Abstract
Purpose
Intravenous maintenance fluid therapy (IV-MFT) prescribing in acute and critically ill children is very variable among pediatric health care professionals. In order to provide up to date IV-MFT guidelines, the European Society of Pediatric and Neonatal Intensive Care (ESPNIC) undertook a systematic review to answer the following five main questions about IV-MFT: (i) the indications for use (ii) the role of isotonic fluid (iii) the role of balanced solutions (iv) IV fluid composition (calcium, magnesium, potassium, glucose and micronutrients) and v) and the optimal amount of fluid.
Methods
A multidisciplinary expert group within ESPNIC conducted this systematic review using the Scottish Intercollegiate Guidelines Network (SIGN) grading method. Five databases were searched for studies that answered these questions, in acute and critically children (from 37 weeks gestational age to 18 years), published until November 2020. The quality of evidence and risk of bias were assessed, and meta-analyses were undertaken when appropriate. A series of recommendations was derived and voted on by the expert group to achieve consensus through two voting rounds.
Results
56 papers met the inclusion criteria, and 16 recommendations were produced. Outcome reporting was inconsistent among studies. Recommendations generated were based on a heterogeneous level of evidence, but consensus within the expert group was high. “Strong consensus” was reached for 11/16 (69%) and “consensus” for 5/16 (31%) of the recommendations.
Conclusions
Key recommendations are to use isotonic balanced solutions providing glucose to restrict IV-MFT infusion volumes in most hospitalized children and to regularly monitor plasma electrolyte levels, serum glucose and fluid balance.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-022-06882-z.
Keywords: Isotonic fluids, Balanced fluids, Hyponatremia, Fluid balance, Intensive care, Acutely ill children
Take-home message
| A systematic review was conducted to produce guidelines on intravenous maintenance fluid therapy in acutely and critically ill children. Sixteen recommendations were produced, which suggest favouring isotonic balanced glucose-containing fluids designed for children, and to infuse them in lower amounts than Holliday and Segar’s formula. |
Introduction
Intravenous maintenance fluid therapy (IV-MFT) has been defined as the water and electrolyte prescription designed to replace anticipated physiologic water and electrolyte losses over the ensuing 24-h period [1]. Maintenance fluid therapy is provided by enteral hydration and/or IV-MFT which comprises both specific IV-MFT prescription, but also additional fluids administered as vectors of various treatments, blood products, or line flush infusions. IV-MFT should be differentiated from fluid boluses and resuscitation fluids that are administered to correct a relative or true fluid deficit, or from replacement fluids that are administered to correct abnormal fluid losses, even if this might be challenging as they are often administered simultaneously. IV-MFT is a standard of care for many hospitalized children in both acute and critical care settings (i.e. emergency department, pediatric wards, surgical wards or intermediate (high dependency) care units and pediatric intensive care units (PICU)). Despite this, maintenance fluid prescribing practices vary considerably [2] and guidelines are scarce [3]. Historically, IV-MFT prescriptions (fluid volume calculation formulas and solution composition) were based on Holliday and Segar’s work [4]. Since then, the use of these guidelines has been associated with potentially severe complications such as hyponatremia, fluid overload and hyperchloremic acidosis, due to inappropriate fluid composition and/or infusion rates/volumes [3, 5–7]. Several established practices are currently questioned. First, the ideal fluid tonicity, i.e. the osmotic pressure gradient of the fluid which is determined by the concentration of osmoactive or non-penetrating solutes, mainly sodium [8]. Second, the chloride content and balanced nature of the fluid (i.e. balanced or buffered solutions are produced after the replacement of part of the chloride anions by organic anions to align to the plasma chloride levels, which may impact on acid–base equilibrium). Finally, the volume of fluid administered, especially in situations where patients are at risk of increased anti-diuretic hormone (ADH) secretion and free water excretion impairment [7]. Over the past decade, several pediatric societies have developed guidelines and recommendations surrounding IV-MFT, but their impact has been limited by a lack of dissemination, a lack of recent systematic reviews and because some important questions remained unanswered [3, 9, 10]. In 2020, the Metabolism, Endocrine and Nutrition (MEN) section of the European Society of Pediatric and Neonatal Intensive care (ESPNIC) formed a working group to develop European guidelines on IV-MFT. These guidelines aim to provide evidence-based recommendations around the indications for IV-MFT, the tonicity of IV-MFT, the electrolyte or glucose content and the volume of IV-MFT administered to children from term to 18 years of age.
Method
To generate these evidence-based guidelines, we followed the Scottish Intercollegiate Guidelines Network (SIGN) 50 methodology [11, 12] and results are presented in line with the EQUATOR PRISMA checklist for systematic reviews [13] and the AGREE guideline reporting checklist [14]. The systematic review was registered on PROSPERO on December 15, 2020, when the research protocol was finalized by the working group (CRD42020218847) [15].
Selection of members
In September 2020, under ESPNIC, three project leaders (DB, LT and FVV) formed a working group of members within the MEN section. This group comprised medical doctors, nurses, pharmacists and dieticians, and was created following a call for interested candidates. Selection by the project leaders was based on expertise in the methods and experience in the field of pediatric acute and intensive care, metabolism, and research. This work group also included an academic librarian specialized in systematic reviews, a systematic review methodologist, an epidemiologist and a biostatistician specialized in meta-analyses. There was no industry input into the guidelines development. No member of the working group received honoraria for any role in the process and all members have declared any potential conflicts of interest.
Question development
The content and wording of the clinical questions was agreed at the first online meeting. All questions were structured in the Population, Intervention, Control, and Outcome(s) (PICO) format as follows:
Population: acute and critically ill children aged term to 18 years. Critically ill children are those presenting with severe organ failure(s) or requiring pediatric intensive care admission; acutely ill children are those presenting with an acute non-critical condition and requiring in hospital care (e.g. admitted to the emergency department, to pediatric wards, intermediate or high dependency units, or post-surgery). Preterm babies (< 37 weeks gestational age) and the intra-operative setting were outside the scope of the guidelines.
The agreed questions were as follows:
PICO1—Indication: Does IV-MFT versus other hydration therapies (none, oral or enteral route) impact on clinical outcomes?
PICO2—Tonicity: Do isotonic solutions versus hypotonic solutions (as IV-MFT) impact on clinical outcomes?
PICO3—Balanced fluids: Do balanced solutions versus non-balanced solutions (as IV-MFT) impact on clinical outcomes?
PICO4—Composition: Does the composition of IV-MFT in terms of glucose, electrolytes (P, Mg, Ca, K), vitamins and trace elements impact on clinical outcomes?
PICO5—Amounts: Does the use of a restrictive IV-MFT volume versus the standard Holliday and Segar calculated volume impact on clinical outcomes?
Outcomes: Standard outcomes (i.e. mortality and length of hospital or PICU stay) were selected. PICO specific outcomes were further identified: hypo or hyper -natremia for PICO 2; hyper-chloremia and acidosis for PICO 3; hypo or hyper -glycemia, -kalemia, -phosphoremia and -magnesemia for PICO 4; fluid balance for PICO 5. However, the preliminary search revealed a large inconsistency in outcome reporting among studies and among PICOs, with discrepancy between outcome definition, presentation, and time of assessment. Consequently, all reported outcomes in reviewed studies were extracted and later included in meta-analysis whenever possible.
Each member of the working group was allocated to a sub-group dedicated to one of the five PICO questions. Allocation of the members to these subgroups was decided by the project leaders according to each member’s preferences and expertise, ensuring a balanced distribution by countries, between professional disciplines, between junior and senior clinicians and between researchers. In each of the five subgroups, group leaders were allocated based on their methodological experience in conducting previous systematic reviews and guideline development.
Literature search and inclusion criteria
After an initial scoping search, each group identified MESH terms for their PICO questions. Subsequently, the medical librarian undertook the literature search on five databases (Pubmed/Medline; Web of Science; Scopus; Cochrane; Embase) for each PICO question. The inclusion criteria for papers were as follows: (1) all papers published until November 2020; (2) written in English, French, Spanish or German with an English abstract; (3) inclusion of critically ill or acutely ill (in the hospital setting) children aged from 37 weeks’ gestational age (GA) to 18 years of age; (4) study designs were randomized controlled trials, cohort studies, before and after studies and case/control studies. To ensure an exhaustive search, literature reviews and editorials were sought and their reference lists checked, but they were not included in the data extraction. Animal studies, case studies, conference abstracts and letters were excluded. The search equations for each PICO question are presented in Supplementary Material 1.
After the removal of duplicates, the librarian uploaded the paper abstract for each PICO question, into the review online software Rayyan [Rayyan Systems Inc. Cambridge, Mass, USA], which access was shared with all the members of the working group.
Selection of relevant studies
Using Rayyan software, each PICO group was responsible for screening for relevance, by title and abstract, the articles for their PICO search, as per the inclusion criteria. At least, two group members, blinded to each other, performed this screening, to reduce subjectivity. In the case of disagreement, differences in decision were resolved via a discussion. If it could not be resolved, a third member was involved. Once all relevant papers were agreed, the full text papers were retrieved by the librarian and shared with each PICO group. A similar screening process was applied to full text manuscripts (double blind screening,) based on inclusion/exclusion criteria, until a final list of papers was agreed for data extraction.
Data extraction and assessment of methodological quality
Once papers were agreed for inclusion for each PICO, papers were independently analyzed by two group members (excluding any authors of the paper). Each member extracted key data and summarized the main findings in a standardized data extraction form, according to the study design. Concurrently the same two reviewers assessed the risk of bias according to the Cochrane risk-of-bias tool for randomized trials [16] and to the Newcastle–Ottawa Quality Assessment Form for cohort studies [17]. In case of disagreement, differences in assessment were resolved via discussion, involving the group leader(s) if required.
Data analysis
When appropriate, data were combined statistically in a meta-analysis. To be combined the data had to meet the following criteria: (1) more than one study; (2) the combined studies were of one study design either randomized trials or observational studies; (3) the population and the intervention were sufficiently similar; (4) the outcomes were the same, or for continuous outcome variables, data on the distribution of the variable was available; (5) the risk of bias was not considered critical according to the SIGN grading system. If two or more groups of patients in a same study were independent, we analyzed each sample as an independent study. Two biostatisticians (AB, JJP) conducted the meta-analyses, but did not participate in development of the recommendations or the voting process.
To compare experimental and control groups of randomised controlled trials (RCTs), we calculated the effect sizes and their 95% confidence intervals using the mean difference for quantitative endpoints and odds ratio for qualitative endpoints, weighted by the inverse of the variance. The analyses were performed using a random effects model. The heterogeneity of outcomes was determined using chi-squared and Higgins I2 tests: a p-value < 0.05 or an I2 ≥ 50% indicated significant heterogeneity. If the heterogeneity was significant and the conditions for the validity of the analysis were verified, a sensitivity analysis was performed to assess the robustness of the results, excluding the most influential studies. The publication bias was explored for meta-analyses with four or more included studies using the funnel plots.
A p value less than 0.05 was considered significant; all p values were two-tailed. Meta-analyses were performed using Review Manager software (RevMan, Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Consensus methodology, grading of the recommendations and voting rounds
Two online consensus meetings took place in October and November 2021. At these meetings, each group presented their conclusions based on the results from the systematic review and the meta-analyses. A first draft of recommendations was proposed to the whole study team. The wording of the recommendations was discussed with the study team and reworded if required. The classification of the grades of recommendation (A–D, Good Clinical Practice) was undertaken according to the SIGN grading system [11] (supplemental material 2). After those meetings, the recommendations, the supporting rationales, and the recording of the meetings were made available online to the whole team before the online voting. Every member of the study team attended the meetings or watched the meeting recordings and read the proposed rational before voting.
In December 2021, all the PICO recommendations were combined in an online survey (survey Monkey, Inc. (Palo Alto, CA)) for the members to vote, the intention being to gain consensus using a modified Delphi method [18]. Members were required to indicate “agreement”, “partial agreement with a suggested minor wording change” or “disagreement, with free comments” for each recommendation.
Consensus was defined as “strong consensus” (> 95%), as “consensus” (75–95%) and “no consensus” (< 75%) agreement. In the case of less than 95% agreement during the first online voting, each PICO group was asked to modify the wording of the recommendation according to members suggestions or, if they chose to make no amendment, to provide an explanation. A second and final online voting round took place in January 2022 to attempt to reach strong consensus on the set of recommendations. Members were required to indicate “agreement” or “disagreement”, with explanations and proposal.
Results
A total of 18,399 abstracts were screened (Fig. 1 PRISMA flow chart). Subsequently 56 publications from 1969 to 2021 were included [19–74] for data extraction, which included 11,689 patients in both acute and critical care, medical and surgical care settings and used in the development of 16 recommendations (Table 1).
Fig. 1.
PRISMA flow chart of literature search and study screening
Table 1.
Intravenous maintenance fluid therapy (IV-MFT) recommendations, level of evidence according to SIGN grading, and consensus within the expert group
| Recommendations | Level of evidence | Consensus |
|---|---|---|
| PiCO 1: IV-MFT indications | ||
| In acutely ill children, the enteral or oral route for the delivery of maintenance fluid therapy should be considered, if tolerated, to reduce the failure rate of hydration access and costs | C | Strong consensus |
| In critically ill children with improving hemodynamic state, the enteral or oral route for the delivery of maintenance fluid therapy should be considered, if tolerated, to reduce length of stay in term neonates | GCP | Strong consensus |
| PiCO 2: use of isotonic fluids | ||
| In acutely and critically ill children, isotonic maintenance fluid should be used to reduce the risk of hyponatremia | A | Strong consensus |
| PiCO 3: use of balanced solutions | ||
| In critically ill children, balanced solutions should be favoured when prescribing intravenous maintenance fluid therapy to slightly reduce length of stay | B | Strong consensus |
| In acutely ill children, balanced solutions should be used when prescribing intravenous maintenance fluid therapy to slightly reduce length of stay | A | Strong consensus |
| In acutely and critically ill children, lactate buffer solution should not be considered in the case of severe liver dysfunction to avoid lactic acidosis | D | Consensus |
| PiCO 4: IV-MFT fluid composition (Ca, Mg, P, Micronutrients, Glucose) | ||
| In acutely and critically ill children, glucose provision in intravenous maintenance fluid therapy should be considered in sufficient amount and guided by blood glucose monitoring (at least daily) to prevent hypoglycaemia | GCP | Consensus |
| In critically ill children, glucose provision in intravenous maintenance fluid therapy should not be excessive and guided by blood glucose monitoring (at least daily) to prevent hyperglycaemia | B | Consensus |
| In acutely and critically ill children, there is insufficient evidence to recommend routine supplementation of magnesium, calcium and phosphate in intravenous maintenance fluid therapy | GCP | Strong consensus |
| In acutely and critically ill children, an appropriate amount of potassium should be considered and added to intravenous maintenance fluid therapy, based on the child's clinical status and regular potassium level monitoring to avoid hypokalemia | GCP | Consensus |
| In acutely and critically ill children, there is insufficient evidence to recommend routine supplementation of vitamins and trace elements in intravenous maintenance fluid therapy, in the absence of signs of deficiency | GCP | Strong consensus |
| PiCO 5: volume of IV-MFT administered | ||
| In acutely and critically ill children, in order to prevent fluid creep and reduce fluid intake, the total daily amount of maintenance fluid therapy should be considered including: IV fluids, blood products, all IV medications (both infusions and bolus drugs), arterial and venous line flush solutions and enteral intake, but does not include replacement fluids and massive transfusion | D | Strong consensus |
| In acutely and critically ill children, avoidance of fluid overload and cumulative positive fluid balance should be considered, to avoid prolonged mechanical ventilation and length of stay | D | Strong consensus |
| In acutely and critically ill children, who are at risk of increased endogenous secretion of ADH, restriction of total intravenous maintenance fluid therapy volume (calculated by Holliday and Segar formula) should be considered to some extent, to avoid a decrease in natremia but the amount and duration of this restriction is uncertain | C | Strong consensus |
|
In acutely and critically ill children who are at risk of increased endogenous secretion of ADH, restricting maintenance fluid therapy volume to between 65–80% of the volume calculated by the Holliday and Segar formula should be considered to avoid fluid overload In children at greater risk of oedematous states, e.g., heart failure, renal failure or hepatic failure, restricting maintenance fluid therapy volume to between 50% to 60% of the volume calculated with the Holiday and Segar formula should be considered to avoid fluid overload |
GCP | Strong consensus |
| Whilst receiving intravenous maintenance fluid therapy, re-assessment of acutely and critically ill children should be considered at least daily in terms of fluid balance and clinical status and regularly regarding electrolytes, especially sodium level | D | Consensus |
ADH anti-diuretic hormone; GCP good clinical practice; IV-MFT intravenous maintenance fluid therapy; Consensus (expert votes): 90% < agreement < 95%; Strong consensus: > 95% agreement
Long rationales are available for each recommendation in supplemental materials 7–11
Overall, the level of evidence was low [11] with most studies graded 1 or lower with a serious risk of bias (Table 2 and supplemental files 3–6).
Table 2.
Summary table of studies included to produce recommendations and their SIGN level of evidence
| Study: author and year of publication | Study examined | Study design settings and location | Patient population | Summary of results | Risk of bias | Applicability to our question/patients | SIGN level of evidence |
|---|---|---|---|---|---|---|---|
| Mackenzie et al. (1991) |
PICO 1 IV vs enteral hydration with electrolyte solutions |
RCT Single centre Australia |
104 dehydrated acutely ill children with gastroenteritis | No difference | Serious risk | High | 1− |
| Nager et al. (2002) |
PICO 1 IV vs enteral hydration with electrolyte solutions |
RCT Single Centre United states of America (USA) |
90 dehydrated acutely ill children with gastroenteritis | Higher costs in IV group | Serious risk | High | 1− |
| Sharifi et al. (1985) |
PICO 1 IV rehydration vs enteral rehydration |
RCT Single Centre Iran |
470 dehydrated acutely ill children with gastroenteritis | Less hyponatremia, acidosis hypokalaemia, diarrhoea, and higher weight gain in enteral group | Serious risk | High | 1− |
| Spandorfer et al. (2005) |
PICO 1 IV rehydration vs enteral rehydration |
RCT Single Centre USA |
73 dehydrated acutely ill children with gastroenteritis | No difference | Serious risk | Moderate | 1− |
| Rao et al. (2020) |
PICO 1 IV hydration vs enteral feeding |
RCT Single Centre India |
186 Critically ill term neonates on inotropes | No difference | Low risk | High | 1 + + |
| Oakley et al. (2013) |
PICO 1 IV vs enteral hydration or enteral feeds |
RCT Multicentre Australia and New Zealand |
759 acutely ill bronchiolitis children | No difference | Serious risk | High | 1− |
| Oakley et al. (2017) |
PICO 1 IV vs enteral hydration or enteral feeds |
RCT Multicentre Australia and New Zealand |
759 acutely ill bronchiolitis children | Higher costs in IV group | Serious risk | High | 1− |
| Duke et al. (2002) |
PICO 1 100% 0,45%NaCl-G5 IV, vs 60% breast milk |
RCT Multicentre Papua New Guinea |
357 acutely ill children with meningitis | Improved outcomes in IV hydration group | Serious risk | Moderate | 1− |
| Saeidi et al. (2009) |
PICO 1 Breast milk + IV hydration, vs breast milk |
RCT Single Centre Iran |
100 term neonates requiring phototherapy for hyper-bilirubinemia | Bilirubin levels decreased faster in the IV group | Serious risk | Moderate | 1− |
| Wilson et al. (1990) |
PICO 1 IV vs no IV hydration |
RCT Single Centre United Kingdom |
50 children undergoing tonsillectomy | No difference | Serious risk | High | 1− |
| Easa et al. (2013) |
PICO 1 EN feeds with supplemental IV hydration, vs EN feeds or EN hydration |
RCT Single Centre Iraq |
64 term neonates requiring phototherapy for hyper-bilirubinemia | No difference | Serious risk | Moderate | 1− |
| Szabo et al. (2015) |
PICO 1 NPO + low IV hydration, Vs NPO + high IV hydration, Vs PO + low IV hydration, Vs PO + high IV hydration |
Retrospective cohort study Single Centre USA |
201 acutely and critically ill children with pancreatitis | Improved outcomes in the PO + high IV hydration group | Good quality | Moderate | 2 + |
| McNab et al. (2015) |
PICO 2 Isotonic (Plasmalyte-G5%®) vs Hypotonic ((G5%- NaCl 0,45%) |
RCT Single Centre Australia |
641 acutely ill children | Lower risk of hyponatremia in isotonic group | Serious risk | Moderate | 1− |
| Lehtiranta et al. (2021) |
PICO 2 Isotonic (Plasmalyte-G5%®) vs Hypotonic (G5%- NaCl 80 mmol/L) |
RCT Single Centre Finland |
614 acutely ill children | No significant difference in natremia | Serious risk | Moderate | 1− |
| Coulthard et al. (2012) |
PICO 2 and PICO 3 and PICO 5 Hartmann-G5% full maintenance vs 0.45NaCl-G5%, 2/3 of Holliday and Segar formula |
RCT Single Centre Australia |
82 critically ill children after neurosurgery |
smaller postoperative fall in plasma sodium in Hartmann-G5% group No difference in Cl and HCO3 plasma levels No data support the effect of rate/amount due to mixed intervention |
Serious risk | Low | 1− |
| Almeida et al. (2015) |
PICO 2 Isotonic (0.9%NaCl) vs hypotonic (0.45%NaCl) IV-MFT |
RCT Single Centre Portugal |
233 critically ill children | Lower risk of hypernatremia with 0.9% saline than hyponatremia with 0.45% | Serious risk | Moderate | 1− |
| Bagri et al. (2019) |
PICO 2 Isotonic (0.9%NaCl) vs hypotonic (0.45%NaCl) IV-MFT |
RCT Single Centre India |
150 acutely ill children | lower serum osmolarity at 48 h in the hypotonic group | Serious risk | High | 1− |
| Castilla et al. (2019) |
PICO 2 Isotonic (0.9%NaCl) vs hypotonic (0.30%NaCl) IV-MFT |
RCT Single Centre Spain |
130 critically ill children after surgery | Lower risk of hyponatremia in isotonic group | Serious risk | High | 1− |
| Choong et al. (2011) |
PICO 2 Isotonic (0.9%NaCl) vs hypotonic (0.45%NaCl) IV-MFT |
RCT Single Centre Canada |
258 acutely and critically ill children after surgery | Lower risk of hyponatremia in isotonic group | Low risk | High | 1 + + |
| Flores et al. (2016) |
PICO 2 Isotonic (G5%-NaCl 0,9%) vs Hypotonic (G5%- NaCl 0,45%) vs Hypotonic (G5%- NaCl 0,3%) |
RCT Single Centre Mexico |
163 acutely ill children | Lower risk of hyponatremia in isotonic group | Low risk | Moderate | 1 + |
| Friedman et al. (2015) |
PICO 2 Isotonic (G5%-NaCl 0,9%) vs Hypotonic (G5%- NaCl 0,45%) |
RCT Single Centre Canada |
110 acutely ill children with acute respiratory diagnosis | No significant differences | Low risk | High | 1 + |
| Kannan et al. (2010) |
PICO 2 & PICO 5 Isotonic (G5%-NaCl 0.9%) vs Hypotonic (G5%- NaCl 0.18%) vs Hypotonic (G5%- NaCl 0.18%%) lower infusion rate |
RCT Single Centre India |
167 acutely ill children |
Less hyponatremia in isotonic group Less hyponatremia in the restrictive group |
Moderate risk (serious) | High (moderate) |
1 + (1−) |
| Kumar et al. (2020) |
PICO 2 Isotonic (G5%-NaCl 0,9%) vs Hypotonic (G5%- NaCl 0,45%) |
RCT Single Centre India |
168 acutely ill children | No difference | Moderate risk | Moderate | 1− |
| Montanana et al. (2008) |
PICO 2 Isotonic (NaCl 140 mmol/L) vs Hypotonic (20–100 mmol/L Na) |
RCT Single Centre Spain |
122 critically ill children | Increased risk of hyponatremia in hypotonic group | Serious risk | High | 1− |
| Pemde et al. (2015) |
PICO 2 Isotonic (NaCl 0,9%) vs Hypotonic (G5%- NaCl 0,45%) |
RCT Single Centre India |
92 acutely ill children with central nervous system infection | Less Hyponatremia in the isotonic group | Serious risk | Low | 1− |
| Ramanathan et al. (2016) |
PICO 2 Isotonic (NaCl 0,9%) vs Hypotonic (NaCl 0,18%) |
RCT Single Centre India |
119 acutely ill children with pneumonia | Increased risk of hyponatremia in hypotonic group | Serious risk | Moderate | 1− |
| Rey et al. (2011) |
PICO 2 Isotonic (NaCl 156 mmol/L) vs Hypotonic (50–70 mmol/L Na) |
RCT Multicentre Spain |
125 critically ill children | Increased risk of hyponatremia in hypotonic group | Serious risk | Low | 1− |
| Saba et al. (2011) |
PICO 2 Isotonic (G5%-NaCl 0,9%) vs Hypotonic (G5%- NaCl 0,45%) |
RCT Single Centre Canada |
37 acutely ill children requiring IV-MFT | No difference | Serious risk | Moderate | 1− |
| Torres et al. (2019) |
PICO 2 Isotonic (G5%-NaCl 0,9%) vs Hypotonic (G5%- NaCl 0,45%) |
RCT Single Centre Argentina |
294 acutely and critically ill children requiring IV-MFT | Increased risk of hyponatremia in hypotonic group | Serious risk | Low | 1− |
| Jorro Baron et al. (2013) |
PICO 2 Isotonic (NaCl 154 mmol/L) vs Hypotonic (77 mmol/L Na) |
RCT Single Centre Argentina |
63 critically ill children | Higher Na Plasma levels in isotonic group | Serious risk | High | 1− |
| Tuzun et al. (2020) |
PICO 2 Isotonic (G5%-NaCl 0,9%) vs Hypotonic (G5%- NaCl 0,45%) |
RCT Single Centre Turkey |
108 critically ill term neonates | Lower plasma Na Change in isotonic group | Serious risk | Low | 1− |
| Velasco et al. (2018) |
PICO 2 Isotonic (NaCl 154 mmol/L) vs Hypotonic (51–77 mmol/L Na) |
Retrospective cohort Single Centre Spain |
111 critically ill children | Less hyponatremia risk in isotonic group | Good quality | High | 2− |
| Da Silva Vadalao et al. (2015) |
PICO 2 Isotonic (NaCl 0,9%) vs Hypotonic (NaCl 0,18%) |
RCT Single Centre Brazil |
50 acutely ill children after appendicectomy | No difference | Critical risk | Low | 1− |
| Carandang et al. (2013) |
PICO 2 Isotonic (any type) vs Hypotonic (any type) |
Retrospective cohort Single Centre USA |
1048 acutely ill children | Increased risk of hyponatremia in the hypotonic group | Poor quality | Low | 2− |
| Golshekan et al. (2016) |
PICO 2 Isotonic (G5%-NaCl 0,9%) vs Hypotonic (G5%- NaCl 0,45%) |
RCT Single Centre Iran |
75 acutely ill children | Increased risk of hyponatremia in the hypotonic group | Critical risk | Low | 1− |
| Karageorgos et al. (2018) |
PICO 2 Isotonic (NaCl 0,9%) vs Hypotonic (Na 0.45%, 0.675% or 0.225%) |
Retrospective cohort Multicentre USA |
472 acutely ill children | Increased risk of hyponatremia in the hypotonic group | Poor quality | Moderate | 2 + |
| Lima et al. (2019) |
PICO 3 Plasma-Lyte A® vs NaCl0.9% |
RCT Single Centre Brazil |
53 acute and critically ill children after brain tumour surgery | Higher chloremia in the NaCl group, and lower natremia in the balanced group | Serious risk | High | 1− |
| Naseem et al. (2020) |
PICO 3 Ringer Lactate vs NaCl 0.9% |
RCT Single Centre India |
70 acutely ill children with dehydration and gastroenteritis | Faster resolution of metabolic acidosis occurs with Ringer lactate | Moderate risk | Moderate | 1− |
| Mahajan et al. (2012) |
PICO 3 Ringer Lactate vs NaCl 0.9% |
RCT Single Centre India |
22 acutely ill children with dehydration and gastroenteritis | No difference | Serious risk | Low | 1− |
| Kartha et al. (2017) |
PICO 3 Ringer Lactate vs NaCl 0.9% |
RCT Single Centre India |
68 acutely ill children with dehydration and gastroenteritis | Clinical and pH improvement increased in the Ringer lactate group | Low risk | Moderate | 1 + |
| Gutman et al. (1969) |
PICO 3 Ringer Lactate vs Cholera designed replacement solution (45 mmol/L acetate) |
RCT Single Centre Taiwan |
27 acutely ill children with dehydration and gastroenteritis (cholera) | Faster normalisation of HCO3 in the cholera buffer enriched solution | Serious risk | Moderate | 1− |
| Farrell et al. (2020) |
PICO 3 Ringer Lactate vs NaCl 0.9% |
Retrospective cohort Multicentre USA |
1581 acutely ill children with pancreatitis | Shorter length of stay and lower costs in the Ringer lactate group | Fair quality | Moderate | 2− |
| Yung et al. (2017) |
PICO 3 Ringer Lactate vs NaCl 0.9% |
RCT Single Centre Australia |
77 acute and critically ill children with moderate and severe diabetic ketoacidosis | No difference | Low risk | Moderate | 1 + + |
| Williams et al. (2020) |
PICO 3 Plama-Lyte A® vs NaCl 0.9% |
RCT Single Centre India |
66 acutely and critically ill children with moderate and severe diabetic ketoacidosis | No difference | Low risk | Moderate | 1 + + |
| Balamuth et al. (2019) |
PICO 3 Ringer Lactate vs NaCl 0.9% |
RCT Single Centre USA |
50 acutely ill children with septic shock | No difference (pilot study) | Serious risk | Low | 1− |
| Bulfon et al. (2019) |
PICO 3 0.9% NaCl, 0,45% NaCl Vs Ringer lactate |
Retrospective cohort Multicentre Canada |
543 critically ill children | Lower risk of Hyperchloremic metabolic acidosis in the ringer lactate group | Good quality | High | 2 + + |
| Martinez Carapeto et al. (2018) |
PICO 4 G3.3% vs G5% MV-MFT |
RCT Single Centre Spain |
130 critically ill children after surgery | No difference | Serious risk | Moderate | 1− |
| De Betue et al. (2012) |
PICO 4 Glucose infusion: 2.5 mg/kg/min vs 5 mg/kg/min |
RCT Single Centre The Netherlands |
11 critically ill children after cardiac surgery | Lower glycemia in the 2.5 mg group, without hypoglycemia | Low risk | High | 1 + |
| Verbruggen et al. (2011) |
PICO 4 Glucose infusion: 2.5 mg/kg/min vs 5 mg/kg/min |
RCT Single Centre The Netherlands |
8 critically ill children after craniosynostosis surgery | Higher hyperglycemia risk in the 5 mg group | Low risk | High | 1 + |
| Lex et al. (2014) |
PICO 4 G10% vs G5% MV-MFT |
Case control study Single Centre Hungary |
596 critically ill children after cardiac surgery | Hospital length of stay was longer in the 10% group | Fair quality | Moderate | 2− |
| Diaz et al. (2018) |
PICO 5 100% (standard) vs 50% (pre-emptive) of Holliday Segar formula |
Case control study Single Centre Chile |
76 critically ill children with sepsis or ARDS | Fluid overload, ventilation duration, length of stay were significantly lower in pre-emptive group | Poor quality | Moderate | 2− |
| Ingelse et al. (2019) |
PICO 5 85% (standard) vs < 70% (conservative) of Holliday Segar formula |
RCT Single Centre The Netherlands |
23 critically ill children respiratory infection on mechanical ventilation | No difference | Critical Risk | High | 1− |
| Yung et al. (2009) |
PICO 5 100% (standard) vs < 2/3 (conservative) of Holliday Segar formula sub studies based on fluid type: A. Normal saline 0.9% B. 4% Dextrose – 0.18% saline |
RCT Single Centre Australia |
50 critically ill children | No difference | Serious risk | Moderate | 1− |
| Raksha et al. (2017) |
PICO 5 0.18% saline in 5%G at 2/3 standard rate vs 0.9% saline in 5%G at standard IV maintenance rate |
RCT Single Centre India |
240 critically ill children |
Less hyponatremia and shorter length of ICU stay in isotonic group No data support the effect of rate/amount due to mixed intervention |
Serious risk | Low | 1− |
| Neville et al. (2010) |
PICO 5 100% (standard) vs < 50% (conservative) of Holliday Segar formula sub studies based on fluid type: A. 5% dextrose + normal saline 0.9% B. 5% dextrose + half normal saline 0.45% |
RCT Single Centre Australia |
62 acutely ill children after surgery | No difference | Serious risk | Moderate | 1− |
| Singhi et al. (1995) |
PICO 5 Standard rate (100% Holliday Segar formula) vs Restrictive rate (65% of standard, liberalization after 24 h with an increase of 10 ml/kg/8 h) Sub studies based on patient status at inclusion: A. Patients without hyponatremia B. Patients with hyponatremia |
RCT Single Centre India |
50 acutely ill children with meningitis | No difference | Serious risk | High | 1− |
EN enteral nutrition; IV intravenous; IV-MFT intravenous maintenance fluid therapy; NPO nil per oral; PO per os; RCT randomised controlled trial
Furthermore, the data were only suitable to combine in a meta-analysis (Figs. 2–5) for some outcomes in four of the PICO questions (PICO 4 on composition did not allow for any meta-analysis). However, outcomes varied significantly between studies and limited the scope of the meta-analysis. All forest plots are available in Supplement files 3, 4, 5 and 6 with the respective heterogeneity assessment and relative risks.
Fig. 2.
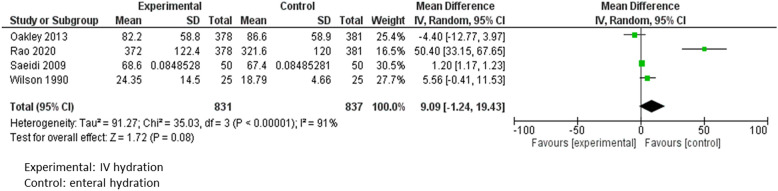
Meta-analysis of studies comparing the impact on length of stay of intravenous versus enteral hydration
Fig. 5.
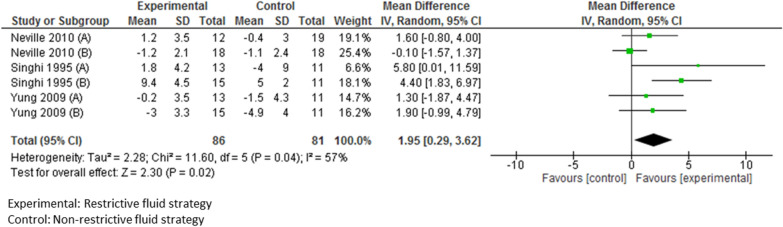
Meta-analysis of studies comparing the impact on natremia of a restrictive versus a non-restrictive fluid strategy
Table 2 provides the list of included studies and their main characteristics; Table 3 summarizes for each PICO the profile of the patients recruited in these studies.
Table 3.
Characteristics of the patients recruited in the included studies
| Acutely ill children | Critically ill children | No distinction possible between acute and critically ill children | |
|---|---|---|---|
| PICO 1 |
11 studies Total: 2268 patients Gastroenteritis n = 737 Bronchiolitis n = 759 Meningitis n = 357 Newborns with hyperbilirubinemia n = 164 Post tonsillectomy n = 50 Pancreatitis n = 201 |
1 study Total: 186 term newborns |
0 |
| PICO 2 |
14 studies Total: 3444 patients Respiratory failure n = 229 Surgery n = 229 Central nervous system infection n = 92 Various diagnosis n = 2894 |
10 studies Total: 1692 patients Surgery n = 207 Neurosurgery n = 82 Term neonates n = 108 Various diagnosis n = 1295 |
1 study Total: 294 patients Various diagnosis |
| PICO 3 |
7 studies Total: 1849 patients Gastroenteritis n = 187 Pancreatitis n = 1581 Sepsis n = 50 DKA n = 31 |
3 studies Total: 660 patients DKA n = 35 Neurosurgery n = 82 Various diagnosis n = 543 |
2 studies Total: 130 patients Neurosurgery n = 53 DKA n = 77 |
| PICO 4 | 0 |
4 studies Total: 745 patients Post-surgery n = 130 Post cardiac surgery n = 607 Post craniosynostosis n = 8 |
0 |
| PICO 5 |
3 studies Total: 279 patients Meningitis n = 50 Post-surgery n = 62 Various diagnosis n = 167 |
5 studies Total: 471 patients Neurosurgery n = 82 Respiratory failure n = 23 Various diagnosis n = 366 |
0 |
DKA diabetic keto acidosis
For PICO 1 on indications for IV-MFT, a meta-analysis included 1668 children. It showed no significant difference in length of stay between enteral and parenteral hydration (mean difference = 9.09, 95% CI [−1.24–19.43], p = 0.08) (Fig. 2) but a trend towards a reduction in length of hospital stay in the enteral group [23, 24, 27, 28].
For PICO 2 on isotonic IV-MFT solutions, a meta-analysis of 17 RCTs (involving 3356 patients) assessing the risk of developing hyponatremia showed that isotonic solutions significantly reduced this risk compared with hypotonic fluids, with an odds ratio of 0.41 (95% CI [0.26–0.67], p = 0.0003), although the heterogeneity was high between studies [31–34, 36–40, 42, 45, 46] (Fig. 3).
Fig. 3.
Meta-analysis of studies comparing the impact on hyponatremia occurrence of isotonic versus hypotonic solutions
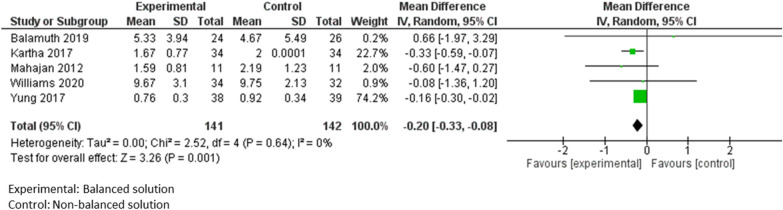
In PICO 3 on balanced IV-MFT solutions, the length of acute care or PICU stay were slightly but significantly decreased in children receiving balanced solutions in a meta-analysis of 5 studies, including 283 patients (mean difference: −0.20 days; 95% CI [−0.33; −0.08], p = 0.001 [57, 58, 61–63] (Fig. 4).
Fig. 4.
Meta-analysis of studies comparing the impact on acute or critical care stay of balanced versus non-balanced solutions
For PICO 4, the data did not allow the conduct of any meta-analysis.
In PICO 5 on the amount of IV-MFT, a restrictive strategy was significantly associated with a lower change in plasma sodium (mean difference = 1.95; 95% CI [0.29; 3.62], p = 0.02) after 12 h or more of treatment, in a meta-analysis pooling 167 patients in six subgroups in three RCTs, including patients in the PICU [71, 73, 74] (Fig. 5).
Figure 6 provides a short answer to each PiCO question.
Fig. 6.
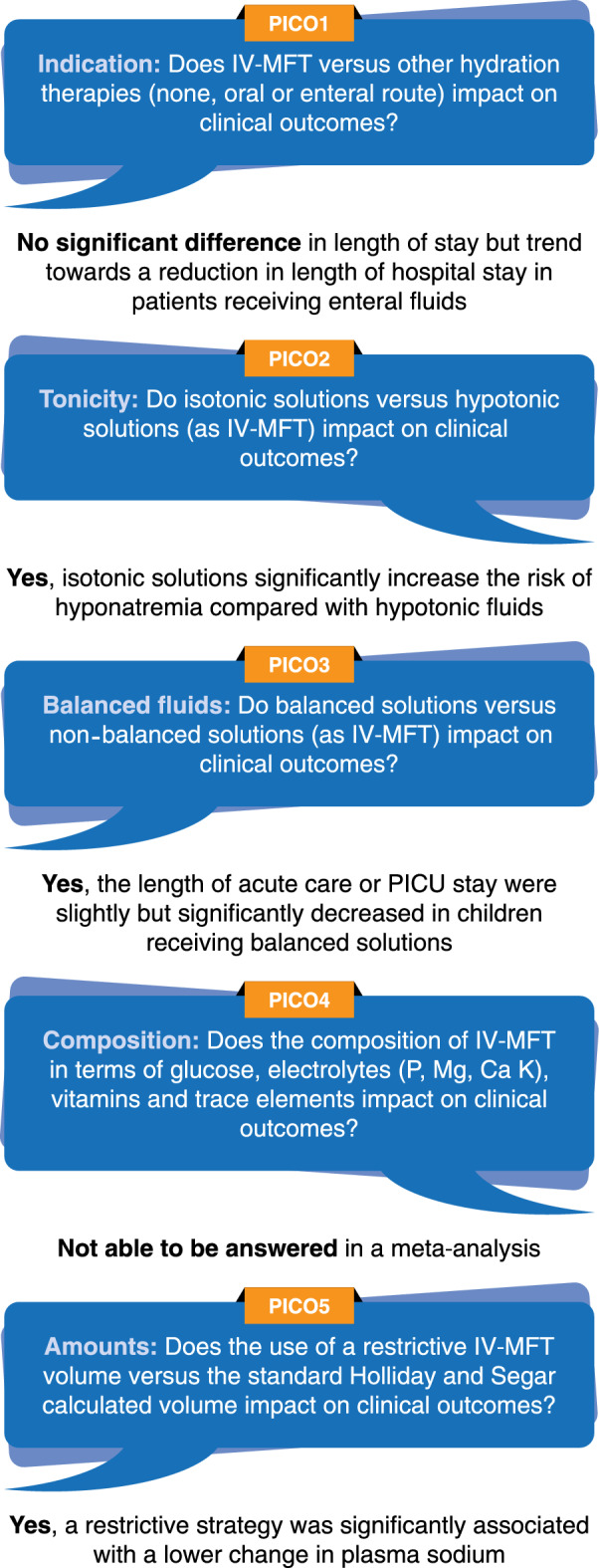
Short answers to PICO questions
The grading of the 16 recommendations was heterogenous (ranging from good clinical practice and expert opinion to Grade A), according to the SIGN grading [11] and the quality of the meta-analysis (Table 1). After the 2-round voting process, strong consensus (> 95% agreement) was reached for 11/16 (69%) and consensus (90% < agreement < 95%) for 5/16 (31%) of the recommendations.
A long rationale supporting each recommendation is provided for each PICO question in supplemental files 7–11. This details the underlying background and pathophysiology of the PICO question, summarizes current European practice [2], discusses available recommendations in adults or other pediatric settings, provides further details of our literature search, data extraction and analysis of the data, analyzes the volume of evidence, its applicability, generalizability, consistency and clinical impact of the recommendations and finally provides the recommendation.
Discussion
Summary of findings
These ESPNIC evidence-based recommendations provide guidance for clinicians using IV-MFT in children both in acute hospital and intensive care settings. They are based on a comprehensive literature search, including literature up to November 2020.
We were able to propose 16 recommendations corresponding to our questions with a high level of consensus, and to provide an extensive rationale to support each of them, available in supplemental material. Unfortunately, the general level of evidence remains low, both in terms of quantity and quality. Few well conducted and low risk of bias RCTs were available. Furthermore, the study populations, settings and interventions were heterogeneous and, in many cases, made it difficult to pool and to compare results. Thus, even with a strong consensus, the majority of the recommendations 12/16 (75%) might be considered as weak (C or under) as they are based on a low level of evidence.
Consistency with other guidelines and recent publications
Our recommendations are consistent with the 2018 American Academic of Pediatrics clinical practice IV-MFT guidelines regarding the use of isotonic fluids [3]. Indeed, the American Academic of Pediatrics guidelines strongly recommended the use of isotonic fluids in children between 28 days to 18 years of age in acute or intensive care settings. However, our guidelines extend beyond the American guidelines as they also make recommendations on the indications for IV-MFT, on the composition of IV-MFT, and on the amount of IV-MFT that should be prescribed. In 2021, Leung and colleagues [75] published a consensus statement on IV-MFT in hospitalized children, based on a review of the literature (2000–2019). Their recommendations are similar to ours regarding prioritization of the enteral route, the use of isotonic fluids, the reduced amount of fluids administered and the type of monitoring. With the exception of isotonic fluids (strong recommendation with high quality of evidence), recommendations were all consensus based on low-quality evidence and expert opinion. The use of balanced fluids was not addressed.
Our study protocol included literature until November 2020 only, we used the same search equations and inclusion criteria to extract recent IV-MFT studies (December 2020–June 2022) to ensure the consistency of their findings with our guidelines. Twenty-one new studies (and 3 ongoing trials) were identified and are summarized in supplemental digital content 12. Their findings are globally consistent with our recommendations, especially regarding the use of isotonic fluids, reduced infusion volumes and the use of the enteral route when possible. The impact of isotonic fluids has now been studied in specific sub-groups of children such as newborns and patients with diabetic keto acidosis. Balanced solutions are currently being studied, and we have identified three ongoing RCTs comparing them to non-balanced solutions which may help revising our guidelines or adapting their level of evidence in the future.
Implications for practice
The reporting of key outcomes was inconsistent within studies, which prevented us from conducting other meta-analyses. For example, the impact of isotonic and/or balanced solutions on Na and Cl plasma levels was presented as absolute values in some papers, or changes over time, or absolute difference in others. Improving the standardization of outcome reporting in future trials is essential to allow pooling of the data. In the future, our expert group aims to conduct an international Delphi study to gain expert consensus and develop a core outcome set to be reported in future studies on IV-MFT in children. Despite this, these guidelines highlight several important points that must be considered in daily practice when prescribing IV-MFT for children. First, the intravenous route for hydration is not required in every clinical situation. Second, isotonic IV-MFT should be preferred over hypo or hypertonic solutions. Third, balanced fluids should be the standard IV-MFT solution used in children. Fourth, the IV fluid composition is important, and glucose and plasma electrolyte monitoring is essential. Finally, the harm of excessive fluids and volume overload is highlighted, and the daily calculation of fluid balance is essential for any child receiving IV-MFT.
Future implementation challenges
As per its definition, IV-MFT needs to be differentiated from IV replacement therapy, which aims to compensate for abnormal losses (e.g. skin losses in case of major wounds or burns, intestinal losses in case of severe enteropathy, losses through drains, etc.). However, IV-MFT and replacement fluids may be administered through one single IV fluid prescription which then needs to combine both IV-MFT recommendations (in terms of amounts and composition) and adapt to the rate of loss and composition of fluid losses. The implementation of these recommendations into clinical practice may be challenged by the lack of availability of ready-to-use solutions adapted for children in some European countries. A recent survey by Morice et al. [2] reported some centers having to reconstitute isotonic glucose IV-MFT solutions within clinical care units. The use of ready-to-use IV-MFT is beneficial to avoid reconstitution errors, physico-chemical stability issues and microbiological contaminations. Furthermore, products designed for adults do not usually provide glucose and, therefore, do not meet the requirements of younger children. Perioperative maintenance fluids designed as per Sümpelmann et al. recommendations [10] (Glucose 1–2.5%) may not provide sufficient amounts of glucose if used outside the perioperative setting. Isotonic balanced solutions, providing some glucose (4 to 10%) and limited amounts of potassium (+/- 4 mmol/L), would meet most children’s requirements in terms of IV-MFT. Solutions such as these can be found in certain countries in various amounts (250, 500 and 1000 mL bags) and have an osmolarity compatible with peripheral infusion. Nevertheless, such solutions are inconsistently available through Europe. Availability of ready-to-use IV-MFT solutions that are tailored to childrens needs would help facilitate the implementation of these new ESPNIC recommendations.
Limitations
The main limitation of these evidence-based recommendations is the general paucity of evidence and the low level of evidence around some of the questions. We have included all studies published until 2021 due to the paucity of studies available, but most (80.0%) were published in the last decade, which reduces the bias from studies published within a large time period. Our definition of acute and critical illness may slightly differ from the one used in some studies as PICU admission policies vary within institutions. For most of the recommendations, apart from PICO 1, sparse evidence prevented us from translating this to specific pediatric populations or specific clinical situations; sub populations should be further studied in future trials to secure extrapolation of these guidelines to specific groups of patients and for different age ranges. Apart from the trials conducted on IV-MFT during phototherapy, term neonates were rarely analyzed as a specific group or a subgroup in most of the studies, and extrapolation of the results to this specific population should be considered with caution. Cardiac patients were included in some of the study populations and recommendations for PICO 2 and 5 are likely to apply to this specific population. Some studies assessed both IV-MFT and replacement or bolus fluid therapy, as these treatments were administered simultaneously or consequently over the study period. Consequently, this may have introduced a bias in the interpretation of the results, even if the results were consistent with other studies focusing on IV-MFT. However, a consistent fluid strategy considering resuscitation, replacement and maintenance fluid therapy seems reasonable. Furthermore, due to inconsistencies among the interventions (fluids and volumes used) and outcomes, meta-analyses were not possible to conduct for many questions and heterogeneity between studies may have resulted in some bias. Finally, in these evidence-based recommendations, the PICO questions and consensus voting only reflects the view of our ESPNIC expert group. Service users (acute and critically ill children) and their parents were not involved in the design of the project. Despite these limitations, this is the most up to date and extensive review of IV-MFT in children both in acute and critical care settings.
Conclusions
These evidence-based recommendations provide a ‘best-available-evidence’ guide for both pediatric and intensive care clinicians around the prescription of IV-MFT. Due to a paucity of robust evidence, the evidence level for most of the recommendations is low, even when strong consensus was reached among the expert group. Thus, many recommendations are based on expert opinion. This review clearly identifies the urgent need and gaps for future research in this field. Each of our PICO questions deserves further robust research, and new RCTs should be conducted specifically to clarify the impact of the use of isotonic solutions or balanced solutions in various age groups (term neonates, infants and older children) and a variety of clinical conditions. The lack of evidence regarding optimal electrolyte compositions (K, P, Mg, Ca) of IV-MFT is striking and so is the need for micronutrient additions in IVMFT. Consistent reporting of relevant outcome is mandatory to enable future metanalysis. ESPNIC intends to update these guidelines every 5 years, following the same methodology. In the future, our expert group also aims to produce tools to help implement these guidelines into clinical practice and promote auditing and monitoring of their implementation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Authors would like to gratefully thank Mrs Florence Bouriot (Documentation centrale—Hospices Civils de Lyon—France), who helped as an academic librarian to build the search equations, to search the databases, to exclude the duplicates and to get the full papers.
Author contributions
FV, DB and LT initiated and led the project. All authors contributed substantially to the conception and design of the study. CJC helped as a methodologist to design the study and present the results. ARB and J-JP performed the meta-analyses. FV, DB, LT, SV, CJC, CM, LM and SR led the PICO groups. All authors contributed to the acquisition of data, or the analysis and interpretation of the data; DB, FV and LT drafted the manuscript. All authors provided critical revision of the article and provided final approval of the version submitted for publication.
Funding
No funding was received for the conduct of the review.
Declarations
Conflicts of interest
LRB and FVV declare consultant fees received from Baxter. IG received consultant fees from BBraun medical. Other authors declare no conflicting interest.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Footnotes
The original online version of this article was revised: In this article Fig. 3 has been updated.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/25/2022
A Correction to this paper has been published: 10.1007/s00134-022-06933-5
Change history
7/24/2023
A Correction to this paper has been published: 10.1007/s00134-023-07119-3
Contributor Information
David W. Brossier, Email: brossier-d@chu-caen.fr
Lyvonne N. Tume, Email: Lyvonne.Tume@edgehill.ac.uk
Anais R. Briant, Email: briant-a@chu-caen.fr
Corinne Jotterand Chaparro, Email: corinne.jotterand@hesge.ch.
Clémence Moullet, Email: clemence.moullet@hesge.ch.
Shancy Rooze, Email: shancy.rooze@huderf.be.
Sascha C. A. T. Verbruggen, Email: s.verbruggen@erasmusmc.nl
Luise V. Marino, Email: luise.marino@uhs.nhs.uk
Fahad Alsohime, Email: fahad.alsohime@gmail.com.
Sophie Beldjilali, Email: beldjilali.sophie@gmail.com.
Fabrizio Chiusolo, Email: fabrizio.chiusolo@opbg.net.
Leonardo Costa, Email: leonardo.costa@aosp.bo.it.
Capucine Didier, Email: capucined@gmail.com.
Stavroula Ilia, Email: stavroula.ilia@uoc.gr.
Nyandat L. Joram, Email: jnyandat@gmail.com
Martin C. J. Kneyber, Email: m.c.j.kneyber@umcg.nl
Eva Kühlwein, Email: Eva.Kuehlwein@kispi.uzh.ch.
Jorge Lopez, Email: jlopezgonz82@gmail.com.
Jesus López-Herce, Email: pielvi@hotmail.com.
Huw F. Mayberry, Email: huw.mayberry@alderhey.nhs.uk
Fortesa Mehmeti, Email: fortesa.Mehmeti@hcuge.ch.
Magdalena Mierzewska-Schmidt, Email: mcdosia@gmail.com.
Maria Miñambres Rodríguez, Email: mariamiro@gmail.com.
Claire Morice, Email: Claire.Morice@hcuge.ch.
John V. Pappachan, Email: jvp@soton.ac.uk
Florence Porcheret, Email: florence.porcheret@chu-nantes.fr.
Leonor Reis Boto, Email: boto.leonor@gmail.com.
Luregn J. Schlapbach, Email: Luregn.Schlapbach@kispi.uzh.ch
Hakan Tekguc, Email: tekguchakan@gmail.com.
Konstantinos Tziouvas, Email: ktziouvas@yahoo.com.
Jean-Jacques Parienti, Email: Parienti-jj@chu-caen.fr.
Isabelle Goyer, Email: goyer-i@chu-caen.fr.
Frederic V. Valla, Email: frederic.valla@chu-lyon.fr
References
- 1.Holliday MA, Ray PE, Friedman AL. Fluid therapy for children: facts, fashions and questions. Arch Child. 2007;92:546–550. doi: 10.1136/adc.2006.106377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morice C, Alsohime F, Mayberry H, et al. Intravenous maintenance fluid therapy practice in the pediatric acute and critical care settings: a European and Middle Eastern survey. Eur J Pediatr. 2022 doi: 10.1007/s00431-022-04467-y. [DOI] [PubMed] [Google Scholar]
- 3.Feld LG, Neuspiel DR, Foster BA, et al. Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics. 2018;142:e20183083. doi: 10.1542/peds.2018-3083. [DOI] [PubMed] [Google Scholar]
- 4.Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19:823–832. [PubMed] [Google Scholar]
- 5.McNab S, Ware RS, Neville KA, et al (2014) Isotonic versus hypotonic solutions for maintenance intravenous fluid administration in children. Cochrane Database Syst Rev CD009457 [DOI] [PMC free article] [PubMed]
- 6.Wang J, Xu E, Xiao Y. Isotonic versus hypotonic maintenance IV fluids in hospitalized children: a meta-analysis. Pediatrics. 2014;133:105–113. doi: 10.1542/peds.2013-2041. [DOI] [PubMed] [Google Scholar]
- 7.Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely Ill patients. N Engl J Med. 2015;373:1350–1360. doi: 10.1056/NEJMra1412877. [DOI] [PubMed] [Google Scholar]
- 8.Bhave G, Neilson EG. body fluid dynamics: back to the future. J Am Soc Nephrol. 2011;22:2166–2181. doi: 10.1681/ASN.2011080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NICE Recommendations (2020) Intravenous fluid therapy in children and young people in hospital Guidance | NICE. Available at https://www.nice.org.uk/guidance/ng29/chapter/Recommendations. Accessed 4 June 2021
- 10.Sümpelmann R, Becke K, Crean P, et al. European consensus statement for intraoperative fluid therapy in children. Eur J Anaesthesiol. 2011;28:637–639. doi: 10.1097/EJA.0b013e3283446bb8. [DOI] [PubMed] [Google Scholar]
- 11.Scottish Intercollegiate Guidelines Network, Harbour RT, Forsyth L (2008) SIGN 50: a guideline developer’s handbook. Scottish Intercollegiate Guidelines Network, Ediburgh, Scotland
- 12.SIGN 50: a guideline developer. In: SIGN. https://www.sign.ac.uk/assets/sign50_2019.pdf. Accessed 29 Jun 2022
- 13.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brouwers MC, Kerkvliet K, Spithoff K, AGREE Next Steps Consortium (2016) The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. BMJ i1152. 10.1136/bmj.i1152 [DOI] [PMC free article] [PubMed]
- 15.Booth A, Clarke M, Ghersi D, et al. An international registry of systematic-review protocols. Lancet. 2011;377:108–109. doi: 10.1016/S0140-6736(10)60903-8. [DOI] [PubMed] [Google Scholar]
- 16.Cochrane Handbook for Systematic Reviews of Interventions. http://handbook-5-1.cochrane.org/. Accessed 25 Jan 2021
- 17.Wells G, Shea B, O’Connell D et al The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 20 May 2022
- 18.Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32:1008–1015. doi: 10.1046/j.1365-2648.2000.t01-1-01567.x. [DOI] [PubMed] [Google Scholar]
- 19.Mackenzie A, Barnes G. Randomised controlled trial comparing oral and intravenous rehydration therapy in children with diarrhoea. BMJ. 1991;303:393–396. doi: 10.1136/bmj.303.6799.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nager AL, Wang VJ. Comparison of nasogastric and intravenous methods of rehydration in pediatric patients with acute dehydration. Pediatrics. 2002;109:566–572. doi: 10.1542/peds.109.4.566. [DOI] [PubMed] [Google Scholar]
- 21.Sharifi J, Ghavami F, Nowrouzi Z, et al. Oral versus intravenous rehydration therapy in severe gastroenteritis. Arch Dis Child. 1985;60:856–860. doi: 10.1136/adc.60.9.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spandorfer P, Alessandrini E, Joffe M, et al. Oral versus intravenous rehydration of moderately dehydrated children: a randomized, controlled trial. Pediatrics. 2005;115:295–301. doi: 10.1542/peds.2004-0245. [DOI] [PubMed] [Google Scholar]
- 23.Rao YK, Saxena R, Midha T, et al. Clinical outcome of enteral nutrition versus IV fluids in newborns on inotropes: a randomized study. J Clin Neonatol. 2020;9:261–265. [Google Scholar]
- 24.Oakley E, Borland M, Neutze J, et al. Nasogastric hydration versus intravenous hydration for infants with bronchiolitis: a randomised trial. Lancet Respir Med. 2013;1:113–120. doi: 10.1016/S2213-2600(12)70053-X. [DOI] [PubMed] [Google Scholar]
- 25.Oakley E, Carter R, Murphy B, et al. Economic evaluation of nasogastric versus intravenous hydration in infants with bronchiolitis: economic evaluation of hydration methods in bronchiolitis. Emerg Med Australas. 2017;29:324–329. doi: 10.1111/1742-6723.12713. [DOI] [PubMed] [Google Scholar]
- 26.Duke T, Mokela D, Frank D, et al. Management of meningitis in children with oral fluid restriction or intravenous fluid at maintenance volumes: a randomised trial. Ann Trop Paediatr. 2002;22:145–157. doi: 10.1179/027249302125000878. [DOI] [PubMed] [Google Scholar]
- 27.Saeidi R, Heydarian F, Fakehi V. Role of intravenous extra fluid therapy in icteric neonates receiving phototherapy. Saudi Med J. 2009;30:1176–1179. [PubMed] [Google Scholar]
- 28.Wilson PS, Snow DG, O’Connel J, et al. Role of routine fluid replacement in children undergoing tonsillectomy. J Laryngol Otol. 1990;104:801–802. doi: 10.1017/s0022215100113933. [DOI] [PubMed] [Google Scholar]
- 29.Easa Z. Effect of intravenous fluid supplementation on serum bilirubin level during conventional phototherapy of term infants with severe hyperbilirubinemia. Al-Qadisiah Med J. 2013;9:36–45. [Google Scholar]
- 30.Szabo FK, Fei L, Cruz LA, Abu-el-Haija M. Early enteral nutrition and aggressive fluid resuscitation are associated with improved clinical outcomes in acute pancreatitis. J Pediatr. 2015;167:397–402.e1. doi: 10.1016/j.jpeds.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 31.McNab S, Duke T, South M, et al. 140 mmol/L of sodium versus 77 mmol/L of sodium in maintenance intravenous fluid therapy for children in hospital (PIMS): a randomised controlled double-blind trial. Lancet Lond Engl. 2015;385:1190–1197. doi: 10.1016/S0140-6736(14)61459-8. [DOI] [PubMed] [Google Scholar]
- 32.Lehtiranta S, Honkila M, Kallio M, et al. Risk of electrolyte disorders in acutely Ill children receiving commercially available plasmalike isotonic fluids: a randomized clinical trial. JAMA Pediatr. 2021;175:28–35. doi: 10.1001/jamapediatrics.2020.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coulthard MG, Long DA, Ullman AJ, Ware RS. A randomised controlled trial of Hartmann’s solution versus half normal saline in postoperative paediatric spinal instrumentation and craniotomy patients. Arch Dis Child. 2012;97:491–496. doi: 10.1136/archdischild-2011-300221. [DOI] [PubMed] [Google Scholar]
- 34.Almeida HI, Mascarenhas MI, Loureiro HC, et al. The effect of NaCl 0.9% and NaCl 0.45% on sodium, chloride, and acid-base balance in a PICU population. J Pediatr (Rio J) 2015;91:499–505. doi: 10.1016/j.jped.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Bagri NK, Saurabh VK, Basu S, Kumar A. Isotonic versus hypotonic intravenous maintenance fluids in children: a randomized controlled trial. Indian J Pediatr. 2019;86:1011–1016. doi: 10.1007/s12098-019-03011-5. [DOI] [PubMed] [Google Scholar]
- 36.Castilla J, Carapeto I, Gutierrez R, et al. Efficacy and safety of isotonic saline serum as a maintenance therapy serum after general surgery in pediatrics patients. Acta Pediatr Esp. 2019;77:181–187. [Google Scholar]
- 37.Choong K, Arora S, Cheng J, et al. Hypotonic versus isotonic maintenance fluids after surgery for children: a randomized controlled trial. Pediatrics. 2011;128:857–866. doi: 10.1542/peds.2011-0415. [DOI] [PubMed] [Google Scholar]
- 38.Flores Robles CM, Cuello García CA. A prospective trial comparing isotonic with hypotonic maintenance fluids for prevention of hospital-acquired hyponatraemia. Paediatr Int Child Health. 2016;36:168–174. doi: 10.1179/2046905515Y.0000000047. [DOI] [PubMed] [Google Scholar]
- 39.Friedman JN, Beck CE, DeGroot J, et al. Comparison of isotonic and hypotonic intravenous maintenance fluids: a randomized clinical trial. JAMA Pediatr. 2015;169:445–451. doi: 10.1001/jamapediatrics.2014.3809. [DOI] [PubMed] [Google Scholar]
- 40.Kannan L, Lodha R, Vivekanandhan S, et al. Intravenous fluid regimen and hyponatraemia among children: a randomized controlled trial. Pediatr Nephrol. 2010;25:2303–2309. doi: 10.1007/s00467-010-1600-4. [DOI] [PubMed] [Google Scholar]
- 41.Kumar M, Mitra K, Jain R. Isotonic versus hypotonic saline as maintenance intravenous fluid therapy in children under 5 years of age admitted to general paediatric wards: a randomised controlled trial. Paediatr Int Child Health. 2020;40:44–49. doi: 10.1080/20469047.2019.1619059. [DOI] [PubMed] [Google Scholar]
- 42.Montañana PA, Modesto i Alapont V, Ocón AP,, et al. The use of isotonic fluid as maintenance therapy prevents iatrogenic hyponatremia in pediatrics: a randomized, controlled open study. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2008;9:589–597. doi: 10.1097/PCC.0b013e31818d3192. [DOI] [PubMed] [Google Scholar]
- 43.Pemde HK, Dutta AK, Sodani R, Mishra K. Isotonic intravenous maintenance fluid reduces hospital acquired hyponatremia in young children with central nervous system infections. Indian J Pediatr. 2015;82:13–18. doi: 10.1007/s12098-014-1436-1. [DOI] [PubMed] [Google Scholar]
- 44.Ramanathan S, Kumar P, Mishra K, Dutta AK. Isotonic versus hypotonic parenteral maintenance fluids in very severe pneumonia. Indian J Pediatr. 2016;83:27–32. doi: 10.1007/s12098-015-1791-6. [DOI] [PubMed] [Google Scholar]
- 45.Rey C, Los-Arcos M, Hernández A, et al. (2011) Hypotonic versus isotonic maintenance fluids in critically ill children: a multicenter prospective randomized study. Acta Paediatr Oslo Nor. 1992;100:1138–1143. doi: 10.1111/j.1651-2227.2011.02209.x. [DOI] [PubMed] [Google Scholar]
- 46.Saba TG, Fairbairn J, Houghton F, et al. A randomized controlled trial of isotonic versus hypotonic maintenance intravenous fluids in hospitalized children. BMC Pediatr. 2011;11:82. doi: 10.1186/1471-2431-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torres SF, Iolster T, Schnitzler EJ, et al. Hypotonic and isotonic intravenous maintenance fluids in hospitalised paediatric patients: a randomised controlled trial. BMJ Paediatr Open. 2019;3:e000385. doi: 10.1136/bmjpo-2018-000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jorro Barón FA, Meregalli CN, Rombolá VA, et al. Hypotonic versus isotonic maintenance fluids in critically ill pediatric patients: a randomized controlled trial. Arch Argent Pediatr. 2013;111:281–287. doi: 10.5546/aap.2013.eng.281. [DOI] [PubMed] [Google Scholar]
- 49.Tuzun F, Akcura Y, Duman N, Ozkan H. Comparison of isotonic and hypotonic intravenous fluids in term newborns: is it time to quit hypotonic fluids. J Matern Fetal Neonatal Med. 2020;35:356–361. doi: 10.1080/14767058.2020.1718094. [DOI] [PubMed] [Google Scholar]
- 50.Velasco P, Alcaraz Romero AJ, Oikonomopoulou N, et al. Hospital-acquired hyponatremia: does the type of fluid therapy affect children admitted to intensive care? [Hiponatremia adquirida en el hospital: ¿Influye el tipo de fluidoterapia en los niños ingresados a cuidados intensivos?] Rev Chil Pediatr. 2018;89:42–49. doi: 10.4067/S0370-41062018000100042. [DOI] [PubMed] [Google Scholar]
- 51.Da Silva Valadão MC, Piva JP, Santana JCB, Garcia PCR. Comparison of two maintenance electrolyte solutions in children in the postoperative appendectomy period: a randomized, controlled trial. J Pediatr (Rio J) 2015;91:428–434. doi: 10.1016/j.jped.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Carandang F, Anglemyer A, Longhurst CA, et al. Association between maintenance fluid tonicity and hospital-acquired hyponatremia. J Pediatr. 2013;163:1646–1651. doi: 10.1016/j.jpeds.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Golshekan K, Badeli H, Miri M, et al. Suitable intravenous fluid for preventing dysnatremia in children with gastroenteritis; a randomized clinical trial. J Ren Inj Prev. 2016;5:69–73. doi: 10.15171/jrip.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karageorgos S, Kratimenos P, Landicho A, et al. Hospital-acquired hyponatremia in children following hypotonic versus isotonic intravenous fluids infusion. Children. 2018;5:139. doi: 10.3390/children5100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lima MF, Neville IS, Cavalheiro S, et al. Balanced crystalloids versus saline for perioperative intravenous fluid administration in children undergoing neurosurgery: a randomized clinical trial. J Neurosurg Anesthesiol. 2019;31:30–35. doi: 10.1097/ANA.0000000000000515. [DOI] [PubMed] [Google Scholar]
- 56.Naseem M, Dubey AP, Mishra TK, Singh R. Effect of rehydration with normal saline versus ringer lactate on serum sodium level of children with acute diarrhea and severe dehydration: a randomized controlled trial. Indian Pediatr. 2020;57:519–522. [PubMed] [Google Scholar]
- 57.Mahajan V, Sajan SS, Sharma A, Kaur J. Ringers lactate vs Normal saline for children with acute diarrhea and severe dehydration—a double blind randomized controlled trial. Indian Pediatr. 2012;49:963–968. doi: 10.1007/s13312-012-0251-x. [DOI] [PubMed] [Google Scholar]
- 58.Kartha GB, Rameshkumar R, Mahadevan S. Randomized double-blind trial of ringer lactate versus normal saline in pediatric acute severe diarrheal dehydration. J Pediatr Gastroenterol Nutr. 2017;65:621–626. doi: 10.1097/MPG.0000000000001609. [DOI] [PubMed] [Google Scholar]
- 59.Gutman RA, Drutz DJ, Whalen GEJ, Watten RH. Double blind fluid therapy evaluation in pediatric cholera. Pediatrics. 1969;44:922–931. [PubMed] [Google Scholar]
- 60.Farrell PR, Farrell LM, Hornung L, Abu-El-Haija M. Use of lactated ringers solution compared with normal saline is associated with shorter length of stay in pediatric acute pancreatitis. Pancreas. 2020;49:375–380. doi: 10.1097/MPA.0000000000001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yung M, Letton G, Keeley S. Controlled trial of Hartmann’s solution versus 0.9% saline for diabetic ketoacidosis. J Paediatr Child Health. 2017;53:12–17. doi: 10.1111/jpc.13436. [DOI] [PubMed] [Google Scholar]
- 62.Williams V, Jayashree M, Nallasamy K, et al. 0.9% saline versus plasma-lyte as initial fluid in children with diabetic ketoacidosis (SPinK trial): a double-blind randomized controlled trial. Crit Care Lond Engl. 2020 doi: 10.1186/s13054-019-2683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balamuth F, Kittick M, McBride P, et al. Pragmatic Pediatric Trial of balanced versus normal saline fluid in sepsis: the PRoMPT BOLUS randomized controlled trial pilot feasibility study. Acad Emerg Med Off J Soc Acad Emerg Med. 2019;26:1346–1356. doi: 10.1111/acem.13815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bulfon AF, Alomani HL, Anton N, et al. Intravenous fluid prescription practices in critically Ill children: a shift in focus from natremia to chloremia? J Pediatr Intensive Care. 2019;8:218–225. doi: 10.1055/s-0039-1692413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martínez Carapeto I, López Castilla JD. Fresneda Gutiérrez R (2018) [A comparison of post-surgical plasma glucose levels in patients on fluids with different glucose concentrations] An Pediatr Barc Spain. 2003;89:98–103. doi: 10.1016/j.anpedi.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 66.de Betue CTI, Verbruggen SCAT, Schierbeek H, et al. Does a reduced glucose intake prevent hyperglycemia in children early after cardiac surgery? a randomized controlled crossover study. Crit Care Lond Engl. 2012;16:R176. doi: 10.1186/cc11658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verbruggen SCAT, de Betue CTI, Schierbeek H, et al. Reducing glucose infusion safely prevents hyperglycemia in post-surgical children. Clin Nutr Edinb Scotl. 2011;30:786–792. doi: 10.1016/j.clnu.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 68.Lex D, Szántó P, Breuer T, et al. Impact of the insulin and glucose content of the postoperative fluid on the outcome after pediatric cardiac surgery. Interv Med Appl Sci. 2014;6:160–169. doi: 10.1556/IMAS.6.2014.4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Díaz F, Nuñez MJ, Pino P, et al. Implementation of preemptive fluid strategy as a bundle to prevent fluid overload in children with acute respiratory distress syndrome and sepsis. BMC Pediatr. 2018 doi: 10.1186/s12887-018-1188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ingelse SA, Geukers VG, Dijsselhof ME, et al. Less is more?—a feasibility study of fluid strategy in critically ill children with acute respiratory tract infection. Front Pediatr. 2019;7:496. doi: 10.3389/fped.2019.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yung M, Keeley S. Randomised controlled trial of intravenous maintenance fluids. J Paediatr Child Health. 2009;45:9–14. doi: 10.1111/j.1440-1754.2007.01254.x. [DOI] [PubMed] [Google Scholar]
- 72.Raksha SK, Dakshayani B, Premalatha R. Full volume isotonic (0.9%) vs. two-thirds volume hypotonic (0.18%) intravenous maintenance fluids in preventing hyponatremia in children admitted to pediatric intensive care unit—a randomized controlled study. J Trop Pediatr. 2017;63:454–460. doi: 10.1093/tropej/fmx012. [DOI] [PubMed] [Google Scholar]
- 73.Neville KA, Sandeman DJ, Rubinstein A, et al. Prevention of hyponatremia during maintenance intravenous fluid administration: a prospective randomized study of fluid type versus fluid rate. J Pediatr. 2010;156(313–319):e1–2. doi: 10.1016/j.jpeds.2009.07.059. [DOI] [PubMed] [Google Scholar]
- 74.Singhi SC, Singhi PD, Srinivas B, et al. Fluid restriction does not improve the outcome of acute meningitis. Pediatr Infect Dis J. 1995;14:495–503. doi: 10.1097/00006454-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 75.Leung LC, So L, Ng Y et al (2021) Initial intravenous fluid prescription in general paediatric in-patients aged >28 days and <18 years: consensus statements. Hong Kong Med J 27:276–286. 10.12809/hkmj209010 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.