Abstract
Alopecia is a common and distressing medical condition that has affected a majority of people worldwide, which leads to great effects on the quality of life and self-esteem. Numerous treatments had been used to cure alopecia, including hair growth stimulants, herbal products, and hair transplantation. However, these treatments have their side effects, such as hypertrichosis, edema, and even cardiovascular adverse effects, which lead to the urgent requirement to explore a new hair-follicle (HF) regeneration approach. Tissue engineering could be the potential way for HF regeneration by simulating the epithelial-mesenchymal interaction and cell-extracellular matrix interactions. This review summarized the potential cells that are used in tissue engineering, commonly used tissue engineering techniques, and most importantly, the biomaterials that have been applied for in vitro three-dimensional cell culture or in vivo co-transplantation in HF regeneration. The literature shows that advances in this field toward functional HF development have progressively increased. Although the clinical application of biomaterial co-transplantation for HF regeneration still faces various challenges, numerous studies have proved that this is a promising direction that could be achieved in the future.
Keywords: Dermal papilla cells, Hair follicle stem cells, Skin-derived precursors, Epithelial-mesenchymal interaction, 3D cell cultures
1. Introduction
Alopecia is a common and distressing medical condition that has affected a majority of people worldwide, especially the middle-aged [1]. Various types of alopecia had been known, including alopecia universalis, androgenic alopecia (AGA), alopecia areata (AA), syphilitic alopecia, telogen effluvium, stress, immune-related alopecia, and chemotherapy-related alopecia. For simplicity, alopecia can be primarily divided into 2 general sub-types, namely, scarring (cicatricial) and non-scarring (non-cicatricial) [2,3]. Clinically, frontal fibrosing alopecia, lichen planopilaris, folliculitis decalvans, and discoid lupus erythematosus are some of the common causes of cicatricial; whereas, AGA, AA, and telogen effluvium are the most common causes of non-cicatricial subtype [4]. Regardless of the etiology, people who suffer from alopecia lose the physiological and aesthetic function of hair follicles (HFs), which greatly affects the quality of life, self-esteem, and even the individual's psychosocial well-being [5]. Various kinds of treatments had been used to cure alopecia, including hair growth stimulants, herbal products, and hair transplantation [6,7]. However, these treatments all have certain limitations. Drugs have drastic side effects, such as hypertrichosis, edema, and even cardiovascular adverse effects [[8], [9], [10]]. Hair transplant is a surgery that moves hair to bald or thinning scalp areas, which requires patients to have good self-conditions, including good HF quality, numerous residual HFs [11], and good recipient area conditions [12]. Therefore, limited patients can meet the requirements for hair transplantation [6].
HFs are essential mini organs in the skin that are comprised of multiple layers that encapsulate and produce the hair shaft, which protrudes through the epidermis [13]. As a unique mammal characteristic, HFs exert multiple functions, including physical protection, thermoregulation, sensory perception, and social interactions [14,15]. Thus, HF regeneration is crucial for patients who suffer from alopecia. In the process of HF regeneration, the intensive cooperation of epithelial and hair-inducive mesenchymal, named epithelial-mesenchymal interaction (EMI), plays a critical role in HF morphogenesis and regeneration [14], which is a prerequisite for functional HF formation, regeneration, and cycling [16]. Healthy HFs undergo life-long cyclical transformations through stages of rapid growth (anagen), regression (catagen), and relative “quiescence” (telogen). Most hair disorders are caused by HF cycling abnormalities [17], which has led to urgent requirements to explore a new approach that has the potential to produce functional HFs, especially for patients with cicatricial alopecia whose HFs had been replaced by fibrous scar tissue [18,19] and HFs were completely dysfunctional [14].
Tissue engineering was a field that applies the principles of engineering and biology to repair the damaged tissue by developing the functional substitutes [20]. For patients with alopecia, regenerating HFs in vivo or in vitro could be a potential approach using tissue engineering to simulate EMI (Fig. 1). Current techniques for cell isolation and culture in vitro make it possible to obtain potential cells, such as dermal papilla cells (DPCs), skin-derived precursors (SKPs), and hair follicle stem cells (HFSCs) [[21], [22], [23]]. These cells can be enmeshed in a biomaterial matrix, which mimics the chemical structure of HF extracellular matrix (ECM) and provides specific effects in regulating cell behaviors, such as cell survival, proliferation, and differentiation [24,25]. This review summarized: (1) The potential cells that are used in tissue engineering for HF regeneration and their underlying molecular mechanisms; (2) Commonly used techniques for 3D cell spheroid formation; (3) Biomaterials that have been used for in vitro 3D cell culture to promote HF regeneration-related gene expression or in vivo co-transplantation to induce de novo HF regeneration. Additionally, the current limitations for the application of tissue engineering in HF regeneration and the future investigation direction are also discussed.
Fig. 1.
The application of tissue engineering in HF regeneration, including materials, models, cells, and growth factors.
2. Growth factors and functional cells involved in HFs regeneration
HF is a dynamic mini organ that undergoes continuous cycling and regeneration throughout its lifetime. Several cells and growth factors with the potential for HF formation have been identified (Fig. 2), which greatly expanded the cell seed bank, reduced the problem of inadequate cell sources, and promoted the development of HF regenerative strategies [14]. This section mainly focuses on the in vitro investigations of functional growth factors and representative cells that involved in the HF regeneration.
Fig. 2.
Growth factors and functional cells involved in HF regeneration. DPCs are located at the bottom of the hair follicle. SKPs are located in the dermis of the skin. HFSCs are located in the bulge region of the outer root sheath of HF.
2.1. Growth factors
Wnt signaling is one of the most critical signaling pathways that controls cell fate and tissue patterning during early embryonic and later development. During HF cycling, Wnt is also considered to be the core pathway [26], which is activated in the dermis [27,28] and could regulate hair shaft differentiation [29]. The overexpression of Wnt ligand in the epidermis could increase the number of regenerated HFs, which could be a novel window for manipulation of HF formation [30]. The upregulation of Wnt10b is found to inhibit the bone morphogenetic protein 6 (BMP6) expression for quicker telogen-anagen transition and accelerate HF regeneration [31]. Lymphoid enhancer factor 1 (LEF1) is a transcription factor that mediates Wnt signaling pathway and also deeply involves in embryonic and stem cell development [32]. The recombination experiments performed by Kratochwil et al. revealed that the LEF1 is critically needed for the development of mouse vibrissa follicles, and LEF1 is transiently expressed in the epithelium and required for morphogenesis [33]. Research also finds that the transgenic mice that bear a TOPGAL reporter gene is responsive to LEF/β-catenin signaling and gives a unique method of identifying cells that are affected by WNT signaling. A WNT-responsive TOPGAL reporter gene is expressed in hair shaft precursor cells that also express LEF1, while adjacent cells express Wnt3 [34].
The sonic hedgehog (SHH) pathway is one of the intricate signal transduction mechanisms that regulates the developmental processes of multicellular organisms, including HF [35]. SHH has shown its crucial role for follicular morphogenesis and cycling, which is dependent on WNT signaling, and is needed for follicular epithelial cell proliferation and dermal papilla formation [36]. The deregulation of SHH signaling could alters HF formation and generates epidermal neoplasia [37]. During the anagen, SHH is also highly expressed in the follicle matrix and acts as a mitogen that drives anagen regeneration [38].
Transforming growth factor-β (TGF-β) is a multifunctional cytokine that controls cell proliferation, differentiation, morphogenesis, tissue homeostasis and regeneration [39]. The TGF-β2 can be found in both the placode and the dermal condensate [40,41]. Chick embryo mesenchyme that is stripped of epithelium and mouse embryo skin explants that are incubated with TGF-β2 can produce dermal papillae and HFs, which suggest TGF-β2 is a HF promoter [42]. Consistently, TGF-β2 knockout mice have a reduced number of HFs and a delayed HF development [43]. Further investigation also found that TGF-β2 promotes HF morphogenesis through the activation of Snail transcription factor and mitogen-activated protein kinase activity, resulting in local E-cadherin downregulation [44].
Moreover, Vitamin D3 (VitD3) is also found to significantly enhance DPCs function, as well as hair folliculogenesis through the activation of Wnt10b, alkaline phosphatase (ALP), and TGF-β2 expression [45]. A recent study suggested that platelet-rich plasma (PRP) could enhance HF regeneration by downregulating MYC and CCAAT/enhancer-binding proteins β and E2F transcription factor-1 gene as VitD3 does [46,47]. PTEN regulates skin development since its deficiency causes epidermal hyperplasia and HF morphogenesis. The BMP2 was found to promote HFSC differentiation, wound contraction, and epidermal regeneration when combined with PTEN in a mouse wound model [15].
Neurotrophic growth factors are also found to involve in the HF regeneration, where neurotrophin receptor p75 (P75NTR) is highly expressed and affect the rate of HF morphogenesis in the placode and dermal condensate of developing HFs, suggesting that neurotrophins may also be involved in the early steps of HF regeneration [48,49]. Neurotrophin-3 (NT-3) and its high-affinity-receptor tyrosine kinase C are also correlated with HF regeneration. The early stages of HF morphogenesis are significantly accelerated in newborn NT-3 overexpressing mice, while retarding in newborn heterozygous NT-3 knockout mice [50].
2.2. Dermal papilla cells
DPC is a kind of differentiated dermal cell that locates at the HF base and is believed to be originated from blimp1+ fibroblasts during the embryonic development [51]. Hair growth, development, and cyclization are largely regulated by DPCs [52,53]. The diameter of the DPC cluster is directly proportional to the width of the hair shaft, and the decrease of DPC cell number and cluster size have been observed in hair loss conditions, like androgenetic alopecia.
CD133 and versican are the specific marker molecules that have been identified for DPCs to possess HF-inducive capacity. CD133+ DPCs are able to secret Wnt ligands and regulate the interaction between the mesenchyme and the epithelial to further promote HF growth and regeneration [54]. The HF regeneration is also highly dependent on DPC aggregation growth, which was mainly composed of versican+ DPCs [55]. Other functional molecules that involved in the positive regulation of DPC HF-inducive capacity also include but not limited to Wnt/β-catenin, SHH/platelet-derived growth factor-A (PDGF-A), fibroblast growth factor (FGF), BMP, TGF-β, and Edar/nuclear factor-kappa B (NF-κB) signaling pathways [[56], [57], [58]].
DPCs have showed promising potential to regenerate HFs, however, the freshly extracted human DPCs are not efficient to regenerate de novo HFs when they are directly transplanted [59], which could due to the loss of spheroids formation [60]. To restore the HF regeneration ability of DPCs, three-dimensional (3D) cell culture could be used to promote the cell–cell and cell–matrix interaction for better HF formation, which will be further discussed in the following sections. It is also reported that the HF-inducive capacity of DPCs may be preserved using keratinocyte-conditioned medium, coculture with keratinocytes, or the addition of BMP6 and basic FGF were able to promote the HF regeneration capacity of DPCs [[61], [62], [63]]. Therefore, HF inducibility is believed as an intrinsic DPC property, which is also controlled by milieu. Interestingly, DPCs could also regulate the cellular behavior of other HF regeneration cells. The proliferation, differentiation, and follicle formation in HF cycling and embryonic development of epithelial HFSCs are correlated with the paracrine of DPCs [64,65]. Moreover, DPCs even have a multilineage potential since they can undergo reprogramming to become pluripotent and then become induced pluripotent stem cells (iPSCs) [66].
2.3. Hair follicle stem cells
HFSCs, which exist in the outer root sheath of HF, are known to possess multi-potency and self-renewal capacity and can differentiate into smooth muscle cells, keratin cells, melanocytes, and neurons [[67], [68], [69]]. Additionally, HFSCs are also capable of differentiating into epidermal and sebaceous gland lineages, which contribute to skin wound healing and make them ideal candidates for cutaneous regeneration and repair. HFSCs can be divided into two subtypes, quiescent HFSCs and primed HFSCs. Quiescent HFSCs are localized in the niche that known as the bulge region and are able to transiently activate, and proliferate to form the hair germ [70]. Primed HFSCs form a tight, ball-like structure in hair germ, underneath quiescent HFSCs in the bulge, which have faster activation dynamics in the secondary hair germ of telogen HF [71,72]. The activation of primed HFSCs could induce the subsequent activation of quiescent HFSCs, and the coordination of these two subtypes of HFSCs promotes the HF regeneration from telogen to anagen [73,74].
Researchers have combined a range of markers to identify HFSCs in recent studies to improve isolation accuracy. CK19, CD15, and CD200 are positive markers that have been widely used for HFSCs identification and are expressed in the anagen and telogen phases of the HF cycle [[75], [76], [77]]. Other HFSC markers have also been identified, including CD71, CD146, connexin 43, Spinocerebellar ataxia type 1, Bcrp1, and P75NTR [77,78]. HFSCs are the most beneficial donor cells for skin regenerative medicine under their strong proliferative capacity and multipotency [79]. A subpopulation of HFSCs actively proliferates and promotes new hair shaft production under the pretreatment of Axin2 [80], Sox9, transcription factor 3, Lhx2, nuclear factor of activated t cells 1, and several signaling pathways, including BMP and PTEN, in the new hair cycle process [63,74,81,82].
2.4. Skin-derived precursors
SKPs, firstly described by Toma et al. in 2001, are novel multipotent precursors resident in the mammalian dermis [83]. Mesenchymal differentiation of SKPs has been reported [84], where SKPs are able to differentiate into adipocytes, osteocytes and chondrocytes, smooth muscle cells, and myofibroblast [[85], [86], [87], [88]]. SKPs also have been found to induce hair follicle formation, where the SKPs subcutaneously injected in nude mice are found to move out of their niche to contribute cells for dermal maintenance and induce hair genesis [89].
There are several chemocytocines that could influence the cellular behavior of SKPs. Trichostatin A (TSA) is a specific histone deacetylase inhibitor, which is found to significantly alleviate the SKP senescence induced by in vitro culture expansion and restore the hair inductive capacity of SKPs through the upregulation of ALP expression [90,91]. Sox2 is a transcription factor that is essential for maintaining self-renewal and pluripotency of undifferentiated stem cells, which is also one of the major regulators that contribute to the hair regeneration capacity for SKPs. The Sox2+ cells are able to induce hair morphogenesis and differentiate into dermal cell types [92]. SKPs that express Sox2 and nestin are also found to exhibit long-term proliferation potential when being cultured in suspension [93]. Moreover, the suspension medium that supplemented with basic FGF and epidermal growth factor is also found to promote SKPs to expand into self-renewing colonies and differentiated into multilineage cells [94]. Age may be an important factor for SKPs. Nurita et al. tested SKPs that are derived from the foreskin of healthy subjects who are aged from 8 months to 85 years and found a negative correlation between sphere-forming potential and age. The samples from subjects over the age of 50 years lose their ability to form spheres, while the samples from younger subjects exhibit larger dermospheres and better differentiation potential than the samples from elderly subjects [95].
3. Cell-only hair follicle regeneration
EMI is a common biological activity that happens in teeth generation, lung organogenesis, and mammary gland morphogenesis, which is also the prerequisite for functional HF formation, regeneration, and cycling [96]. EMI includes the exchanging of soluble molecules between cells, altering cellular pathways, and cell–ECM transduction, which is the theoretical basis for the application of tissue engineering for HF regeneration [16]. In this section, the cell-based transplantation for HF regeneration without the application of biomaterials is reviewed.
3.1. Cell-only transplantation using two-dimension culture
Cell-only transplantation is one of the most used approaches for in vivo HF regeneration. Zhang et al. injected a mixture of primary epidermal stem cells and DPCs in nude mice (Fig. 3a) [97]. New HF was formed with correct structures in the experiment group compared with the control group that was injected with a keratinocyte serum-free medium. Additionally, the mixture injection has induced multilayered stratified epidermis and epidermis that contains HF-like structures, suggesting that EMI rearrangement was necessary for HF regeneration. Young HFSCs periodically switch between active and inactive stages during the HF cycles to generate new HF, while the aged HFSCs would lose this potential [98]. However, when aged HFSCs were co-transplanted with young DPCs in vivo, new HFs remained generated, suggesting the importance of EMI interactions and niche microenvironment youthfulness as a dominant role in HF regeneration compared with HFSCs [99], which could be a promising new avenue for HF regenerative and drug development. Asakawa et al. created tissue-engineered HF germs with embryonic skin-derived epithelial and dermal papilla mixtures for ectopic transplantation. Histologically correct HFs were generated, which successfully connected to the host skin epithelium by intracutaneous transplantation and reproduced the stem-cell niche and hair cycles. Furthermore, the generated HF autonomously connected with nerves and the arrector pili muscle at the permanent region and exhibited piloerection ability, suggesting that the tissue-engineered HF is applicable to surgical alopecia treatments (Fig. 3b) [100]. More advanced investigations conducted by Toyoshima et al. demonstrated a fully functional hair organ regeneration through bioengineered pelage and vibrissa follicle germ transplantation that is reconstituted with SKPs and adult vibrissa stem cells (Fig. 3c) [101]. The tissue-engineered HF developed correct structures and formed proper connections with surrounding host tissues, including the arrector pili muscle, epidermis, and nerve fibers, which suggested that adult tissue-derived follicular stem cells have the potential HF regeneration applications.
Fig. 3.
Cell-only transplantation in vivo. (a) Injection of primary Epi-SCs and DPCs in nude mice. Adapted with permission [97]. Copyright 2020 Springer (open access); (b) Tissue engineered HF germs with embryonic skin-derived epithelial and dermal papilla mixtures. Adapted with permission [100]. Copyright 2012 Springer Nature (open access); (c) Fully functional hair organ regeneration through the transplantation of a bioengineered pelage and vibrissa follicle germ reconstituted with SKPs and adult vibrissa stem cells. Adapted with permission [101]. Copyright 2012 Springer Nature (open access).
DPCs and dermal fibroblasts have highly correlated gene expression since both cells originate from the fibroblast progenitors during embryonic skin development [102]. Collins et al. had induced adult dermal fibroblasts to acquire similar molecular, cellular, and structural characteristics as DPCs through the epidermal β-catenin activation [103]. Zhao et al. used hybrid induction factors, which contain platelet-derived growth factor, fibroblast growth factor-2, and 6-bromoindirubin-3′-oxime (BIO), to induce adult foreskin fibroblasts into hair-inducing DP-like cells and successfully generated HF in nude mice [104]. A similar study was conducted by Yang et al. that used a combination of microphthalmia-associated transcription factor (MITF), Sox10, and PAX3 induction factors to convert mouse and human fibroblasts to induced melanocytes (iMels), which joined the dermal–epidermal junction and generated pigmented epidermis and HFs in skin reconstitution assays in vivo [105].
3.2. Cell-only transplantation using three-dimension culture
3D cell cultures have been widely used in tissue engineering since they could provide a more promising platform to mimic and study the complexity of in vivo microenvironment, including the growth factor gradients and direct cell–cell contacts, such as cellular spheroids, which is critical for maintaining hair-inducing properties [106]. The hanging drop is one of the most commonly used 3D culture approaches that could also lead to a controllable 3D spheroid formation, which is based on surface tension and the interaction between surface tension and a gravity field that causes the convergence of liquid drops [107]. The high-passaged DPC spheroids retained many similarities to primary DPCs through hanging-drop culture. Additionally, the subcutaneous DPC spheroids implantation mixed with epidermal cells successfully reproduced HF in nude mice, suggesting that this model could provide the potential to produce controllable and scalable DPC spheroids for future follicle regeneration (Fig. 4a) [108]. Non-adhesive microwell plate is also an efficient approach to produce 3D cellular spheroids. Kageyama et al. cultured mouse epithelial and mesenchymal cell mixture in lab-made microwells [109]. Cells initially formed a randomly distributed single-cell aggregate and then spatially separated to exhibit dumbbell-like morphology, which has typical HF germ morphological features. The additive of activated PRP further promoted the follicular gene expression and the hair regeneration ability upon intracutaneous transplantation, which indicate that PRPr could be potentially used for autologous hair regenerative medicine. Human adipose-derived stem cells (hADSCs) were mixed with human DPCs and murine embryonic epithelial cells to form aggregates, where the hADSCs were localized on the hDPC aggregate side, using the same lab-made microwells [110]. The hADSC involvement significantly increased gene expression that is associated with hair morphogenesis and promoted hair shaft generation upon nude mice transplantation. A similar study performed by the same research group mixed vascular endothelial cells, DPCs, and epithelial together (Fig. 4b), where the containing of vascular endothelial cells exhibited higher hair morphogenesis-related gene expression and greater hair shaft regeneration levels upon transplantation to the dorsal side of nude mice [111].
Fig. 4.
3D cellular spheroids formation. (a) The hanging drop approaches for a controllable 3D spheroid formation. Adapted with permission [107]. Copyright 2020 Frontiers (open access); (b) HUVECs increase gene expression associated with hair morphogenesis and promoted the hair shafts generation. Adapted with permission [111]. Copyright 2021 Springer Nature (open access).
4. Biomaterial-assisted hair follicle regeneration
Cell–ECM interactions in the HF are also crucial in addition to the critical cell–cell interaction since it maintains the specific cell phenotype and is involved in the hair cycle and hair-associated disorders [112]. The epithelial and mesenchymal compartments of the HF are separated by an ECM basement membrane, including chemical components, such as collagen IV, fibronectin, and laminin [113]. The basement membrane is particularly relevant for epithelial cell differentiation state [5]. The activation of the differentiation pathway for HF basal keratinocytes requires detachment from the basement membrane, while the epidermal reservoir maintenance is retained by anchored stem cells [114]. Therefore, the application of biomaterials to simulate the cell-ECM interaction in vitro could also promote the understanding of the mechanisms underlying HF regeneration.
4.1. Commonly used biomaterials in HF tissue engineering
Collagen is the most abundant protein in mammals and is widely observed in the hair growth cycles. Type I, type III, and type IV collagens were observed in the dermal sheath and the ECM of dermal papilla. In the telogen follicles, where the ECM is significantly reduced in dermal papilla, the collagen IV is still highly expressed [115]. The existence of collagen in skin not only physically support the hair growth, but also chemically correlated with hair growth cycle. COL17A1 is highly expressed in bulge Epi-SCs and maintains the epithelial and melanocyte stem-cell quiescence. The COL17A1-null mice have suffered from premature hair loss and hair graying [116]. Therefore, collagen was widely used in the HF tissue engineering. Mouse embryonic mesenchymal cells or human DPCs were cultured in collagen microgels and could induce HF in nude mice after transplantation [117]. Collagen could also be used to prepare in vitro reconstructed human skin (RHS) through culturing of fibroblasts to induce normal human keratinocytes to form hair shafts and stimulate hamartomatous change [118,119]. Gelatin is a natural form of hydrolyzed collagen, which retains collagen's high biocompatibility and cellular adhesion properties. Native gelatin can also form hydrogel by thermo-crosslinking like collagen, while the it was often mixed with other biomaterials to promote the mechanical properties [120]. Gelatin-alginate nanogels were developed to encapsulate single DPC to create controllable DPC spheroids for HF regeneration [121].
Hyaluronic acid (HA) is also an ECM component in basement membrane, which plays an important role in holding water, maintains osmotic balance in tissues, and regulates the human hair follicle growth cycle [122]. Due to the low gelation rate, HA could be mixed with collagen to mimic the natural niche of hSKPs in the dermis and supported hSKP proliferation as spheres [123]. DPC and skin epidermal keratinocytes cultured in mixed hydrogel containing HA, aggrecan, biglycan, and fibronectin had significantly higher cellular proliferation compared with 2D culture [124]. HA could also be methacrylated (HAMA) to promote the gelation rate and further mixed with gelatin methacryloyl to mimic the compositions of collagen and glycosaminoglycan in native skin [125].
Matrigel is a commercialized natural biomaterial that extracted from the basement membrane of Engelbreth-Holm-Swarm (EHS) mouse sarcoma and contains numerous ECM and growth factors that could closely mimic the chemical properties of natural ECM [126]. The epidermis and neogenic HF were successfully formed by culturing Epi-SCs and SKPs in Matrigel and implanted in nude mice [127]. However, the safety in clinical application of Matrigel for HF regeneration is still unclear due to the tumor cell-derived source. Decellularized ECM (dECM) has been widely used due to its similar structural, biochemical, and biophysical properties as native tissues, allowing adequate mechanical and chemical support for tissue engineering [128,129]. Human placenta dECM (HPECM) hydrogel cultured with DPCs restored the protein expression that observed in vivo and the co-transplantation in nude mice regenerated de novo HF [25].
Self-assembling peptide made of natural amino acids with similar biochemical structure as natural ECMs. Taking the advantages of the tailor-made, self-assembling peptides have shown promising applications in tissue engineering through incorporating with specific ligands for cell receptors [[130], [131], [132]]. RADA16 (Ac-(RADA)4-CONH2) that consists of 16 alternating hydrophobic and hydrophilic amino acids had shown to facilitate SKPs attachment, proliferation, and HF neogenesis in vivo [133]. Laminin-511 is another protein that is prominently expressed in the hair germ basement membrane. Its blockage in mice impairs hair development, contained fewer hair germs, and exhibits a defective basement membrane compared to wild type [134]. The further laminin-511 investigation by Gao et al. revealed that laminin-511 is required for the noggin and downstream regulator expressions, which are essential for murine DP maturation during HF development [135]. Although laminin has not been used in the tissue engineering of HF regeneration, it should be an ideal biomaterial for these applications in the future development.
Polyvinyl alcohol (PVA) is synthetic resin prepared by the polymerization of vinyl acetate, and the physical characteristics could be modified as the degree of polymerization and the degree of hydrolysis. PVA has been used as a biomaterial for biomedical applications, including HF regeneration, due to its good biocompatibility. DPCs cultured on PVA could aggregate into single spheroids with progressive compaction and the further transplantation in nude mice could induce HF regeneration [136]. PVA could also be mixed with natural biomaterials to improve the physical and chemical properties. Zhang et al. used chitosan-PVA to construct porous scaffolds and improved the secretory activity of high-passaged DPCs compared with 2D cultures [137].
4.2. In vitro cell culture with biomaterials
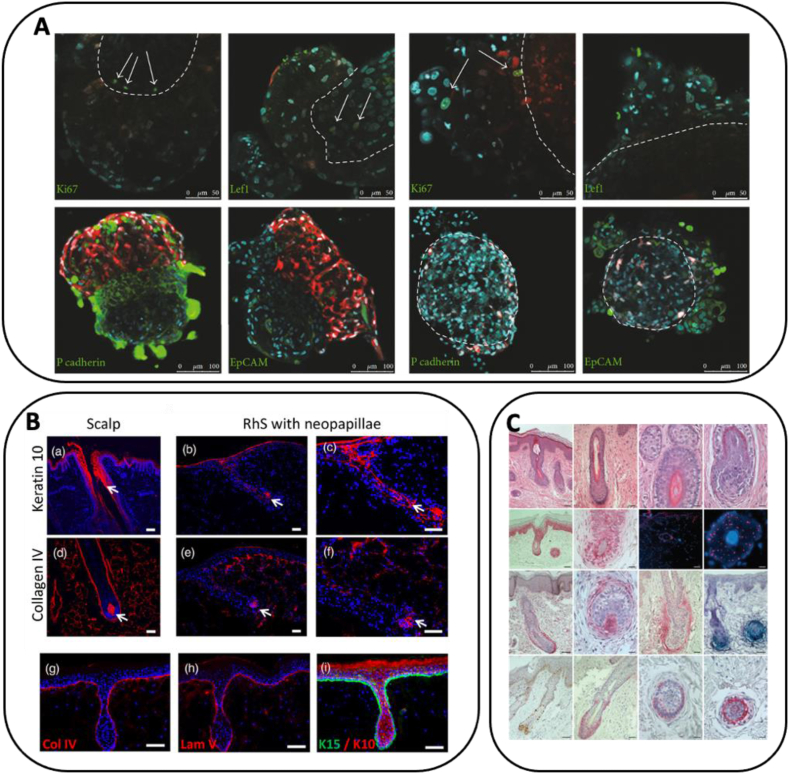
Recent studies have used biomaterials to mimic the HF microenvironment for the 3D cell culture of HF regeneration cells in vitro due to the importance of ECM in HF regeneration and the quick loss of hair-inducing functions in 2D culture to promote HF regeneration ability and investigate the HF mechanisms. Wang et al. [123] developed HA-collagen hydrogel to mimic the natural niche of hSKPs in the dermis, which alleviated hSKP senescence and supported hSKP proliferation as spheroids, while largely retaining their properties and potential, due to the deficiency of adult hSKPs expansion in vitro [138,139]. Kalabusheva et al. cultured DPC and skin epidermal keratinocytes on aggrecan, biglycan, fibronectin, and HA, which significantly promoted the cellular proliferation compared with 2D culture and did not affect cellular identity (Fig. 5a) [124]. Additionally, aggrecan, biglycan, and fibronectin prevented the cell aggregate formation, while HA promoted larger organoid formation. Zhou et al. cultured DP cells in modified Extracel® Hydrogel that can constantly release β-catenin to promote clonal growth of CD133 positive DP cells in vitro, where the CD133 is expressed by a DP cell subpopulation that is capable of inducing HF formation in vivo [140]. Further CD133-positive DP cell transplantation in mice demonstrated accelerated in vivo hair growth compared to control cells. Dong et al. prepared a collagen-chitosan (C-CS) scaffold and cultured it with epithelial cells and DPCs, followed by Wnt-conditional media (CM) treatment from the supernatant of Wnt1α+ bone marrow mesenchymal stem cells [141]. The cell mixtures induced hair regeneration in nude mice and Wnt-CM promoted the hair induction ability of DPCs through Wnt/β-catenin signaling pathway activation, which suggests that these 3D culture models have the potential ability to prepare large-scale cells for HF regeneration. Synthetic polymers could also be used to promote the HF neogenesis of DPCs in vitro. PVA also induced DPCs to aggregate into single spheroids with progressive compaction [136]. The cell spheroid size could be regulated through cell number modification, and both human and rat DPC spheroids were able to induce HF regeneration in nude mice. Additionally, larger DP spheroids exhibit higher HF inductivity, while the thickness of regenerated hair fiber did not have a significant difference by changing with the transplanted DPC spheroid size. A further study prepared chitosan-PVA scaffolds to construct efficient and functional multicellular spheroids [137]. High-passaged DPCs demonstrated improved secretory activity and restored their intrinsic properties compared with 2D cultures. Transplantation in nude mice showed that cultured DP multicellular spheroids could effectively enhance HF-inducing ability. Vahav et al. prepared RHS using fibroblasts and multi-layer collagen hydrogel, followed by seeding with neopapillae, which resulted in spheroid engulfment (Fig. 5b) [118]. A similar study was also performed by Li et al. [119], wherein tuberous sclerosis complex 2 (TSC2)-null fibroblast-like cells were grown on a collagen matrix to prepare RHS, which induced normal human keratinocytes to form sebaceous glands and hair shafts and stimulate hamartomatous changes (Fig. 5c).
Fig. 5.
In vitro 3D cell culture using biomaterials as matrix. (a) 3D cell culture promoted the DPC, and skin epidermal keratinocytes proliferation compared with 2D culture and did not affect cellular identity. Adapted with permission [124]. Copyright 2017 Hindawi (open access); (b) RHS seeded with neopapillae resulting in spheroid engulfment. Adapted with permission [118]. Copyright 2020 Wiley (open access); (c). TSC2-null fibroblast-like cells grew on RHS and induced normal human keratinocytes to form sebaceous glands. Adapted with permission [119]. Copyright 2011 Springer Nature (open access).
3D printed skin equivalents made with GelMA/HAMA was used to mimic the hair follicle structures and epidermal/papillary dermal layers. The composition of GelMA and HAMA was adjusted to recapitulate the collagen and glycosaminoglycan constitutions in native skin. The bioprinted scaffolds successfully maintained the hair-inductive potency of HaCaT cell and facilitate the spontaneous hair pore development, suggesting it may serve as useful models for HF tissue engineering and regeneration.
4.3. Co-transplantation of cells and biomaterials
The co-transplantation of cells with cultured hydrogels was also investigated because the hydrogels could provide physical protection for cells after transplantation and continue to induce HF regeneration in vivo.
Among different hydrogel types, collagen is most used. Kageyama et al. mixed epidermal and mesenchymal cells in suspension and seeded them in non-adhesive microwells to induce the 3D spheroid formation [142]. Mixed cells formed dumbbell-like morphology and the further encapsulation in collagen and co-transplantation in nude mice resulted in spatially aligned HF generation. Kageyama et al. further improved the approach and encapsulated mouse embryonic mesenchymal cells or human DPCs in collagen microgels through the spontaneous constriction of cell-encapsulated collagen drops and named hair beads (HBs) [117]. The intracutaneous HB transplantation in nude mice efficiently generated HF, which represented a robust and practical approach for germ-like tissue preparation for hair regenerative medicine (Fig. 6a). Using this approach, phosphoinositide 3-kinase and Akt (PI3K/Akt) signaling pathways were investigated and were significantly upregulated in HBs, which was crucial for hair inductivity maintenance and restoration of DPCs [143].
Fig. 6.
Cell-hydrogels co-transplantation in vivo. (a) Transplantation of HBs in nude mice efficiently generated hair follicles. Adapted with permission [117]. Copyright 2019 Elsevier; (b) Self-assembling peptide hydrogels facilitate SKPs attachment, proliferation, and HF neogenesis in vivo. Adapted with permission [133]. Copyright 2016 Elsevier; (c) LbL self-assembly nanogel encapsulates single DPC and assembled to create controllable DPC spheroids. Adapted with permission [121]. Copyright 2017 Wiley; (d) 3D printed models that recapitulated the physiological 3D organization of cellular distribution in the HF microenvironment. Adapted with permission [144]. Copyright 2018 Springer Nature (open access).
Other hydrogels have also been used for HF regeneration co-transplantation. Wang et al. mixed Epi-SCs and SKPs derived from the adult human, cultured in Matrigel, and implanted into an excisional wound in nude mice [127]. The epidermis and HF were successfully formed by Epi-SCs, and SKPs contributed to dermal papilla in the neogenic HF, which suggests that a combination of Epi-SCs and SKPs reconstituted functional HFs and sebaceous glands, which implied the great potential for novel bioengineered skin substitute development. The same research group further prepared self-assembling peptide hydrogels by combining the RADA16 (Ac-(RADA)4-CONH2) and PRG (RADA-PRG) to facilitate SKPs attachment, proliferation, and HF neogenesis in vivo (Fig. 6b) [133]. RADA-PRG-cell transplantation, including neonatal epidermal cells, resulted in a significantly increased number of neogenic hairs compared with Matrigel, RADA-only hydrogels, and PRG-only hydrogels, which may be attributed to chemical property similarities between RADA-PRG hydrogels and HF ECM molecules [145].
Layer-by-layer self-assembly nanogel that is made with gelatin and alginate was used to encapsulate the single DPC and assembled using physical crosslinking to create controllable DPC spheroids due to the promotion effect of DPC spheroid formation on HF regeneration (Fig. 6c) [121]. The expression of ALP, versican, and neural cell adhesion molecule (NCAM) was restored in high-passaged DPC spheroids, while lost in 2D culture. The co-transplantation of nanogel-encapsulated DPC spheroids and epidermal cells successfully regenerated HFs in vivo. A similar study was performed by the same research group using HPECM hydrogel because dECM retains the ligands for cellular receptor recognition and active growth factors that enhance the cellular adhesion and growth [128]. The DPCs in HPECM hydrogel also formed cellular spheroid and restored the expression levels of Versican, ALP, and β-catenin high-passaged DPC. The co-grafted with newborn mouse epidermal cells regenerated de novo HF [25]. Additionally, 3D printing technology has been widely used in tissue engineering [146] and could fabricate models that recapitulated the physiological 3D organization of the HF microenvironment cellular distribution (Fig. 6d) [144]. After combining with collagen hydrogel, HF-like extensions were prepared with a diameter of 500 μm and a length of 4 mm, which can be seeded with various hair densities, including 19, 81, and 255 HFs/cm2. The co-transplantation in nude mice led to HF-bearing human skin construct vascularization, increased graft survival, and efficient human hair growth.
5. Discussion
Much progress has been made in the tissue engineering for HF regeneration in the past decades. A variety of cells with the potential to regenerate HF have been identified and the application of biomaterials for either in vitro 3D cell culture or co-transplantation in vivo has also made excellent progress to overcome the loss of HF regeneration ability for potential cells that caused by 2D culture. However, numerous challenges remained to tackle for the clinical applications in the future.
5.1. Challenges in the HF regeneration cellular isolation and maintenance
The identifications of HF regeneration cells, including DPCs, SKPs, HFSCs, and reprogrammed cells significantly expanded the cell sources, however, the problems that these cells have in common are the difficulty to isolate. There are three techniques that have been routinely used for the isolation of cells that have HF regeneration ability: microdissection, enzymatic digestion, and fluorescence-activated cell sorting (FACS) [147]. Microdissection is commonly applied for the isolation of cells from DP [148] or the bulge [149], where fine forceps and blades are used to isolate of the area of interest and samples are subsequently transferred onto tissue culture plates to allow cells migrate out of the tissue. Microdissection could preserve the whole tissue and improve the efficiency of cell isolation, while it is quite laborious and requires experienced technicians. Enzymatic digestion is a relatively easier approach for cellular isolation. The ECM presents around the follicle could be digested using enzymes, including collagenase, dispase, or enzymes combinations, and the cells can be further isolated with centrifugation [150]. The disadvantage of enzymatic digestion is the little control over the type of cells that obtained, which causes the batch-to-batch variation between isolations [151]. FACS is a common method for stem cell isolation, where cells are sorted using the fluorescently labeled antibodies that tag to the cellular surface [152,153]. The yielded cells are highly purified populations that can be further expanded or directly used for cellular experiments or analyzed for mRNA or protein expression. Recently, numerous markers have been discovered for DPCs and HFSCs, which makes FACS a more promising approach for HF regeneration cell isolation.
Except the direct isolation from in vivo tissues, HF regeneration cells can also be obtained from the reprogram of other stem cells, like induced pluripotent stem cells (iPSCs), which have similar characteristics to embryonic stem cells in self-renewal and differentiation capacity. Therefore, reprogramming of iPSCs into potential HF regeneration cells could be a promising approach to expand the cell sources for HF regeneration. The mixture of iPSC ectodermal precursor cells in keratinocyte culture medium supplemented with BMP could transform into new DPCs [154]. iPSCs could also differentiate to induced mesenchymal cells (iMCs), where a highly proliferative and plastic subset of iMCs (LNGFR+ and THY-1+) could be subsequently programmed to acquire DP properties using retinoic acid. The further co-transplantation with keratinocytes in vivo gave rise to hair shaft structures [155]. Additionally, iPSCs cultured on Matrigel could differentiate into keratinocytes with high efficiency after treatment of keratinocyte serum-free media supplemented with retinoic acid and BMP4 [156]. Other than iPSCs, human dermal fibroblasts can also be transformed into DPCs through the treatment of medium that supplemented with FGF2, PDGF and BIO [104]. SKPs are also found to be produced from healthy adult fibroblasts via lineage reprogramming [157]. Therefore, the cellular reprogramming could also be a promising approach to obtain HF regeneration cells for tissue engineering.
Ideally, a cell-based regenerative therapy would be autologous, where the therapeutical cells are derived from patients' tissue biopsies. However, HF was proved to be an immune-privileged site during anagen phase, since it has low expression of the major histocompatibility complex class I antigens and immunosuppressive agents like α-MSH and TGF-β1, which made allogeneic cell be an alternative source for HF regenerative therapy [[158], [159], [160]]. Transgender transplantation of a microdissected dermal sheath tissue from the base of scalp skin follicles of a male to a female recipient was shown to successfully induce HFs [161]. The mixture of human-derived cells (hADSCs and DPCs) with mouse cells could also promote hair shaft generation upon nude mice transplantation [110]. These studies pointed the possibility of using an allogeneic cell source for HF regeneration.
Even though the cells can be successfully obtained, these cells are still easy to loss the potential for HF regeneration during the long-term culture, due to the cell aging and inefficient reprogramming in vitro. Freshly extracted human DPCs are not efficient to regenerate de novo HFs when they are directly transplanted [59]. SKPs cultured in vitro senesce and lost the self-renewal ability soon after separation from their physiological microenvironments [138]. To overcome this problem, various cell culture methods and customized culture medium are applied. The activating of PI3K-Akt pathway in SKPs with PDGF-AA and a PTEN inhibitor, promoted the cellular proliferation, enhanced spheroid formation, and alleviated senescence, while retaining the differentiation potential [162]. The application of VitD3 and PRP on DPCs could also significantly improve the functionality in HF regeneration [[45], [46], [47]].
The optimization of the in vitro culture system is also investigated to maintain the HF regeneration functionality. Computer-controlled stirred suspension bioreactors are developed to culture SKPs to overcome the problem, where SKPs cultured in bioreactor proliferate faster and share similar expression profile to the in vivo SKPs compare with static 2D cultures and are able to induce de novo HFs and repopulate their cellular niches [163]. The construction of 3D culture systems that better mimic the ECM components of in vivo microenvironment also provide an alternative approach to maintain or even promote the HF regeneration ability, which are reviewed in the previous sections.
5.2. Limitations of development for HF tissue engineering scaffolds
Current strategies in simulating EMI remained insufficient, which lacks the microenvironment of connective tissue and remained quite different from the physiological environment of normal tissues and organs. Collagen is the most used hydrogel for in vitro cell culture and co-transplantation, since collagen is most abundant in skin and researchers have shown the advanced HF regeneration compared with cell-only as mentioned in the previous section. However, the basement membrane of HF also includes other ECM components, such as chondroitin sulfate, heparan sulfate, fibronectin, and laminin [113]. Various components have their unique HF regeneration properties, such as the laminin is essential for the expression of noggin and downstream regulators development [135], while the inclusion of laminin in HF tissue engineering scaffold was not developed yet. Therefore, interpenetrating polymer network (IPN) hydrogels that contain multiple independent biomaterials need to be developed to achieve the desired properties and hierarchical structures that better mimics the microenvironment of HF. Self-assembling peptide could be a potential choice to reach such achievement since they could represent the functional groups on the hydrogel long chain [164]. Most importantly, they are remarkable biomolecules that are generally easy to synthesize by chemical routes and are easily conjugated or mixed in the material matrix with limited influence on the original properties. However, high cost and limited production currently restrict the uptake of these materials as synthetic ECM. Other critical aspects except for the chemical diversity of the hydrogel used for HF regeneration include safety, toxicity, and stability. Ideal hydrogels need to be kept in a stable state without biological degradation for a short period and then degrade harmlessly and easily metabolized after hair regeneration.
The co-transplanted hydrogels not only need to physically support and protect the cellular growth in vivo but also need to induce and promote HF regeneration. Therefore, the tissue engineering scaffolds for HF regeneration should also be included with functional growth factors, such as Wnt, BMP, and TGF-β. Recent research found the growth factors that covalently conjugated to the biomaterials could enhance the cellular spreading and viability compared with soluble growth factors [165]. Therefore, biomaterials contain binding sites for growth factors have been widely investigated, such as heparin, which can form stable complexes with various growth factors through electrostatic interactions [166]. Synthetic binding peptides for biomaterials were also developed to achieve specific growth factor binding, like BMP-2 [167] and TGF-β [168], which also have promising potentials for HF regeneration. Further investigations also developed synthetic peptides with specific sequences that mimic the functional groups of certain growth factors to allow facile chemical modification and preserve natural bioactivity of biomaterials. A stable supramolecular nanostructure with the capacity to activate FGF-2 receptor was developed and assemble into a scaffold to promote the proliferation and migration of the human umbilical vein endothelial cells in vitro [169]. Similar studies were also performed for BMP-2 [170] and TGF-β [171]. Therefore, the development of tissue engineering scaffolds for HF regeneration should also be more diversifiable to include various types of growth factors using different approach to better support the HF regeneration.
6. Conclusion
Overall, many challenges remained to be tackled for clinical applications. Future works should focus on biomaterial development that better mimics the ECM components of HF in vivo and should also consist of the growth factors or drugs that can induce HF regeneration. Additionally, investigations should be concentrated on the length of de novo hair growth and how long they can last, since these are the key factors that patients mostly consider. The clinical application of hydrogel co-transplantation for HF regeneration still faces various challenges; however, evidence has accumulated from various studies to prove that this is a promising direction and could be achieved in the future.
Funding
This work was supported by the National Key Research and Development Program of China (Grant No. 2018YFA0703000) and the National Natural Science Foundation of China (Grant Nos. 52075482).
Declaration of Competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled “The Potential Applications of Biomaterials in Tissue Engineering for Hair Follicles Regeneration”.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Peng Wei, Email: weipeng@nbu.edu.cn.
Jun Yin, Email: junyin@zju.edu.cn.
References
- 1.Jamerson T.A., Aguh C. An approach to patients with alopecia. Med Clin North Am. 2021;105(4):599–610. doi: 10.1016/j.mcna.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Roohaninasab M., Goodarzi A., Ghassemi M., Sadeghzadeh-Bazargan A., Behrangi E., Najar Nobari N. Systematic review of platelet-rich plasma in treating alopecia: focusing on efficacy, safety, and therapeutic durability. Dermatol Ther. 2021;34(2) doi: 10.1111/dth.14768. [DOI] [PubMed] [Google Scholar]
- 3.Phillips T.G., Slomiany W.P., Allison R. Hair loss: common causes and treatment. Am Fam Physician. 2017;96(6):371–378. [PubMed] [Google Scholar]
- 4.Alessandrini A., Bruni F., Piraccini B.M., Starace M. Common causes of hair loss—clinical manifestations, trichoscopy and therapy. J Eur Acad Dermatol Venereol. 2021;35(3):629–640. doi: 10.1111/jdv.17079. [DOI] [PubMed] [Google Scholar]
- 5.Abreu C.M., Marques A.P. Recreation of a hair follicle regenerative microenvironment: successes and pitfalls. Bioeng Transl Med. 2022;7(1) doi: 10.1002/btm2.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jimenez F., Alam M., Vogel J.E., Avram M. Hair transplantation: basic overview. J Am Acad Dermatol. 2021;85(4):803–814. doi: 10.1016/j.jaad.2021.03.124. [DOI] [PubMed] [Google Scholar]
- 7.Likhitkar M., Shakur A.A., Bansal K.K., Pande M. Alopecia‒reason and possible treatments. MOJ Drug Des Dev Ther. 2018;2(5):198–208. [Google Scholar]
- 8.Randolph M., Tosti A. Oral minoxidil treatment for hair loss: a review of efficacy and safety. J Am Acad Dermatol. 2021;84(3):737–746. doi: 10.1016/j.jaad.2020.06.1009. [DOI] [PubMed] [Google Scholar]
- 9.Sinclair R.D. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57(1):104–109. doi: 10.1111/ijd.13838. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez-Cauhe J., Saceda-Corralo D., Rodrigues-Barata R., Hermosa-Gelbard A., Moreno-Arrones O.M., Fernandez-Nieto D., et al. Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. J Am Acad Dermatol. 2019;81(2):648–649. doi: 10.1016/j.jaad.2019.04.054. [DOI] [PubMed] [Google Scholar]
- 11.Avram M.R., Finney R., Rogers N. Hair transplantation controversies. Dermatol Surg. 2017;43(Suppl 2):S158–S162. doi: 10.1097/DSS.0000000000001316. [DOI] [PubMed] [Google Scholar]
- 12.Agaoglu G., Ozer F., Karademir S., Agaoglu E., Erol O. Hair transplantation in burn scar alopecia after combined non-ablative fractional laser and microfat graft treatment. Aesthetic Surg J. 2021;41(11):NP1382–NP1390. doi: 10.1093/asj/sjab225. [DOI] [PubMed] [Google Scholar]
- 13.Ji J., Ho B.S., Qian G., Xie X.M., Bigliardi P.L., Bigliardi-Qi M. Aging in hair follicle stem cells and niche microenvironment. J Dermatol. 2017;44(10):1097–1104. doi: 10.1111/1346-8138.13897. [DOI] [PubMed] [Google Scholar]
- 14.Ji S., Zhu Z., Sun X., Fu X. Functional hair follicle regeneration: an updated review. Signal Transduct Targeted Ther. 2021;6(1):66. doi: 10.1038/s41392-020-00441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider M.R., Schmidt-Ullrich R., Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19(3):R132–R142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Rezza A., Wang Z., Sennett R., Qiao W., Wang D., Heitman N., et al. Signaling networks among stem cell precursors, transit-amplifying progenitors, and their niche in developing hair follicles. Cell Rep. 2016;14(12):3001–3018. doi: 10.1016/j.celrep.2016.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh J.W., Kloepper J., Langan E.A., Kim Y., Yeo J., Kim M.J., et al. A guide to studying human hair follicle cycling in vivo. J Invest Dermatol. 2016;136(1):34–44. doi: 10.1038/JID.2015.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanti V., Rowert-Huber J., Vogt A., Blume-Peytavi U. Cicatricial alopecia. J Dtsch Dermatol Ges. 2018;16(4):435–461. doi: 10.1111/ddg.13498. [DOI] [PubMed] [Google Scholar]
- 19.Bernardez C., Molina-Ruiz A.M., Requena L. Histologic features of alopecias: part II: scarring alopecias. Actas Dermosifiliogr. 2015;106(4):260–270. doi: 10.1016/j.ad.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Langer R., Vacanti J. Tissue engineering. Science (New York, NY) 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 21.Lichti U., Anders J., Yuspa S.H. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3(5):799–810. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Topouzi H., Logan N.J., Williams G., Higgins C.A. Methods for the isolation and 3D culture of dermal papilla cells from human hair follicles. Exp Dermatol. 2017;26(6):491–496. doi: 10.1111/exd.13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai R., Hua W., Chen W., Xiong L., Li L., Li Y. Isolation, characterization, and safety evaluation of human skin-derived precursors from an adherent monolayer culture system. Stem Cell Int. 2019;2019 doi: 10.1155/2019/9194560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Couchman J.R. Rat hair follicle dermal papillae have an extracellular matrix containing basement membrane components. J Invest Dermatol. 1986;87(6):762–767. doi: 10.1111/1523-1747.ep12456955. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X., Xiao S., Liu B., Miao Y., Hu Z. Use of extracellular matrix hydrogel from human placenta to restore hair-inductive potential of dermal papilla cells. Regen Med. 2019;14(8):741–751. doi: 10.2217/rme-2018-0112. [DOI] [PubMed] [Google Scholar]
- 26.Cai B., Zheng Y., Yan J., Wang J., Liu X., Yin G. BMP2-mediated PTEN enhancement promotes differentiation of hair follicle stem cells by inducing autophagy. Exp Cell Res. 2019;385(2) doi: 10.1016/j.yexcr.2019.111647. [DOI] [PubMed] [Google Scholar]
- 27.Luscan A., Shackleford G., Masliah-Planchon J., Laurendeau I., Ortonne N., Varin J., et al. The activation of the WNT signaling pathway is a Hallmark in neurofibromatosis type 1 tumorigenesis. Clin Cancer Res Off J Am Assoc Cancer Res. 2014;20(2):358–371. doi: 10.1158/1078-0432.CCR-13-0780. [DOI] [PubMed] [Google Scholar]
- 28.Hu X., Li Z., Zhang D., Yang Y., Fu S., Zhang Z., et al. A systematic summary of survival and death signalling during the life of hair follicle stem cells. Stem Cell Res Ther. 2021;12(1):453. doi: 10.1186/s13287-021-02527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith A., Li J., Liu B., Hunter D., Pyles M., Gillette M., et al. Activating hair follicle stem cells via R-spondin2 to stimulate hair growth. J Invest Dermatol. 2016;136(8):1549–1558. doi: 10.1016/j.jid.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 30.Ito M., Yang Z., Andl T., Cui C., Kim N., Millar S.E., et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447(7142):316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 31.Wu P., Zhang Y., Xing Y., Xu W., Guo H., Deng F., et al. The balance of Bmp6 and Wnt10b regulates the telogen-anagen transition of hair follicles. Cell Commun Signal. 2019;17(1):16. doi: 10.1186/s12964-019-0330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakatomi M., Ida-Yonemochi H., Ohshima H. Lymphoid enhancer-binding factor 1 expression precedes dentin sialophosphoprotein expression during rat odontoblast differentiation and regeneration. J Endod. 2013;39(5):612–618. doi: 10.1016/j.joen.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 33.Kratochwil K., Dull M., Farinas I., Galceran J., Grosschedl R. Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Gene Dev. 1996;10(11):1382–1394. doi: 10.1101/gad.10.11.1382. [DOI] [PubMed] [Google Scholar]
- 34.DasGupta R., Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development (Cambridge, England) 1999;126(20):4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 35.Varjosalo M., Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22(18):2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 36.Qu R., Gupta K., Dong D., Jiang Y., Landa B., Saez C., et al. Decomposing a deterministic path to mesenchymal niche formation by two intersecting morphogen gradients. Dev Cell. 2022;57(8):1053–1067. doi: 10.1016/j.devcel.2022.03.011. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMahon A.P., Ingham P.W., Tabin C.J. Developmental roles and clinical significance of Hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- 38.Oro A.E., Higgins K. Hair cycle regulation of Hedgehog signal reception. Dev Biol. 2003;255(2):238–248. doi: 10.1016/s0012-1606(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 39.Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13(10):616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horesh E., Chéret J., Paus R. Growth hormone and the human hair follicle. Int J Mol Sci. 2021;22(24) doi: 10.3390/ijms222413205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim H., Choi N., Kim D., Kim S., Song S., Sung J. TGF-β2 and collagen play pivotal roles in the spheroid formation and anti-aging of human dermal papilla cells. Aging. 2021;13(16):19978–19995. doi: 10.18632/aging.203419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oshimori N., Fuchs E. Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell. 2012;10(1):63–75. doi: 10.1016/j.stem.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun T., Huang Z., Liang W., Yin J., Lin W., Wu J., et al. TGFβ2 and TGFβ3 isoforms drive fibrotic disease pathogenesis. Sci Transl Med. 2021;13(605) doi: 10.1126/scitranslmed.abe0407. [DOI] [PubMed] [Google Scholar]
- 44.Jamora C., Lee P., Kocieniewski P., Azhar M., Hosokawa R., Chai Y., et al. A signaling pathway involving TGF-beta2 and snail in hair follicle morphogenesis. PLoS Biol. 2005;3(1):e11. doi: 10.1371/journal.pbio.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aoi N., Inoue K., Chikanishi T., Fujiki R., Yamamoto H., Kato H., et al. 1α, 25-dihydroxyvitamin D3 modulates the hair-inductive capacity of dermal papilla cells: therapeutic potential for hair regeneration. Stem Cell Transl Med. 2012;1(8):615–626. doi: 10.5966/sctm.2012-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miao Y., Feng C., Zhang Z., Li Z., Xiao S., Jiang J., et al. [Effect of PRP on the proliferation of dermal papilla cells and hair follicle regeneration in mice] Zhonghua zheng xing wai ke za zhi = Zhonghua zhengxing waike zazhi = Chin J Plast Surg. 2013;29(2):131–135. [PubMed] [Google Scholar]
- 47.Shen H., Cheng H., Chen H., Zhang J. Identification of key genes induced by platelet-rich plasma in human dermal papilla cells using bioinformatics methods. Mol Med Rep. 2017 doi: 10.3892/mmr.2016.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Botchkarev V., Botchkareva N., Albers K., Chen L., Welker P., Paus R. A role for p75 neurotrophin receptor in the control of apoptosis-driven hair follicle regression. FASEB J Off Pub Fed Am Soc Exp Biol. 2000;14(13):1931–1942. doi: 10.1096/fj.99-0930com. [DOI] [PubMed] [Google Scholar]
- 49.Botchkarev V., Botchkareva N., Peters E., Paus R. Epithelial growth control by neurotrophins: leads and lessons from the hair follicle. Prog Brain Res. 2004;146:493–513. doi: 10.1016/S0079-6123(03)46031-7. [DOI] [PubMed] [Google Scholar]
- 50.Botchkarev V.A., Botchkareva N.V., Albers K.M., van der Veen C., Lewin G.R., Paus R. Neurotrophin-3 involvement in the regulation of hair follicle morphogenesis. J Invest Dermatol. 1998;111(2):279–285. doi: 10.1046/j.1523-1747.1998.00277.x. [DOI] [PubMed] [Google Scholar]
- 51.Jahoda C., Whitehouse J., Reynolds A., Hole N. Hair follicle dermal cells differentiate into adipogenic and osteogenic lineages. Exp Dermatol. 2003;12(6):849–859. doi: 10.1111/j.0906-6705.2003.00161.x. [DOI] [PubMed] [Google Scholar]
- 52.Millar S.E. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118(2):216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- 53.Daszczuk P., Mazurek P., Pieczonka T., Olczak A., Boryń Ł., Kobielak K. An intrinsic oscillation of gene networks inside hair follicle stem cells: an additional layer that can modulate hair stem cell activities. Front Cell Dev Biol. 2020;8 doi: 10.3389/fcell.2020.595178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou L., Yang K., Carpenter A., Lang R.A., Andl T., Zhang Y. CD133-positive dermal papilla-derived Wnt ligands regulate postnatal hair growth. Biochem J. 2016;473(19):3291–3305. doi: 10.1042/BCJ20160466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng M., Yang G., Wu J. Versican targeting by RNA interference suppresses aggregative growth of dermal papilla cells. Clin Exp Dermatol. 2011;36(1):77–84. doi: 10.1111/j.1365-2230.2010.03917.x. [DOI] [PubMed] [Google Scholar]
- 56.Christiano A.M. Epithelial stem cells: stepping out of their niche. Cell. 2004;118(5):530–532. doi: 10.1016/j.cell.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 57.Andl T., Reddy S., Gaddapara T., Millar S. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2(5):643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- 58.de Groot S.C., Ulrich M.M.W., Gho C.G., Huisman M.A. Back to the future: from appendage development toward future human hair follicle neogenesis. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.661787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lawrence A.J.R.C., Cserhalmi-Friedman P.B., Christiano A.M., Jahoda C.A.B. Trans-gender induction of hair follicles. Narure. 1999;402:33–34. doi: 10.1038/46938. [DOI] [PubMed] [Google Scholar]
- 60.Kang B.M., Kwack M.H., Kim M.K., Kim J.C., Sung Y.K. Sphere formation increases the ability of cultured human dermal papilla cells to induce hair follicles from mouse epidermal cells in a reconstitution assay. J Invest Dermatol. 2012;132(1):237–239. doi: 10.1038/jid.2011.250. [DOI] [PubMed] [Google Scholar]
- 61.Rheinwald J., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 62.Harel S., Higgins C., Cerise J., Dai Z., Chen J., Clynes R., et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1(9) doi: 10.1126/sciadv.1500973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rendl M., Polak L., Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Gene Dev. 2008;22(4) doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamao M., Inamatsu M., Ogawa Y., Toki H., Okada T., Toyoshima K., et al. Contact between dermal papilla cells and dermal sheath cells enhances the ability of DPCs to induce hair growth. J Invest Dermatol. 2010;130(12):2707–2718. doi: 10.1038/jid.2010.241. [DOI] [PubMed] [Google Scholar]
- 65.Choi N., Kim W., Oh S., Sung J. Epiregulin promotes hair growth via EGFR-medicated epidermal and ErbB4-mediated dermal stimulation. Cell Prolif. 2020;53(9) doi: 10.1111/cpr.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsai S.Y., Clavel C., Kim S., Ang Y.S., Grisanti L., Lee D.F., et al. Oct4 and klf4 reprogram dermal papilla cells into induced pluripotent stem cells. Stem Cell. 2010;28(2):221–228. doi: 10.1002/stem.281. [DOI] [PubMed] [Google Scholar]
- 67.Chacón-Martínez C., Klose M., Niemann C., Glauche I., Wickström S. Hair follicle stem cell cultures reveal self-organizing plasticity of stem cells and their progeny. EMBO J. 2017;36(2):151–164. doi: 10.15252/embj.201694902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hsu Y., Pasolli H., Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144(1):92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fantauzzo K., Christiano A. There and back again: hair follicle stem cell dynamics. Cell Stem Cell. 2011;8(1):8–9. doi: 10.1016/j.stem.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 70.Quist S.R., Quist J. Keep quiet-how stress regulates hair follicle stem cells. Signal Transduct Targeted Ther. 2021;6(1):364. doi: 10.1038/s41392-021-00772-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee S.A., Li K.N., Tumbar T. Stem cell-intrinsic mechanisms regulating adult hair follicle homeostasis. Exp Dermatol. 2021;30(4):430–447. doi: 10.1111/exd.14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hsu Y.C., Li L., Fuchs E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell. 2014;157(4):935–949. doi: 10.1016/j.cell.2014.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greco V., Chen T., Rendl M., Schober M., Pasolli H.A., Stokes N., et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4(2):155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsu Y.C., Pasolli H.A., Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144(1):92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bühring H., Treml S., Cerabona F., de Zwart P., Kanz L., Sobiesiak M. Phenotypic characterization of distinct human bone marrow-derived MSC subsets. Ann N Y Acad Sci. 2009;1176:124–134. doi: 10.1111/j.1749-6632.2009.04564.x. [DOI] [PubMed] [Google Scholar]
- 76.Inoue K., Aoi N., Sato T., Yamauchi Y., Suga H., Eto H., et al. Differential expression of stem-cell-associated markers in human hair follicle epithelial cells. Lab Invest. 2009;89(8):844–856. doi: 10.1038/labinvest.2009.48. [DOI] [PubMed] [Google Scholar]
- 77.Ohyama M., Terunuma A., Tock C.L., Radonovich M.F., Vogel J.C. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006;116(1):249–260. doi: 10.1172/JCI26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nath M., Offers M., Hummel M., Seissler J. Isolation and in vitro expansion of Lgr6-positive multipotent hair follicle stem cells. Cell Tissue Res. 2011;344(3):435–444. doi: 10.1007/s00441-011-1165-y. [DOI] [PubMed] [Google Scholar]
- 79.Ohyama M. Hair follicle bulge: a fascinating reservoir of epithelial stem cells. J Dermatol Sci. 2007;46(2):81–89. doi: 10.1016/j.jdermsci.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 80.Xinhong Lim, Si Hui, Tan Ka, Lou Yu, Sophia Beng. Axin2 marks quiescent hair follicle bulge stem cells that are maintained by autocrine Wnt/β-catenin signaling. Proceed Nat Acad Sci USA. 2016 doi: 10.1073/pnas.1601599113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rhee H., Polak L., Fuchs E. Lhx2 maintains stem cell character in hair follicles. Science. 2006;312(5782):1946–1949. doi: 10.1126/science.1128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vidal V., Chaboissier M.C., Lützkendorf S., Cotsarelis G., Mill P., Hui C.C., et al. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol. 2005;15(15):1340–1351. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 83.Toma J., Akhavan M., Fernandes K., Barnabé-Heider F., Sadikot A., Kaplan D., et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3(9):778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 84.Kishi K., Hayashi R. Hair induction by cultured mesenchymal cells using sphere formation. Method Mol Biol (Clifton, N.J.) 2016;1453:85–92. doi: 10.1007/978-1-4939-3786-8_10. [DOI] [PubMed] [Google Scholar]
- 85.Krause M., Dworski S., Feinberg K., Jones K., Johnston A., Paul S., et al. Direct genesis of functional rodent and human schwann cells from skin mesenchymal precursors. Stem Cell Rep. 2014;3(1):85–100. doi: 10.1016/j.stemcr.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kang H., Min S., Jung S., Jung K., Jang D., Kim O., et al. The potential of mouse skin-derived precursors to differentiate into mesenchymal and neural lineages and their application to osteogenic induction in vivo. Int J Mol Med. 2011;28(6):1001–1011. doi: 10.3892/ijmm.2011.785. [DOI] [PubMed] [Google Scholar]
- 87.Lavoie J., Biernaskie J., Chen Y., Bagli D., Alman B., Kaplan D., et al. Skin-derived precursors differentiate into skeletogenic cell types and contribute to bone repair. Stem Cell Dev. 2009;18(6):893–906. doi: 10.1089/scd.2008.0260. [DOI] [PubMed] [Google Scholar]
- 88.Hill R., Gledhill K., Gardner A., Higgins C., Crawford H., Lawrence C., et al. Generation and characterization of multipotent stem cells from established dermal cultures. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0050742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Biernaskie J., Paris M., Morozova O., Fagan B.M., Marra M., Pevny L., et al. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell. 2009;5(6):610–623. doi: 10.1016/j.stem.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo L., Wang X., Yuan J., Zhu M., Fu X., Xu R., et al. TSA restores hair follicle-inductive capacity of skin-derived precursors. Sci Rep. 2019;9(1):2867. doi: 10.1038/s41598-019-39394-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frye M., Fisher A., Watt F. Epidermal stem cells are defined by global histone modifications that are altered by Myc-induced differentiation. PLoS One. 2007;2(8):e763. doi: 10.1371/journal.pone.0000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Biernaskie J., Paris M., Morozova O., Fagan B., Marra M., Pevny L., et al. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell. 2009;5(6):610–623. doi: 10.1016/j.stem.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hunt D.P., Morris P.N., Sterling J., Anderson J.A., Joannides A., Jahoda C., et al. A highly enriched niche of precursor cells with neuronal and glial potential within the hair follicle dermal papilla of adult skin. Stem Cell. 2008;26(1):163–172. doi: 10.1634/stemcells.2007-0281. [DOI] [PubMed] [Google Scholar]
- 94.Caldwell M., He X., Wilkie N., Pollack S., Marshall G., Wafford K., et al. Growth factors regulate the survival and fate of cells derived from human neurospheres. Nat Biotechnol. 2001;19(5):475–479. doi: 10.1038/88158. [DOI] [PubMed] [Google Scholar]
- 95.Gago N., Pérez-López V., Sanz-Jaka J., Cormenzana P., Eizaguirre I., Bernad A., et al. Age-dependent depletion of human skin-derived progenitor cells. Stem cells (Dayton, Ohio) 2009;27(5):1164–1172. doi: 10.1002/stem.27. [DOI] [PubMed] [Google Scholar]
- 96.Mao M.Q., Jing J., Miao Y.J., Lv Z.F. Epithelial-mesenchymal interaction in hair regeneration and skin wound healing. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.863786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang M., Ye Y., Zhao P., Bai L., Li X. Preliminary studies of hair follicle regeneration by injections of epidermal stem cells and dermal papilla cells into nude mice. Cell Tissue Bank. 2020;21(2):321–327. doi: 10.1007/s10561-020-09825-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Matsumura H., Mohri Y., Binh N.T., Morinaga H., Fukuda M., Ito M., et al. Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science. 2016;351(6273):aad4395. doi: 10.1126/science.aad4395. [DOI] [PubMed] [Google Scholar]
- 99.Ge Y., Miao Y., Gur-Cohen S., Gomez N., Yang H., Nikolova M., et al. The aging skin microenvironment dictates stem cell behavior. Proc Natl Acad Sci U S A. 2020;117(10):5339–5350. doi: 10.1073/pnas.1901720117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Asakawa K., Toyoshima K.E., Ishibashi N., Tobe H., Iwadate A., Kanayama T., et al. Hair organ regeneration via the bioengineered hair follicular unit transplantation. Sci Rep. 2012;2:424. doi: 10.1038/srep00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Toyoshima K.E., Asakawa K., Ishibashi N., Toki H., Ogawa M., Hasegawa T., et al. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat Commun. 2012;3:784. doi: 10.1038/ncomms1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rendl M., Lewis L., Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3(11):e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Collins C.A., Kretzschmar K., Watt F.M. Reprogramming adult dermis to a neonatal state through epidermal activation of beta-catenin. Development. 2011;138(23):5189–5199. doi: 10.1242/dev.064592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao Q., Li N., Zhang H., Lei X., Cao Y., Xia G., et al. Chemically induced transformation of human dermal fibroblasts to hair-inducing dermal papilla-like cells. Cell Prolif. 2019;52(5) doi: 10.1111/cpr.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang R., Zheng Y., Li L., Liu S., Burrows M., Wei Z., et al. Direct conversion of mouse and human fibroblasts to functional melanocytes by defined factors. Nat Commun. 2014;5:5807. doi: 10.1038/ncomms6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim H.S., Sun X., Lee J.H., Kim H.W., Fu X., Leong K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv Drug Deliv Rev. 2019;146:209–239. doi: 10.1016/j.addr.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 107.Cho C.Y., Chiang T.H., Hsieh L.H., Yang W.Y., Hsu H.H., Yeh C.K., et al. Development of a novel hanging drop platform for engineering controllable 3D microenvironments. Front Cell Dev Biol. 2020;8:327. doi: 10.3389/fcell.2020.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin B., Miao Y., Wang J., Fan Z., Du L., Su Y., et al. Surface tension guided hanging-drop: producing controllable 3D spheroid of high-passaged human dermal papilla cells and forming inductive microtissues for hair-follicle regeneration. ACS Appl Mater Interfaces. 2016;8(9):5906–5916. doi: 10.1021/acsami.6b00202. [DOI] [PubMed] [Google Scholar]
- 109.Kageyama T., Nanmo A., Yan L., Nittami T., Fukuda J. Effects of platelet-rich plasma on in vitro hair follicle germ preparation for hair regenerative medicine. J Biosci Bioeng. 2020;130(6):666–671. doi: 10.1016/j.jbiosc.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 110.Nakajima R., Tate Y., Yan L., Kageyama T., Fukuda J. Impact of adipose-derived stem cells on engineering hair follicle germ-like tissue grafts for hair regenerative medicine. J Biosci Bioeng. 2021;131(6):679–685. doi: 10.1016/j.jbiosc.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 111.Kageyama T., Chun Y.S., Fukuda J. Hair follicle germs containing vascular endothelial cells for hair regenerative medicine. Sci Rep. 2021;11(1):624. doi: 10.1038/s41598-020-79722-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Watt F.M. Engineered microenvironments to direct epidermal stem cell behavior at single-cell resolution. Dev Cell. 2016;38(6):601–609. doi: 10.1016/j.devcel.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 113.Messenger A.G., Elliott K., Westgate G.E., Gibson W.T. Distribution of extracellular matrix molecules in human hair follicles. Ann N Y Acad Sci. 2006;642(1):253–262. doi: 10.1111/j.1749-6632.1991.tb24392.x. [DOI] [PubMed] [Google Scholar]
- 114.Rippa A.L., Vorotelyak E.A., Vasiliev A.V., Terskikh V.V. The role of integrins in the development and homeostasis of the epidermis and skin appendages. Acta Naturea. 2013;5 [PMC free article] [PubMed] [Google Scholar]
- 115.Messenger A.G., Elliott K., Temple A., Randall V.A. Expression of basement membrane proteins and interstitial collagens in dermal papillae of human hair follicles. J Invest Dermatol. 1991;96(1):93–97. doi: 10.1111/1523-1747.ep12515907. [DOI] [PubMed] [Google Scholar]
- 116.Tanimura S., Tadokoro Y., Inomata K., Binh N.T., Nishie W., Yamazaki S., et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011;8(2):177–187. doi: 10.1016/j.stem.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 117.Kageyama T., Yan L., Shimizu A., Maruo S., Fukuda J. Preparation of hair beads and hair follicle germs for regenerative medicine. Biomaterials. 2019;212:55–63. doi: 10.1016/j.biomaterials.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 118.Vahav I., van den Broek L.J., Thon M., Monsuur H.N., Spiekstra S.W., Atac B., et al. Reconstructed human skin shows epidermal invagination towards integrated neopapillae indicating early hair follicle formation in vitro. J Tissue Eng Regen Med. 2020;14(6):761–773. doi: 10.1002/term.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li S., Thangapazham R.L., Wang J.A., Rajesh S., Kao T.C., Sperling L., et al. Human TSC2-null fibroblast-like cells induce hair follicle neogenesis and hamartoma morphogenesis. Nat Commun. 2011;2:235. doi: 10.1038/ncomms1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang F., Qu K., Li X., Liu C., Ortiz L.S., Wu K., et al. Gelatin-based hydrogels combined with electrical stimulation to modulate neonatal rat cardiomyocyte beating and promote maturation. Bio Des Manuf. 2020;4:100–110. [Google Scholar]
- 121.Wang J., Miao Y., Huang Y., Lin B., Liu X., Xiao S., et al. Bottom-up nanoencapsulation from single cells to tunable and scalable cellular spheroids for hair follicle regeneration. Adv Healthc Mater. 2018;7(3) doi: 10.1002/adhm.201700447. [DOI] [PubMed] [Google Scholar]
- 122.Fernandez-Martos S., Calvo-Sanchez M., Garcia-Alonso K., Castro B., Hashtroody B., Espada J. Sustained human hair follicle growth Ex vivo in a glycosaminoglycan hydrogel matrix. Int J Mol Sci. 2019;20(7) doi: 10.3390/ijms20071741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang X., Liu S., Zhao Q., Li N., Zhang H., Zhang X., et al. Three-dimensional hydrogel scaffolds facilitate in vitro self-renewal of human skin-derived precursors. Acta Biomater. 2014;10(7):3177–3187. doi: 10.1016/j.actbio.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 124.Kalabusheva E., Terskikh V., Vorotelyak E. Hair germ model in vitro via human postnatal keratinocyte-dermal papilla interactions: impact of hyaluronic acid. Stem Cell Int. 2017;2017 doi: 10.1155/2017/9271869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kang M.S., Kwon M., Lee S.H., Kim W.H., Lee G.W., Jo H.J., et al. 3D printing of skin equivalents with hair follicle structures and epidermal-papillary-dermal layers using gelatin/hyaluronic acid hydrogels. Chem Asian J. 2022 doi: 10.1002/asia.202200620. [DOI] [PubMed] [Google Scholar]
- 126.Kleinman H.K., Martin G.R. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15(5):378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 127.Wang X., Wang X., Liu J., Cai T., Guo L., Wang S., et al. Hair follicle and sebaceous gland de novo regeneration with cultured epidermal stem cells and skin-derived precursors. Stem Cells Transl Med. 2016;5(12):1695–1706. doi: 10.5966/sctm.2015-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang X., Chen X., Hong H., Hu R., Liu J., Liu C. Decellularized extracellular matrix scaffolds: recent trends and emerging strategies in tissue engineering. Bioact Mater. 2022;10:15–31. doi: 10.1016/j.bioactmat.2021.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.D'Alessandro C.C., Dimopoulos A., Andriopoulou S., Messaris G.A.T., Korossis S., Koutsoukos P., et al. In vitro calcification studies on bioprosthetic and decellularized heart valves under quasi-physiological flow conditions. Bio Des Manuf. 2020;4:10–21. [Google Scholar]
- 130.Luo Z., Yue Y., Zhang Y., Yuan X., Gong J., Wang L., et al. Designer D-form self-assembling peptide nanofiber scaffolds for 3-dimensional cell cultures. Biomaterials. 2013;34(21):4902–4913. doi: 10.1016/j.biomaterials.2013.03.081. [DOI] [PubMed] [Google Scholar]
- 131.Cheng T.Y., Chen M.H., Chang W.H., Huang M.Y., Wang T.W. Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering. Biomaterials. 2013;34(8):2005–2016. doi: 10.1016/j.biomaterials.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 132.Najafi H., Jafari M., Farahavar G., Abolmaali S.S., Azarpira N., Borandeh S., et al. Recent advances in design and applications of biomimetic self-assembled peptide hydrogels for hard tissue regeneration. Bio Des Manuf. 2021;4:735–756. doi: 10.1007/s42242-021-00149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang X., Wang J., Guo L., Wang X., Chen H., Wang X., et al. Self-assembling peptide hydrogel scaffolds support stem cell-based hair follicle regeneration. Nanomedicine. 2016;12(7):2115–2125. doi: 10.1016/j.nano.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 134.Li J., Tzu J., Chen Y., Zhang Y.-P., Nguyen N.T., Gao J., et al. Laminin-10 is crucial for hair morphogenesis. EMBO J. 2003;22:2400–2410. doi: 10.1093/emboj/cdg239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gao J., DeRouen M.C., Chen C.H., Nguyen M., Nguyen N.T., Ido H., et al. Laminin-511 is an epithelial message promoting dermal papilla development and function during early hair morphogenesis. Genes Dev. 2008;22(15):2111–2124. doi: 10.1101/gad.1689908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huang Y.C., Chan C.C., Lin W.T., Chiu H.Y., Tsai R.Y., Tsai T.H., et al. Scalable production of controllable dermal papilla spheroids on PVA surfaces and the effects of spheroid size on hair follicle regeneration. Biomaterials. 2013;34(2):442–451. doi: 10.1016/j.biomaterials.2012.09.083. [DOI] [PubMed] [Google Scholar]
- 137.Zhang K., Bai X., Yuan Z., Cao X., Jiao X., Qin Y., et al. Cellular nanofiber structure with secretory activity-promoting characteristics for multicellular spheroid formation and hair follicle regeneration. ACS Appl Mater Interfaces. 2020;12(7):7931–7941. doi: 10.1021/acsami.9b21125. [DOI] [PubMed] [Google Scholar]
- 138.Gago N., Perez-Lopez V., Sanz-Jaka J.P., Cormenzana P., Eizaguirre I., Bernad A., et al. Age-dependent depletion of human skin-derived progenitor cells. Stem Cell. 2009;27(5):1164–1172. doi: 10.1002/stem.27. [DOI] [PubMed] [Google Scholar]
- 139.Joannides A., Gaughwin P., Schwiening C., Majed H., Sterling J., Compston A., et al. Efficient generation of neural precursors from adult human skin-astrocytes promote neurogenesis from skin-derived. Lancet. 2004;364:172–178. doi: 10.1016/S0140-6736(04)16630-0. [DOI] [PubMed] [Google Scholar]
- 140.Zhou L., Yang K., Xu M., Andl T., Millar S.E., Boyce S., et al. Activating beta-catenin signaling in CD133-positive dermal papilla cells increases hair inductivity. FEBS J. 2016;283(15):2823–2835. doi: 10.1111/febs.13784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dong L., Hao H., Liu J., Tong C., Ti D., Chen D., et al. Wnt1a maintains characteristics of dermal papilla cells that induce mouse hair regeneration in a 3D preculture system. J Tissue Eng Regen Med. 2017;11(5):1479–1489. doi: 10.1002/term.2046. [DOI] [PubMed] [Google Scholar]
- 142.Kageyama T., Yoshimura C., Myasnikova D., Kataoka K., Nittami T., Maruo S., et al. Spontaneous hair follicle germ (HFG) formation in vitro, enabling the large-scale production of HFGs for regenerative medicine. Biomaterials. 2018;154:291–300. doi: 10.1016/j.biomaterials.2017.10.056. [DOI] [PubMed] [Google Scholar]
- 143.Yamane M., Seo J., Zhou Y., Asaba T., Tu S., Nanmo A., et al. Effects of the PI3K/Akt signaling pathway on the hair inductivity of human dermal papilla cells in hair beads. J Biosci Bioeng. 2022 doi: 10.1016/j.jbiosc.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 144.Abaci H.E., Coffman A., Doucet Y., Chen J., Jackow J., Wang E., et al. Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat Commun. 2018;9(1):5301. doi: 10.1038/s41467-018-07579-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kumada Y., Zhang S. Significant type I and type III collagen production from human periodontal ligament fibroblasts in 3D peptide scaffolds without extra growth factors. PLoS One. 2010;5(4) doi: 10.1371/journal.pone.0010305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.He Y., Gu Z., Xie M., Fu J., Lin H. Why choose 3D bioprinting? Part II: methods and bioprinters. Bio Des Manuf. 2020;3(1):1–4. [Google Scholar]
- 147.Mistriotis P., Andreadis S.T. Hair follicle: a novel source of multipotent stem cells for tissue engineering and regenerative medicine. Tissue Eng B Rev. 2013;19(4):265–278. doi: 10.1089/ten.teb.2012.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jahoda C.A.B., Whitehouse C., Reynolds A., Hole N. Hair follicle dermal cells differentiate into adipogenic and osteogenic lineages. Exp Dermatol. 2003;12:849–859. doi: 10.1111/j.0906-6705.2003.00161.x. [DOI] [PubMed] [Google Scholar]
- 149.Ohyama M., Terunuma A., Tock C.L., Radonovich M.F., Pise-Masison C.A., Hopping S.B., et al. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006;116(1):249–260. doi: 10.1172/JCI26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bajpai V.K., Mistriotis P., Andreadis S.T. Clonal multipotency and effect of long-term in vitro expansion on differentiation potential of human hair follicle derived mesenchymal stem cells. Stem Cell Res. 2012;8(1):74–84. doi: 10.1016/j.scr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu J.J., Liu R.Q., Lu Y.G., Zhu T.Y., Cheng B., Men X. Enzyme digestion to isolate and culture human scalp dermal papilla cells: a more efficient method. Arch Dermatol Res. 2005;297(2):60–67. doi: 10.1007/s00403-005-0554-z. [DOI] [PubMed] [Google Scholar]
- 152.Snippert H.J., Haegebarth A., Kasper M., Jaks V., Es J.H., Barker N., et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327(5971):1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- 153.Jaks V., Barker N., Kasper M., van Es J.H., Snippert H.J., Clevers H., et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40(11):1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 154.Veraitch O., Kobayashi T., Imaizumi Y., Akamatsu W., Sasaki T., Yamanaka S., et al. Human induced pluripotent stem cell-derived ectodermal precursor cells contribute to hair follicle morphogenesis in vivo. J Invest Dermatol. 2013;133(6):1479–1488. doi: 10.1038/jid.2013.7. [DOI] [PubMed] [Google Scholar]
- 155.Veraitch O., Mabuchi Y., Matsuzaki Y., Sasaki T., Okuno H., Tsukashima A., et al. Induction of hair follicle dermal papilla cell properties in human induced pluripotent stem cell-derived multipotent LNGFR(+)THY-1(+) mesenchymal cells. Sci Rep. 2017;7 doi: 10.1038/srep42777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Itoh M., Kiuru M., Cairo M.S., Christiano A.M. Generation of keratinocytes from normal and recessive dystrophic epidermolysis bullosa-induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2011;108(21):8797–8802. doi: 10.1073/pnas.1100332108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wenzel V., Roedl D., Gabriel D., Gordon L.B., Herlyn M., Schneider R., et al. Naive adult stem cells from patients with Hutchinson-Gilford progeria syndrome express low levels of progerin in vivo. Biol Open. 2012;1(6):516–526. doi: 10.1242/bio.20121149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Christoph T., Müller-Röver S., Audring H., Tobin D.J., Hermes B., Cotsarelis G., et al. The human hair follicle immune system: cellular composition and immune privilege. Br J Dermatol. 2002;142:862–873. doi: 10.1046/j.1365-2133.2000.03464.x. [DOI] [PubMed] [Google Scholar]
- 159.Westgate G.E., Craggs R.I., Gibson W.T. Immune privilege in hair growth. J Invest Dermatol. 1991;97(3):417–420. doi: 10.1111/1523-1747.ep12481002. [DOI] [PubMed] [Google Scholar]
- 160.Znidaric M., Zurga Z.M., Maver U. Design of in vitro hair follicles for different applications in the treatment of alopecia-A review. Biomedicines. 2021;9(4) doi: 10.3390/biomedicines9040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Reynolds A.J., Lawrence C., Cserhalmi-Friedman P.B., Christiano A.M., Jahoda C.A. Trans-gender induction of hair follicles. Nature. 1999;402:33–34. doi: 10.1038/46938. [DOI] [PubMed] [Google Scholar]
- 162.Liu S., Liu S., Wang X., Zhou J., Cao Y., Wang F., et al. The PI3K-Akt pathway inhibits senescence and promotes self-renewal of human skin-derived precursors in vitro. Aging Cell. 2011;10(4):661–674. doi: 10.1111/j.1474-9726.2011.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Agabalyan N.A., Borys B.S., Sparks H.D., Boon K., Raharjo E.W., Abbasi S., et al. Enhanced expansion and sustained inductive function of skin-derived precursor cells in computer-controlled stirred suspension bioreactors. Stem Cells Transl Med. 2017;6(2):434–443. doi: 10.5966/sctm.2016-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hamley I.W. Small bioactive peptides for biomaterials design and therapeutics. Chem Rev. 2017;117(24):14015–14041. doi: 10.1021/acs.chemrev.7b00522. [DOI] [PubMed] [Google Scholar]
- 165.Fan V.H., Tamama K., Au A., Littrell R., Richardson L.B., Wright J.W., et al. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cell. 2007;25(5):1241–1251. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- 166.Jha A.K., Tharp K.M., Ye J., Santiago-Ortiz J.L., Jackson W.M., Stahl A., et al. Enhanced survival and engraftment of transplanted stem cells using growth factor sequestering hydrogels. Biomaterials. 2015;47:1–12. doi: 10.1016/j.biomaterials.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lee S.S., Hsu E.L., Mendoza M., Ghodasra J., Nickoli M.S., Ashtekar A., et al. Gel scaffolds of BMP-2-binding peptide amphiphile nanofibers for spinal arthrodesis. Adv Healthc Mater. 2015;4(1):131–141. doi: 10.1002/adhm.201400129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lewis J.A., Freeman R., Carrow J.K., Clemons T.D., Palmer L.C., Stupp S.I. Transforming growth factor β-1 binding by peptide amphiphile hydrogels. ACS Biomater Sci Eng. 2020;6(8):4551–4560. doi: 10.1021/acsbiomaterials.0c00679. [DOI] [PubMed] [Google Scholar]
- 169.Rubert Perez C.M., Alvarez Z., Chen F., Aytun T., Stupp S.I. Mimicking the bioactivity of fibroblast growth factor-2 using supramolecular nanoribbons. ACS Biomater Sci Eng. 2017;3(9):2166–2175. doi: 10.1021/acsbiomaterials.7b00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Madl C.M., Mehta M., Duda G.N., Heilshorn S.C., Mooney D.J. Presentation of BMP-2 mimicking peptides in 3D hydrogels directs cell fate commitment in osteoblasts and mesenchymal stem cells. Biomacromolecules. 2014;15(2):445–455. doi: 10.1021/bm401726u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Su J., Satchell S.C., Wertheim J.A., Shah R.N. Poly(ethylene glycol)-crosslinked gelatin hydrogel substrates with conjugated bioactive peptides influence endothelial cell behavior. Biomaterials. 2019;201:99–112. doi: 10.1016/j.biomaterials.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]