Abstract
Bioactive molecules of microbial origin are finding increasing biotechnological applications. Their sources range from the terrestrial, marine, and endophytic to the human microbiome. These biomolecules have unique chemical structures and related groups, which enable them to improve the efficiency of the bioprocesses. This review focuses on the applications of biomolecules in bioremediation, agriculture, food, pharmaceutical industries, and human health.
Keywords: Bioactive products, Metagenomics, Antibacterials, Therapeutics, Anticancer
Introduction
Naturally produced products have been exploited mainly for their therapeutic potential as they possess essential pharmacokinetic characteristics necessary for clinical works [1]. Most bioactive compounds are known for their growth-promoting, antimicrobial, antiviral, and antitumor activities [2, 3]. In addition to these major targets, other molecules are being searched with activities to counter parasites, nematodes, inflammation, and neurological issues [4]. Chemically, these bioactive molecules are small peptides, polyketides, terpenoids, and alkaloids [5]. Diverse environmental niches are actively explored through metagenomic approaches for bioactive compounds [6–8]. The prospecting for bioactive molecules through regular procedures is quite a tedious process, which has been proving to be inefficient and uneconomical. Molecular biology tools, especially metagenomics accompanied by bioinformatics techniques has revolutionized this search for novel biomolecules. A combined approach involving culture-independent analysis, whole genome sequencing, high-throughput data analysis is expected to help search for biomolecules of diverse origins for applications in agriculture, aquaculture, and human health. Most review articles have dealt with one aspect or field at a time. Here, we have comprehensively assessed their applications in diverse fields.
Biomolecules of Diverse Origins
Terrestrial Microbes
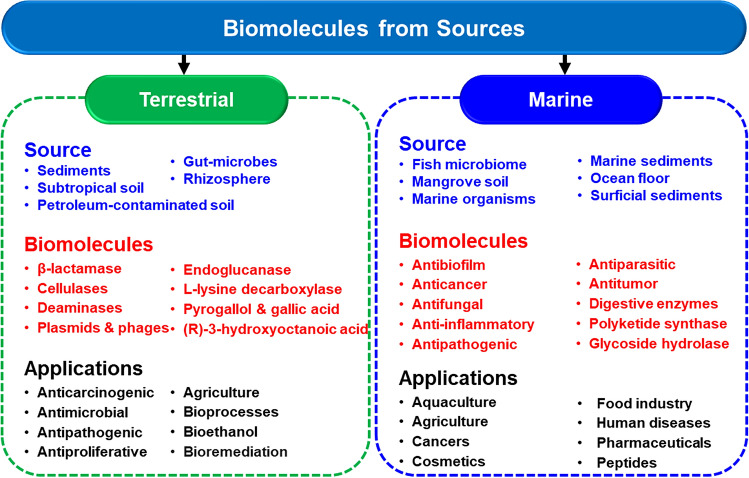
Microbes from terrestrial habitats are among the most frequently reported to produce bioactive molecules (Table 1; Fig. 1). Bacterially produced polyhydroxyalkanoate was used to produce 18 compounds through (R)-3-hydroxyoctanoic acid. The ten compounds had antimicrobial and antiproliferative effects on mammalian cell lines. Hyphae formation by Candida ablicans was inhibited by 3-halogenated octanoic acids, whereas quorum sensing (QS)-mediated pyocyanin production by Pseudomonas aeruginosa was inhibited by (E)-oct-2-enoic acids and (R)-3-hydroxyoctanoic [9]. Compounds derived from gut microbes can generate anti-carcinogenic compounds such as pyrogallol and gallic acid [10]. The need to develop novel enzymes, processes and products for biotechnological applications seems to be achievable through metagenomic approaches [11]. Functional metagenomic assays were used to search microbes from soil samples for inhibiting the growth of phytopathogen—Bacillus subtilis DSM10. It helped to identify a putative β-lactamase-like gene, abgT, which had esterase-like hydrolytic activity [12]. Rhizospheric soil microbial DNA-based metagenomic library proved effective in identifying genes encoding for 1-aminocyclopropane-1-carboxylate deaminase [13]. Soil microbiome was reported to be a reservoir containing plasmids, phages, and transposable elements. Antibiotic resistance was among the most prevalent traits in these mobilomes [14]. Using a metagenomic analysis strategy, gene coding for endoglucanase could be cloned from agricultural residues of rice crop. The enzyme was effective against carboxymethylcellulose, showing that it has an endoactive lytic property. The enzyme was used for metabolizing cellulose into sugars for further metabolism to ethanol by Saccharomyces cervisiae with a yield of 8 g/L over a period of 7 days [15]. Metagenomic sources were used to isolate clones carrying genes encoding cellulase with properties that enabled them to function in non-natural and extreme conditions such as high pH, high temperature, and in the presence of solvents [16]. Efforts to obtain new genes through metagenomic approach have been quite fruitful. The enzyme—L-lysine decarboxylase necessary for the decarboxylation of L-lysine to 1,5-pentanediamine was identified from an uncultured organism [17]. Green technologies are being developed to produce it commercially [18]. Bioremediation of soils contaminated with petroleum products is one of the most studied ecosystems. Microbes for hydrocarbon degradation have direct biotechnological applications. Microbes primarily α- and γ- proteobacteria with the ability to degrade xenobiotic aromatic compounds such as bisphenol, xylene, methylnaphthalene, aminobenzoate, chlorobenzoate, DDT, toluene were reported through metagenomic analysis [19]. Compared to aerobic degradation of crude oil, where Actinobacteria, Firmicutes, and Proteobacteria are critical [19], Alcanivorax, Lactococcus, and Bacteroidetes are crucial under anoxic conditions [20, 21]. An in silico analysis of the genome of Corynebacteriums glutamicum, a soil-dwelling microbe, revealed its ability to degrade nitrile-based herbicides [22].
Table 1.
Biomolecules of diverse origins obtained through metagenomic approaches from terrestrial sources
| Genomic source | Biomolecule | Applications | References |
|---|---|---|---|
| Bacteria | Polyhydroxyalkanoate to (R)-3-hydroxyoctanoic acid | Antimicrobial and antiproliferative effects on mammalian cell lines | [9] |
| Gutmicrobes | Pyrogallol and gallic acid | Anti-carcinogenic compounds | [10] |
| Sediments | Enzymes | Bioprocesses and products | [11] |
| Microbes from soil samples | β-lactamase-like gene, abgT | Inhibiting the growth of phytopathogen—Bacillus subtilis DSM10 | [12] |
| Rhizospheric soil microbial DNA | Identifying genes encoding for 1-aminocyclopropane-1-carboxylate deaminase | Agricultural productivity | [13] |
| Soil microbiome | Plasmids, phages, and transposable elements | Prevalent traits (antibiotic resistance) in these mobilomes | [14] |
| Metagenomic analysis strategy | Gene coding for endoglucanase | Effective against carboxymethylcellulose, showing that it has an endoactive lytic property. Used for metabolizing cellulose (from agricultural residues of rice crop) into sugars to ethanol by Saccharomyces cervisiae | [15] |
| Metagenomic libraries | Isolated clones carrying genes encoding cellulase | Function in non-natural and extreme conditions such as high pH, high temperature, and in the presence of solvents | [16] |
| Metagenomic of subtropical soil microbes | New genes from an uncultured organism | The enzyme—L-lysine decarboxylase (for decarboxylation of L-lysine to 1,5-pentanediamine) | [17, 18] |
| Metagenomics of petroleum-contaminated soil microbiome | Microbes (α- and γ- proteobacteria) | Bioremediation of soils contaminated with petroleum products: aerobic and anoxic conditions | [19] |
| Whole genome analysis | Genome of Corynebacteriums glutamicum | Bioremediation: ability to degrade nitrile-based herbicides | [22] |
Figure 1 Biomolecules of diverse origins obtained through metagenomic approaches from terrestrial and marine sources
Marine Microbes
Among the diverse environmental niches, oceans have the highest diversity of microbes [23]. Marine organisms have been reported to produce compounds anti-inflammatory molecules and anti-pathogenic molecules against fungi, bacteria, parasites, nematodes, and tumors [24] (Table 2; Fig. 1). Microbial genome analysis of Flaviramulus ichthyoenteri isolated from fish intestine showed that it contains genes encoding for AHL lactonase FiaL. This enzyme had little similarity to known lactonases. In addition, other digestive enzymes such as lipases and alginate lyases were also observed [25]. High microbial diversity was found to inhabit the surficial sediments of the Arabian Sea. The presence of secondary metabolites such as PKS biosynthetic genes was reported from 40% of the isolates belonging to Bacillales, γ-proteobacteria and Micrococcaceae. Organic extracts proved bactericidal to human pathogens and cytotoxic to human breast cancer cells (MCF-7) [26]. Marine fungus Oceanitis cincinnatula was found to protect shrimp against acute hepatopancreatic necrosis disease (AHPND) caused by biofilm-forming Vibrio parahaemolyticus [27]. The interest in marine sources for using bioactive compounds for biotechnological applications through metagenomic approaches has been increasing rapidly [28, 29]. Metagenomic library prepared from mangrove soil enable the isolation of β-agarase gene. Polypeptide sequenced deduced from the gene had a high identity with glycoside hydrolase. The purified recombinant protein was active at pH 7.0 and 50 °C. The biochemical activities of the enzyme showed that it could be used in cosmetic, food, and pharmaceutical industries [30].
Table 2.
Biomolecules of diverse origins obtained through metagenomic approaches from marine sources
| Genomic source | Biomolecule | Applications | References |
|---|---|---|---|
| Marine organisms | Anti-inflammatory molecules and anti-pathogenic molecules | Against fungi, bacteria, parasites, nematodes, and tumors | [24] |
| Bacterial whole genome Flaviramulus ichthyoenteri (isolated from fish intestine) | Acylhomoserine lactone lactonase (AHLase) FiaL along with digestive enzymes such as lipases and alginate lyases | Quorum sensing inhibitors as antipathogen in aquaculture | [25] |
| Microbes from surficial sediments of the Arabian Sea | Secondary metabolites such as PKS biosynthetic genes | Bactericidal to human pathogens and cytotoxic to human breast cancer cells (MCF-7) | [26] |
| Marine fungus Oceanitis cincinnatula | Antibiofilm (of Vibrio parahaemolyticus) | Aquaculture: Protect shrimp against acute hepatopancreatic necrosis disease (AHPND) | [27] |
| Metagenomic library from mangrove soil | Isolation of β-agarase gene for glycoside hydrolase | Recombinant protein was active at pH 7.0 and 50 °C. Useful in cosmetic, food, and pharmaceutical industries | [30] |
| Marine library | QS inhibitors—AHLase | Inhibit biofilm formation by Pseudomonas aeruginosa | [31] |
| Screening Metagenomic libraries | Secondary metabolite gene clusters for phosphopantetheinyl transferase (PPTase). | Necessary for activation of polyketide synthase (PKS) and Non-ribosomal peptide synthetase | [33, 34] |
| Metagenomic library from marine water sample | Identification of a novel peptide with antifungal properties | Peptide had a chitin-binding characteristic | [35] |
| metagenomic approach using ‘Candidatus Endoecteinascidia frumentensis’ | Ecteinascidin 743 (ET-743) | Anti-cancer compound | [36] |
| Metagenomic fosmid library from marine sediments | alkaline esterase, Est12 | Active at low temperatures | [37] |
| Metagenomic fosmid library from marine sediments | Class 3 lipase | Specific towards short and medium-length p-nitrophenyl esters especially p-nitrophenyl acetate | [38] |
| Ocean floor Petroleum reservoir | Cellulase | Source of thermostable enzymes | [39] |
Using marine library, QS inhibitors—acylhomoserine lactonases (AHLase) with the ability to inhibit QS mediated biofilm formation by P. aeruginosa were identified using a lacZ reporter fused to AHL synthase promoter [31]. A wide range of natural products of microbial origin have been reportedly discovered through genomic libraries from marine environments [28, 32]. Gene clusters responsible for producing natural products were recovered from metagenomic libraries prepared for screening phosphopantetheinyl transferase (PPTase). These enzymes are necessary for activation of enzymes—polyketide synthase (PKS) and Non-ribosomal peptide synthetase, thus are important for activities of secondary metabolite gene clusters [33, 34]. Screening of a metagenomic library from marine water sample helped in the identification of a novel peptide with antifungal properties. The peptide had a chitin-binding characteristic [35]. Efforts to extract natural products from marine microbes have been based on metagenomics, genomics, and synthetic biology [29]. Another anti-cancer compound -Ecteinascidin 743 (ET-743), was detected using metagenomic approach using ‘Candidatus Endoecteinascidia frumentensis’ [36]. Marine sediment samples used for preparing metagenomic fosmid library was productive in identifying an alkaline esterase, Est12, active at low temperatures [37], class 3 lipase was reported to be more specific towards short and medium length p-nitrophenyl esters especially p-nitrophenyl acetate. The enzyme had an optimum pH of 8.0 and a temperature optimum of 35 °C [38]. A petroleum reservoir below the ocean floor near Norway was reported to be a good source of thermostable cellulase enzymes [39].
The initial gains achieved through metagenomic studies have been appearing to slow down. The investments made so far have not proven to be effective, especially when compared to those derived from terrestrial plants and animals [40]. Metagenomics as a tool has been instrumental in elucidating natural secondary metabolite producers from terrestrial samples, however, ecological niches in marine sources are yet to be explored further for proving beneficial to the pharmaceutical industry [41, 42].
Plant Endophytes
Endophytes existing within plant tissues are most abundant in rainforests. Endophytes have been a source of novel bioactive metabolites [43, 44]. Endophytes as sources of bioactive molecules such as emodin, hypericin, deoxypodophyllotoxin, podophylloyoxin, camptothecin and its analogs, and paclitaxel have been widely reported [45]. However, the production of these compounds on commercial scale is awaited. To overcome the difficulties encountered in reproducing endophytes under laboratory conditions, genome mining has been proposed. It is expected to elucidate the genes responsible for biosynthesis of already known compounds [46]. Multiple genes encoding for the enzymes anthranilase synthases, responsible for the synthesis of acridine, quinoline, and quinazoline alkaloids were reported to be present in the genome of Aspergillus nidulans [47]. Metagenomic DNA extract of endophytic fungi, endophytic to Rhododendron tomentosum was found to code for genes responsible for producing antioxidant and antibacterial compounds [48]. Metagenomic analysis of fungal endophytic communities associated with Paulownia spp. revealed that the most abundant was Olpidiomycota (82.66%) [49]. A similar study has shown that the fungal community was dominated by Dysphania ambrosioides (Zygomycota) and Ascomycota (100%) [50]. Recent work has revealed culturable endophytic bacterial taxa generally belong to Enterobacter, Pontea, Pseudomonas, Stenotrophomonas, Bacillus, Paenibacillus, Staphylococcus, Microbacterium, and Curtobacterium [51].
Human Microbiome
A diversity of bioactive microbial products (BAMPs), primarily metabolites and proteins, play key functional roles in the metabolisms of living organisms [52, 53]. Around 80% of the anti-cancer drugs and nearly 50% of FDA-approved drugs released between 1981 and 2010 have originated from natural products [54]. Bioactives within the human gut include molecules such as short-chain fatty acids, secreted and surface peptides and sugars and processes like antimicrobial activity, microbial metabolisms, microbial signaling, immune activation and inflammation, and host-microbe cross-talk [55, 56]. In fact, natural products have been instrumental in the advancement of basic research [57]. Human health has been shown to be greatly influenced by the microbiome it carries within the body and on skin surface [58]. The symbiotic relationships between humans and bacteria harbored by them extend to numerous biosynthetic genes and important biological functions. Bacterial populations in a niche are regulated by post-translationally modified peptides as natural products [59]. These compounds include thiopeptides, thiazole/oxazole-modified microcins, microcins, bacteriocins, and lantibiotics [60]. Biosynthetic gene clusters from microbial genomes associated with a human were identified through 752 metagenomic samples. Antibiotics like thiopeptides were reported to be widely distributed in metagenomes and genomes of the human microbiota. Lactocilin—a thiopeptide antibiotic, was produced by microbes associated with the vagina. It was effective in inhibiting a wide range of Gram-positive pathogens. The study demonstrated that bacteria with the ability to produce drug-like molecules are produced by human microbiota [61]. The role of gut microbiota on human health has been gaining importance. Microbial interaction with human host metabolism affects a diversity of functions such as development, immunity, behavior, and dysbiosis leading to disease conditions [53]. Among the diverse community of microbiota present in a human host, the search for specific effectors that influence the bacteria-host interaction was analyzed by examining clones of 3000 Mb of metagenomic DNA. The search was targeted toward those which activate NF-kB transcription factor, which is instrumental in mediating the response to environmental factors [62]. One of the effector gen family (Cbeg 12) was reported to encode for producing commendamide, which resembled a mammalian signaling molecule. It was found to activate G-protein-coupled receptor G2A/GPR132, which was implicated in diseases such as autoimmunity and atherosclerosis [62].
The Prospects
The progress in producing bioactive compounds in large quantities is blocked at the clinical stage since the amount of drug required for clinical trials is large [63]. Extensive efforts in high throughput sequencing have still not been able to facilitate the recovery of bioactive molecules. It is primarily due to the tremendous diversity and the complexity of soil microbiomes. To overcome these challenges, in situ enrichment techniques and metagenomic tools need to be combined to recover and reconstruct microbial genomes [64]. Primarily, in the case of marine organisms, we cannot rely on bioprospecting alone, and we may need to focus on metagenomics [65]. Predictive bioinformatic tools and high-throughput fluorescence methods were used to mine novel constitutive promoter sequences, which belonged to diverse phylogenetic groups [66]. In addition, metabolic engineering and heterologous expression in different hosts with high growth rates can prove helpful [67]. Although gene expression in E. coli has been widely reported, however, other organisms such B. subtilis, Pseudomonas spp., Streptomyces spp., or some eukaryotic expression systems could be employed as alternatives [68].
The human microbiome is being studied quite vigorously. However, there are still wide gaps in our comprehension of the functions of natural products which have been reported to be made by the human microbiome [69]. The need is to prioritize the targets. Secondary metabolites produced by skin, lungs and various other organs need to be analysed and co-related with microbes associated with human systems [70, 71]. The need is to integrate the abilities of researchers from diverse fields and use diverse approaches to resolve the actual potential of natural bioproducts.
Conclusion
Despite persistent efforts, a majority of the microbial diversity is still unknown. It, however, enthuses the necessary confidence in researchers that much is still untapped. Another feature that enhances this enthusiasm among researchers is the discovery of novel biotechnological tools which unravel the non-culturable microbial diversity. High throughput sequencing facilities integrated with predictive bioinformatic tools to analyze an enormous amount of data are proving instrumental in meeting the challenges posed during the prospecting of diverse sources for isolating novel bioactive compounds. We can envisage that the multi-disciplinary approach will help us realize the potential of natural bioproducts in various biotechnological applications.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2021H1D3A2A0109705, NRF-2021R1I1A1A01060963, NRF-2020R1I1A1A01073483). This paper was supported by Konkuk University Researcher Fund in 2021. The sponsor(s) had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Declarations
Conflict of interest
All authors declare that there is no actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within five years of beginning the submitted work that could inappropriately influence, or be perceived to influence, their work.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Vipin Chandra Kalia and Chunjie Gong contributed equally.
References
- 1.Scherlach K, Hertweck C. Mining and unearthing hidden biosynthetic potential. Nat Commun. 2021;12:3864. doi: 10.1038/s41467-021-24133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matuszewska A, Jaszek M, Stefaniuk D, et al. Anticancer, antioxidant, and antibacterial activities of low molecular weight bioactive subfractions isolated from cultures of wood degrading fungus Cerrena unicolor. PLoS ONE. 2018;13:e0197044. doi: 10.1371/journal.pone.0197044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adeleke BS, Babalola OO. Biotechnological overview of agriculturally important endophytic fungi. Hortic Environ Biotechnol. 2021;62:507–520. doi: 10.1007/s13580-021-00334-1. [DOI] [Google Scholar]
- 4.Abdel-Mageed W, Milne B, Wagner M, et al. Dermacozines, a new phenazine family from deep-sea dermacocci isolated from a Mariana Trench sediment. Org Biomol Chem. 2010;8:2352–2362. doi: 10.1039/c001445a. [DOI] [PubMed] [Google Scholar]
- 5.Graça A, Bondoso J, Gaspar H, et al. Antimicrobial activity of heterotrophic bacterial communities from the marine sponge Erylus discophorus (astrophorida, geodiidae) PLoS ONE. 2013;8:e78992. doi: 10.1371/journal.pone.0078992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalia VC. Mining metagenomes for novel bioactive molecules. In: Kalia VC, Shouche Y, Purohit HJ, Rahi P, editors. Mining of microbial wealth and metagenomics. Singapore: Springer; 2017. pp. 1–9. [Google Scholar]
- 7.Kalia VC. The dawn of the era of bioactive compounds. In: Kalia VC, editor. Metabolic engineering for bioactive compounds. Singapore: Springer; 2017. pp. 3–10. [Google Scholar]
- 8.Saini AK, Kalia VC. Potential challenges and alternative approaches in metabolic engineering of bioactive compounds in industrial set up. In: Saini AK, Kalia VC, editors. Metabolic engineering for bioactive compounds. Singapore: Springer; 2017. pp. 405–412. [Google Scholar]
- 9.Radivojevic J, Skaro S, Senerovic L, et al. Polyhydroxyalkanoate-based 3-hydroxyoctanoic acid and its derivatives as a platform of bioactive compounds. Appl Microbiol Biotechnol. 2016;100:161–172. doi: 10.1007/s00253-015-6984-4. [DOI] [PubMed] [Google Scholar]
- 10.Mazzoli R, Riedel K, Pessione E. Bioactive compounds from microbes. Front Microbiol. 2017;8:392. doi: 10.3389/fmicb.2017.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elfeki M, Alanjary M, Green SJ, et al. Assessing the efficiency of cultivation techniques to recover natural product biosynthetic gene populations from sediment. ACS Chem Biol. 2018;13:2074–2081. doi: 10.1021/acschembio.8b00254. [DOI] [PubMed] [Google Scholar]
- 12.O’Mahony MM, Henneberger R, Selvin J, et al. Inhibition of the growth of Bacillus subtilis DSM10 by a newly discovered antibacterial protein from the soil metagenome. Bioengineered. 2015;6:89–98. doi: 10.1080/21655979.2015.1018493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin Z, Di Rienzi SC, Janzon A, et al. Novel rhizosphere soil alleles for the enzyme 1-aminocyclopropane-1-carboxylate deaminase queried for function with an in vivo competition assay. Appl Environ Microbiol. 2015;82:1050–1059. doi: 10.1128/AEM.03074-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo W, Xu Z, Riber L, et al. Diverse gene functions in a soil mobilome. Soil Biol Biochem. 2016;101:175–183. doi: 10.1016/j.soilbio.2016.07.018. [DOI] [Google Scholar]
- 15.Meneses C, Silva B, Medeiros B, et al. A metagenomic advance for the cloning and characterization of a cellulase from red rice crop residues. Molecules. 2016;21:e831. doi: 10.3390/molecules21070831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ilmberger N, Streit WR. Screening for cellulase encoding clones in metagenomic libraries. Methods Mol Biol. 2017;1539:205–217. doi: 10.1007/978-1-4939-6691-2_12. [DOI] [PubMed] [Google Scholar]
- 17.Deng J, Gao H, Gao Z, et al. Identification and molecular characterization of a metagenome-derived L-lysine decarboxylase gene from subtropical soil microorganisms. PLoS ONE. 2017;12:e0185060. doi: 10.1371/journal.pone.0185060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y, Huang Y, Ji X, et al. Green chemical and biological synthesis of cadaverine: recent development and challenges. RSC Adv. 2021;11:23922–23942. doi: 10.1039/d1ra02764f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bao YJ, Xu Z, Li Y, et al. High-throughput metagenomic analysis of petroleum-contaminated soil microbiome reveals the versatility in xenobiotic aromatics metabolism. J Environ Sci (China) 2017;56:25–35. doi: 10.1016/j.jes.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q, Tang J, Liu X, et al. Vertical response of microbial community and degrading genes to petroleum hydrocarbon contamination in saline alkaline soil. J Environ Sci (China) 2019;81:80–92. doi: 10.1016/j.jes.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Gao Y, Yuan L, Du J, et al. Bacterial community profile of the crude oil-contaminated saline soil in the Yellow River Delta Natural Reserve, China. Chemosphere. 2022;289:133207. doi: 10.1016/j.chemosphere.2021.133207. [DOI] [PubMed] [Google Scholar]
- 22.Amrutha M, Nampoothiri KM. In silico analysis of nitrilase-3 protein from Corynebacterium glutamicum for bioremediation of nitrile herbicides. J Genet Eng Biotechnol. 2022;20:51. doi: 10.1186/s43141-022-00332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ameen F, AlNadhari S, Al-Homaidan AA. Marine microorganisms as an untapped source of bioactive compounds. Saudi J Biol Sci. 2021;28:224–231. doi: 10.1016/j.sjbs.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karthikeyan A, Joseph A, Nair BG. Promising bioactive compounds from the marine environment and their potential effects on various diseases. J Genet Eng Biotechnol. 2022;20:14. doi: 10.1186/s43141-021-00290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Liu J, Tang K, et al. Genome analysis of Flaviramulus ichthyoenteri Th78(T) in the family Flavobacteriaceae: insights into its quorum quenching property and potential roles in fish intestine. BMC Genomics. 2015;16:38. doi: 10.1186/s12864-015-1275-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anas A, Nilayangod C, Jasmin C, et al. Diversity and bioactive potentials of culturable heterotrophic bacteria from the surficial sediments of the Arabian Sea. 3 Biotech. 2016;6:238. doi: 10.1007/s13205-016-0556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soowannayan C, Teja NC, Yatip P, et al. Vibrio biofilm inhibitors screened from marine fungi protect shrimp against acute hepatopancreatic necrosis disease (AHPND) Aquaculture. 2019;499:1–8. doi: 10.1016/j.aquaculture.2018.09.004. [DOI] [Google Scholar]
- 28.Liu X, Ashforth E, Ren B, et al. Bioprospecting microbial natural product libraries from the marine environment for drug discovery. J Antibiot (Tokyo) 2010;63:415–422. doi: 10.1038/ja.2010.56. [DOI] [PubMed] [Google Scholar]
- 29.Xiong ZQ, Wang JF, Hao YY, et al. Recent advances in the discovery and development of marine microbial natural products. Mar Drugs. 2013;11:700–717. doi: 10.3390/md11030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mai Z, Su H, Zhang S. Isolation and characterization of a glycosyl hydrolase family 16 β-agarase from a mangrove soil metagenomic library. Int J Mol Sci. 2016;17:e1360. doi: 10.3390/ijms17081360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schipper C, Hornung C, Bijtenhoorn P, et al. Metagenome-derived clones encoding two novel lactonase family proteins involved in biofilm inhibition in Pseudomonas aeruginosa. Appl Environ Microbiol. 2009;75:224–233. doi: 10.1128/AEM.01389-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Guo J, Dai S, et al. Exploring and exploiting microbial diversity through metagenomics for natural product drug discovery. Curr Top Med Chem. 2009;9:1525–1535. doi: 10.2174/156802609789909849. [DOI] [PubMed] [Google Scholar]
- 33.Owen J, Robins K, Parachin N, et al. A functional screen for recovery of 4’-phosphopantetheinyl transferase and associated natural product biosynthesis genes from metagenome libraries. Environ Microbiol. 2012;14:1198–1209. doi: 10.1111/j.1462-2920.2012.02699.x. [DOI] [PubMed] [Google Scholar]
- 34.Wei Y, Zhang L, Zhou Z, Yan X. Diversity of gene clusters for polyketide and nonribosomal peptide biosynthesis revealed by metagenomic analysis of the Yellow Sea sediment. Front Microbiol. 2018;9:295. doi: 10.3389/fmicb.2018.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pushpanathan M, Rajendhran J, Jayashree S, et al. Identification of a novel antifungal peptide with chitin-binding property from marine metagenome. Protein Pept Lett. 2012;19:1289–1296. doi: 10.2174/092986612803521620. [DOI] [PubMed] [Google Scholar]
- 36.Schofield MM, Jain S, Porat D, et al. Identification and analysis of the bacterial endosymbiont specialized for production of the chemotherapeutic natural product ET-743. Environ Microbiol. 2015;17:3964–3975. doi: 10.1111/1462-2920.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu Y, Liu Y, Li J, et al. Structural and functional analysis of a low-temperature-active alkaline esterase from South China Sea marine sediment microbial metagenomic library. J Ind Microbiol Biotechnol. 2015;42:1449–1461. doi: 10.1007/s10295-015-1653-2. [DOI] [PubMed] [Google Scholar]
- 38.De Santi C, Altermark B, Pierechod MM, et al. Characterization of a cold-active and salt tolerant esterase identified by functional screening of Arctic metagenomic libraries. BMC Biochem. 2016;17:1. doi: 10.1186/s12858-016-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewin A, Zhou J, Pham VTT, et al. Novel archaeal thermostable cellulases from an oil reservoir metagenome. AMB Express. 2017;7:183. doi: 10.1186/s13568-017-0485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burgess JG. New and emerging analytical techniques for marine biotechnology. Curr Opin Biotechnol. 2012;23:29–33. doi: 10.1016/j.copbio.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Trindade M, van Zyl LJ, Navarro-Fernández J, et al. Targeted metagenomics as a tool to tap into marine natural product diversity for the discovery and production of drug candidates. Front Microbiol. 2015;6:890. doi: 10.3389/fmicb.2015.00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindequist U. Marine-derived pharmaceuticals-challenges and opportunities. Biomol Ther. 2016;24:561–571. doi: 10.4062/biomolther.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gouda S, Das G, Sen SK, et al. Endophytes: a treasure house of bioactive compounds of medicinal importance. Front Microbiol. 2016;7:1538. doi: 10.3389/fmicb.2016.01538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amirzakariya BZ, Shakeri A. Bioactive terpenoids derived from plant endophytic fungi: an updated review (2011–2020) Phytochemistry. 2022;197:113130. doi: 10.1016/j.phytochem.2022.113130. [DOI] [PubMed] [Google Scholar]
- 45.Kusari S, Spiteller M. Are we ready for industrial production of bioactive plant secondary metabolites utilizing endophytes? Nat Prod Rep. 2011;28:1203. doi: 10.1039/c1np00030f. [DOI] [PubMed] [Google Scholar]
- 46.Challis GL. Genome mining for novel natural product discovery. J Med Chem. 2008;51:2618–2628. doi: 10.1021/jm700948z. [DOI] [PubMed] [Google Scholar]
- 47.Scherlach K, Hertweck C. Discovery of aspoquinolones A-D, prenylated quinoline-2-one alkaloids from Aspergillus nidulans, motivated by genome mining. Org Biomol Chem. 2006;4:3517–3520. doi: 10.1039/B607011F. [DOI] [PubMed] [Google Scholar]
- 48.Tejesvi MV, Kajula M, Mattila S, et al. Bioactivity and genetic diversity of endophytic fungi in Rhododendron tomentosum Harmaja. Fungal Divers. 2011;47:97. doi: 10.1007/s13225-010-0087-4. [DOI] [Google Scholar]
- 49.Woźniak M, Grządziel J, Gałązka A, et al. Metagenomic analysis of bacterial and fungal community composition associated with Paulownia elongate × Paulownia fortunei. BioRes. 2019;14:8511–8529. doi: 10.15376/biores.14.4.8511-8529. [DOI] [Google Scholar]
- 50.Parmar S, Li Q, Wu Y, et al. Endophytic fungal community of Dysphania ambrosioides from two heavy metal-contaminated sites: evaluated by culture-dependent and culture-independent approaches. Microb Biotechnol. 2018;11:1170–1183. doi: 10.1111/1751-7915.13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riva V, Mapelli F, Bagnasco A, et al. A meta-analysis approach to defining the culturable core of plant endophytic bacterial communities. Appl Environ Microbiol. 2022;88:e02537–e02521. doi: 10.1128/aem.02537-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joice R, Yasuda K, Shafquat A, et al. Determining microbial products and identifying molecular targets in the human microbiome. Cell Metab. 2014;20:731–741. doi: 10.1016/j.cmet.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharon G, Garg N, Debelius J, et al. Specialized metabolites from the microbiome in health and disease. Cell Metab. 2014;20:719–730. doi: 10.1016/j.cmet.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lemon KP, Armitage GC, Relman DA, et al. Microbiota-targeted therapies: an ecological perspective. Sci Transl Med. 2012;4:137rv5. doi: 10.1126/scitranslmed.3004183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lopez CA, Kingsbury DD, Velazquez EM, et al. Collateral damage: microbiota-derived metabolites and immune function in the antibiotic era. Cell Host Microbe. 2014;16:156–163. doi: 10.1016/j.chom.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Milshteyn A, Schneider JS, Brady SF. Mining the metabiome: identifying novel natural products from microbial communities. Chem Biol. 2014;21:1211–1223. doi: 10.1016/j.chembiol.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koppel N, Balskus EP. Exploring and understanding the biochemical diversity of the human microbiota. Cell Chem Biol. 2016;23:18–30. doi: 10.1016/j.chembiol.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 59.Donia MS, Cimermancic P, Schulze CJ, et al. A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics. Cell. 2014;158:1402–1414. doi: 10.1016/j.cell.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arnison PG, Bibb MJ, Bierbaum G, et al. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat Prod Rep. 2013;30:108–160. doi: 10.1016/j.cell.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Donia MS, Fischbach MA. Small molecules from the human microbiota. Science. 2015;349:1254766. doi: 10.1126/science.1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen LJ, Kang HS, Chu J, et al. Functional metagenomic discovery of bacterial effectors in the human microbiome and isolation of commendamide, a GPCR G2A/132 agonist. Proc Natl Acad Sci USA. 2015;112:E4825–E4834. doi: 10.1073/pnas.1508737112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsukimoto M, Nagaoka M, Shishido Y, et al. Bacterial production of the tunicate-derived antitumor cyclic depsipeptide didemnin B. J Nat Prod. 2011;74:2329–2331. doi: 10.1021/np200543z. [DOI] [PubMed] [Google Scholar]
- 64.Delmont TO, Eren AM, Maccario L, et al. Reconstructing rare soil microbial genomes using in situ enrichments and metagenomics. Front Microbiol. 2015;6:358. doi: 10.3389/fmicb.2015.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McGenity TJ. 2038–When microbes rule the Earth. Environ Microbiol. 2018;20:4213–4220. doi: 10.1111/1462-2920.14449. [DOI] [PubMed] [Google Scholar]
- 66.Westmann CA, Alves LF, Silva-Rocha R, et al. Mining novel constitutive promoter elements in soil metagenomic libraries in Escherichia coli. Front Microbiol. 2018;9:1344. doi: 10.3389/fmicb.2018.01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J, Neubauer P. Escherichia coli as a cell factory for heterologous production of nonribosomal peptides and polyketides. New Biotechnol. 2014;31:1–7. doi: 10.1016/j.nbt.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 68.Lorenz P, Eck J. Metagenomics and industrial applications. Nat Rev Microbiol. 2005;3:510–516. doi: 10.1038/nrmicro1161. [DOI] [PubMed] [Google Scholar]
- 69.Wilson MR, Zha L, Balskus EP. Natural product discovery from the human microbiome. J Biol Chem. 2017;292:8546–8552. doi: 10.1074/jbc.R116.762906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kalia VC, Gong G, Shanmugam R, et al. The emerging biotherapeutic agent. Akkermansia Indian J Microbiol. 2022;62:1–10. doi: 10.1007/s12088-021-00993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kalia VC, Shim WY, Patel SKS, et al. Recent developments in antimicrobial growth promoters in chicken health: Opportunities and challenges. Sci Total Environ. 2022;834:155300. doi: 10.1016/j.scitotenv.2022.155300. [DOI] [PubMed] [Google Scholar]