Abstract
Purpose
To determine the accuracy of bedside sonographic measurements of optic nerve sheath diameter (ONSD) and ONSD/eyeball transverse (ETD) diameter ratios to predict space-occupying lesions (SOLs) or elevated intracranial pressure (ICP) in pediatric head trauma.
Methods
Children who presented to the emergency department with head trauma and underwent cranial computed tomography (CT) were enrolled and examined by ocular ultrasonography (US), and the ONSD was measured at 3 mm posterior to the globe and ETD were measured. Ratios of ONSD at 3 mm/ETD were calculated. All ONSD measurements and ratios were calculated from cranial CT images.
Results
Subjects with elevated ICP had increased ONSD measurements and ratios. To predict elevated ICP, the AUC for ONSD at 3 mm was 0.956 (95% CI 0.896–1). At a cut-off level of 5.1 mm, the sensitivity and specificity of ONSD 3 mm values for elevated ICP were 92.9% and 94.0%. For the ONSD 3 mm/ETD ratio, it was 0.980 (95% CI 0.959–1). At a cut-off level of 0.22, the sensitivity and specificity were 100% sensitivity and 88.0%. All sonographic ONSD measurements and ratios were significantly correlated with readings calculated from cranial CT images.
Conclusion
Sonographic ONSD measurements and ratios were found to be quite sensitive to detect elevated ICP on cranial CT images. Additionally, there was a significant correlation between measurements calculated by ocular US and cranial CT scans. Bedside ocular US seems to be a promising and useful tool to determine ICP in children with head trauma.
Keywords: Children, Intracranial pressure, Optic nerve sheath diameter, Trauma, Ultrasound
Introduction
Head trauma is responsible for a significant rate of emergency department (ED) visits and hospitalizations for children, and traumatic brain injury (TBI) is a leading cause of pediatric disability and mortality [1].
After TBI, the intracranial compensatory reserve may deteriorate due to space-occupying lesions (SOLs) or cerebral edema, resulting in elevated intracranial pressure (ICP), which is associated with poor neurological outcomes. Management of severe TBI is mainly based on achieving optimal cerebral perfusion pressure [2, 3]. Clinical findings are often nonspecific in pediatric patients. Funduscopic examination is helpful but the child may not stay calm during examination and it remains uncertain when papilledema occurs after an acute elevation of ICP [4, 5]. Neuroimaging techniques such as cranial computed tomography (CT), magnetic resonance imaging (MRI), and transcranial Doppler ultrasonography (US) are also used, but they have some disadvantages such as exposure to ionizing radiation, prolonged transport time, high cost, and need for sedation, and they may not be readily available in some emergency settings [6, 7]. The gold standard for ICP measurement includes invasive techniques such as intraventricular catheterization or intraparenchymal microtransducers, requiring a neurosurgeon and an intensive care unit. Therefore, a noninvasive and more practical tool is required for evaluating ICP.
Bedside sonographic assessment of the optic nerve sheath has gained popularity for estimating ICP in recent years [8–21]. It is easy to learn and perform, rapid, repeatable, and cost-effective [12–14]. The optic nerve sheath distends as the ICP increases due to the enlargement of the underlying subarachnoid space and it is stated that this change occurs within minutes of acute increase in ICP [2, 10]. But few studies have evaluated optic nerve sheath diameter (ONSD) measurements of pediatric subjects [12–16].
Eyeball transverse diameter (ETD) was found to correlate with ONSD, and the ONSD measured at 3 mm posterior to the globe/ETD ratio was suggested to be a useful value to avoid the overlapping of standard deviations of normal and pathologic ONSD values and changes of the globe in individuals. But there are no data evaluating ONSD/ETD ratios for children [22–24].
The primary outcome of our study was to determine the accuracy of bedside sonographic measurements of ONSD at 3 mm behind the globe and ONSD/ETD ratios to predict SOLs or elevated ICP in children who presented to the emergency department with head trauma and underwent cranial CT. The secondary outcome was to assess the correlation of ONSD measurements calculated from ocular US and cranial CT images.
Materials and methods
Study design
The study was performed between April 2018 and November 2018 in the pediatric ED of a tertiary level hospital with approximately 120,000 pediatric emergency department admissions annually. The study protocol was approved by the Local Ethics Committee (approval number: 2018/18–31). Written informed consent was obtained from the parents or legal guardians of each participant before enrollment in the study. All procedures followed were in accordance with the ethical standards of the Declaration of Helsinki.
Previously healthy children aged between 1 month and 18 years, who presented to the pediatric emergency department with head trauma and underwent cranial CT according to the Pediatric Emergency Care Applied Research Network (PECARN) rules, were included [10]. Patients with chronic neuromuscular disease, metabolic or genetic disorders, ocular trauma, glaucoma, refraction errors, optic disc drusen, or a history of prematurity were excluded. The demographic data, trauma mechanism, vital signs, clinical findings, and Glasgow Coma Scale (GCS) scores were recorded. The GCS scores of the patients were divided into three groups: 15, 9–14, and ≤ 8.
Measurements
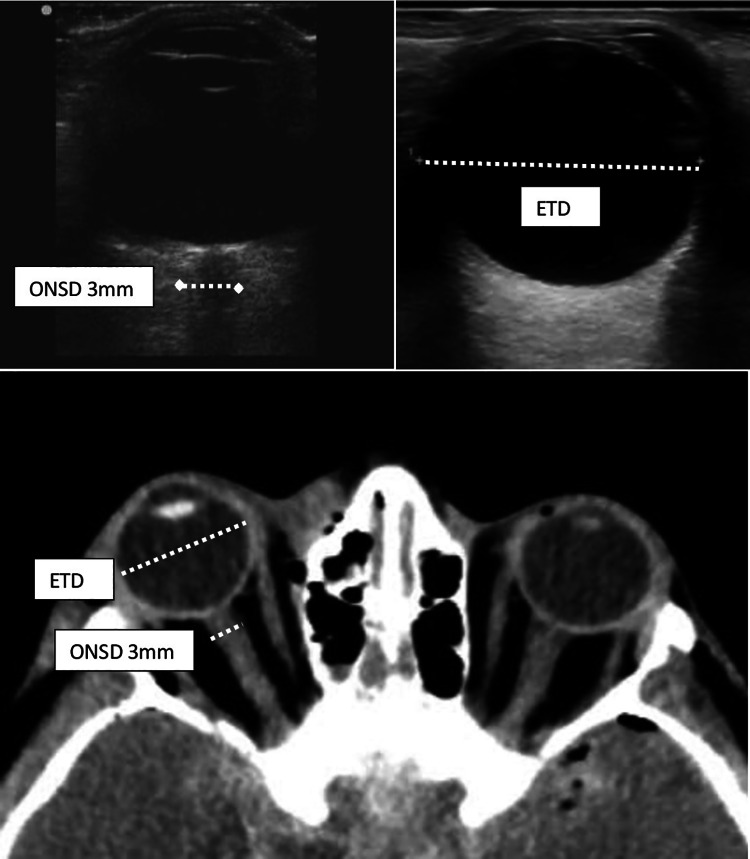
After clinical evaluation and immediate intervention if needed, subjects were examined by ocular US by a single pediatric emergency fellow who was unaware of the clinical/radiological findings and was well trained on didactic and goal-directed ocular sonography. In the supine position with eyelids closed, patients were instructed to remain calm and keep their gaze at the midline. Infants were kept waiting until they seemed calm or fell asleep, using pacifiers or distraction techniques during the examination if they were uncooperative. A transparent barrier was placed on the eyelid before applying the ultrasound gel to minimize discomfort. Sonographic examination was performed with a Philips ClearVue 350 portable system with an L12-4 MHz linear transducer. A large amount of gel was applied to the outside of each eyelid. Measurements were taken in the transverse plane with the transducer horizontal. The sheath was demonstrated as a bilateral hypoechoic line, cursors were placed on the outer edge, and the ONSD was calculated perpendicular to the vertical axis as the horizontal distance between the two cursors. The ONSD was measured at 3 mm posterior to the globe in the anterior axial transbulbar view. The ETD was measured as the maximal transverse diameter of the eyeball (retina to retina). The ratio of ONSD measurement at 3 mm/ETD was calculated (Fig. 1).
Fig. 1.
Measurements of ONSD at 3 mm and eyeball diameters on US and cranial CT scan. ONSD optic nerve sheath diameter, US ultrasound, CT computed tomography
All cranial CT scans were obtained by a 128-slice CT scanner (Toshiba Aquilion Prime). Images were evaluated and reported by a pediatric radiologist, and elevated ICP was considered positive if one or more of the following were present: midline shift (at least 3 mm), effacement of sulci, collapse of the third ventricle, hydrocephalus, or abnormal mesencephalic cistern. According to cranial CT findings, patients were divided into three groups: Group 1: Elevated ICP (+). Group 2: Space-occupying lesion (SOL) (+) and elevated ICP (−): Depressed skull fracture and/or intracranial hemorrhage (epidural, subdural, subarachnoid, intraparenchymal) or cerebral contusion without elevated ICP. Group 3: SOL (−) and elevated ICP (−).
The ONSD at 3 mm posterior to the globe, the ETD measurements, and the ratios were determined from cranial CT images (Fig. 1). Finally, the mean values of right and left eyes were recorded.
Statistical analysis
All statistical analyses were performed using SPSS 22.0 for Windows. Categorical and continuous variables were reported as frequencies and percentiles, and means with standard deviations (SDs) or medians with interquartile ranges (IQRs). The Mann–Whitney U test was used to compare nonparametric variables and Student’s t test was performed for parametric data. The nonparametric Kruskal–Wallis test was used to compare the ONSD values and ratios according to the GCS groups. Correlations between measurements calculated from US and CT images were assessed with Spearman’s rank correlation coefficient. To determine cut-off values of ONSD measurements and ratios to predict elevated ICP and intracranial lesions, the receiver operating characteristic (ROC) curve was drawn and sensitivity and specificity were calculated. Values of p < 0.05 were considered statistically significant.
Results
Study population
One hundred forty-seven children were enrolled in the study. The median age was 8.0 years (IQR 4.0–13.0). Of the patients, 100 (68.1%) were female. The most common trauma mechanism was falling from a height. Table 1 details the trauma mechanisms of our patients. For 107 (72.8%) patients, the GCS score was 15; for 23 (15.6%), it was 9–14; and for 17 (11.6%), it was ≤ 8. Of the 147 children, 59 had abnormal cranial CT findings. Skull fracture was detected in 40 (27.2%) patients, being depressed in 22 (15.0%) cases and non-depressed in 18 (12.2%). Intracranial hemorrhage was determined in 33 (22.4%) of the subjects; 17 (11.6%) patients had subdural hemorrhage (SDH), 12 (8.2%) had intraparenchymal hemorrhage (IPH), 11 (7.5%) had epidural hemorrhage (EDH), 10 (6.8%) had subarachnoid hemorrhage (SAH), and 6 (4.1%) had cerebral contusion. Elevated ICP was found in 14 (9.5%) of the patients. The numbers of subjects according to cranial CT findings divided into three groups were as follows: Group 1: Elevated ICP (+): 14 patients (9.5%). Group 2: SOL (+) and elevated ICP (−): 27 patients (18.4%). Group 3: SOL (−) and elevated ICP (−): 106 patients (72.1%) (Table 1).
Table 1.
Trauma mechanisms, hospital admissions and cranial CT findings of the patients (n 147)
| Trauma mechanism | n (%) |
|---|---|
| Falling from a height | 71 (48.3%) |
| Traffic accident (out of vehicle) | 46 (31.3%) |
| Traffic accident (in vehicle) | 16 (10.9%) |
| Hitting of the head | 9 (6.1%) |
| Falling of an object onto head | 5 (3.4%) |
| Cranial CT | |
| Normal | 88 (59.9%) |
| Abnormal | 59 (40.1%) |
| Elevated ICP | 14 (9.5%) |
| Intracranial hemorrhage | 33 (22.4%) |
| SDH | 17 (11.6%) |
| IPH | 12 (8.2%) |
| EDH | 11 (7.5%) |
| SAH | 10 (6.8%) |
| Contusion | 6 (4.1%) |
| Skull fracture | 40 (27.2%) |
| Depressed | 22 (15.0%) |
| Non-depressed | 18 (12.2%) |
| According to elevated ICP or SOL on cranial CT findings (n, %) | |
| Elevated ICP (+) | 14 (9.5%) |
| SOL (+), elevated ICP (−) | 27 (18.4%) |
| SOL (−), elevated ICP (−) | 106 (72.1%) |
CT computed tomography, ICP intracerebral pressure, ICH intracranial hemorrhage, SDH subdural hemorrhage, EDH epidural hemorrhage, SAH subarachnoid hemorrhage, IPH intraparenchymal hemorrhage, SOL space-occupying lesion
Ultrasound findings
The patients with abnormal cranial CT findings had higher sonographic ONSD measurements and ratios than those with normal CT results (p < 0.01) (Table 2). As the GCS scores decreased, a significant increase was observed in ONSD measurements and ratios (p < 0.001) (Table 3). Subjects with elevated ICP had increased ONSD measurements and ratios (p < 0.001) compared to those without elevated ICP according to the cranial CT reports (Table 2). To predict elevated ICP, ROC analysis was performed and the value of the area under the curve (AUC) for ONSD at 3 mm was 0.956 (95% CI 0.896–1). At a cut-off level of 5.1 mm, the sensitivity and specificity of ONSD 3 mm values for elevated ICP were 92.9% and 94.0%, respectively. For the ONSD 3 mm/ETD ratio, the AUC was 0.980 (95% CI 0.959–1). At a cut-off level of 0.22, the sensitivity and specificity were 100% and 88.0%, respectively (Fig. 2).
Table 2.
Sonographic ONSD measurements and ratios of the patients according to cranial CT findings (n 147)
| Ultrasound findings | Cranial CT findings | p | |
|---|---|---|---|
| Normal (n 88) | Abnormal (n 59) | ||
| ONSD 3 mm | 4.09 ± 0.45 | 4.67 ± 1.05 | 0.001 |
| ONSD 3 mm/ETD ratio | 0.18 ± 0.02 | 0.21 ± 0.04 | < 0.001 |
| Ultrasound findings | Elevated ICP (+) (n 14) | Elevated ICP (−) (n 133) | p |
|---|---|---|---|
| ONSD 3 mm | 5.95 ± 0.87 | 4.15 ± 0.57 | < 0.001 |
| ONSD 3 mm/ETD ratio | 0.27 ± 0.39 | 0.19 ± 0.24 | < 0.001 |
| Ultrasound findings | SOL (+), elevated ICP (−) (n 27) | SOL (−), elevated ICP (−) (n 106) | p |
|---|---|---|---|
| ONSD 3 mm | 4.52 ± 0.81 | 4.05 ± 0.45 | < 0.001 |
| ONSD 3 mm/ETD ratio | 0.20 ± 0.03 | 0.18 ± 0.02 | 0.004 |
Variables are expressed as mean ± SD
CT computed tomography, ICP intracerebral pressure, SOL space-occupying lesion, ONSD optic nerve sheath diameter, ETD eyeball transverse diameter
Table 3.
Sonographic ONSD measurements and ratios of the patients according to the GCS groups (n 147)
| Ultrasound findings | Glasgow Coma Scale Score | p | ||
|---|---|---|---|---|
| 15 (n 107) | 9–14 (n 23) | ≤ 8 (n 17) | ||
| ONSD 3 mm | 4.06 ± 0.52 | 4.50 ± 0.70 | 5.72 ± 0.89 | < 0.001 |
| ONSD 3 mm/ETD ratio | 0.18 ± 0.02 | 0.21 ± 0.03 | 0.25 ± 0.04 | < 0.001 |
Variables are expressed as mean ± SD
ONSD optic nerve sheath diameter, ETD eyeball transverse diameter, GCS Glasgow Coma Scale Score
Fig. 2.
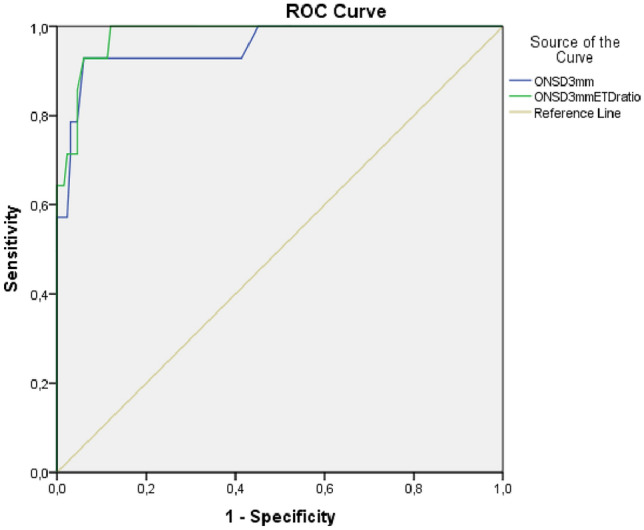
Receiver operating characteristic curve analyses of ONSD at 3 mm and ONSD 3 mm/ETD ratio in patients with elevated ICP. To predict elevated ICP, the AUC for ONSD at 3 mm was 0.956 (95% CI 0.896–1) and for the ONSD 3 mm/ETD ratio it was 0.980 (95% CI 0.959–1). ICP intracerebral pressure, ONSD optic nerve sheath diameter, ETD eyeball transverse diameter
In the group without elevated ICP findings on CT, subjects with SOLs (n 27) had higher sonographic ONSD measurements and ratios (p < 0.001) compared with SOL (−) patients (n 106) (Table 2). To predict SOLs, the AUC for ONSD at 3 mm was 0.648 (95% CI 0.525–0.771). At a cut-off level of 4.0 mm, the sensitivity and specificity of ONSD 3 mm values for SOL were 75.0% and 63.0%, respectively (Fig. 3). There were 18 patients who had non-depressed skull fractures without any SOL. We compared them with patients with normal cranial CT findings and found no difference in ONSD measurements or ratios.
Fig. 3.
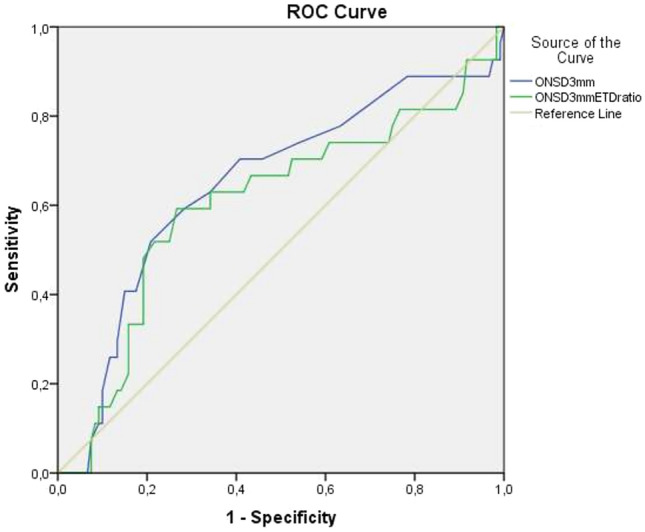
Receiver operating characteristic curve analyses of ONSD at 3 mm and ONSD 3 mm/ETD ratio in patients with SOL (+) and elevated ICP (−). To predict SOL, the AUC for ONSD at 3 mm was 0.648 (95% CI 0.525–0.771) and for the ONSD 3 mm/ETD ratio it was 0.606 (95% CI 0.477–0.730). SOL space-occupying lesion, ICP intracerebral pressure, ONSD optic nerve sheath diameter, ETD eyeball transverse diameter
Sonographic ONSD measurements and ratios were significantly correlated with ONSD readings calculated based on cranial CT images (p < 0.001) (Table 4).
Table 4.
Correlations between ONSD measurements and ratios calculated from ocular US and cranial CT images
| Characteristics | r | p |
|---|---|---|
| ONSD 3 mm | 0.941 | < 0.001 |
| ONSD 3 mm/ETD ratio | 0.891 | < 0.001 |
ONSD optic nerve sheath diameter, US ultrasound, CT computed tomography, ETD eyeball transverse diameter
The subjects with normal cranial CT scans had an increase in measurements of ONSD at 3 mm and ETD by age (p < 0.05), but the ratios of ONSD 3 mm/ETD did not statistically differ, respectively.
Discussion
Most of the trials about ONSD including children were performed by pediatric radiologists or ophthalmologists, but the experience of a pediatric emergency fellow to demonstrate the utility of ocular sonography as a screening tool is crucial in an emergency department. It is easy and practical to perform, suggesting it would be a useful tool for the management of pediatric TBI [25, 26]. The ocular US in pediatric head trauma remains a first-level examination. Its practical value could be high in well-appearing children with suspected history and physical examination findings with low risk for clinically important TBI.
To our knowledge, this is the first study to evaluate sonographic ONSD measurements and ONSD/eyeball diameter ratios in pediatric head trauma patients.
We demonstrated the sonographic cut-off value to predict elevated ICP as 5.1 mm for ONSD measured at 3 mm behind the globe. For children, cut-off values were stated differently for different age groups: 4 mm for < 1 year and 4.5 mm for 1–16 years, or 4 mm for < 4 years and 5 mm for > 4 years, or 4.5 mm for < 1 year and 5 mm for > 1 year (measured at 3 mm behind the globe) [15–20]. We obtained 5.1 mm as the cut-off value for all ages because 14 children enrolled in our study had elevated ICP findings and, among them, only one patient was under 1 year and 4 were under 4 years, so we could not divide the subjects into age groups.
We also found the ONSD 3 mm/ETD ratios to successfully predict elevated ICP and detected the threshold values as 0.22. Du et al. assessed adult TBI patients and stated the thresholds for predicting elevated ICP as 5.53 mm (80% sensitivity, 79.3% specificity) for sonographic ONSD 3 mm and as 0.25 (90% sensitivity, 82.3% specificity) for the ONSD 3 mm/ETD ratio [21]. Kim et al. evaluated healthy adults and reported that the normal ONSD/ETD ratio ranged between 0.14 and 0.23 [18]. We are not aware of any previous data evaluating the ONSD/ETD ratio for children.
In the present study, ocular sonography was sensitive to predict SOLs even without elevated ICP findings on cranial CT images. We could also demonstrate thresholds for SOLs without elevated ICP findings as 4 mm for ONSD measured at 3 mm, suggesting that the increase in ICP could be recognized in the early period by using sonographic ONSD measurements without reflecting on cranial CT scans. In an adult study including 26 patients (trauma and non-trauma cases), ONSD had sensitivity of 60% (95% CI 27–86) and specificity of 100% (95% CI 76–100) for detecting any acute pathology on cranial CT [14]. Vaiman et al. concluded that the ONSD/ETD ratio was higher in subjects with intracranial hemorrhage compared with healthy adults [17].
The ratio of ONSD/ETD was found to correlate with ONSD but not with weight, height, sex, body mass index, or head circumference in adults and was suggested to be a useful ratio to avoid the overlapping of standard deviations of normal and pathologic ONSD values and changes of the globe in individuals [18]. In the present study, children with normal cranial CT results had an increase in measurements of ONSD at 3 mm and ETD by age but the ratios of ONSD 3 mm/ETD did not correlate, and considering these ratios could help exclude changes by age for children. Although these patients had normal cranial CT results, they all experienced head trauma and emergency physicians were needed for neuroimaging. We think that these measurements have limited generalizability for all children, so data about the ONSD ratios of healthy individuals are also required. Fontanel et al. concluded that the ONSD measurement at 3 mm had an increasing curve until approximately 10 years of age and then remained stable until 18 years with an upper limit of 4.5 mm [22].
Lee et al. assessed ONSD readings from 3 mm in adults with TBI on CT images and found measurements to increase in subjects with a positive CT scan compared with negative results. They stated that the ONSD at 3 mm was an independent factor to differentiate positive CT scans and found the threshold of 4.13 mm to predict TBI [16]. We demonstrated the sonographic cut-off value of ONSD 3 mm behind the globe as 4 mm to predict SOLs without elevated ICP findings. In our study, sonographic ONSD 3 mm ratios were also sensitive for predicting elevated ICP or SOL.
Our sonographic measurements were significantly correlated with measurements calculated from cranial CT images. Bhandari et al. assessed patients aged between 2 and 60 years old who underwent VPS surgery, and they found that ocular US and cranial CT had good correlation for ONSD readings at 3 mm [23]. Driessen et al. evaluated 128 children with craniosynostosis; although cranial CT scans were obtained for only 35 subjects, measurements on CT were correlated with ocular US results [38]. In another study including adult TBI patients, US and CT scans had good agreement for the ONSD 3 mm/ETD ratio [24].
Our study has some limitations. First, we used cranial CT findings to differentiate elevated ICP. Although this is not the gold standard, performing invasive ICP measurements is quite difficult in an emergency department. Second, the number of patients with elevated ICP was insufficient to obtain cut-off values of ONSD measurements and ratios according to age groups. Third, we did not record the weight, height, body mass index, or head circumference of our patients to evaluate the correlation between measurements. Finally, all US and CT scan measurements and ratios were calculated by one pediatric emergency fellow. Studies with the measurements of multiple physicians are needed to demonstrate the utility of bedside ocular US in emergency settings.
In conclusion, sonographic ONSD measurements and ratios were found to be quite sensitive to detect elevated ICP or SOLs on cranial CT images. Additionally, there was a significant correlation between measurements calculated by ocular US and cranial CT scans. It was shown that sonographic ONSD measurements and ratios were significantly increased in patients with intracranial lesions even without elevated ICP, suggesting that the increase in ICP could be recognized in the early period by using ocular US without reflecting on cranial CT images. Bedside ocular US seems to be a promising and useful tool to determine ICP in children with head trauma.
Author contributions
NŞ and MD contributed to the conception and design of this study; NŞ, EU, HÇ, AÖ and AE performed the statistical analysis and drafted the manuscript; DY and MD critically reviewed the manuscript and supervised the whole study process. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The data that support the findings of this study are available from the corresponding author, upon request.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The study protocol was approved by the Local Ethics Committee (Approval number: 2018/18–31). All procedures followed were in accordance with the ethical standards of the Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from the parents or legal guardians of each participant before enrollment in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sekhon MS, Griesdale DE, Robba C, et al. Optic nerve sheath diameter on computed tomography is correlated with simultaneously measured intracranial pressure in patients with severe traumatic brain injury. Intensive Care Med. 2014;40(9):1267–1274. doi: 10.1007/s00134-014-3392-7. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal S, Brierley J. Optic nerve sheath measurement and raised intracranial pressure in paediatric traumatic brain injury. Eur J Trauma Emerg Surg. 2012;38(1):75–77. doi: 10.1007/s00068-011-0093-6. [DOI] [PubMed] [Google Scholar]
- 3.Launey Y, Nesseler N, Le Maguet P, Mallédant Y, Seguin P. Effect of osmotherapy on optic nerve sheath diameter in patients with increased intracranial pressure. J Neurotrauma. 2014;31(10):984–988. doi: 10.1089/neu.2012.2829. [DOI] [PubMed] [Google Scholar]
- 4.Rigi M, Almarzouqi SJ, Morgan ML, Lee AG. Papilledema: epidemiology, etiology, and clinical management. Eye Brain. 2015;17(7):47–57. doi: 10.2147/EB.S69174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le A, Hoehn ME, Smith ME, Spentzas T, Schlappy D, Pershad J. Bedside sonographic measurement of optic nerve sheath diameter as a predictor of increased intracranial pressure in children. Ann Emerg Med. 2009;53(6):785–791. doi: 10.1016/j.annemergmed.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Hall MK, Spiro DM, Sabbaj A, Moore CL, Hopkins KL, Meckler GD. Bedside optic nerve sheath diameter ultrasound for the evaluation of suspected pediatric ventriculoperitoneal shunt failure in the emergency department. Childs Nerv Syst. 2013;29(12):2275–2280. doi: 10.1007/s00381-013-2172-y. [DOI] [PubMed] [Google Scholar]
- 7.Helmke K, Hansen HC. Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension. I Experimental study. Pediatr Radiol. 1996;26(10):701–705. doi: 10.1007/BF01383383. [DOI] [PubMed] [Google Scholar]
- 8.Moretti R, Pizzi B. Ultrasonography of the optic nerve in neurocritically ill patients. Acta Anaesthesiol Scand. 2011;55(6):644–652. doi: 10.1111/j.1399-6576.2011.02432.x. [DOI] [PubMed] [Google Scholar]
- 9.Soldatos T, Chatzimichail K, Papathanasiou M, Gouliamos A. Optic nerve sonography: a new window for the non-invasive evaluation of intracranial pressure in brain injury. Emerg Med J. 2009;26(9):630–634. doi: 10.1136/emj.2008.058453. [DOI] [PubMed] [Google Scholar]
- 10.Nakhjavan-Shahraki B, Yousefifard M, Hajighanbari MJ, Oraii A, Safari S, Hosseini M. Pediatric Emergency Care Applied Research Network (PECARN) prediction rules in identifying high risk children with mild traumatic brain injury. Eur J Trauma Emerg Surg. 2017;43(6):755–762. doi: 10.1007/s00068-017-0811-9. [DOI] [PubMed] [Google Scholar]
- 11.Körber F, Scharf M, Moritz J, Dralle D, Alzen G. Sonography of the optical nerve—experience in 483 children. Rofo. 2005;177(2):229–235. doi: 10.1055/s-2004-813936. [DOI] [PubMed] [Google Scholar]
- 12.Ballantyne J, Hollman AS, Hamilton R, et al. Transorbital optic nerve sheath ultrasonography in normal children. Clin Radiol. 1999;54(11):740–742. doi: 10.1016/S0009-9260(99)91176-5. [DOI] [PubMed] [Google Scholar]
- 13.Newman WD, Hollman AS, Dutton GN, Carachi R. Measurement of optic nerve sheath diameter by ultrasound: a means of detecting raised intracranial pressure in hydrocephalus. Br J Ophthalmol. 2002;86(10):1109–1113. doi: 10.1136/bjo.86.10.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu D, Kahn M. Measurement and relationship of subarachnoid pressure of the optic nerve to intracranial pressures in fresh cadavers. Am J Ophthalmol. 1993;116(5):548–556. doi: 10.1016/S0002-9394(14)73195-2. [DOI] [PubMed] [Google Scholar]
- 15.Hansen HC, Helmke K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: ultrasound findings during intratechal infusion tests. J Neurosurg. 1997;87(1):34–40. doi: 10.3171/jns.1997.87.1.0034. [DOI] [PubMed] [Google Scholar]
- 16.Lee SH, Yun SJ (2018) Optic nerve sheath diameter on facial CT: a tool to predict traumatic brain injury. Eur J Trauma Emerg Surg. 10.1007/s00068-018-1035-3 [Epub ahead of print] [DOI] [PubMed]
- 17.Vaiman M, Sigal T, Kimiagar I, Bekerman I. Noninvasive assessment of the intracranial pressure in non-traumatic intracranial hemorrhage. J Clin Neurosci. 2016;34:177–181. doi: 10.1016/j.jocn.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Kim DH, Jun JS, Kim R. Ultrasonographic measurement of the optic nerve sheath diameter and its association with eyeball transverse diameter in 585 healthy volunteers. Sci Rep. 2017;7(1):15906. doi: 10.1038/s41598-017-16173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaiman M, Gottlieb P, Bekerman I. Quantitative relations between the eyeball, the optic nerve, and the optic canal important for intracranial pressure monitoring. Head Face Med. 2014;17(10):32. doi: 10.1186/1746-160X-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaiman M, Sigal T, Kimiagar I, Bekerman I. Intracranial pressure assessment in traumatic head injury with hemorrhage via optic nerve sheath diameter. J Neurotrauma. 2016;33(23):2147–2153. doi: 10.1089/neu.2015.4293. [DOI] [PubMed] [Google Scholar]
- 21.Du J, Deng Y, Li H et al (2019) Ratio of optic nerve sheath diameter to eyeball transverse diameter by ultrasound can predict intracranial hypertension in traumatic brain injury patients: a prospective study. Neurocrit Care. 10.1007/s12028-019-00762-z [Epub ahead of print] [DOI] [PubMed]
- 22.Fontanel L, Pensiero S, Ronfani L, Rosolen V, Barbi E. Optic nerve sheath diameter ultrasound: optic nerve growth curve and its application to detect intracranial hypertension in children. Am J Ophthalmol. 2019;208:439. doi: 10.1016/j.ajo.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Bhandari D, Udupi Bidkar P, Adinarayanan S, Narmadhalakshmi K, Srinivasan S. Measurement of changes in optic nerve sheath diameter using ultrasound and computed tomography scan before and after the ventriculoperitoneal shunt surgery in patients with hydrocephalus—a prospective observational trial. Br J Neurosurg. 2019;33(2):125–130. doi: 10.1080/02688697.2019.1576856. [DOI] [PubMed] [Google Scholar]
- 24.Driessen C, van Veelen ML, Lequin M, Joosten KF, Mathijssen IM. Nocturnal ultrasound measurements of optic nerve sheath diameter correlate with intracranial pressure in children with craniosynostosis. Plast Reconstr Surg. 2012;130(3):448e–e451. doi: 10.1097/PRS.0b013e31825dc1f1. [DOI] [PubMed] [Google Scholar]
- 25.Lin SD, Kahne KR, El Sherif A, et al. The use of ultrasound-measured optic nerve sheath diameter to predict ventriculoperitoneal shunt failure in children. Pediatr Emerg Care. 2019;35(4):268–272. doi: 10.1097/PEC.0000000000001034. [DOI] [PubMed] [Google Scholar]
- 26.Robba C, Cardim D, Czosnyka M, et al. Ultrasound non-invasive intracranial pressure assessment in paediatric neurocritical care: a pilot study. Childs Nerv Syst. 2020;36(1):117–124. doi: 10.1007/s00381-019-04235-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon request.