with L. major (2 × 106 promastigotes) in each hind foot. Where indicated, infected mice were treated with 0.3 mg of anti-CTLA4 MAb on days 0 and 7 of infection. Data represent weekly footpad thicknesses ± the SEM for control and anti-CTLA4-treated BALB/c mice and for control and anti-CTLA4-treated IL-4 KO BALB/c mice. Transient exacerbation and long-term recovery of anti-CTLA4-treated IL-4 KO mice was confirmed in two additional experiments.
Anti-CTLA4 alters the developing cytokine response in IL-4 KO BALB/c mice.
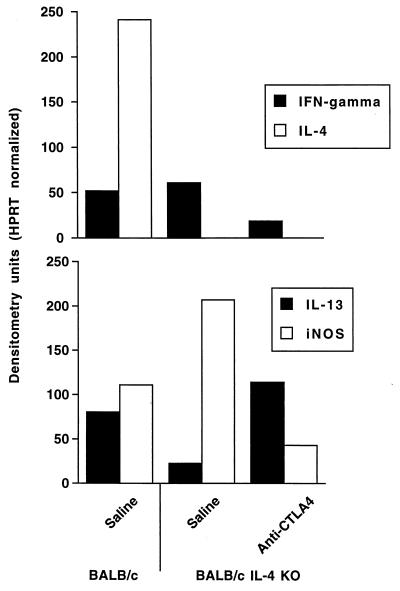
In repeated experiments, the draining lymph node cells of IL-4 KO BALB/c mice failed to produce increased amounts of IFN-γ or IL-13 at 1 and 2 weeks of infection (data not shown). This is consistent with previous reports demonstrating both kinetic and quantitative deficiencies in the local immune responses of IL-4 KO mice infected with L. major (22). At 4 weeks of infection, when exacerbation of disease was maximal and cytokine mRNA expression in IL-4 KO mice was readily detectable, anti-CTLA4-treated IL-4 KO mice expressed fivefold more IL-13 and fivefold less IFN-γ mRNA than control IL-4 KO mice (Fig. 5). Consistent with an inverted ratio of Th1 and Th2 cytokine activities in vivo, the expression of iNOS mRNA was decreased fivefold in anti-CTLA4-treated IL-4 KO mice compared to infected, control IL-4 KO mice.
FIG. 5.
Effects of anti-CTLA4 treatment on cytokine mRNA expression in the draining lymph nodes of BALB/c and IL-4 KO BALB/c mice at 4 weeks of infection. Groups of four BALB/c and IL-4 KO mice each were infected with L. major as described above. Where indicated, IL-4 KO mice were injected with saline or 0.5 mg of anti-CTLA4 MAb on days 0 and 7 of infection. After 4 weeks, lymph nodes were harvested into STAT-60 and RNA obtained for reverse transcriptase PCR analysis. Shown are the results of comparative reverse transcriptase PCR analysis for the indicated cytokine mRNA as measured in arbitrary densitometry units that are corrected for hypoxanthine phosphoribosyl transferase (HPRT) expression. Expression of these mRNA was not detected in uninfected lymph nodes at the levels of amplification used.
DISCUSSION
These studies were intended to identify whether perturbed CTLA4 activity could influence CD4+ T-cell differentiation and disease outcomes in susceptible BALB/c mice infected with L. major. We found that anti-CTLA4 antibody markedly accelerated the development of IL-4 and IL-13 cytokine responses in Th2-biased BALB/c strain during infection with L. major and that disease was more rapidly progressive in these animals. Injection with neutralizing anti-CD86 MAb had the opposite effect, delaying the onset of Th2 cytokine production until the second week of infection and promoting the increased production of IFN-γ over that of IL-4. Although the anti-CTLA4 MAb used had been previously shown to antagonize CTLA4 regulation of superantigen-driven responses in vivo when used as either Fab or intact antibody (24), recent findings in the L. major system indicate that intact and Fab fragments of anti-CTLA4 separately promote Th2- and Th1-dominant cytokine responses, respectively (37). This contrasts sharply with the dual Th1- and Th2-promoting effects of this same anti-CTLA4 MAb when used to treat BALB/c mice infected with L. donovani (32). Although the increase in both Th2 activities and in lymph node cellularity observed in our anti-CTLA4-treated mice is consistent with neutralization of CTLA4-dependent T-cell-inhibitory activities, the pronounced decrease in antigen-specific IFN-γ production in these same animals suggests complex regulatory effects of CTLA4 on Th1 cell development that may be unique to this model of disease.
Novel findings in this report include insights into the interactions between CTLA4 and the IL-4-independent expression and potential function of the Th2 cytokine, IL-13. IL-13 is capable of mediating IL-4-like biologic activities through activation of shared IL-13 and IL-4 receptors and Stat-6-dependent signal transduction pathways (1, 41). Our data indicate that antigen-specific IL-13 responses develop in both BALB/c and C57BL/6 mice infected with L. major but that production decreases in disease-resistant mice late in infection. Like IL-4, IL-13 synthesis by lymph node cells from both control and anti-CTLA4-treated mice was abrogated by anti-MHC II antibodies, confirming that CD4+ T cells were the likely source for these cytokines. However, IL-4 receptor blockade in antigen-stimulated cultures did not affect IL-13 synthesis, a finding consistent with the IL-4-independent development of IL-13-producing T cells in other models of parasitic disease (8, 38). Since both IL-13 and IL-4 suppress macrophage iNOS mRNA expression necessary for mediating nitric oxide-dependent killing of leishmanias (10), the increased production of IL-13 might be predicted to promote nonhealing disease. Consistent with this, BALB/c mice genetically deficient in the IL-4 receptor alpha chain, which is necessary for both IL-4- and IL-13-dependent signaling, are more resistant to L. major than BALB/c mice deficient only in IL-4 (34).
The more rapid appearance and greater production of IL-4-independent IL-13 in anti-CTLA4 mice during L. major infection therefore assumes greater interest in view of the transient IL-4-independent exacerbation of leishmaniasis that resulted. We first observed that doses of anti-IL-4 MAb 11B11 otherwise sufficient to restore curative immunity in infected BALB/c mice could not ameliorate anti-CTLA4 MAb-dependent disease exacerbation. Furthermore, anti-CTLA4 MAb induced a pronounced, if transient, exacerbation of disease in BALB/c mice that were genetically deficient in IL-4. The ability of wild-type mice to produce small amounts of IL-4 late in disease after clearance of the anti-IL-4 antibody may account for the persistent exacerbation seen relative to the transient effects observed in IL-4 KO mice. Although IL-4 KO BALB/c mice are fully susceptible to specific strains of L. major, such as the Friedlin strain (33), they were resistant to the 173 strain employed in our studies. The markedly delayed onset of antigen-dependent cytokine responses in this strain made early comparisons with wild-type BALB/c mice difficult, but we observed increased IL-13 and decreased IFN-γ mRNA expression in anti-CTLA4-treated IL-4 KO mice at later times of infection. The corresponding decrease in iNOS mRNA confirms the in vivo effects of combined IFN-γ deficiency and Th2 cytokine excess, while suggesting the mechanism responsible for the observed increases in parasite load and lesion size. We tentatively propose that anti-CTLA4 antibody enhanced the differentiation and expansion of IL-13-producing cells and that increased IL-13 responses in vivo functionally substituted for IL-4 in mediating progression of murine leishmaniasis. Alternatively, IL-13 may only be a marker for other Th2 activities more directly involved in disease progression. In this regard, preliminary studies with neutralizing anti-IL-13 MAb (R&D) as cotreatment failed to reverse the exacerbative effects of anti-CTLA4 in IL-4 KO mice (data not shown). Additional studies are needed to confirm the pathologic relevance of IL-13 in anti-CTLA4-treated mice and to determine the relative significance of decreased IFN-γ in mediating these outcomes.
Although both IFN-γ deficiency and Th2 cytokine excess may have contributed to disease exacerbation, the mechanism by which anti-CTLA4 antibody promotes Th2 hyperpolarization remains unresolved. The data of Saha et al. (37) suggests that intact UC10-4F10 antibody activates CTLA4 function, an idea consistent with the curative and Th1-promoting effects of CTLA4 inactivation by CD80/CD86 blockade or by neutralizing anti-CTLA4 Fab fragments. Since anti-CTLA4 has diverse effects on Th1 and Th2 outcomes in different disease models, CTLA4 activation presumably amplifies preexisting biases in Th cell differentiation through differential effects on expanding Th2 and Th1 cells. This suggests proliferation-enhancing functions for CTLA4 that conflict with many experimental studies of this costimulatory molecule (23, 42, 43), although others have identified lymphocyte-activating effects similar to that of CD28 (29, 30, 46). The role of CD28 in coregulating the effects of CTLA4 in murine leishmaniasis is also uncertain in view of these contradictory experimental data. Functional inactivation of both CTLA4 and CD28 by CTLA4-Ig or by anti-CD86 antibodies consistently inhibits Th2 responses and cures L. major infection in wild-type BALB/c mice (6, 9). However, Th2 development and disease progression in infected CD28 KO BALB/c mice are paradoxically unaffected by CTLA4-Ig, yet inhibited by Fab anti-CTLA4 antibody (11, 37). Further studies are needed to exclude the possibility that anti-CTLA4 MAb activates CTLA4 via unique intermolecular interactions distinct from binding to CD86/CD80. Other findings in this report additionally extend our understanding of IL-13 production in the mouse model of leishmaniasis by demonstrating IL-4-independent exacerbation of disease in association with enhanced IL-13 synthesis after anti-CTLA4 administration. Since IL-13 functionally substitutes for IL-4 in other models of Th2 immunopathology (15, 41, 44), we tentatively propose that the variable susceptibility of IL-4 KO mice to different strains of L. major may reflect parasite-specific effects on T-cell costimulation that lead to preferential development of T cells secreting disease-promoting IL-13. We conclude that antibodies against CTLA4 enhance the genetic predisposition toward Th2 development in BALB/c mice infected with L. major and that CTLA4-modulated immune responses are characterized by increased production of alternative Th2 cytokines in association with IL-4-independent disease exacerbation.
ACKNOWLEDGMENTS
This work was supported by the VA Medical Research Service and by National Institute of Allergy and Infectious Diseases grants RO1 AI35979 and K04 AI01229.
We thank J. Bluestone for his generous donation of the UC10-4F10 hybridoma. We also gratefully acknowledge the technical assistance of Ronald M. Rerko and Andrea Hujer in some of these studies.
REFERENCES
- 1.Bancroft A J, McKenzie A N, Grencis R K. A critical role for IL-13 in resistance to intestinal nematode infection. J Immunol. 1998;160:3453–3461. [PubMed] [Google Scholar]
- 2.Belkaid Y, Kamhawi S, Modi G, Valenzuela J, Noben-Trauth N, Rowton E, Ribeiro J, Sacks D L. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania majorinfection in the mouse ear dermis. J Exp Med. 1998;188:1941–1953. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bird J J, Brown D R, Mullen A C, Moskowitz N H, Mahowald M A, Sider J R, Gajewski T F, Wang C R, Reiner S L. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 4.Blair P J, Riley J L, Levine B L, Lee K P, Craighead N, Francomano T, Perfetto S J, Gray G S, Carreno B M, June C H. CTLA-4 ligation delivers a unique signal to resting human CD4 T cells that inhibits interleukin-2 secretion but allows Bcl-X(L) induction. J Immunol. 1998;160:12–15. [PubMed] [Google Scholar]
- 5.Brown D R, Green J M, Moskowitz N H, Davis M, Thompson C B, Reiner S L. Limited role of CD28-mediated signals in T helper subset differentiation. J Exp Med. 1996;184:803–810. doi: 10.1084/jem.184.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown L A, Titus R G, Nabavi N, Glimcher L H. Blockade of CD86 ameliorates Leishmania majorinfection by down-regulating the Th2 response. J Infect Dis. 1996;174:1303–1308. doi: 10.1093/infdis/174.6.1303. [DOI] [PubMed] [Google Scholar]
- 7.Calvo C R, Amsen D, Kruisbeek A M. Cytotoxic T lymphocyte antigen 4 (CTLA-4) interferes with extracellular signal-regulated kinase (ERK) and Jun NH2-terminal kinase (JNK) activation, but does not affect phosphorylation of T cell receptor zeta and ZAP70. J Exp Med. 1997;186:1645–1653. doi: 10.1084/jem.186.10.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiaramonte M G, Schopf L R, Neben T Y, Cheever A W, Donaldson D D, Wynn T A. IL-13 is a key regulatory cytokine for Th2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansonieggs. J Immunol. 1999;162:920–930. [PubMed] [Google Scholar]
- 9.Corry D B, Reiner S L, Linsley P S, Locksley R M. Differential effects of blockade of CD28-B7 on the development of Th1 or Th2 effector cells in experimental leishmaniasis. J Immunol. 1994;153:4142–4148. [PubMed] [Google Scholar]
- 10.Doherty T M, Kastelein R, Menon S, Andrade S, Coffman R L. Modulation of murine macrophage function by IL-13. J Immunol. 1993;151:7151–7160. [PubMed] [Google Scholar]
- 11.Elloso M M, Scott P. Expression and contribution of B7-1 (CD80) and B7-2 (CD86) in the early immune response to Leishmania majorinfection. J Immunol. 1999;162:6708–6715. [PubMed] [Google Scholar]
- 12.Fallarino F, Fields P E, Gajewski T F. B7-1 engagement of cytotoxic T lymphocyte antigen 4 inhibits T cell activation in the absence of CD28. J Exp Med. 1998;188:205–210. doi: 10.1084/jem.188.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gause W C, Chen S J, Greenwald R J, Halvorson M J, Lu P, Zhou X D, Morris S C, Lee K P, June C H, Finkelman F D, Urban J F, Abe R. CD28 dependence of T cell differentiation to IL-4 production varies with the particular type 2 immune response. J Immunol. 1997;158:4082–4087. [PubMed] [Google Scholar]
- 14.Gause W C, Mitro V, Via C, Linsley P, Urban J F, Jr, Greenwald R J. Do effector and memory T helper cells also need B7 ligand costimulatory signals? J Immunol. 1997;159:1055–1058. [PubMed] [Google Scholar]
- 15.Grunig G, Warnock M, Wakil A E, Venkayya R, Brombacher F, Rennick D M, Sheppard D, Mohrs M, Donaldson D D, Locksley R M, Corry D B. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Heinzel, F. P. Unpublished observations.
- 16.Heinzel F P, Rerko R M, Ahmed F, Pearlman E. Endogenous interleukin-12 (IL-12) is required for control of Th2 CD4+T cell responses capable of exacerbating leishmaniasis in normally resistant C57BL/6 mice. J Immunol. 1995;155:730–739. [PubMed] [Google Scholar]
- 17.Heinzel F P, Sadick M D, Holaday B J, Coffman R L, Locksley R M. Reciprocal expression of interferon-γ or interleukin-4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Himmelrich H, Parra-Lopez C, Tacchini-Cottier F, Louis J A, Launois P. The IL-4 rapidly produced in BALB/c mice after infection with Leishmania major down-regulates IL-12 receptor beta 2-chain expression on CD4+T cells resulting in a state of unresponsiveness to IL-12. J Immunol. 1998;161:6156–6163. [PubMed] [Google Scholar]
- 19.Karandikar N J, Vanderlugt C L, Walunas T L, Miller S D, Bluestone J A. CTLA-4: a negative regulator of autoimmune disease. J Exp Med. 1996;184:783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keane M A, Gause W C, Linsley P S, Chen S J, Wills K M. B7-CD28/CTLA-4 costimulatory pathways are required for the development of T helper cell 2-mediated allergic airway responses to inhaled antigens. J Immunol. 1997;158:2042–2049. [PubMed] [Google Scholar]
- 21.Kearney E R, Walunas T L, Karr R W, Morton P A, Loh D Y, Bluestone J A, Jenkins M K. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J Immunol. 1995;155:1032–1036. [PubMed] [Google Scholar]
- 22.Kropf P, Etges R, Schopf L, Chung C, Sypek J, Muller I. Characterization of T-cell-mediated responses in nonhealing and healing Leishmania majorinfections in the absence of endogenous IL-4. J Immunol. 1997;159:3434–3443. [PubMed] [Google Scholar]
- 23.Krummel M F, Allison J P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krummel M F, Sullivan T J, Allison J P. Superantigen responses and co-stimulation: CD28 and CTLA-4 have opposing effects on T cell expansion in vitro and in vivo. Int Immunol. 1996;8:519–523. doi: 10.1093/intimm/8.4.519. [DOI] [PubMed] [Google Scholar]
- 25.Kuchroo V K, Das M P, Brown J A, Ranger A M, Zamvil S S, Sobel R A, Weiner H L, Nabavi N, Glimcher L. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 26.Lee K M, Chuang E, Griffin M, Khattri R, Hong D K, Zhang W, Straus D, Samelson L E, Thompson C B, Bluestone J A. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 27.Liew F, Li Y, Severn A, Millott S, Schmidt J, Salter M, Moncada S. A possible novel pathway of regulation by murine T helper type-2 cells of a Th1 cell activity via the modulation of the induction of nitric oxide synthase on macrophages. Eur J Immunol. 1991;21:2489–2494. doi: 10.1002/eji.1830211027. [DOI] [PubMed] [Google Scholar]
- 28.Lin H, Rathmell J C, Gray G S, Thompson C B, Leiden J M, Alegre M L. Cytotoxic T lymphocyte antigen 4 (CTLA4) blockade accelerates the acute rejection of cardiac allografts in CD28-deficient mice: CTLA4 can function independently of CD28. J Exp Med. 1998;188:199–204. doi: 10.1084/jem.188.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linsley P S, Greene J L, Tan P, Bradshaw J, Ledbetter J A, Anasetti C, Damle N K. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992;176:1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y. Is CTLA-4 a negative regulator for T-cell activation? Immunol Today. 1997;18:569–572. doi: 10.1016/s0167-5699(97)01170-5. [DOI] [PubMed] [Google Scholar]
- 31.McCoy K, Camberis M, Gros G L. Protective immunity to nematode infection is induced by CTLA-4 blockade. J Exp Med. 1997;186:183–187. doi: 10.1084/jem.186.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy M L, Cotterell S E, Gorak P M, Engwerda C R, Kaye P M. Blockade of CTLA-4 enhances host resistance to the intracellular pathogen, Leishmania donovani. J Immunol. 1998;161:4153–4160. [PubMed] [Google Scholar]
- 33.Noben-Trauth N, Kropf P, Muller I. Susceptibility to Leishmania majorinfection in interleukin-4-deficient mice. Science. 1996;271:987–990. doi: 10.1126/science.271.5251.987. [DOI] [PubMed] [Google Scholar]
- 34.Noben-Trauth N, Paul W E, Sacks D L. IL-4- and IL-4 receptor-deficient BALB/c mice reveal differences in susceptibility to Leishmania majorparasite substrains. J Immunol. 1999;162:6132–6140. [PubMed] [Google Scholar]
- 35.Reiner S L, Locksley R M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 36.Sadick M D, Locksley R M, Tubbs C, Raff H V. Murine cutaneous leishmaniasis: resistance correlates with the capacity to generate interferon-γ in response to leishmania antigens in vitro. J Immunol. 1986;136:655–661. [PubMed] [Google Scholar]
- 37.Saha B, Chattopadhyay S, Germond R, Harlan D M, Perrin P J. CTLA4 (CD152) modulates the Th subset response and alters the course of experimental Leishmania majorinfection. Eur J Immunol. 1998;28:4213–4220. doi: 10.1002/(SICI)1521-4141(199812)28:12<4213::AID-IMMU4213>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 38.Subramanian G, Kazura J W, Pearlman E, Jia X, Malhotra I, King C L. B7-2 requirement for helminth-induced granuloma formation and CD4 type 2 T helper cell cytokine expression. J Immunol. 1997;158:5914–5920. [PubMed] [Google Scholar]
- 39.Szabo S J, Dighe A S, Gubler U, Murphy K M. Regulation of the interleukin (IL)-12R β2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tivol E A, Borriello F, Schweitzer A N, Lynch W P, Bluestone J A, Sharpe A H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 41.Urban J F, Jr, Noben-Trauth N, Donaldson D D, Madden K B, Morris S C, Collins M, Finkelman F D. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 42.Walunas T L, Bakker C Y, Bluestone J A. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waterhouse P, Penninger J M, Timms E, Wakeham A, Shahinian A, Lee K P, Thompson C B, Griesser H, Mak T W. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 44.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben T Y, Karp C L, Donaldson D D. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 45.Wu Y, Zhou Q, Zheng P, Liu Y. CD28-independent induction of T helper cells and immunoglobulin class switches requires costimulation by the heat-stable antigen. J Exp Med. 1998;187:1151–1156. doi: 10.1084/jem.187.7.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng P, Wu Y, Guo Y, Lee C, Liu Y. B7-CTLA4 interaction enhances both production of antitumor cytotoxic T lymphocytes and resistance to tumor challenge. Proc Natl Acad Sci USA. 1998;95:6284–6289. doi: 10.1073/pnas.95.11.6284. [DOI] [PMC free article] [PubMed] [Google Scholar]