Abstract
In this article we wish to provide MAKO robotic knee users a surgical guide including tips and tricks on performing MAKO robotic-assisted patellofemoral joint replacements. The senior authors in this paper from the Exeter Knee Reconstruction Unit, United Kingdom are highly experienced MAKO users who have been performing MAKO assisted Patellofemoral joint replacements since 2017.
Keywords: Patellofemoral Joint Replacements, Robotic Surgery, Patellofemoral Joint Replacements Tips and Tricks, MAKO PatelloFemoral Joint Replacements, Robotic Patellofemoral Joint Surgical Technique
Introduction
Approximately 10% of patients above the age of 40 are affected by patellofemoral joint (PFJ) osteoarthritis (OA) [1]. Patients with this condition often present with anterior knee pain, stiffness, functional impairment and discrete pain when walking up and downstairs [2].
Among the knee specific risk factors are; trochlear dysplasia, excessive femoral anteversion, valgus knee alignment, PFJ malalignment and previous anterior cruciate ligament reconstruction [3–5]. Patients with quadriceps and hip abductor weakness as well as iliotibial band and hamstrings tightness are also at higher risk of developing PFJ OA [3]. Non-knee specific factors include; increased body mass index (BMI), previous PFJ trauma (patella dislocation or fracture) and female gender [6–8].
Indications for PFJ Replacement
Patient selection for PFJ replacement is crucial. Patients must have a normal PFJ alignment with an isolated non-inflammatory PFJ arthritis which causes persistent pain and daily activity limitations. There should be evidence of significant OA on plain radiographs AP, lateral and skyline (Fig. 1). Most patients will have a MRI scan pre-operatively to ensure this is isolated disease (Fig. 2). The clinical picture should fit the radiological findings. They should have undergone a period of non-operative management such as analgesia and physiotherapy. Contra-indications or PFJ replacements are active infection elsewhere, inflammatory arthritis, significant OA (bone on bone) in the medial or lateral compartments. Some degree of lateral or medial compartment wear is acceptable if the PFJ replacement is in a young patient as part of a staged life long compartmental resurfacing strategy. Obesity has been shown to be a poor prognostic indicator [9]. There is no evidence age influences the outcomes of PFJ replacements, therefore there is no age limit to performing a PFJ replacement and in fact the lower morbidity and mortality associated with partial knee replacement makes this an attractive solution in the older patient as long as the inclusion criteria are met.
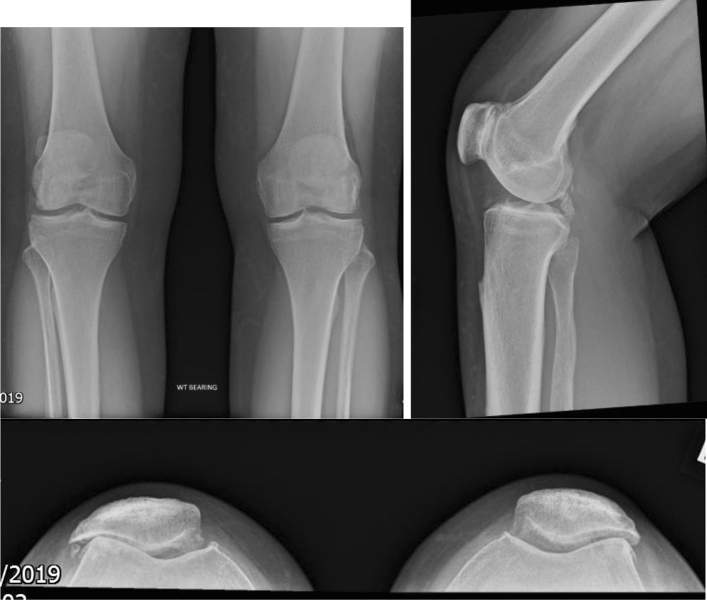
Fig. 1.
AP, lateral and skyline views of PFJ OA
Fig. 2.

Axial MRI view of a right knee showing PFJ OA
History of PFJ and Evolution
Patellofemoral arthroplasty (PFA) was first proposed in 1955 by McKeever. Despite the initial encouraging results, it was quickly abandoned due to excessive wear in the trochlear groove [10]. The first-generation PFA known as the inlay style that used trochlear components inset into the native trochlear was designed in the 1970s. This design did not account for the patient’s anatomical variability. Studies have shown a high incidence of patellar maltracking and revision rate with this design [11–13].
The second generation PFA known as the onlay style was developed in the 1990s. This design was introduced to address anatomical mismatch that was present in the first-generation implant designs. It replaced the entire anterior trochlear surface and the incidence of patellar maltracking (< 1%) and revision rate was reduced [14–17].
In an attempt to further reduce patellar maltracking and implant mismatch, navigation and robotics surgery were introduced and computer assisted unicompartmental arthroplasty has been shown to improve implant alignment [18].
Successful clinical outcomes following joint replacement surgery depends on component placement and restoration of natural knee kinematics. The MAKO robotic system was designed to improve component placement accuracy and reproducibility in PFA. It also helps the surgeon to accurately assess the cartilage implant interface and in medial and lateral compartmental replacement dynamically balance knee soft tissue tension. Clinical studies have shown that MAKO partial knee has the potential to produce accurate and reproducible component placement in accordance with the pre-operative plans and to re-establish soft tissue balance [19, 20]. We recently published our outcomes of MAKO assisted PFJ replacements performed at the Exeter Knee Reconstruction Unit, UK with a mean follow-up of 30 months [21].
Surgical Technique
Pre-operative Planning
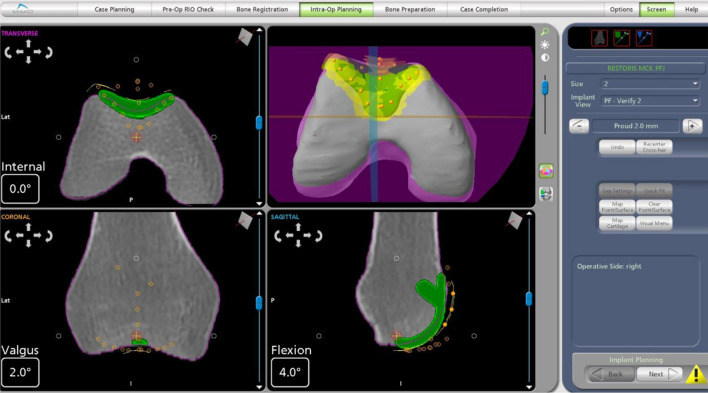
The aims of pre-operative implant planning are to determine the size, orientation and implant position required to fit the patient’s anatomy. The patient has a CT scan. The Mako Product Specialist (MPS) segments the scans and plans using anatomical landmarks to determine different axis in the lower limb. The largest trochlear component that does not overhang the bone on the medial to lateral direction should be selected. It should not be more than 2 mm inset on either side. If the plan is between two sizes plan for a smaller rather than a larger implant. The distal tip of the trochlear component should not be anterior to Blumensaat’s line (Fig. 3). This is to ensure that the component does not impinge on the anterior cruciate ligament (ACL). In the sagittal plane the trochlear groove of the implant should be 1–2 mm proud (approximate thickness of the cartilage) of the cortical bone margins (Fig. 4). This takes into consideration the thickness of the healthy cartilage. It is important to remember the CT scan shows cortical bone margins not cartilage thickness and the resection levels will be reassessed intraoperatively after cartilage mapping.
Fig. 3.
This is a pre-op plan. On the transverse plane the largest trochlear implant is used without overhang on the medial and lateral direction. On the sagittal plane the distal tip of the trochlear component should be anterior to Blumensaat’s line. The proudness of this implant was 2.5 mm. On the coronal view, the component is positioned so that it is centred on the notch. Medial/lateral and varus/valgus are adjusted as necessary
Fig. 4.

In the sagittal plane the trochlear groove of the implant is 1–2 mm proud (approximate thickness of the cartilage)
The recommended implant position is:
Four degrees of internal to 0° of external rotation relative to the epicondylar axis; implant proudness of 1–2 mm from bone at the trochlear groove (the preferred rotation is 0° external)
Three degrees varus to 2° valgus in the coronal plane; with the implants distal tongue being centre on the intercondylar notch (the preferred rotation is 0°–2° of varus)
Five degrees of flexion to 5° of extension in the sagittal plane.
The rotation needs to be optimised in relation to the epicondylar axis and the anterior femoral cortex to avoid notching.
Extremely careful preparation of the bed for trochlear especially in the area of cartilage implant transition is crucial. If not careful, there could be a gap between the implant and the cartilage. To avoid this and to achieve smooth transition:
Burr carefully at the transition zone, angling the burr from the opposite direction i.e. if burring the medial margin, bring the burr in from the lateral side
The new 6 mm conical burr allows for a better transition compared to the previous circular burr
Burr the bone but be less aggressive on the cartilage side as any overlapping cartilage can be gently and easily removed with a scalpel to fine tune the transition
Theatre Set Up
The Mako robotic arm (MRA) can be positioned on either side of the patient, but the set-up is best with the screen on the opposite side to the surgeon and the arm therefore is on the surgeon’s side (Fig. 5). The scrub nurse and the MPS drape the MRA and register the probe and MRA prior to patient coming into theatre (Fig. 6).
Fig. 5.

Theatre set-up. MRA positioned on the operating side and camera positioned on the opposite side
Fig. 6.

Scrub nurse and the MPS drape the MRA and register the probe and MRA prior to the patient coming into theatre
The patient should be positioned as far lateral as possible on the operating side. A knee support is used to stabilise the leg (Fig. 7). A standard midline incision is used but this can be shorter than for a total knee replacement. The incision should extend from the superior border of the patella to the level of the tibial plateau. It can be extended proximally or distally as required. A medial parapatellar approach is used. All compartments are inspected and the integrity of ACL and PCL is assessed. The patella is reflected laterally and fat pad and suprapatellar synovium is elevated as necessary for trochlear exposure.
Fig. 7.

The patient is as far lateral as possible and knee support is used to position the leg
Insert two bicortical bone pins slightly medially about four finger breaths superior to the superior pole of the patella with the knee in 90° of flexion. Secure the array tightly to the bone pins. Ensure it is visible to the camera on the opposite side. Insert a checkpoint on the outer side of the medial femoral condyle away from the resection site so it is easily accessible (Fig. 8).
Fig. 8.

Midline incision and medial parapatellar approach performed with two bicortical bone pins and medial femoral condyle checkpoint
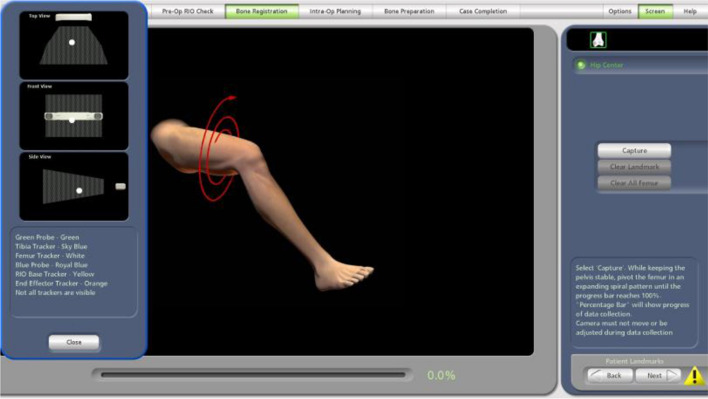
The next step is to cross reference the patient’s landmarks with the CT model. The hip centre is collected by moving the leg in concentric circles (Fig. 9). Femoral check points are verified with the green probe (Fig. 10). The blue probe is used to register the bone reference checks. Thirty-five points that are five millimetres apart are identified (Fig. 11). With the blue probe used to pierce through soft tissue and cartilage in three planes reaching the cortex. Registration is finally verified with blue spheres. Osteophytes should not be removed until after registration.
Fig. 9.
Collect patients hip centre by moving the leg in concentric circles
Fig. 10.
Femoral checkpoint is verified with the green probe
Fig. 11.
Blue probe is used to register the bone at 35 points, 5 mm apart
Cartilage mapping is then undertaken. Accurate cartilage mapping is crucial especially in the area of implant cartilage transition. A minimum of five cartilage points should be collected along the deepest points of the trochlear groove. Three cartilage points are collected on each of the medial and lateral distal patellofemoral transition zones. The implant position and orientation is fine-tuned for proper implant proudness, and smooth transition from the component to the mapped cartilage surfaces (Fig. 12). Fine tuning the orientation of the implant in sagittal, coronal and axial plane is important so that:
There is a smooth cartilage transition (need to adjust depth and varus/valgus alignment in the coronal plane).
To avoid impingement of the implant on ACL. We need to make sure that the tip of the implant does not extend beyond the Blumensaat’s line.
Fig. 12.
Fine tuning of implant positioning and orientation for proper implant proudness and smooth transition from the component to the mapped cartilage surfaces
Once the virtual implant position is optimised the burr is brought into the haptic boundary. Use the hand trigger or foot pedal to engage the burr. The burr should start off the bone and all the green segment on the virtual model should be burred. The burr is used from inside the resection area to cut the edges so it creates vertical edges rather than rolled up edges. The burr is then brought into the haptic boundary of the peg hole. The burr is lined up looking top down on the peg hole and the peg holes burred with the burr starting off the bone. Gently plunge the burr only once then release the trigger to create a proper press fit with the implant peg (Fig. 13). Plunging more than once can create a larger diameter hole and prevent the peg from fitting tightly. The joint is washed in preparation for the trial implant.
Fig. 13.
Final trochlear resection performed utilizing MAKO robotic arm. Green on screen signifies more resection is needed, white is the appropriate depth of resection and red signifies a resection of Xmm beyond the planned white level
The trochlear trial implant is inserted (Fig. 14). Check that the position is satisfactory. It can be re-positioned and the bone re-burred if needed. Impaction can damage the bone if the fit is too tight. The patella is resurfaced manually in a standard fashion either free hand or using a patella cutting jig. This obviously comes with its own set of errors but it is important to recreate the correct patella thickness to ensure correct tension within the anterior compartment. The trial implants are inserted and tracking is checked. The implant sizes may have to be changed intraoperatively depending on the results of the trial. Wash the joint with pulse lavage and dry it before cementing the final implants. Ensure that there is good pressurisation of cement to achieve good interdigitation. Remove extra cement and keep the joint stable until the cement sets. If the leg is moved during cementation, the components may move. Once the cement sets, reassess joint and patella tracking (Fig. 15). Remove all checkpoints, bone pins and arrays. The surgical wound is closed in the normal fashion.
Fig. 14.

Trial trochlear component inserted
Fig. 15.

Post cementation of trochlear component and patella button
Implants
The implants used with Stryker Robotic Arm System (MAKO) are the Restoris MCK Patellofemoral implant system. The trochlear component is side specific. All trochlear component sizes are compatible with all patella component sizes with this system. The trochlear component in this system ranges from size 2–8. The patella dome component in this system ranges from 26–41 mm with 3 mm increments. The patellofemoral (trochlear) component trochlear groove pathway is curved proximally 6° to align with the anatomical axis (when the implant is planned at 0° varus/valgus). The trochlear implant contains a trochlear sulcus groove which is a reference to the potential patella tracking pathway (Fig. 16).
Fig. 16.

Arrow shows the potential patella tracking pathway
Post Operatively
Routine post-operative arthroplasty protocol (Fig. 17). Follow routine local thromboembolic prophylaxis. Weight bearing as tolerated with crutches as needed. Radiographs are obtained before discharge (Fig. 18).
Fig. 17.

Patient at 6 weeks follow-up
Fig. 18.
AP, lateral and skyline views of post op PFJ
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standard statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed consent
For this type of study informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Davies AP, Vince AS, Shepstone L, Donell ST, Glasgow MM. The radiologic prevalence of patellofemoral osteoarthritis. Clinical Orthopaedics and Related Research. 2002;402:206–212. doi: 10.1097/00003086-200209000-00020. [DOI] [PubMed] [Google Scholar]
- 2.Duncan, R., Peat, G., Thomas, E., Wood, L., Hay, E., & Croft, P. (2009). Does isolated patellofemoral osteoarthritis matter? Osteoarthritis Cartilage [Internet]. 17(9):1151–1155. http://www.ncbi.nlm.nih.gov/pubmed/19401244(cited 2020 Apr 13) [DOI] [PubMed]
- 3.Mills, K., & Hunter, D. J. (2014). Patellofemoral joint osteoarthritis: an individualised pathomechanical approach to management. Best Pract Res Clin Rheumatol [Internet]. 28(1):73–91. http://www.ncbi.nlm.nih.gov/pubmed/24792946. (cited 2020 Apr 13) [DOI] [PubMed]
- 4.Culvenor, A. G., Cook, J. L., Collins, N. J., & Crossley, K. M. (2013). Is patellofemoral joint osteoarthritis an under-recognised outcome of anterior cruciate ligament reconstruction? A narrative literature review. Br J Sports Med [Internet]. 47(2):66–70. http://www.ncbi.nlm.nih.gov/pubmed/23038783. (cited 2020 Apr 13) [DOI] [PubMed]
- 5.van Jonbergen, H. P. W., Poolman, R. W., & van Kampen, A. (2010). Isolated patellofemoral osteoarthritis. Acta Orthop [Internet]. 81(2):199–205. http://www.ncbi.nlm.nih.gov/pubmed/20175647. (cited 2020 Apr 13) [DOI] [PMC free article] [PubMed]
- 6.Grelsamer, R. P., & Stein, D. A. (2006). Patellofemoral arthritis. J Bone Jt Surg Am [Internet]. 88(8):1849–1860. http://www.ncbi.nlm.nih.gov/pubmed/16882912. (cited 2020 Apr 13) [DOI] [PubMed]
- 7.Cooper C, McAlindon T, Snow S, Vines K, Young P, Kirwan J, et al. Mechanical and constitutional risk factors for symptomatic knee osteoarthritis: Differences between medial tibiofemoral and patellofemoral disease. Journal of Rheumatology. 1994;21(2):307–313. [PubMed] [Google Scholar]
- 8.Argenson, J. N. A., Guillaume, J. M., Aubaniac, J. M. (1995). Is there a place for patellofemoral arthroplasty? Clin Orthop Relat Res. (321):162–167. [PubMed]
- 9.Parratte, S., Pauly, V., Aubaniac, J. M., Argenson, J. N. A. (2010). Survival of bicompartmental knee arthroplasty at 5 to 23 years. Clin Orthop Relat Res, 468(1), 64–72. [DOI] [PMC free article] [PubMed]
- 10.Levitt RL. A long term evaluation of patellar prostheses. CLINORTHOP. 1973;97:153–157. doi: 10.1097/00003086-197311000-00024. [DOI] [PubMed] [Google Scholar]
- 11.de Winter WEAE, Feith R, van Loon CJM. The Richards type II patellofemoral arthroplasty: 26 cases followed for 1–20 years. Acta Orthopaedica Scandinavica. 2001;72(5):487–490. doi: 10.1080/000164701753532826. [DOI] [PubMed] [Google Scholar]
- 12.Tauro, B., Ackroyd, C. E., Newman, J. H., & Shah, N. A. (2001). The Lubinus patellofemoral arthroplasty. A five- to ten-year prospective study. J Bone Jt Surg Br [Internet]. 83(5):696–701. http://www.ncbi.nlm.nih.gov/pubmed/11476308. (cited 2018 Sep 16) [DOI] [PubMed]
- 13.Blazina, M. E., Fox, J. M., del Pizzo, W., Broukhim, B., & Ivey, F. M. (2005). Patellofemoral replacement. 1979. Clin Orthop Relat Res [Internet]. (436):3–6. http://www.ncbi.nlm.nih.gov/pubmed/15995413. (cited 2020 Apr 13) [PubMed]
- 14.Amis, A. A., Senavongse, W., & Darcy, P. (2005). Biomechanics of patellofemoral joint prostheses. Clin Orthop Relat Res [Internet]. (436):20–29. http://www.ncbi.nlm.nih.gov/pubmed/15995416. (cited 2020 Apr 13) [DOI] [PubMed]
- 15.Starks, I., Roberts, S., & White, S. H. (2009). The Avon patellofemoral joint replacement: independent assessment of early functional outcomes. J Bone Jt Surg Br [Internet]. 91(12):1579–1582. http://www.ncbi.nlm.nih.gov/pubmed/19949120. (cited 2020 Apr 13) [DOI] [PubMed]
- 16.Beitzel, K., Schöttle, P. B., Cotic, M., Dharmesh, V., & Imhoff, A. B. (2013). Prospective clinical and radiological two-year results after patellofemoral arthroplasty using an implant with an asymmetric trochlea design. Knee Surg Sports Traumatol Arthrosc [Internet]. 21(2):332–339. http://www.ncbi.nlm.nih.gov/pubmed/22547249. (cited 2020 Apr 13) [DOI] [PubMed]
- 17.Mont, M. A., Haas, S., Mullick, T., & Hungerford, D. S. (2002). Total knee arthroplasty for patellofemoral arthritis. J Bone Jt Surg Am [Internet].;84-A(11):1977–81. http://www.ncbi.nlm.nih.gov/pubmed/12429758. (cited 2018 Sep 16) [DOI] [PubMed]
- 18.Zhang, Z., Zhu, W., Zhu, L., & Du, Y. (2016). Superior alignment but no difference in clinical outcome after minimally invasive computer-assisted unicompartmental knee arthroplasty (MICA-UKA). Knee Surg Sports Traumatol Arthrosc [Internet]. 24(11):3419–24. http://www.ncbi.nlm.nih.gov/pubmed/25423875. (cited 2020 Apr 13) [DOI] [PubMed]
- 19.Plate JF, Mofidi A, Mannava S, Smith BP, Lang JE, Poehling GG, et al. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Advances in Orthopedics. 2013;2013:1–6. doi: 10.1155/2013/837167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell SW, Anthony I, Jones B, MacLean A, Rowe P, Blyth M. Improved accuracy of component positioning with robotic-assisted unicompartmental knee arthroplasty. Journal of Bone and Joint Surgery American Volume. 2016;98(8):627–635. doi: 10.2106/JBJS.15.00664. [DOI] [PubMed] [Google Scholar]
- 21.Selvaratnam, V., Cattell, A., Eyres, K. S., Toms, A. D., Phillips, J. R. P., & Mandalia, V. I. Robotic-assisted patellofemoral replacement-correlation of preoperative planning with intraoperative implant position and early clinical experience: a minimum 2-year follow-up. J Knee Surg [Internet]. 35(7). https://pubmed.ncbi.nlm.nih.gov/33126284/. (cited 2022 Aug 26) [DOI] [PubMed]