Abstract
BACKGROUND
Metastatic cancer may involve the central and peripheral nervous system, usually in the late stages of disease. At this point, most patients have been diagnosed and treated for widespread systemic disease. Rarely is the involvement of the peripheral nervous system the presenting manifestation of malignancy. One reason for this is a proposed “blood-nerve barrier” that renders the nerve sheath a relatively privileged site for metastases.
OBSERVATIONS
The authors presented a novel case of metastatic melanoma presenting as intractable leg pain and numbness. Further workup revealed concurrent disease in the brain and breast, prompting urgent treatment with radiation and targeted immunotherapy.
LESSONS
This case highlights the rare presentation of metastatic melanoma as a mononeuropathy. Although neurological complications of metastases tend to occur in later stages of disease after initial diagnosis and treatment, one must remember to consider malignancy in the initial differential diagnosis of mononeuropathy.
Keywords: melanoma, cancer, metastasis, nerve metastasis, peripheral nervous system, central nervous system
ABBREVIATIONS : MRI = magnetic resonance imaging, PET = positron emission tomography, PNS = peripheral nervous system
Despite rising rates of melanoma worldwide1 and the frequency of metastasis, it is uncommon for malignant melanoma to involve nerves.2 Furthermore, metastases to the peripheral nerves are exceedingly rare.3 This could potentially be explained by the nerve sheath functioning as a barrier to tumor invasion or by the existence of a “blood-nerve barrier,” which may block tumor spread via the vascular system.4 These challenges to tumor cell dissemination serve to explain why metastases to the peripheral nervous system (PNS) most often present in the late stages of disease.5
Metastases to the PNS occur far less frequently than to the central nervous system and tend to involve the proximal aspect of nerves, namely the nerve roots and plexi.3,6 One study cites the incidence of metastasis to nerve plexuses in the PNS as 0.43%–0.71%.5 The few cases reported in the literature of melanoma malignancies in the PNS involve the brachial plexus and trigeminal nerve, exclusively.3,5,7,8 Here, we present a novel case of concurrent melanoma metastases to the brain and femoral nerve in a patient presenting with leg pain and weakness.
This case illustrates the potential for melanoma to disseminate to peripheral nerves and highlights the importance of including malignancy in the differential diagnosis of mononeuropathy.
Illustrative Case
A 67-year-old man with a history of recurrent skin lesions previously treated by a dermatologist (unknown pathology) presented to his primary care provider with two recently discovered lumps on the lateral aspect of his right breast and a complaint of pain and paresthesias along the lateral aspect of the right leg. He described first noticing numbness and pruritis along the distal right leg that progressed to burning pain and weakness over several days, eventually ascending to his right hip and lateral thigh. The pain was severe, limiting his ability to sleep. The largest breast mass was 3 cm, multilobulated, and slightly painful on palpation. He was prescribed muscle relaxants and gabapentin and sent for an ultrasound-guided biopsy of his breast mass, which revealed malignant melanoma. He was referred to an oncologist, who ordered contrast magnetic resonance imaging (MRI) of his brain and lumbar spine, revealing numerous intracranial metastases (Fig. 1) but no lumbar disease or malignancy. The patient received Gamma Knife stereotactic radiosurgery to nine intracranial lesions 6 weeks after his initial symptom onset.
FIG. 1.
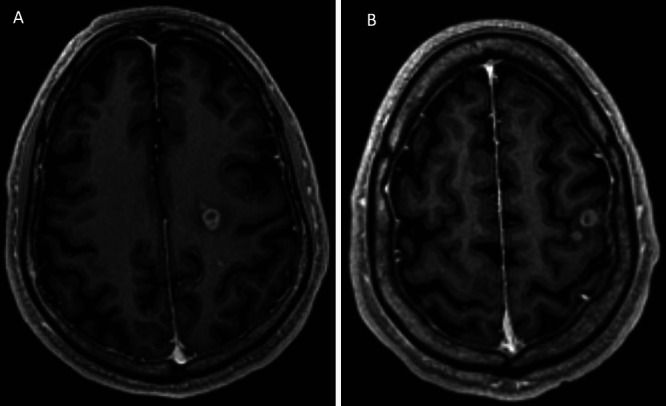
Axial T1-weighted postcontrast brain MRI demonstrating two of nine intracranial metastases. A: Contrast-enhancing mass in the left centrum semiovale. B: Contrast-enhancing mass with satellite nodule anterior to the left central sulcus, adjacent to the motor strip.
On further workup, a positron emission tomography (PET) with computed tomography was ordered, revealing disseminated disease in the lungs, liver, inferior right axilla, and right pelvis (Fig. 2). Follow-up imaging with a pelvic MRI revealed a 1.4-cm focally enhancing mass on the right femoral nerve between the iliacus and psoas muscle (Fig. 3), corresponding to the focal uptake in the right pelvis seen on the PET scan. There was no significant pelvic adenopathy.
FIG. 2.
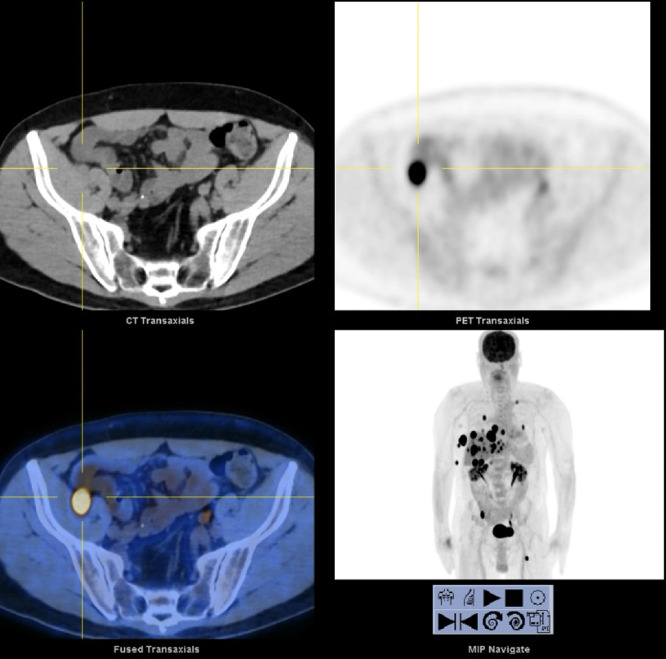
PET computed tomography depicting focally increased uptake in the right pelvis (crosshairs).
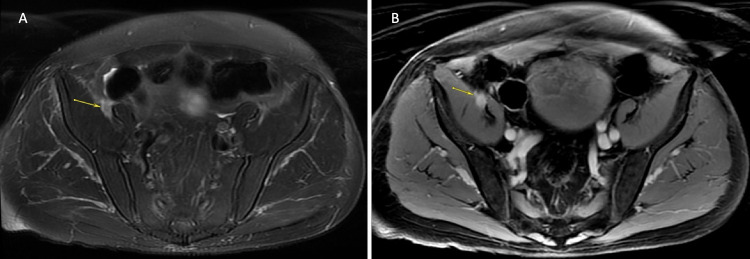
FIG. 3.
MRI of the pelvis demonstrating an enhancing metastasis to the right femoral nerve. A: Axial fat-saturated image depicting focal enlargement of the femoral nerve as it courses between the iliacus and psoas muscles (arrow). B: Axial T1-weighted postcontrast image depicting diffuse enhancement of the mass and the femoral nerve (arrow), likely secondary to melanin deposition.
The patient’s pain became more severe, eventually requiring hospitalization for pain management. A focused neurological examination revealed weakness in his right iliopsoas and quadriceps (Medical Research Council grade 2/5), decreased sensation to light touch and temperature in the right L4 dermatome, and a complete loss of the patellar reflex in the right leg. He therefore received palliative hypofractionated radiation to the right femoral nerve (25 Gy in five fractions). Although his weakness persisted, the leg pain improved significantly after radiation.
Despite two cycles of targeted immunotherapy (ipilimumab and nivolumab) and radiotherapy, the patient’s interval imaging 2 months after initial treatment demonstrated rapid intracranial progression. Whole brain radiation therapy was delivered (30 Gy in 10 fractions). Serial imaging continued to demonstrate systemic disease progression. After careful discussion with family, the patient elected to enroll in hospice 5 months after initial presentation.
Discussion
Cancer metastasis can affect any part of the nervous system. Up to 25% of patients with cancer develop neurological complications.6 When the PNS is affected, it is usually a result of cancer treatment, particularly chemotherapy.6 However, cancers may also infiltrate and metastasize directly to the PNS, including the cranial nerves, nerve roots, cervical, brachial and lumbosacral plexuses, and seldom, the peripheral nerves.3,6 Compression or infiltration of cancerous cells can potentially harm peripheral nerves, leading to progressive neuropathies. Patients with neoplastic mononeuropathies initially present with pain, followed by focal sensory and eventually motor deficits in a dermatomal distribution, similar to our patient’s presentation.5,6 Solid tumors in adjacent organs or lymphatic tissue typically cause these neuropathies via direct extension. However, metastasis from lymphoma, leukemia, or distant solid tumors (breast or prostate cancer) has also been demonstrated.3,6,9
Neoplastic lesions affect peripheral nerves far less frequently than toxic, metabolic, or even paraneoplastic processes.9 When a mononeuropathy is present in the setting of malignancy, it is often due to mechanical damage inflicted by recent surgical procedures.10 Other causes include toxic effects of chemotherapy and direct invasion of surrounding tissue. Metastases to individual nerves distal to the plexus are rare. One suspected rationale for this phenomenon is the existence of a distinct blood-nerve barrier.
Many studies have demonstrated that peripheral nerves have an abundant blood supply.11,12 The mesoneurium, a web of connective tissue surrounding the nerves, contains many small arteries. Numerous anastomoses arise between arteries and veins as they pass through the mesoneurium or just next to the nerve surface. Within the nerve itself lies a dense network of blood vessels that form an intricate intraneural capillary network.12 The establishment of a blood-nerve barrier, analogous to the blood-brain barrier, may limit the spread of tumor cells through vascular channels, which may account for peripheral nerve resistance to metastatic infiltration.12 The nerve sheath, or layer of myelin and connective tissue that insulates peripheral nerve fibers, also represents a separate line of defense against neoplastic infiltration.
The functional and structural integrity of a peripheral nerve can be affected by neoplastic tissue in three direct structural-anatomical ways: (1) the tumor mass can stretch the nerve trunk by pushing it without actually invading the sheath; (2) the mass can compress or strangulate the nerve by engulfing it without actually invading the sheath; and (3) the tumor can directly perforate the nerve sheath and invade in between the fascicles. It appears that nerve sheaths act as significant barriers in each of these cases.13 The integrity of a peripheral nerve may also be affected by external bony compression, similar to nonneoplastic compressive mononeuropathies.
Nerves that cross over or through the bone may sustain damage as a result of malignant bony infiltration. Obturator nerve compression in the obturator canal due to pelvic cancer is one instance of this.14 An obturator mononeuropathy has been linked to various cancers, including lymphoma, pelvic papillary carcinoma, transitional cell cancer of the bladder, and prostate adenocarcinoma.14 The ulnar nerve at the axilla or elbow, the intercostal nerves between ribs, the sciatic nerve in the pelvis, and the peroneal nerve near the fibular head are other documented locations of malignant compressive mononeuropathies.6,14 Unfortunately, neoplasms do not rely solely on external bony compression to cause neoplastic mononeuropathies. In rare occasions, solid tumors metastasize and invade individual peripheral nerves.
Intraneural metastases from solid tumors are uncommon. There are scattered examples in the literature of neoplastic mononeuropathies caused by intraneural metastasis. A case report by Grisold et al.15 demonstrated a mononeuropathy caused by intraneural metastasis from a carcinoid tumor. A separate case published by Humphries et al.16 demonstrated a patient with metastatic renal cell carcinoma to the ulnar nerve presenting as a painful mass in the right volar wrist. Another patient with a delayed sciatic nerve metastasis from a surgically removed renal cell carcinoma has been reported by Varin et al.17 Additionally, there is a rare instance of peripheral neuropathy brought on by infiltrative metastatic adenocarcinoma with no apparent primary lesion.18 Not reported in the literature, however, is our case of a melanoma metastasis to the femoral nerve causing the initial clinical presentation of painful mononeuropathy.
Observations
We believe our patient experienced a rare intraneural metastasis of melanoma to the femoral nerve, evidenced by the intrinsic T1 hyperintensity of the femoral nerve on MRI, indicating melanin deposition within the nerve, and the absence of adjacent lymphatic involvement. It was this focal metastasis that led to the patient’s clinical presentation and diagnostic workup.
Lessons
Isolated mononeuropathies are abundantly encountered in clinical practice. However, in a patient with a history of recurrent skin lesions, an isolated mononeuropathy may be indicative of something far more sinister. The practitioner must harbor a high index of suspicion for malignancy in such instances. In our case, the patient was swiftly referred for biopsy and began treatment within 6 weeks.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Guerrero, Bhenderu, Sadrameli, Shkedy, Faraji, Rostomily. Acquisition of data: Guerrero, Sadrameli, Faraji. Analysis and interpretation of data: Guerrero, Bhenderu, Sadrameli, Faraji. Drafting the article: Guerrero, Taghlabi, Meyer, Bhenderu, Sadrameli, Faraji. Critically revising the article: Guerrero, Taghlabi, Meyer, Bhenderu, Faraji, Rostomily. Reviewed submitted version of manuscript: Guerrero, Taghlabi, Faraji, Rostomily. Approved the final version of the manuscript on behalf of all authors: Guerrero. Administrative/technical/material support: Guerrero, Faraji, Rostomily. Study supervision: Faraji.
References
- 1. Damsky WE, Theodosakis N, Bosenberg M. Melanoma metastasis: new concepts and evolving paradigms. Oncogene. 2014;33(19):2413–2422. doi: 10.1038/onc.2013.194. [DOI] [PubMed] [Google Scholar]
- 2. Hughes TA, McQueen IN, Anstey A, Laidler P. Neurotropic malignant melanoma presenting as a trigeminal sensory neuropathy. J Neurol Neurosurg Psychiatry. 1995;58(3):381–382. doi: 10.1136/jnnp.58.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gwathmey KG. Plexus and peripheral nerve metastasis. Handb Clin Neurol. 2018;149:257–279. doi: 10.1016/B978-0-12-811161-1.00017-7. [DOI] [PubMed] [Google Scholar]
- 4. Meller I, Alkalay D, Mozes M, Geffen DB, Ferit T. Isolated metastases to peripheral nerves. Report of five cases involving the brachial plexus. Cancer. 1995;76(10):1829–1832. doi: 10.1002/1097-0142(19951115)76:10<1829::aid-cncr2820761023>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5. Mendez JS, DeAngelis LM. Metastatic complications of cancer involving the central and peripheral nervous systems. Neurol Clin. 2018;36(3):579–598. doi: 10.1016/j.ncl.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramchandren S, Dalmau J. Metastases to the peripheral nervous system. J Neurooncol. 2005;75(1):101–110. doi: 10.1007/s11060-004-8102-9. [DOI] [PubMed] [Google Scholar]
- 7. Kim DH, Murovic JA, Tiel RL, Moes G, Kline DG. A series of 146 peripheral non-neural sheath nerve tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102(2):256–266. doi: 10.3171/jns.2005.102.2.0256. [DOI] [PubMed] [Google Scholar]
- 8. Gachiani J, Kim DH, Nelson A, Kline D. Management of metastatic tumors invading the peripheral nervous system. Neurosurg Focus. 2007;22(6):E14. [PubMed] [Google Scholar]
- 9. Recht L, Mrugala M. Neurologic complications of hematologic neoplasms. Neurol Clin. 2003;21(1):87–105. doi: 10.1016/s0733-8619(02)00030-0. [DOI] [PubMed] [Google Scholar]
- 10. Grisold W, Briani C, Vass A. Malignant cell infiltration in the peripheral nervous system. Handb Clin Neurol. 2013;115:685–712. doi: 10.1016/B978-0-444-52902-2.00040-0. [DOI] [PubMed] [Google Scholar]
- 11.Prahm C, Heinzel J, Kolbenschlag J. Blood supply and microcirculation of the peripheral nerve. In: Phillips JB, Hercher D, Hausner T, editors. Peripheral Nerve Tissue Engineering and Regeneration. Reference Series in Biomedical Engineering. Springer; 2022. pp. 35–36. [Google Scholar]
- 12. Kanda T. Peripheral neuropathy and blood-nerve barrier. Article in Japanese. Rinsho Shinkeigaku. 2009;49(11):959–962. doi: 10.5692/clinicalneurol.49.959. [DOI] [PubMed] [Google Scholar]
- 13. Best TJ, Mackinnon SE. Intraneural vascular investigative techniques. J Reconstr Microsurg. 1991;7(3):245–248. doi: 10.1055/s-2007-1006785. [DOI] [PubMed] [Google Scholar]
- 14. Patel DK, Gwathmey KG. Neoplastic nerve lesions. Neurol Sci. 2022;43(5):3019–3038. doi: 10.1007/s10072-022-05951-x. [DOI] [PubMed] [Google Scholar]
- 15. Grisold W, Piza-Katzer H, Jahn R, Herczeg E. Intraneural nerve metastasis with multiple mononeuropathies. J Peripher Nerv Syst. 2000;5(3):163–167. doi: 10.1046/j.1529-8027.2000.00016.x. [DOI] [PubMed] [Google Scholar]
- 16. Humphries LS, Baluch DA, Nystrom LM, Borys D, Bednar MS. Interfascicular renal cell carcinoma metastasis to the ulnar nerve: a case report. Hand (N Y) 2016;11(2):NP1–NP4. doi: 10.1177/1558944715627620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Varin S, Faure A, Bouc P, Maugars Y, Berthelot JM. Endoneural metastasis of the sciatic nerve disclosing the relapse of a renal carcinoma, four years after its surgical treatment. Joint Bone Spine. 2006;73(6):760–762. doi: 10.1016/j.jbspin.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 18. Saikumar J, Li G, Chaudhary RT. Reversible neuropathy after chemotherapy for metastatic adenocarcinoma from an unknown primary tumor to the sural nerve. Am J Ther. 2011;18(6):e264–e268. doi: 10.1097/MJT.0b013e3181d8d9aa. [DOI] [PubMed] [Google Scholar]