Abstract
Ultrasound (US) represents the first-level imaging technique in the assessment of breast in young patients, whereas it is complementary to mammography in adult ones. It is not uncommon to encounter non-glandular mass during either screening or diagnostic breast US; sometimes the evaluation of palpable lump may be the reason of clinician's US request. The breast US field-of-view includes not only the glandular parenchyma, but also the tissues located anterior and posterior to it, from the skin to the ribs. In this setting, the radiologist must be familiar with the non-glandular breast diseases, which can occur in the superficial layers as well as in the chest wall. The differential diagnosis varies according to anatomic layer, so the anatomic origin is the first feature to assess and the correct localization is needed to avoid misdiagnosis and to choose, when requested, the second diagnostic step, imaging or histologic analysis. This paper is the first of two focused on non-glandular breast lesions; characterization, differential diagnosis, and pitfalls of superficial lesions are reviewed. They may be located in the dermis or hypodermis: the former are usually benign skin lesions, whereas the latter, although usually benign, may arise also from the anterior terminal lobular units, hence the papilloma, fibroadenomas, and breast cancers should be included in the differential diagnosis. US is more sensitive than CT and MRI in the assessment of superficial lesions due to higher spatial resolution.
Keywords: Breast Ultrasound, Superficial non-glandular Lesions, Skin Lesions, Hypodermal Lesions, Characterization, Differential Diagnosis, Pitfalls
Introduction
Non-glandular breast imaging findings are often encountered incidentally during screening or diagnostic breast ultrasound (US). Sometimes clinical or self-examination of a breast lump may be the reason of the US request, because erroneously any mammary lump is considered to be of glandular relevance, without considering its possible origin from the other tissues present in this region. On the other hand, not all breast sonographers are expert in US features of non-glandular abnormalities or, in case of superficial findings, use high-frequency transducers coupled with a step-off pad or a blob of acoustic gel to increase the accuracy of US assessment.
The US field-of-view includes the glandular parenchyma and the tissues located either anterior or posterior to it, hence any lesions which arise from them. In particular, superficial lesions may originate within the dermis, hypodermis, or superficial mammary tissue. The correct identification of the layer of origin is the first finding useful in the differential diagnosis and it influence the radiologic management. An in-depth anatomic knowledge as well as the US features of lesions which arise within the above-mentioned layers are required for the correct diagnosis. In particular, US is more sensitive than Computed Tomography and Magnetic Resonance in the assessment of superficial lesions, so, when conventional US coupled with color-Doppler does not allow a final diagnosis, US-guided biopsy should be needed.
The aim of our manuscript is to illustrate the wide spectrum of superficial non-glandular entities which may develop in tissues located anterior to breast glandular parenchyma and included in the US field-of-view. We will focus on the diagnostic role of conventional US and the additional value of color-Doppler, also highlighting pitfalls and differential diagnosis.
Sonographic anatomy of the breast
The breast region is covered by the skin and it can be divided in three main anatomical zones from superficial to deep planes: the pre-mammary, mammary, and retro-mammary zones (Fig. 1). Their US identification is necessary to correctly evaluate any imaging finding, because the location and relationship with the glandular parenchyma are the first features to be evaluated in the differential diagnosis. The skin is composed of three layers: epidermis, dermis, and hypodermis [1, 2]. The first two may not be distinguished at imaging, their combined normal thickness is 0.5–2 mm and they appear as a single echogenic layer. The thin epidermis is composed of several cell layers and does not contain blood or lymphatic vessels, which, contrariwise, are present within the dermis, as well as hair follicles, sebaceous glands, sweat glands, and nerve endings. The hypodermis, also called subcutaneous fat layer, represents the pre-mammary zone, located between the dermis and the anterior mammary fascia. It contains nerves, lymphatics, larger blood vessels, and fatty tissue. This layer is crossed by superficial extension of Cooper ligaments, known as the retinacula cutis, which anchor to the dermis. Cooper ligaments represent the structural support of the breast, they transverse the parenchyma, incompletely compartmentalizing it, and connect the anterior to the posterior fascia, which envelop the glandular parenchyma. The anterior fascia lies at the base of the hypodermis, while the posterior one on the pectoral muscle. It is to underline that terminal duct lobular units (TDLUs), located in superficial breast tissue, may extend into Cooper ligaments at the base of the hypodermis or be entrapped within them over time as the fibroglandular tissue atrophies (Fig. 2). Hence, breast lesions, that arise from TDLUs entrapped in the retinacula cutis, such as fibroadeonoma, papilloma or breast cancer, may be located within the subcutaneous fat and they should be considered in the differential diagnosis with hypodermic lesions. A subcutaneous localization does not allow to exclude with certainty a glandular disease, either benign or malignant. The US appearance of subcutaneous fat is an isoechogenic layer, consisting of oval fat lobules separated by echogenic septa, the thickness varies according to patient’s body habitus. Cooper’s ligaments are virtually always demonstrable at US [3].
Fig. 1.
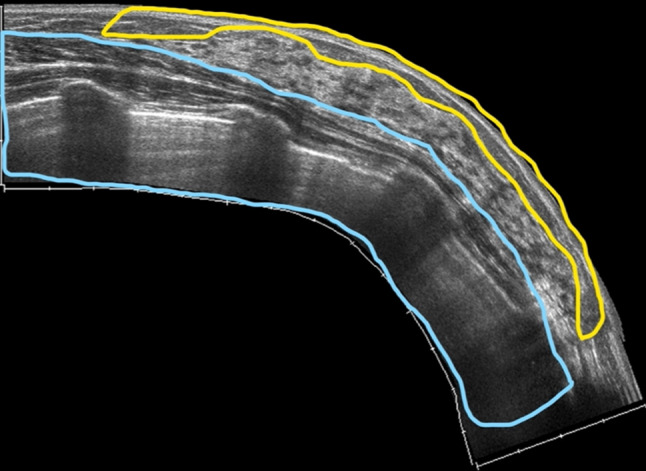
US appearance of the breast region: pre-mammary zone (yellow box), mammary zone (between yellow and light blue boxes), and retro-mammary zones (light blue box)
Fig. 2.
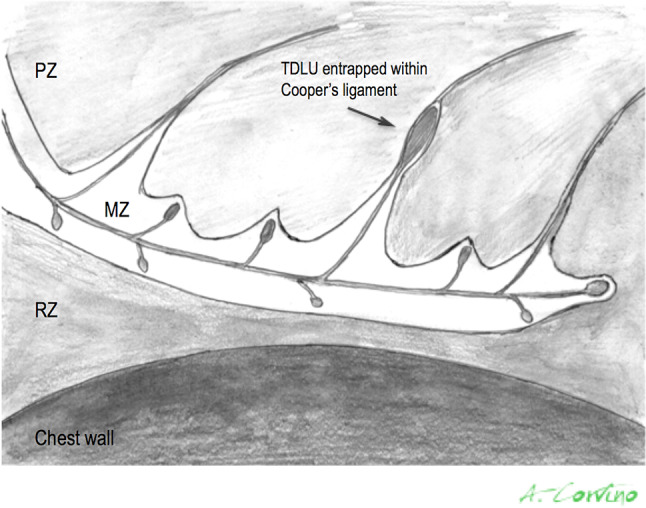
Schematic picture of the breast region: pre-mammary zone (PZ), mammary zone (MZ), and retro-mammary zones (RZ). The superficial extensions of Cooper ligaments, structural support of the breast, cross the hypodermis and they may entrap some TDLUs
A special mention is required for nipple-areolar complex: the skin is normally slightly thicker than 2 mm and pigmented. Numerous nerve endings, sebaceous and apocrine sweat glands are present in the nipple; the areola contains also a particular type of sebaceous glands, the Montgomery glands. The Montgomery ducts are specific of this area, they are modified lactiferous ducts which join sebaceous ducts within the areola and open at the Montgomery tubercles. This explains why malignant lesions of nipple-areolar complex may reach and/or arise in the dermal plane. The lactiferous ducts, that drain the several TDLUs, converge in five to ten lactiferous sinuses, which open at the nipple. Smooth muscle contributes to contractile function during lactation [1].
The mammary zone lies between the superficial pre-mammary and the deeper retro-mammary layers. It contains almost all of the mammary ducts and TDLUs as well as various amounts of stromal fat and fibrous tissue. This region is the most interested by glandular diseases, however, pathologic processes may extend out into either the pre-mammary or the retro-mammary zone, particularly malignant and infiltrating lesions. As mentioned-above, glandular parenchyma is enveloped by the mammary fascia [4].
The retro-mammary zone, composed mainly by fat, lies between the mammary zone and the pectoralis muscle as well as other chest wall structures. The involvement of this layer by pathological processes is usually secondary to those occurring within the mammary area and rarely by those originating from the structures of the chest wall.
US imaging findings of superficial abnormalities
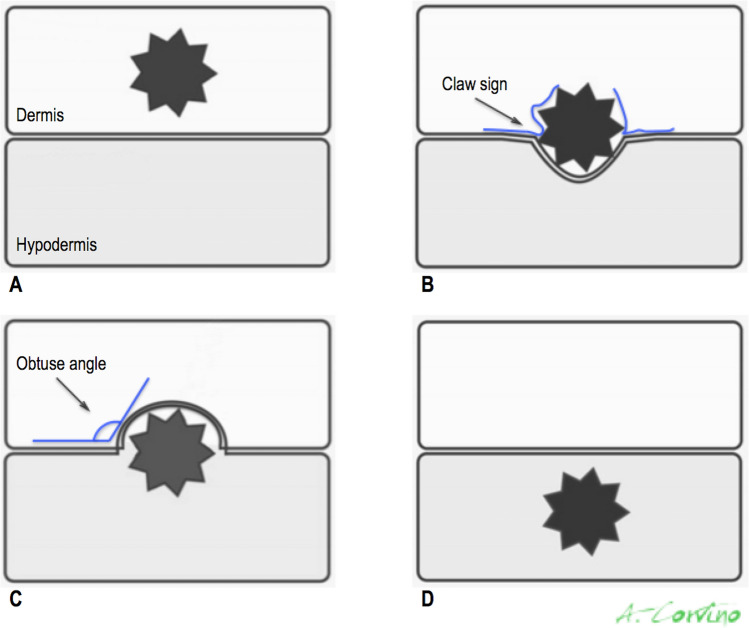
US is characterized by excellent spatial resolution, this makes it the optimal modality for localizing superficial palpable or non-palpable breast lesions, either glandular or non-glandular. The use of high-frequency transducers (L17-5) coupled with a step-off pad or a blob of acoustic gel is recommended [5, 6]. Lesions completed located within the echogenic dermal layer may be confidently identified as dermal, they are generally benign and, in any case, they cannot originate from breast glandular tissue. Lesions which lie within the hypoechoic hypodermal layer, but show a tract communicating to surface, may be considered of dermal origin [1]. The sonographer must be able to distinguish lesions completely contained in the dermis from those completely included in the hypodermis as well as from those that also involve either the hypodermis or the dermis to varying degrees, respectively (Fig. 3). In detail, regarding lesions that are located either partially or wholly within the hypodermal layer, the assessment of the angles made between the lesion margin and the dermis may be helpful to hypothesize the dermal or hypodermal origin. Lesions that are completely within the hypodermis form an obtuse angle with the dermis as they push upward, whilst lesions that are partially within the dermis and partially within the subcutaneous fat form an acute or 90° degrees angle with the dermis [1]. In addition, when a lesion is visualized in the hypodermic layer, several US findings may help to determine if the lesion derives in the dermis: a “claw” of dermal tissue enveloping the lesion margin as well as the presence of a tract from the lesion to the epidermal skin surface, representing extension of the hair follicle, these findings are indicative of a sebaceous or epidermal inclusion cyst [1]. In absence of these US criteria, a glandular origin of lesions contained in the hypodermic layer cannot be excluded with certainty. As stated above, TDLUs may extend into Cooper ligaments at the base of the hypodermis, so glandular lesions, both benign or malignant, may arise from them and be located in the hypodermic layer. For these reasons, glandular or hypodermic non-glandular masses must be considered in the differential diagnosis of lesions placed in the hypodermal. Biopsy may be requested in presence of suspicious imaging features.
Fig. 3.
Diagram illustrates how to determine whether a lesion is dermal (A and B) or hypodermal (C and D) on the basis of US location and findings. Claw sign is determined by a “claw” of hypoechoic skin invaginating around the margins and supports dermal origin (B). Hypodermal lesions form obtuse angles with dermal line (C)
Skin abnormalities
Skin tumors are by far the most common worldwide malignancy and they are classified according to cells of origin, which can be located in the epidermis, dermis or skin appendages [7]. Malignant tumors arising from the epidermis include cutaneous melanoma and non-melanoma cancers, such as basal cell carcinoma, squamous cell carcinoma, and Merkel cell carcinoma; whereas those arising from dermal components are atypical fibroxantoma, dermatofibrosarcoma protuberans, angiosarcoma, and Kaposi’s sarcoma; finally, those developing from skin appendages include sebaceous carcinoma, eccrine porocarcinoma and microcystic adnexal carcinoma. In the list of skin cancer primary and secondary cutaneous lymphoma as well as skin metastases must also be added. Cutaneous melanoma accounts 4% of all skin cancer and it is by far the most important because its lethality. Basal and squamous cell carcinoma represent up to 95% of all skin malignancies, the remaining skin tumors are uncommon [8]. The scanning methodology is crucial in the suspect of skin cancer: high frequency (> 15 MHz) transducer is recommended, beam focus should be placed at level of or immediately below the lesion, and gain should be regulated to maximize image contrast. The use of an abundant amount of gel or gel pad spacers are suggested, the hand pressure applied on the probe should be minimized [5].
Cutaneous melanoma occurs in pale-skinned Caucasians and clinically present as pigmented macules or nodules with irregular borders and ulcerations. At US it appears as an oval/fusiform, well-defined, homogeneous, hypervascular, and echo-poor lesion. The hyperechoic epidermis line is seen above the tumor, except for ulcerated melanoma [9].
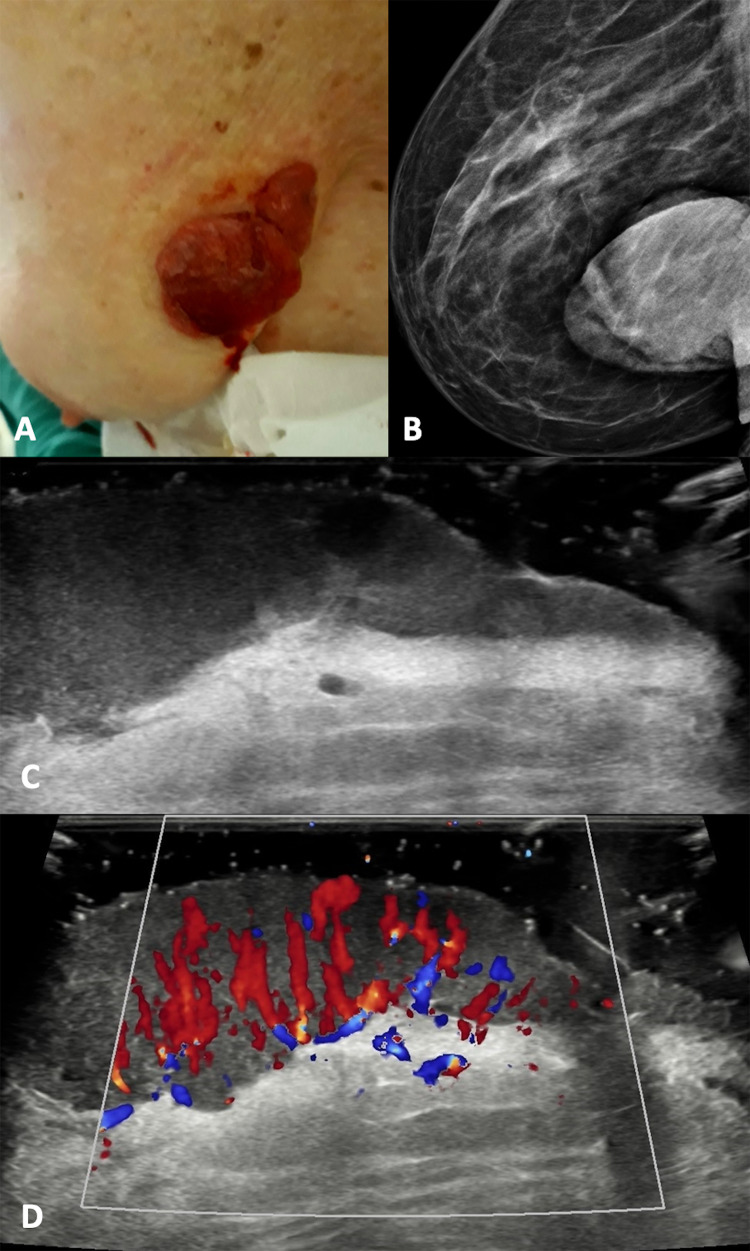
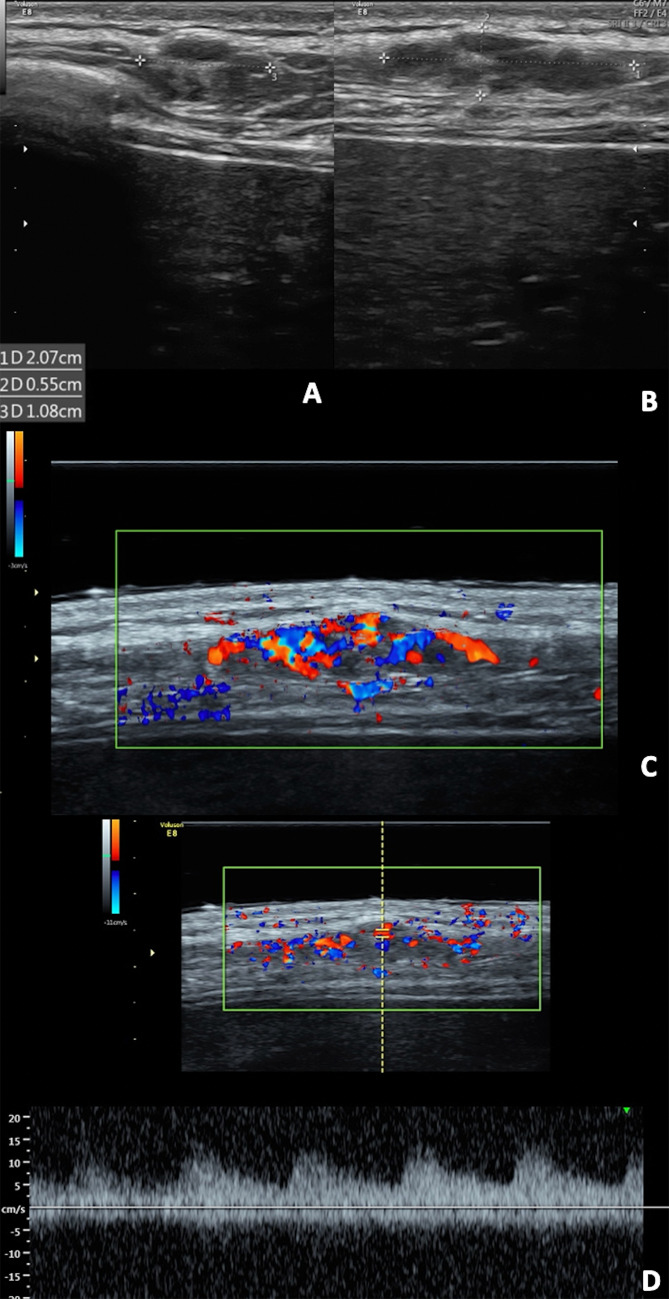
Regarding non-melanoma cancers, basal cell and squamous cell carcinomas usually occur in sun-exposed areas, but they may appear on the breast; the first are locally destructive, whereas the second usually metastasize to local lymph nodes [7]. Squamous cell carcinoma appears as red patches, plaques, hyperkeratotic or ulcerated lesions. The ultrasonographic appearance is a heterogeneously hypoechoic lesion with irregular borders, tending to involve the deeper layers. The low-flow vascular pattern is increased diffusely throughout the entire lesion, but particularly at the periphery (Fig. 4). Finally, Basal cell carcinoma presents as a pearlscent nodule or ulcer. On US, it appears as well-defined, oval or slightly irregular, hypoechoic or heterogeneous dermal structure, although it can also affect deep layers. This lesion usually presents hyperechoic spots, which may be a useful sign in the differential diagnosis with other types of skin cancers. They have a cotton flower-like appearance and seem not to show the posterior acoustic shadowing artifact, correlating with the presence of horn cysts, microcalcifications, or clusters of apoptotic cells in the center of tumor nests [10]. Low flow arterial and venous vessels are detected within or at the bottom of the lesion [11].
Fig. 4.
Clinical presentation, mammography (B), B-mode (C) and color Doppler (D) scans with a gel spacer. (A) Squamous cell carcinoma presents as red and raised nodule with some bleeding areas. (B) The mammography shows a large radiopaque nodule. (B, C) At US, it appears as an irregular skin thickness tending to involve the deeper layer with diffuse increased vascular signals.
Epidermoid cysts
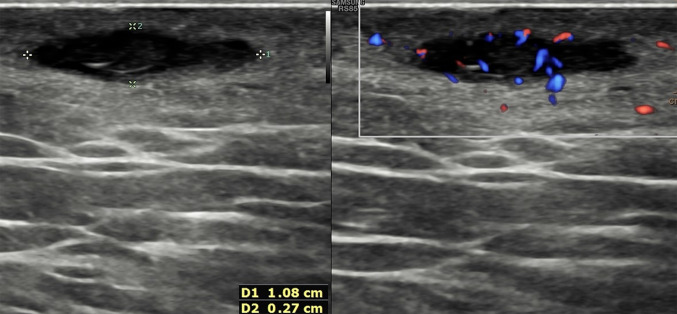
Epidermoid cysts, also known as epidermal inclusion cysts, are caused by the dermic or hypodermic implantation of epidermal components. They are thought to arise from the infundibulum (the uppermost portion) of the hair follicle and they have a wall lined by a true epidermis and contain keratinous material [12]. Epidermoid cysts may arise spontaneously or result from a prior trauma, such as breast core biopsy or reduction mammoplasty; in the latter, they may result from epidermal displacement during surgery, so they may occur in the dermis or ectopically [13]. Clinically, epidermal inclusion cysts appear as palpable skin nodules, ranging in size from a few millimeters to several centimeters, sometimes associated with redness or oily/creamy discharge from a very tiny visible opening on the skin, in these cases a subsequent decompression of the palpable mass occurs. Possible complications are represented by inflammation or rupture [14].
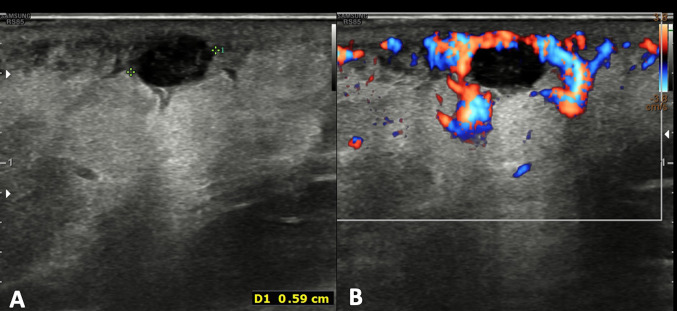
At US, the epidermal inclusion cysts appear as a well-circumscribed lesion with internal echotexture varying from anechoic to hypoechoic or heterogenous, depending on the internal contents (Fig. 5) [1]. Lee et al. correlated the US appearance of the internal contents with the pathologic analysis and noticed that a more complex or solid appearance was associated with variable amounts of internal keratinous debris and granulation tissue; in addition, they reported that epidermal inclusion cysts, which contain lamellated keratinous material, have a characteristic internal whorled or onion-ring appearance [15]. When the sloughed keratin forms a denser nodule within the cyst, it may have a pearl-like appearance, which may cause acoustic shadowing, even when not calcified [3]. Epidermal inclusion cysts do not show vascular signals at color Doppler, unless it is inflamed or ruptured. In these cases, the margin become indistinct, the surrounding dermal layer is thickened, edematous, and shows an increase of vascular signal at color Doppler (Fig. 6) [1]. Differential diagnosis with superficial malignancies may be difficult at imaging; for that, antibiotic therapy and imaging follow-up after treatment are recommended.
Fig. 5.
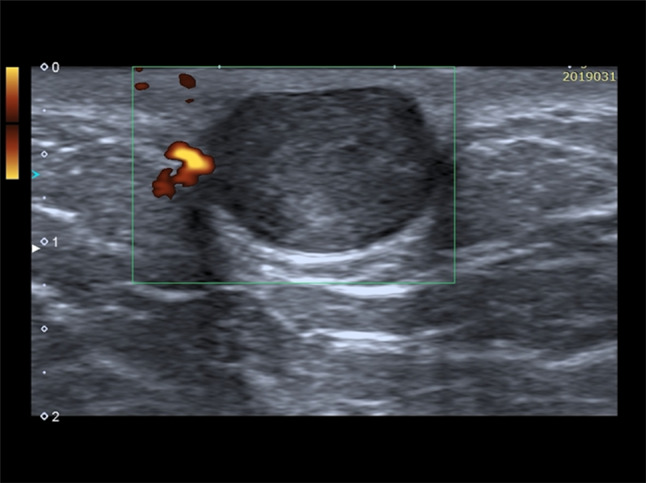
Power Doppler scan of an epidermoid cyst. Oval, well-defined epidermoid cyst with slightly hypoechoic echotexture compared to dermal layer. Power Doppler does not show intralesional vascular signal. The “claw” sign confirms the dermal origin of the lesion
Fig. 6.
B-mode (A) and color Doppler (B) scans of the breast region. Oval epidermoid cyst with slightly irregular margins and hypo-anechoic internal echotexture. The surrounding dermal layer is thickened and hyperechoic due to edema, with increased vascular signal at color Doppler assessment
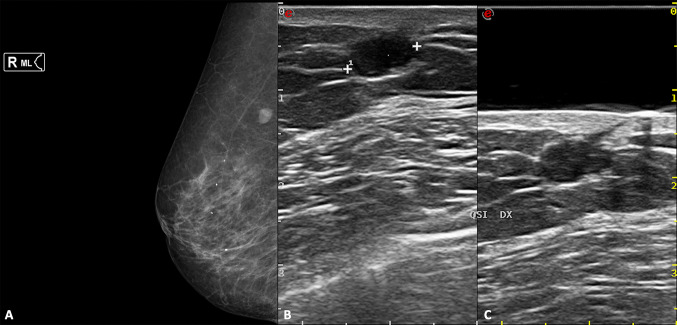
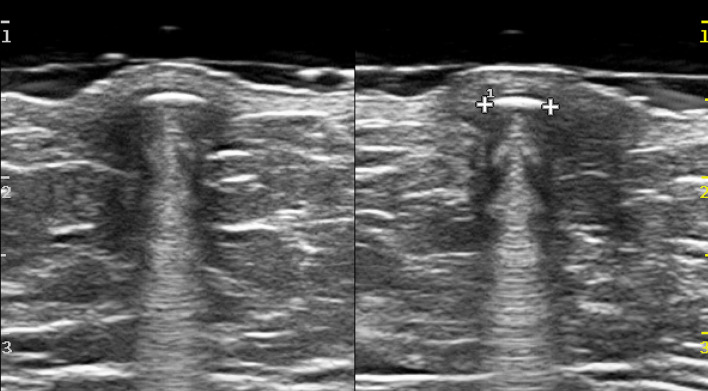
The certain diagnosis is possible when the "punctum" is identified: a thin hypoechoic tract that passes through the dermis and opens on the skin surface. The high-frequency probe and the use of a spacer may aid in its assessment (Fig. 7).
Fig. 7.
Right medio-lateral mammography (A), B-mode scans of the right breast without (B) and with (C) a gel stand-off pad. Oval, well-defined epidermoid cyst, which appears as an opacity at mammography, while a hypoechoic nodule at US, located in the hypodermal layer. The presence of the “punctum” (C) confirm the diagnosis of epidermoid cyst
Sebaceous cysts
They are less common than epidermal inclusion cysts and are not readily distinguishable from these on the basis of imaging or clinical features. Sebaceous cysts origin from the outer root sheath of the hair follicle. Steatocystomas are related to sebaceous cysts and are typically found in the derma of the axillae or sternum; they are characterized histologically by flattened sebaceous gland lobules in relation to the cyst wall and filled with an oil fluid and keratinous material. At US, they are usually located in the dermis, but may expand in the hypodermis, and they vary from cystic to more solid in appearance, depending on the internal contents [16].
Hidradenitis suppurative
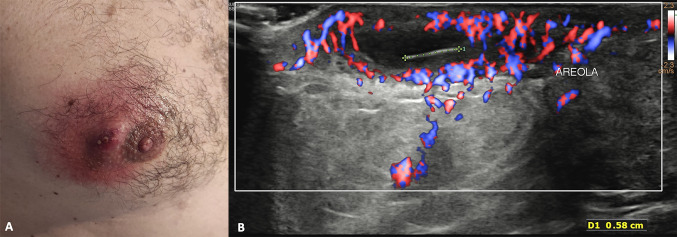
Hidradenitis suppurative (HS) is a chronic-recurrent inflammatory disease, probably with autoimmune etiology, which affects the intertriginous apocrine gland-bearing areas of the axillary, perineum, inframammary regions, groins, perineum, vulva, and scrotum [17]. HS typically develops in otherwise healthy post-puberal males and females, with a prevalence rate of up to 4% [18]. The disease process is thought to begin with occlusion of hair follicles, which subsequently rupture and re-epithelize, resulting in sinus tract that can house foreign material and bacteria. Over time, these tracts can coalesce into large regions of subcutaneous honeycombing; they can potentially dissect into deep structures including muscle, fascia, and lymph nodes. The diagnosis of HS is made clinically: patients often present tender subcutaneous nodules, which may spontaneously rupture or coalesce, forming painful, deep dermal abscesses without central necrosis or pointing. Even though this may sometimes occur, in these cases they exude a purulent, foul-smelling discharge. Eventually, the formation of extensive sinus tracts may result. The inflammatory abscesses ultimately heal, producing fibrosis, dermal contractures, and induration of the skin [19].
In this setting, US has a complementary role in the diagnosis, balance of severity, therapeutic planning and response evaluation to medical therapy. The US appearance of HS is characterized by dermal fluid collection of different sizes, numbers, and locations with echogenic internal debris and fragments of hair, which appear as thin echogenic striae (Fig. 8). In detail, an increase of skin dermal thickness is seen (3.3 ± 1.0 mm vs 1.4 ± 0.3) accompanied by an apparent decrease of echogenicity in the affected regions [20]. The echo lucent areas reflect edema and inflamed tissue, these changes affect not only the skin, but also the subcutaneous tissue, reflecting the clinically invasive behavior of the disease. Multiple fistulas of the dermis and/or hypodermis may be seen, they may be linear or branched, blinded or with multiple skin orifices. Furthermore, hypo-anechoic pseudocystic lesions of the dermis and multiple reactive lymphadenopathies may also be present. Peripheral hyperemia is described at color Doppler assessment. The hair follicles appear enlarged in nonaffected skin [20]. Furthermore, the US exam reveals subclinical lesions also in clinically unaffected regions, this is fundamental to correctly assess the true extent of the disease in the pre-operative planning [21]. Currently, surgery offers the best chances of cure for patients suffering from extensive and scarring disease; it is of the utmost importance that excision of HS lesions is sufficiently large.
Fig. 8.
Split screen US image showing B-mode scan of the axillary on the left and corrisponding color Doppler one on the right. A dermal fluid collection with echogenic fragments of hair is typical of hidradenitis suppurative. The surrounding tissues show an increased echogenicity and vascular signals at color Doppler due to inflammation and invasive behavior of the disease
Pilomatrixoma
The pilomatrixoma, also called calcifying epithelioma of Malherbe, is benign, deep dermal or subcutaneous tumor, which arises from hair follicle. A traumatic origin is hypothesized. It slowly enlarges and develops usually during the first 2 decades of lives with a female predominance [22]. They are commonly asymptomatic but are occasionally tender. Some may be inflamed, presenting as a granulomatous swelling, with occasional extrusion of calcium as a thick, white, gritty discharge. Surgical excision is usually recommended, as a foreign body reaction due to calcification of the lesion may occur and cause a vigorous inflammatory response with risk of scarring. In this setting, US supports or excludes the clinical diagnosis of pilomatrixoma and contribute to appropriate preoperative assessment. The US exam begins with a preliminary gray scale evaluation with sufficient coupling gel or a standoff to define the skin and subcutaneous tissue and is completed with color Doppler evaluation. Pilomatrixomas usually range in size from 0.5 to 3.0 cm, they appear as an ovoid complex mass, parallel to the skin, located at the junction of the dermis and hypodermis with focal thinning of the overlying dermis. It consistently presents as a target lesion with a hypoechoic rim and an echogenic center, which correspond to the connective tissue capsule and the central islands of epithelial cells, respectively [23]. The calcifications may occur and show a posterior shadow [23]. The color Doppler evaluation shows a hyper-vascular lesion (Fig. 9).
Fig. 9.
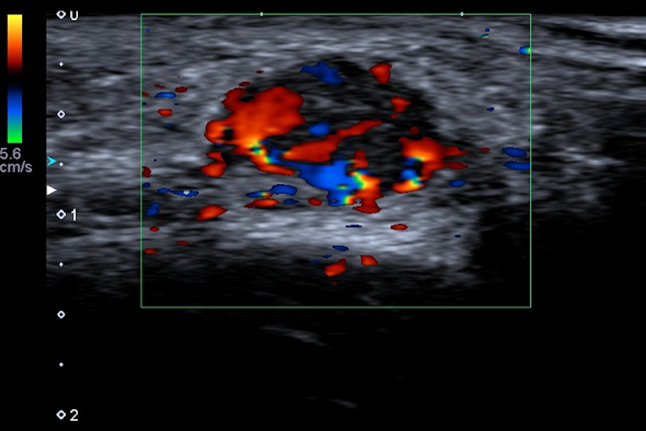
Color Doppler scan of axillary in a child. Pilomatrixoma appears as an ovoid, hyper-vascular lesion, parallel to the skin and located at the dermis-hypodermis junction; the internal echotexture is heterogeneous, predominantly hypoechoic
Hematoma
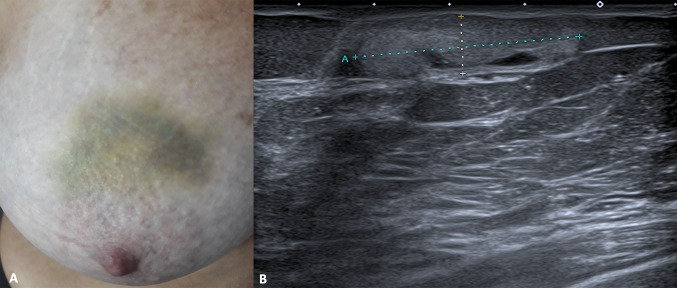
Breast hematoma is a localized blood collection, may result from preceding trauma, surgery, biopsy or contusion and it may be easily misinterpreted as breast malignancy, if the correct clinical context is not taken into account. The history of trauma, sometimes the presence of skin hematoma and regression within weeks are the clues to diagnosis [24]. They rarely occur spontaneously, especially in patients with coagulopathy or on anticoagulant therapy. Hematomas may be hyperacute to chronic in duration, therefore their internal contents vary from fresh hemorrhage to chronic hemoglobin degradation products, often there is a mixture of different aged components. These changes over time explain the various US appearances, varying from heterogeneous ill-defined area to hyperechoic or hypoechoic organizing collection (Fig. 10). The inflammatory reaction in the interested area may develop into fat necrosis, which progresses over 1–2 months in lipid cysts, then fibrotic changes occur, which may become calcified over 3–4 years. Fat necrosis usually appear as solid area but may have complex or cystic echotexture. They may be well- or ill-defined and may have posterior acoustic shadowing or enhancement [25].
Fig. 10.
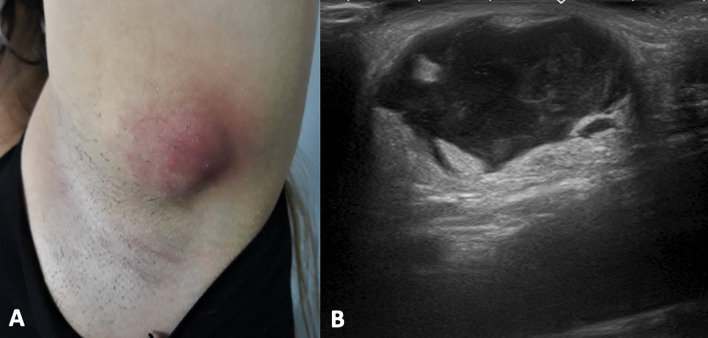
Clinical presentation (A) and B-mode scan (B) of breast skin hematoma. The US appearance is represented by hyperechoic collection located in the hypodermis with some anechoic areas
Hematomas are usually sterile when first formed and the majority remain uninfected. However, superinfection is seen forming a hemorrhagic abscess and is one of the well-recognized complications, which may require drainage.
Foreign bodies
Foreign bodies may be retained in the breast soft tissue, such as piercing fragments or ingrown hair, and it is mandatory to locate them before infected. All foreign bodies appear hyperechoic and they may or may not manifest with posterior acoustic shadowing and/or reverberation artifact, depending on their composition and the angle of insonation (Figs. 11, 12) [26]. The probe should be as parallel as possible to the foreign body to maximize reflection and consequent detectability. The inflammatory reaction of the surrounding tissues can create a hypoechoic halo, which represents edema, abscess, fibrous tissue, or granulation tissue and increase the conspicuity of the lesion [27]. Hypervascularity around the foreign body can be depicted at power Doppler beginning 24 h after the object enters the soft tissue [28]. A disadvantage of US is false-positive results, which may occur because of calcifications, fresh hematomas, or air trapped in the soft tissues, which may cause acoustic shadowing large enough to obscure deeper foreign bodies [26].
Fig. 11.
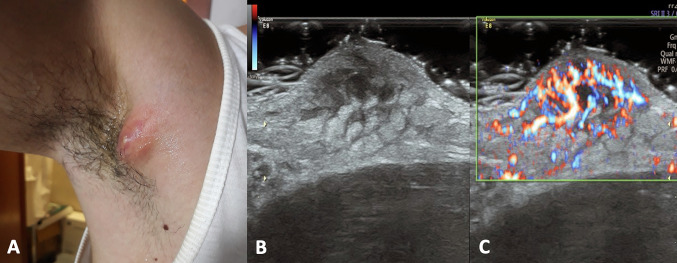
Clinical presentation (A) and color Doppler scan (B) of an abscess due to ingrown hair. Physical examination shows a red bump and the skin around feel painful and warm to touch. US shows an anechoic fluid collection located in the hypodermis with a thin hyperechoic stria inside (hair ingrown). The surrounding tissues are thickened and inflamed with increased vascular signal at color Doppler
Fig. 12.
B-mode scans of the breast. A piercing fragment retained in the subcutaneous tissues appears hyperechoic with reverberation artifact and postero-lateral acoustic shadowings
Subcutaneous abscess
The subcutaneous abscess is a manifestation of a spectrum of soft tissue skin infection, developing within the dermis and/or hypodermis. Patients typically present with an acute or subacute history of a focal swelling or lump with accompanying signs of cellulitis. If there is bacteremia, systemic signs of sepsis may be present, such as fever and raised inflammatory markers. They are overwhelmingly caused by bacteria, which spread into the subcutaneous tissues through breaches in the epidermis, more frequently during lactation. The US findings of subcutaneous abscess are various: anechoic or hypoechoic spherical fluid collections, well-defined by echogenic capsule or with indistinct margins. The internal echotexture may be heterogenous because of the presence of septae, sediment, or gas. Cobblestone appearance of the surrounding subcutaneous tissues is due to edema of associated cellulitis (Figs. 13, 14) [29].
Fig. 13.
Clinical presentation (A), B-mode (B) and color Doppler scans (C) of right axillary. Physical examination shows a slight red, painful bump. At US, the subcutaneous abscess appears as an anechoic area with indistinct margins without vascular signal at color Doppler; the surrounding tissues are very thickened, heterogenous, slightly hyperechoic due to edema (cobblestone appearance) and hypervascularized
Fig. 14.
Clinical presentation (A) and B-mode scan (B) of left axillary. Physical examination shows a red, painful bump, the surrounding area is swelled and warm to touch. Subcutaneous abscess presents as a fluid collection with irregular margins and heterogeneous internal echotexture because of the presence of debris. The surrounding tissue are slightly hyperechoic due to inflammation
Mondor's disease
Mondor’s disease is a rare benign condition, a superficial thrombophlebitis of a subcutaneous vein of the anterior chest or abdomen wall. Risk factors include female gender, age between 30 and 50, recent thoracic trauma or surgery, intense physical activity, local infections, and intravenous drug consumption. Diagnosis is first based on clinical presentation and physical examination, US usually confirms it and excludes other conditions, like cancer. The most common clinical features include lateral chest wall tension and pain as well as the presence of a tender subcutaneous linear or winding cord-like structure, corresponding to the affected vessel, often with skin redness, edema or retraction (Fig. 15) [25]. No homolateral adenopathies are found. US shows a hypoechoic subcutaneous and uncompressible linear or “rosary-like” structure with or without echogenic thickening of the surrounding fat. No flow with color Doppler is visible inside the lesion [30]. This finding should not be confused with a dilated duct, which always has a radial orientation towards the areola. Mondor’s disease resolves spontaneously within a period from 1 to 6 months.
Fig. 15.
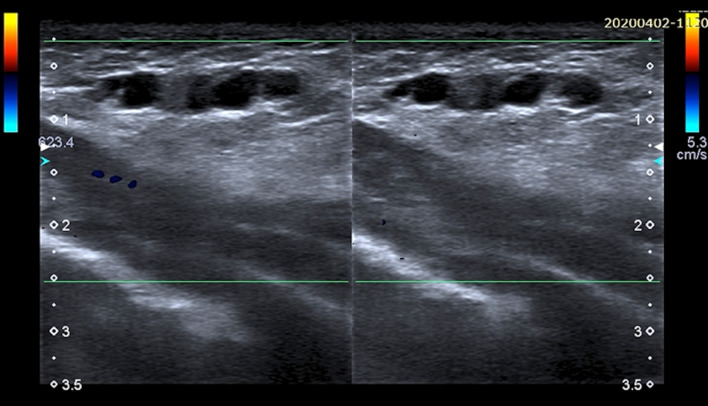
Color Doppler scans of the breast. US appearance of Mondor’s disease is characterized by hypoechoic subcutaneous and uncompressible “rosary-like” structure with some internal hyperechoic area due to blood clots. No vascular flow is visible with Color Doppler
Other common hypodermal lesions
The hypodermis is composed mainly of fat, but also nerves, vascular and lymphatic vessels are present. Furthermore, it is crossed by superficial extension of Cooper ligaments, the retinacula cutis, which may contain TDLUs. Hence, glandular lesions, both benign or malignant, may develop in this layer and should be considered in the differential diagnosis with lesions arising from other hypodermal tissues.
Regarding lesions developing from the TDLUs, adenosis, fibroadenomas, peripheral papillomas, and superficial breast cancers should be considered, whereas, regarding fat-containing lesions, fat necrosis and lipoma should be mentioned. In detail, fat necrosis may have a mixed echotexture, whilst lipoma, a typical subcutaneous lesion, appears as a well-defined, solid-appearing nodule with a variable echotexture, hyper-, iso-, or slightly hypoechoic relative to adjacent fat [1]. Sometimes lipoma may be occult within the rest of the fatty tissue [31].
Regarding vascular lesions, soft tissue hemangiomas should be mentioned; they are subdivided into five categories depending on the predominant type of vascular channel: capillary, cavernous, arteriovenous, venous, and mixed variations. Clinically, they usually appear with skin bruising. The US appearance is a lobulated, well-circumscribed, solid mass, which are predominantly hypoechoic and may contain calcifications [32]. They are characterized by dilated tortuous thin-walled vessels and Doppler evaluation may show hypervascularity and low-resistance arterial flow (Figs. 16, 17). If phleboliths are abundant, acoustic shadowing may also document [33]. Angiolipomas, rare lesions in the breast, present as well-circumscribed hyperechoic masses without posterior acoustic enhancement at US [32].
Fig. 16.
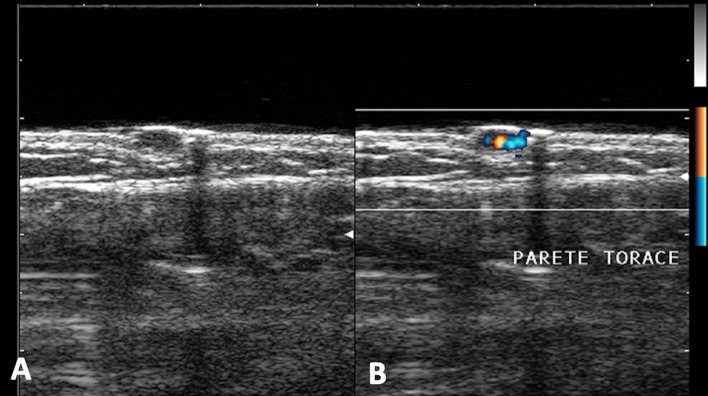
B-mode (A) and color Doppler (B) of the pectoral region in a child. Well-defined, hypoechoic cutaneous angioma with rich vascularization at color Doppler
Fig. 17.
B-mode (A and B), color Doppler (C) and spectral Doppler (D) scans. Lobulated, well-circumscribed and predominantly hypoechoic hemangioma with rich intralesional vascularization on color Doppler and low resistance arterial flow on spectral Doppler
Regarding lesions arising from lymphatic vessels, lymphangiomas are congenital disorders of the lymphatic channels, which they are localized, they appear as a well-demarcated cysts mass with lobulations and septae of variable thickness. The fluid inside can appear completely anechoic, hypoechoic, or hyperechoic, because of infections, hemorrhages, or a high lipid content [3].
Finally, granular cell tumor, a rare usually benign lesion, is thought to arise from Schwann cells of peripheral nerves; about 5% of them occur within the breast, in glandular parenchyma, hypodermis, or dermis. The lesion usually manifests as a painless, firm, usually mobile lump in the upper aspect of the breast. When they are located close to the dermis, skin thickening or retraction may be associated. It may appear at US as a well-defined nodule with posterior acoustic enhancement, or as a solid mass with indistinct or frankly spiculated margins and marked posterior acoustic shadowing [34].
Conclusions
Superficial non-glandular lesions may present as breast lump and be the reason of clinician's US request or they may be an incidental finding during diagnostic or screening breast US. In both circumstances, careful evaluation of imaging features and a thorough knowledge of skin anatomy play a crucial role in the assessment of these superficial non-glandular lesions. The sonographer must be able to adequately identify and characterize them. With a high-frequency linear transducer, US provides higher spatial resolution than mammography and MRI for lesions predominantly visualizing in the superficial layers, it may aid in their identification, localization and characterization. Color-Doppler and spectral tracings can provide information regarding vascular flow, which is particularly important in the evaluation of vascular malformations and hemangiomas.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Declarations
Conflict of interest
We confirm that this work is original and has not been published elsewhere nor is it currently under consideration for publication elsewhere. Publication is approved by all authors and by the responsible authorities where the work was carried out. Each author have participated sufficiently in any submission to take public responsibility for its content. The authors have no conflicts of interest. Written informed consent was obtained from all patients, and the study was approved by the ethics committee of the institution.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Martina Caruso, Email: carumart@yahoo.it.
Orlando Catalano, Email: orlcat@tin.it.
Robert Bard, Email: rbar@cancer.com.
Carlo Varelli, Email: varcar@tin.it.
Fabio Corvino, Email: effecorvino@gmail.com.
Corrado Caiazzo, Email: corraca@yahoo.it.
Antonio Corvino, Email: an.cor@hotmail.it.
References
- 1.Giess CS, Raza S, Birdwell RL. Distinguishing breast skin lesions from superficial breast parenchymal lesions: Diagnostic criteria, imaging characteristics, and pitfalls. Radiographics. 2011;31:1959–1972. doi: 10.1148/rg.317115116. [DOI] [PubMed] [Google Scholar]
- 2.Draghi F, Cocco G, Richelmi FM, Schiavone C. Abdominal wall sonography: a pictorial review. J Ultrasound. 2020;23:265–278. doi: 10.1007/s40477-020-00435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji HY, Kim EK, Min JK, Ki KO. Imaging findings of chest wall lesions on breast sonography. J Ultrasound Med. 2008;27:125–138. doi: 10.7863/jum.2008.27.1.125. [DOI] [PubMed] [Google Scholar]
- 4.Pandya S, Moore RG. Breast development and anatomy. Clin Obstet Gynecol. 2011;54:91–95. doi: 10.1097/GRF.0b013e318207ffe9. [DOI] [PubMed] [Google Scholar]
- 5.Corvino A, Sandomenico F, Corvino F, et al. Utility of a gel stand-off pad in the detection of Doppler signal on focal nodular lesions of the skin. J Ultrasound. 2020;23:45–53. doi: 10.1007/s40477-019-00376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cocco G, Delli Pizzi A, Scioscia M, et al. Ultrasound imaging of abdominal wall endometriosis: a pictorial review. Diagnostics (Basel, Switzerland) 2021 doi: 10.3390/diagnostics11040609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon R. Skin cancer: an overview of epidemiology and risk factors. Semin Oncol Nurs. 2013;29:160–169. doi: 10.1016/j.soncn.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:237–247. doi: 10.1016/j.jaad.2017.08.059. [DOI] [PubMed] [Google Scholar]
- 9.Catalano O, Caracò C, Mozzillo N, Siani A. Locoregional spread of cutaneous melanoma: Sonography findings. Am J Roentgenol. 2010;194:735–745. doi: 10.2214/AJR.09.2422. [DOI] [PubMed] [Google Scholar]
- 10.Bobadilla F, Wortsman X, Muñoz C, et al. Pre-surgical high resolution ultrasound of facial basal cell carcinoma: correlation with histology. Cancer Imaging. 2008;8:163–172. doi: 10.1102/1470-7330.2008.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wortsman X. Sonography of facial cutaneous basal cell carcinoma. J Ultrasound Med. 2013;32:567–572. doi: 10.7863/jum.2013.32.4.567. [DOI] [PubMed] [Google Scholar]
- 12.Davies JD, Nonni A, D'Costa HF. Mammary epidermoid inclusion cysts after wide-core needle biopsies. Histopathology. 1997;31:549–551. doi: 10.1046/j.1365-2559.1997.3290905.x. [DOI] [PubMed] [Google Scholar]
- 13.Herreros-Villaraviz M, Mallo-Alonso R, Santiago-Freijanes P, Díaz-Veiga MJ. Epidermal inclusion cysts of the breast. Breast J. 2008;14:599–600. doi: 10.1111/j.1524-4741.2008.00659.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim HS, Cha ES, Kim HH, Yoo JY. Spectrum of sonographic findings in superficial breast masses. J Ultrasound Med. 2005;24:663–680. doi: 10.7863/jum.2005.24.5.663. [DOI] [PubMed] [Google Scholar]
- 15.Lee HS, Bin JK, Song HT, et al. Relationship between sonographic and pathologic findings in epidermal inclusion cysts. J Clin Ultrasound. 2001;29:374–383. doi: 10.1002/jcu.1052. [DOI] [PubMed] [Google Scholar]
- 16.Park KY, Oh KK, Noh TW. Steatocystoma multiplex: mammographic and sonographic manifestations. Am J Roentgenol. 2003;180:271–274. doi: 10.2214/ajr.180.1.1800271. [DOI] [PubMed] [Google Scholar]
- 17.Slade DE, Powell B, Mortimer P. Hidradenitis suppurativa: pathogenesis and management. Br J Plast Surg. 2003;56:451–461. doi: 10.1016/S0007-1226(03)00177-2. [DOI] [PubMed] [Google Scholar]
- 18.Wiseman MC. Hidradenitis suppurativa: a review. Dermatol Ther. 2004;17:50–54. doi: 10.1111/j.1396-0296.2004.04007.x. [DOI] [PubMed] [Google Scholar]
- 19.Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539–561. doi: 10.1016/j.jaad.2008.11.911. [DOI] [PubMed] [Google Scholar]
- 20.Wortsman X, Jemec GBE. Real-time compound imaging ultrasound of hidradenitis suppurativa. Dermatol Surg. 2007;33:1340–1342. doi: 10.1111/j.1524-4725.2007.33286.x. [DOI] [PubMed] [Google Scholar]
- 21.Jemec GB, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol. 1996;34:994–999. doi: 10.1016/S0190-9622(96)90277-7. [DOI] [PubMed] [Google Scholar]
- 22.Marino MA, Ascenti G, Cardia R, et al. Pilomatrixoma of the right thigh: Sonographic-pathologic correlation in a young man. Radiol Case Rep. 2020;15:230–233. doi: 10.1016/j.radcr.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes J, Lam A, Rogers M. Use of ultrasonography in the diagnosis of childhood pilomatrixoma. Pediatr Dermatol. 1999;16:341–344. doi: 10.1046/j.1525-1470.1999.00090.x. [DOI] [PubMed] [Google Scholar]
- 24.Cocco G, Ricci V, Boccatonda A, et al. Sonographic demonstration of a spontaneous rectus sheath hematoma following a sneeze: a case report and review of the literature. J Ultrasound. 2021;24:125–130. doi: 10.1007/s40477-020-00493-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gatta G, Pinto A, Romano S, et al. Clinical, mammographic and ultrasonographic features of blunt breast trauma. Eur J Radiol. 2006;59:327–330. doi: 10.1016/j.ejrad.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Carneiro BC, Cruz IAN, Chemin RN, et al. Multimodality imaging of foreign bodies: new insights into old challenges. Radiographics. 2020;40:1965–1986. doi: 10.1148/rg.2020200061. [DOI] [PubMed] [Google Scholar]
- 27.Bradley M. Image-guided soft-tissue foreign body extraction. Success and pitfalls. Clin Radiol. 2012;67:531–534. doi: 10.1016/j.crad.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 28.Davae KC, Sofka CM, DiCarlo E, Adler RS. Value of power doppler imaging and the hypoechoic halo in the sonographic detection of foreign bodies. J Ultrasound Med. 2003;22:1309–1313. doi: 10.7863/jum.2003.22.12.1309. [DOI] [PubMed] [Google Scholar]
- 29.Tayal VS, Hasan N, Norton HJ, Tomaszewski CA. The effect of soft-tissue ultrasound on the management of cellulitis in the emergency department. Acad Emerg Med. 2006;13:384–388. doi: 10.1197/j.aem.2005.11.074. [DOI] [PubMed] [Google Scholar]
- 30.Amano M, Shimizu T. Mondor’s disease: a review of the literature. Intern Med. 2018;57:2607–2612. doi: 10.2169/internalmedicine.0495-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pui MH, Movson IJ. Fatty tissue breast lesions. Clin Imaging. 2003;27:150–155. doi: 10.1016/S0899-7071(02)00536-3. [DOI] [PubMed] [Google Scholar]
- 32.Glazebrook KN, Morton MJ, Reynolds C. Vascular tumors of the breast: mammographic, sonographic, and MRI appearances. Am J Roentgenol. 2005;184:331–338. doi: 10.2214/ajr.184.1.01840331. [DOI] [PubMed] [Google Scholar]
- 33.Olsen KI, Stacy GS, Montag A. Soft-tissue cavernous hemangioma. Radiographics. 2004;24:849–854. doi: 10.1148/rg.243035165. [DOI] [PubMed] [Google Scholar]
- 34.Feder JM, de Paredes ES, Hogge JP, Wilken JJ. Unusual breast lesions: radiologic-pathologic correlation. Radiographics. 1999;19:S11–S26. doi: 10.1148/radiographics.19.suppl_1.g99oc07s11. [DOI] [PubMed] [Google Scholar]