Abstract
Mycobacterium tuberculosis is the etiologic agent of human tuberculosis and is estimated to infect one-third of the world's population. Control of M. tuberculosis requires T cells and macrophages. T-cell function is modulated by the cytokine environment, which in mycobacterial infection is a balance of proinflammatory (interleukin-1 [IL-1], IL-6, IL-8, IL-12, and tumor necrosis factor alpha) and inhibitory (IL-10 and transforming growth factor β [TGF-β]) cytokines. IL-10 and TGF-β are produced by M. tuberculosis-infected macrophages. The effect of IL-10 and TGF-β on M. tuberculosis-reactive human CD4+ and γδ T cells, the two major human T-cell subsets activated by M. tuberculosis, was investigated. Both IL-10 and TGF-β inhibited proliferation and gamma interferon production by CD4+ and γδ T cells. IL-10 was a more potent inhibitor than TGF-β for both T-cell subsets. Combinations of IL-10 and TGF-β did not result in additive or synergistic inhibition. IL-10 inhibited γδ and CD4+ T cells directly and inhibited monocyte antigen-presenting cell (APC) function for CD4+ T cells and, to a lesser extent, for γδ T cells. TGF-β inhibited both CD4+ and γδ T cells directly and had little effect on APC function for γδ and CD4+ T cells. IL-10 down-regulated major histocompatibility complex (MHC) class I, MHC class II, CD40, B7-1, and B7-2 expression on M. tuberculosis-infected monocytes to a greater extent than TGF-β. Neither cytokine affected the uptake of M. tuberculosis by monocytes. Thus, IL-10 and TGF-β both inhibited CD4+ and γδ T cells but differed in the mechanism used to inhibit T-cell responses to M. tuberculosis.
Mycobacterium tuberculosis remains a major cause of morbidity and mortality worldwide, infecting approximately one-third of the world's population (50). Cellular immune responses control M. tuberculosis infection in most healthy individuals, resulting in fewer than 10% of infected persons developing active tuberculosis (13). T cells and mononuclear phagocytes are required for successful control of M. tuberculosis infection. Mycobacterial antigens are recognized by a variety of T-cell populations, including CD4+ αβ T-cell receptor (TCR)-positive (TCR+) T cells (CD4+ T cells) and Vδ2+ γδ T cells (γδ T cells) (reviewed in reference 41). CD4+ T cells have critical regulatory and effector functions in protective immunity to M. tuberculosis (5, 10, 57). γδ T cells are readily activated by M. tuberculosis, but their role in protective immunity to M. tuberculosis is less well defined (3, 9, 30, 40, 51). CD4+ and γδ T cells differ in the antigens that they recognize and the manner in which these antigens are processed and presented (68).
Infection of macrophages with M. tuberculosis results in the secretion of both proinflammatory (interleukin 1 [IL-1], IL-12, and tumor necrosis factor alpha) and inhibitory (IL-10 and transforming growth factor β [TGF-β]) cytokines (4, 67, 71). The balance of proinflammatory and inhibitory cytokines influences T-cell activation. Overproduction of IL-10 and TGF-β has been documented in tuberculosis patients and implicated as a cause of depressed T-cell function in these individuals (21, 34).
IL-10 is an 18-kDa homodimeric cytokine secreted by activated T cells, B cells, and monocytes (65). IL-10 inhibits proliferation and IL-2 production by activated human T cells and secretion of proinflammatory cytokines by lipopolysaccharide-activated monocytes (25, 64). IL-10 also down-regulates cell adhesion and costimulatory molecules (CD54/ICAM-1, CD80, and CD86) as well as major histocompatibility complex (MHC) class II glycoproteins (18, 52, 60). TGF-β is a member of a family of pleiotropic 25-kDa homodimeric proteins representing signaling molecules with potent immunoregulatory properties (48). TGF-β is produced by lymphocytes, macrophages, and dendritic cells. TGF-β can, under certain conditions, stimulate T-cell proliferation and differentiation and prevent T-cell apoptosis (11, 12, 42). TGF-β can modulate the expression of adhesion molecules and induce chemotaxis of leukocytes as well as other inflammatory cells. In addition, TGF-β inhibits macrophage activation, T-cell proliferation, and the generation of cytotoxic T lymphocytes and can down-regulate the expression of class II MHC molecules (17, 27, 47, 61, 69). Few studies have compared the differential sensitivity to IL-10 and TGF-β of human T cells having similar cytokine patterns (Th-1) but different surface phenotypes (CD4, CD8, and γδ). Differential sensitivity to the effects of IL-10 and TGF-β might be one mechanism for explaining why multiple T-cell subsets participate in the immune response to M. tuberculosis. The aims of the present work were to determine the effects of IL-10 and TGF-β on M. tuberculosis-reactive human CD4+ and γδ T cells, the two major T-cell subsets activated by mycobacterial antigens, and to investigate the mechanisms of action of these cytokines on the interaction between T cells and M. tuberculosis-infected monocytes.
MATERIALS AND METHODS
Monoclonal antibodies and cytokines.
To identify T-cell subsets and up-regulated CD25 expression, phycoerythrin (PE)-conjugated Leu-4 (CD3-PE), anti-IL-2 receptor alpha chain (CD25-PE), fluorescein isothiocyanate (FITC)-conjugated Leu-3a (CD4-FITC), FITC-conjugated Leu-2a (CD8-FITC), and PE- and FITC-conjugated isotypic controls were purchased from Becton Dickinson, San Jose, Calif. PE-conjugated anti-human γδ TCR was purchased from Caltag, Burlingame, Calif. FITC-conjugated anti-human γδ TCR (TCRδ1) was obtained from T cell Diagnostics, Inc., Woburn, Mass., and FITC-conjugated anti-Vδ2 TCR was obtained from Pharmigen, San Diego, Calif. Monocytes were identified with FITC-conjugated anti-CD14 (Becton Dickinson).
Costimulator and MHC molecule expression on monocytes was measured by indirect immunofluorescence with the following antibodies: rat anti-class II MHC (LO-DRa) (ARP, Belmont, Mass.), rat anti-class I MHC (YTH 76.3) (Serotec, Raleigh, N.C.), and mouse anti-CD40 (5C3) and anti-CD86 (2331 and FUN-1) (Pharmingen) and anti-CD80 (P1.H5.A1.A1) (Ancell, Bayport, Minn.). Isotypic controls were purchased from Sigma Chemical Co., St. Louis, Mo. PE-conjugated goat anti-rat immunoglobulin G (IgG) (Caltag) and FITC-conjugated goat anti-mouse antibody (Cappel) were used as secondary antibodies.
IL-10 and IL-2 were purchased from Pharmingen, and TGF-β was purchased from Research and Diagnostic Systems Inc., Minneapolis, Minn. Plate-bound unconjugated mouse anti-CD3 antibody (CRIS-7) (Biosource, Camarillo, Calif.) was used to stimulate T cells.
Bacteria.
M. tuberculosis H37Ra was cultured in Middlebrook 7H9 with albumin-dextrose-catalase enrichment, and frozen stocks were prepared as described previously (30). Bacterial counts and viability were determined by light microscopy and by counting CFU on 7H10 medium. M. tuberculosis H37Ra stocks were tested periodically for viability with an M. tuberculosis complex-specific DNA probe (AccuProbe; Gen-Probe, San Diego, Calif.) to ensure their purity. Before use in T-cell assays, mycobacteria were washed three times in RPMI 1640, sonicated for 40 s, and passed multiple times through a 25-gauge needle to disrupt clumps; they were used at 106/ml. FITC-labeled bacteria were prepared by incubating H37Ra cells (109/ml) with 1 mg of FITC (Sigma) per ml in 0.1 M carbonate buffer (pH 9) at 37°C for 1 h. FITC-labeled bacteria were washed twice with phosphate-buffered saline, and cells were resuspended in fresh RPMI and used on the same day.
Isolation of PBMC and monocytes.
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation over sodium diatrizoate-Hypaque, and monocytes were obtained by adherence from PBMC as previously described (9). Briefly, PBMC were incubated on plastic tissue culture dishes precoated with pooled human serum (PHS), nonadherent cells were removed, and adherent cells were collected by scraping with a plastic policeman. PBMC were isolated from healthy tuberculin-positive persons (18 to 45 years old). They were selected for consistency of γδ T-cell expansion (20 to 60% γδ TCR+ T cells) after stimulation with live M. tuberculosis.
Expansion of resting CD4+ and γδ T cells stimulated by M. tuberculosis.
Purified PBMC (2 × 106) were cultured with live mycobacteria (2.5 × 106/ml) and with or without different concentrations of IL-10 or TGF-β in a final volume of 2 ml. The culture medium consisted of RPMI 1640 supplemented with 10% PHS, 20 mM HEPES, 2 mM l-glutamine, and antibiotics. Cultures were incubated for 7 days, cells were harvested, and viable cells were counted before determination of the percentage of CD25+ γδ and CD4+ T cells by flow cytometry. On day 3 of culturing, 50 μl of supernatants was harvested to measure the levels of secreted gamma interferon (IFN-γ).
Purification of M. tuberculosis-activated CD4+ and γδ T-cell populations.
PBMC stimulated with live M. tuberculosis for 7 to 9 days were used to obtain CD4+ and γδ T-cell lines by positive selection. Viable cells were harvested by density sedimentation on sodium diatrizoate-Hypaque gradients. CD4+ and γδ T-cell subsets were purified by positive selection with magnetic microbead-coated antibodies (Militenyi Biotec, Gladbach, Germany). For CD4 enrichment, cells were incubated with beads conjugated to monoclonal mouse anti-human CD4 antibody (Leu-3a). For γδ T-cell purification, cells were first incubated with a hapten-modified anti-γδ TCR antibody and then treated with FITC-conjugated anti-hapten microbeads. The purity of M. tuberculosis-activated CD4+ and γδ TCR+ T cells after positive selection was confirmed by fluorescence-activated cell sorting (FACS). One cycle of selection was sufficient to obtain ≥95% CD4+ T cells or γδ TCR+ T cells (Vδ2+ Vγ9+, >90%). Ninety-eight percent of CD4+ T cells coexpressed αβ TCR, as detected by FACS. Less than 1% of CD4+ T cells were found in the purified γδ TCR+ fractions.
Immunofluorescence analysis.
CD25-PE was used with FITC-conjugated anti-γδ TCR and CD4-FITC to measure IL-2 receptor alpha chain (CD25) expression on CD4+ and γδ T cells by two-color FACS to determine the percentage of CD25+ γδ and CD4+ T cells activated by M. tuberculosis.
Cells were analyzed on a FACScan (Becton Dickinson) with LYSISTMII software. Cells were gated in a two-parameter plot of 90° versus forward-angle scatter. The gate for lymphocytes or monocytes was set widely. Five thousand events were recorded for each cell surface marker. The cutoff lines for positive and negative fluorescence were set manually based on the distribution of cells stained with FITC- and PE-conjugated isotypic controls alone and were kept constant within each experiment. The percentage reported for a given surface marker represents the proportion of gated cells with a positive signal minus the percentage of cells staining positive with isotypic controls alone.
Proliferation and IFN-γ assays.
Positively selected CD4+ and γδ T cells (5 × 104 cells per 200-μl well) were cocultured with irradiated autologous monocytes as antigen-presenting cells (APC) (105 cells per 200-μl well), M. tuberculosis, and different concentrations of IL-10 or TGF-β for 72 h in 96-well plates. Alternatively, CD4+ and γδ T cells (1 × 105 cells per 200-μl well) were cultured in 96-well plates coated with mouse (monoclonal) anti-human CD3 antibody (1 μg/ml) and with or without IL-10 or TGF-β. Cells were pulsed with 1 μCi of [3H]thymidine (ICN, Costa Mesa, Calif.) for 12 to 16 h before being harvested on glass fiber filters. [3H]thymidine incorporation was measured by liquid scintillation counting and expressed as counts per minute. Before pulsing of the cultures with [3H]thymidine, 50 μl of supernatant was harvested from each well for measurement of IFN-γ by a sandwich enzyme-linked immunosorbent assay (ELISA) with M70-A and M70-B antibodies purchased from Endogen (Cambridge, Mass.).
Paraformaldehyde fixation of monocytes.
Freshly isolated monocytes were placed in 96-well flat-bottom plates (106 cells/well) and infected with M. tuberculosis (106 bacteria/well) in the presence or absence of IL-10 or TGF-β. After overnight incubation, plates were washed with RPMI 1640 and incubated with 1% paraformaldehyde for 15 min at room temperature. Plates then were washed twice with RPMI 1640 before the addition of 0.2 M lysine. After 20 min, lysine was discarded, and plates were washed four times with RPMI 1640. Following fixation, 2.5 × 104 to 5 × 104 purified CD4+ or γδ T cells were added to the wells. After 3 days, supernatants were harvested for IFN-γ measurement by the ELISA.
Measurement of intracellular bacteria.
To evaluate the phagocytosis of M. tuberculosis, adherence-purified monocytes were treated with either IL-10 or TGF-β (10 ng/ml) or medium alone for 24 h at 37°C. Cytokine-treated monocytes and nontreated monocytes were infected with H37Ra (bacterium/cell ratio, 20:1) for 2 h at 37°C in RPMI 1640 supplemented with 20% non-heat-inactivated PHS. Infected monocytes were scraped, washed, and stained by the Ziehl-Neelsen method, and acid-fast bacilli were counted by direct microscopy. The percentage of infected cells and the number of bacteria per cell were determined.
The percentage of infected cells was confirmed by a fluorescence-quenching technique as described previously (23, 32). Briefly, cytokine-treated and nontreated monocytes were incubated with FITC-labeled bacteria for 2 h at 37°C. To quench the fluorescence of noningested but membrane-associated bacteria, cells were washed and resuspended in sodium acetate buffer (0.05 M, pH 4.5) containing 0.06% trypan blue for 5 min at 4°C. After two washes with sodium acetate buffer, the number of cells loaded with bacteria was measured by flow cytometry. Appropriate controls were included to validate the quenching of surface fluorescence.
Statistical analysis.
Statistical analysis was done by Student's t test, and a P value of <0.05 was considered significant.
RESULTS
Inhibition of resting CD4+ and γδ T-cell expansion in response to M. tuberculosis by IL-10 and TGF-β.
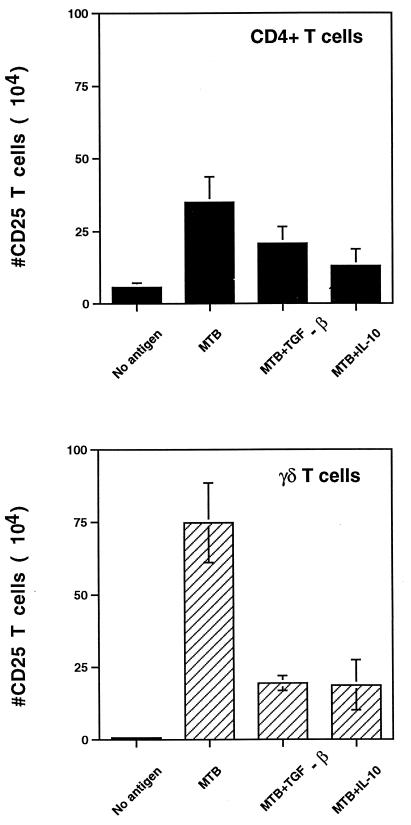
In preliminary studies, 1 to 10 ng of IL-10 and TGF-β per ml inhibited proliferation ([3H]thymidine incorporation) and IFN-γ production by PBMC in response to M. tuberculosis. The concentrations of IL-10 and TGF-β in these experiments were based on the amounts of IL-10 and TGF-β produced by M. tuberculosis-infected monocytes (28). To determine the effect of IL-10 and TGF-β on memory CD4+ and γδ T-cell activation, PBMC from healthy tuberculin skin test-positive individuals were stimulated with live M. tuberculosis bacilli in the presence or absence of IL-10 and TGF-β. After 7 days, viable cells were harvested, counted, and analyzed by two-color flow cytometry for CD25 expression on CD4+ and γδ T cells. Results are expressed as the number of CD25+ T cells for each subset on day 7. As shown in Fig. 1, IL-10 and TGF-β inhibited the expansion of CD25+ CD4+ and γδ T cells in response to M. tuberculosis (P value for cytokine- versus non-cytokine-treated cultures, <0.05; n = 7 for TGF-β; n = 3 for IL-10). IL-10 and TGF-β were equally effective for each T-cell subset; however, CD25+ γδ T cells were inhibited more than CD25+ CD4+ T cells.
FIG. 1.
CD25 expression on CD4+ and γδ T cells analyzed by two-color flow cytometry after M. tuberculosis stimulation. PBMC from purified protein derivative-positive donors (2 × 106 cells/well) were stimulated with M. tuberculosis (MTB) (2.5 × 106 bacilli/well) in the presence or absence of IL-10 or TGF-β. After 1 week, cells were harvested, counted, stained with CD25-PE and CD4-FITC or TCRδ1, and analyzed by two-color flow cytometry. Results represent the number (mean ± standard error of the mean) of CD25+ CD4+ and CD25+ γδ TCR+ T cells on day 7 (P value for cytokine-treated versus nontreated wells, <0.05).
IL-2 rescues IL-10- and TGF-β-mediated inhibition of M. tuberculosis-reactive CD4+ IL-2R+ cells and γδ IL-2R+ T-cell expansion.
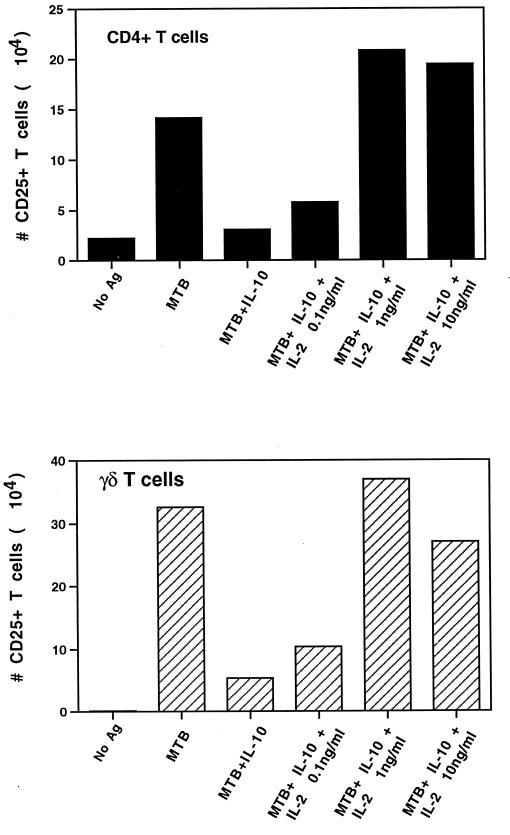
To further elucidate the mechanism of inhibition of M. tuberculosis-reactive CD4+ and γδ T-cell expansion by TGF-β and IL-10, the ability of recombinant IL-2 to rescue the inhibition of both T-cell subsets by IL-10 and TGF-β was analyzed. Human PBMC were cultured with IL-10 or TGF-β (10 ng/ml) and with different doses of recombinant IL-2. Supernatants were harvested at day 3 to measure IFN-γ, and at day 7 cells were harvested and analyzed for the expansion of CD4+ IL-2 receptor-positive (IL-2R+) and γδ IL-2R+ T cells. As shown in Fig. 2, recombinant IL-2 effectively rescued the inhibition of both CD4+ and γδ T-cell expansion by IL-10. Similar results were obtained when IL-2 was added to TGF-β-treated cultures. Accordingly, recombinant IL-2 rescued the inhibition of IFN-γ production by M. tuberculosis-stimulated PBMC by IL-10 and TGF-β (data not shown).
FIG. 2.
CD25 expression on CD4+ and γδ T cells analyzed by two-color flow cytometry after M. tuberculosis stimulation and treatment with IL-10 and IL-2. PBMC from purified protein derivative-positive donors (2 × 106 cells/well) were stimulated with M. tuberculosis (MTB) (2.5 × 106 bacilli/well) in the presence or absence of IL-10 (10 ng/ml) and different doses of IL-2. After 1 week, cells were harvested, counted, stained with CD25-PE and CD4-FITC or TCRδ1, and analyzed by two-color flow cytometry. Results represent the number of CD25+ CD4+ and CD25+ γδ TCR+ T cells on day 7 in one representative experiment. A similar result was obtained when IL-2 was added to TGF-β-treated cultures. Standard error of the mean did not exceed 10%. Ag, antigen.
Effect of IL-10 and TGF-β on proliferation and IFN-γ production by CD4+ and γδ T cells in response to M. tuberculosis.
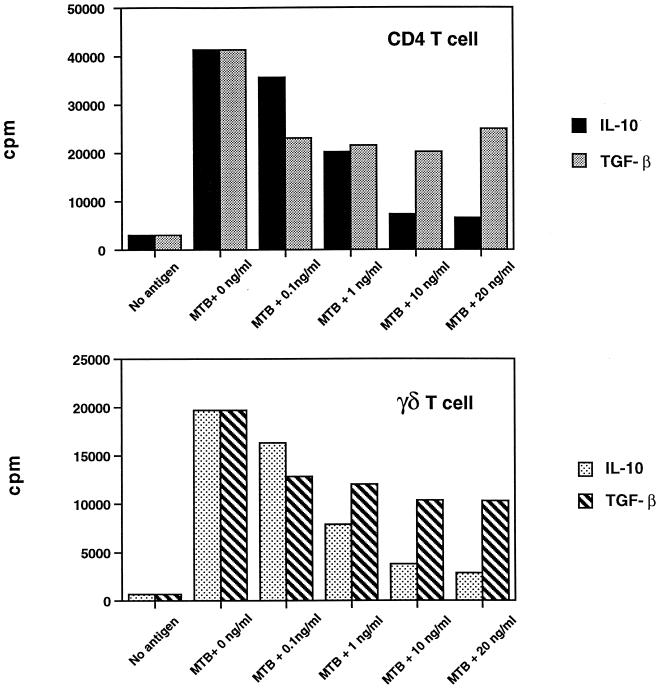
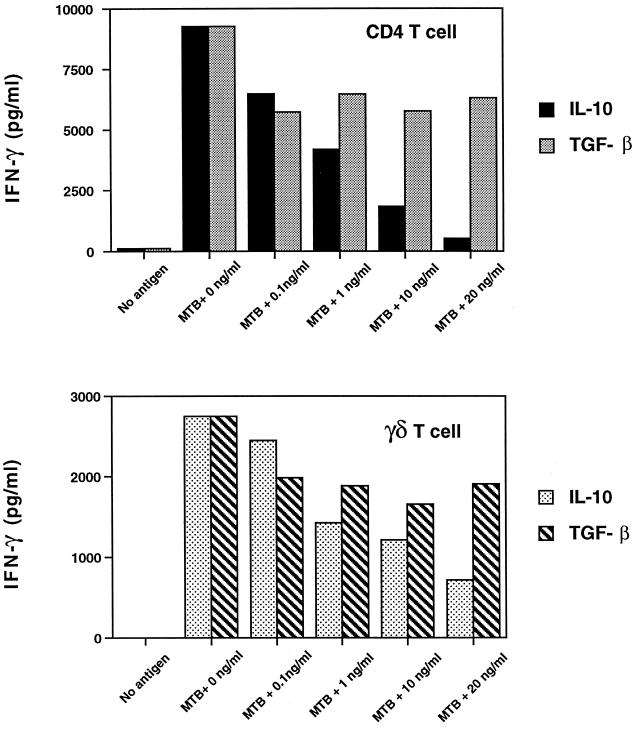
To study the effect of IL-10 and TGF-β on different T-cell subsets, M. tuberculosis-activated CD4+ and γδ T cells were purified from the same donor by positively selecting T cells with magnetic microbeads from PBMC stimulated by M. tuberculosis for 7 days. Positively selected T cells were restimulated with M. tuberculosis, with autologous irradiated monocytes as APC, and with or without IL-10 or TGF-β. After 3 days, proliferation and IFN-γ production were measured. As shown in a representative experiment in Fig. 3, both IL-10 and TGF-β inhibited CD4+ and γδ T-cell proliferation (P, <0.05; n = 4). IL-10 was a more potent inhibitor than TGF-β at 10 to 20 ng/ml for both T-cell subsets (P, <0.05; n = 4). IL-10 and TGF-β inhibited IFN-γ production by both CD4+ and γδ T cells to the same extent as proliferation (Fig. 4). CD4+ T cells were as sensitive to the inhibitory effects of IL-10 and TGF-β as γδ T cells. In contrast to the results obtained with IL-10, inhibition was maximal at 0.1 to 1 ng of TGF-β per ml. Higher concentrations of TGF-β did not enhance inhibition (Fig. 3 and 4). No synergistic inhibition was observed when IL-10 and TGF-β were added together (data not shown).
FIG. 3.
Effect of IL-10 and TGF-β on M. tuberculosis-stimulated CD4+ and γδ TCR+ T-cell proliferation. Positively selected CD4+ and γδ T cells (5 × 104 cells/well) from M. tuberculosis-stimulated PBMC were cocultured with irradiated monocytes (105 cells/well), M. tuberculosis (MTB) (106 bacilli/well), and different concentrations of IL-10 or TGF-β. After 3 days, [3H]thymidine incorporation was measured and expressed as counts per minute. One representative experiment of four is shown. The standard error of the mean of triplicates did not exceed 10%. Both IL-10 and TGF-β inhibited CD4+ and γδ T-cell proliferation (n = 4; P, <0.05), but IL-10 was a more potent inhibitor than TGF-β for both CD4+ and γδ T cells.
FIG. 4.
Effect of IL-10 and TGF-β on IFN-γ production by M. tuberculosis-stimulated CD4+ and γδ TCR+ T cells. Positively selected CD4 and γδ T cells (5 × 104 cells/well) from M. tuberculosis-stimulated PBMC were cocultured with irradiated monocytes (105 cells/well), M. tuberculosis (MTB) (106 bacilli/well), and different concentrations of IL-10 or TGF-β. After 3 days, supernatants were collected and IFN-γ levels were measured by an ELISA. Data represent the mean of triplicates from a representative experiment; the standard error of the mean did not exceed 10%. IL-10 inhibited IFN-γ production by both CD4+ and γδ T cells (n = 4; P, <0.05), but TGF-β was a less potent inhibitor than IL-10 for CD4+ and γδ T cells.
Effect of IL-10 and TGF-β on anti-CD3 antibody stimulation of CD4+ and γδ T cells.
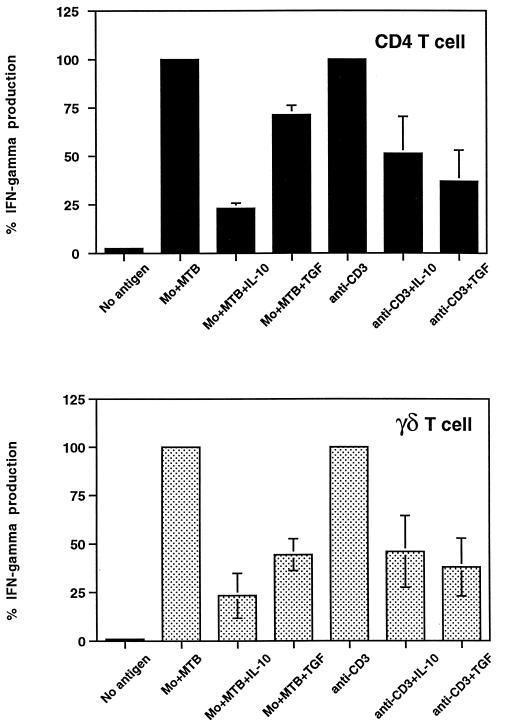
To determine if IL-10 and TGF-β inhibited CD4+ and γδ T cells directly, T cells alone were stimulated with plate-bound anti-CD3 antibody in the presence or absence of 10 ng of IL-10 and TGF-β per ml. In parallel, T cells were stimulated with M. tuberculosis-infected APC for comparison. As shown in Fig. 5, IL-10 and TGF-β inhibited IFN-γ secretion by CD4+ and γδ T cells when T cells were activated directly through the TCR in the absence of APC. The results shown are the average percent inhibition for three experiments.
FIG. 5.
Direct inhibition by IL-10 and TGF-β of CD4+ and γδ TCR+ T-cell IFN-γ production. Positively selected CD4+ and γδ T cells (5 × 104 or 5 × 105 cells/well) were stimulated with either M. tuberculosis-infected macrophages (Mo+MTB) (105 cells/well) or plate-bound anti-CD3 antibodies (1 μg/ml). IL-10 or TGF-β (10 ng/ml) was added on day 0; on day 3, supernatants were harvested and IFN-γ levels were measured by an ELISA. Inhibition of IFN-γ production in the presence of cytokines is expressed as the percentage of maximal production in response to M. tuberculosis or anti-CD3 antibodies. Results represent the mean of three different experiments ± the standard error of the mean. Inhibition by IL-10 and TGF-β was significant for both M. tuberculosis- and anti-CD3 antibody-stimulated IFN-γ production by CD4+ and γδ T cells (n = 3; P, <0.05).
When anti-CD3 antibody stimulation was compared to stimulation by M. tuberculosis-infected monocytes, the degrees of inhibition by TGF-β were similar for both T-cell subsets. IL-10 tended to have a greater inhibitory effect on infected monocyte-mediated activation of CD4+ T cells than on anti-CD3 antibody activation. The same patterns of inhibition by IL-10 and TGF-β were observed when T-cell proliferation was measured (data not shown).
Effect of IL-10 and TGF-β on monocyte APC function for CD4+ and γδ T cells.
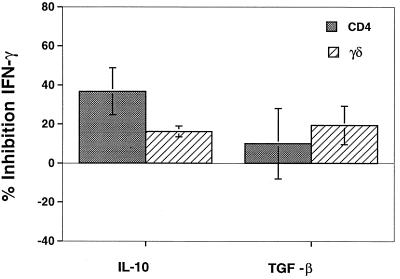
To determine the effect of IL-10 and TGF-β on the APC function of M. tuberculosis-infected monocytes, freshly isolated MHC-matched monocytes were infected with M. tuberculosis in the presence or absence of 10 ng of IL-10 or TGF-β per ml for 24 h. Monocytes then were fixed in 1% paraformaldehyde and added to T cells, and IFN-γ in 72-h supernatants was measured by an ELISA. Figure 6 shows the mean percent inhibition for three experiments in which cytokine-treated infected monocytes were compared to nontreated cells. IL-10 inhibited APC function significantly (P, <0.05) for both CD4+ and γδ T cells. In contrast, TGF-β treatment had no significant effect on APC function for either T-cell subset. In these experiments, all cytokines were removed before the addition of fixed cells to T cells; therefore, only events preceding fixation were influenced by IL-10 and TGF-β.
FIG. 6.
Effect of IL-10 and TGF-β on APC function. Monocytes were infected with M. tuberculosis in the presence or absence of IL-10 or TGF-β for 24 h and then fixed with 1% paraformaldehyde. Fixed APC were cocultured with CD4+ or γδ T cells for 3 days, and IFN-γ levels were measured by an ELISA. The percent inhibition of IFN-γ secretion by CD4+ or γδ T cells after stimulation with IL-10- or TGF-β-treated APC is shown. IL-10 inhibited IFN-γ production by CD4+ and γδ T cells (P value for cytokine-treated versus non-cytokine-treated monocytes, <0.05; n = 3), while TGF-β did not affect monocyte APC function (P >0.05; n = 3). Error bars show the standard error of the mean.
One mechanism for decreased APC function could be inhibition of mycobacterial uptake by monocytes. As shown in Table 1, IL-10 and TGF-β treatment did not affect uptake and infection of M. tuberculosis by monocytes. These results, obtained by microscopy, were confirmed with a fluorescence-quenching method in which extracellular bacteria are quenched by trypan blue (0.06%) and the percentage of infected cells is measured by flow cytometry (data not shown).
TABLE 1.
Quantification of intracellular bacteria in monocytes treated or not treated with IL-10 or TGF-βa
| Cytokine | Expt | % of cells
that:
|
||||
|---|---|---|---|---|---|---|
| Were uninfected | Contained
|
Were infected (total) | ||||
| 1–2 b/c | 3–10 b/c | >10 b/c | ||||
| None | 1 | 32 | 21 | 21 | 26 | 68 |
| 2 | 14 | 36 | 29 | 21 | 86 | |
| 3 | 12 | 11 | 21 | 56 | 88 | |
| Mean ± SE | 19 ± 6.3 | 23 ± 7.5 | 24 ± 2.9 | 34 ± 11 | 81 ± 6.3 | |
| Il-10 | 1 | 29 | 13 | 26 | 32 | 71 |
| 2 | 14 | 36 | 29 | 21 | 86 | |
| 3 | 25 | 6 | 20 | 49 | 75 | |
| Mean ± SE | 23 ± 4.6 | 18 ± 9.2 | 25 ± 2.9 | 34 ± 8 | 77 ± 4.6 | |
| TGF-β | 1 | 32 | 17 | 20 | 31 | 68 |
| 2 | 23 | 32 | 31 | 14 | 77 | |
| 3 | 22 | 9 | 20 | 49 | 78 | |
| Mean ± SE | 26 ± 3.5 | 19 ± 6.9 | 24 ± 3.5 | 31 ± 10 | 74 ± 3.5 | |
Monocytes were purified by adherence and treated with IL-10 or TGF-β (10 ng/ml). After 24 h, cells were washed and infected with M. tuberculosis for 2 h. Infected monocytes were stained by the Ziehl-Neelsen method, and acid-fast bacilli were counted by direct microscopy. No significant differences were observed in the percentages of infected cells between cytokine-treated and non-cytokine-treated monocytes (P, >0.05; n = 3). b/c, bacilli per cell.
Class I and class II MHC, CD40, CD80 (B7-1), and CD86 (B7-2) expression on M. tuberculosis-infected monocytes treated with IL-10 and TGF-β.
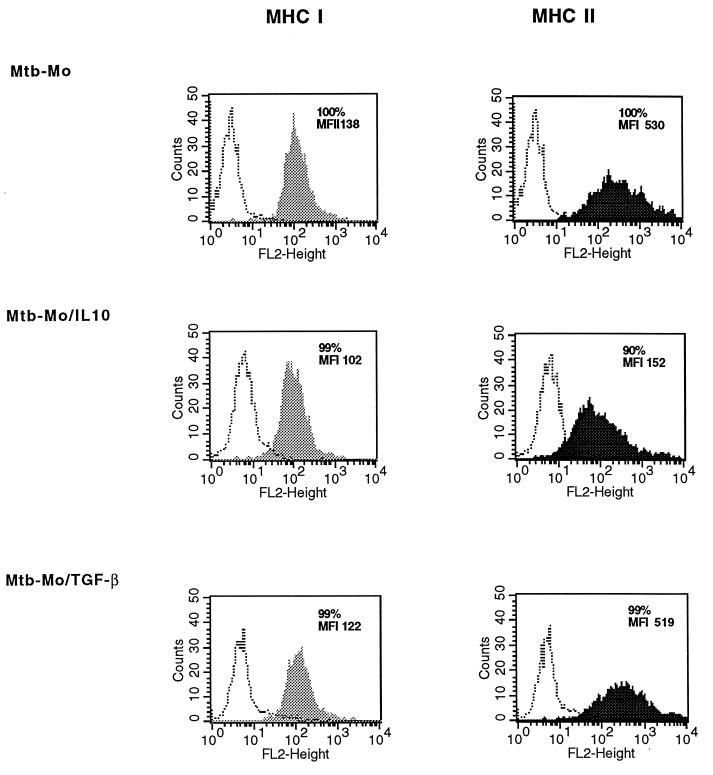
The effects of IL-10 and TGF-β on the expression of MHC and costimulatory molecules on monocytes was measured by flow cytometry to determine the mechanism for the differential effects of IL-10 and TGF-β on APC function. Constitutive and M. tuberculosis-induced expression of cell surface molecules important in APC function on monocytes was measured by flow cytometry in the presence or absence of 10 ng of IL-10 and TGF-β per ml. As shown in a representative experiment, treatment with IL-10 for 24 h resulted in the down-regulation of class II MHC expression on M. tuberculosis-infected monocytes (mean fluorescence index [MFI], 730 ± 106 versus 467 ± 175; n = 3; P, <0.05) (Fig. 7). A similar pattern was noted for constitutive class II MHC expression. IL-10 did not significantly down-regulate class I MHC expression (MFI, 287 ± 84 versus 178 ± 40; n = 3; P, >0.05).
FIG. 7.
Class I and class II MHC expression on monocytes analyzed by flow cytometry. M. tuberculosis-infected macrophages (Mtb-Mo) were treated with IL-10 or TGF-β for 24 h. Cells were stained with rat anti-class I MHC or rat anti-class II MHC IgG followed by PE-conjugated goat anti-rat IgG and analyzed by one-color flow cytometry. Monocytes were gated according to their side scatter and forward angle scatter parameters and were 85 to 95% CD14+ CD3−. The percentages of class I MHC- and class II MHC-positive cells and the MFI (mean fluorescence of cells with specific antibody − mean fluorescence of cells with matched isotype IgG) are indicated. One representative experiment of three is shown. FL2 height represents PE fluorescence (log scale). Open histograms represent fluorescence with matched isotype control antibody; shaded and closed histograms represent staining for MHC I and MHC II, respectively.
TGF-β did not significantly down-regulate class II MHC expression on infected monocytes (MFI, 730 ± 106 versus 644 ± 107; n = 3; P, >0.05). TGF-β also did not affect constitutive class II MHC or class I MHC expression.
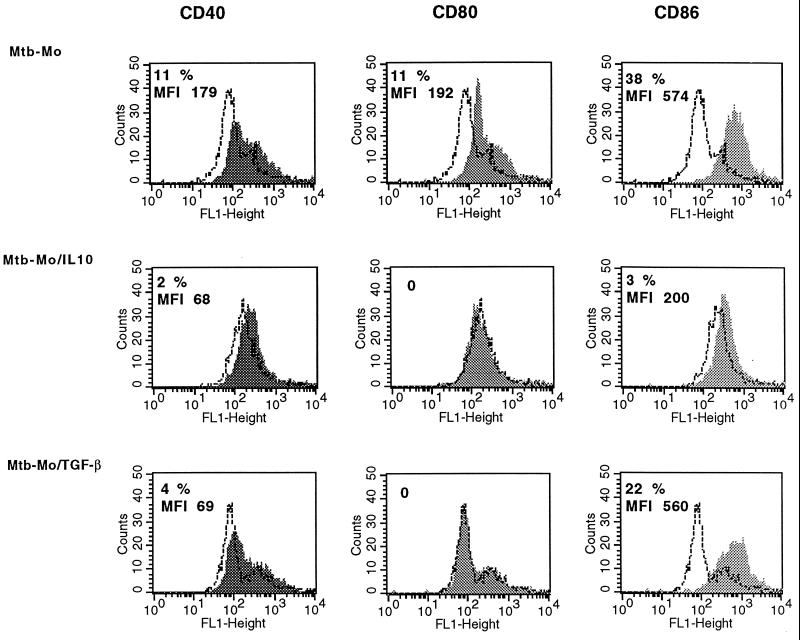
When the effect of IL-10 on CD40, CD80, and CD86 expression was measured after M. tuberculosis infection, inhibition of expression of all three molecules was observed (Fig. 8). TGF-β down-regulated CD40 and CD80 expression but did not have any significant effect on CD86 expression.
FIG. 8.
CD40, CD80 (B7-1), and CD86 (B7-2) surface expression on monocytes analyzed by flow cytometry. M. tuberculosis-infected macrophages (Mtb-Mo) were treated with IL-10, TGF-β or no cytokine for 24 h. Cells were stained with mouse anti-CD40, anti-CD80, or anti-CD86 IgG followed by PE-conjugated goat anti-mouse IgG and analyzed by one-color flow cytometry. Monocytes were gated according to their SSC and FSC parameters and were 85 to 95% CD14+ CD3−. The percentages of CD40-, CD80-, and CD86-positive cells and the MFI (mean fluorescence of cells with specific antibody − mean fluorescence of cells with matched isotype IgG) are indicated. One representative experiment of three is shown. FL1 height represents FITC fluorescence. Open histograms represent fluorescence with isotype control antibody. Shaded histograms represent staining for CD40, CD80, or CD86.
DISCUSSION
The activation of T cells in response to M. tuberculosis-infected macrophages takes place in a complex cytokine environment where proinflammatory cytokines produced by macrophages and T cells are balanced by inhibitory cytokines, such as IL-10 and TGF-β. Increased production of IL-10 and TGF-β in tuberculosis patients has been observed and could contribute to the inability of macrophages and T cells to control M. tuberculosis infection (20, 21, 33, 35, 67). Little is known about the effect of these inhibitory cytokines on T-cell subset function in response to M. tuberculosis. Our results suggest that both CD4+ and γδ T-cell responses to M. tuberculosis are inhibited by IL-10 and TGF-β. However, IL-10 and TGF-β differed in the degree of inhibition and in the mechanisms used to inhibit T-cell responses.
Inhibitory activities of TGF-β and IL-10 for human and mouse T-cell responses have been documented in numerous studies (1, 18, 22, 24, 43, 52, 55). However most of these studies have been performed with PBMC or CD4+ T-cell lines and clones. There have been few studies on the effect of IL-10 or TGF-β on non-CD4+ T cells and no studies comparing T-cell subsets. We found that the M. tuberculosis-stimulated expansion of resting γδ T cells was particularly sensitive to inhibition by both IL-10 and TGF-β. Inhibition of γδ T-cell expansion has been reported previously for IL-10 but not for TGF-β (58). In tuberculin-sensitized individuals, γδ T cells expand dramatically upon stimulation with mycobacteria in vitro (8, 9, 30, 31, 38, 39, 51, 68). Furthermore, BCG vaccination enhances γδ T-cell responses to mycobacterial antigens (36). Since γδ T-cell expansion is dependent in part on IL-2 produced by mycobacterial antigen-specific CD4+ T cells, inhibition of γδ T-cell expansion by IL-10 and TGF-β could be mediated in part by diminished CD4+ T-cell help (58). The finding that exogenous IL-2 counteracted the inhibitory effects of IL-10 and TGF-β on M. tuberculosis-stimulated CD4+ and γδ TCR+ T cells is consistent with previous reports (19, 43, 64).
M. tuberculosis-activated CD4+ T cells were as sensitive to the inhibitory effects of IL-10 and TGF-β as γδ T cells. IL-10 was more potent overall than TGF-β. No synergy between IL-10 and TGF-β was observed, suggesting that these cytokines could modulate but not completely shut down T-cell responses to M. tuberculosis. Others have reported inhibition by IL-10 and TGF-β of T-cell proliferation and cytokine secretion (6, 37, 43, 62, 64, 70), but few have used APC-free systems (1, 19, 29, 63). We found that IL-10 and TGF-β directly inhibited M. tuberculosis-activated CD4+ and γδ T cells with anti-CD3 antibodies as a stimulus in an APC-free system. Both T-cell proliferation and IFN-γ secretion were affected. We did not observe discordance between proliferation and IFN-γ production, as was noted in one study in which IL-10 treatment of T-cell clones decreased proliferation and IL-2 production but not IFN-γ secretion after stimulation with anti-CD3 or anti-CD2 antibodies or mitogens (19). The mechanisms for direct T-cell inhibition by IL-10 and TGF-β include down-regulation of IL-2 gene transcription (43, 64) and of IL-2 receptor expression (44). In addition, TGF-β inhibits TCR- and IL-2 receptor-mediated tyrosine phosphorylation (1), and IL-10 inhibits MAP kinases (59), facts which may explain why CD4+ and γδ T cells were equally sensitive to direct inhibition by IL-10 and TGF-β.
In addition to direct effects on T cells, IL-10 affected monocyte APC function for both CD4+ and γδ T cells. The first step in antigen processing, i.e., mycobacterial uptake by monocytes, was not affected by pretreatment or treatment during uptake with IL-10 or TGF-β and therefore could not explain decreased APC function, according to our data. Up-regulation of endocytosis in IL-10-treated dendritic cells has been described (49, 53), and there are contradictory findings on the role of TGF-β in regulating phagocytosis by macrophages (3, 35, 72).
APC function for CD4+ T cells was more sensitive to IL-10 than APC function for γδ T cells. The effect of TGF-β was not significant. IL-10 and TGF-β have been implicated in the regulation of many aspects of macrophage function, including bacterial uptake, costimulatory function, and antigen processing and presentation. The effect of IL-10 on monocyte APC for M. tuberculosis antigens to CD4+ T cells correlated with the down-regulation of class II MHC expression on monocytes, consistent with previous reports on the role of IL-10 in down-regulating class II MHC expression on monocytes (18, 26, 45, 56). The IL-10-mediated effects on class II MHC expression on monocytes do not exclude the possibility that IL-10 inhibits the intracellular processing of M. tuberculosis and presentation to for CD4+ T cells. The minimal effect of TGF-β on APC function for CD4+ T cells correlated with the minimal inhibition of surface class II MHC expression. The effect of TGF-β on class II MHC expression may depend on the type of mononuclear phagocyte population used. Czarniecki et al. (16) noted decreased class II MHC expression on monocytes, and Bonham et al. (7) noted no effect on human bone marrow macrophages.
Down-regulation of class I and class II MHC expression on monocytes does not explain the inhibition of APC function for γδ T cells by IL-10, since processing and presentation of mycobacterial antigens for γδ T cells are not dependent on these molecules. A unique aspect of human γδ T cells is their ability to recognize structurally defined nonpeptide antigens (14, 15, 65, 66). Recognition of these phosphorylated molecules or isoprenoids by γδ T cells does not require antigen processing or known antigen-presenting elements. However, T-cell–APC contact is required for optimal activation of γδ T cells with these small phosphate-containing molecules (46, 54). Furthermore, a previous study by our group with whole M. tuberculosis bacilli indicated that antigens for γδ T cells are processed and remain stably associated on the surface of monocytes (2). Therefore, the effect of IL-10 on APC function for γδ T cells may be attributable to the inhibition of whole bacterial processing, the inhibition of antigen presentation on some still-unknown molecule, or the down-regulation of APC costimulatory functions. We showed here that IL-10 and, to a lesser extent, TGF-β down-regulated the expression of the costimulatory molecules CD80, CD86, and CD40 on APC. For costimulatory molecule expression, IL-10 also had a greater inhibitory effect than TGF-β. Therefore, the down-regulation of APC costimulatory functions may be one mechanism to explain the reduction of T-cell responses.
In summary, IL-10 and TGF-β both can inhibit CD4+ and γδ T-cell responses to M. tuberculosis without preferentially inhibiting one T-cell subset over another. Overall, IL-10 was a more potent inhibitor than TGF-β, and the two cytokines differed in the predominant mechanism used to inhibit the interaction of T cells and M. tuberculosis-infected monocytes. TGF-β was primarily a direct inhibitor of T-cell proliferation and IFN-γ production, with minimal inhibition of APC function. IL-10, on the other hand, affected both T cells directly and monocyte APC function. These results support a model in which IL-10 and TGF-β produced by M. tuberculosis-infected macrophages can down-regulate T-cell responses without completely shutting them down. In health, these cytokines would diminish the deleterious inflammatory responses associated with T-cell immunity to mycobacterial antigens once infection has been controlled. In disease, the overproduction of IL-10 and TGF-β renders T-cell responses ineffectual for adequate control of mycobacterial growth. Thus, the balance of proinflammatory and inhibitory cytokines can regulate the interaction between T cells and macrophages in M. tuberculosis infection. Shifting the balance away from inhibitory cytokine pathways may improve immunotherapeutic and vaccine development strategies for tuberculosis.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants AI-27243, HL-55967, and AI-41717.
REFERENCES
- 1.Ahuja S S, Paliogianni F, Yamada H, Balow J E, Boumpas D T. Effect of transforming growth factor-beta on early and late activation events in human T cells. J Immunol. 1993;150:3109–3118. [PubMed] [Google Scholar]
- 2.Balaji K N, Boom W H. Processing of Mycobacterium tuberculosis bacilli by human monocytes for CD4+αβ and γδ T cells: role of particulate antigen. Infect Immun. 1998;66:98–106. doi: 10.1128/iai.66.1.98-106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balaji K N, Schwander S, Rich E A, Boom W H. Alveolar macrophages as accessory cells for human γδ T cells activated by M. tuberculosis. J Immunol. 1995;154:5959–5968. [PubMed] [Google Scholar]
- 4.Barnes P F, Lu S, Abrams J S, Wang E, Yamamura M, Modlin R L. Cytokine production at the site of disease in human tuberculosis. Infect Immun. 1993;61:3482–3489. doi: 10.1128/iai.61.8.3482-3489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes P F, Mistry S D, Cooper C L, Pirmez C, Rea T H, Modlin R L. Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J Immunol. 1989;142:1114–1119. [PubMed] [Google Scholar]
- 6.Bejarano M T, de Waal Malefyt R, Abrams J S, Bigler M, Bacchetta R, de Vries J E, Roncarolo M G. Interleukin 10 inhibits allogeneic proliferative and cytotoxic T cell responses generated in primary mixed lymphocyte cultures. Int Immunol. 1992;4:1389–1397. doi: 10.1093/intimm/4.12.1389. [DOI] [PubMed] [Google Scholar]
- 7.Bonham C A, Lu L, Banas R A, Fontes P, Rao A S, Starzl T E, Zeevi A, Thomson A W. TGF-beta 1 pretreatment impairs the allostimulatory function of human bone marrow-derived antigen-presenting cells for both naive and primed T cells. Transplant Immunol. 1996;4:186–191. doi: 10.1016/s0966-3274(96)80015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boom W H, Balaji K N, Nayak R, Tsukaguchi K, Chervenak K A. Characterization of a 10- to 14-kilodalton protease-sensitive Mycobacterium tuberculosisH37Ra antigen that stimulates human γδ T cells. Infect Immun. 1994;62:5511–5518. doi: 10.1128/iai.62.12.5511-5518.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boom W H, Chervenak K A, Mincek M A, Ellner J J. Role of the mononuclear phagocyte as an antigen-presenting cell for human gamma delta T cells activated by live Mycobacterium tuberculosis. Infect Immun. 1992;60:3480–3488. doi: 10.1128/iai.60.9.3480-3488.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boom W H, Wallis R S, Chervenak K A. Human Mycobacterium tuberculosis-reactive CD4+T-cell clones: heterogeneity in antigen recognition, cytokine production, and cytotoxicity for mononuclear phagocytes. Infect Immun. 1991;59:2737–2743. doi: 10.1128/iai.59.8.2737-2743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerwenka A, Bevec D, Majdic O, Knapp W, Holter W. TGF-beta 1 is a potent inducer of human effector T cells. J Immunol. 1994;153:4367–4377. [PubMed] [Google Scholar]
- 12.Cerwenka A, Kovar H, Majdic O, Holter W. Fas- and activation-induced apoptosis are reduced in human T cells preactivated in the presence of TGF-beta 1. J Immunol. 1996;156:459–464. [PubMed] [Google Scholar]
- 13.Comstock G W. Epidemiology of tuberculosis. Am Rev Respir Dis. 1982;125:8–15. doi: 10.1164/arrd.1982.125.3P2.8. [DOI] [PubMed] [Google Scholar]
- 14.Constant P, Davodeau F, Peyrat M A, Poquet Y, Puzo G, Bonneville M, Fournie J J. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 15.Constant P, Poquet Y, Peyrat M-A, Davodeau F, Bonneville M, Fournie J-J. The antituberculous Mycobacterium bovisBCG vaccine is an attenuated mycobacterial producer of phosphorylated nonpeptidic antigens for human gamma delta T cells. Infect Immun. 1995;63:4628–4633. doi: 10.1128/iai.63.12.4628-4633.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czarniecki C W, Chiu H H, Wong G H, McCabe S M, Palladino M A. Transforming growth factor-beta 1 modulates the expression of class II histocompatibility antigens on human cells. J Immunol. 1988;140:4217–4223. [PubMed] [Google Scholar]
- 17.de Jong R, van Lier R A, Ruscetti F W, Schmitt C, Debre P, Mossalayi M D. Differential effect of transforming growth factor-beta 1 on the activation of human naive and memory CD4+ T lymphocytes. Int Immunol. 1994;6:631–638. doi: 10.1093/intimm/6.4.631. [DOI] [PubMed] [Google Scholar]
- 18.de Waal Malefyt R, Haanen J, Spits H, Roncarolo M G, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries J E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Waal Malefyt R, Yssel H, de Vries J E. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993;150:4754–4765. [PubMed] [Google Scholar]
- 20.Dlugovitzky D, Bay M L, Rateni L, Urizar L, Rondelli C F, Largacha C, Farroni M A, Molteni O, Bottasso O A. In vitro synthesis of interferon-gamma, interleukin-4, transforming growth factor-beta and interleukin-1 beta by peripheral blood mononuclear cells from tuberculosis patients: relationship with the severity of pulmonary involvement. Scand J Immunol. 1999;49:210–217. doi: 10.1046/j.1365-3083.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- 21.Dlugovitzky D, Torres-Morales A, Rateni L, Farroni M A, Largacha C, Molteni O, Bottasso O. Circulating profile of Th1 and Th2 cytokines in tuberculosis patients with different degrees of pulmonary involvement. FEMS Immunol Med Microbiol. 1997;18:203–207. doi: 10.1111/j.1574-695X.1997.tb01046.x. [DOI] [PubMed] [Google Scholar]
- 22.Espevik T, Waage A, Faxvaag A, Shalaby M R. Regulation of interleukin-2 and interleukin-6 production from T-cells: involvement of interleukin-1 beta and transforming growth factor-beta. Cell Immunol. 1990;126:47–56. doi: 10.1016/0008-8749(90)90299-7. [DOI] [PubMed] [Google Scholar]
- 23.Fattorossi A, Nisini R, Pizzolo J G, D'Amelio R. New, simple flow cytometry technique to discriminate between internalized and membrane-bound particles in phagocytosis. Cytometry. 1989;10:320–325. doi: 10.1002/cyto.990100311. [DOI] [PubMed] [Google Scholar]
- 24.Fiorentino D F, Bond M W, Mosmann T R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiorentino D F, Zlotnik A, Mosmann T R, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 26.Fiorentino D F, Zlotnik A, Vieira P, Mosmann T R, Howard M, Moore K W, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 27.Fontana A, Frei K, Bodmer S, Hofer E, Schreier M H, Palladino M A, Jr, Zinkernagel R M. Transforming growth factor-beta inhibits the generation of cytotoxic T cells in virus-infected mice. J Immunol. 1989;143:3230–3234. [PubMed] [Google Scholar]
- 28.Fulton S A, Cross J V, Toossi Z T, Boom W H. Regulation of interleukin-12 by interleukin-10, transforming growth factor-beta, tumor necrosis factor-alpha, and interferon-gamma in human monocytes infected with Mycobacterium tuberculosis H37Ra. J Infect Dis. 1998;178:1105–1114. doi: 10.1086/515698. [DOI] [PubMed] [Google Scholar]
- 29.Groux H, Bigler M, de Vries J E, Roncarolo M G. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Havlir D V, Ellner J J, Chervenak K A, Boom W H. Selective expansion of human gamma delta T cells by monocytes infected with live Mycobacterium tuberculosis. J Clin Investig. 1991;87:729–733. doi: 10.1172/JCI115053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Havlir D V, Wallis R S, Boom W H, Daniel T M, Chervenak K, Ellner J J. Human immune response to Mycobacterium tuberculosisantigens. Infect Immun. 1991;59:665–670. doi: 10.1128/iai.59.2.665-670.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hed J, Hallden G, Johansson S G, Larsson P. The use of fluorescence quenching in flow cytofluorometry to measure the attachment and ingestion phases in phagocytosis in peripheral blood without prior cell separation. J Immunol Methods. 1987;101:119–125. doi: 10.1016/0022-1759(87)90224-9. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch C S, Ellner J J, Blinkhorn R, Toossi Z. In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosis by natural inhibitors of transforming growth factor beta. Proc Natl Acad Sci USA. 1997;94:3926–3931. doi: 10.1073/pnas.94.8.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirsch C S, Hussain R, Toossi Z, Dawood G, Shahid F, Ellner J J. Cross-modulation by transforming growth factor beta in human tuberculosis: suppression of antigen-driven blastogenesis and interferon gamma production. Proc Natl Acad Sci USA. 1996;93:3193–3198. doi: 10.1073/pnas.93.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirsch C S, Yoneda T, Averill L, Ellner J J, Toossi Z. Enhancement of intracellular growth of Mycobacterium tuberculosis in human monocytes by transforming growth factor-beta 1. J Infect Dis. 1994;170:1229–1237. doi: 10.1093/infdis/170.5.1229. [DOI] [PubMed] [Google Scholar]
- 36.Hoft D F, Brown R M, Roodman S T. Bacille Calmette-Guerin vaccination enhances human gamma delta T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J Immunol. 1998;161:1045–1054. [PubMed] [Google Scholar]
- 37.Hsu D H, de Waal Malefyt R, Fiorentino D F, Dang M N, Vieira P, de Vries J, Spits H, Mosmann T R, Moore K W. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science. 1990;250:830–832. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- 38.Janis E M, Kaufmann S H, Schwartz R H, Pardoll D M. Activation of gamma delta T cells in the primary immune response to Mycobacterium tuberculosis. Science. 1989;244:713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- 39.Kabelitz D, Bender A, Schondelmaier S, Schoel B, Kaufmann S H. A large fraction of human peripheral blood gamma/delta + T cells is activated by Mycobacterium tuberculosis but not by its 65-kD heat shock protein. J Exp Med. 1990;171:667–679. doi: 10.1084/jem.171.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kabelitz D, Da Silva Lobo M L, Schurmann G, Hofmann W J, Otto G. Presence of gamma/delta T-cell receptor-expressing T cells in liver biopsies following liver transplantation. Hum Immunol. 1990;28:167–169. doi: 10.1016/0198-8859(90)90014-g. [DOI] [PubMed] [Google Scholar]
- 41.Kaufmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 42.Kehrl J H. Transforming growth factor-beta: an important mediator of immunoregulation. Int J Cell Cloning. 1991;9:438–450. doi: 10.1002/stem.1991.5530090502. [DOI] [PubMed] [Google Scholar]
- 43.Kehrl J H, Wakefield L M, Roberts A B, Jakowlew S, Alvarez-Mon M, Derynck R, Sporn M B, Fauci A S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163:1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim S J, Kehrl J H, Burton J, Tendler C L, Jeang K T, Danielpour D, Thevenin C, Kim K Y, Sporn M B, Roberts A B. Transactivation of the transforming growth factor beta 1 (TGF-beta 1) gene by human T lymphotropic virus type 1 tax: a potential mechanism for the increased production of TGF-beta 1 in adult T cell leukemia. J Exp Med. 1990;172:121–129. doi: 10.1084/jem.172.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koppelman B, Neefjes J J, de Vries J E, de Waal Malefyt R. Interleukin-10 down-regulates MHC class II alphabeta peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity. 1997;7:861–871. doi: 10.1016/s1074-7613(00)80404-5. [DOI] [PubMed] [Google Scholar]
- 46.Lang F, Peyrat M A, Constant P, Davodeau F, David-Ameline J, Poquet Y, Vie H, Fournie J J, Bonneville M. Early activation of human V gamma 9V delta 2 T cell broad cytotoxicity and TNF production by nonpeptidic mycobacterial ligands. J Immunol. 1995;154:5986–5994. [PubMed] [Google Scholar]
- 47.Lee Y J, Han Y, Lu H T, Nguyen V, Qin H, Howe P H, Hocevar B A, Boss J M, Ransohoff R M, Benveniste E N. TGF-beta suppresses IFN-gamma induction of class II MHC gene expression by inhibiting class II transactivator messenger RNA expression. J Immunol. 1997;158:2065–2075. [PubMed] [Google Scholar]
- 48.Letterio J J, Roberts A B. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 49.Longoni D, Piemonti L, Bernasconi S, Mantovani A, Allavena P. Interleukin-10 increases mannose receptor expression and endocytic activity in monocyte-derived dendritic cells. Int J Clin Lab Res. 1998;28:162–169. doi: 10.1007/s005990050037. [DOI] [PubMed] [Google Scholar]
- 50.Miller B, Schieffelbein C. Tuberculosis. Bull W H O. 1998;76:141–143. [PMC free article] [PubMed] [Google Scholar]
- 51.Modlin R L, Pirmez C, Hofman F M, Torigian V, Uyemura K, Rea T H, Bloom B R, Brenner M B. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature. 1989;339:544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- 52.Moore K W, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 53.Morel A S, Quaratino S, Douek D C, Londei M. Split activity of interleukin-10 on antigen capture and antigen presentation by human dendritic cells: definition of a maturative step. Eur J Immunol. 1997;27:26–34. doi: 10.1002/eji.1830270105. [DOI] [PubMed] [Google Scholar]
- 54.Morita C T, Beckman E M, Bukowski J F, Tanaka Y, Band H, Bloom B R, Golan D E, Brenner M B. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 55.Mosmann T R. Role of a new cytokine, interleukin-10, in the cross-regulation of T helper cells. Ann N Y Acad Sci. 1991;628:337–344. doi: 10.1111/j.1749-6632.1991.tb17266.x. [DOI] [PubMed] [Google Scholar]
- 56.Murray P J, Wang L, Onufryk C, Tepper R I, Young R A. T cell-derived IL-10 antagonizes macrophage function in mycobacterial infection. J Immunol. 1997;158:315–321. [PubMed] [Google Scholar]
- 57.Orme I M, Lee B Y, Appelberg R, Miller E S, Chi D L, Griffin J P, Roberts A D. T cell response in acquired protective immunity to Mycobacterium tuberculosis infection. Bull Int Union Tuberc Lung Dis. 1991;66:7–13. [PubMed] [Google Scholar]
- 58.Pechhold K, Wesch D, Schondelmaier S, Kabelitz D. Primary activation of V gamma 9-expressing gamma delta T cells by Mycobacterium tuberculosis. Requirement for Th1-type CD4 T cell help and inhibition by IL-10. J Immunol. 1994;152:4984–4992. [PubMed] [Google Scholar]
- 59.Perrin G O, Subnamanian P S, Johnson H M. Interleukin 10 (IL-10) directly inhibits the proliferation of CD4+ T cells at the level of the cell cycle by suppressing the MAP kinase cascade. FASEB J. 1999;13:A654. [Google Scholar]
- 60.Ralph P, Nakoinz I, Sampson-Johannes A, Fong S, Lowe D, Min H Y, Lin L. IL-10, T lymphocyte inhibitor of human blood cell production of IL-1 and tumor necrosis factor. J Immunol. 1992;148:808–814. [PubMed] [Google Scholar]
- 61.Schmitt E, Hoehn P, Huels C, Goedert S, Palm N, Rude E, Germann T. T helper type 1 development of naive CD4+ T cells requires the coordinate action of interleukin-12 and interferon-gamma and is inhibited by transforming growth factor-beta. Eur J Immunol. 1994;24:793–798. doi: 10.1002/eji.1830240403. [DOI] [PubMed] [Google Scholar]
- 62.Sher A, Gazzinelli R T, Oswald I P, Clerici M, Kullberg M, Pearce E J, Berzofsky J A, Mosmann T R, James S L, Morse H C D. Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev. 1992;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 63.Taga K, Mostowski H, Tosato G. Human interleukin-10 can directly inhibit T-cell growth. Blood. 1993;81:2964–2971. [PubMed] [Google Scholar]
- 64.Taga K, Tosato G. IL-10 inhibits human T cell proliferation and IL-2 production. J Immunol. 1992;148:1143–1148. [PubMed] [Google Scholar]
- 65.Tanaka Y, Morita C, Tanaka Y, Nieves E, Brenner M B, Bloom B M. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 66.Tanaka Y, Sano S, Nieves E, De-Libero G, Rosa D, Modlin R L, Brenner M B, Bloom B R, Morita C T. Nonpeptide ligands for human gamma delta T cells. Proc Natl Acad Sci USA. 1994;91:8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toossi Z, Gogate P, Shiratsuchi H, Young T, Ellner J J. Enhanced production of TGF-beta by blood monocytes from patients with active tuberculosis and presence of TGF-beta in tuberculous granulomatous lung lesions. J Immunol. 1995;154:465–473. [PubMed] [Google Scholar]
- 68.Tsukaguchi K, Balaji K N, Boom W H. CD4+ alpha-beta T cell and gamma delta T cell responses to Mycobacterium tuberculosis: similarities and differences in antigen recognition, cytotoxic effector function, and cytokine production. J Immunol. 1995;154:1786–1796. [PubMed] [Google Scholar]
- 69.Tsunawaki S, Sporn M, Ding A, Nathan C. Deactivation of macrophages by transforming growth factor-beta. Nature. 1988;334:260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 70.Vieira P, de Waal-Malefyt R, Dang M N, Johnson K E, Kastelein R, Fiorentino D F, deVries J E, Roncarolo M G, Mosmann T R, Moore K W. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci USA. 1991;88:1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wallis R S, Amir T M, Ellner J J. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins: the monocyte Western blot. Proc Natl Acad Sci USA. 1990;87:3348–3352. doi: 10.1073/pnas.87.9.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Warwick-Davies J, Lowrie D B, Cole P J. Selective deactivation of human monocyte functions by TGF-beta. J Immunol. 1995;155:3186–3193. [PubMed] [Google Scholar]