Abstract
The large-scale usage of petro-chemical-based plastics has proved to be a significant source of environmental pollution due to their non-biodegradable nature. Microbes-based enzymes such as esterases, cutinases, and lipases have shown the ability to degrade synthetic plastic. However, the degradation of plastics by enzymes is primarily limited by the unavailability of a robust enzymatic system, i.e., low activity and stability towards plastic degradation. Recently, the machine learning strategy involved structure-based and deep neural networks show desirable potential to generate functional, active stable, and tolerant polyethylene terephthalate (PET) degrading enzyme (FAST-PETase). FAST-PETase showed the highest PET hydrolytic activity among known enzymes or their variants and degraded broad ranges of plastics. The development of a closed-loop circular economy-based system of plastic degradation to monomers by FAST-PETase followed by the re-polymerization of monomers into clean plastics can be a more sustainable approach. As an alternative to synthetic plastics, diverse microbes can produce polyhydroxyalkanoates, and their degradation by microbes has been well-established. This article discusses recent updates in the enzymatic degradation of plastics for sustainable development.
Keywords: Bio-degradation process, Machine learning strategy, Plastic degrading enzyme, Polyhydroxyalkanoates, Synthetic plastic
Plastics are hydrocarbons and petroleum-based high molecular weight polymers and play a crucial role in daily life. Due to their high durability and long-lasting properties, these are broadly used in diverse activities, including agriculture, municipal, and industrial sectors [1, 2]. Unfortunately, regardless of their unique properties, limited natural degradation of plastics causes massive environmental accumulation. Approximately plastics are produced in more than 350 million tons worldwide (European Commission, 2018) [2]. Nearly 2% of the produced plastics are recycled. Plastic wastes (~ 70%) are burnt/incinerated or disposed in landfills. The rest remains in the ecosystem [1]. To avoid the high accumulation of plastics on land and aquatic systems, efficient degradation via sustainable approaches is required. Plastic is primarily composed of silicon (Si), chloride (Cl), oxygen (O), nitrogen (N), carbon (C), and hydrogen (H). The degradation of plastics can potentially generate microparticles (< 5 mm) that are toxic to humans and the environment i.e., as potent carcinogenic and mutagenic component such as styrene oxide and bisphenol A [3, 4]. Up to 102,000 microparticles as micro-plastics can be generated in incineration per metric ton of plastics [1]. In addition, the incineration of plastics can lead to a generation of toxic compounds such as 2-dichloroethane, 1,3-butadiene, benzene, styrene, and toluene [5].The plastic particle size < 100 nm is described as nano-plastics that can be formed in the aquatic environment and lead to adverse influence on health. In the atmosphere, plastics can be degraded via photo-, hydrolytic-, thermo-oxidative, or bio-degradation processes. Photo-degradation of plastics can generate volatile organic toxic compounds such as benzene, acrolein, methyl vinyl ketone, and propanal that can be linked to hepato-, nephro, and neuro-toxicity in human and animals [6, 7]. The plastics degradation rates can be improved in the presence of photo- or thermo-oxidant-degrading agents. Polyethylene has a monomeric formula of CnH2n, consisting of ~ 64% of total plastics. Based on chemical properties, plastics can be classified as non-degradable or degradable. Degradable plastics are produced from renewable resources [1]. Therefore, polymers such as poly(γ-thiobutyrolactone) made from renewable assets are desirable alternatives to non-degradable polymers of petroleum origin i.e., low-density polyethylene (plastics). The ultrafast degradation of poly(γ-thiobutyrolactone) can be easily achieved by an external commercially available organocatalyst (1,5,7-triazabicyclo[4.4.0]dec-5-ene). However, a simple and robust strategy for producing poly(γ-thiobutyrolactone) using monomers derived from renewable origin holds great potential to generate degradable polymeric materials [7].
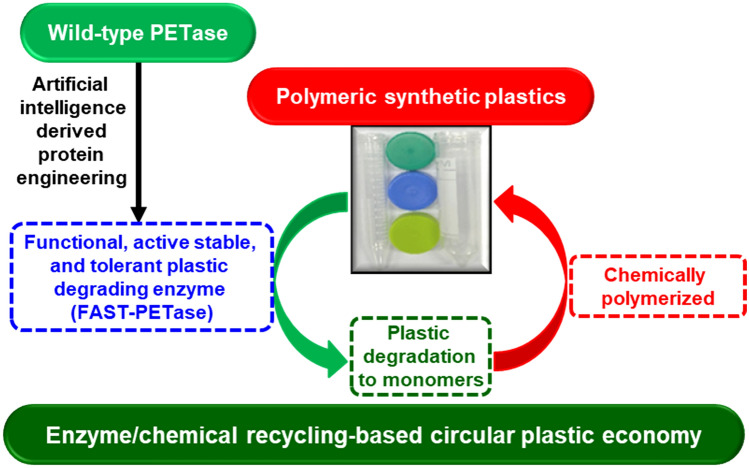
Polyethylene terephthalate (PET) is the most widely used plastic worldwide requires centuries to break down [8]. Globally, PET contributes about 12% of total solid waste. Degradation of PET by enzymes such as hydrolases is limited by low activity, selectivity, or stability. In the past few years, research has focused on areas to degrade PET by improving the properties of PET-hydrolyzing enzymes such as esterases, cutinases, or lipases [8, 9]. PETase is an enzyme that can break down PET. Ideonella sakaiensis assimilate PET at ambient conditions by PETase activity that is less stable (24 h at 37 °C) [10]. The computational and rational protein engineering strategies have proved beneficial for developing variants of PETase such as Dura- and Thermo-PETases with enhanced stability and activity under certain conditions [10, 11]. Hu et al. suggested that the machine learning approach consists of a structure-based and deep neural network that can improve PETase function than the focused protein engineering strategies for the overall enzyme stability and activity [8] (Fig. 1). Initially, a self-supervised three-dimensional convolutional neural network (Compute24) strategy was employed to identify stable mutations within wild-type (WT)- and thermos-PETases. This strategy resulted in 159 single or other predicted mutations, and these variants showed enhanced activity (especially in rates of plastic degradation and esterase activity) and stability (melting temperature of PETase). The prediction-based mutant PETaseS121E/R224Q/N233K exhibited up to 29-fold enhancement of PET-hydrolytic activity compared to WT-PETase. The combination of mutant and scaffold generated super functional active stable, and tolerant PETase (FAST-PETase) that exhibited the highest PET degradation among all mutants. FAST-PETase contains five mutations compared to WT-PETase, including from prediction (N233K, R224Q, and S121E) and the scaffold (D186H and R280A) [8]. A total of 51 plastic products available at local grocery stores with diverse crystallinity, thickness and additives, molecular weight, and hole-punched were fully degraded by FAST-PETase within a week, including a short period of 24 h at 50 °C. Apart from PET, FAST-PETase potentially degrades polyester products from commercial sources. FAST-PETase can efficiently degrade textile fabrics-based PET into monomers that can substantially minimize the environmental leaching of microfibers [8]. For the circular economy, PET depolymerization can be considered a half cycle. The combined strategies of FAST-PETase-based depolymerization of plastic waste followed by re-polymerization into virgin PET can be adapted for sustainable development. FAST-PETase-based degradation of plastic recovered terephthalic acid with a yield and purity of 95 and 97%, respectively. Subsequently, the chemical polymerization of monomers regenerated virgin PET. These degradations to re-polymerization processes are based on enzymatic and chemical cycling that can complete in a few days. This finding demonstrated the closed-loop practicality of virgin PET clean film generation and overcame the challenges of mixed-colored PET products recycling. Overall, these processes are in the initial state; more research is required to develop them for the industry [8]. The details of various types of plastic degrading enzymes are presented in Table 1 [12, 13].
Fig. 1.
Strategy to generate efficient plastic-degrading enzyme (FAST-PETase) by artificial intelligence-derived protein engineering for developing sustainable enzyme/chemical recycling-based circular plastic economy [8]
Table 1.
| Type of plastic | Degrading enzymes |
|---|---|
| Low-density polyethylene | Cutinase, laccase, and peroxidase |
| Poly-Ɛ-caprolactone | Cutinase, lipase, and PETase |
| Poly(1,4-butylene 2,5-furandicarboxylate) | Cutinase |
| Polybutylene succinate | Cutinase, PHB-depolymerase, and lipase |
| Polyethylene succinate | PHB-depolymerase |
| PET | Cutinase, and PETase |
| Polyhydroxybutyrate | Cutinase, and PHB-depolymerase |
| Polylactic acid | Cutinase, esterase, lipase, protease, and proteinase K |
| Polyurethane | Cutinase |
| Polyvinyl chloride | Laccase, and peroxidase |
The environmental and health concerns of plastics are increasing, which is supported by government regulations to minimize their use and promote safer plastic products such as biodegradable plastics [14, 15]. In addition, nature biopolymers such as cellulose, chitosan, alginates, polyhydroxybutyrate (PHB), PHB co-polymers as polyhydroxyalkanoates (PHAs), and carrageenan or their derivatives have received more importance due to environmental worries caused by the use of synthetic plastics. Bioplastics, especially PHAs are a possible solution for switching linear economy into the circular model due to their broad biotechnological applications [1, 16]. PHAs can be produced by more than 300 microbes belonging to diverse phylogenetic groups. Bacteria can accumulate PHAs up to 90% of total cell dry mass [17]. Almost complete degradation of PHAs occurs between 4 to 8 days [18]. The bioplastics market is expected to increasing at an annual growth rate above 15% by 2025. This information supports that PHA-based product demands will be ~ 100 million USD in the present scenario [1]. Various elements regulating commercial microbial PHAs production include feedstock and facilities that have resulted in reducing the high cost of PHAs over synthetic plastics [19]. Shortly, the use of biowastes as a feedstock and up-scaling of bioprocess by high-yielding PHAs can be produced economically leading to a more desirable and sustainable approach as an alternative to synthetic plastics.
Acknowledgements
Basic Science Research Program supported this study through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2019R1C1C11009766). This work was supported by the KU Research Professor Program of Konkuk University.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kalia VC, Patel SKS, Shanmugam R, et al. Polyhydroxy alkanoates: trends and advances towards biotechnological applications. Bioresour Technol. 2021;326:124737. doi: 10.1016/j.biortech.2021.124737. [DOI] [PubMed] [Google Scholar]
- 2.Yu L, Zhao D, Wang W. Mechanical properties and long-term durability of recycled polysulfone plastic. Waste Manag. 2019;84:402–412. doi: 10.1016/j.wasman.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Patel SKS, Otari SV, Li J, et al. Synthesis of cross-linked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. J Hazard Mater. 2018;347:442–450. doi: 10.1016/j.jhazmat.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Patel SKS, Choi H, Lee J-K. Multi-metal based inorganic–protein hybrid system for enzyme immobilization. ACS Sustain Chem Eng. 2019;7:13633–13638. doi: 10.1021/acssuschemeng.9b02583. [DOI] [Google Scholar]
- 5.Jang M, Yang H, Park S-A, et al. Analysis of volatile organic compounds produced during incineration of non-degradable and biodegradable plastics. Chemosphere. 2022;303:134956. doi: 10.1016/j.chemosphere.2022.134946. [DOI] [PubMed] [Google Scholar]
- 6.Lomonaco T, Macro E, Corti A, et al. Release of harmful volatile organic compounds (VOCs) from photo-degraded plastic debris: a neglected source of environmental pollution. J Hazard Mater. 2020;394:122596. doi: 10.1016/j.jhazmat.2020.122596. [DOI] [PubMed] [Google Scholar]
- 7.Guillaume SM. Sustainable and degradable plastics. Nat Chem. 2022;14:245–246. doi: 10.1038/s41557-022-00901-8. [DOI] [PubMed] [Google Scholar]
- 8.Lu H, Diaz DJ, Czarnecki NJ, et al. Machine learning-aided engineering of hydrolases for PET depolymerization. Nature. 2022;604:662–667. doi: 10.1038/s41586-022-04599-z. [DOI] [PubMed] [Google Scholar]
- 9.Son HF, Cho IJ, Joo S, et al. Rational protein engineering of thermo-stable PETase from Ideonella sakaiensis for highly efficient PET degradation. ACS Catal. 2019;9:3519–3526. doi: 10.1021/acscatal.9b00568. [DOI] [Google Scholar]
- 10.Cui Y, Chen Y, Liu X, et al. Computational redesign of a PETase for plastic biodegradation under ambient condition by the GRAPE strategy. ACS Catal. 2021;11:1340–1350. doi: 10.1021/acscatal.0c05126. [DOI] [Google Scholar]
- 11.Chen CC, Dai L, Ma L, et al. Enzymatic degradation of plant biomass and synthetic polymers. Nat Rev Chem. 2020;4:114–126. doi: 10.1038/s41570-020-0163-6. [DOI] [PubMed] [Google Scholar]
- 12.Kaushal J, Khatri M, Arya SK. Recent insight into enzymatic degradation of plastics prevalent in the environment: a mini-review. Clean Eng Technol. 2021;2:100083. doi: 10.1016/j.clet.2021.100083. [DOI] [Google Scholar]
- 13.Andler R, Tiso T, Blank L, et al. Current progress on the biodegradation of synthetic plastics: from fundamentals to biotechnological applications. Rev Environ Sci Biotechnol. 2022 doi: 10.1007/s11157-022-09631-2. [DOI] [Google Scholar]
- 14.Kalia VC, Raizada N, Sonakya V. Bioplastics. J Sci Ind Res. 2000;59:433–445. [Google Scholar]
- 15.Kumar P, Singh M, Mehariya S, et al. Ecobiotechnological approach for exploiting the abilities of Bacillus to produce co-polymer of polyhydroxyalkanoate. Indian J Microbiol. 2014;54:151–157. doi: 10.1007/s12088-014-0457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel SKS, Singh M, Kumar P, et al. Exploitation of defined bacterial cultures for production of hydrogen and polyhydroxybutyrate from pea-shells. Biomass Bioenergy. 2012;36:218–225. doi: 10.1016/j.biombioe.2011.10.027. [DOI] [Google Scholar]
- 17.Singh M, Kumar P, Patel SKS, et al. Production of polyhydroxyalkanoate co-polymer by Bacillus thuringiensis. Indian J Microbiol. 2013;53:77–83. doi: 10.1007/s12088-012-0294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray S, Kalia VC. Polyhydroxyalkanoate production and degradation patterns in Bacillus species. Indian J Microbiol. 2017;57:387–392. doi: 10.1007/s12088-017-0676-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel SKS, Das D, Kim SC, et al. Integrating strategies for sustainable conversion of waste biomass into dark-fermentative hydrogen and value-added products. Renew Sustain Energy Rev. 2021;150:111491. doi: 10.1016/j.rser.2021.111491. [DOI] [Google Scholar]