Abstract
BACKGROUND
Epilepsy-associated psychoses are poorly understood, and management is focused on treating epilepsy. Chronic, interictal psychosis that persists despite seizure control is typically treated with antipsychotics. Whether resection of a mesial temporal lobe lesion may improve interictal psychotic symptoms that persist despite seizure control remains unknown.
OBSERVATIONS
In a 52-year-old man with well-controlled epilepsy and persistent comorbid psychosis, brain magnetic resonance imaging (MRI) revealed an infiltrative, intraaxial, T2 fluid-attenuated inversion recovery intense mass of the left amygdala. The patient received an amygdalectomy for oncological diagnosis and surgical treatment of a presumed low-grade glioma. Pathology was ganglioglioma, World Health Organization grade I. Postoperatively, the patient reported immediate resolution of auditory hallucinations. Patient has remained seizure-free on 2 antiepileptic drugs and no antipsychotic pharmacotherapy and reported lasting improvement in his psychotic symptoms.
LESSONS
This report discusses improvement of psychosis symptoms after resection of an amygdalar glioma, independent of seizure outcome. This case supports a role of the amygdala in psychopathology and suggests that low-grade gliomas of the limbic system may represent, at minimum, partially reversible etiology of psychotic symptoms.
Keywords: amygdala, epilepsy-associated psychosis, interictal psychosis, psychosis, temporal lobe epilepsy
ABBREVIATIONS : FDG = fluorodeoxyglucose, MRI = magnetic resonance imaging
Psychoses associated with epilepsy are well-described but incompletely understood phenomena.1 Low-grade gliomas are a relatively common cause of epilepsy2,3 but are less frequently associated with psychotic symptoms.4–7 One schema for categorizing epilepsy-associated psychoses focuses on the temporal relationship of psychotic symptoms to seizures: ictal psychosis, postictal psychosis, and chronic interictal psychosis.8 There may be bidirectionality to the symptomatology, as studies have suggested that people with epilepsy are at increased risk of developing schizophrenia, whereas people with schizophrenia have a higher prevalence of epilepsy than the general population.9,10
Psychotic symptoms may be independently associated with brain tumors, although such symptoms are not clearly localizing signs.4–6,11 The association of psychiatric pathology with tumors of the limbic system and temporal lobe has been documented.12,13 The interactions between the limbic system and targets of antipsychotic drugs are manifold,14–17 and limbic dysfunction (e.g., in encephalitis)18 can present with psychosis.
Although there is general agreement that limbic tumors can cause both epilepsy and occasionally psychotic symptoms, whether resection can relieve psychotic symptoms in chronic interictal psychosis is not clear.19 We report an illustrative case of a patient with longstanding epilepsy, chronic interictal psychosis, and a nonenhancing amygdalar tumor who had immediate improvement of psychotic symptoms after removal of his amygdalar tumor.
Illustrative Case
We present the case of a 52-year-old man with history of epilepsy and psychosis who was referred for neurosurgical evaluation for management of a left mesial temporal lesion after thorough epilepsy workup and pharmacological seizure treatment at an outside institution, as described below.
History of Present Illness
The patient’s main psychiatric complaints began as paranoia in his 20’s, progressing to paranoid and grandiose delusions in his early 30’s. Auditory hallucinations and intrusive thoughts also developed. Additional psychosocial history includes persistent challenges with aggression since childhood, chronic difficulty holding jobs, and development of hoarding behavior in young adulthood. Prior to the surgery described in this report, the patient was experiencing daily auditory hallucinations.
The patient described auditory hallucinations as “voices” that he described as “not me.” They were predominately negative in content and at times consisted of command hallucinations instructing self-harm. He described the belief that the voices he heard were transmitted through small subcutaneous lipomas on his back. He also endorsed the feeling of others being aware of his private thoughts. The patient had a brief history of psychotherapy at another institution. He discontinued the treatment because he did not feel comfortable with his psychotherapy provider. He has had a sporadic history of antipsychotic pharmacotherapy, which he had not engaged in for more than a year at the time of engagement with our medical center. The patient believed that psychiatric medications were not helpful and had undesirable side effects.
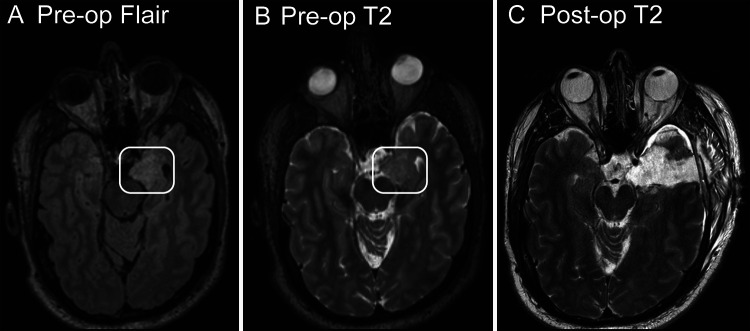
His first witnessed seizure occurred in 2012 while in an inpatient psychiatric unit for management of an exacerbation of chronic auditory hallucinations. Diagnostic workup included contrast-enhanced magnetic resonance imaging (MRI) of the brain, which revealed a T2-intense lesion, without gadolinium enhancement, located within the left amygdala (Fig. 1A and B). The patient was started on oral divalproex at that time. After several medication trials, his seizure control remained poor until 2019, when he was transitioned to lamotrigine and brivaracetam, resulting in resolution of seizures. At most recent follow-up in 2022, he has remained seizure-free for 3 years on a stable dose of lamotrigine 200 mg twice daily and brivaracetam 50 mg twice daily.
FIG. 1.
A and B: Axial MRI of the brain showing the T2-fluid-attenuated inversion recovery nonsuppression (A) and native T2 hyperintensity (B) of the asymmetrically expanded left amygdala prior to surgery. The white box outlines the abnormal amygdala. C: T2-weighted MRI postoperatively showing gross total resection of the amygdalar lesion.
Typical seizure semiology consisted of an aura with feeling of intense anxiety and electric shock–like somatic paresthesias before progressing to a period of impaired awareness with lip smacking and grunting vocalizations, consistent with temporal lobe seizures. These ictal events lasted 30 to 60 seconds and were followed by a brief postictal period, with an expressive predominant aphasia but preserved verbal comprehension. Prior to beginning dual medication therapy in 2019, these focal seizures occurred at least daily and progressed to generalized tonic-clonic seizures 1 to 4 times per month.
Additional epilepsy workup included epilepsy monitoring unit admission for video-electroencephalography monitoring during which multiple seizures were captured and all exhibited onset in left temporal electrodes. None of the captured seizures were associated with psychotic symptoms. A fluorodeoxyglucose (FDG) positron emission tomography scan showed moderately decreased FDG uptake in the left mesial temporal region. Neuropsychological testing was positive for mild impairments in learning and encoding of verbal information as well as difficulty discriminating target information from semantically related distractors. The patient scored at or above average in auditory attention, working memory, processing speed, and visuospatial memory. Overall, findings on these neuropsychological evaluations, including weakness in verbal memory relative to visual memory, were supportive of left (dominant) hemisphere dysfunction.
After the patient relocated medical care to our institution, repeat MRI showed expansion of the known amygdalar lesion, and he was referred for surgical evaluation. The patient was offered resection of this brain tumor for tissue diagnosis, in line with known evidence that resection of low-grade gliomas is associated with long-term increases in overall survival.20–22 The patient was counseled on the risks and benefits of surgery. Because his seizures were well-controlled with pharmacotherapy, drug-resistant epilepsy was not an indication. Moreover, while the patient was interested in whether the surgery could help with his ongoing psychiatric symptoms, which were his most disruptive medical issue because the link between his psychiatric symptoms and either the tumor or epilepsy was not clear, the patient was advised that improvement in psychiatric symptoms was not an outcome that could be predicted or expected. The patient agreed to these risks and benefits and decided to proceed with resection.
Surgical Management and Outcome
We proceeded with a left temporal craniotomy for a transmiddle temporal gyrus approach for resection of the tumor. The corticectomy was restricted to the anterior 5 cm of the middle temporal gyrus to minimize risk to language centers. The temporal horn of the lateral ventricle was encountered and opened to expose the mesial temporal structures. Meticulous subpial resection was performed. The amygdala was abnormally firm in texture and consistency. Intraoperative frozen samples were consistent with low-grade glioma. The grossly abnormal amygdala and portions of the hippocampal head were resected until normal parenchyma was encountered. Adequate extent of resection was further confirmed with the use of intraoperative neuronavigation and direct visualization of surrounding neurovascular structures, including the crural and carotid cisterns.
Postoperatively, the patient returned to his neurological baseline with intact naming, repetition, and sentence production. Immediate postoperative MRI showed a gross total resection of the T2-intense lesion. Final pathology confirmed the diagnosis of ganglioglioma, World Health Organization grade I. On postoperative day 2, he reported the subjective feeling of his “head [being] clearer” and excitedly reported he was no longer hearing voices. At 1 month postoperatively, he reported complete absence of auditory hallucinations that had previously been daily features. He denied seizures and has remained on his preoperative antiepileptic medication regimen. These improvements were verified by the patient’s spouse, who also reported improvement in his mood and interpersonal communication. On most current neuropsychiatric evaluation (5 months postoperatively), the patient reported occasional distressing/intrusive thoughts but reiterated that his hallucinations were significantly reduced compared to his preoperative baseline, estimated by the patient as a 90% reduction in symptoms.
Discussion
Observations
We report the case of a patient with a longstanding history of psychosis with auditory hallucinations and left temporal–onset epilepsy whose psychotic symptoms showed significant improvement after resection of a left amygdalar glioma. This case is important to formally document in the literature given the limited data available to guide preoperative counseling of patients regarding possible psychiatric implications of brain tumor surgery. These findings underscore the important role of temporal lobe structures in subserving both physiological and pathologic auditory processing and further suggest that psychiatric symptoms (namely auditory hallucinations) can benefit from removal of irritative intraaxial lesions in this location.
A great deal of time and care was taken in our preoperative counseling to ensure that the patient and family understood unequivocally that surgery was not being offered to improve his psychiatric symptoms. The clinical indication was oncological: specifically, growth of a nonenhancing lesion that had been managed conservatively at another institution. While this single case study should be treated with the same caveats that any case study deserves, it allows for a reference for other surgeons with patients harboring the clinical triad of well-controlled epilepsy, amygdalar tumor, and psychotic symptoms.
Psychosis and Epilepsy
The increased prevalence of psychiatric disease and neurobehavioral disorders in epilepsy is well described.9,23 The epilepsy-associated psychosis of the patient in this report may best be described as chronic interictal psychosis.1,8 The primary treatment of other forms of epilepsy-associated psychoses such as ictal psychosis and postictal psychosis are focused around treating the epilepsy.8 Treatment of chronic interictal psychosis is generally similar to that of treating psychosis independent of epilepsy.1,8 The preoperative persistence of the patient’s psychotic symptoms despite adequate preoperative control of seizures makes the connection between these phenomena complicated but highlights how interictal psychosis may require antipsychotic treatment independent of AEDs.
Limbic Structural Pathology and Psychoses
The link between limbic structural pathology and psychotic syndromes is not new. Prior neuroimaging studies have linked hippocampal atrophy and amygdalar enlargement to psychotic syndromes24 as well as to psychosis associated with epilepsy25 in patients without evidence of neoplasia.
Limbic tumors also have a history of association with psychiatric disturbance.13,26 Case series prior to the advent of modern neuroimaging found that significant numbers of patients with mental illness had undiagnosed intracranial tumors.27 Speaking broadly, the possibility of a limbic system tumor contributing to a psychotic syndrome is not at odds with the literature. What is difficult clinically is predicting when the associated psychoses will improve with resection of the lesion.19 If the lesion and symptoms are both acute in onset, the temporal association may support a causal link. However, in a case such as discussed here, in which the psychotic symptoms and tumor are both chronic in nature and time of onset may be variable, subjective, and/or unknown, the conundrum of causality remains.
Lessons
In summary, we report the case of resection of an amygdalar tumor in a patient with longstanding epilepsy and psychosis. Surgery led to immediate reprieve from his psychotic symptoms. Chronic interictal-psychosis, in the presence of well-controlled epilepsy, may respond to resection of an amygdalar tumor.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Andrews. Acquisition of data: Andrews, Wozny. Analysis and interpretation of data: Andrews, Wozny, Yue. Drafting the article: all authors. Critically revising the article: Andrews, Wozny, Yue. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Andrews. Study supervision: Andrews.
References
- 1. Maguire M, Singh J, Marson A. Epilepsy and psychosis: a practical approach. Pract Neurol. 2018;18(2):106–114. doi: 10.1136/practneurol-2017-001775. [DOI] [PubMed] [Google Scholar]
- 2. Chang EF, Potts MB, Keles GE, et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008;108(2):227–235. doi: 10.3171/JNS/2008/108/2/0227. [DOI] [PubMed] [Google Scholar]
- 3. Englot DJ, Berger MS, Barbaro NM, Chang EF. Predictors of seizure freedom after resection of supratentorial low-grade gliomas. A review. J Neurosurg. 2011;115(2):240–244. doi: 10.3171/2011.3.JNS1153. [DOI] [PubMed] [Google Scholar]
- 4. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors: diagnostic implications. Expert Rev Neurother. 2007;7(4):343–349. doi: 10.1586/14737175.7.4.343. [DOI] [PubMed] [Google Scholar]
- 5. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529–1536. doi: 10.1586/ern.10.94. [DOI] [PubMed] [Google Scholar]
- 6. Andrews JP, Taylor J, Saunders D, Qayyum Z. Peduncular psychosis. BMJ Case Rep. 2016;2016:bcr2016216165. doi: 10.1136/bcr-2016-216165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sherman SJ, Tanaka R, Qaddoumi I. Psychiatric symptoms in children with low-grade glioma and craniopharyngioma: a systematic review. J Psychiatr Res. 2022;148:240–249. doi: 10.1016/j.jpsychires.2022.01.056. [DOI] [PubMed] [Google Scholar]
- 8. Nadkarni S, Arnedo V, Devinsky O. Psychosis in epilepsy patients. Epilepsia. 2007;48(suppl 9):17–19. doi: 10.1111/j.1528-1167.2007.01394.x. [DOI] [PubMed] [Google Scholar]
- 9. Qin P, Xu H, Laursen TM, Vestergaard M, Mortensen PB. Risk for schizophrenia and schizophrenia-like psychosis among patients with epilepsy: population based cohort study. BMJ. 2005;331(7507):23. doi: 10.1136/bmj.38488.462037.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wotton CJ, Goldacre MJ. Coexistence of schizophrenia and epilepsy: record-linkage studies. Epilepsia. 2012;53(4):e71–e74. doi: 10.1111/j.1528-1167.2011.03390.x. [DOI] [PubMed] [Google Scholar]
- 11. Gupta RK, Kumar R. Benign brain tumours and psychiatric morbidity: a 5-years retrospective data analysis. Aust N Z J Psychiatry. 2004;38(5):316–319. doi: 10.1080/j.1440-1614.2004.01357.x. [DOI] [PubMed] [Google Scholar]
- 12. Keschner M, Bender MB, Strauss I. Mental symptoms in cases of tumor of the temporal lobe. Arch Neurol Psychiatry. 1936;35(3):572–596. [Google Scholar]
- 13. Malamud N. Psychiatric disorder with intracranial tumors of limbic system. Arch Neurol. 1967;17(2):113–123. doi: 10.1001/archneur.1967.00470260003001. [DOI] [PubMed] [Google Scholar]
- 14. Joyce EM. Organic psychosis: the pathobiology and treatment of delusions. CNS Neurosci Ther. 2018;24(7):598–603. doi: 10.1111/cns.12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laruelle M, Kegeles LS, Abi-Dargham A. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci. 2003;1003(1):138–158. doi: 10.1196/annals.1300.063. [DOI] [PubMed] [Google Scholar]
- 16. Murray GK, Corlett PR, Clark L, et al. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatry. 2008;13(3):239, 267–276. doi: 10.1038/sj.mp.4002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pralong E, Magistretti P, Stoop R. Cellular perspectives on the glutamate-monoamine interactions in limbic lobe structures and their relevance for some psychiatric disorders. Prog Neurobiol. 2002;67(3):173–202. doi: 10.1016/s0301-0082(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 18. Ganguli A, Fitzgerald R, Walker L, Beadsworth M, Mwandumba HC. Voltage-gated, potassium-channel antibody-associated limbic encephalitis presenting as acute psychosis. J Neuropsychiatry Clin Neurosci. 2011;23(2):E32–E34. doi: 10.1176/jnp.23.2.jnpe32. [DOI] [PubMed] [Google Scholar]
- 19. Marchetti RL, Fiore LA, Valente KD, Gronich G, Nogueira AB, Tzu WH. Surgical treatment of temporal lobe epilepsy with interictal psychosis: results of six cases. Epilepsy Behav. 2003;4(2):146–152. doi: 10.1016/s1525-5050(03)00021-0. [DOI] [PubMed] [Google Scholar]
- 20. Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 21. Hervey-Jumper SL, Berger MS. Role of surgical resection in low- and high-grade gliomas. Curr Treat Options Neurol. 2014;16(4):284. doi: 10.1007/s11940-014-0284-7. [DOI] [PubMed] [Google Scholar]
- 22. Brown TJ, Bota DA, van Den Bent MJ, et al. Management of low-grade glioma: a systematic review and meta-analysis. Neurooncol Pract. 2019;6(4):249–258. doi: 10.1093/nop/npy034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin JJ, Mula M, Hermann BP. Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. Lancet. 2012;380(9848):1180–1192. doi: 10.1016/S0140-6736(12)61455-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Velakoulis D, Wood SJ, Wong MT, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63(2):139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- 25. Tebartz Van Elst L, Baeumer D, Lemieux L, et al. Amygdala pathology in psychosis of epilepsy: a magnetic resonance imaging study in patients with temporal lobe epilepsy. Brain. 2002;125(Pt 1):140–149. doi: 10.1093/brain/awf008. [DOI] [PubMed] [Google Scholar]
- 26. Mulder DW, Daly D. Psychiatric symptoms associated with lesions of temporal lobe. J Am Med Assoc. 1952;150(3):173–176. doi: 10.1001/jama.1952.03680030005003. [DOI] [PubMed] [Google Scholar]
- 27. Klotz M. Incidence of brain tumors in patients hospitalized for chronic mental disorders. Psychiatr Q. 1957;31(4):669–680. doi: 10.1007/BF01568758. [DOI] [PubMed] [Google Scholar]